Abstract
Basal cell carcinoma (BCC) is a common disease of the skin caused principally by prolonged solar radiation exposure. It is normally a malignancy with favorable prognostic features and is potentially curable by standard excision. In White populations with high disease incidence, general practitioners (GPs) play a vital role in diagnosing and managing BCC, including surgical excision. Dedicated care at the primary care level by adequately trained GPs is conceivably cost effective for the health system and more convenient for the patient.
In Asia and other parts of the world with low incidence, this valuable role of GPs may appear to be inconsequential. In this regard, any justification for the involvement of local GPs in BCC surgery is debatable. This article aims to provide a clinical update on essential information relevant to BCC surgery and advance understanding of the intricate issues of making a treatment decision at the primary care level.
Case Report
Madam Tan, a 71-year-old Malaysian Chinese lady, otherwise healthy, presented to her local GP with a complaint of a nodule over the left cheek that had been there for more than a decade. Her concern was that the lesion was growing and had become conspicuous. She had spent most of her life as a farmer working in her orchard.
Upon examination, she had an obvious dome-shaped nodule over the left cheek measuring approximately 1.8 cm in diameter. The lesion was firm, pigmented, well-demarcated, and slightly ulcerated at the top. Clinically, she was diagnosed with a pigmented nodular basal cell carcinoma of the left cheek. Examination of the systems was unremarkable.
She requested that the consulting GP remove the growth. The cost for specialist treatment and waiting time at the local hospital were her concerns.
Clinical Questions
Can the basal cell skin cancer be excised safely and effectively in the local primary care setting? What are the crucial preoperative concerns?
Keywords: Basal Cell Carcinoma, Surgical Excision, General Practice, GPwSI
Introduction
Basal cell carcinoma surgery in general practice is gaining a foothold. The pivotal role of general practitioners (GPs) in the diagnosis and management of basal cell carcinoma (BCC) is well established in a number of countries with high incidence of the disease, primarily in countries such as the UK and Australia.1,2 In the US, the role of primary care physicians in the diagnosis and management of skin cancers is increasingly important as the disease rises towards epidemic proportion.3 In other health systems, low-risk BCC is the target of substitution of care, from secondary to primary care.4
BCCs are often treated successfully as office-based procedures in primary care with surgical excision, which remains the gold standard of treatment.5 The involvement of GPs may potentially result in earlier diagnosis, reduced health expenditure, and lessened demand for the over-stretched dermatology services.1
In Asia, where the prevalence is much lower, these benefits may be less evident. However, with the aging population in the tropical environment, we are seeing an increasing number of older patients with BCCs. We believe that local GPs with special interest (GPwSI)6 in skin surgery and dermatology may play a role in the surgical treatment of the relatively straightforward or uncomplicated BCCs (low-risk BCCs) or managing patients who prefer primary care to specialist treatment due to circumstantial constraints. To explore this view further, we examine and present the evidence for or against GPs performing BCC surgery and discuss the challenges and controversies involved.
Methods
To seek answers to the questions posed, we conducted a review on the subject of BCC surgery in general practice. The review includes summaries of the pertinent characteristics of this disease with regard to the epidemiology, clinical characteristics, diagnostic challenges, and preoperative risk assessment. We analyzed published articles on BCC examining the involvement of GPs and other specialties and made a list of relevant Clinical Practice Guidelines developed for BCC evaluation and management. Local studies and any characteristics of disease unique to Asians were inspected.
We performed a systematic literature search, on MEDLINE, using the National Library of Medicine PubMed interface, and a general search on Google using the keywords, Basal cell carcinoma, epidemiology, clinical features, diagnosis and management, Non-melanoma skin cancers, Keratinocyte cancers, Basal cell carcinoma and General Practitioners, Surgical excision of BCCs, Incomplete excision of BCCs, and Clinical Practice Guidelines on BCC. A secondary search for relevant local publications was done. Studies involving randomized control trials, cohort, case series, reviews, and miscellaneous clinical reports were retrieved and evaluated from 1990 to 2020. The study was carried out between Jan 2019 and Jan 2020. A total of 1022 articles containing abstracts and full-texts were screened and evaluated, of which 58 articles were cited in this review.
Results
Terminology
Non-melanoma skin cancers (NMSCs) is a term that encompasses a mixture of skin cancers including BCCs, squamous cell carcinomas (SCCs), cutaneous lymphomas, adnexal tumors, Merkel cell carcinoma, and other more rare tumors. Keratinocyte cancer comprises both BCC and SCC because they share the same cellular lineage from the epidermis. Keratinocyte cancers, of which approximately 80% are BCC, and 20% are SCC,7 form the major kinds of non-melanoma skin cancers.
The term keratinocyte cancer is more definitive and practical and will be increasingly encountered.
Epidemiology
NMSCs commonly affect Caucasian or White populations. Ultraviolet radiation is the main etiological agent associated with BCC. A light-skinned phototype is the most susceptible constitutional risk factor.8 Global warming and depletion of the ozone layer may have a role in the increasing incidence of skin cancers.9
NMSC incidence is increasing worldwide. BCC, the major component of NMSCs, is the most common malignancy in many countries worldwide.10,11 In Caucasian populations, BCCs are ubiquitous. For example, in the US, it was estimated that more than 3 million persons were treated for keratinocyte cancers in 2012.12 Australia has the highest incidence of NMSCs in the world.13
In contrast, according to the Malaysian Cancer Registry Report 2012-2016, NMSCs only ranked as the ninth most common cancer in males and tenth in females.14 (Note that NMSCs were ranked tenth most common in males and fourteenth most common in females in the Malaysian Cancer Registry Report 2007-2011). BCC incidence rates may be significantly underreported because data for this tumor are not routinely collected by cancer registries, and notification is not mandatory.
