Abstract
Classical collectins (surfactant protein A and D) play a significant role in innate immunity and host defence in uropathogenic Escherichia coli (UPEC)-induced urinary tract infection (UTI). However, the functions of collectin-11 (CL-11) with respect to UPEC and UTI remain largely unexplored. This study aimed to investigate the effect of CL-11 on UPEC and its role in UTI. We further examined its modulatory effect on inflammatory reactions in proximal tubular epithelial cells (PTECs). The present study provides evidence for the effect of CL-11 on the growth, agglutination, binding, epithelial adhesion and invasion of UPEC. We found increased basal levels of phosphorylated p38 MAPK and human cytokine homologue (keratinocyte-derived chemokine) expression in CL-11 knockdown PTECs. Furthermore, signal regulatory protein α blockade reversed the increased basal levels of inflammation associated with CL-11 knockdown in PTECs. Additionally, CL-11 knockdown effectively inhibited UPEC-induced p38 MAPK phosphorylation and cytokine production in PTECs. These were further inhibited by CD91 blockade. We conclude that CL-11 functions as a mediator of innate immunity via direct antibacterial roles as well as dual modulatory roles in UPEC-induced inflammatory responses during UTI. Thus, the study findings suggest a possible function for CL-11 in defence against UTI.
Keywords: Collectin-11, innate immunity, urinary tract infection, uropathogenic Escherichia coli
Introduction
Urinary tract infection (UTI) is one of the most common bacterial infections, affecting 150 million people each year worldwide.1 Nearly half of the total number of women develop a UTI in their lifetime, and up to 50% of these suffer from recurrent infection within six months of the initial infection.2 Uropathogenic Escherichia coli (UPEC) is the most common pathogen causing UTIs.1 Recent studies have highlighted the importance of innate immunity in UTI.3 The innate immune system, consisting of PRRs and their downstream effectors, helps protect against bacteria in the urinary tract.1 PRRs can be classified into two major families: TLRs and C-type lectins.1
Collectins are a subtype of the C-type lectin family that have a collagen-like domain (CLD) and a calcium-dependent carbohydrate-recognition domain (CRD).4 Our previous studies and other reports found that classical collectins, surfactant protein A (SP-A) and surfactant protein D (SP-D) play a significant role in innate immunity and host defence in UTIs.5–10 Both SP-A and SP-D can directly bind to UPEC and inhibit its growth.5–7 Additionally, SP-D can agglutinate UPEC.5 Previous studies, including our own, showed that SP-A and SP-D function as PRRs, interact with a variety of receptors and possess both pro- and anti-inflammatory signalling functions.7,11–13 Under healthy conditions, SP-A and SP-D bind to signal regulatory protein α (SIRPα) at its CRD domain, which inhibits excessive activation of p38 MAPK, thus showing anti-inflammatory properties.11 However, in the presence of microbes (such as UPEC), the CRD domain of SP-A and SP-D interacts with the microbial surface,14 and the CLD domain binds to calreticulin/CD91 of the epithelial cells, thereby stimulating p38 MAPK phosphorylation and exerting pro-inflammatory effects that help in bacterial clearance.7,11,12
Collectin-11 (CL-11), also known as collectin kidney 1 (CL-K1), is a novel collectin identified by Keshi et al. in 2006.15 CL-11 exhibits calcium-dependent sugar binding activity to l-fucose and d-Man, which can be inhibited by EDTA.15,16 CL-11 recognises and binds to a broad range of bacteria, including E. coli O:126 and E. coli O:60 (smooth E. coli strains with known LPS structures that contain l-fucose and d-Man in their O-Ags), rough E. coli HB:101 and Pseudomonas aeruginosa owing to its specific sugar-binding character,16,17 and possesses antibacterial activity.18 Further studies have reported that calreticulin/CD91 and SIRPα are involved in CL-11-mediated opsonophagocytosis and cytokine production in epithelial cells.19 However, the functions of CL-11 with respect to UPEC and UTI remain unclear.
This study aimed to investigate the possible modulatory effect of CL-11 on UPEC and its role in UTI. Thus, we examined whether CL-11 directly interacts with UPEC. We further examined its modulatory effect on inflammatory reactions in proximal tubular epithelial cells (PTECs).
Methods
Reagents and buffers
Recombinant mouse CL-11 protein, anti-SIRPα and anti-CD91 Abs were purchased from R&D Systems (Minneapolis, MN). Anti-CL-11 Ab was purchased from Invitrogen (Carlsbad, CA). Anti-p38 MAPK, anti-phosphorylated p38 MAPK and anti-GAPDH Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). UPEC (strain CFT 073, a smooth strain with the O6:H1 serotype of LPS) isolated from a human pyelonephritis patient, was purchased from the American Type Culture Collection (ATCC, Manassas, VA). CL-11 small interfering RNA (siRNA) and negative control siRNA were purchased from Qiagen (Hilden, Germany). The following buffers were used: TBS (10 mM Tris and 145 mM NaCl), TBS/Ca (TBS with 2 mM CaCl2, pH 7.4), TBST (TBS with 0.1% Tween-20) and TBS/EDTA (TBS with 2 mM EDTA, pH 7.4).
