Abstract
Cancer chemoprevention is the most effective approach to control cancer in the population. Despite significant progress, chemoprevention has not been widely adopted because agents that are safe tend to be less effective and those that are highly effective tend to be toxic. Thus, there is an urgent need to develop novel and effective chemopreventive agents, such as mitochondria-targeted agents, that can prevent cancer and prolong survival. Mitochondria, the central site for cellular energy production, have important functions in cell survival and death. Several studies have revealed a significant role for mitochondrial metabolism in promoting cancer development and progression, making mitochondria a promising new target for cancer prevention. Conjugating delocalized lipophilic cations such triphenylphosphonium cation (TPP+) to compounds of interest is an effective approach for mitochondrial targeting. The hyperpolarized tumor cell membrane and mitochondrial membrane potential allow for selective accumulation of TPP+ conjugates in tumor cell mitochondria versus those in normal cells. This could enhance direct killing of pre-cancerous, dysplastic, and tumor cells while minimizing potential toxicities to normal cells.
Keywords: Mitochondria, Targeting, Triphenylphosphonium, Cancer, Chemoprevention
1. Introduction
1.1. Role of mitochondria in cell growth and death
Mitochondria efficiently produce ATP via cellular respiration that is essential to fulfill cellular bioenergetic needs. The mitochondrial electron transport chain (ETC) generates a transmembrane proton gradient that is used to generate ATP (1). Some electrons may be prematurely shunted to O2, mainly by ETC complexes I and III, which results in the generation of superoxide (O2•−). Mitochondrial superoxide dismutase can dismutate O2•− to hydrogen peroxide (H2O2) (1). Therefore, mitochondria are well recognized for their potential to generate reactive oxygen species (ROS). In addition, many mitochondrial TCA cycle metabolites can be used as building blocks to produce nucleotides, amino acids, lipids, heme, and others. Mitochondria also have important roles in redox signaling.
Mitochondria can also initiate the intrinsic pathway that promotes apoptosis and necrosis-like non-apoptotic cell death. Once receiving proapoptotic stimuli, such as from Bax and Bak proteins, Ca2+ overload, or other signals, mitochondrial outer membrane permeabilization (MOMP) is induced. Once MOMP is initiated, cytochrome c is irreversibly released from the mitochondrial intermembrane space to the cytosol (2), where it facilitates apoptosome formation, which acts as a platform to activate caspase-9 and the resulting cascades that promote apoptosis (2). Once the mitochondrial permeability transition pore complex (mPTPC) is opened, large amounts of solutes enter the mitochondria matrix, resulting in osmotic swelling which can lead to rupture of the inner and outer membranes, accompanied by loss of mitochondrial membrane potential (ΔΨm), decreased ATP generation and excess ROS production (2).
1.2. Mitochondrial metabolism is required for cancer development
Long ago, Otto Warburg and colleagues observed that cancer cells ferment large amounts of glucose to lactate even in the presence of oxygen (3). In the 1970s, Efraim Racker named this phenomenon ‘aerobic glycolysis’ or the ‘Warburg Effect’ (4). The ‘Warburg Effect’ led to historical viewpoints that cancer cell mitochondria are dysfunctional and that these defects in cancer cell mitochondria render them inconsequential for cancer development. While glycolysis plays an important role in tumorigenesis, mitochondria provide ATP and building blocks (for pyrimidine, amino acid and heme biosynthesis) for cancer cells (5), increasing evidence supports a pivotal role for mitochondrial function in cancer viability. For example, tumor cells lacking mitochondrial DNA (mtDNA) display significantly slower growth and lower tumorigenesis potential (6–8).
Mutations that inactivate tumor suppressor genes and activate oncogenes also alter mitochondrial metabolism. For example, amplification of the Myc oncogene leads to activation of genes that are essential for mitochondrial biogenesis and function (9). Loss of the retinoblastoma tumor suppressor retinoblastoma (RB1) induces mitochondrial protein translation and increases oxidative phosphorylation (OXPHOS) (10). A consequence of increased OXPHOS is the accumulation of low levels of mitochondrial ROS which have been shown to contribute to neoplastic transformation, cell proliferation and survival (11). Some cancer subtypes (e.g. sarcoma, liver cancer, lung cancer and skin cancer) rely predominantly on OXPHOS for ATP production (12). Fatty acid beta-oxidation (FAO), a series of multi-step catabolic reactions that shorten fatty acids, generates NADH and FADH2 that shuttle into mitochondria to support the ETC. FAO enzymes are dysregulated in cancer; elevated key FAO enzymes and/or high FAO activities are seen in multiple cancer types, including triple negative breast cancer, ovarian cancer, glioma, and mutant KRAS-driven lung cancer (13). FAO has been implicated in contributing to cancer cell survival and proliferation, metastasis, drug resistance (13) and cancer stemness (14). Although a majority of cancer cells have functional mitochondria, some cancers have defects that result from mutations in mtDNA or nuclear DNA that encode mitochondrial proteins (15). Interestingly, malfunctioning mitochondria can ultimately contribute to the formation and progression of cancer via ROS-induced nuclear gene instability, a process called retrograde signaling (15). Collectively, these findings indicate that mitochondrial metabolism plays a critical role in tumorigenesis and cancer development.
1.3. Mitochondrial targeting using ligand-drug conjugates
There are basic two strategies for mitochondrial targeting, non-peptide based and peptide- and amino acid-based. The non-peptide based strategies include delocalized lipophilic cations, and others such as benzothiazepines, modified phospholipids or sulfonylurea-related compounds (16).
1.3.1. Mitochondrial targeting using delocalized lipophilic cations
Compared to normal cells, tumor cells have notable transformations in bioenergetics, biosynthesis, and modulated signal transduction which support non-stop growth (17). For some time, scientists have been working on mitochondrial-targeted agents with high selectivity for cancer cells versus normal cells. The mitochondrial membrane potential in cancer cells (–220 mV) is more hyperpolarized than that in normal cells (–140mV) (18). This discrepancy can be exploited with compounds such as delocalized lipophilic cations (DLCs) that selectively accumulate in cancer cell mitochondria (19). The mitochondrial membrane potential (negative inside) can drive a 100- to 1000-fold uptake of cations. Another advantage of lipophilic cations is that their lipophilicity promotes their ability to cross the plasma membrane and the mitochondrial outer and inner membranes. Moreover, the altered redox homeostasis and elevated ROS production in cancer cells (20) renders them potentially more vulnerable to ROS-generating agents (including DLCs), and/or could further weaken their cellular antioxidant defense systems (Figure 1).
Figure 1. The basis for mitochondrial targeting using mito-compounds for cancer cell killing.
(A) Cancer cells have a more hyperpolarized membrane, which serves to drive the uptake of TPP+-conjugated compounds up to 100–1000-fold compared to extracellular compounds. Moreover, elevated ROS production in cancer cells renders them potentially more vulnerable to be killed by ROS-generating agents (like TPP+-conjugated compounds). (B) Normal cells have a less hyperpolarized mitochondrial membrane and comparatively lower levels of ROS, which leads to less uptake of mito-compounds and insufficient ROS to initiate cell death.
Mitochondria-targeting DLCs include triphenylphosphonium cation (TPP+), Rhodamine 123 and dequalinium (DQA). These lipophilic cations readily accumulate in the mitochondrial matrix (21, 22). Originally, these DLCs were research tools to study mitochondrial structure and metabolism, and to monitor mitochondrial membrane potential (23, 24). Ligation of these DLCs to various parental compounds for de novo drug design led to the development of mitochondria-targeting antioxidants (25). Linking anti-cancer agents with mitochondria-targeting moieties was done to improve anticancer efficacy, minimize multidrug resistance (MDR), and damage cancer cell mitochondria to trigger cell death (26).
Triphenylphosphonium cation (TPP+) is the best characterized and most widely used lipophilic cation among the nonpeptide-based strategies for delivering conjugated compounds into mitochondria. TPP+ contains a positively charged phosphorus surrounded by three lipophilic phenyl groups. Due to greater uptake of lipophilic cations into cancer cells, phosphonium salts by themselves have some anti-proliferative activities by disrupting mitochondrial membrane integrity and inhibiting respiration in several cancer cell lines in vitro and in human ovarian cancer models in vivo (27, 28). However, the effects of these TPP+ cations are limited given that their uptake into mitochondria is reversible (29). More importantly, TPP+ salts themselves don’t have anti-tumor effects. However, there are many advantages to using TPP+ as a ligand to deliver other compounds into cancer cell mitochondria. TPP+-based carriers are relatively simple to synthesize and purify, and they have low reactivity towards their conjugates.
TPP+ has been used to deliver various potential anticancer compounds including, but not limited to, antagonists of heat shock proteins, polyphenolic compounds, metabolic modulating agents, triterpernoids, and others (30–33). Parental drugs can be conjugated to mitochondria-targeted ligands via various linkers including esters, amides, carbamates, carbonates, phosphate and others (34). These complexes may be given directly or can be encapsulated in nanoparticle or liposomal vesicles. Recently, their delivery using nanoformulation-based carriers has attracted interest due to potential superior pharmacokinetic properties and improved solubility (35), and relatively higher tumor accumulation (36). Due to the advantage of using TPP+ for mitochondrial-targeting and the significant success of this approach for cancer prevention and treatment, we will focus on TPP+-based conjugates in this review.
1.3.2. Mitochondrial targeting using peptide-drug conjugates
Tethering peptides or short sequences to bioactive compounds is another type of major strategy for mitochondrial targeting. Common examples include modified gramicidin S (GS)-based pentapeptide sequences(37–41), D-(KLAKLAK)2 pro-apoptotic peptides (42) and Szeto-Schiller tetrapeptides (43).
Modified forms of antibacterial peptide GS have been successfully used as a mitochondrial-targeting moiety; a β-turn within the peptide motif inserts into the mitochondrial membrane and this insertion does not rely on the mitochondrial membrane potential. Examples of this approach include GS-based nitroxide ROS scavenger conjugates that can protect cells from oxidative damage and apoptosis and can prolong survival in a rat model of hemorrhagic shock (37, 38). One of these conjugates (XJB-5–131) suppresses DNA damage and motor decline in a mouse model of Huntington’s disease (41). A GS-based PARP inhibitor (XJB-veliparib) inhibits mitochondrial PARP which prevents hypoxia/ischemia-induced death in neurons (39). A GS-conjugated ROS generator, XJB-Lapachone, can stimulate mitochondrial ROS and promote autophagic cell death in cancer cells (40).
1.4. TPP+-based compounds for cancer chemoprevention
Chemoprevention is the use of agents to prevent cancer in healthy persons (Primary prevention), to prevent pre-cancerous lesions from becoming cancer (Secondary prevention), and to prevent cancer recurrence or metastasis (Tertiary prevention). It would be ideal to use them for primary or secondary prevention. However, their use in tertiary prevention could also be highly valuable as conventional cancer therapeutics are often unable to stop recurrence or metastasis. Chemopreventive efficacy is generally estimated by preclinical in vitro cell culture and in vivo animal studies followed by phase I clinical trials. Toxicity is determined in animals and clinical trials. An ideal chemoprevention agent should be highly effective, cause minimal side effects, and have known mechanisms of action. The selective accumulation of TPP+ conjugates in tumor cell mitochondria make them promising agents for chemoprevention with enhanced efficacy and minimal toxicity for normal tissues.
This review will summarize and recent published approaches in using mitochondrial-targeted TPP+ based-compounds (Figure 2 and Table 1) as well as modifications or improvements to classical mito-compounds (Table 2) for cancer chemoprevention and treatment. We will also discuss their mechanisms of action and propose future perspectives.
Figure 2.
Chemical structures of representative mitochondria-targeted compounds.
Table 1.
