Mycobacteroides abscessus (Mab) is an opportunistic environmental pathogen that can cause chronic pulmonary disease in the setting of structural lung conditions such as bronchiectasis, chronic obstructive pulmonary disease, and cystic fibrosis. These infections are often incurable and associated with rapid lung function decline. Mab is naturally resistant to most of the antibiotics available today, and current treatment guidelines require at least 1 year of daily multidrug therapy, which is often ineffective and is associated with significant toxicities. β-Lactams are the most widely used class of antibiotics and have a demonstrated record of safety and tolerability.
KEYWORDS: Mycobacteroides abscessus, synergy, β-lactams, drug resistance
ABSTRACT
Mycobacteroides abscessus (Mab) is an opportunistic environmental pathogen that can cause chronic pulmonary disease in the setting of structural lung conditions such as bronchiectasis, chronic obstructive pulmonary disease, and cystic fibrosis. These infections are often incurable and associated with rapid lung function decline. Mab is naturally resistant to most of the antibiotics available today, and current treatment guidelines require at least 1 year of daily multidrug therapy, which is often ineffective and is associated with significant toxicities. β-Lactams are the most widely used class of antibiotics and have a demonstrated record of safety and tolerability. Here, using a panel of recent clinical isolates of Mab, we evaluated the in vitro activities of dual-β-lactam combinations to identify new treatments with the potential to treat infections arising from a wide range of Mab strains. The Mab clinical isolates were heterogeneous, as reflected by the diversity of their genomes and differences in their susceptibilities to various drugs. Cefoxitin and imipenem are currently the only two β-lactams included in the guidelines for treating Mab disease, yet they are not used concurrently in clinical practice. However, this dual-β-lactam combination exhibited synergy against 100% of the isolates examined (n = 21). Equally surprising is the finding that the combination of two carbapenems, doripenem and imipenem, exhibited synergy against the majority of Mab isolates. In the setting of multidrug-resistant Mab disease with few therapeutic options, these combinations may offer viable immediate treatment options with efficacy against the broad spectrum of Mab strains infecting patients today.
INTRODUCTION
Patients with chronic lung diseases such as cystic fibrosis (CF) and bronchiectasis are at high risk for development of recurrent pulmonary infections, largely due to poor clearance of respiratory secretions resulting in persistent colonization with pathogenic bacteria (1–4). Some species of nontuberculous mycobacteria (NTM) are capable of causing invasive infections in this patient population. The prevalence of NTM pulmonary disease in the United States has steadily risen over the past several decades and currently far outweighs the prevalence of tuberculosis in this country (5, 6).
Mycobacteroides abscessus (Mab) (formerly Mycobacterium abscessus) (7) is considered one of the most virulent NTMs, and Mab lung disease is associated with significant decline in pulmonary function (8–10), with cure rates as low as 25 to 40% with antibiotic treatment alone (11, 12). Mab has been described as an “antibiotic nightmare” and “an environmental bacterium turned clinical nightmare” because it is intrinsically resistant to several antibiotic classes (13–16), with acquired resistance further limiting therapeutic options (17). Additionally, Mab subspecies abscessus and bolletii readily develop resistance to macrolide antibiotics upon exposure to these drugs (18), which are considered the cornerstone of treatment of Mab lung disease (8, 19). Current treatment guidelines for pulmonary Mab infections include at least 12 to 18 months of multidrug therapy, with several agents requiring intravenous administration and causing substantial cytotoxicity (20, 21). There are no FDA-approved antibiotics for Mab disease based on clinical trials, and current treatment recommendations were developed from expert consensus and empirical experience (22). β-Lactams are among the antibiotics included in these guidelines and have proved to be useful in treating this disease (22–24).
β-Lactams represent the most widely used class of antibiotics for treatment of bacterial infections (25) and are generally well tolerated, with minimal comparative cytotoxicity. β-Lactams exert their activity via inhibition of the synthesis of peptidoglycan, the exoskeleton of the bacterial cell wall that is essential for cellular growth and survival (26). The final step of peptidoglycan synthesis involves polymerization of disaccharide-peptide monomers to generate a single macromolecule that encapsulates the plasma membrane (27). Unlike most bacteria whose peptidoglycan synthesis requires only d,d-transpeptidases (also known as penicillin-binding proteins), in Mab the majority of monomer polymerization is undertaken by nonclassical peptidases, the l,d-transpeptidases (28). An initial survey of the Mab genome has identified five putative l,d-transpeptidase-encoding genes (29). These l,d-transpeptidases are differentially susceptible to β-lactam subclasses, with carbapenems exhibiting the strongest inhibition, followed by cephalosporins, and only a select few penicillins exhibiting limited inhibition of these enzymes (30–32). Based on these observations, the hypothesis that synergistic activity is likely from a combination of β-lactams, one that optimally inhibits d,d-transpeptidases and another that optimally inhibits l,d-transpeptidases, was proposed (32, 33). Subsequent findings using in vitro (31, 34, 35) and in vivo (36) assessments have supported this hypothesis.
These initial proof-of-concept studies were performed using the Mab reference strain (ATCC 19977). Mab strains isolated from patients exhibit extensive genotypic and phenotypic heterogeneity, resulting in widely variable antimicrobial susceptibility profiles and clinical responses to treatment regimens (37–39); this often necessitates tailoring of treatment regimens based on in vitro drug susceptibility testing. Due to inherent diversity among Mab clinical isolates, the question of whether studies using any single Mab strain can be predictive of treatment success in the clinic is raised. Therefore, preclinical studies including multiple isolates are warranted to better represent the diversity of strains with which patients present. Furthermore, the ATCC 19977 reference strain that was used in the initial proof-of-concept studies was isolated in 1953 from a synovial fluid culture (40) and may not be the most relevant reference to represent a patient population presenting primarily with pulmonary Mab infections occurring in an era of ubiquitous antibiotic use that has resulted in extensive antimicrobial resistance.
Therefore, inclusion of multiple diverse Mab clinical isolates in preclinical studies is rigorous and is likely to generate data that are more representative and predictive of clinical outcomes. Because several β-lactam combinations exhibit synergy against Mab reference strain ATCC 19977 in vitro (34) and in vivo (36), β-lactam combinations present an untapped resource whose further preclinical evaluation could lead to repurposing of these drugs to treat Mab infections. A prior investigation identified synergy between ceftazidime and ceftaroline or imipenem against a panel of Mab clinical isolates (35). Here, we have assessed the activities of 13 dual-β-lactam combinations that have not been previously studied against 21 Mab strains that were recently isolated from pulmonary exudates from CF patients (41). These β-lactams are commercially available and thus have the potential to serve as novel therapeutic options pending efficacy trials. The main objective of this study was to identify dual-β-lactam combinations that exhibit synergy against the largest number of recent Mab clinical isolates. Such combinations are likely to harbor greater potential for clinical efficacy against the broad spectrum of Mab strains infecting patients today.
RESULTS
Mab clinical isolates and genomic diversity.