Animals
The C57BL/6 mice were bred and maintained in the animal facility at Hubei University of Arts and Science. Mice were housed in a temperature-controlled room at 22°C under specific pathogen-free conditions. In this study, we used 8- to 10-wk-old female mice. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Hubei University of Arts and Science (approval number #2018-071). Procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication no. 8023, revised 1978). Murine urine and kidney samples were collected as previously described.7
Immunohistochemistry
Immunohistochemistry (IHC) was performed on paraffin-embedded normal murine kidney sections as per our previously published protocol.20 In brief, sections were incubated with Ab against mouse CL-11 overnight at 4°C, followed by incubation with biotinylated secondary Ab and avidin–biotin peroxidase complex. Peroxidase activity was visualised by diaminobenzidine (DAB). As a negative control, the samples were incubated with non-immune serum instead of the primary Ab.
Primary PTEC isolation, culture and treatment
Primary PTECs from mice were isolated using our previously described methods.21 In brief, the renal cortex was dissected and digested with type I collagenase and subsequently passed through a 70 μm nylon sieve. The pellet was overlaid on an OptiPrepTM density gradient solution (Sigma–Aldrich, St Louis, MO) and centrifuged. Purified PTECs were collected and cultured in DMEM/F12 medium supplemented with 5% FBS, non-essential amino acids and insulin-transferrin-selenium (Sigma–Aldrich). For the identification of PTECs, the cells were placed on glass coverslips after fixing with 4% paraformaldehyde, following which they were double stained with anti-megalin Ab (Santa Cruz Biotechnology) to confirm the species of the cultured cells and to determine the purity of PTECs. For the fluorescence visualisation of primary Abs, slides were stained with FITC-conjugated IgG for 1 h at room temperature. The nuclei were counterstained with DAPI (Antgene, Wuhan, PR China) for 5 min. For experiments, confluent layers of PTECs (control; CL-11 knockdown and pretreated with 2 µg/ml anti-SIRPα Ab, 2 µg/ml anti-CD91 Ab or control IgG for 30 min) were infected with CFT 073 for 3 h. To assess if the effects of CL-11 knockdown could be rescued with the addition of CL-11 protein to the assay medium, CL-11-knockdown PTECs via siRNA were incubated with CL-11 (10 µg/ml) for 1 h, then infected with or without CFT 073. Supernatant and protein were collected and stored at –80°C until further analysis.
Western blot analysis
PTECs, murine renal tissue and murine urine samples were lysed with RIPA buffer containing a cocktail of protein inhibitors and phosphatase inhibitors (Roche, Mannheim, Germany). The samples were centrifuged, and the supernatant was recovered for Western blot analysis. In brief, protein samples were subjected to electrophoresis using a Tris-glycine gel and transferred to polyvinylidene fluoride membranes (Thermo Fisher Scientific, Waltham, MA). Membranes were probed with primary Abs (Abs against mouse CL-11, p38 MAPK, phosphorylated p38 MAPK or GAPDH), followed by secondary IRDye 800CW labelled Abs (LI-COR Biosciences, Lincoln, NE). Membranes were scanned using an Odyssey CLx infrared imaging system (LI-COR Biosciences), and Quantity One analysis software (Bio-Rad, Hercules, CA) was used to quantify the band intensity of the blotted proteins.
Preparation of bacteria
CFT 073 cells were cultured in lysogeny broth (LB) at 37°C. Bacteria were harvested by centrifugation at 2000 g for 10 min at 4°C and re-suspended in PBS. UV-treated CFT 073 cells were prepared for binding experiments. The cultured medium was UV irradiated for 5 min. The bacteria were then washed, re-suspended and diluted to a final OD of 0.1 at 600 nm in sterile TBS for further experiments.
Binding of CL-11 to UPEC CFT073
Because both SP-A and SP-D can directly bind to UPEC CFT073 in a calcium-dependent manner through CRD, and this binding is strongly inhibited by EDTA,5,6 UV-treated CFT 073 were incubated with CL-11 (10 µg/ml) in TBS/Ca or TBS/EDTA buffer at 37°C for 1 h. After incubation, CFT 073 was collected by centrifugation at 5000 g for 5 min and washed with TBST. The bacterial pellet obtained after centrifugation was re-suspended in SDS-loading buffer. Samples were analysed using SDS-PAGE and Western blotting using the anti-CL-11 Ab as described above.
Bacterial viability assay
Bacterial viability assays were carried out using a LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen, Molecular Probes, Carlsbad, CA) according to the manufacturer’s instructions. Stained bacteria were incubated with different concentrations of CL-11 (0, 5 or 10 µg/ml) in TBS/Ca for 4 h. After incubation, the bacteria were observed by fluorescence microscopy. Changes in fluorescence intensity were measured using the fluorescence microplate readers (Thermo Fisher Scientific) and compared to a standard curve that was generated using increasing concentrations of live/dead bacteria.