Features of direct conjugation of mitochondria-targeted compounds
| Parental drug | Bond | Conjugates name | In vitro model and IC50 or other values | In vivo model and antitumor efficacy | Mode of actions | Reference |
|---|---|---|---|---|---|---|
| Pyriplatin | C-C | OPT | IC50 (A549): 8.7±1.6 μM; | A549 xenograft; Dosage: 5mg/kg BW; i.v.; TGII: > 50% | nDNA Damage, OXPHOS and glycolysis inhibition, apoptosis | (112) |
| Terpyridine platinum (II) | C-C | TTP | IC50 (CaoV): 9.0 ± 0.2 μM; IC50 (A549): 9.5 ±1.2 μM IC50 (A549/DDP): 24.1 ±1.4 μM |
- | Mitochondrial TrxR inhibition, OXPHOS and glycolysis inhibition, ΔΨm decrease | (113) |
| Pt (IV) complexes | C-C | PDT | IC50 (A549): 12.20 ± 2.29 μM; IC50 (MCF7): 7.54 ±2.27 μM IC50 (HeLa): 6.42 ±1.03 μM |
- | Apoptosis, ΔΨm decrease, OXPHOS and glycolysis inhibition | (115) |
| Chlorambucil | Amide | Mito-Chlor | IC50 (MCF7): 7.0 ± 1.2 μM IC50 (MIA PaCa-2): 1.6 ±0.9 μM IC50 (BXPC3): 2.5 ± 2.8 μM |
MIA PaCa-2 xenograft; Dosage: 10mg/kg BW; TGII: 61.5% |
mtDNA and nDNA Damage, cell cycle arrest, ROS production | (118) |
| Betulinic acid (BA) | Triethylene glycol | Mito-BA compound 9 | IC50 (TET21N): 0.74 ± 0.14 μM IC50 (MCF-7): 0.70 ±0.11 μM |
- | Apoptosis, OXPHOS inhibition | (93) |
| Glycyrrhetinic acid (GA) | Alkyl | Mito-GA compound 2f | IC50 (HeG-2): 7.58 ± 0.89 μM IC50 (A549): 5.25± 0.84 μM IC50 (MCF07): 7.23 ± 1.01 μM IC50 (HT-29): 8.71 ± 1.06 μM Inhibit A549 cell migration @ 5 μM |
- | G2 cell cycle arrest, apoptosis, cell migration inhibition, ΔΨm decrease | (96) |
| Desi-iodo analog of PU-H71 | C-C | PU-H71-TPP-2 | Reduced the viability of AML cell lines (HL-60, THP-1 and MV-411) | - | OXPHOS inhibition, apoptosis, ATP decrease | (30) |
| Apoptozole | Ester | Az-TPP-O3 | A panel of cancer cell lines with IC50 ranges from 0.5 to 1.5 μM | - | Mortalin ATPase activities inhibition, interaction between mortalin and p53 disruption, MOMP formation, apoptosis | (152) |
| Curcumin | Ester | Mito-curcumin | IC80 (A549): 5 μM | - | Mitochondrial TrxR2 decease, ROS overproduction, ΔΨm decrease, mtDNA damage, apoptosis | (69) |
| Honokiol | C-C | Mito-HNK | IC50 (H2030): 0.26 μM | H2030-BrM3 and DMS-273 orthotropic model; Dosage: 3.75 μmol/kg BW; oral gavage; TGII: 70% and 40%, respectively |
ETC complex I inhibition, ROS overproduction, mitoSTAT3 phosphorylation suppression | (31) |
| Phenol | C-C | Phenol TPP-derivative 2 | IC50 (A549): 0.55 ± 0.07 μM IC50 (MDA-MB-231): 0.34 ± 0.11 μM IC50 (U-02OS): 0.63 ± 0.10 μM |
- | Mitochondrial mass decrease, nuclear PGC-1α levels decrease, electron transport rate decrease, ROS overproduction, ΔΨm decrease, apoptosis | (79) |
| Caffeic acid | Ester | Mito- caffeic acid | IC50 (HepG2): 9.19 ± 0.25 μM | - | mPTP open, ΔΨm decrease, apoptosis | (73) |
| Gentisic acid (GA) | C-C | GA-TPP+C10 | IC50 (MCF-7): 2.42 ± 0.43 μM IC50 (MCF-7-rho0): 14.72 ± 0.37 μM IC50 (MCF-TAMR): 11.47± 0.30 μM |
- | Mitochondrial ABC transporter increase, ETC complex I inhibition, αKGDHC activity inhibition, cell cycle arrest in G1 phase | (75) |
| Lonidamine | Aliphatic chain | Mito-LND | IC50 (H2030BrM3): 0.74 μM IC50 (A549): 0.69 μM |
H2030-BrM3 orthotropic model; Dosage: 7.5 μmol/kg BW; oral gavage; TGIIprimary tumor: >40% TGIILN metastasis: >50% H2030-BrM3 brain metastasis model; Dosage: 7.5 μmol/kg BW; oral gavage | ETC complex I and II inhibition, ROS overproduction, AKT/mTOR/p70S6K axis disruption, autophagic cell death | (32) |
| Pyruvate dehydrogenase kinase 1 (PDK1) inhibitors | Alkyl | TPP-PDK1–1f | IC50 (MCF-7): 0.50 ± 0.11 μM IC50 (A375): 0.51 ± 0.09 μM IC50 (NCI-H1650): 0.21 ± 0.04 μM |
- | PDK activity inhibition, pyruvate glycolysis decrease, ΔΨm decrease | (85) |
| Dichloroacetate | Ester bond | Mito-DCA | IC50 (PC-3): 17 ± 1 μM IC50 (DU145): 42 ± 2 μM IC50 (LNCaP): 30 ± 6 μM |
- | Glycolysis decrease, cellular respiration and ATP decrease, anti-tumor immunity increase | (87) |
| Tamoxifen | C-C | Mito-Tam | IC50 (MCF-7): 1.25 μM IC50 (MDA-MB-436): 3.4 μM IC50 (SK-BR-3): 3.5 μM |
NeuTL syngeneic model; Dosage:0.54 μmol/mouse/dose; oral gavage; TGII: 80% MCFHer2high model; Dosage:0.25 μmol/mouse/dose; oral gavage; Tumor didn’t grow | ETC complex I inhibition, ROS overproduction, tumor-derived senescent cells elimination | (99, 101) |
| Peptide nucleic acids (PNA) | Amide | PNAs-mito | IC50 (A549): 15.6 μM | A549 xenograft model; intratumoral injection; TGII: > 50% | Mitochondrial fusion disruption, ΔΨm, ATP decrease | (109) |
| Triethoxypropylsilane | Amide | Triethoxypropylsilane–TPP (1) | IC50 (SCC7): 3 μM IC50 (PC3): 10 μM IC50 (MCF-7/ADR): 21 μM |
Xenograft model; Dose: 50 mg kg−1 in PBS; Tumor growth was inhibited | Mitochondrial biomineralization and morphology disruption, ΔΨm decrease, apoptosis | (156) |
| Furocoumarin Derivatives | C-C | PAPTP | Induce more than half of B16F10 cells death at 1 μM; Induce up to 85% of B-CLL cells death at 10 μM |
Orthotopic mouse B16F10 melanoma model; Dosage:5nmol/g BW; i.p.; TGII: 90% when combine used with cisplatin Orthotopic xenograft human pancreatic ductal adenocarcinoma; Dosage: 5nmol/g BW; i.p; Tumor sizes were significantly reduced. |
Kv1.3 channel inhibition, mitochondrial damage, ROS overproduction, apoptosis | (155) |
| Imidazolium salt | C-C | TPP1 | For 1-hour of exposure: IC50 (RT112): 200 μM IC50 (SW780): 200 μM IC50 (UMUC3): 80 μM |
- | ΔΨm decrease, apoptosis | (185) |
Abbreviation: TGII: Tumor growth inhibition index; αKGDHC: α-ketoglutarate dehydrogenase complex; BW: body weight
Note: Targeting moieties are all TPP cations
Table 2.
Features of mitochondria-targeted compounds assembled in nano-formulations
| Nanocarrier | Parental drug | In vitro model and IC50 value | In vivo model and antitumor efficacy | Mode of actions | Reference |
|---|---|---|---|---|---|
| PLGA-b-PEG NPs | Platin-Cbl | IC50 (A2780/CP70): 1.0 ± 0.5 μM IC50 (PC3): 0.22 ± 0.04 μM |
- | ΔΨm decrease, mitochondrial respiration disruption, citrate synthase activity inhibition | (119) |
| PLGA/CPT/ PEI-DMMA NPs | Doxorubicin | IC50 (MCF-7/ADR): 19.82 μM at pH 6.5 | MCF-7/ADR xenograft; Dosage: 5mg/kg BW; i.v.; TGII: 84.9% |
ΔΨm decrease, OXPHOS inhibition, mtDNA damage, apoptosis | (125) |
| Folate-cholesteryl albumin NPs | Docetaxel | IC50 (B16F10): around 0.1 μM IC50 (MCF-7): around 1 μM |
MCF xenograft; Dosage: 5mg/kg BW; i.v.; TGII: 69.5% |
ΔΨm decrease, apoptosis | (133) |
| NCs with stabilizing agent (TPP+-Brij98) | Paclitaxel | A mortality rate of 73.5% ± 3.7% (MCF-7/ADR): 50% B-TPP/PTX NCs @ 5 μg/ml; The diameters for MCF-7 ADR spheroid in control and 50% B-TPP/PTX NCs on days 5: 633.1 ± 17.8 μm vs. 416.0 ± 30.1 μm |
- | ΔΨm decrease, apoptosis | (139) |
| DSPE-PEG liposomes | Resveratrol | IC50 (B16F10): around 10 μg/ml | - | ΔΨm decrease | (54) |
| PEG-NPs with pH-sensitive de-PEGylation | Quercetin | IC50 (HeLa and HepG2): 50 μM ~ 100 μM | H22 xenograft; TGII: 65.7 % | ΔΨm decrease, apoptosis | (59) |
Abbreviation: NPs: Nanoparticles; NCs: Nanocrystals; PLGA: Poly (lactic-co-glycolic acid); PEG: Polyethylene glycol; CPT: Camptothecin; PEI: Polyethylenimine; DMMA: 2,3-Dimethylmaleic anhydride; DSPE: Distearoylphosphatidylethanolamine; BW: body weight
Note: Targeting moieties are all TPP cations, except for special indication
2. Mitochondria-Targeted Chemopreventive Agents
Known chemopreventive drugs and natural polyphenolic compounds have been intensively studied for their preventive and therapeutic effects on cancer. Their anticancer efficacies are largely attributed to their roles in cell cycle regulation, anti-proliferation, pro-apoptosis and modulating redox state. To enhance their potential chemopreventive effects, several compounds have been modified to enhance mitochondrial targeting. While several examples have direct evidence for cancer prevention, other examples represent in vitro and in vivo studies showing enhanced efficacy and decreased toxicity and strong potential for further testing.
2.1. TPP+-based natural phenolic compounds
2.1.1. Mito-Honokiol
Honokiol (HNK), a phenolic compound from magnolia bark, has chemopreventive/chemotherapeutic effects in many cancer types by suppressing tumor initiation, retarding malignant progression, reducing tumor load, preventing therapy-resistance and primary tumor metastasis (44). Its chemopreventive effects have been attributed to its potent anti-proliferative, anti-inflammatory, anti-angiogenic, anti-oxidative, cell cycle arrest, and anti-migration properties (44, 45). Recent studies have pointed to potential mitochondrial effects, with high-dose HNK decreasing cancer mitochondrial bioenergetics and inhibiting the development of squamous cell carcinoma in a mouse model (46). These findings led to the hypothesis that creating mitochondrial-targeted HNK could further increase its anticancer and chemopreventive efficacy and potency.
Mito-HNK was synthesized by linking TPP+ to HNK via a long alkyl chain (31). Mito-HNK is 100-fold more potent than HNK in suppressing mitochondrial bioenergetics and retarding cell proliferation/invasion of human lung cancer cells derived from brain metastases and a small cell lung cancer line with high metastatic potential. Mito-HNK causes pronounced inhibition of respiratory chain complex I which stimulates ROS generation (31). Further evidence of its mitochondrial effects were its promotion of mitochondrial Prx3 oxidation, its suppression of the phosphorylation of mitochondrial STAT3, and its inability to significantly inhibit cancer cells that lack mitochondria (31). In mice, Mito-HNK suppresses lung cancer development and brain metastases, whereas the same dose of HNK failed to have an effect (31). Since Mito-HNK was administered starting 1 day after engrafting metastatic lung cancer cells into the arterial circulation, its ability to suppress brain metastases indicates chemopreventive activity. Mito-HNK crosses the blood-brain barrier. RNA-seq analysis of brain metastatic lesions showed that Mito-HNK strongly induced mediators of cell death in the malignant cells but not in the non-malignant tumor stroma (31), and downregulated pathways involved in tumor invasion and proliferation. In the tumor stroma, Mito-HNK downregulated inflammatory and angiogenic pathways which typically support metastasis (31). Mito-HNK is effective against models with mutant EGFR and mutant KRas, suggesting efficacy regardless of driver mutation status. Similar to the good safety profile of HNK (47), Mito-HNK showed no toxicity in mice even when tested at ~20-fold higher than the effective dose (31). These findings make Mito-HNK a highly attractive agent for the prevention and treatment of lung cancer and its brain metastases, and warrant its further development.
2.1.2. Mito-resveratrol
Resveratrol, a phytoalexin in fruit skins, has diverse activities including anti-tumorigenic, anti-viral, cell-cycle inhibitory and cardiovascular and neurological protective effects, which make it ideal for chemoprevention (48). Although it has anti-cancer potential (49), its poor bioavailability and rapid metabolism are major impediments (50). Resveratrol was one of the first natural polyphenols to be modified for mitochondrial targeting (51, 52) which greatly improved its water solubility. These resveratrol show selectivity for fast-growing and tumoral cells (51, 52) and their effects on mitochondrial complexes I and III could contribute to ROS generation (53). Resveratrol liposomes modified with TPP-polyethylene glycol ((TLS (Res)) have increased blood circulation time, enhanced mitochondrial uptake (>5-fold), enhanced ROS generation and dissipation of the mitochondrial membrane potential, and enhanced anti-tumor effects (54). Further investigation of its in vivo activity is warranted.
2.1.3. Mito-quercetin
Quercetin, a natural polyphenolic flavonoid, has well-documented chemopreventive/chemotherapeutic properties in many tumor models (55, 56). Its mechanisms include redox-modulation, cell-cycle arrest, anti-metastatic and direct pro-apoptotic effects on many cancer cells via mitochondrial- and caspase-dependent pathways (55, 56). Low dose quercetin has selectivity for cancer cells versus normal cells (57). However, its poor water solubility is a major obstacle.
Mattarei et al. (58) synthesized a TPP derivative of quercetin (compound 7) which accumulated in mitochondria while maintaining its ability to inhibit mitochondrial ATPase. Compound 7 was markedly more effective on colon cancer CT26 cells, but did not affect slow-growing normal fibroblasts (58). To diminish its rapid clearance by the reticuloendothelial system, Xing et al. (59) developed novel self-assembled quercetin PEGylated nanoparticles (TPP-Que-NP) which enhances mitochondrial localization and disruption of mitochondrial membrane potential, increases caspase activation, and greatly improves anti-proliferative effects on four cancer lines (MCF-7, A459, HepG2, and HeLa) (59). In vivo, these quercetin nanoparticles showed greater tumor suppressive activity than doxorubicin in H22 tumor bearing mice, with no signs of toxicity (59).
2.1.4. Mito-curcuminoids
Curcumin, a major polyphenol in turmeric, has long been recognized for its chemoprevention potential for various cancer lines in vitro (60). However, its low bioavailability and limited intracellular accumulation limit its in vivo potential. Selective DLC-based mitochondria targeting (61) and nanoparticle-mediated delivery (62) may improve its potential. Linking curcumin with mitochondriotropic molecules greatly enhanced its potency for cancer cells including cell cycle arrest and apoptosis.
Recent attention has examined how mito-curcumin analogs alter redox homeostasis. Mammalian thioredoxin reductases (TrxRs) have central roles in maintaining redox homeostasis (63), and in promoting cell growth, preventing cell apoptosis and supporting angiogenesis (64). The TrxR/Trx system can greatly influence cancer development, progression and chemoresistance (65). Therefore, TrxRs are considered potential chemopreventive/therapeutic targets (65).
Curcumin irreversibly inhibits TrxR by alkylation of its active site (66). Qiu et al. (67) found that curcumin analogues also inhibit TrxR. One such analog (compound 2a) has 100-fold more TrxR inhibitory activity than curcumin, although its anti-tumor effect was not significantly improved. In order to enhance anti-tumor effects, compound 2a was tethered to TPP+ via a decyl linker to create TPP2a (68). TPP2a is a potent inhibitor of mitochondrial TrxR (IC50 =1.44 μM) and promotes mitochondrial-dependent apoptosis (68) in multiple human cancer cells (A549 lung adenocarcinoma, HeLa cervical cancer, PC3 prostate cancer, MDA-MB-231 triple-negative breast cancer, and SW480 colon cancer) (IC50 range 0.91–2.77 μM). Jayakumar et al. (69) found that mito-curcumin also greatly decreased the frequency of cancer stem cells (69); it increased ROS generation, decreased mitochondrial TrxR2 activity by >50%, decreased glutathione, and induced the loss of membrane potential. Overexpression of TrxR2 in cancer cells accentuated its anti-tumor effects (69). Mito-curcumin analogues are highly promising agents for primary and tertiary cancer prevention.