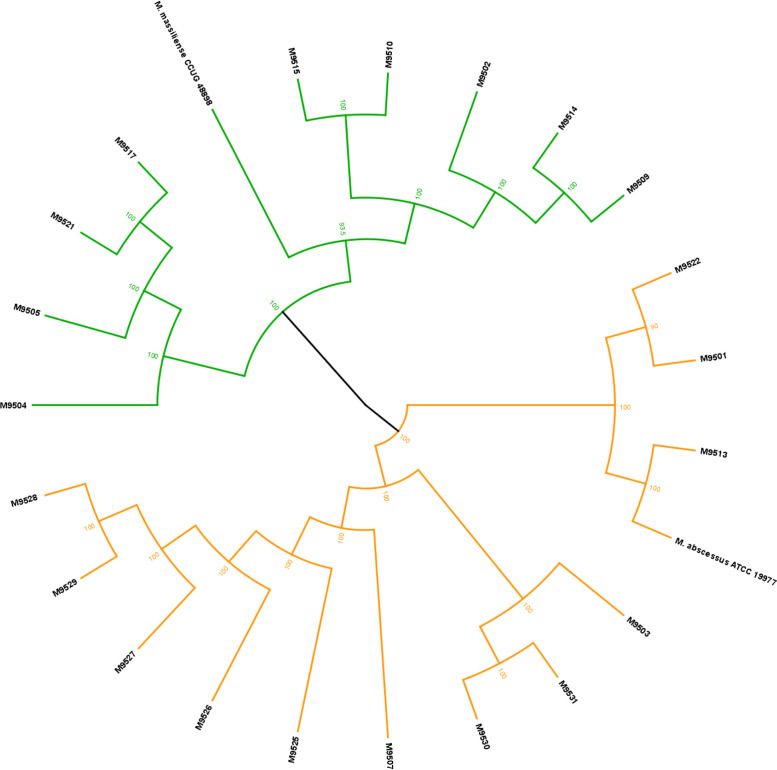
The clinical strains were obtained through the Johns Hopkins Clinical Mycobacteriology Laboratory and were collected from lung exudate specimens from CF patients at the Johns Hopkins Hospital over the past 15 years (41). The whole-genome sequences of 21 clinical isolates were determined, and six genes were used as genetic markers to identify the isolates to the subspecies level as described (42, 43). This assessment identified 12 strains as belonging to Mab subspecies abscessus and the remaining 9 as belonging to Mab subspecies massiliense (Fig. 1). To verify these subspecies identification results, we also queried the sequence for the erm(41) gene for all strains and observed that the identification of strains as Mab subsp. massiliense by the phylogenetic approach was correct, based on the previously described truncation of the gene (43, 44). Lastly, a recent publication reported that subspecies identification can be achieved by using a single gene, gnD (MAB_0003) (45). We generated a phylogenetic tree using the same approach as described above but with gnD as the marker, and we found this tree to be nearly identical to the tree built with six genes (see Fig. S1 in the supplemental material). Strains that clustered together in the six-gene approach also clustered together in this single-gene approach, successfully validating this method for subspecies identification of Mab strains.
FIG 1.
Phylogenetic tree for clinical Mab isolates based on six marker genes. The genes used were hsp65 (MAB_0650), secA (MAB_2397), rpoB (MAB_3869c), polC (MAB_2696c), hoa (MAB_0626), and ftsZ (MAB_2009). We used the Geneious Tree Builder with the Tamura-Nei genetic distance model and the UPGMA tree-building method bootstrapped to 1,000 iterations. Of the 21 clinical strains, 12 strains were revealed to be Mab subspecies abscessus, while 9 strains were Mab subspecies massiliense.
Based on the phylogenetic tree illustrated in Fig. 1 and Fig. S1, the clinical isolates in this study are genetically diverse, even those that clustered together within the tree. Due to the limitations of short-read sequencing, we mapped the reads to the reference strain ATCC 19977 to compare the patterns of the locations at which each clinical isolate’s reads would map to the reference strain. We found that there were many different patterns, indicating our clinical isolates to be diverse. For further verification, we randomly selected 12 strains for long-read sequencing (PacBio) to attempt to generate de novo a circularized genome for these isolates. Results from the whole-genome alignment showed many insertions/deletions, inversions, and even shuffling of whole sections of the genomes, compared to each other (Fig.S2 and S3).
MICs of β-lactams against Mab clinical isolates.
We began by determining the MICs of 12 β-lactams against all 21 Mab clinical isolates to establish baseline susceptibility (Table 1). The β-lactams evaluated in this study included the cephalosporins cefuroxime, cefadroxil, cefdinir, cefditoren, cefpodoxime, and cefoxitin, the carbapenems ertapenem, imipenem, doripenem, biapenem, and tebipenem, and the penem faropenem. The majority of cephalosporins exhibited high baseline MICs of 256 to 512 μg/ml, with the exception of cefoxitin and cefdinir, which had more variable MICs, ranging from 32 to 256 μg/ml. The MICs of ertapenem, tebipenem, and faropenem were also consistently high, at 256 to 512 μg/ml, for the majority of isolates. Imipenem, doripenem, and biapenem, in contrast, tended to have lower MICs but with high levels of variability between strains, ranging from 8 μg/ml to 256 μg/ml (Table 1). Compared to the Mab reference strain ATCC 19977 (34), the MICs of most cephalosporins, carbapenems, and penem against the clinical isolates were higher, with a few exceptions (Table S1). In addition, the clinical isolates had distinct MIC profiles among themselves. Any two Mab isolates differed in MICs of at least three (for instance, M9501 versus M9502) and to up to seven (M9501 versus M9529) β-lactams. Based on these results, we established that the Mab clinical isolates were intrinsically diverse in their susceptibilities to β-lactams.
TABLE 1.
MICs of each individual β-lactam tested against 21 clinical Mab strains
| Mab clinical strain (subspecies) | MIC (μg/ml) ofa: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cephalosporins |
Carbapenems/penem |
|||||||||||
| CXM | CFR | CDR | CDN | CPD | FOX | ETP | IPM | DOR | BIA | FAR | TEB | |
| M9501 (abscessus) | 512 | 512 | 128 | 512 | 512 | 64 | 512 | 8 | 32 | 16 | 256 | 256 |
| M9502 (massiliense) | 512 | 512 | 64 | 512 | 512 | 128 | 512 | 8 | 16 | 16 | 256 | 256 |
| M9503 (abscessus) | 256 | 512 | 64 | 256 | 512 | 64 | 512 | 8 | 8 | 8 | 256 | 256 |
| M9504 (massiliense) | 256 | 512 | 64 | 512 | 512 | 128 | 256 | 16 | 16 | 16 | 256 | 512 |
| M9505 (massiliense) | 256 | 512 | 64 | 256 | 256 | 32 | 256 | 16 | 16 | 8 | 512 | 256 |
| M9507 (abscessus) | 512 | 512 | 256 | 512 | 512 | 256 | 512 | 32 | 128 | 128 | 512 | 512 |
| M9509 (massiliense) | 256 | 512 | 64 | 512 | 512 | 32 | 256 | 16 | 16 | 16 | 64 | 128 |
| M9510 (massiliense) | 512 | 512 | 256 | 512 | 512 | 256 | 512 | 64 | 128 | 128 | 512 | 256 |
| M9513 (abscessus) | 512 | 512 | 64 | 512 | 512 | 64 | 512 | 16 | 32 | 16 | 512 | 512 |
| M9514 (massiliense) | 512 | 512 | 64 | 512 | 512 | 128 | 512 | 8 | 32 | 32 | 256 | 256 |
| M9515 (massiliense) | 512 | 512 | 256 | 512 | 512 | 256 | 512 | 32 | 256 | 128 | 512 | 512 |
| M9517 (massiliense) | 512 | 512 | 128 | 512 | 512 | 64 | 512 | 16 | 16 | 16 | 256 | 512 |
| M9521 (massiliense) | 512 | 512 | 128 | 512 | 512 | 32 | 256 | 8 | 16 | 16 | 128 | 256 |
| M9522 (abscessus) | 512 | 512 | 256 | 512 | 512 | 64 | 512 | 16 | 32 | 32 | 512 | 512 |
| M9525 (abscessus) | 512 | 512 | 256 | 512 | 512 | 128 | 512 | 32 | 64 | 64 | 512 | 512 |
| M9526 (abscessus) | 512 | 512 | 256 | 512 | 512 | 64 | 512 | 16 | 32 | 32 | 512 | 512 |
| M9527 (abscessus) | 512 | 512 | 128 | 512 | 512 | 128 | 512 | 16 | 64 | 32 | 512 | 512 |
| M9528 (abscessus) | 512 | 512 | 256 | 512 | 512 | 128 | 512 | 32 | 64 | 32 | 512 | 512 |
| M9529 (abscessus) | 512 | 512 | 256 | 512 | 512 | 128 | 512 | 256 | 256 | 128 | 512 | 512 |
| M9530 (abscessus) | 512 | 512 | 128 | 512 | 512 | 64 | 512 | 16 | 64 | 16 | 512 | 512 |
| M9531 (abscessus) | 512 | 256 | 64 | 512 | 512 | 64 | 512 | 16 | 64 | 32 | 256 | 512 |
Based on CLSI-defined breakpoints, the following extrapolations can be made: cephalosporins: ≤16 μg/ml, susceptible; ≤64 μg/ml, intermediately susceptible; carbapenems: ≤4 μg/ml, susceptible; ≤8 μg/ml, intermediately susceptible. CXM, cefuroxime; CFR, cefadroxil; CDR, cefdinir; CDN, cefditoren; CPD, cefpodoxime; FOX, cefoxitin; ETP, ertapenem; IPM, imipenem; DOR, doripenem; BIA, biapenem; FAR, faropenem; TEB, tebipenem.