Adherence and invasion assay
PTECs (105 cells/well) were seeded onto a 24-well plate and allowed to adhere to the surface overnight. The PTECs were then infected with CL-11-pre-treated (10 µg/ml for 1 h at 37°C) or vehicle-treated (control, DMEM/F12 medium) UPEC CFT073 at 37°C in DMEM/F12 medium. For bacterial adherence, after incubation with UPEC CFT073 for 1 h, cells were vigorously washed with PBS to remove unattached bacteria and lysed with 0.1% Triton X-100 for 10 min.22 For bacterial invasion, after incubation with UPEC CFT073 for 1 h, PTECs were washed with PBS three times and then incubated with 100 µg/ml gentamicin for 1 h to kill extracellular bacteria. Cells were then washed and lysed with TBST as previously described.22 The lysate was serially diluted and plated onto LB agar plates for a colony count.
CL-11 siRNA transfection
PTECs were transfected with 10 nM CL-11 siRNA or control siRNA using HiperFect transfection reagent (Qiagen) as previously described.23 Cells were then cultured in normal growth media for 48 h before treatment. To assess the knockdown efficiency, transfected cells (CL-11 siRNA or control siRNA) were collected for subsequent Western blot analysis using specific CL-11 Ab 48 h following transfection.
Urinary CL-11 levels in patients with recurrent UTI
Forty-two non-pregnant women (aged 18–65 yr) with recurrent UTI and 42 age-matched healthy women were included in this study. All patients were recruited at the Xiangyang Central Hospital. Each patient’s first morning urine specimen was collected for analysis. Recurrent UTI was defined according to our previously published definition24 and the European Association of Urology Guidelines on Urological Infections (presented at the European Association of Urology Annual Congress Copenhagen 2018) as three or more recurrences per yr or two or more recurrences within 6 mo. Exclusion criteria included urinary tract obstruction, vesicoureteral reflux and diabetes. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Xiangyang Central Hospital, Xiangyang, China (approval number #ZXYYLL-2017-006). All participants gave written informed consent.
ELISA
Supernatants were collected after CFT 073 infection of PTECs (control; CL-11 knockdown and pre-treated with either 2 µg/ml anti-SIRPα Ab, 2 µg/ml anti-CD91 Ab or control IgG for 30 min) and centrifuged at 5000 g for 5 min and stored at –80°C. The levels of released keratinocyte-derived chemokine (KC; the functional homolog of human IL-8) from PTECs were measured by KC ELISA assay Kit (R&D) according to the manufacturer’s instructions. Urine samples were centrifuged at 2000 g for 5 min, and the levels of CL-11 were measured using the Human CL-11 BioAssay™ ELISA Kit (USBiological, Swampscott, MA) according to the manufacturer’s instructions.
Statistical analyses
All data are presented as means±SEM. Statistical analysis of the data was performed using GraphPad Prism v7.0 (GraphPad Software, San Diego, CA). Comparison among/between groups was performed using one-way ANOVA or Student’s t-test. For all comparisons, P < 0.05 was considered statistically significant.
Results
CL-11 expression in murine kidney tissue and urine
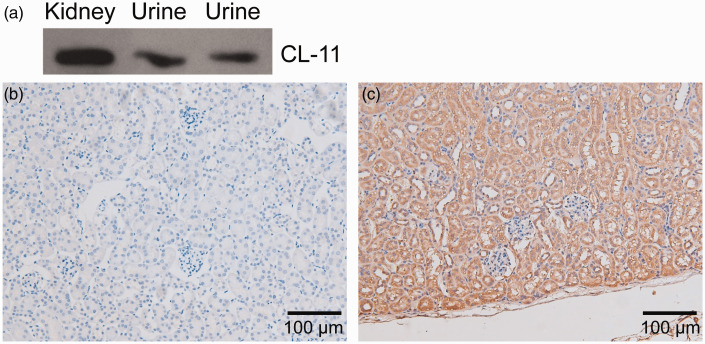
To examine the expression and distribution of CL-11 in murine kidney and urine, Western blot analysis and IHC were performed. CL-11 protein expression was detected using Western blotting in the kidneys and urine of mice (Figure 1a). Immunoreactivity for CL-11 was detected in the proximal tubules and medullary collecting tubules of mice by IHC (Figure 1b and c).
Figure 1.
Collectin-11 (CL-11) expression in the kidney and urine of mice. (a) CL-11 proteins were detected in the kidney and urine by Western blot analysis. (b) and (c) Positive staining of CL-11 expression was detected in the tubular epithelial cells of the kidney (original magnification, 200×).