In Asians, females are as likely to be affected as males, in contrast to Caucasian populations, where BCC is seen more commonly in men.15 Asians also develop BCCs at an older age and have fewer of these BCCs over their lifetime than Caucasians.16 The incidence rate of disease is inversely related to skin color (Fitzpatrick Skin Types I–VI); therefore, in the local multiracial mix, the incidence of BCCs is higher in Chinese than Malays or Indians.11,15,17,18
The lower incidence of BCC locally suggests that local GPs may have relatively less experience with diagnosing and treating the disease. Potential for late diagnosis and delayed treatment of BCCs, particularly in the trunk and limbs, has been highlighted.15
Clinical features and diagnosis
BCC is derived from the basal layer of the epidermis. Its local destructive potential but rare metastasis may be explained by the characteristic presence of an angiogenic response in the stroma but a lack of microvessels in the body of the tumor.19 Incidence rates of metastasis have been reported to be from 0.0028% to 0.55%.20 A retrospective cohort US study found that locally advanced BCC accounted for only 0.8% of all BCC cases.21 These excellent prognostic features present opportunities for appropriately trained primary care doctors to perform a gatekeeper’s role by managing early and uncomplicated cases.
Recognizable clinical variants of the common BCC include the nodular, superficial, morphoiec, and ulcerated (rodent ulcer) types. The clinical and histological features of the main BCC types are tabulated in Table 1 for easy reference. Note that the histopathological classification of BCC by Rippey22 is used. Some of the common findings in HPE reports are also given.
Table 1. Clinical and histopathological features of the main basal cell carcinoma types.
| Histopathological Classification (Rippey) | Distinctive clinical features | Histological features | |
|---|---|---|---|
| 1. | Nodular type |
Classical variant ‘Pearly’ translucent papule or nodule Reddish, smooth surface, firm Distinct border Telangiectasia Bleeds easily with contact |
Tumor cells with scanty cytoplasm with round large nucleus (basaloid cells) Basaloid cells arranged in nests, lobules or nodular sheets with peripheral palisading Nuclear pleomorphism Retraction artifact of tumor cells from surrounding stroma Increased mitosis Chronic inflammatory cellular infiltrate in subepithelial stroma Other features: myxoid changes, calcifications |
|
Ulcerative variant Central atrophy Rolled border is distinct |
Epidermis missing Tumor infiltrating into epidermis causing ulceration. |
||
|
Pigmented variant A range of stippling of melanin pigmentation may impart a bluish, brown or black color May resemble melanoma but a rolled translucent border gives away the diagnosis. |
Melanocytes seen Melanin pigments seen in the tumor cells |
||
| 2. | Superficial type | Well-demarcated reddish scaly plaques. Looks like eczema but does not itch. Characteristic raised border may be retained | Sheets of tumor cells attached and confined to the undersurface of the epidermis only |
| 3. | Infiltrative/morphearorm/sclerosing type |
Morphea-like Scar-like Ill-defined border Mixed skin colored, whitish and pigmentation |
Basaloid strands of tumor cells spreading in a spiky irregular fashion. Cords, strands, islands of tumor cells infiltrating into the dermis Dense fibrous stroma seen Perineural infiltration Perivascular infiltration |
GP surgeons would be most interested in the nodular type, which is most amenable to curing with standard excision. In general, the nodular type of BCCs is the most common form, occurring in up to 75% of cases.22 The majority of BCCs seen locally, as well as in other parts of Asia, are also pigmented and have relatively distinct borders.15,23 This is in contrast with non-pigmented lesions, which are predominant in Caucasians.24 According to the European consensus-based guidelines for diagnosis and treatment of basal cell carcinoma, more than 95% of BCCs can be classified as “easy to treat” with standard surgery or a range of alternative blind treatments early in the disease course.21
When diagnostic uncertainty occurs, it is best to refer the patient to a dermatologist, or a biopsy can be arranged. The best approach to biopsy is complete lesion excision if appropriate.1 However, it is often safer for the GP to first perform an incisional biopsy for diagnostic and classification purposes, particularly if more aggressive subtypes are considered. This approach is necessary if the patient requires more complex surgery for clearance and reconstruction by more specialized doctors. It is mandatory that the GPs are trained in the correct technique of performing a skin biopsy.25
Dermoscopy is a useful adjunct in the clinical diagnosis of keratinocyte cancers but is not commonly available in the GP’s office. Evaluation with a dermatoscope is more accurate than visual inspection alone for BCC detection.26 A dermatoscope is essentially a handheld instrument containing a high-quality magnifying lens and a powerful light source (both polarized and non-polarized). During dermoscopy, the area to be examined is typically covered with some oil and the dermatoscope applied to the skin when viewing. Optimum use requires training. Dermatoscopic criteria for BCC21 includes:
Absence of pigment network
Arborizing and superficial telangiectasia
Multiple erosions
Ulceration
Ovoid nests and globules and focused dots
Leaf-like areas
Spoke-wheel areas
Concentric structures.