Antibiotic synergy against clinical isolates.
Based on a prior study that demonstrated synergy of dual-β-lactam combinations against Mab reference strain ATCC 19977 (34), we determined the activity of 13 distinct dual-β-lactam combinations against the panel of 21 distinct Mab clinical isolates. These combinations were cefoxitin and imipenem, cefuroxime and imipenem, doripenem and imipenem, biapenem and imipenem, cefdinir and imipenem, faropenem and imipenem, cefadroxil and tebipenem, ertapenem and imipenem, cefditoren and imipenem, cefpodoxime and imipenem, cefditoren and tebipenem, cefuroxime and cefditoren, and cefditoren and biapenem. Each combination was tested against the 21 clinical Mab strains in vitro using a checkerboard titration assay, as described in Materials and Methods. The checkerboard assay allows for calculation of a fractional inhibitory concentration index (FICI) for each drug pair against each isolate. The FICI is a mathematical representation of the degree to which each drug in a combination contributes to synergy (46, 47). A stringent interpretation of FICI values was used, with FICIs of ≤0.5 indicating synergy, FICIs of >0.5 to <4 indifference, and FICIs of >4 antagonism (48). The fractional inhibitory concentration (FIC) was used to extrapolate the decrease in the MIC of each drug in a pair as a result of synergy. As may be expected from the MIC results, FICIs of the dual-β-lactam combinations were highly varied among clinical strains (Table 2). Similarly, the number of strains against which each drug combination exhibited synergy also varied, ranging from 14% to 100% of isolates. For example, the combination of cefditoren and biapenem exhibited synergy against only 3/21 isolates, while the combination of cefoxitin and imipenem was synergistic against all 21 strains; this was the only combination that exhibited synergy against 100% of isolates. In general, 7/13 drug pairs exhibited synergy against >50% of the clinical strains (Table 3).
TABLE 2.
Comparison of MICs of single drugs and MICs resulting from dual-β-lactam synergy
| Mab clinical strain (subspecies)a | Data forb: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOX/IPM | CXM/IPM | DOR/IPM | BIA/IPM | CDR/IPM | FAR/IPM | CFR/TEB | ETP/IPM | CDN/IPM | CPD/IPM | CDN/TEB | CXM/CDN | CDN/BIA | |
| M9501 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 64/8 | 512/8 | 32/8 | 512/512 | 512/8 | ||||||||
| Extrapolated MIC (μg/ml) | 4/1 | 112/2 | 7/2 | 128/128 | 68/2 | ||||||||
| FICI | 0.292 | 0.414 | 0.438 | 0.500 | 0.328 | ||||||||
| M9502 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 128/8 | 512/8 | 512/256 | 512/8 | 512/256 | 512/512 | |||||||
| Extrapolated MIC (μg/ml) | 28/2 | 112/1 | 96/64 | 112/2 | 76/48 | 78/43 | |||||||
| FICI | 0.422 | 0.321 | 0.438 | 0.422 | 0.336 | 0.235 | |||||||
| M9503 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 64/8 | 256/8 | 8/8 | 8/8 | 64/8 | 256/8 | 512/256 | 512/8 | 256/8 | 512/8 | 256/256 | ||
| Extrapolated MIC (μg/ml) | 14/2 | 51/2 | 2/2 | 2/2 | 14/2 | 56/1 | 112/56 | 83/1 | 64/2 | 112/2 | 56/50 | ||
| FICI | 0.438 | 0.357 | 0.422 | 0.438 | 0.422 | 0.360 | 0.438 | 0.287 | 0.500 | 0.422 | 0.414 | ||
| M9504 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | 256/16 | 16/16 | 8/16 | 32/16 | 256/16 | 512/512 | 512/16 | 256/16 | 512/16 | |||
| Extrapolated MIC (μg/ml) | 10/3 | 67/2 | 3/3 | 3/4 | 8/3 | 48/3 | 86/86 | 80/2 | 83/2 | 112/3 | |||
| FICI | 0.315 | 0.267 | 0.360 | 0.438 | 0.438 | 0.360 | 0.334 | 0.352 | 0.297 | 0.360 | |||
| M9505 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | 256/16 | 16/16 | 512/16 | 256/256 | 256/16 | 256/256 | ||||||
| Extrapolated MIC (μg/ml) | 12/4 | 40/2 | 4/4 | 82/3 | 67/43 | 72/4 | 48/64 | ||||||
| FICI | 0.438 | 0.321 | 0.500 | 0.286 | 0.334 | 0.422 | 0.375 | ||||||
| M9507 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 256/32 | 512/32 | 128/32 | 128/32 | 512/32 | ||||||||
| Extrapolated MIC (μg/ml) | 37/6 | 128/6 | 26/6 | 22/6 | 128/8 | ||||||||
| FICI | 0.332 | 0.438 | 0.378 | 0.360 | 0.500 | ||||||||
| M9509 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 32/16 | 256/16 | 16/16 | 16/16 | 64/16 | 64/16 | 256/16 | 512/16 | 512/16 | 512/128 | |||
| Extrapolated MIC (μg/ml) | 4/2 | 23/7 | 3/2 | 3/2 | 11/2 | 16/1 | 48/1 | 112/1 | 96/2 | 98/28 | |||
| FICI | 0.240 | 0.248 | 0.320 | 0.320 | 0.301 | 0.290 | 0.258 | 0.301 | 0.289 | 0.410 | |||
| M9510 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 256/64 | 128/64 | 128/64 | 256/64 | 512/128 | ||||||||
| Extrapolated MIC (μg/ml) | 43/10 | 32/10 | 28/8 | 56/9 | 128/32 | ||||||||
| FICI | 0.326 | 0.407 | 0.344 | 0.348 | 0.500 | ||||||||
| M9513 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | 512/16 | 32/16 | 64/16 | 512/16 | 512/512 | 512/512 | 512/512 | |||||
| Extrapolated MIC (μg/ml) | 14/4 | 112/3 | 8/3 | 16/2 | 112/3 | 78/72 | 56/12 | 96/72 | |||||
| FICI | 0.438 | 0.360 | 0.438 | 0.344 | 0.391 | 0.291 | 0.329 | 0.328 | |||||
| M9514 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 128/8 | 512/8 | 32/8 | 32/8 | 512/256 | 512/8 | |||||||
| Extrapolated MIC (μg/ml) | 25/2 | 83/1 | 8/2 | 6/2 | 112/40 | 128/2 | |||||||
| FICI | 0.388 | 0.279 | 0.500 | 0.438 | 0.375 | 0.438 | |||||||
| M9515 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 256/32 | 512/32 | 256/32 | 128/32 | 256/32 | 128/32 | |||||||
| Extrapolated MIC (μg/ml) | 48/5 | 96/4 | 35/5 | 18/6 | 43/5 | 80/6 | |||||||
| FICI | 0.328 | 0.293 | 0.284 | 0.306 | 0.306 | 0.438 | |||||||