CL-11 agglutinates UPEC CFT073 and exhibits antimicrobial activity against UPEC CFT073
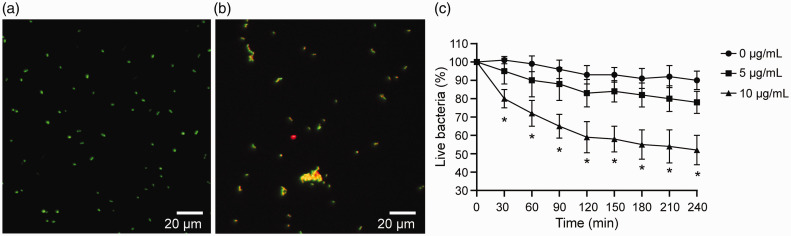
We conducted cell viability assays to determine the agglutination and antimicrobial activity of recombinant mouse CL-11 against UPEC CFT073. Through LIVE/DEAD cell staining, we documented a clear reduction in UPEC CFT073 and higher agglutination in UPEC CFT073 treated with CL-11 compared to negative control (Figure 2a and b, respectively). Additionally, our results demonstrated that mouse CL-11 at 10 µg/ml exhibits rapid, dose-dependent bactericidal activity towards UPEC CFT 073 (Figure 2c).
Figure 2.
CL-11 shows bactericidal activity towards uropathogenic E. coli (UPEC) CFT 073. CFT 073 was stained using a LIVE/DEAD BacLight Bacterial Viability Kit. (a) Bacteria were incubated with CL-11 (10 µg/ml). Untreated bacteria served as the control. Bacterial viability was analysed integrating fluorescent changes from viable (green) and dead (red) cells. (b) CFT 073 was incubated without or with CL-11 (10 µg/ml) in the presence of CaCl2. After incubation, the bacteria were observed by fluorescence microscopy (original magnification, 400×). (c) The percentage of live bacteria was plotted against time. *P < 0.05.
CL-11 directly interacts with UPEC CFT073
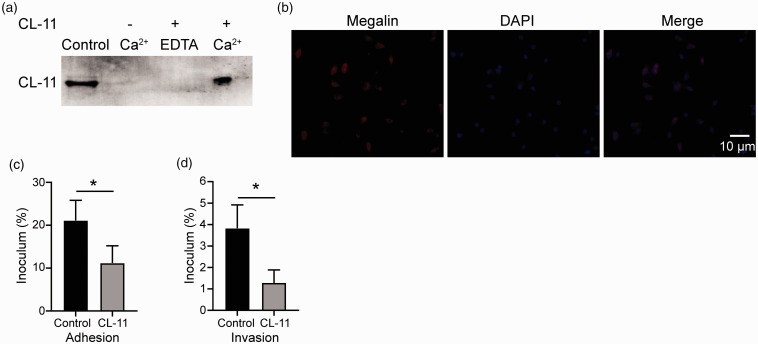
The binding of CL-11 to CFT 073 was investigated using a co-incubation assay. Unbound proteins were removed with several washes, and CL-11 bound to the CFT 073 was detected using Western blotting. We found that CL-11 binds in a calcium-dependent manner to CFT 073 (Figure 3a). As CL-11 is considered to interact with specific complex carbohydrates of bacteria in a calcium-dependent manner via the CRD, we performed an inhibition analysis using EDTA to identify the binding specificities of CL-11 against CFT 073 and found that EDTA inhibited the binding of CL-11 to CFT 073 (Figure 3a). These results indicated that CL-11 recognised carbohydrate patterns of CFT 073 in a calcium-dependent manner via the CRD.
Figure 3.
CL-11 binds to UPEC and inhibits their adhesion and invasion into proximal tubular epithelial cells (PTEC) CFT 073 in vitro. (a) CFT 073 was incubated with or without CL-11 (10 µg/ml) in the presence of CaCl2 or EDTA. After incubation, the mixture of CFT 073 and protein was washed and centrifuged. The bacterial pellet obtained was subjected to SDS-PAGE, and blotting analysis was performed to detect CL-11 co-sedimented with the bacteria. As a positive control, recombinant CL-11 was loaded. (b) PTECs from mice were isolated and identified as described in the Methods. More than 95% of the isolated cells showed megalin-positive staining. (c) and (d) CFT 073 was pre-treated with CL-11 (10 µg/ml) or vehicle control. PTECs were then incubated with CFT 073. The media was aspirated, and PTECs were washed, treated without or with gentamicin (adhesion or invasion) and finally lysed with TBST to quantitate intracellular bacteria (expressed as % of initial inoculum). *P < 0.05 compared to 0 and 10 µg/ml.