2.1.5. Mito-hydroxycinnamic acid derivatives (Mito-HCAs)
Hydroxycinnamic acids are plant phenolic acids with excellent chemopreventive potential in preclinical models (70). When administered at high dose, they may trigger mitochondrial malfunction and mitochondria-mediated cell death in various cancer lines (71, 72). Li et al. (73) synthesized a series of TPP-conjugated hydroxycinnamic acid derivatives (mito-HCAs), including p- coumaric (p- CoA), caffeic (CaA), ferulic (FA) and cinnamic acid (CA). At relatively high concentrations, these mito-HCAs selectively inhibit the proliferation of human hepatoma HepG2 cells with minimal effects on non-cancerous cells (73). Mito-caffeic acid dose-dependently caused fragmented mitochondria morphology, collapsed ΔΨm, and caused apoptotic cell death in cancer cells (73). Cyclosporin A, which inhibits the opening of the mitochondria permeability transition pore (mPTP), partially rescued cancer cells from mito-caffeic acid, which implied that mPTP could contribute to its anti-tumor activity. Unlike most of the mitochondria-targeted compounds discussed above, for which their pro-oxidant properties induce cell killing, these Mito-HCAs increased antioxidant enzymes (catalase, glutathione peroxidase) and decreased mitochondrial lipid peroxidation (73).
2.1.6. TPP+-based polyhydroxybenzoates
Breast cancer is a heterogenous disease with different subtypes. Sandoval-Acuña et al. (74) studied the anticancer effects of mitochondria-targeted polyhydroxybenzoates on breast cancer lines representing four cancer subtypes. They linked TPP+ with various decyl-polyhydroxybenzoates (SA-TPP+ C10, GA-TPP+ C10, PIA-TPP+ C10, and PCA-TPP+ C10). All of these compounds were effective on all breast cancer lines tested, which implied that estrogen receptor or HER2/neu status was inconsequential for efficacy (74). MCF-7 cells were more prone to apoptosis induction by these agents than MDA-MB-231 cells (74). The different basal metabolic states in these cells may account for this difference. Their efficacy corresponded with an initial loss of mitochondrial membrane potential, and a modest decline in ATP. These TPP+-polydroxybenzoates also inhibited the migratory ability of MDA-MB-231 cells, which are highly metastatic (74). Subsequent mechanistic studies (75) on GA-TPP+ C10 demonstrated its time-dependent effects on mitochondrial bioenergetics, including a significant drop in Complex I dependent respiration, and late-stage inhibition of the α-Ketoglutarate Dehydrogenase Complex. Cells responded to this treatment with several adaptations that could promote survival, including AMPK activation, metabolic remodeling towards glycolysis, increased expression of for mitochondrial biogenesis and electron transport, and decreased expression of uncoupling proteins. Combined treatment with doxorubicin and GA-TPP+ C10 showed synergistic effects against breast cancer cells (75). While additional in vivo studies are needed to to fully evaluate GA-TPP+ C10’s chemopreventive potential, its parental compound gentisic acid has anti-neoplastic effects (76, 77) and may protect against the cardiac myofibrillary and endothelial damage induced by doxorubicin (78).
2.1.7. Phenol TPP-derivatives
Gazzano et al. (79) developed two phenol TPP derivatives of 4-hydroxybenzaldehyde (compounds 1 and 2). Both compounds were effective against various human cancer cell lines and had much higher IC50 values for their normal cell counterparts. The differential effect was the greatest for human lung cancer A549 cells versus immortalized human bronchial epithelial cells (BEAS-2B) (79). Both compounds accumulated in lung cancer cell mitochondria, but not in BEAS-2B cell mitochondria. These TPP-phenols decreased mitochondrial mass, decreased levels of nuclear PGC-1α (the transcriptional regulator for mitobiogenesis), decreased electron transport, increased ROS generation, and triggered mitochondrial depolarization and caspase 3/9 dependent apoptotic cell death of tumor cells (79). These characteristics warrant further examination of their chemoprevention potential.
2.2. TPP+-based metabolic regulation agents
2.2.1. Mito-lonidamine
The potential of lonidamine (LND; 1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid), in combination with other agents, to prevent and treat multiple cancers has been explored (80). Although it has the potential to reverse resistance and enhance sensitivity to standard-of-care therapies, LND by itself has limited anti-cancer efficacy. Historically, LND’s anti-neoplastic properties were proposed to result from effects on energy metabolism including glycolysis (81) and complex II inhibition (82). To enhance its mitochondrial effects, our group developed Mito-LND by linking LND with TPP+ via an aliphatic chain (32). In vitro, Mito-LND is 180- and 300-fold more potent than LND in blocking the growth of H2030BrM3 cells (lung cancer cells isolated from brain metastases) and A549 adenocarcinoma cells, respectively (32). In vitro, Mito-LND is ~100-fold more potent in suppressing invasion of highly metastatic lung cancer lines (32). Compared with LND, Mito-LND is 370- and 162-fold more potent at inhibiting mitochondrial complexes I and II, respectively. Mito-LND enhances ROS generation and the oxidation of mitochondrial Prx3, but not cytosolic Prx1, implying that the ROS is primarily mitochondrial. Mito-LND cause decreased phosphorylation of energy sensing proteins (AKT and P70S6K), and induces autophagy in lung cancer cells (32). In orthotopic models of lung adenocarcinoma, a low dose Mito-LND significantly reduced primary tumor growth and metastasis to lymph nodes, and suppressed the development of brain metastasis (32). Given the timing of Mito-LND administration, its ability to suppress brain metastases is consistent with chemopreventive activity. To the best of our knowledge, Mito-LND is the one of the least toxic mitochondria-targeted compounds, causing no toxic effects in mice that were treated for 8 weeks with doses 50 times above the effective anti-cancer dose. Mito-LND induced autophagy in vivo in mouse lung tumors and their brain metastases, but not in normal mouse lung and brain (32). The low toxicity and pronounced anti-cancer and anti-invasive effects of Mito-LND make it a highly promising agent for the tertiary prevention and treatment of lung cancer or its metastases to lymph nodes and the brain.
2.2.2. Mitochondria-targeted pyruvate dehydrogenase kinase inhibitors
Mitochondrial pyruvate dehydrogenase kinase (PDK) is activated in several cancers (but not in normal tissues) where it inhibits (through phosphorylation) the pyruvate dehydrogenase enzyme complex (PDC) which converts pyruvate into acetyl-CoA to support the TCA cycle and OXPHOS. PDK therefore favors glycolysis over OXPHOS, and contributes to the Warburg effect. Inhibition of PDK, and especially PDK1 (the most common isoform in many cancer types) (83), could provide a promising anti-cancer strategy to reverse the Warburg effect. Several PDK inhibitors can retard cancer cell proliferation (84) and overcome chemotherapeutic drug resistance (83). However, these inhibitors have some undesirable off-target effects.
To improve the selectivity for PDK1, Xu et al. (85) designed a series of mitochondria-targeted and tumor-specific PDK1 inhibitors (compounds 1a-f). Unlike the parental drugs, which were equally effective on cancer and normal cells, all these TPP+-conjugated PDK1 inhibitors were selective for cancer cells. Compounds 1c-f showed both improved antiproliferative effects and anti-PDK ability. IC50 values of their antiproliferative effects against NCI-H1650 cells ranged from 0.21 μM to 0.88 μM. As expected, the most potent compound, 1f, converted the glucose metabolic profile in NCI-H1650 cancer cells from lactate production to increased oxygen consumption (85). While TPP-PDK1 inhibitors did not sabotage OXPHOS, they exhibited profound anti-cancer properties including disruption of mitochondrial membrane potential and promoting apoptotic cell death (85).
Dichloroacetate (DCA) is so far the only PDK inhibitor tested in clinical trials; it was well-tolerated in patients with recurrent malignant brain tumors and brain metastases (86). However, its anionic nature limits its cellular and mitochondrial uptake. To circumvent this problem, Pathak et al. (87) conjugated TPP+ to DCA via labile ester bonds. Mito-DCA was ~1000-fold more active than DCA against multiple prostate cancer cells, with greater effects on highly glycolytic cells (PC3) than less glycolytic cells (LNCaP). In addition to suppressing glycolysis, mito-DCA reduced basal cellular respiration and ATP synthesis (87). Since lactic acid can be immunosuppressive (88), mito-DCA’s ability to decrease lactate production can improve anti-cancer immunity. Mito-DCA did not affect metabolism in non-malignant cells (87).
2.3. Mitochondria-targeted triterpenoids
Pentacyclic triterpenes are plant metabolites that include oleanolic acids, ursolic acids, betulinic acids, betulonic acids, glycyrrhetinic acids, and others. These compounds possess many activities including anti-proliferative, pro-apoptotic, anti-angiogenic anti-oxidative and anti-cancer effects (89). Their anti-tumor effects are mainly mediated through activation of mitochondrial apoptosis (90, 91). However, their poor bioavailability and limited intracellular accumulation restrain their use in vivo (92).
To improve cellular uptake and mitochondria targeting, Nedopekina et al. (93) synthesized a series of TPP+ conjugates of ursolic and betulinic acids. In vitro, the conjugates were more effective on three human cancer lines (MCF-7, HCT-116 and TET21N) than with the parental compounds. Betulinic acid conjugate 9 exhibited the highest efficacy. Its superior cell killing was mediated through mitochondria-mediated caspase-dependent apoptosis (93). Tsepaeva et al. (94) developed a series of TPP-betulin derivatives, of which compound 8–13 had the highest efficacy (IC50 as low as 0.045 μM) against various cancer cell lines, with ~10-fold specificity for cancer cells. The length of the alkyl linker and the location of the TPP conjugate affected anti-tumor activity. Compounds 12 and 13, bearing two phosphonium groups, reduced mitochondrial membrane potential more than compounds with only one phosphonium. When tested against PC-3 prostate adenocarcinoma cells, no changes in the cell cycle phase were observed (94). Recently, the same group developed a series of C-28-TPP-conjugated triterpenoids with variable length alkyl linkers and C-3 oxygen-containing groups (33). Elongation of the linker generally lowered their anti-tumor effects, whereas variation in C-3 oxygen groups did not considerably influence efficacy. TPP+-betulinic conjugates 6a-d lowered ΔΨm ~3-fold (33). Compounds with short propylene and butylene linkers caused greater ROS production. In summary, the C-28-TPP-triterpenoid conjugates with shorter alkyl linkers have the most promising anticancer activity within this class (33).
Derivatives of Glycyrrhetinic acid (GA), the pharmacologically active metabolite of licorice, have extensively substantial chemopreventive potential (95). Jin et al. (96) recently designed a series of GA-TPP conjugates, of which compound 2f had excellent activity against human cancer cells and improved selectivity for cancer cells, inhibiting proliferation and migration, and triggering mitochondrial-dependent apoptosis via collapse of the mitochondrial membrane potential (96). The potential of GA-TPP conjugates for tertiary chemoprevention warrants further evaluation.
2.4. Other types of TPP+-based compounds
2.4.1. Mitochondria-targeted tamoxifen
Tamoxifen is FDA-approved for primary breast chemoprevention in premenopausal and postmenopausal women at high risk, and for the adjuvant treatment of early stage estrogen receptor (ER)-positive breast cancer and ER-positive metastatic breast cancer (to suppress recurrence or metastasis). However, tamoxifen is insufficient for HER2-positive breast cancer. Doses of tamoxifen well above typical therapeutic levels can compromise mitochondrial complex I in breast cancer cells (97, 98). Mitochondria-targeted tamoxifen (Mito-Tam) was designed to enhance its mitochondrial effects (99). Mito-Tam kills not only ER-positive, but also HER2-positive and triple-negative breast cancer, including lines that are tamoxifen-resistant. Mito-Tam does not cause systemic toxicity. Mito-Tam is particularly effective against HER2high tumors (99). Molecular modeling predicts that Mito-Tam may block the interaction between the ubiquinone-binding pocket and Complex I. It has also been found that a fraction of HER2 protein is localized at the mitochondrial inner membrane, and HER2high cells have elevated OXPHOS. These findings provide new potential for using Mito-Tam for the tertiary prevention of HER2high breast cancer.
Many chemotherapeutics often eventually induce cell senescence, which can later contribute to tumor relapse (100). However, Mito-Tam suppresses tumor progression without inducing senescence (101). Furthermore, Mito-Tam eliminates both tumor-derived senescent cells and tumor-unrelated senescent cells. The effects of Mito-Tam on senescent cells were distinct from those of other agents, including tamoxifen, and inhibitors of Complex I (rotenone and pieridicin A), and Complex II (Mito-VES and atpenin 5). The mechanisms by which Mito-Tam kills senescent cells may involve suppression of adenine nucleotide translocase 2 (ANT2), which transfers ATP into mitochondria that are faced with ATP crisis (101). The forced overexpression of ANT2 in senescent cells renders them less sensitive to Mito-Tam.
In summary, Mito-Tam is a promising agent for tertiary prevention of multiple types of breast cancer and tumor recurrence. To the best of our knowledge, Mito-Tam’s potential for primary or secondary chemoprevention remains to be determined. Due to its excellent efficacy and safety profile, it is entering Phase I clinical trials in the Czech Republic.
2.4.2. TPP+-based plastoquinone (SkQ1)
Recent studies have shown that anti-cancer effects of mitochondria-targeted antioxidants are mostly mediated though their pro-oxidant properties when used at higher concentrations (102). Titova et al. (103) investigated how low nanomolar concentrations of the mitochondria-targeted antioxidant SKQ1 regulates the cell cycle of fibrosarcoma and rhabdomyosarcoma cells. SkQ1 suppressed the growth of fibrosarcoma and rhabdomyosarcoma cells and caused cell cycle arrest during transition from mitosis to cytokinesis (103). This effect could partly be attributed to decreased levels of Aurora family and Rb proteins, both of which play important roles in regulating mitosis progression and the cell cycle (104, 105). Since tumorigenesis is highly regulated by cell cycle disorders, the cytostatic properties of mitochondria-targeted SkQ1 are considered to be protective against tumor development. Appropriate animal models are required to test its preventive potential.
2.4.3. TPP+-based peptide nucleic acids (PNA-mito)
Mitochondrial architecture changes such as mitochondrial fusion and fission have roles in tumor development, stemness of tumor cells (106), and chemotherapy drug response (107). Peptide nucleic acids (PNAs) are synthetic DNA analogues in which the deoxyribose phosphate backbone is replaced with a neutral peptide-like N-(2-aminoethyl) glycine skeleton. PNAs hybridize with complementary DNA or RNA with high specificity, and can disrupt DNA replication and transcription (108). In order to interfere with the displacement loop regulatory region of mitochondrial DNA, Chen et al. (109) developed a mitochondria-targeting PNA by conjugating TPP to a PNA that targets the D-loop (PNA-mito). In A549 lung cancer cells, PNA-mito blocked mitochondrial gene expression, disrupted mitochondrial fusion and the mitochondrial membrane potential, and decreased ATP generation. PNA-mito inhibited lung tumor growth in mice by >50%. Low dose (both 1 μM and 5 μM) PNA-mito significantly enhanced the sensitivity of drug-resistant A549 tumor spheres to cisplatin (109). These findings suggest tertiary lung cancer prevention potential for PNA-mito including tumors that are cisplatin-resistant.