Assessment of risk factors for recurrence
Outcomes of surgical excision can be studied in terms of completeness of excision or of tumor recurrence rates. Factors affecting these outcomes may be categorized as:
Tumor-related factors, which include location, size, histologic types, borders, and primary or previously excised lesions.
Patient-related factors, which include patient’s immunity (immunosuppression), coexisting medical conditions, and site of previous radiotherapy.
Operator-related factors, which can be analyzed in terms of personnel (GP, dermatologist, plastic surgeon, etc.) experience, surgical techniques (standard excision, Mohs micrographic surgery), and surgical margin width (applicable for standard excision).
Although there are a considerable number of studies in the literature addressing these issues, good-quality research in terms of randomized controlled trials is scarce.5
Tumor factors
Location
Certain anatomical sites solely, barring histologic types, confer a higher risk of tumor recurrence after excision than other areas. BCCs occur most frequently in sun-exposed areas; as a consequence, the head and neck region is the most common site. 75–85% of lesions occur in the head and neck region27 and the rest in the trunk and extremities. Tumors in the head and neck generally have a higher risk of recurrence than the rest of the body. Within the head and neck region itself, there are areas that are at higher risk than others. These high-risk zones or H-zones are depicted in Figure 1. The risk profiles are also related to the lesion size.28
Figure 1. The H-zone or high-risk areas-includes the central face, eyelids, periorbital, nose, lips, chin, mandible, preauricular and postauricular skin/sulci, temple, ear.
The surgeon must also consider other aspects of the surgical risks, principally injuries to vital structures such as nerves, tear ducts, eyelids, and mouth. A few areas where BCC commonly occurs present problems with direct surgical closure. For example, the skin around the tip of the nose and nasal alae are thick, noncompliant, and unforgiving, precluding the ease of primary closure of any defect. Similarly, the anterior and lateral part of the ears contains little subcutaneous tissue between the skin and cartilage, resulting in extremely tight attachment and, therefore, direct closure of the surgical defect will be difficult.
Tumors located in the periocular region, nasal, and nasolabial folds and the auricular region are shown to be more aggressive and infiltrative.29 Infiltrative extensions into adjacent vital structures can cause serious complications. Extreme care should be exercised when dealing with tumors in these areas, with a low threshold for referral being a rational thing to do. For example, for tumors in the periocular region, even if less than 1 cm, care needs to be taken to avoid postoperative complications such as ectropion. Facial BCCs in the high-risk zone may require the Mohs microsurgical technique for clearance, particularly those that had a recurrence after previous excision.30,31
Mohs micrographic surgery is a tissue-sparing surgical approach first developed by Frederic E. Mohs in 1938. During surgery, consecutive layers of tissue are excised circumferentially around and deep to the clinical margin of the tumor for frozen section. After each removal of tissue, the surgeon waits for the tissue to be examined by the pathologist for cancer cells. Further tissue layers are removed and studied until all cancer tissue are completely cleared in a process termed complete circumferential peripheral and deep margin assessment (CCPDMA). The final surgical margin is comparatively small, and the cure rate is high.
Size
Any BCC larger than 2.0 cm diameter or of long duration is considered high-risk for extensive subclinical spread.20,29 High-risk criteria for BCC include tumors > 2 cm on the trunk or extremities or > 1 cm on the head and neck, hands, feet, genitalia, or shins.32 The main challenge for GPs in large tumors will be primary closure of sizable post-surgical defect.
Histology
BCCs can be classified histologically by their growth pattern or cell differentiation. Classification by histological growth pattern is the most useful biologically and clinically.22 Rippey’s classification is adopted here for its simplicity and practicality.
The infiltrative/morpheaform/sclerosing type represents the high-risk type with predilection for subclinical spread, aggressive local behavior, and resulting in higher rates of incomplete excision. Aggressive local spread can cause difficulty in total clearance and poor cosmetic results. Deep infiltration into vital structures can lead to severe complications.
The nodular type, which has numerous phenotypic variants, is low-risk. In Asians, pigmentation appears to be a favorable prognostic factor in terms of recurrence risk after excision.23 The local ones are predominantly pigmented and have distinct borders (a low-risk attribute).
Other factors
The risk for tumor recurrence increases for lesions that require recurrent excision. A quick checklist of high-risk features of BCCs for GPs is given in Table 2. These lesions are likely difficult to treat, and referral should be considered. For guidance in matching a low-risk BCC for management by a suitably trained GP, the NICE guidelines (see below) are most useful.
Table 2. High risk features of basal cell carcinoma.
| Patient-related factors | Immunosuppression: eg. AIDS, Transplant recipient |
| Site of previous radiotherapy | |
| Recurrent or incompletely excised lesions | |
| Tumor-related factors | Location: H-zone, independent of size |
|
Size: - >2cm (trunk and extremities) - >1cm (head and neck, hands, feet, genitalia or shins) Border: Indistinct border |
|
| Clinical: growing rapidly, infiltrative or local spread | |
| Histology (result of biopsy) | Infiltrative/morpheaform/sclerosing type |
Guidelines
The following clinical practice guidelines (CPGs) on the management of BCCs provide useful recommendations for treatment decisions, although they are not to be taken as an absolute standard of care. The articles should be consulted for details. The first two references are principally directed to GPs. A brief mention of pertinent points is given.