CL-11 prevents adhesion and invasion of UPEC CFT073 to PTECs
The key events during UPEC CFT073 infection are epithelial adhesion and intracellular invasion of UPEC CFT073. Because the results described above indicate that CL-11 exerts innate immune functions in part via direct binding to UPEC CFT073, we next focused on the effects of CL-11 on the adhesion and invasion of UPEC CFT073 to PTECs. The primary PTECs from mice were identified by immunofluorescence staining for megalin, a proximal tubular specific biomarker, as previously described.21 The results indicated that > 95% of the isolated cells showed positive megalin staining (PTEC biomarker), suggesting that these isolated cells contained > 95% of PTECs (Figure 3b). We observed that UPEC CFT073 pre-incubated with CL-11 exhibited significantly less adherence and invasion to the PTECs (P < 0.05; Figure 3c and d). These data indicate that binding of CL-11 to CFT 073 prevents adhesion and invasion of the bacteria to PTECs.
CL-11 exerts anti-inflammatory effect via SIRPα without UPEC CFT073 stimulation in PTECs
CL-11 siRNA effectively reduced CL-11 expression in PTECs (Figure 4a). Without UPEC CFT073-stimulation, very low levels of basal p38 MAPK, phosphorylated p38 MAPK and KC expression were observed in PTECs (Figure 4). However, basal and phosphorylated levels of p38 MAPK and KC expression were significantly increased in CL-11 siRNA-transfected PTECs (CL-11 knockdown) compared to that in cells without CL-11 siRNA transfection or with negative control siRNA transfection (P < 0.05; Figure 4). Furthermore, the addition of CL-11 to CL-11 siRNA-transfected PTECs (CL-11 knockdown) could reverse the increase observed in KC expression (Figure 4d). These results showed that CL-11 attenuated p38 MAPK phosphorylation and KC expression under normal conditions (without UPEC CFT073 infection). Moreover, SIRPα Ab inhibited the expression of CL-11 to activate p38 MAPK phosphorylation and KC expression (P < 0.05; Figure 4), indicating that without UPEC CFT073 infection (under normal conditions), CL-11 exerts an anti-inflammatory role through SIRPα.
Figure 4.
CL-11 exerts anti-inflammatory effect via SIRPα in uninfected PTECs. (a) CL-11 siRNA effectively reduced CL-11 expression in PTECs. (b) and (c) PTECs were pre-treated with anti-SIPRα Ab or control IgG, and then incubated with or without CL-11 siRNA. Levels of p-p38 MAPK were detected using Western blot analysis. (d) Supernatants were collected and used for measuring keratinocyte-derived chemokine (KC) expression using ELISA. *P < 0.05.
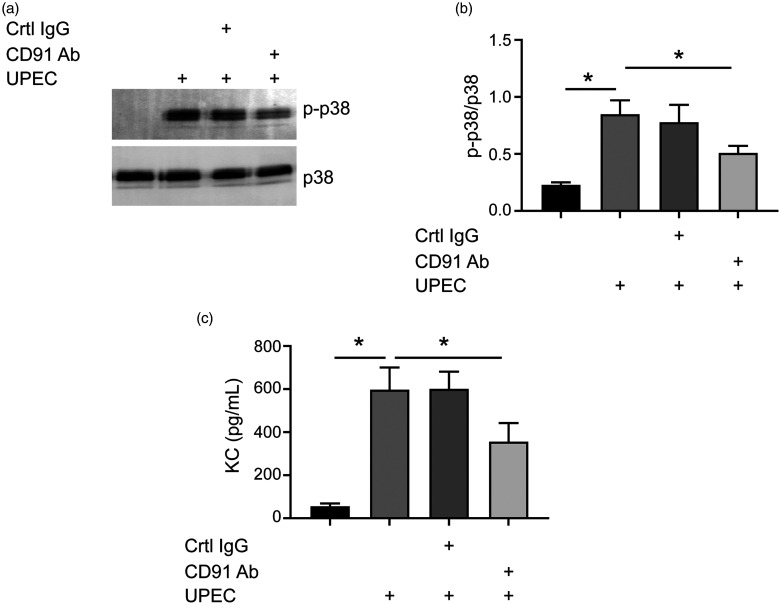
CL-11 exerts a pro-inflammatory role via CD91 in UPEC CFT073-infected PTECs
Although UPEC CFT073 did not change the expression of CL-11 (data not shown), it up-regulated p38 MAPK phosphorylation and KC expression in PTECs compared to untreated PTECs or negative control (Figure 5). Pre-treatment of PTECs with calreticulin/CD91 Ab partially reversed UPEC CFT073-induced p38 MAPK phosphorylation activation and KC expression (Figure 5), indicating that calreticulin/CD91 is involved in UPEC CFT073-induced inflammatory response. CL-11 siRNA transfection also reduced UPEC CFT073-induced p38 MAPK phosphorylation activation and KC expression. The addition of CL-11 to CL-11 knockdown PTECs could reverse the decrease observed in KC expression (Figure 6), indicating a pro-inflammatory role for CL-11 under UPEC CFT073 stimulation. Incubation with the CD91 Ab further blocked p38 MAPK phosphorylation-mediated activation and KC expression under UPEC CFT073 stimulation (Figure 6), indicating that calreticulin/CD91 is involved in UPEC CFT073-induced CL-11 activation of inflammatory response.