3. Mitochondria-Targeted Chemotherapeutic Drugs
TPP+ has been linked with many classical chemotherapy drugs to attempt to enhance mitochondrial targeting and anti-tumor effects. Lipophilic TPP+-based chemotherapeutic drugs are expected to have enhanced efficacy but significantly decreased toxicity. Some of these conjugates may therefore have potential for cancer chemoprevention, especially for tertiary prevention.
3.1. TPP+ based standard chemotherapeutics
3.1.1. Mitochondria-targeted Pt(II) and Pt(IV) based drugs and pro-drugs
Platinum (Pt)-based drugs (cisplatin, carboplatin, and oxaliplatin) have long been a mainstay for many cancer types (110). However, they have well-known serious side effects, low bioavailability and poor stability, and can fail clinically due to acquired resistance (111).
Developing non-conventional monofunctional Pt(II) complexes is one potential way to circumvent these drawbacks. Recently, a series of mitochondria-targeted unconventional monofunctional Pt(II) complexes were developed (112). One of the derivatives, Pt(ortho-PPh3CH2Py) (NH3)2Cl] (NO3)2 (OPT), showed higher efficacy than cisplatin against lung cancer cells in vitro and tumor growth in vivo. In addition to its effects on nuclear and mitochondrial DNA, OPT disrupted mitochondrial structure and respiration, and induced cytochrome c release and apoptosis (112). Another TPP+-based terpyridine platinum(II) complex (113) was developed to inhibit thioredoxin reductase (TrxR). It inhibited cancer cell mitochondrial TrxR activity by 70%, and caused severe damage to mitochondrial morphology and function, which resulted in dual inhibition of mitochondrial and glycolytic bioenergetics. The resulting hypometabolic state was effective in human ovarian cancer cells (113).
Another way to overcome the shortcomings of conventional Pt(II)-based drugs is to develop Pt(IV) pro-drugs which can be conjugated with ligands at the axial positions to render “dual actions” (114). Jin et al. (115) developed and synthesized two mitochondria-targeted TPP+-based Pt(IV) complexes, namely, c,c, t-[Pt(NH3)2Cl2(OCOCH2CH2CH2CH2PPh3)(OH)]Br (PMT) and c,c,t-[Pt(NH3)2Cl2(OCOCH2CH2CH2CH2PPh3)2]Br2 (PDT). The TPP+ moiety on the axial position of Pt(IV) markedly changed the reactivity and anti-tumor effects. Although TPP+ increased their lipophilicity, the generation of Pt(II) species from these Pt(IV) prodrugs was decreased and thus their reactivity with DNA. Although these Pt(IV) compounds caused more mitochondrial damage (disrupted morphology, dissipation of ΔΨm, and decreased ATP production) than cisplatin, PMT and PDT were less effective on cancer cells in vitro (115). These modified Pt(IV) pro-drugs may therefore not be useful candidates.
3.1.2. Mito-Chlor and T-Platin-Cbl-NPs
The DNA alkylating agent chlorambucil is commonly used to treat chronic lymphocytic leukemia/small lymphocytic lymphoma (116). However, it has several severe adverse effects, low stability, non-selective reactivity towards all peripheral cells, and the development of resistance (117). Mitochondria-targeted chlorambucil (Mito-Chlor) developed by Millard et al. (118) preferentially localizes in cancer cell mitochondria where it damages mitochondrial DNA and causes cell cycle perturbation and apoptosis. Mito-Chlor is 80-fold more effective than chlorambucil on breast and pancreatic cancer cell lines that are insensitive to the parent drug. Mito-Chlor significantly retarded the growth of human pancreatic cancer in mice (118). Platin-Cbl (119), a combination of cisplatin and chlorambucil (Cbl), demonstrated efficacy for several tumor cell lines in the NCI60 cell panel, but was unable to completely block energy generation from fatty acid metabolism in cisplatin-resistant cells. To improve its mitochondrial effects, they encapsulated Platin-Cbl inside polymeric nanoparticles and further linked Platin-Cbl with TPP+. These mitochondria-targeted TPP-Platin-Cbl nanoparticles arrested all metabolic pathways in cisplatin-resistant cells, and further enhanced anti-tumor efficacy 5-fold (119).
3.1.3. Mitochondria-targeted doxorubicin
Doxorubicin (DOX), an anthracycline antitumor antibiotic, stabilizes topoisomerase 2 which prevents the resealing of DNA strands (120). Previous attempts to improve its anticancer efficacy and potency involved either directly linking TPP+ to DOX or encapsulating DOX in various liposomal or nanoparticle carriers (121). The efficacy of TPP+-DOX for wild-type cancer cell lines varied between different carrier formulations (121).
Efflux proteins are a major reason for DOX resistance (122). Adding a membrane-permeating peptide (with both hydrophobic and cationic properties) to DOX followed by attachment to copolymers can successfully bypass efflux pumps (123). This strategy enhances mitochondrial accumulation by >11-fold and markedly improves the suppression of DOX-resistant tumor spheroids. Another approach is dual-targeting DOX-loaded carrier systems, such as TPP-functionalized PEG-modified polydopamine (PDA-PEG-TPP-DOX) (124). Compared to DOX nanoparticles without TPP, PDA-PEG-TPP-DOX exhibited much higher mitochondrial penetration and a greater lowering of mitochondrial membrane potential. In MDA-MD-231 human breast cancer cells, PDA-PEG-TPP-DOX overcame drug resistance to a greater extent than PDA-PEG-DOX (124). Yu et al. (125) used an acidity triggered cleavable polyanion PEI-DMMA (PD) to coat lipid-polymer hybrid nanoparticles (DOX-PLGA/CPT) to form DOX-PLGA/CPT/PD. Although the net charge of this compound was negative, it could still target tumor sites, taking advantage of the acidic tumor microenvironment. After it was endocytosed by tumor cells, TPP+ guided DOX-PLGA/CPT to mitochondria, where it disrupted mtDNA and induced apoptosis (125). In vitro, both DOX-PLGA/CPT and DOX-PLGA/CPT/PD overcame DOX resistance of MCF-7/ADR cells (125). However, these two formulations showed completely different anticancer efficacy in mice. DOX-PLGA/CPT/PD caused almost four-fold greater tumor inhibition (84.9%) compared with DOX-PLGA/CPT (19.0%). Such drastic differences between in vitro and in vivo efficacy were ascribed to the overall negative charge of DOX-PLGA/CPT/PD which decreased its rapid clearance from the blood and enhanced its tumor tissue accumulation. Liu et al. recently embedded DOX-TPP into a series of nanoparticle formulations (126, 127). By conjugating DOX-TPP to water-soluble hyaluronic acid (HA) using an acid-cleavable bond to achieve pH-responsive selective targeting of cancer cells (128), HA-hydro-DOX-TPP mainly accumulated inside mitochondria (and not the nucleus) after its separation from the HA-nano carrier. The cellular content of HA-hydro-DOX-TPP in MCF-7/ADR cells was ~7-fold higher than that achieved with free DOX, and HA-hydro-DOX-TPP was much more effective on MCF-7/ADR cells (126). Its antitumor effects were further confirmed in vivo, and higher drug levels were consistently observed in tumors of the HA-hydro-DOX-TPP group (126). This nanoparticle formulation possessed a good safety profile (126).
HA-ionic-TPP-DOX, another hyaluronic acid modified DOX-TPP NC derivative with a different (ionic) linkage(127) showed comparatively inferior in vitro activities (127). However, in vivo HA-ionic-TPP-DOX exhibited much higher efficacy than DOX-TPP. Its higher antitumor efficacy in vivo could result from a higher tumor accumulation of HA-ionic-TPP-DOX (127). These results suggest its potential for future clinical trials.
Kim et al. (129) developed a self-assembled mitochondria-targeted TPP+-based nanoparticle probe (N1) with a cyanostilbene fluorescent tag to visualize the therapeutic effects. N1 was later modified with DOX to form the model drug N1-DOX. N1-DOX inhibited tumor growth by ~60%, exhibited higher cancer cell uptake and predominately mitochondrial accumulation, and caused mitochondrial dysfunction (massive ROS generation, MMP collapse) and eventually apoptosis.
3.1.4. Mitochondria-targeted taxanes
The taxanes, including paclitaxel and docetaxel, block at the G2/M phase of the cell cycle by enhancing the assembly and stability of microtubules (130). While the taxanes have been used clinically for many types of malignancies (131), they cause severe adverse reactions including neurologic toxicity and bone marrow suppression (132). Mitochondrial targeting may reduce these side effects on normal tissues.
Battogtokh et al. (133) synthesized TPP-Docetaxel (TPP-DTX) conjugates and an albumin-nanoparticle formulation TPP-DTX@FA-chol-BSA to provide for better drug stability and longer retention in the circulation. They proposed that drug-loaded nanoparticles would release about 48–60% of TPP-DTX in a low pH environment (such as tumor cell lysosomes). TPP-DTX conjugate and its nanoparticles showed significantly higher accumulation in mitochondria vs. the cytosol in MCF-7 cells, induced 2-fold more ROS, and triggered mitochondrial depolarization (133). However, the selectivity for cancer versus normal cells was not reported. In vivo, TPP-DTX nanoparticles redcued tumor size 4.8-fold. Zhang et al. (134) generated a different liposome system to deliver TPP-DTX. EphA10 is a receptor that is predominately expressed on some breast cancer cells (135). They added an EphA10 antibody onto the surface of the liposome carrier, and also synthesized a pH-sensitive PEG-Schiff base-cholesterol (PSC) to modify the liposome (PSLP) to ensure sustained release of the drug. This final drug-loaded product (EPSLP/TD) exhibited the highest cellular uptake, mitochondrial targeting, and the highest levels of cleaved poly (ADP-ribose) polymerase (PARP) and cytochrome c release to the cytosol (134).
Clinical responses to paclitaxel (PTX) are variable, and resistance mechanisms include multidrug resistance (MDR) transporters (136). Studies have tested the hypothesis that mitochondria-targeted drugs can overcome MDR by suppressing ATP synthesis. TPP+-conjugated PTX-loaded liposomes can improve efficacy against MDR-positive PTX-resistant ovarian cells and cisplatin-resistant lung cancer cells (137, 138). Han et al. (139) developed PTX nanocrystals stabilized with TPP+-modified Brij 98 (a P-glycoprotein inhibitor) to overcome MDR. All of the Brij-TPP/PTX nanocrystals (with different percentages of Brij98 in the formulations) showed much higher anti-proliferative effects on human breast cancer MCF-7 cells and its MDR line MCF-7/ADR (139). The anti-proliferative effects were correlated with loss of mitochondrial membrane potential, and the Brij-TPP/PTX nanocrystals were effective against 3D spheroids (139).
3.2. TPP+-based Hsp inhibitors
3.2.1. TPP+-based Hsp90
Heat shock protein (Hsp90) is a major chaperone protein that assists with proper folding and function of client proteins. Many Hsp90 clients, such as signaling kinases (e.g. MAPK, PI3K, AKT), p53 and telomerase, play crucial roles in cancer development (140). By binding to inactive clients, Hsp90 facilitates their activation. Hence, inhibiting Hsp90 could have anti-cancer effects by indirectly inactivating other oncoproteins (140). Though initial Hsp90-based anti-cancer strategies had modest effects (141). Kang et al. (142) developed the first in class mitochondria-targeted Hsp90 inhibitor, which contains the Hsp90 ATPase antagonist 17-(allylamino)-17-demethoxy-geldanamycin (17-AAG) linked to TPP+ (GA-TPP-OH). GA-TPP-OH induced loss of mitochondrial membrane potential, triggered cytochrome c release, and induced mitochondrial apoptosis (142). Hsp90 protein physically interacts with the PTP component protein CypD to inhibit pore opening to prevent initiation of cell death (142). GA-TPP-OH reduced the physical interaction between Hsp90 chaperones and CypD to potentiate the onset of cell death. GA-TPP-OH was tumor cell selective (142). GA-TPP-OH inhibited lung tumor growth in vivo by ~70%, a much larger effect than the the parental drug 17-AAG. GA-TPP-OH did not cause apparent toxicity to mice (142).
Gamitrinib specifically acts on the mitochondrial Hsp90 protein TRAP1. Mitochondria-targeted Gamitrinib was developed to study its regulation of the protein-folding response inside the mitochondria of tumor cells (143). At high dose, Gamitrinib-TPP causes rapid mitochondria-mediated apoptosis of tumor cells, whereas lower doses trigger an unfolded protein response (UPR), autophagy, and complete inhibition of NF-κB-dependent cell survival. Gamitrinib-TPP’s ability to blunt pro-survival mediators enhanced the sensitivity of tumor cells to death receptor agonists. Gamitrinib-TPP worked synergistically with the apoptosis-inducing agent TRAIL to arrest intracranial glioblastoma growth in mice (143). A recent study (144) focused on how Gamitrinib-TPP alters glutamine metabolism in non-small cell lung cancer (NSCLC) which relies heavily on glutamine metabolism (145). Glutamine-dependent NSCLCs are especially sensitive to Gamitrinib-TPP. TRAP1 inhibition by Gamitrinib-TPP increased the enzymatic activity of glutamine synthetase (GS) which synthesizes glutamine from glutamate. Increased phosphorylation of AMPK in Gamitrinib-TPP-treated cells implied that these cells were deprived of ATP (144). Thus, Gamitrinib-TPP could be a therapeutic strategy for glutamine-dependent cancers.
Bryant et al. (30) developed two variants of mitochondria-targeted Hsp90 inhibitors, PU-H7-TPP1 and PU-H7-TPP2, by linking the purine-based chemical scaffold PU-H71 with TPP+. The concentration of PU-H7-TPP in mitochondria was 17-fold higher than that in the cytosol. Both PU-H7-TPP1 and PU-H7-TPP2 reduced the viability of multiple acute myeloid leukemia (AML) cell lines, with PU-H7-TPP2 having greater efficacy (30). However, both treatments were ineffective for the chronic myeloid leukemia cell line K562. Unmodified PU-H71 was ineffective. PU-H7-TPP’s mechanistic effects include MMP dissipation, OXPHOS inhibition and ATP depletion (30). Mitochondrial metabolism is important for AML progression (146). Since mitochondrial Hsp90 (TRAP1) is upregulated in AML patient samples, and increased TRAP1 correlates with worse outcomes, PU-H7-TPP2 induced apoptosis and disrupted mitochondrial function in patient-derived AML samples (30). Thus, mitochondria-targeted Hsp90 inhibitors have therapeutic potential for AML patients.
All clinically proven Hsp90 inhibitors target the N-terminal part of Hsp90 to disconnect the interaction between Hsp90 and ATP. However, this inhibition ultimately leads to heat shock response, with more Hsp70 which can suppress apoptosis (147). To avoid this pitfall, inhibitors that target the C-terminus of Hsp90 have been developed that do not generate heat shock response. Zhang et al. (148) tcreated mitochondria-targeted Hsp90 C-terminal inhibitors by replacing an amine with TPP+. These modified compounds accumulated in the mitochondria and had a two-fold greater anti-proliferative effect on human breast cancer MCF-7 cells. The TPP+-modified compound only triggered the degradation of Hsp90 client proteins Raf-1 and CDK-4, but not Her2 or AKT (148).