-
National Institute for Health and Clinical Excellence (NICE) guidance on cancer services relating to the management of low-risk BCCs in the community (May2010)2
NICE provides up-to-date guidance for improving outcomes for people with skin tumors, including melanoma. The 2010 update includes recommendations on the management of low-risk BCCs in the community. GPs play the role of gatekeepers with the management of the low-risk BCCs and referral of high-risk lesions to secondary care. The proposals in the CPG suggest models of care aiming to match the skills of the healthcare professionals, including suitably trained GPs to the risks associated with BCC surgery, which are principally inadequate excision and poor cosmetic results. The objective is to identify suitable low-risk BCCs and triage them to be managed by three groups of health professionals in primary care, summarily:
Directed enhanced services/local enhanced services (DES/LES) GP surgeons: These GPs have no special interest or training in skin cancer and are expected to handle low-risk BCCs in anatomical sites where excision is easy, and closure is not difficult. These are generally uncomplicated lesions located anatomically below the clavicle and less than 1cm in diameter with clearly defined margins.
Model 1 practitioners: These practitioners are GPwSI trained and accredited in the management and excision of BCCs in the community. Their spread of coverage of low-risk cases issomewhat expanded.
Model 2 practitioners: These are medical practitioners performing skin surgery in the community setting or specialist nurse operators that have their cases reviewed and approved by a multidisciplinary team member.
-
Cancer Council Australia Keratinocyte Cancers Guideline Working Party. Clinical practice guidelines for keratinocyte cancer. Sydney: Cancer Council Australia (Jan 2020)1
This new keratinocyte cancer guidelines document is a revision of the Clinical Practice Guide for management of BCC, SCC, and related lesions developed by the Australian Cancer Network in 2008. The guide is most useful for Australian GPs because they provide the majority of care for those suffering from non-melanoma skin cancers. Although the guide is not prescriptive per se, its general intention is to provide information for appropriate practice. GPs need to be aware of their own limitations and must be able to demonstrate an ability to refer appropriately. The guide generally indicated what kinds of lesions are within the scope of a GP with experience and confidence in surgical procedures.
-
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology Version 1.2016. Basal cell Skin Cancer (US)28
This CPG is regularly updated. The CPG provides up-to-date and evidence-based recommendations for the evaluation and management of BCC. The section on risk stratification is extremely useful. Risk factors for tumor recurrence are listed and include factors found in history and physical examination and in the tumor’s pathology. Anatomic location has been risk stratified into high, moderate, and low risk:
Area H: The high-risks area – includes the central face, eyelids, periorbital, nose, lips, chin mandible, preauricular and postauricular skin/sulci, temple, ear, genitalia, hands, and feet.
Area M: The moderate-risk area – includes the cheeks, forehead, scalp, neck, and pretibia.
Area L: The low-risk area – includes the trunk and extremities (excluding pretibia, hands, feet, nail units, and ankles).
The tumor size is also factored in when determining risks.
-
Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines 201921
The European consensus-based interdisciplinary guidelines for the diagnosis and treatment of BCC published in 2019 proposed a new approach where BCCs are conveniently and practically categorized as either “easy to treat” (common) BCC or “difficult to treat” BCC taking into account a variety of factors.
Discussion
Case
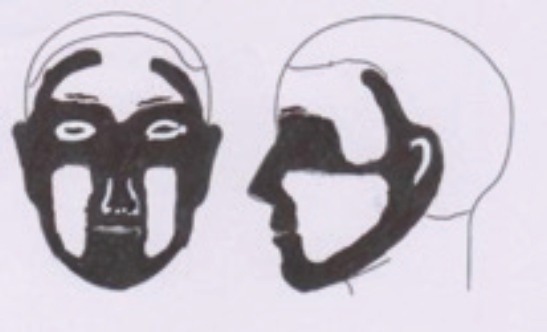
Madam Tan is a typical local patient diagnosed with a BCC, namely an older, Chinese, and outdoor worker with cumulative sun exposure. The location of the tumor at the cheek, and a size of >1.0 cm, put her in the high-risk category, according to NCCN.28 On the other hand, the lesion can be considered relatively “easy to treat” in view of a number of favorable factors. These are the well-defined border, pigmented nodule in clinical appearance, not located in the H-zone, primary not recurrent tumor, lack of co-morbidities, and patient preference/acceptance for primary care treatment. Surgery was performed in the general practice setting, and a 3 mm surgical margin was chosen. Primary closure was possible because the skin in the area was lax. Histology revealed a nodular BCC, and the surgical margins were found to be clear. Surgical outcome was satisfactory (Fig. 2).
Figure 2. Pathology laboratory specimen showing the 1.8 cm pigmented dome-shaped tumor excised from the left cheek. Note the normal post-excision tissue shrinkage obscuring the margin of excision. Postoperative wound healing at 2 weeks is also shown.
Surgery
The majority of low-risk BCCs can be managed by standard excision and direct wound closure.1 Standard BCC excision is a minor operation under local anesthesia in which a piece of elliptical skin is excised containing the lesion together with a surrounding margin of normal tissue. The skin specimen is tagged for orientation and sent for histopathological examination. The pathologist examines the lateral and deep borders of the specimen for adequacy of tumor clearance, determines the histological type of the BCC, and seeks evidence of perineural, vascular, or lymphatic spread. An incomplete excision is defined as the presence of tumor histopathologically at the surgical edge or at a distance of less than 0.5 mm from it.33 The wound defect is typically closed using sutures.