Figure 5.
CD91 is involved in UPEC-induced p-p38 activation. (a) and (b) PTECs were pre-treated with anti-CD91 Ab or control IgG and then incubated with or without UPEC. Levels of phosphorylated p38 MAPK were detected using Western blot analysis. (c) Supernatants were collected and used for measuring KC expression levels using ELISA. *P < 0.05.
Figure 6.
CL-11 exerts pro-inflammatory effect via CD91 in UPEC-infected PTECs. (a) and (b) PTECs were pre-treated with CL-11 siRNA (with or without CD91 Ab) or control siRNA and then incubated with or without UPEC. Levels of p-p38 MAPK were detected in PTECs using Western blot analysis. (c) Supernatants were collected and used for measuring KC expression using ELISA. *P < 0.05.
Urinary CL-11 levels in recurrent-UTI and healthy groups
The levels of CL-11 in the urine of patients were tested by ELISA. Compared to the healthy group, urinary CL-11 levels were significantly lower in the recurrent UTI group (8.7 ± 3.93 µg/ml vs. 4.2 ± 2.3 µg/ml; P < 0.05).
Discussion
UTI is one of the most common bacterial infections in humans. Numerous host factors have been implicated in the defence mechanisms against UTI induced by UPEC. Previous studies revealed that classic collectins (SP-A and SP-D) are involved in the host defence against UPEC-induced UTI.5–7,20 In a recent investigation, CL-11 knockout mice were shown to be more susceptible to lung infection with Streptococcus pneumoniae.25 However, the functions of CL-11 with respect to UPEC and UTI were unclear. In the present study, we provided evidence that CL-11, as one of the novel collectins, affected the growth, agglutination, binding, epithelial adhesion and invasion of UPEC. We found increased basal levels of phosphorylated p38 MAPK and KC expression in CL-11 knockdown PTECs. Moreover, SIRPα blockade reversed the increased basal levels of inflammation via CL-11 knockdown in PTECs. In addition, UPEC induced inflammation in PTECs. However, CL-11 knockdown effectively inhibited UPEC-induced p38 MAPK phosphorylation and KC expression in PTECs. CD91 blockade further inhibited the UPEC-induced p38 MAPK phosphorylation and KC expression in PTECs. These observations suggested that CL-11 exerted a direct antibacterial effect and modulated inflammatory response to UTI.
In this study, we demonstrated direct antibacterial roles of CL-11 in host defence against UPEC. We showed that CL-11 directly bound to and agglutinated UPEC CFT 073, a smooth strain with the O6:H1 serotype of LPS. Previous studies have reported that CL-11 bind to LPS from E. coli EH100 (rough strain), E. coli F583 (rough strain) and Klebsiella pneumoniae.15 Furthermore, CL-11 has been shown to bind, with broad specificity, to bacteria including E. coli O:126 (smooth), E. O:60 (smooth), E. coli HB:101 (rough), S. pneumoniae and P. aeruginosa.16,17,25,26 As previous studies have shown that CL-11 exhibits calcium-dependent sugar-binding activity to l-fucose and d-Man, the O-Ags of E. coli O:126 and E. coli O:60 LPS (which contain l-fucose and d-Man), glycolipids or UPEC surface lipids could be the candidate ligands for CL-11. Although a recent study showed that CL-11 inhibits the growth of non-pathogenic E. coli,18 it was unclear whether it would have the same effects on the growth of pathogenic E. coli, as pathogenic strains usually have a different composition/structure of LPS than that in non-pathogenic strains. Our present study found that CL-11 inhibited the growth of UPEC CFT 073. This may be explained by the binding of collectins to glyco and lipid conjugates on bacterial membranes, leading to increased permeabilisation and lysis of the micro-organisms.27 Incubation of PTECs with a complex of CL-11 and UPEC inhibited bacterial adherence and invasion into the PTECs. It is likely that the binding of CL-11 to UPEC, which agglutinated UPEC, may be responsible for the decreased adhesion and invasion. We detected CL-11 expression in the proximal tubules and medullary collecting tubules of mice. These results were consistent with previous findings.28 In the present study, we also demonstrated that the level of urinary CL-11 was lower in patients with recurrent UTI compared to that in healthy individuals. These results clearly indicated the direct interaction of CL-11 with UPEC.