| M9517 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | 512/16 | 16/16 | 16/16 | 64/16 | 256/16 | 512/512 | 512/16 | |||||
| Extrapolated MIC (μg/ml) | 14/4 | 86/2 | 4/4 | 4/4 | 16/4 | 48/3 | 88/88 | 102/2 | |||||
| FICI | 0.438 | 0.288 | 0.500 | 0.500 | 0.500 | 0.375 | 0.344 | 0.328 | |||||
| M9521 (massiliense) | |||||||||||||
| MIC (μg/ml) of each drug | 32/8 | 512/8 | 16/8 | 128/8 | 128/8 | 256/8 | 512/8 | 512/256 | 512/512 | 512/16 | |||
| Extrapolated MIC (μg/ml) | 5/2 | 75/1 | 4/2 | 24/2 | 32/2 | 64/2 | 112/2 | 96/48 | 74/112 | 96/3 | |||
| FICI | 0.375 | 0.253 | 0.500 | 0.375 | 0.438 | 0.438 | 0.438 | 0.375 | 0.364 | 0.375 | |||
| M9522 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | 512/16 | 32/16 | 32/16 | 256/16 | 512/16 | 512/512 | 512/16 | 512/16 | ||||
| Extrapolated MIC (μg/ml) | 9/3 | 96/2 | 8/4 | 8/4 | 49/3 | 112/4 | 96/72 | 128/4 | 128/3 | ||||
| FICI | 0.279 | 0.317 | 0.414 | 0.422 | 0.388 | 0.438 | 0.328 | 0.500 | 0.500 | ||||
| M9525 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 128/32 | 512/32 | 64/32 | 64/32 | 256/32 | 512/32 | 512/512 | 512/32 | |||||
| Extrapolated MIC (μg/ml) | 22/4 | 112/2 | 12/6 | 9/6 | 56/4 | 128/0.5 | 128/128 | 128/3 | |||||
| FICI | 0.306 | 0.293 | 0.360 | 0.293 | 0.348 | 0.266 | 0.500 | 0.344 | |||||
| M9526 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | ||||||||||||
| Extrapolated MIC (μg/ml) | 13/3 | ||||||||||||
| FICI | 0.396 | ||||||||||||
| M9527 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 128/16 | ||||||||||||
| Extrapolated MIC (μg/ml) | 28/4 | ||||||||||||
| FICI | 0.438 | ||||||||||||
| M9528 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 128/32 | 512/32 | 32/32 | 32/32 | 512/32 | ||||||||
| Extrapolated MIC (μg/ml) | 25/4 | 112/7 | 16/6 | 8/8 | 128/6 | ||||||||
| FICI | 0.328 | 0.422 | 0.438 | 0.500 | 0.438 | ||||||||
| M9529 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 128/256 | 256/256 | 512/256 | 512/256 | |||||||||
| Extrapolated MIC (μg/ml) | 32/64 | 33/44 | 66/51 | 112/18 | |||||||||
| FICI | 0.500 | 0.332 | 0.326 | 0.293 | |||||||||
| M9530 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | 512/16 | 64/16 | 16/16 | 128/16 | 512/16 | 512/512 | 512/16 | 512/16 | 512/512 | 512/512 | ||
| Extrapolated MIC (μg/ml) | 11/3 | 96/2 | 10/3 | 3/4 | 64/4 | 82/2 | 104/64 | 112/3 | 128/1 | 128/128 | 83/83 | ||
| FICI | 0.344 | 0.317 | 0.301 | 0.438 | 0.422 | 0.262 | 0.329 | 0.410 | 0.321 | 0.500 | 0.328 | ||
| M9531 (abscessus) | |||||||||||||
| MIC (μg/ml) of each drug | 64/16 | 512/16 | 64/16 | 32/16 | 64/16 | 512/512 | |||||||
| Extrapolated MIC (μg/ml) | 10/3 | 86/2 | 9/3 | 6/4 | 16/4 | 102/98 | |||||||
| FICI | 0.329 | 0.310 | 0.267 | 0.410 | 0.500 | 0.357 | |||||||
For each clinical strain, the top row of data represents the MIC of each individual drug, whereas the middle row represents the extrapolated MIC of each drug when used in combination. The extrapolated MIC of each drug was determined by averaging the concentrations of the drug in each well in which Mab growth was inhibited. These values were used to determine FICIs. FICIs and MICs in combination were extrapolated using data averaged from at least two biological replicates. The bottom row of data represents the FICI for each synergistic combination. The empty cells indicate lack of synergy.
FOX, cefoxitin; IPM, imipenem; CXM, cefuroxime; DOR, doripenem; BIA, biapenem; CDR, cefdinir; FAR, faropenem; CFR, cefadroxil; TEB, tebipenem; ETP, ertapenem; CDN, cefditoren; CPD, cefpodoxime.
TABLE 3.
Numbers (and percentage) of strains against which each drug pair exhibited synergy
| Drug combinationa | No. (%) of strains for whichb: |
||
|---|---|---|---|
| Synergy was exhibited | MIC of ≥1 drug was brought into therapeutic range | MICs of both drugs were brought into therapeutic range | |
| FOX and IPM | 21 (100) | 21 (100) | 19 (90) |
| CXM and IPM | 17 (81) | 16 (76) | 3 (14) |
| DOR and IPM | 16 (76) | 15 (71) | 9 (43) |
| BIA and IPM | 15 (71) | 14 (67) | 10 (48) |
| CDR and IPM | 12 (57) | 12 (57) | 11 (52) |
| FAR and IPM | 12 (57) | 12 (57) | 0 (0) |
| CFR and TEB | 12 (57) | 0 (0) | 0 (0) |
| ETP and IPM | 10 (48) | 10 (48) | 0 (0) |
| CDN and IPM | 6 (29) | 5 (24) | 1 (5) |
| CPD and IPM | 6 (29) | 6 (29) | 0 (0) |
| CDN and TEB | 6 (29) | 2 (10) | 0 (0) |
| CXM and CDN | 5 (24) | 2 (10) | 1 (5) |
| CDN and BIA | 3 (14) | 1 (5) | 0 (0) |
FOX, cefoxitin; IPM, imipenem; CXM, cefuroxime; DOR, doripenem; BIA, biapenem; CDR, cefdinir; FAR, faropenem; CFR, cefadroxil; TEB, tebipenem; ETP, ertapenem; CDN, cefditoren; CPD, cefpodoxime.
Extrapolated MICs were calculated using the FICI for each drug pair and clinical strain. The therapeutic range was defined as MICs at or below the CLSI breakpoints for cephalosporins (≤16 μg/ml, susceptible; ≤64 μg/ml, intermediately susceptible) and carbapenems (≤4 μg/ml, susceptible; ≤8 μg/ml, intermediately susceptible).
Synergistic combinations were further assessed to determine whether the resulting decrease in MICs was sufficient to bring the isolate into the therapeutic range for each drug (Table 3). Although Clinical and Laboratory Standards Institute (CLSI) guidelines regarding MIC breakpoints for Mab strains are not currently available for most of the antibiotics tested, they have been established for cefoxitin and imipenem (49). Therefore, MIC breakpoints for all cephalosporins and carbapenems were assumed to be the same as those for cefoxitin (≤16 μg/ml, susceptible; ≤64 μg/ml, intermediately susceptible) and imipenem (≤4 μg/ml, susceptible; ≤8 μg/ml, intermediately susceptible), respectively.