Although not the focus of this review, GPs need to be aware of the extensive range of non-surgical treatment alternatives for BCC. Many low-risk tumors can be managed with destructive techniques (curettage alone, curettage with electrodessication, cryosurgery), photodynamic therapy, or topical drugs (e.g., 5-fluorouracil, imiquimod). On the other hand, advanced or inoperable BCC may be treated by radiotherapy or hedgehog pathway inhibitors.34 The indication for these options may need to be individualized, and working with a friendly dermatologist is the most suitable approach.
Objectives
The primary objective of surgery for BCCs is the complete removal of all tumor tissues. The secondary objectives are to ensure minimal sacrifice of surrounding normal healthy tissue, preservation of function, and obtaining optimal cosmetic results. Additionally, the aim for most practitioners is to be able to achieve primary skin closure. It is also economically likely to be most beneficial if surgical treatment can be completed at first contact by an appropriately trained GP.1
Margins
The ability to recognize the clinical margin between normal and diseased tissues is the key to a successful complete excision.35 Estimating the margin of excision is not an exact science. To date, there have been no randomized control trials studying the relationship between surgical excision margins and tumor recurrence rates. The recommendation for lateral surgical excision margin for normal low-risk non-pigmented nodular BCC of Caucasians is a 4-mm margin.36 The Asian pigmented nodular BCCs have well-demarcated or distinct borders, and a 2-3-mm margin was found to be adequate.23,24,37,38 In general, from a meta analysis of the literature of 16,066 BCC excisions, a 3-mm lateral surgical excision was found to be safe for non-morpheaform BCC to achieve a 95 percent cure rate for lesions 2 cm or smaller.39 The rates for incomplete excision are usually determined from specimens sent to the laboratory for histopathological examination. Reported rates of incomplete excision of BCC vary from 5% to 25% among centers worldwide.40
Controversy
Traditionally, skin cancer surgery falls within the confines of dermatology and plastic surgery for the most part. Other involved specialties include general surgery, ENT, maxillofacial surgery, and ophthalmology. Hospital-based BCC management is costly.41 The involvement of general practice in skin cancer management is inevitable in many countries with high disease incidence because the potential for cost savings and reduction of specialist waiting times is evident. Controversies arise when the diagnosis and treatment of the disease are not carried out to expected standards.
Numerous studies have been performed comparing GPs with dermatologists and other specialists in their ability to make precise cutaneous diagnoses. Many of these studies, using real clinical cases and clinical photographs, showed that GPs performed significantly less well than dermatologists or other specialists when diagnosing skin cancers.42,43,44,45,46,47
Solutions to this deficiency have been examined. Firstly, training and ample clinical experience with the disease has been shown to increase the diagnostic skills of practicing GPs.47,48,49 Secondly, the deficiency in diagnostic skills can be offset if GPs can demonstrate the ability to refer and biopsy appropriately.48,50
The concept of GP-led BCC skin cancer management is still evolving in countries with high disease incidence. The surgical skills and training of individual GPs vary considerably due to their diverse backgrounds. Controversy occurs when there is a significant difference in the surgical outcome measures between GPs and specialists. An incomplete excision of a BCC entails the burden of further excision, cost, and anxiety. The preponderance of available studies showed that GPs performed significantly less well than dermatologists or other specialists when excising skin cancers.43,51,52,53,54 Some believe that dermatologists are better at judging the tumor edge and, hence, have the lowest levels of incomplete excision.43,52 Others have found that GPs did not perform as well as dermatologists because GPs tend to excise BCCs with a significantly smaller cut margin.55 It was postulated that GPs excised inadequately, particularly for lesions in the head and neck region, due to cosmetic concerns.43
Competency in surgical expertise comes with training and experience. In Australia, GPs have expanded their surgical capabilities beyond the fundamental aspects. Besides standard excision for skin cancers, GPs are increasingly using skin flaps for repair. In a number of Australian states, the GPs performed more skin cancer surgeries than specialists and nationwide, GPs outperformed specialists.56
In a paper entitled “Are there sufficient numbers of low-risk basal cell carcinoma to justify general practitioners (family physicians) carrying out basal cell carcinoma surgery”, investigators in the UK highlighted that it would be difficult for their GPs to maintain competency to perform effective skin surgery in view of the low numbers of low-risk BCC suitable for GP surgeons.57 In this latter study carried out between Jan 2012 and Sept 2014, out of 1,743 BCCs excised from a population of over 700,000, only 3% met NICE criteria for being low risk and suitable for excision by their DES/LES GP surgeons. The percentage of lesions suitable for excision by Model 1 GP was only 15.1%.
In light of such findings, extrapolated to local settings with low clinical exposure, maintenance of competency for local GPs will be difficult. Hence, in regions with low disease incidence, the question remains whether it is appropriate for GPs to be involved in BCC surgery. Practically speaking, adequately skilled GPwSI may manage the uncomplicated low-risk lesions on an individualized basis. Conceivable benefits include lowering cost and providing comfort and convenience58 to patients who do not mind being treated by GPwSI. There are no local guidelines or controlled trials involving GPs and BCC skin cancer surgery to lend support.