We next focused on the roles of CL-11 in the modulation of the inflammatory response to UPEC. Epithelial cells of the urinary tract not only form a barrier to prevent pathogens, but also cause inflammation in response to UPEC stimulation. Previous studies, including ours, have revealed the dual immunomodulatory effects of classic collectins (SP-A and SP-D).7,11,13 Additionally, the immunoregulatory roles of CL-11 as a novel collectin have recently received more attention. CL-11 has been shown to interact with cell surface receptors, including calreticulin/CD91 and SIPRα, thereby regulating inflammatory signals.19 Murine KC is a functional homolog of human cytokine IL-8. IL-8 expression has been observed to be significantly increased in the kidneys of patients with UTI.29 Human IL-8 production in the urinary tract epithelial cells is dependent on p38 MAPK-mediated NF-κB activation.30 The p38 MAPK signalling pathway plays a central role in the regulation of pro-inflammatory cytokine production. Previous studies have shown that p38 MAPK is phosphorylated in response to UPEC infection and regulates the activation of NF-κB in UTI.31 Interestingly, we found increased basal levels of p38 MAPK phosphorylation and KC expression in CL-11 knockdown PTECs, but SIRPα blockade reversed these increased basal levels of p38 MAPK phosphorylation and KC expression by CL-11 knockdown in PTECs. These results indicated that under normal environmental conditions or in the healthy state, CL-11 binds to SIRPα, which inhibits the activation of p38 MAPK and attenuates the production of inflammatory mediators, thus exerting a pro-inflammatory role.
We also found that UPEC increased p38 MAPK phosphorylation and KC expression. Exposing PTECs to CD91 Ab or CL-11 siRNA prior to UPEC infection significantly dampened UPEC-induced p38 MAPK activation and KC expression. The combination of CD91 blockade and CL-11 knockdown further suppressed UPEC-mediated inflammation. We postulated the following to explain this phenomenon. After infection of PTECs, CL-11 first binds to UPEC via CRD, while its CLD interacts with CD91/calreticulin on PTECs, thereby activating the p38 MAPK signalling pathway and up-regulating the expression of inflammatory mediators. Our findings are similar to those of a recent study on retinal pigment epithelial cell infection models, where CL-11 was found to modulate retinal pigment epithelial cell phagocytosis and cytokine production either via the interaction of its CLD with calreticulin/CD91 or its CRD with SIRPα on retinal pigment epithelial cells under different situations.19 For the benefit of the host, it is important that the immune response is slightly regulated and homeostasis is restored following an infection. UPEC attach to the surface of PTECs, where they rapidly invade epithelial cells. Therefore, during the phase of acute infection, CL-11 could exert pro-inflammatory effects in PTECs and help clear UPEC. When UPEC is cleared, CL-11 exerts anti-inflammatory effects, inhibiting cytokine production to avoid excessive inflammatory reactions that can damage the body.
Our study has a few limitations. First, most of the data in this study were obtained from in vitro experiments with primary PTECs and an analysis of CL-11 in the urine of patients with UTI. Therefore, it lacks in vivo animal model-related evidence in order to confirm the hypothesis. Second, SIRPα and calreticulin/CD91 are important receptors present in epithelial cells, and PTECs express SP-A, SP-D and other proteins that are known to regulate SIRPα and calreticulin/CD91-mediated signalling.32–34 Therefore, blocking SIRPα or calreticulin/CD91 may affect the signalling pathway mediated by proteins other than CL-11. Further studies are required to provide mechanistic insights into this aspect.
Based on the present study findings and previously published reports, CL-11 plays an important role in innate immunity during the pathogenesis of UTI. CL-11 has direct antibacterial roles and is responsible for dual regulation of UPEC-induced inflammatory responses. This study demonstrates that CL-11 could protect PTECs against UPEC infection, thus suggesting a possible function for CL-11 in defence against UTI.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81300617); the Natural Science Foundation of Hubei Province (2019CFB597); and the Hubei Province Health and Family Planning Scientific Research Project (WJ2015Q038).
ORCID iD: Fengqi Hu https://orcid.org/0000-0002-3050-239X
References
- 1.Hamilton C, Tan L, Miethke T, et al. Immunity to uropathogens: the emerging roles of inflammasomes. Nat Rev Urol 2017; 14: 284–295. [DOI] [PubMed] [Google Scholar]
- 2.Sihra N, Goodman A, Zakri R, et al. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol 2018; 15: 750–776. [DOI] [PubMed] [Google Scholar]
- 3.Abraham SN, Miao Y. The nature of immune responses to urinary tract infections. Nat Rev Immunol 2015; 15: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vieira F, Kung JW, Bhatti F. Structure, genetics and function of the pulmonary associated surfactant proteins A and D: the extra-pulmonary role of these C type lectins. Ann Anat 2017; 211: 184–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto J, Takahashi M, Saito A, et al. Surfactant protein A inhibits growth and adherence of uropathogenic Escherichia coli to protect the bladder from infection. J Immunol 2017; 198: 2898–2905. [DOI] [PubMed] [Google Scholar]