In general, synergy between agents with very high initial MICs (i.e., 256 to 512 μg/ml) was often not sufficient to bring the resulting MICs within the therapeutic range. For example, the combination of cefadroxil and tebipenem was unable to reach the therapeutic threshold for either drug, despite exhibiting synergy against 12/21 isolates. This was especially true of the carbapenems with high MICs, as their therapeutic window is much smaller than that of the cephalosporins. Indeed, the combinations of cefpodoxime and imipenem, ertapenem and imipenem, faropenem and imipenem, cefadroxil and tebipenem, cefditoren and biapenem, and cefditoren and tebipenem were unable to achieve the therapeutic range for both drugs against any of the clinical strains.
DISCUSSION
Independent studies have reported the phenomenon of synergy between β-lactams against Mab strains using both in vitro and in vivo approaches (31, 34–36, 50, 51). Although the mechanism behind dual-β-lactam synergy is not fully understood, a hypothesis that two β-lactams that inhibit distinct sets of nonredundant enzymes may exhibit synergy in antibacterial activity has been proposed (32), based on differential inhibition of enzymes involved in peptidoglycan synthesis (30, 32, 52). Because β-lactams are widely available and generally well tolerated by patients, they may be an untapped resource in our desperate fight to treat drug-resistant Mab infections.
In this study, we tested the 13 β-lactam combinations against a library of 21 clinical isolates obtained from CF patients in recent years. The rationale for including these specific dual-β-lactam combinations is a prior proof-of-concept screen against a single Mab strain, the reference strain ATCC 19977, in which they exhibited synergy (34). As may be expected based on prior studies (37–39), this study illustrates the significant heterogeneity that exists among Mab clinical strains with regard to drug susceptibility profiles and potential responses to antibiotics. In many instances, the MICs of several of the antibiotics differed by only a 2-fold dilution. However, these differences were observed at higher drug concentrations, where the amount of drug present differed drastically between the dilutions, and the results were highly reproducible. It also supports our position that study of a single Mab reference strain provides an inadequate assessment of novel treatments. Several clinical isolates harbored much higher MICs for certain drugs, compared to the reference strain, and we observed significant variations in the degree of synergy exhibited by β-lactam pairs.
For example, the MIC of imipenem for ATCC 19977 is 8 μg/ml. Only 5 of the 21 clinical strains had an imipenem MIC of 8 μg/ml; the other MICs ranged from 16 to 256 μg/ml, and the strains were considered resistant to imipenem, based on CLSI breakpoints. This is particularly relevant because imipenem is one of the cornerstone antibiotics used to treat Mab lung infections (21). It is one of only two β-lactams included in the Cystic Fibrosis Foundation and European Cystic Fibrosis Society treatment guidelines, along with cefoxitin (21). In our study, the combination of imipenem and cefoxitin was the only pair that exhibited synergy against 100% of isolates and was capable of reducing MICs to within the therapeutic range for all but one strain (Table 3). However, these two agents are never used concurrently against Mab in clinical practice. This is likely due to the fact that current treatment paradigms were developed using the historical model of β-lactam activity, which was predominantly influenced by observations in Gram-negative organisms that considered only one target in the peptidoglycan synthesis pathway, the d,d-transpeptidases (26). An important and underappreciated reason for this was the availability of only the penicillin and cephalosporin subclasses at the time, which were used to probe enzymes inhibited by β-lactams (53). These two subclasses preferentially bind to d,d-transpeptidases and did not readily permit identification of l,d-transpeptidases (54). According to this historical model, d,d-transpeptidases represented the only enzyme class targeted by β-lactams. Therefore, concurrent use of two β-lactams was considered redundant. The recent discovery of l,d-transpeptidases (55), their dominant role in peptidoglycan synthesis in Mab (28), and their preferential inhibition by the carbapenem subclass of β-lactams (31) have called this historical dogma into question.
Several of the currently recommended first-line agents for treatment of Mab infections have substantial side effect profiles, and regimens often require adjustment due to adverse events or toxicity. For example, amikacin is associated with significant nephrotoxicity and ototoxicity, the latter of which is dose dependent and irreversible. Use of this drug for a prolonged period, as is required for treatment of Mab infections, can eventually cause deafness (56–59). Based on these reports, use of this drug as first-line treatment for Mab disease in CF patients, especially children, is undesirable. Therefore, there is a real potential for dual β-lactams to replace existing first-line agents, which could be a major innovation for our current and future patients.
Despite this, use of dual β-lactams remains a radical approach to treatment and has also raised concern regarding the risk of adverse events. However, a recent meta-analysis comparing dual β-lactams to β-lactam plus aminoglycoside regimens for treatment of Gram-negative infections showed significantly lower rates of nephrotoxicity and ototoxicity among dual β-lactams, with both treatment efficacy and rates of other adverse events being largely equal between the two study arms (60).
The combination of cefoxitin and imipenem exhibited synergy against all 21 of the clinical strains tested, maintaining efficacy despite vast differences in drug resistance profiles. These agents are already used separately in the treatment of Mab lung disease. Given our current deficit of viable therapeutic options against this pathogen, it may be reasonable to consider concurrent use of both agents as an alternative approach in the setting of extensive drug resistance.
Although none of the other β-lactam pairs performed as well as cefoxitin and imipenem, there were three additional combinations that exhibited previously unexpected synergistic activities; these were doripenem and imipenem, biapenem and imipenem, and cefdinir and imipenem, which exhibited synergy against 76%, 71%, and 57% of the isolates, respectively. These agents tended to have slightly lower initial MICs and thus exhibited sufficient synergy to bring the MICs of both drugs to within the therapeutic range for roughly one-half of the isolates (43%, 48%, and 52%, respectively). Both cefdinir and doripenem are commercially available in the United States, and cefdinir’s oral formulation makes it logistically appealing for patients. Biapenem (61) is not currently FDA approved in the United States, but it has been used commercially in Asia for nearly 20 years and has shown efficacy against Mab in vivo (36, 62) and against Mycobacterium tuberculosis in vitro and in vivo (63–66). Given the abysmal cure rates of 25 to 40% with current regimens (11, 12) and our urgent need for tolerable long-term therapies against Mab pulmonary disease, these combinations may be worth consideration in certain clinical cases.
The combination of cefuroxime and imipenem exhibited synergy against the second largest number of clinical strains at 17/21 strains (81%). However, the therapeutic ranges for both drugs were achieved in only 14% of isolates due to the very high initial MIC of cefuroxime. This trend was observed with several of the β-lactam pairs, in which synergy was achieved with a number of isolates but the therapeutic window was not, thus limiting the potential for clinical use of these combinations (Table 3).
As mentioned above, the current Mab reference strain (ATCC 19977), which was derived nearly 70 years ago from a synovial fluid sample, may not be representative of modern trends in Mab pulmonary disease, especially with regard to drug resistance profiles. We speculate that our fully sequenced library of clinical strains may contain potential candidates for one or more updated reference strains. In a recent study, we evaluated six of these clinical isolates (M9505, M9513, M9521, M9522, M9526, and M9529) in a murine model of Mab pulmonary disease to determine whether they were capable of causing invasive infections in vivo (62), as would be required of any candidate reference strain for the purpose of drug treatment studies. All of the clinical strains exhibited similar lung implantation burdens, and three of the isolates (M9513, M9521, and M9529) caused invasive infections, leading to ≥3-log10 unit increases in the bacterial burden at 3 weeks, similar to results seen with ATCC 19977 under the same conditions. Although additional in vivo studies are needed to further characterize these strains, they may have the potential to broaden our current repertoire of reference strains.