3.2.2. TPP+-based Hsp70
Hsp70 expression is highly elevated in various cancers, and correlates strongly with tumor initiation and progression (149). Various small inhibitors have been developed to target various members of Hsp70 that localize to different subcellular compartments. Ko et al. (150) developed a lysosomal Hsp70 antagonist, apoptozole, which binds to Hsp70’s ATPase domain to disable its ATPase activity. The Hsp70 in mitochondria, known as mortalin, promotes cell proliferation and downregulates apoptosis (151). Park et al. (152) modified the lysosomal antagonist apoptozole with TPP+ to create Az-TPP-O3 which enabled the targeting of mitochondrial Hsp70 (mortalin). Its anti-tumor effects for a panel of 20 human cancer cell lines was improved by ~6-fold (152). Mitochondrial-localized Az-TPP-O3 disrupted the interaction of mortalin with p53; the dissociated p53 helped initiate Bak-mediated mitochondrial outer membrane permeabilization, leading to caspase-dependent apoptosis in cancer cells. Apoptozole-TPP-O3 did not affect lysosome-mediated apoptosis or autophagy (152).
3.3. Other types of TPP+-based compounds
3.3.1. TPP+-based mitochondrial K+ channel inhibitors
Blockade of mitochondrial ion channels can result in loss of ΔΨm, ROS production, mitochondrial swelling, cytochrome c release, and cancer cell death induction that bypasses upstream players in intrinsic apoptosis (e.g. Bcl-2 family proteins, p53 status, and others) (153). The voltage-gated potassium channel mtKv1.3 is overexpressed in many cancer cells compared to normal cells, and plays a pivotal role in cancer development (154). The plant-derived furocoumarins are specific Kv1.3 channel inhibitors, but they have only modest tumor-killing effects at pharmacologically relevant concentrations.
Leanza et al. (155) developed two derivatives by directly linking TPP+ with PAP-1’s carbon chain (PAPTP), or via indirect conjugation using a carbamic ester bond (PCARBTP). PAP-1 and its mitochondriotropic analogs only induced apoptotic cell death in cancer cells that highly expressed mtKv1.3, but not in cancer cells with lower expression or healthy counterparts. Both derivatives could induce the death of more than half of the B cells derived from patients with chronic lymphocytic leukemia. Upon accumulating in mitochondria, they induced an initial increase in membrane potential and ROS release, which facilitated opening of the PTP, followed by mitochondrial swelling and structure fragmentation, membrane depolarization and release of cytochrome c (155). The modified compounds showed stunning effects in decreasing tumor volume in both orthotopic B16F10 melanoma and a mouse pancreatic ductal adenocarcinoma model. Their tumor-killing effects were likely mediated by ROS production, as pre-treatment with an anti-oxidant eliminated their efficacy (155). These psoralen derivatives did not affect fast-proliferating normal cells including immune cells or major organs (155). In summary, mitochondria-targeted furocoumarin derivatives are ideal anti-cancer agents for cancers that highly express mtKv1.3 channels and have altered redox states.
3.3.2. TPP+-based trialkoxysilane
Biomineralization is a natural process of living organisms to form mineral-based materials from available elements within the biological matrix. Kim et al. (156) incorporated intracellular mitochondrial-targeting strategies into the biomineralization system to improve selectivity for tumor cells. Trialkoxysilane can cause mitochondrial dysfunction by forming silica particles in response to the alkaline pH of 8 inside the mitochondrial matrix. They linked TPP+ to trialkoxysilane, creating compound 1 which accumulated and initiated biomineralization mostly within tumor cell mitochondria (156). High concentrations of compound 1 within mitochondria formed large silica particles which disrupted mitochondrial morphology and depolarized the mitochondrial membrane (156). These events triggered apoptosis in various cancer cells, including drug-resistant cell lines. In contrast, compound 1 had low toxicity for normal cells. Systemic administration of compound1 inhibited tumor growth, but did not cause toxicity (156).
4. Mitochondria-targeted agents to modulate the tumor microenvironment
Mitochondria have important roles in establishing immune cell phenotypes, modulating their metabolic and physiologic states, and their functionality (157). In fact, almost every mitochondrial component, including catabolic and anabolic metabolism, redox systems, mtDNA and mitochondrial dynamics/turnover has a great impact on the immune system (157). Mitochondrial pathways (TCA, OXPHOS and amino acid metabolism) regulate immune cell activation status and and functional responses. For example, resting T cells (naïve and memory type) require mostly OXPHOS respiration and lipid oxidation, whereas proliferating effector T cells (Teff) switch to aerobic glycolysis and glutamine metabolism (158). Immune suppressive regulatory T cells have distinct metabolism to maintain their suppressive function, including increased complex-III dependent OXPHOS and decreased glycolysis (159). Mitochondria-generated ROS act as second messengers for both inflammasome activation (160) and antigen-specific T cell activation (161). And, mtDNA released from dysfunctional mitochondria can bind to and activate the NLRP3 inflammasome, which plays a critical role in innate immunity by triggering secretion of pro-inflammatory cytokines (e.g. interleukin-1β and IL-18) (162). Mitochondrial dynamics also control T cell activation and differentiation. Activated T cells tend to have more fragmented mitochondrial membrane structure and more expanded cristae to reduce ETC efficiency and enhance glycolytic metabolism, whereas memory T cells have more fused mitochondrial structure that favors OXPHOS and fatty acid oxidation (163).
The immune system is a major determinant of the tumor microenvironment. The dual forces of how the tumor affects immunity and host-protection have established the foundation for the “cancer immunoediting” theory (164). One way tumors restrain host immunity is by rendering immune cells metabolically anergic. Non-stop tumor growth modifies the surrounding environment to be nutrient-deficient/altered, acidic, hypoxic, and with accumulated waste. As a result, immune cells are not properly activated or have lost their battle for resources. Mitochondria-targeted compounds could potentially influence immunosurveillance in a cancer cell-intrinsic or cancer cell-extrinsic fashion. Through selectively inducing energy crisis and death in tumor cells, mitochondria-targeted compounds could potentially make more nutrients available to the microenvironment. Moreover, the release of mtDNA or mtROS from dying tumor cells could help to initiate innate immunity and adaptive immunity. For example, mitochondria-targeted IR-780 was recently developed as an immunogenic cell death inducer through its ability to trigger anticancer immunity (165). After accumulating inside tumor cell mitochondria and killing cancer cells, large amounts of tumor-associated antigens were released and triggered dendritic cell maturation, and more T cells infiltrated into the tumor microenvironment which finally led to suppression of tumor development and the prevention of metastasis (165). Another study developed the TPP+-based Ce6-loaded nanocarrier for mitochondria-targeted anticancer photodynamic therapy (166). This Ce6-loaded nanocarrier exhibited superior in vivo anticancer efficacy, specifically lowering tumor load in two rodent models by ~90%. The immunological mechanisms were increased activation of DC cells and T cells, T cell infiltration and upregulated cytokines (IFN-γ and TNF-α) in tumors (166). Tumor metabolism waste could also negatively affect anticancer immunity, with lactate being the most prominent. Lactate can inhibit activation and maturation of DCs (167) and proliferation of Teff cells (168) in the tumor milieu. After shutting down tumor metabolism (OXPHOS or/and glycolysis) by mito-compounds, metabolism waste would decrease and exert less inhibitory effects on immunosurveillance. As previously discussed, mito-DCA helps dendritic cells to excrete more anticancer-related cytokines (87). Alternatively, mitochondria-targeted compounds could regulate anticancer immunological activities via directly modulating mitochondrial metabolism in immune cells. However, there is not yet direct evidence with TPP+-based mito-compounds to support this viewpoint. However, biguanides (phenformin and metformin), which can inhibit mitochondrial respiratory complex I, can suppress the inhibitory function of granulocytic MDSC ex vivo and their accumulation in tumor microenvironment in vivo through enhanced AMPK phosphorylation (169, 170).
5. Mitochondria-targeted agents in clinical trials
Numerous mitochondria-targeted agents have shown improved anticancer efficacy and potency in preclinical settings. However, most of these mito-compounds have not yet advanced to clinical trials for cancer chemoprevention. To our knowledge, Mito-TAM is the only such compound that has advanced to a Phase I clinical cancer trial.
Regarding human use of mitochondria-targeted TPP+-based agents for diseases other than cancer, the antioxidant mitoquinone (mitoQ) has been the most extensively investigated. It consists of the parental ubiquinone CoQ10 covalently linked with TPP via a ten-carbon chain (171). In many preclinical studies, mitoQ is protective against mitochondrial oxidative stress in wide variety of diseases and conditions (172). Eighteen studies on the diverse actions of mitoQ can be found on http://www.clinicaltrials.gov/. Of these, four have been completed, one was terminated, and 13 are in the recruiting or active stage. Although considered a neuroprotective compound, mitoQ did not slow down the progression of Parkinson’s Disease in a Phase II trial (173). In another phase II trial, mitoQ suppressed the levels of liver enzymes that are indicative of liver damage in hepatitis C patients (174). Recent human trials showed that chronic supplementation with mitoQ could safely ameliorate age-related vascular function deterioration (175). Although conflicting results have been seen in diseases having oxidative damage as a pathological cause, mitoQ may still hold promise as an effective antioxidant in the ongoing trials.
Mitochondria-targeted compounds that are promising candidates for clinical translation for cancer prevention should ideally have good bioavailability, prolonged retention in circulation, selective targeting of tumor cells, and high accumulation in the mitochondria of tumor cells. Oral administration of these agents would be preferred for ease of use. Because of TPP’s lipophilicity, TPP+-conjugates are generally expected to traverse phospholipid bilayers and enter the systemic circulation from the gut. Consistent with this premise, TPP conjugation has been shown to generally enhance bioavailability and cellular uptake (52, 61, 93). However, once in the circulation, the positive charge on TPP+ renders them susceptible to rapid clearance by the reticuloendothelial system. To overcome this barrier, it is ideal to have a negative surface charge when in the circulation, and to later reveal the positive charge once inside tumor cells to target mitochondria; the DOX-PLGA/CPT nanoparticles discussed above are an example of this approach (125). This type of charge conversion can be achieved by coating nanoparticles with an acidity-triggered-cleavable polyanion material; once triggered by the acidic pH in the tumor microenvironment, the negatively charged coating will be stripped off to expose the TPP cations. Another way to prolong the retention time of TPP+ compounds in the circulation and to deliver a sufficient amount is to encapsulate TPP+ compounds inside nanosized carriers. There has been an increasing trend to use of nano-formulations for Enhanced Permeability and Retention (EPR) effect and tumor site targetability. Mitochondrial accumulation is usually enhanced many-fold by the TPP+ moiety as noted for the many examples discussed in this review. However, variations in the alkyl carbon chain that links TPP+ to its cargo group can also affect the rate of mitochondrial uptake, the specific site of action (mitochondrial membrane or matrix), and the corresponding effects on cellular respiration. In general, longer linkers (usually carbon n = 3–10) result in more hydrophobic conjugates, faster mitochondrial localization, and greater ability to inhibit OXPHOS (176, 177).
6. Future Perspectives
Relative to the parental compounds, TPP+ conjugants have improved in vitro efficacy against cancer cell lines on the order of ≥5-fold. The greatest improvement of efficacy has been observed with mitochondria-targeted metformin that has a 10-carbon linker (IC50 of 1.1 μM vs. 1.3 mM for metformin) (178). Although numerous in vitro anti-tumor effects have been shown, only some studies examined their anticancer efficacy in vivo. In fact, not all in vitro data can be translated in vivo. Hence, it will be useful for future studies to include in vivo tumor models to better evaluate chemopreventive efficacy as well as potential toxicity. To date, most in vivo tests have been done using mouse models which omit the potential effect that mitochondria-targeted compounds could have on modulating anticancer immunity. Future studies will need more syngeneic tumor models to fully understand how various mitochondria-targeted species perform in the context of a functional immune system. Although TPP+ has low toxicity, non-specific toxicities have been reported for some TPP-derived compounds that were given systemically (134). To reduce unwanted toxicities, attempts have included modification of the TPP cation and liposome incorporation of the modified compounds to improve selectivity for tumor cells and their mitochondria (134, 179). Regional administration of the compounds, such as intratumoral injection, could also be an alternative method to systemic administration. Advantages of intratumoral injection include the ability to greatly enhance drug concentration around the target injection site, and to lower the exposure of other organs to the drug (180, 181). Moreover, in situ vaccination using mitochondria-targeted compounds could also help to prevent distant/secondary tumor challenge (unpublished data).
Overcoming resistance to standard chemotherapeutics and preventing distant metastasis have been a recent focus for broadening the utility of mitochondria-targeted compounds for cancer prevention and treatment. Besides conjugating parental drugs to TPP+ as a potential way to evade efflux pumps, a recent trend has been to encase the conjugates in nanosized carriers for better drug stability, or to incorporate them with inhibitors of P-glycoprotein pumps (124, 125, 139). Multiple animal studies have demonstrated that some TPP-derived compounds can cross the blood-brain barrier and be taken up into brain tissue, including drugs that have the potential to protect from mitochondrial damage in neurodegenerative diseases (29, 182–184). Therefore, some mitochondria-targeted compounds may be useful to treat or prevent brain metastasis. As lead examples, both Mito-HNK and Mito-LND have shown excellent ability to retard brain metastases (31, 32). It is therefore likely that other agents could be developed to treat brain metastases or neurological tumors.
7. Conclusion
Only in the last decade has mitochondrial metabolism emerged as a promising target for cancer prevention/treatment, thanks to our better understanding of the vital role of mitochondria in cancer development, progression, and regulating the tumor immune microenvironment. A diverse array of mitochondria-targeting drugs has been developed that show good anticancer activities in preclinical settings. In general, many of these mito-targeting compounds have better pharmacological activities (bioavailability, cellular and mitochondrial uptake) and markedly improved anticancer efficacy compared with the parental compounds without harming normal cells. These properties make this class of drugs attractive for development as preventive and therapeutic agents. However, translating these promising preclinical agents to clinical use will require considerably more effort. In this review, we summarized our vision for the next frontier of this field including anti-cancer strategies aimed at mitochondrial metabolic and bioenergetic modifications that are characteristic of many cancers (Figure 3 and Table 3) as well as newly developed drug carrier delivery systems. In addition to suppressing primary tumors, some mito-compounds (Mito-HNK, Mito-LND, and Mito-TAM) can efficiently prevent or suppress tumor metastasis. We also pointed out the potential role of mito-compounds in reshaping the tumor immune microenvironment in a manner that would promote cancer elimination. Although this new direction is in its infancy and needs more in-depth study, we predict that targeting immuno-metabolism will lead to new breakthroughs for prevention and treatment. As this field continues to advance, and with many promising candidates under development, there is considerable potential for several of these candidates to advance to clinical trials and eventual preventive use.