- 6.Kurimura Y, Nishitani C, Ariki S, et al. Surfactant protein D inhibits adherence of uropathogenic Escherichia coli to the bladder epithelial cells and the bacterium-induced cytotoxicity: a possible function in urinary tract. J Biol Chem 2012; 287: 39578–39588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu F, Ding G, Zhang Z, et al. Innate immunity of surfactant proteins A and D in urinary tract infection with uropathogenic Escherichia coli. Innate Immun 2016; 22: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Hu F, Liang W, et al. Polymorphisms in the surfactant protein A gene are associated with the susceptibility to recurrent urinary tract infection in Chinese women. Tohoku J Exp Med 2010; 221: 35–42. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Hu F, Wang G, et al. Lipopolysaccharide-induced expression of surfactant proteins A1 and A2 in human renal tubular epithelial cells. J Inflamm (Lond) 2013; 10: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin Y, Liu J, Liu J, et al. Collectins in urinary tract and kidney diseases. Int Urol Nephrol 2018; 50: 695–703. [DOI] [PubMed] [Google Scholar]
- 11.Gardai SJ, Xiao YQ, Dickinson M, et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003; 115: 13–23. [DOI] [PubMed] [Google Scholar]
- 12.Guo CJ, Atochina-Vasserman EN, Abramova E, et al. S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol 2008; 6: e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colmorten KB, Nexoe AB, Sorensen GL. The dual role of surfactant protein-D in vascular inflammation and development of cardiovascular disease. Front Immunol 2019; 10: 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogasawara Y, McCormack FX, Mason RJ, et al. Chimeras of surfactant proteins A and D identify the carbohydrate recognition domains as essential for phospholipid interaction. J Biol Chem 1994; 269: 29785–29792. [PubMed] [Google Scholar]
- 15.Keshi H, Sakamoto T, Kawai T, et al. Identification and characterization of a novel human collectin CL-K1. Microbiol Immunol 2006; 50: 1001–1013. [DOI] [PubMed] [Google Scholar]
- 16.Hansen S, Selman L, Palaniyar N, et al. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J Immunol 2010; 185: 6096–6104. [DOI] [PubMed] [Google Scholar]
- 17.Ma YJ, Skjoedt MO, Garred P. Collectin-11/MASP complex formation triggers activation of the lectin complement pathway – the fifth lectin pathway initiation complex. J Innate Immun 2013; 5: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Bai L, Chen Y, et al. Identification, expression profile and analysis of the antimicrobial activity of collectin 11 (CL-11, CL-K1), a novel complement-associated pattern recognition molecule, in half-smooth tongue sole (Cynoglossus semilaevis). Fish Shellfish Immunol 2019; 95: 679–687. [DOI] [PubMed] [Google Scholar]
- 19.Dong X, Wu W, Ma L, et al. Collectin-11 is an important modulator of retinal pigment epithelial cell phagocytosis and cytokine production. J Innate Immun 2017; 9: 529–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu F, Liang W, Ren Z, et al. Surfactant protein D inhibits lipopolysaccharide-induced monocyte chemoattractant protein-1 expression in human renal tubular epithelial cells: implication for tubulointerstitial fibrosis. Clin Exp Immunol 2012; 167: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Abdel-Razek O, Shi Q, et al. Surfactant protein D attenuates acute lung and kidney injuries in pneumonia-induced sepsis through modulating apoptosis, inflammation and NF-κB signaling. Sci Rep 2018; 8: 15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu H, Pazgier M, Jung G, et al. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 2012; 337: 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Z, Chen X, Zhu K, et al. Dab1 contributes to angiotensin II-induced apoptosis via p38 signaling pathway in podocytes. Biomed Res Int 2017; 2017: 2484303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Hu F, Liang W, et al. Polymorphisms in the surfactant protein a gene are associated with the susceptibility to recurrent urinary tract infection in Chinese women. Tohoku J Exp Med 2010; 221: 35–42. [DOI] [PubMed] [Google Scholar]
- 25.Hwang I, Mori K, Ohtani K, et al. Collectin kidney 1 plays an important role in innate immunity against Streptococcus pneumoniae infection. J Innate Immun 2017; 9: 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali YM, Lynch NJ, Haleem KS, et al. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog 2012; 8: e1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Kuzmenko A, Wan S, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest 2003; 111: 1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu W, Liu C, Farrar CA, et al. Collectin-11 promotes the development of renal tubulointerstitial fibrosis. J Am Soc Nephrol 2018; 29: 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury P, Sacks SH, Sheerin NS. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin Exp Immunol 2006; 145: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai KW, Lai HT, Tsai TC, et al. Difference in the regulation of IL-8 expression induced by uropathogenic E. coli between two kinds of urinary tract epithelial cells. J Biomed Sci 2009; 16: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chassin C, Goujon JM, Darche S, et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol 2006; 177: 4773–4784. [DOI] [PubMed] [Google Scholar]
- 32.Yao M, Rogers NM, Csányi G, et al. Thrombospondin-1 activation of signal-regulatory protein-α stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol 2014; 25: 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao J, Zhao X, Yu W, et al. Surfactant protein A induces the pathogenesis of renal fibrosis through binding to calreticulin. Exp Ther Med 2019; 17: 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarró E, Durán M, Rico A, et al. Cyclophilins A and B oppositely regulate renal tubular epithelial cell phenotype. J Mol Cell Biol 2020; 12: 499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]