This study was limited by the fact that the dual-β-lactam combinations tested were initially identified as exhibiting synergy against the Mab reference strain; therefore, it is likely that a percentage of the additional 107 β-lactam combinations that were previously deemed nonsynergistic against ATCC 19977 (34) might have exhibited synergy against one or more clinical strains. Additionally, this study was performed in vitro, which does not necessarily correlate with clinical responses in patients with Mab infections. Further studies are needed to assess the in vivo efficacy of these novel combinations in animal models of Mab disease or in preclinical models that approximate certain aspects of drug activity in humans, such as the hollow-fiber model that has been used to model the pharmacokinetics/pharmacodynamics of antibacterials against Mab (67).
In conclusion, Mab pulmonary disease can have devastating effects on patient health, and current treatments are prolonged, poorly tolerated, and too often ineffective. In this study, several dual-β-lactam combinations exhibited synergistic efficacy against a genetically varied library of clinical strains that are resistant to several drugs currently used to treat Mab disease. In particular, cefoxitin and imipenem exhibited synergy against 100% of the isolates, suggesting their potential for widespread clinical efficacy, as these agents are already used separately against Mab. Given the urgent need for novel therapeutics against this pathogen, dual-β-lactam regimens may offer viable immediate treatment options with efficacy against drug-resistant Mab infections.
MATERIALS AND METHODS
Bacterial strains and in vitro growth conditions.
Clinical Mab isolates utilized in this study were obtained by Nicole Parrish from CF patients seen at the Johns Hopkins Hospital between 2004 and 2018 and were biobanked by the Johns Hopkins Clinical Microbiology Laboratory. Strains were grown at 37°C in Middlebrook 7H9 broth (Difco) supplemented with 0.5% glycerol, 10% albumin-dextrose-catalase enrichment, and 0.05% Tween 80, with constant shaking at 220 rpm in an orbital shaker. All drugs were obtained from the following commercial vendors: ertapenem, Toronto Research Chemicals; imipenem, doripenem, biapenem, faropenem, tebipenem, and all cephalosporins, Sigma-Aldrich. To assess the quality of these compounds, a few were randomly selected and assessed by liquid chromatography-mass spectrometry. The purity of compounds ranged from 95% to 99%.
MICs.
The MIC of each drug against each clinical isolate of Mab was determined using the standard broth dilution method (68, 69) in accordance with CLSI guidelines specific for this organism (49). In summary, powdered drug stocks were reconstituted in either dimethyl sulfoxide (DMSO) or sterile deionized water (for imipenem and biapenem, given their poor solubility in DMSO), and 2-fold serial dilutions were prepared in Middlebrook 7H9 broth to obtain final drug concentrations ranging from 512 μg/ml to 2 μg/ml in 96-well plates in a final volume of 200 μl. A total of 105 CFU of Mab from an exponentially growing culture was added to each well. Mab culture without drug and 7H9 broth alone were included in each plate as positive and negative controls, respectively. Plates were incubated at 30°C for 72 h according to CLSI guidelines. Growth of Mab or lack thereof was determined using a Sensititre Manual Viewbox, and the MIC for each drug was recorded as the lowest concentration at which Mab growth was not observed. All MIC assessments were repeated to verify results.
Checkerboard titration assay.
The checkerboard titration assay is a modified broth dilution assay and was performed as described previously (46, 47). To determine the degree of synergy, two drugs were added to Middlebrook 7H9 broth in a 96-well plate, each starting at 2× MIC and serially diluted 2-fold up to 1/64× MIC; therefore, all possible 2-fold dilution combinations from 2× to 1/64× MIC were assayed. A total of 105 CFU of Mab was inoculated into each well. Plates were incubated at 30°C and evaluated for Mab growth by visual inspection at 72 h using a Sensititre Manual Viewbox. The FIC of each drug in combination was determined as described (46, 47). The FIC of a drug in a sample is calculated as the concentration of the drug divided by the MIC of the drug when used alone. The FICI is the sum of the FICs of two drugs in a sample. The FICI was calculated for each combination of drugs that inhibited Mab growth at less than one-half the MIC of each individual drug. FICIs of ≤0.5 were interpreted as synergy, FICIs of >0.5 to 4 as indifference, and FICIs >4 as antagonism, according to the most stringent interpretation recommended (48). As an internal control, the MIC of each individual drug was also assessed via broth microdilution within each plate. All combinations with FICIs of ≤0.5 were tested in duplicate to confirm reproducibility, and an average FICI was calculated and reported here.
DNA extraction, whole-genome sequencing, and assembly.
To extract genomic DNA from Mab, we used the phenol-chloroform extraction method optimized for mycobacteria (70). Briefly, for each strain, 30 ml of culture was centrifuged, resuspended in Tris-EDTA (TE) buffer containing lysozyme and RNase A, and incubated overnight at 37°C. SDS (10%) with proteinase K was added, and the mixture was incubated for 10 min at 65°C; 5 M NaCl was then added together with cetyltrimethylammonium bromide (CTAB)/NaCl solution, and the mixture was incubated again for 10 min at 65°C. A solution of 25:24:1 phenol/chloroform/isoamyl alcohol was then added to the suspension and gently mixed before centrifugation for 10 min. The supernatant was transferred to a new tube, which contained a solution of 24:1 chloroform/isoamyl alcohol, and was centrifuged again for 10 min. Next, the top layer of the suspension was added to a tube containing ice-cold isopropanol and was incubated for 20 min at –20°C. This suspension was then centrifuged, the supernatant was discarded, 70% ethanol was added, and the mixture was centrifuged for another 5 min. The supernatant was discarded, the tubes containing the DNA were air dried overnight, and the DNA was resuspended with 300 μl of deionized water the following day.
The genomic DNA obtained was then sequenced using the Illumina PE150 platform (Novogene, CA, USA) and the PacBio Sequel platform (Genewiz, NJ, USA). To identify to the subspecies level the clinical strains at our disposal, we used Geneious v11.1.5 (Biomatters) to de novo assemble the reads into contigs using SPAdes (bundled in Geneious). Subsequently, the Geneious read-mapping algorithm was used to produce longer contigs. We then used the “map to reference” feature in Geneious to map the six different genetic markers that were validated previously (42, 43) for subspecies identification of Mab strains; these genes were hsp65 (MAB_0650), secA (MAB_2397), rpoB (MAB_3869c), polC (MAB_2696c), hoa (MAB_0626), and ftsZ (MAB_2009). Once we obtained the sequence of each of the six genes for each of our strains, we concatenated them in the same order to create a single sequence for each strain, including the reference strain ATCC 19977 for Mab and the reference strain CCUG 48898 for Mab subspecies massiliense. Each sequence was aligned using MUSCLE to create a phylogenetic tree using Geneious’ own tree builder. The parameters set for the tree building included the Tamura-Nei genetic distance model with the UPGMA tree-building method, bootstrapped with 1,000 iterations (43). To compare the genomes generated de novo, we used the Mauve aligner in Geneious to generate an interactive map that allowed for direct comparison of the genomes.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants R21 AI137720 and R01 AI155664 to G.L. E.S.-R. was supported by a Pearl M. Stetler Fund Research Award.