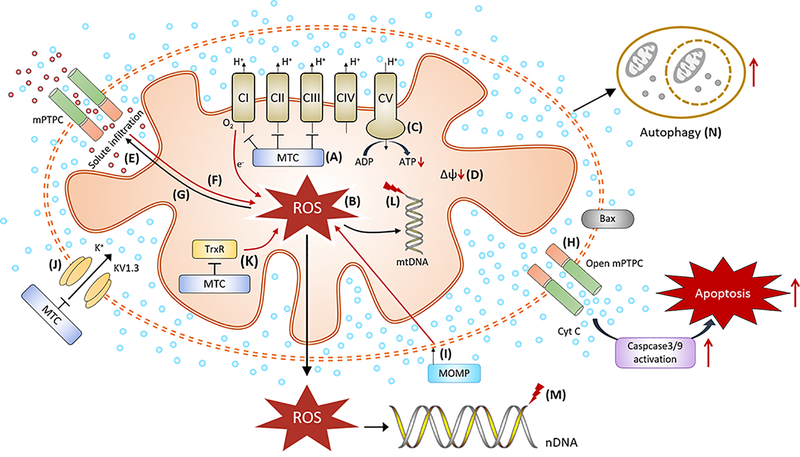
Figure 3. Potential anticancer activities of mitochondria-targeted compounds (MTC).
Inhibition on ETC by MTC (A) leads to mitochondrial uncoupling, with overproduction of mitochondrial ROS (B) (31, 32), and decreases in ATP generation (C) (30, 109). The initial production of mitochondrial ROS can lead to membrane potential dissipation (D) (69, 96, 115), and opening of the mitochondrial permeability transition pore (mPTPC) (E) (73). The opening of the mPTPC could intensify mitochondrial ROS production (F) (191), allowing large amounts of solutes to enter the mitochondrial matrix and cause necrosis-like cell death (G) (191), and release of cytochrome c (Cyt C) into cytosol to trigger apoptosis (H) (73, 115, 156). Induction of mitochondrial outer membrane permeabilization (MOMP, I) (152), inhibition of Kv1.3 channels (J) (155) and TrxR activity (K) (69) by MTC could also promote more ROS production. Excessive mitochondrial ROS can damage mitochondrial DNA (L) (118) and telomeric nuclear DNA (nDNA) (M) (192). These mitochondrial stressors can potentially lead to autophagy-induced cell death (N) (32).
Table 3.
Regulation on mitochondrial ETC complexes and glycolysis
| Mitochondrial- targeted compounds | ETC complexes | Glycolysis | Reference |
|---|---|---|---|
| Mito-honokiol | Complex I inhibition | Inhibition | (31) |
| Mito-lonidamine | Complex I inhibition | Inhibition | (32) |
| Mito-metformin | Complex I inhibition | Inhibition | (178) |
| Mito-resveratrol | Complex I and III inhibition | - | (53) |
| GA-TPP+C10 | Complex I inhibition | Increase | (75) |
| Mito-tamoxifen | Complex I inhibition | - | (99) |
| Mito-DCA | - | Inhibition | (87) |
| TPP- pyruvate dehydrogenase kinase inhibitors | - | Inhibition | (85) |
| MitoVit2 E | Complex I inhibition | - | (186) |
| Mito vitamin E succinate | Complex II inhibition | - | (187) |
| MitoVE11S | Complex II and III inhibition | - | (188) |
| Mito-Coenzyme Q10 | Complex I inhibition | - | (189) |
| Mito-3-bromopyruvate | - | Inhibition | (190) |
Acknowledgements:
This work was supported by National Cancer Institute grants R01CA232433 (M.Y.) and R01CA208648 (M.Y.).
Footnotes
Competing Interests: M. Y. is a co-founder of OncoImmune, Inc. No potential conflicts of interest were disclosed by other authors.
References
- 1.Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). International journal of molecular medicine. 2019;44(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tait SWG, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 2013;5(9):a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O The Metabolism of Carcinoma Cells. The Journal of Cancer Research. 1925;9(1):148. [Google Scholar]
- 4.Racker E. Bioenergetics and the Problem of Tumor Growth: An understanding of the mechanism of the generation and control of biological energy may shed light on the problem of tumor growth. American Scientist. 1972;60(1):56–63. [PubMed] [Google Scholar]
- 5.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morais R, Zinkewich-Peotti K, Parent M, Wang H, Babai F, Zollinger M. Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA. Cancer research. 1994;54(14):3889–96. [PubMed] [Google Scholar]
- 7.Cavalli LR, Varella-Garcia M, Liang BC. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1997;8(11):1189–98. [PubMed] [Google Scholar]
- 8.Magda D, Lecane P, Prescott J, Thiemann P, Ma X, Dranchak PK, et al. mtDNA depletion confers specific gene expression profiles in human cells grown in culture and in xenograft. BMC Genomics. 2008;9:521-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrish F, Hockenbery D. MYC and mitochondrial biogenesis. Cold Spring Harb Perspect Med. 2014;4(5):a014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zacksenhaus E, Shrestha M, Liu JC, Vorobieva I, Chung PED, Ju Y, et al. Mitochondrial OXPHOS Induced by RB1 Deficiency in Breast Cancer: Implications for Anabolic Metabolism, Stemness, and Metastasis. Trends in cancer. 2017;3(11):768–79. [DOI] [PubMed] [Google Scholar]
- 11.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nature reviews Cancer. 2014;14(11):709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. The FEBS journal. 2007;274(6):1393–418. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, et al. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer letters. 2018;435:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo C-Y, Ann DK. When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Commun (Lond). 2018;38(1):47-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C-C, Tseng L-M, Lee H-C. Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (Maywood). 2016;241(12):1281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frantz M-C, Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen. 2010;51(5):462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porporato PE, Filigheddu N, Pedro JMB-S, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Research. 2018;28(3):265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrest MD. Why cancer cells have a more hyperpolarised mitochondrial membrane potential and emergent prospects for therapy. bioRxiv. 2015:025197. [Google Scholar]
- 19.Modica-Napolitano JS, Aprille JR. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Advanced drug delivery reviews. 2001;49(1–2):63–70. [DOI] [PubMed] [Google Scholar]
- 20.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy. Molecular aspects of medicine. 2010;31(2):145–70. [DOI] [PubMed] [Google Scholar]
- 21.Weiss MJ, Wong JR, Ha CS, Bleday R, Salem RR, Steele GD Jr., et al. Dequalinium, a topical antimicrobial agent, displays anticarcinoma activity based on selective mitochondrial accumulation. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(15):5444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baracca A, Sgarbi G, Solaini G, Lenaz G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2003;1606(1):137–46. [DOI] [PubMed] [Google Scholar]
- 23.Terada H, Nagamune H, Morikawa N, Ichikawa T. The cyanine dye triS-C4(5) as a cationic uncoupler of oxidative phosphorylation: interaction with mitochondria detected by derivative spectrophotometry. Cell structure and function. 1983;8(2):161–70. [DOI] [PubMed] [Google Scholar]
- 24.Rottenberg H Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. The Journal of membrane biology. 1984;81(2):127–38. [DOI] [PubMed] [Google Scholar]
- 25.Zinovkin RA, Bakeeva LE, Chernyak BV, Egorov MV, Isaev NK, Kolosova NG, et al. Mitochondrial Targeting of Antioxidants. In: Laher I, editor. Systems Biology of Free Radicals and Antioxidants. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. p. 323–54. [Google Scholar]
- 26.Ralph SJ, Neuzil J. Mitocans, a class of emerging anti-cancer drugs. Molecular nutrition & food research. 2009;53(1):7–8. [DOI] [PubMed] [Google Scholar]
- 27.Dhanya D, Palma G, Cappello AR, Mariconda A, Sinicropi MS, Francesca G, et al. Phosphonium Salt Displays Cytotoxic Effects Against Human Cancer Cell Lines. Anti-cancer agents in medicinal chemistry. 2017;17(13):1796–804. [DOI] [PubMed] [Google Scholar]
- 28.Manetta A, Gamboa G, Nasseri A, Podnos YD, Emma D, Dorion G, et al. Novel phosphonium salts display in vitro and in vivo cytotoxic activity against human ovarian cancer cell lines. Gynecologic oncology. 1996;60(2):203–12. [DOI] [PubMed] [Google Scholar]
- 29.Smith RAJ, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryant KG, Chae YC, Martinez RL, Gordon JC, Elokely KM, Kossenkov AV, et al. A Mitochondrial-targeted purine-based HSP90 antagonist for leukemia therapy. Oncotarget. 2017;8(68):112184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J, Lee Y, Cheng G, Zielonka J, Zhang Q, Bajzikova M, et al. Mitochondria-Targeted Honokiol Confers a Striking Inhibitory Effect on Lung Cancer via Inhibiting Complex I Activity. iScience. 2018;3:192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng G, Zhang Q, Pan J, Lee Y, Ouari O, Hardy M, et al. Targeting lonidamine to mitochondria mitigates lung tumorigenesis and brain metastasis. Nature communications. 2019;10(1):2205-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsepaeva OV, Nemtarev AV, Grigor Eva LR, Salikhova TI, Khozyainova SA, Abdullin TI, et al. Synthesis, Anticancer and Antibacterial Activity of Betulinic and Betulonic Acid C-28-Triphenylphosphonium Conjugates with Variable Alkyl Linker Length. Anti-cancer agents in medicinal chemistry. 2019. [DOI] [PubMed] [Google Scholar]
- 34.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, et al. Prodrugs: design and clinical applications. Nature reviews Drug discovery. 2008;7(3):255–70. [DOI] [PubMed] [Google Scholar]
- 35.Malam Y, Lim EJ, Seifalian AM. Current trends in the application of nanoparticles in drug delivery. Current medicinal chemistry. 2011;18(7):1067–78. [DOI] [PubMed] [Google Scholar]
- 36.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Advanced drug delivery reviews. 2013;65(1):71–9. [DOI] [PubMed] [Google Scholar]
- 37.Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, et al. Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted antioxidants. Crit Care Med. 2007;35(9 Suppl):S461–7. [DOI] [PubMed] [Google Scholar]
- 38.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Amoscato AA, et al. Structural Requirements for Optimized Delivery, Inhibition of Oxidative Stress, and Antiapoptotic Activity of Targeted Nitroxides. Journal of Pharmacology and Experimental Therapeutics. 2007;320(3):1050–60. [DOI] [PubMed] [Google Scholar]
- 39.Krainz T, Lamade AM, Du L, Maskrey TS, Calderon MJ, Watkins SC, et al. Synthesis and Evaluation of a Mitochondria-Targeting Poly(ADP-ribose) Polymerase-1 Inhibitor. ACS chemical biology. 2018;13(10):2868–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, Lim C, Sacher JR, Van Houten B, Qian W, Wipf P. Mitochondrial targeted β-lapachone induces mitochondrial dysfunction and catastrophic vacuolization in cancer cells. Bioorg Med Chem Lett. 2015;25(21):4828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xun Z, Rivera-Sánchez S, Ayala-Peña S, Lim J, Budworth H, Skoda EM, et al. Targeting of XJB-5–131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington’s disease. Cell reports. 2012;2(5):1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton KL, Kelley SO. Engineered apoptosis-inducing peptides with enhanced mitochondrial localization and potency. Journal of medicinal chemistry. 2009;52(10):3293–9. [DOI] [PubMed] [Google Scholar]
- 43.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochemical pharmacology. 2005;70(12):1796–806. [DOI] [PubMed] [Google Scholar]
- 44.Arora S, Singh S, Piazza GA, Contreras CM, Panyam J, Singh AP. Honokiol: a novel natural agent for cancer prevention and therapy. Curr Mol Med. 2012;12(10):1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauf A, Patel S, Imran M, Maalik A, Arshad MU, Saeed F, et al. Honokiol: An anticancer lignan. Biomedicine & Pharmacotherapy. 2018;107:555–62. [DOI] [PubMed] [Google Scholar]
- 46.Pan J, Zhang Q, Liu Q, Komas SM, Kalyanaraman B, Lubet RA, et al. Honokiol inhibits lung tumorigenesis through inhibition of mitochondrial function. Cancer prevention research (Philadelphia, Pa). 2014;7(11):1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Duan X, Yang G, Zhang X, Deng L, Zheng H, et al. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PloS one. 2011;6(4):e18490–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer letters. 2009;284(1):1–6. [DOI] [PubMed] [Google Scholar]
- 49.Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21(3):R209–R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gambini J, Inglés M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Medicine and Cellular Longevity. 2015;2015:837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biasutto L, Mattarei A, Marotta E, Bradaschia A, Sassi N, Garbisa S, et al. Development of mitochondria-targeted derivatives of resveratrol. Bioorg Med Chem Lett. 2008;18(20):5594–7. [DOI] [PubMed] [Google Scholar]
- 52.Sassi N, Mattarei A, Azzolini M, Bernardi P, Szabo I, Paradisi C, et al. Mitochondria-targeted resveratrol derivatives act as cytotoxic pro-oxidants. Current pharmaceutical design. 2014;20(2):172–9. [DOI] [PubMed] [Google Scholar]
- 53.Sassi N, Mattarei A, Azzolini M, Szabo I, Paradisi C, Zoratti M, et al. Cytotoxicity of mitochondria-targeted resveratrol derivatives: interactions with respiratory chain complexes and ATP synthase. Biochimica et biophysica acta. 2014;1837(10):1781–9. [DOI] [PubMed] [Google Scholar]
- 54.Kang JH, Ko YT. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics. 2019;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauf A, Imran M, Khan IA, ur-Rehman M, Gilani SA, Mehmood Z, et al. Anticancer potential of quercetin: A comprehensive review. Phytotherapy Research. 2018;32(11):2109–30. [DOI] [PubMed] [Google Scholar]
- 56.Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, et al. Quercetin and cancer chemoprevention. Evidence-based complementary and alternative medicine : eCAM. 2011;2011:591356-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeong J-H, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. Journal of cellular biochemistry. 2009;106(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mattarei A, Biasutto L, Marotta E, De Marchi U, Sassi N, Garbisa S, et al. A mitochondriotropic derivative of quercetin: a strategy to increase the effectiveness of polyphenols. Chembiochem : a European journal of chemical biology. 2008;9(16):2633–42. [DOI] [PubMed] [Google Scholar]
- 59.Xing L, Lyu JY, Yang Y, Cui PF, Gu LQ, Qiao JB, et al. pH-Responsive de-PEGylated nanoparticles based on triphenylphosphine-quercetin self-assemblies for mitochondria-targeted cancer therapy. Chemical communications (Cambridge, England). 2017;53(62):8790–3. [DOI] [PubMed] [Google Scholar]
- 60.Pongrakananon V, Rojanasakul Y. Anticancer Properties of Curcumin. 2011.