Conclusion
The value of early diagnosis and management of low-risk BCCs by GPs has been demonstrated in healthcare institutions with a high volume of skin cancers. The evidence does support the notion that BCCs can be safely and effectively excised by GPs as office-based procedures, provided the GP surgeon is adequately experienced and skilled.
In the local setting, the experience of GPs is limited by low caseloads and, therefore, involvement would likely be confined to GPs with the relevant skills and interest. There is a notable lack of local studies or guidelines to support involvement of GPs in BCC surgery. Currently, it appears preferable that GPs should refer all cases for specialist management except for specific circumstances, whereby the arrangement is not appropriate, or the patient prefers primary care management. An alternative possible view is for local GPwSI to independently match their own skills and experience to the risk level of the BCC cases to be operated upon. Clinical Practice Guidelines such as those of NICE can be used as a model of care. However, the ultimate verdict regarding the propriety of this approach must be made by the doctor and the patient.
At this point of time, we could at best present the evidence and hope that this review will broaden the theoretical and practical perspectives of our local GPs and will position them to make a rational decision for the patient who requires BCC surgery. Increased primary care involvement in the diagnosis and management of skin cancers is a potential area for advancement in the local healthcare scene.
How does this paper make a difference to general practice?
Basal cell carcinomas (BCCs) are often seen in general practice. Patients may seek advice for a lesion, or the doctor noticed the disease while treating the patient for some other complaint.
BCCs typically have favorable prognostic features, and surgical excision is the mainstay of treatment.
In Caucasian populations where incidence is high, BCC surgery is a common procedure in primary care. BCC incidence is much lower in the Asian setting, and primary care practitioners may have less experience in managing BCC, resulting in neglect or delayed treatment.
We discuss BCC surgery’s performance when carried out by local general practitioners with special interest (GPwSI) in minor surgery/dermatology. Is there an identifiable role?
The article creates awareness, provides an update for GPs, and gives evidence-based data to inform treatment decisions in BCC’s surgical management.
References
- 1.Cancer Council Australia Keratinocyte Cancers Guideline Working Party . Clinical practice guidelines for keratinocyte cancer. Sydney: Cancer Council Australia; [Jan 4;2020 ]. https://wiki.cancer.org.au/australiawiki/index.php?oldid=208400https://wiki.cancer.org.au/australia/Guidelines:Keratinocyte_carcinoma Version URL. Available from: [Google Scholar]
- 2.National Institute for Health and Clinical Excellence The management of low-risk basal cell carcinomas in the community. NICE guidance on cancer services update to CSG8. May, 2010. https://www.nice.org.uk/guidance/csg8/resources/improving-outcomes-for-people-with-skin-tumours-including-melanoma-2010-partial-update-pdf-773380189
- 3.Martinez JC, Otley CC. The management of melanoma and nonmelanoma skin cancer: a review for the primary care physician. Mayo Clin Proc. 2001 Dec;76(12):1253–65. doi: 10.4065/76.12.1253. [DOI] [PubMed] [Google Scholar]
- 4.Noels EC, Wakkee M, van den Bos RR, et al. Substitution of low-risk skin cancer hospital care towards primary care: A qualitative study on view of general practitioners and dermatologists. PLoS One. 2019 Mar 19;14(3) doi: 10.1371/journal.pone.0213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bath-Hextall FJ, Perkins W, Bong J, et al. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003412. doi: 10.1002/14651858.CD003412.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Yellamaty V, Ball L, Crossland L, Jackson C. General practitioners with special interests: An integrative review of their role, impact and potential for the future. Aust J Gen Pract. 2019 Sep;48(9):639–643. doi: 10.31128/AJGP-02-19-4849. [DOI] [PubMed] [Google Scholar]
- 7.Firnhaber JM. Diagnosis and treatment of Basal cell and squamous cell carcinoma. Am Fam Physician. 2012 Jul 15;86(2):161–8. [PubMed] [Google Scholar]
- 8.Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An. Bras. Dermatol. 2011 Mar-Apr;86(2):292–305. doi: 10.1590/S0365-05962011000200013. English, Portuguese. [DOI] [PubMed] [Google Scholar]
- 9.Bharath AK, Turner RJ. Impact of climate change on skin cancer. J R Soc Med. 2009 Jun;102(6):215–8. doi: 10.1258/jrsm.2009.080261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012 May;166(5):1069–80. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 11.Han WH, Yong SS, Tan LL, et al. Characteristics of skin cancers among adult patients in an urban Malaysian population. Australas J Dermatol. 2019 Nov;60(4):e327–329. doi: 10.1111/ajd.13106. [DOI] [PubMed] [Google Scholar]
- 12.Rogers HW, Weinstock MA, Feldman SR, et al. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015 Oct;151(10):1081–6. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 13.Perera E, Gnaneswaran N, Staines C, et al. Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas J Dermatol. 2015 Nov;56(4):258–67. doi: 10.1111/ajd.12282. [DOI] [PubMed] [Google Scholar]
- 14.Malaysian Cancer Registry Report 2012-2016 https://nci.moh.gov.my/index.php/ms/pengumuman/340-national-cancer-registry-report Available at.