We acknowledge the generous gift of Mab clinical isolates from Nicole Parrish, Clinical Microbiology, Johns Hopkins Hospital.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Angrill J, Agustí C, de Celis R, Rañó A, Gonzalez J, Solé T, Xaubet A, Rodriguez-Roisin R, Torres A. 2002. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 57:15–19. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratjen FA. 2009. Cystic fibrosis: pathogenesis and future treatment strategies. Respir Care 54:595–605. doi: 10.4187/aarc0427. [DOI] [PubMed] [Google Scholar]
- 3.Purcell P, Jary H, Perry A, Perry JD, Stewart CJ, Nelson A, Lanyon C, Smith DL, Cummings SP, De Soyza A. 2014. Polymicrobial airway bacterial communities in adult bronchiectasis patients. BMC Microbiol 14:130. doi: 10.1186/1471-2180-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acosta N, Whelan FJ, Somayaji R, Poonja A, Surette MG, Rabin HR, Parkins MD. 2017. The evolving cystic fibrosis microbiome: a comparative cohort study spanning 16 years. Ann Am Thorac Soc 14:1288–1297. doi: 10.1513/AnnalsATS.201609-668OC. [DOI] [PubMed] [Google Scholar]
- 5.Adjemian J, Frankland TB, Daida YG, Honda JR, Olivier KN, Zelazny A, Honda S, Prevots DR. 2017. Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USA. Emerg Infect Dis 23:439–447. doi: 10.3201/eid2303.161827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng C, Fanta CH. 2013. Non-tuberculous mycobacterial pulmonary infection in the immunocompetent host. QJM 106:307–315. doi: 10.1093/qjmed/hct022. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RS, Lo B, Son J. 2018. Phylogenomics and comparative genomic studies robustly support division of the genus Mycobacterium into an emended genus Mycobacterium and four novel genera. Front Microbiol 9:67. doi: 10.3389/fmicb.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, Von Reyn CF, Wallace RJ, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 9.Esther CR, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benwill JL, Wallace RJ. 2014. Mycobacterium abscessus: challenges in diagnosis and treatment. Curr Opin Infect Dis 27:506–510. doi: 10.1097/QCO.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 11.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 12.Diel R, Ringshausen F, Richter E, Welker L, Schmitz J, Nienhaus A. 2017. Microbiological and clinical outcomes of treating non-Mycobacterium avium complex nontuberculous mycobacterial pulmonary disease: a systematic review and meta-analysis. Chest 152:120–142. doi: 10.1016/j.chest.2017.04.166. [DOI] [PubMed] [Google Scholar]
- 13.Brown-Elliott BA, Wallace RJ. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 15:716–746. doi: 10.1128/cmr.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 15.van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. 2012. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat 15:149–161. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Lopeman R, Harrison J, Desai M, Cox J. 2019. Mycobacterium abscessus: environmental bacterium turned clinical nightmare. Microorganisms 7:90. doi: 10.3390/microorganisms7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flume PA. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. J Cyst Fibros 15:139–140. doi: 10.1016/S1569-1993(16)00018-7. [DOI] [PubMed] [Google Scholar]
- 18.Maurer FP, Ruegger V, Ritter C, Bloemberg GV, Bottger EC. 2012. Acquisition of clarithromycin resistance mutations in the 23S rRNA gene of Mycobacterium abscessus in the presence of inducible erm(41). J Antimicrob Chemother 67:2606–2611. doi: 10.1093/jac/dks279. [DOI] [PubMed] [Google Scholar]
- 19.Brown BA, Wallace RJ, Onyi GO, De Rosas V, Wallace RJ. 1992. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob Agents Chemother 36:180–184. doi: 10.1128/aac.36.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace RJ, Swenson JM, Silcox VA, Bullen MG. 1985. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis 152:500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- 21.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71:i1–i22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, Ormerod P, Shingadia D, Smith D, Whitehead N, Wilson R, Floto RA. 2017. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 72(Suppl 2):ii1–ii64. doi: 10.1136/thoraxjnl-2017-210927. [DOI] [PubMed] [Google Scholar]
- 23.Jeon K, Kwon OJ, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park YK, Kim CK, Koh W-J. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease. Am J Respir Crit Care Med 180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 24.Lavollay M, Dubée V, Heym B, Herrmann JL, Gaillard JL, Gutmann L, Arthur M, Mainardi JL. 2014. In vitro activity of cefoxitin and imipenem against Mycobacterium abscessus complex. Clin Microbiol Infect 20:O297–O300. doi: 10.1111/1469-0691.12405. [DOI] [PubMed] [Google Scholar]
- 25.Hamad B. 2010. The antibiotics market. Nat Rev Drug Discov 9:675–676. doi: 10.1038/nrd3267. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann R, Holtje J-V, Schwarz U. 1972. Targets of penicillin action in Escherichia coli. Nature 235:426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- 27.Walsh C, Wencewicz TA. 2016. Antibiotics: challenges, mechanisms, opportunities, p 37–68. American Society for Microbiology, Washington, DC. [Google Scholar]
- 28.Lavollay M, Fourgeaud M, Herrmann JL, Dubost L, Marie A, Gutmann L, Arthur M, Mainardi JL. 2011. The peptidoglycan of Mycobacterium abscessus is predominantly cross-linked by l,d-transpeptidases. J Bacteriol 193:778–782. doi: 10.1128/JB.00606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattoo R, Lloyd EP, Kaushik A, Kumar P, Brunelle JL, Townsend CA, Lamichhane G. 2017. LdtMav2, a nonclassical transpeptidase and susceptibility of Mycobacterium avium to carbapenems. Future Microbiol 12:595–607. doi: 10.2217/fmb-2016-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubée V, Triboulet S, Mainardi JL, Ethève-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet JE, Arthur M. 2012. Inactivation of Mycobacterium tuberculosis l,d-transpeptidase LdtMt1 by carbapenems and cephalosporins. Antimicrob Agents Chemother 56:4189–4195. doi: 10.1128/AAC.00665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P, Chauhan V, Silva JRA, Lameira J, d'Andrea FB, Li S-G, Ginell SL, Freundlich JS, Alves CN, Bailey S, Cohen KA, Lamichhane G. 2017. Mycobacterium abscessus l,d-transpeptidases are susceptible to inactivation by carbapenems and cephalosporins but not penicillins. Antimicrob Agents Chemother 61:e00866-17. doi: 10.1128/AAC.00866-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar P, Kaushik A, Lloyd EP, Li SG, Mattoo R, Ammerman NC, Bell DT, Perryman AL, Zandi TA, Ekins S, Ginell SL, Townsend CA, Freundlich JS, Lamichhane G. 2017. Non-classical transpeptidases yield insight into new antibacterials. Nat Chem Biol 13:54–61. doi: 10.1038/nchembio.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta R, Lavollay M, Mainardi JL, Arthur M, Bishai WR, Lamichhane G. 2010. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med 16:466–469. doi: 10.1038/nm.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Select β-lactam combinations exhibit synergy against Mycobacterium abscessus in vitro. Antimicrob Agents Chemother 63:e02613-18. doi: 10.1128/AAC.02613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey R, Chen L, Manca C, Jenkins S, Glaser L, Vinnard C, Stone G, Lee J, Mathema B, Nuermberger EL, Bonomo RA, Kreiswirth BN. 2019. Dual β-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 10:e02895-18. doi: 10.1128/mBio.02895-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Synergistic efficacy of β-lactam combinations against Mycobacterium abscessus pulmonary infection in mice. Antimicrob Agents Chemother 63:e00614-19. doi: 10.1128/AAC.00614-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreutzfeldt KM, McAdam PR, Claxton P, Holmes A, Seagar AL, Laurenson IF, Fitzgerald JR. 2013. Molecular longitudinal tracking of Mycobacterium abscessus spp. during chronic infection of the human lung. PLoS One 8:e63237. doi: 10.1371/journal.pone.0063237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choo SW, Wee WY, Ngeow YF, Mitchell W, Tan JL, Wong GJ, Zhao Y, Xiao J. 2014. Genomic reconnaissance of clinical isolates of emerging human pathogen Mycobacterium abscessus reveals high evolutionary potential. Sci Rep 4:4061. doi: 10.1038/srep04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansen MD, Herrmann J-L, Kremer L. 2020. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 18:392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 40.Moore M, Frerichs JB. 1953. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region. J Invest Dermatol 20:133–169. doi: 10.1038/jid.1953.18. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz M, Fisher S, Story-Roller E, Lamichhane G, Parrish N. 2018. Activities of dual combinations of antibiotics against multidrug-resistant nontuberculous mycobacteria recovered from patients with cystic fibrosis. Microb Drug Resist 24:1191–1197. doi: 10.1089/mdr.2017.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelazny AM, Root JM, Shea YR, Colombo RE, Shamputa IC, Stock F, Conlan S, McNulty S, Brown-Elliott BA, Wallace RJ, Olivier KN, Holland SM, Sampaio EP. 2009. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J Clin Microbiol 47:1985–1995. doi: 10.1128/JCM.01688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan JL, Khang TF, Ngeow YF, Choo SW. 2013. A phylogenomic approach to bacterial subspecies classification: proof of concept in Mycobacterium abscessus. BMC Genomics 14:879. doi: 10.1186/1471-2164-14-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HY, Kim BJ, Kook Y, Yun YJ, Shin JH, Kim BJ, Kook YH. 2010. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol Immunol 54:347–353. doi: 10.1111/j.1348-0421.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 45.Ng HF, Ngeow YF. 2020. A single-gene approach for the subspecies classification of Mycobacteroides abscessus. Pathog Dis 78:ftaa055. doi: 10.1093/femspd/ftaa055. [DOI] [PubMed] [Google Scholar]
- 46.Rohner P, Herter C, Auckenthaler R, Pechère JC, Waldvogel FA, Lew DP. 1989. Synergistic effect of quinolones and oxacillin on methicillin-resistant Staphylococcus species. Antimicrob Agents Chemother 33:2037–2041. doi: 10.1128/aac.33.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard. a critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-n. [DOI] [PubMed] [Google Scholar]
- 48.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 49.Clinical and Laboratory Standards Institute. 2018. Performance standards for susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes. Supplement M62. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 50.Meir M, Barkan D. 2020. Alternative and experimental therapies of Mycobacterium abscessus infections. Int J Mol Sci 21:6793. doi: 10.3390/ijms21186793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopeman RC, Harrison J, Rathbone DL, Desai M, Lambert PA, Cox JAG. 2020. Effect of amoxicillin in combination with Imipenem-relebactam against Mycobacterium abscessus. Sci Rep 10:928. doi: 10.1038/s41598-020-57844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordillot M, Dubée V, Triboulet S, Dubost L, Marie A, Hugonnet J-E, Arthur M, Mainardi J-L. 2013. In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by l,d-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 57:5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blumberg PM, Strominger JL. 1974. Interaction of penicillin with the bacterial cell: penicillin binding proteins and penicillin sensitive enzymes. Bacteriol Rev 38:291–335. doi: 10.1128/BR.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blumberg PM, Strominger JL. 1971. Inactivation of d-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A 68:2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mainardi JL, Fourgeaud M, Hugonnet JE, Dubost L, Brouard JP, Ouazzani J, Rice LB, Gutmann L, Arthur M. 2005. A novel peptidoglycan cross-linking enzyme for a β-lactam-resistant transpeptidation pathway. J Biol Chem 280:38146–38152. doi: 10.1074/jbc.M507384200. [DOI] [PubMed] [Google Scholar]
- 56.Lerner SA, Matz GJ. 1980. Aminoglycoside ototoxicity. Am J Otolaryngol 1:169–179. doi: 10.1016/s0196-0709(80)80012-3. [DOI] [PubMed] [Google Scholar]
- 57.Mulheran M, Degg C, Burr S, Morgan DW, Stableforth DE. 2001. Occurrence and risk of cochleotoxicity in cystic fibrosis patients receiving repeated high-dose aminoglycoside therapy. Antimicrob Agents Chemother 45:2502–2509. doi: 10.1128/aac.45.9.2502-2509.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prayle A, Smyth AR. 2010. Aminoglycoside use in cystic fibrosis: therapeutic strategies and toxicity. Curr Opin Pulm Med 16:604–610. doi: 10.1097/MCP.0b013e32833eebfd. [DOI] [PubMed] [Google Scholar]
- 59.Chen KS, Bach A, Shoup A, Winick NJ. 2013. Hearing loss and vestibular dysfunction among children with cancer after receiving aminoglycosides. Pediatr Blood Cancer 60:1772–1777. doi: 10.1002/pbc.24631. [DOI] [PubMed] [Google Scholar]
- 60.Jiao Y, Moya B, Chen M-J, Zavascki AP, Tsai H, Tao X, Sutaria DS, Louie A, Boyce JD, Deveson Lucas D, Kim TH, Tsuji BT, Bonomo RA, Drusano GL, Bulitta JB. 2019. Comparable efficacy and better safety of double β-lactam combination therapy versus β-lactam plus aminoglycoside in Gram-negative bacteria in randomized, controlled trials. Antimicrob Agents Chemother 63:e00425-19. doi: 10.1128/AAC.00425-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perry CM, Ibbotson T. 2002. Biapenem. Drugs 62:2221–2234. doi: 10.2165/00003495-200262150-00005. [DOI] [PubMed] [Google Scholar]
- 62.Maggioncalda EC, Story-Roller E, Mylius J, Illei P, Basaraba RJ, Lamichhane G. 2020. A mouse model of pulmonary Mycobacteroides abscessus infection. Sci Rep 10:3690. doi: 10.1038/s41598-020-60452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushik A, Makkar N, Pandey P, Parrish N, Singh U, Lamichhane G. 2015. Carbapenems and rifampin exhibit synergy against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob Agents Chemother 59:6561–6567. doi: 10.1128/AAC.01158-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang D, Wang Y, Lu J, Pang Y. 2016. In vitro activity of β-lactams in combination with β-lactamase inhibitors against multidrug-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 60:393–399. doi: 10.1128/AAC.01035-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaushik A, Ammerman NC, Tasneen R, Story-Roller E, Dooley KE, Dorman SE, Nuermberger EL, Lamichhane G. 2017. In vitro and in vivo activity of biapenem against drug-susceptible and rifampicin-resistant Mycobacterium tuberculosis. J Antimicrob Chemother 72:2320–2325. doi: 10.1093/jac/dkx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo ZY, Zhao WJ, Zheng MQ, Liu S, Yan CX, Li P, Xu SF. 2019. Activities of biapenem against Mycobacterium tuberculosis in macrophages and mice. Biomed Environ Sci 32:235–241. doi: 10.3967/bes2019.033. [DOI] [PubMed] [Google Scholar]
- 67.Ferro BE, Srivastava S, Deshpande D, Sherman CM, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2015. Amikacin pharmacokinetics/pharmacodynamics in a novel hollow-fiber Mycobacterium abscessus disease model. Antimicrob Agents Chemother 60:1242–1248. doi: 10.1128/AAC.02282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tilton RC, Lieberman L, Gerlach EH. 1973. Microdilution antibiotic susceptibility test: examination of certain variables. Appl Microbiol 26:658–665. doi: 10.1128/AM.26.5.658-665.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cynamon MH, Speirs RJ, Welch JT. 1998. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob Agents Chemother 42:462–463. doi: 10.1128/AAC.42.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsen M. 2000. Some common methods in mycobacterial genetics, p 313–320. In Hatfull GF, Jacobs WRJ (ed), Molecular genetics of mycobacteria. American Society for Microbiology, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.