- 61.Reddy CA, Somepalli V, Golakoti T, Kanugula AK, Karnewar S, Rajendiran K, et al. Mitochondrial-targeted curcuminoids: a strategy to enhance bioavailability and anticancer efficacy of curcumin. PloS one. 2014;9(3):e89351–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slika L, Patra D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert opinion on drug delivery. 2019:1–15. [DOI] [PubMed] [Google Scholar]
- 63.Arner ES. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochimica et biophysica acta. 2009;1790(6):495–526. [DOI] [PubMed] [Google Scholar]
- 64.Rundlof AK, Arner ES. Regulation of the mammalian selenoprotein thioredoxin reductase 1 in relation to cellular phenotype, growth, and signaling events. Antioxid Redox Signal. 2004;6(1):41–52. [DOI] [PubMed] [Google Scholar]
- 65.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Current opinion in pharmacology. 2007;7(4):392–7. [DOI] [PubMed] [Google Scholar]
- 66.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. The Journal of biological chemistry. 2005;280(26):25284–90. [DOI] [PubMed] [Google Scholar]
- 67.Qiu X, Liu Z, Shao WY, Liu X, Jing DP, Yu YJ, et al. Synthesis and evaluation of curcumin analogues as potential thioredoxin reductase inhibitors. Bioorganic & medicinal chemistry. 2008;16(17):8035–41. [DOI] [PubMed] [Google Scholar]
- 68.Liang B, Shao W, Zhu C, Wen G, Yue X, Wang R, et al. Mitochondria-Targeted Approach: Remarkably Enhanced Cellular Bioactivities of TPP2a as Selective Inhibitor and Probe toward TrxR. ACS chemical biology. 2016;11(2):425–34. [DOI] [PubMed] [Google Scholar]
- 69.Jayakumar S, Patwardhan RS, Pal D, Singh B, Sharma D, Kutala VK, et al. Mitochondrial targeted curcumin exhibits anticancer effects through disruption of mitochondrial redox and modulation of TrxR2 activity. Free radical biology & medicine. 2017;113:530–8. [DOI] [PubMed] [Google Scholar]
- 70.Rocha L, Monteiro M, Teodoro A. Anticancer Properties of Hydroxycinnamic Acids -A Review. Cancer and Clinical Oncology. 2012;1. [Google Scholar]
- 71.Chang WC, Hsieh CH, Hsiao MW, Lin WC, Hung YC, Ye JC. Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwanese journal of obstetrics & gynecology. 2010;49(4):419–24. [DOI] [PubMed] [Google Scholar]
- 72.Shailasree S, Venkataramana M, Niranjana SR, Prakash HS. Cytotoxic effect of p-Coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Molecular neurobiology. 2015;51(1):119–30. [DOI] [PubMed] [Google Scholar]
- 73.Li J, He D, Wang B, Zhang L, Li K, Xie Q, et al. Synthesis of hydroxycinnamic acid derivatives as mitochondria-targeted antioxidants and cytotoxic agents. Acta Pharm Sin B. 2017;7(1):106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandoval-Acuña C, Fuentes-Retamal S, Guzmán-Rivera D, Peredo-Silva L, Madrid-Rojas M, Rebolledo S, et al. Destabilization of mitochondrial functions as a target against breast cancer progression: Role of TPP+-linked-polyhydroxybenzoates. Toxicology and Applied Pharmacology. 2016;309:2–14. [DOI] [PubMed] [Google Scholar]
- 75.Fuentes-Retamal S, Sandoval-Acuna C, Peredo-Silva L, Guzman-Rivera D, Pavani M, Torrealba N, et al. Complex Mitochondrial Dysfunction Induced by TPP(+)-Gentisic Acid and Mitochondrial Translation Inhibition by Doxycycline Evokes Synergistic Lethality in Breast Cancer Cells. Cells. 2020;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma S, Khan N, Sultana S. Study on prevention of two-stage skin carcinogenesis by Hibiscus rosa sinensis extract and the role of its chemical constituent, gentisic acid, in the inhibition of tumour promotion response and oxidative stress in mice. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 2004;13(1):53–63. [DOI] [PubMed] [Google Scholar]
- 77.Sharma S, Khan N, Sultana S. Modulatory effect of gentisic acid on the augmentation of biochemical events of tumor promotion stage by benzoyl peroxide and ultraviolet radiation in Swiss albino mice. Toxicology letters. 2004;153(3):293–302. [DOI] [PubMed] [Google Scholar]
- 78.Altinoz M, Elmaci I, Cengiz S, Emekli-Alturfan E, Ozpinar A. From epidemiology to treatment: Aspirin’s prevention of brain and breast-cancer and cardioprotection May associate with its metabolite gentisic acid. Chemico-Biological Interactions. 2018;291. [DOI] [PubMed] [Google Scholar]
- 79.Gazzano E, Lazzarato L, Rolando B, Kopecka J, Guglielmo S, Costamagna C, et al. Mitochondrial Delivery of Phenol Substructure Triggers Mitochondrial Depolarization and Apoptosis of Cancer Cells. Frontiers in pharmacology. 2018;9:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Cosimo S, Ferretti G, Papaldo P, Carlini P, Fabi A, Cognetti F. Lonidamine: efficacy and safety in clinical trials for the treatment of solid tumors. Drugs of today (Barcelona, Spain : 1998). 2003;39(3):157–74. [DOI] [PubMed] [Google Scholar]
- 81.Floridi A, Paggi MG, Marcante ML, Silvestrini B, Caputo A, De Martino C. Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. Journal of the National Cancer Institute. 1981;66(3):497–9. [PubMed] [Google Scholar]
- 82.Guo L, Shestov AA, Worth AJ, Nath K, Nelson DS, Leeper DB, et al. Inhibition of Mitochondrial Complex II by the Anticancer Agent Lonidamine. The Journal of biological chemistry. 2016;291(1):42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emmanouilidi A, Falasca M. Targeting PDK1 for Chemosensitization of Cancer Cells. Cancers (Basel). 2017;9(10):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sradhanjali S, Reddy MM. Inhibition of Pyruvate Dehydrogenase Kinase as a Therapeutic Strategy against Cancer. Current topics in medicinal chemistry. 2018;18(6):444–53. [DOI] [PubMed] [Google Scholar]
- 85.Xu B, Yu Z, Xiang S, Li Y, Zhang SL, He Y. Rational design of mitochondria-targeted pyruvate dehydrogenase kinase 1 inhibitors with improved selectivity and antiproliferative activity. Eur J Med Chem. 2018;155:275–84. [DOI] [PubMed] [Google Scholar]
- 86.Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, et al. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Investigational new drugs. 2014;32(3):452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pathak RK, Marrache S, Harn DA, Dhar S. Mito-DCA: a mitochondria targeted molecular scaffold for efficacious delivery of metabolic modulator dichloroacetate. ACS chemical biology. 2014;9(5):1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morrot A, da Fonseca LM, Salustiano EJ, Gentile LB, Conde L, Filardy AA, et al. Metabolic Symbiosis and Immunomodulation: How Tumor Cell-Derived Lactate May Disturb Innate and Adaptive Immune Responses. Frontiers in oncology. 2018;8:81-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghante MH, Jamkhande PG. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J Pharmacopuncture. 2019;22(2):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen CJ, Shih YL, Yeh MY, Liao NC, Chung HY, Liu KL, et al. Ursolic Acid Induces Apoptotic Cell Death Through AIF and Endo G Release Through a Mitochondria-dependent Pathway in NCI-H292 Human Lung Cancer Cells In Vitro. In vivo (Athens, Greece). 2019;33(2):383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar P, Bhadauria AS, Singh AK, Saha S. Betulinic acid as apoptosis activator: Molecular mechanisms, mathematical modeling and chemical modifications. Life sciences. 2018;209:24–33. [DOI] [PubMed] [Google Scholar]
- 92.J C Furtado NA, Pirson L, Edelberg H, M Miranda L, Loira-Pastoriza C, Preat V, et al. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules (Basel, Switzerland). 2017;22(3):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nedopekina DA, Gubaidullin RR, Odinokov VN, Maximchik PV, Zhivotovsky B, Bel’skii YP, et al. Mitochondria-targeted betulinic and ursolic acid derivatives: synthesis and anticancer activity. Medchemcomm. 2017;8(10):1934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsepaeva OV, Nemtarev AV, Abdullin TI, Grigor’eva LR, Kuznetsova EV, Akhmadishina RA, et al. Design, Synthesis, and Cancer Cell Growth Inhibitory Activity of Triphenylphosphonium Derivatives of the Triterpenoid Betulin. Journal of natural products. 2017;80(8):2232–9. [DOI] [PubMed] [Google Scholar]
- 95.Roohbakhsh A, Iranshahy M, Iranshahi M. Glycyrrhetinic Acid and Its Derivatives: Anti-Cancer and Cancer Chemopreventive Properties, Mechanisms of Action and Structure- Cytotoxic Activity Relationship. Current medicinal chemistry. 2016;23(5):498–517. [DOI] [PubMed] [Google Scholar]
- 96.Jin L, Dai L, Ji M, Wang H. Mitochondria-targeted triphenylphosphonium conjugated glycyrrhetinic acid derivatives as potent anticancer drugs. Bioorganic chemistry. 2019;85:179–90. [DOI] [PubMed] [Google Scholar]
- 97.Cardoso CM, Custodio JB, Almeida LM, Moreno AJ. Mechanisms of the deleterious effects of tamoxifen on mitochondrial respiration rate and phosphorylation efficiency. Toxicol Appl Pharmacol. 2001;176(3):145–52. [DOI] [PubMed] [Google Scholar]
- 98.Moreira PI, Custodio J, Moreno A, Oliveira CR, Santos MS. Tamoxifen and estradiol interact with the flavin mononucleotide site of complex I leading to mitochondrial failure. The Journal of biological chemistry. 2006;281(15):10143–52. [DOI] [PubMed] [Google Scholar]
- 99.Rohlenova K, Sachaphibulkij K, Stursa J, Bezawork-Geleta A, Blecha J, Endaya B, et al. Selective Disruption of Respiratory Supercomplexes as a New Strategy to Suppress Her2(high) Breast Cancer. Antioxid Redox Signal. 2017;26(2):84–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. Journal of the National Cancer Institute. 2010;102(20):1536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hubackova S, Davidova E, Rohlenova K, Stursa J, Werner L, Andera L, et al. Selective elimination of senescent cells by mitochondrial targeting is regulated by ANT2. Cell Death & Differentiation. 2019;26(2):276–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(12):4766–71. [DOI] [PubMed] [Google Scholar]
- 103.Titova E, Shagieva G, Ivanova O, Domnina L, Domninskaya M, Strelkova O, et al. Mitochondria-targeted antioxidant SkQ1 suppresses fibrosarcoma and rhabdomyosarcoma tumour cell growth. Cell cycle (Georgetown, Tex). 2018;17(14):1797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Willems E, Dedobbeleer M, Digregorio M, Lombard A, Lumapat PN, Rogister B. The functional diversity of Aurora kinases: a comprehensive review. Cell division. 2018;13:7-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25(38):5220–7. [DOI] [PubMed] [Google Scholar]
- 106.Trotta AP, Chipuk JE. Mitochondrial dynamics as regulators of cancer biology. Cellular and molecular life sciences : CMLS. 2017;74(11):1999–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kong B, Tsuyoshi H, Orisaka M, Shieh D-B, Yoshida Y, Tsang BK. Mitochondrial dynamics regulating chemoresistance in gynecological cancers. Annals of the New York Academy of Sciences. 2015;1350(1):1–16. [DOI] [PubMed] [Google Scholar]
- 108.Pellestor F, Paulasova P. The peptide nucleic acids (PNAs), powerful tools for molecular genetics and cytogenetics. European Journal of Human Genetics. 2004;12(9):694–700. [DOI] [PubMed] [Google Scholar]
- 109.Chen S-S, Tu X-Y, Xie L-X, Xiong L-P, Song J, Ye X-Q. Peptide nucleic acids targeting mitochondria enhances sensitivity of lung cancer cells to chemotherapy. Am J Transl Res. 2018;10(9):2940–8. [PMC free article] [PubMed] [Google Scholar]
- 110.Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton transactions (Cambridge, England : 2003). 2010;39(35):8113–27. [DOI] [PubMed] [Google Scholar]
- 111.Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton transactions (Cambridge, England : 2003). 2018;47(19):6645–53. [DOI] [PubMed] [Google Scholar]
- 112.Zhu Z, Wang Z, Zhang C, Wang Y, Zhang H, Gan Z, et al. Mitochondrion-targeted platinum complexes suppressing lung cancer through multiple pathways involving energy metabolism. Chemical science. 2019;10(10):3089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang K, Zhu C, He Y, Zhang Z, Zhou W, Muhammad N, et al. Restraining Cancer Cells by Dual Metabolic Inhibition with a Mitochondrion-Targeted Platinum(II) Complex. Angewandte Chemie (International ed in English). 2019;58(14):4638–43. [DOI] [PubMed] [Google Scholar]
- 114.Gibson D Platinum(iv) anticancer prodrugs - hypotheses and facts. Dalton transactions (Cambridge, England : 2003). 2016;45(33):12983–91. [DOI] [PubMed] [Google Scholar]
- 115.Jin S, Hao Y, Zhu Z, Muhammad N, Zhang Z, Wang K, et al. Impact of Mitochondrion-Targeting Group on the Reactivity and Cytostatic Pathway of Platinum(IV) Complexes. Inorganic chemistry. 2018;57(17):11135–45. [DOI] [PubMed] [Google Scholar]
- 116.Vidal L, Gurion R, Ram R, Raanani P, Bairey O, Robak T, et al. Chlorambucil for the treatment of patients with chronic lymphocytic leukemia (CLL) - a systematic review and meta-analysis of randomized trials. Leukemia & lymphoma. 2016;57(9):2047–57. [DOI] [PubMed] [Google Scholar]
- 117.Singh RK, Kumar S, Prasad DN, Bhardwaj TR. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. European Journal of Medicinal Chemistry. 2018;151:401–33. [DOI] [PubMed] [Google Scholar]
- 118.Millard M, Gallagher JD, Olenyuk BZ, Neamati N. A selective mitochondrial-targeted chlorambucil with remarkable cytotoxicity in breast and pancreatic cancers. Journal of medicinal chemistry. 2013;56(22):9170–9. [DOI] [PubMed] [Google Scholar]
- 119.Pathak RK, Wen R, Kolishetti N, Dhar S. A Prodrug of Two Approved Drugs, Cisplatin and Chlorambucil, for Chemo War Against Cancer. Molecular cancer therapeutics. 2017;16(4):625–36. [DOI] [PubMed] [Google Scholar]
- 120.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nature Reviews Cancer. 2009;9(5):338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, et al. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem Rev. 2017;117(15):10043–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Broxterman HJ, Gotink KJ, Verheul HMW. Understanding the causes of multidrug resistance in cancer: a comparison of doxorubicin and sunitinib. Drug Resistance Updates. 2009;12(4):114–26. [DOI] [PubMed] [Google Scholar]