- 15.Yap FB. Clinical characteristics of basal cell carcinoma in a tertiary hospital in Sarawak, Malaysia. Int J Dermatol. 2010 Feb;49(2):176–9. doi: 10.1111/j.1365-4632.2009.04342.x. [DOI] [PubMed] [Google Scholar]
- 16.Moore MG, Bennett RG. Basal cell carcinoma in Asians: a retrospective analysis of ten patients. J Skin Cancer. 2012;2012:741397. doi: 10.1155/2012/741397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abishegam S, Yasir A, Hussin I, Noran I. Pilot epidemiological study of basal cell carcinoma in Melaka. Asian Journal of Medical Science. 2019;10(6):18–21. doi: 10.3126/ajms.v10i6.25675. [DOI] [Google Scholar]
- 18.Chng Chin Chwen. A 7-year Retrospective Review of Skin Cancer at University of Malaya Medical Centre: A Tertiary Centre Experience. Malaysian Journal of Dermatology. 2012;29:16–22. [Google Scholar]
- 19.Chin CW, Foss AJ, Stevens A, et al. Differences in the vascular patterns of basal and squamous cell skin carcinomas explain their differences in clinical behaviour. J Pathol. 2003 Jul;200(3):308–13. doi: 10.1002/path.1363. [DOI] [PubMed] [Google Scholar]
- 20.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl Journal of Med. 2005 Nov 24;353(21):2262–9. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 21.Peris K, Fargnoli MC, Garbe C, et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019 Sep;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Vantuchova Y, Curik R. Histological types of basal cell carcinoma. Scripta Medica (Brno) 2006;79(5-6):261–70. [Google Scholar]
- 23.Lin SH, Cheng YW, Yang YC, et al. Treatment of Pigmented Basal Cell Carcinoma with 3mm Surgical Margin in Asians. Biomed Res Int. 2016;2016:7682917. doi: 10.1155/2016/7682917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow VL, Chan JY, Chan RC, et al. Basal cell carcinoma of the head and neck region in ethnic Chinese. Int J Surg Oncol. 2011;2011:890908. doi: 10.1155/2011/890908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JJ. A biopsy is more than a biopsy. J Gen Intern Med. 1998 Jan;13(1):62–3. doi: 10.1046/j.1525-1497.1998.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinnes J, Deeks JJ, Chuchu N, et al. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018 Dec 4;12(12):CD011901. doi: 10.1002/14651858.CD011901.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demirseren DD, Ceran C, Aksam B, et al. Basal cell carcinoma of the head and neck region: a retrospective analysis of completely excised 331 cases. J Skin Cancer. 2014;2014:858636. doi: 10.1155/2014/858636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bichakjian CK, Olencki T, Aasi SZ, et al. Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016 May;14(5):574–97. doi: 10.6004/jnccn.2016.0065. [DOI] [PubMed] [Google Scholar]
- 29.Wollina U, Pabst F, Kronert C, et al. High-risk basal cell carcinoma: An update. Expert Rev Dermatol. 2010;5(3):357–68. doi: 10.1586/edm.10.27. [DOI] [Google Scholar]
- 30.Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008 Dec;9(12):1149–56. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- 31.van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014 Nov;50(17):3011–20. doi: 10.1016/j.ejca.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Nehal KS, Bichakjian CK. Update on Keratinocyte Carcinomas. N Engl J Med. 2018 Jul 26;379(4):363–374. doi: 10.1056/NEJMra1708701. [DOI] [PubMed] [Google Scholar]
- 33.Miszczyk J, Charytonowicz M, Dçbski T, et al. Incomplete excision of basal cell carcinoma (BCC) in the head and neck region: to wait, or not to wait? Postepy Dermatol Allergol. 2017 Dec;34(6):607–611. doi: 10.5114/ada.2017.72467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paoli J, Gyllencreutz JD, Fougelberg J, et al. Nonsurgical Options for the Treatment of Basal Cell Carcinoma. Dermatol Pract Concept. 2019 Apr 30;9(2):75–81. doi: 10.5826/dpc.0902a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gualdi G, Monari P, Crotti S, et al. Matter of margins. J Eur Acad Dermatol Venereol. 2015 Feb;29(2):255–61. doi: 10.1111/jdv.12504. [DOI] [PubMed] [Google Scholar]
- 36.Weinstein MC, Brodell RT, Bordeaux J, et al. The art and science of surgical margins for the dermatopathologist. Am J Dermatopathol. 2012 Oct;34(7):737–45. doi: 10.1097/DAD.0b013e31823347cb. [DOI] [PubMed] [Google Scholar]
- 37.Ito T, Inatomi Y, Nagae K, et al. Narrow-margin excision is a safe, reliable treatment for well-defined, primary pigmented basal cell carcinoma: an analysis of 288 lesions in Japan. J Eur Acad Dermatol Venereol. 2015 Sep;29(9):1828–31. doi: 10.1111/jdv.12689. [DOI] [PubMed] [Google Scholar]
- 38.Lalloo MT, Sood S. Head and neck basal cell carcinoma: treatment using a 2-mm clinical excision margin. Clin Otolaryngol Allied Sci. 2000 Oct;25(5):370–3. doi: 10.1046/j.1365-2273.2000.00376.x. [DOI] [PubMed] [Google Scholar]
- 39.Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a Basal cell carcinoma: a meta-analysis of the literature. Plast Reconstr Surg. 2010 Oct;126(4):1222–31. doi: 10.1097/PRS.0b013e3181ea450d. [DOI] [PubMed] [Google Scholar]
- 40.Su SY, Giorlando F, Ek EW, et al. Incomplete Excision of Basal Cell Carcinoma: a prospective trial. Plast Reconstr Surg. 2007 Oct;120(5):1240–8. doi: 10.1097/01.prs.0000279148.67766.e1. [DOI] [PubMed] [Google Scholar]
- 41.Housman TS, Feldman SR, Wilford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003 Mar;48(3):425–9. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 42.Tran H, Chen K, Lim AC, et al. Assessing diagnostic skill in dermatology: a comparison between general practitioners and dermatologists. Australas J Dermatol. 2005 Nov;46(4):230–4. doi: 10.1111/j.1440-0960.2005.00189.x. [DOI] [PubMed] [Google Scholar]
- 43.Murchie P, Delaney E, Thompson W, Lee A. Excising basal cell carcinomas: comparing the performance of general practitioners, hospital skin specialists and other hospital specialists. Clin Exp Dermatol. 2008 Aug;33(5):565–71. doi: 10.1111/j.1365-2230.2008.02710.x. [DOI] [PubMed] [Google Scholar]
- 44.Morrison A, O’Loughlin S, Powell FC. Suspected skin malignancy: a comparison of diagnoses of family practitioners and dermatologists in 493 patients. Int J Dermatol. 2001 Feb;40(2):104–7. doi: 10.1046/j.1365-4362.2001.01159.x. [DOI] [PubMed] [Google Scholar]
- 45.Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. A review of the literature. Arch Fam Med. 1999 Mar-Apr;8(2):170–2. doi: 10.1001/archfami.8.2.170. [DOI] [PubMed] [Google Scholar]
- 46.Chen SC, Pennie LM, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006 Jul;21(7):678–82. doi: 10.1111/j.1525-1497.2006.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brochez L, Verhaeghe E, Bleyen L, Naeyaert JM. Diagnostic ability of general practitioners and dermatologists in discriminating pigmented skin lesions. J Am Acad Dermatol. 2001 Jun;44(6):979–86. doi: 10.1067/mjd.2001.113442. [DOI] [PubMed] [Google Scholar]
- 48.Heal CF, Satayaputra F, Raasch BA, et al. Comparison of diagnostic accuracy of skin lesions by general practitioners and specialists. J Rural Trop Pub Health. 2010 Nov;9:109–13. [Google Scholar]
- 49.Youl PH, Raasch BA, Janda M, et al. The effect of an educational programme to improve the skills of general practitioners in diagnosing melanocytic/pigmented lesions. Clin Exp Dermatol. 2007 Jul;32(4):365–70. doi: 10.1111/j.1365-2230.2007.02414.x. [DOI] [PubMed] [Google Scholar]
- 50.Jackson AM, Morgan DR, Ellison R, et al. Diagnosis of malignant melanoma by general practitioners and hospital specialists. Postgrad Med J. 2000 May;76(895):295–8. doi: 10.1136/pmj.76.895.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salmon P, Mortimer M, Rademaker M, et al. Surgical excision of skin cancer: the importance of training. Br J Dermatol. 2010 Jan;162(1):117–22. doi: 10.1111/j.1365-2133.2009.09548.x. [DOI] [PubMed] [Google Scholar]
- 52.Goulding JM, Levine S, Blizard RA, et al. Dermatological surgery: a comparison of activity and outcomes in primary and secondary care. Br J Dermatol. 2009 Jul;161(1):110–4. doi: 10.1111/j.1365-2133.2009.09228.x. [DOI] [PubMed] [Google Scholar]
- 53.Haw WY, Rakvit P, Fraser SJ, et al. Skin cancer excision performance in Scottish primary and secondary care: a retrospective analysis. Br J Gen Pract. 2014 Aug;64(625):e465–70. doi: 10.3399/bjgp14X680929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramdas K, van Lee C, Beck S, et al. Differences in Rate of Complete Excision of Basal Cell Carcinoma by Dermatologists, Plastic Surgeons and General Practitioners: A Large CrossSectional Study. Dermatology. 2018;234(3-4):86–91. doi: 10.1159/000490344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahim S. Assessment of excision of basal cell carcinoma: did GPs really ‘underperform? Clin Med (Lond) 2016 Jun 1;16(Suppl 3):s20. doi: 10.7861/clinmedicine.16-3-s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Askew DA, Wilkinson D, Schluter PJ, et al. Skin cancer surgery in Australia 2001-2005: the changing role of the general practitioner. Med J Aust. 2007 Aug 20;187(4):210–4. doi: 10.5694/j.1326-5377.2007.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 57.Fremlin GA, Gomez P, Halpern J. Are there sufficient numbers of low-risk basal cell carcinomas to justify general practitioners (family physicians) carrying out basal cell carcinoma surgery? Clin Exp Dermatol. 2016 Mar;41(2):138–41. doi: 10.1111/ced.12718. [DOI] [PubMed] [Google Scholar]
- 58.George S, Pockney P, Primrose J, et al. A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial. Health Technol Assess. 2008 May;12(23):iii–iv. doi: 10.3310/hta12230. ix-38. [DOI] [PubMed] [Google Scholar]