- 123.Zhou M, Li L, Li L, Lin X, Wang F, Li Q, et al. Overcoming chemotherapy resistance via simultaneous drug-efflux circumvention and mitochondrial targeting. Acta Pharm Sin B. 2019;9(3):615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li WQ, Wang Z, Hao S, He H, Wan Y, Zhu C, et al. Mitochondria-Targeting Polydopamine Nanoparticles To Deliver Doxorubicin for Overcoming Drug Resistance. ACS applied materials & interfaces. 2017;9(20):16793–802. [DOI] [PubMed] [Google Scholar]
- 125.Yu H, Li JM, Deng K, Zhou W, Wang CX, Wang Q, et al. Tumor acidity activated triphenylphosphonium-based mitochondrial targeting nanocarriers for overcoming drug resistance of cancer therapy. Theranostics. 2019;9(23):7033–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu HN, Guo NN, Wang TT, Guo WW, Lin MT, Huang-Fu MY, et al. Mitochondrial Targeted Doxorubicin-Triphenylphosphonium Delivered by Hyaluronic Acid Modified and pH Responsive Nanocarriers to Breast Tumor: in Vitro and in Vivo Studies. Molecular pharmaceutics. 2018;15(3):882–91. [DOI] [PubMed] [Google Scholar]
- 127.Liu HN, Guo NN, Guo WW, Huang-Fu MY, Vakili MR, Chen JJ, et al. Delivery of mitochondriotropic doxorubicin derivatives using self-assembling hyaluronic acid nanocarriers in doxorubicin-resistant breast cancer. Acta Pharmacol Sin. 2018;39(10):1681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018;11(1):64-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim KY, Jin H, Park J, Jung SH, Lee JH, Park H, et al. Mitochondria-targeting self-assembled nanoparticles derived from triphenylphosphonium-conjugated cyanostilbene enable site-specific imaging and anticancer drug delivery. Nano Research. 2018;11(2):1082–98. [Google Scholar]
- 130.Florian S, Mitchison TJ. Anti-Microtubule Drugs. Methods in molecular biology (Clifton, NJ). 2016;1413:403–21. [DOI] [PubMed] [Google Scholar]
- 131.Thirumaran R, Prendergast GC, Gilman PB. Chapter 7 - Cytotoxic Chemotherapy in Clinical Treatment of Cancer. In: Prendergast GC, Jaffee EM, editors. Cancer Immunotherapy. Burlington: Academic Press; 2007. p. 101–16. [Google Scholar]
- 132.Markman M Managing taxane toxicities. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2003;11(3):144–7. [DOI] [PubMed] [Google Scholar]
- 133.Battogtokh G, Gotov O, Kang JH, Cho J, Jeong TH, Chimed G, et al. Triphenylphosphine-docetaxel conjugate-incorporated albumin nanoparticles for cancer treatment. Nanomedicine (London, England). 2018;13(3):325–38. [DOI] [PubMed] [Google Scholar]
- 134.Zhang J, Yang C, Pan S, Shi M, Li J, Hu H, et al. Eph A10-modified pH-sensitive liposomes loaded with novel triphenylphosphine-docetaxel conjugate possess hierarchical targetability and sufficient antitumor effect both in vitro and in vivo. Drug delivery. 2018;25(1):723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nagano K, Maeda Y, Kanasaki S-i, Watanabe T, Yamashita T, Inoue M, et al. Ephrin receptor A10 is a promising drug target potentially useful for breast cancers including triple negative breast cancers. Journal of Controlled Release. 2014;189:72–9. [DOI] [PubMed] [Google Scholar]
- 136.Barbuti AM, Chen Z-S. Paclitaxel Through the Ages of Anticancer Therapy: Exploring Its Role in Chemoresistance and Radiation Therapy. Cancers (Basel). 2015;7(4):2360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Solomon MA, Shah AA, D’Souza GGM. In Vitro assessment of the utility of stearyl triphenyl phosphonium modified liposomes in overcoming the resistance of ovarian carcinoma Ovcar-3 cells to paclitaxel. Mitochondrion. 2013;13(5):464–72. [DOI] [PubMed] [Google Scholar]
- 138.Zhou J, Zhao WY, Ma X, Ju RJ, Li XY, Li N, et al. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials. 2013;34(14):3626–38. [DOI] [PubMed] [Google Scholar]
- 139.Han X, Su R, Huang X, Wang Y, Kuang X, Zhou S, et al. Triphenylphosphonium-modified mitochondria-targeted paclitaxel nanocrystals for overcoming multidrug resistance. Asian journal of pharmaceutical sciences. 2019;14(5):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Targeting HSP90 for cancer therapy. British journal of cancer. 2009;100(10):1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P. Maximizing the Therapeutic Potential of HSP90 Inhibitors. Mol Cancer Res. 2015;13(11):1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, et al. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. The Journal of clinical investigation. 2009;119(3):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Siegelin MD, Dohi T, Raskett CM, Orlowski GM, Powers CM, Gilbert CA, et al. Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. The Journal of clinical investigation. 2011;121(4):1349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vo VTA, Choi JW, Phan ANH, Hua TNM, Kim MK, Kang BH, et al. TRAP1 Inhibition Increases Glutamine Synthetase Activity in Glutamine Auxotrophic Non-small Cell Lung Cancer Cells. Anticancer research. 2018;38(4):2187–93. [DOI] [PubMed] [Google Scholar]
- 145.van den Heuvel AP, Jing J, Wooster RF, Bachman KE. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer biology & therapy. 2012;13(12):1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Basak NP, Banerjee S. Mitochondrial dependency in progression of acute myeloid leukemia. Mitochondrion. 2015;21:41–8. [DOI] [PubMed] [Google Scholar]
- 147.Jolly C, Morimoto RI. Role of the Heat Shock Response and Molecular Chaperones in Oncogenesis and Cell Death. JNCI: Journal of the National Cancer Institute. 2000;92(19):1564–72. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Z, Banerjee M, Davis RE, Blagg BSJ. Mitochondrial-targeted Hsp90 C-terminal inhibitors manifest anti-proliferative activity. Bioorganic & Medicinal Chemistry Letters. 2019;29(22):126676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34(32):4153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ko SK, Kim J, Na DC, Park S, Park SH, Hyun JY, et al. A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chemistry & biology. 2015;22(3):391–403. [DOI] [PubMed] [Google Scholar]
- 151.Yun C-O, Bhargava P, Na Y, Lee J-S, Ryu J, Kaul SC, et al. Relevance of mortalin to cancer cell stemness and cancer therapy. Scientific reports. 2017;7(1):42016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park SH, Baek KH, Shin I, Shin I. Subcellular Hsp70 Inhibitors Promote Cancer Cell Death via Different Mechanisms. Cell chemical biology. 2018;25(10):1242–54.e8. [DOI] [PubMed] [Google Scholar]
- 153.Bachmann M, Costa R, Peruzzo R, Prosdocimi E, Checchetto V, Leanza L. Targeting Mitochondrial Ion Channels to Fight Cancer. International journal of molecular sciences. 2018;19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Felipe A, Bielanska J, Comes N, Vallejo A, Roig S, Ramón YCS, et al. Targeting the voltage-dependent K(+) channels Kv1.3 and Kv1.5 as tumor biomarkers for cancer detection and prevention. Current medicinal chemistry. 2012;19(5):661–74. [DOI] [PubMed] [Google Scholar]
- 155.Leanza L, Romio M, Becker KA, Azzolini M, Trentin L, Managò A, et al. Direct Pharmacological Targeting of a Mitochondrial Ion Channel Selectively Kills Tumor Cells In Vivo. Cancer cell. 2017;31(4):516–31.e10. [DOI] [PubMed] [Google Scholar]
- 156.Kim S, Palanikumar L, Choi H, Jeena MT, Kim C, Ryu J-H. Intra-mitochondrial biomineralization for inducing apoptosis of cancer cells. Chemical science. 2018;9(9):2474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Angajala A, Lim S, Phillips JB, Kim J-H, Yates C, You Z, et al. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Frontiers in immunology. 2018;9(1605). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annual review of immunology. 2013;31:259–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi H, Chi H. Metabolic Control of Treg Cell Stability, Plasticity, and Tissue-Specific Heterogeneity. Frontiers in immunology. 2019;10:2716-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martinon F Signaling by ROS drives inflammasome activation. European journal of immunology. 2010;40(3):616–9. [DOI] [PubMed] [Google Scholar]
- 161.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2011;12(3):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang C-H, Sanin DE, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166(1):63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002;3(11):991–8. [DOI] [PubMed] [Google Scholar]
- 165.Jiang Q, Zhang C, Wang H, Peng T, Zhang L, Wang Y, et al. Mitochondria-Targeting Immunogenic Cell Death Inducer Improves the Adoptive T-Cell Therapy Against Solid Tumor. Frontiers in oncology. 2019;9:1196-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peng N, Yu H, Yu W, Yang M, Chen H, Zou T, et al. Sequential-targeting nanocarriers with pH-controlled charge reversal for enhanced mitochondria-located photodynamic-immunotherapy of cancer. Acta biomaterialia. 2020;105:223–38. [DOI] [PubMed] [Google Scholar]
- 167.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–21. [DOI] [PubMed] [Google Scholar]
- 168.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–9. [DOI] [PubMed] [Google Scholar]
- 169.Kim SH, Li M, Trousil S, Zhang Y, Pasca di Magliano M, Swanson KD, et al. Phenformin Inhibits Myeloid-Derived Suppressor Cells and Enhances the Anti-Tumor Activity of PD-1 Blockade in Melanoma. Journal of Investigative Dermatology. 2017;137(8):1740–8. [DOI] [PubMed] [Google Scholar]
- 170.Xu P, Yin K, Tang X, Tian J, Zhang Y, Ma J, et al. Metformin inhibits the function of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Biomedicine & Pharmacotherapy. 2019;120:109458. [DOI] [PubMed] [Google Scholar]
- 171.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. The Journal of biological chemistry. 2001;276(7):4588–96. [DOI] [PubMed] [Google Scholar]
- 172.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Annals of the New York Academy of Sciences. 2010;1201:96–103. [DOI] [PubMed] [Google Scholar]
- 173.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(11):1670–4. [DOI] [PubMed] [Google Scholar]
- 174.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver international : official journal of the International Association for the Study of the Liver. 2010;30(7):1019–26. [DOI] [PubMed] [Google Scholar]
- 175.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension (Dallas, Tex : 1979). 2018;71(6):1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ross MF, Prime TA, Abakumova I, James AM, Porteous CM, Smith RA, et al. Rapid and extensive uptake and activation of hydrophobic triphenylphosphonium cations within cells. The Biochemical journal. 2008;411(3):633–45. [DOI] [PubMed] [Google Scholar]
- 177.Cheng G, Zielonka J, Ouari O, Lopez M, McAllister D, Boyle K, et al. Mitochondria-Targeted Analogues of Metformin Exhibit Enhanced Antiproliferative and Radiosensitizing Effects in Pancreatic Cancer Cells. Cancer research. 2016;76(13):3904–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Boukalova S, Stursa J, Werner L, Ezrova Z, Cerny J, Bezawork-Geleta A, et al. Mitochondrial Targeting of Metformin Enhances Its Activity against Pancreatic Cancer. Molecular cancer therapeutics. 2016;15(12):2875–86. [DOI] [PubMed] [Google Scholar]
- 179.Guzman-Villanueva D Symbiosis Influence of Triphenylphosphonium (TPP) Cation Hydrophobization with Phospholipids on Cellular Toxicity and Mitochondrial Selectivity. SOJ Pharmacy & Pharmaceutical Sciences. 2015;2. [Google Scholar]
- 180.Voulgaris S, Partheni M, Karamouzis M, Dimopoulos P, Papadakis N, Kalofonos HP. Intratumoral doxorubicin in patients with malignant brain gliomas. American journal of clinical oncology. 2002;25(1):60–4. [DOI] [PubMed] [Google Scholar]
- 181.Duvillard C, Romanet P, Cosmidis A, Beaudouin N, Chauffert B. Phase 2 study of intratumoral cisplatin and epinephrine treatment for locally recurrent head and neck tumors. The Annals of otology, rhinology, and laryngology. 2004;113(3 Pt 1):229–33. [DOI] [PubMed] [Google Scholar]
- 182.Rodriguez-Cuenca S, Cochemé HM, Logan A, Abakumova I, Prime TA, Rose C, et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radical Biology and Medicine. 2010;48(1):161–72. [DOI] [PubMed] [Google Scholar]
- 183.Ghosh A, Chandran K, Kalivendi SV, Joseph J, Antholine WE, Hillard CJ, et al. Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free radical biology & medicine. 2010;49(11):1674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31(44):15703–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stromyer ML, Southerland MR, Satyal U, Sikder RK, Weader DJ, Baughman JA, et al. Synthesis, characterization, and biological activity of a triphenylphosphonium-containing imidazolium salt against select bladder cancer cell lines. Eur J Med Chem. 2020;185:111832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dhanasekaran A, Kotamraju S, Kalivendi S, Matsunaga T, Shang T, Keszler A, et al. Supplementation of Endothelial Cells with Mitochondria-targeted Antioxidants Inhibit Peroxide-induced Mitochondrial Iron Uptake, Oxidative Damage, and Apoptosis. The Journal of biological chemistry. 2004;279:37575–87. [DOI] [PubMed] [Google Scholar]
- 187.Yan B, Stantic M, Zobalova R, Bezawork-Geleta A, Stapelberg M, Stursa J, et al. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC cancer. 2015;15:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dong LF, Jameson VJ, Tilly D, Cerny J, Mahdavian E, Marin-Hernandez A, et al. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. The Journal of biological chemistry. 2011;286(5):3717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Plecitá-Hlavatá L, Ježek J, Ježek P. Pro-oxidant mitochondrial matrix-targeted ubiquinone MitoQ10 acts as anti-oxidant at retarded electron transport or proton pumping within Complex I. The International Journal of Biochemistry & Cell Biology. 2009;41(8):1697–707. [DOI] [PubMed] [Google Scholar]
- 190.Marrache S, Dhar S. The energy blocker inside the power house: Mitochondria targeted delivery of 3-bromopyruvate. Chemical science. 2015;6(3):1832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Green DR, Reed JC. Mitochondria and Apoptosis. Science (New York, NY). 1998;281(5381):1309–12. [DOI] [PubMed] [Google Scholar]
- 192.Qian W, Kumar N, Roginskaya V, Fouquerel E, Opresko PL, Shiva S, et al. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proceedings of the National Academy of Sciences. 2019;116(37):18435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]