Abstract
Purpose
To investigate the retinal vessel density (VD) in healthy and normal-tension glaucoma (NTG) eyes through optical coherence tomography angiography (OCTA) and to determine the correlation between VD and the retinal nerve fiber layer (RNFL) thickness and functional visual field (VF) defects for different locations.
Methods
A total of 74 NTG eyes and 24 healthy eyes were included. OCTA VD at 4.5 × 4.5 mm peripapillary region and 3.0 × 3.0 mm macula area, RNFL thickness, and VF pattern deviation results were individually analyzed on the basis of the Garway-Heath sectorization. Correlations between VD and VF/RNFL and VF were compared.
Results
In the NTG group, peripapillary VD, superficial macula VD, RNFL thickness, and ganglion cell complex thickness were significantly lower. In the whole peripapillary area and inferotemporal sector, anatomic correlations between VD and VF pattern deviation values were significantly higher than those between the RNFL thickness and VF values. In the subgroup analysis, VD was anatomically correlated with VF in early-, moderate-, and severe-stage NTG eyes, whereas the RNFL thickness was correlated with VF in moderate- and severe-stage NTG eyes.
Conclusions
We observed VD reduction in the peripapillary retina and superficial macula area in NTG eyes. The microvascular dropout of VD in the peripapillary retina, especially in the inferotemporal sector, provided a more accurate anatomic correlation with functional VF defects than that of the RNFL thickness, especially in early-stage NTG eyes.
Translational Relevance
In patients who cannot comply VF exam, VD is a good tool for disease detection.
Keywords: Optical coherence tomography angiography, Normal-tension glaucoma, Vessel density, Visual field defect
Introduction
Normal-tension glaucoma (NTG) is chronic, progressive optic neuropathy with glaucomatous visual field (VF) defects.1 Several studies have indicated that ocular vascular contributions are crucial in NTG eyes.2 Although exact mechanisms underlying abnormal ocular blood flow (OBF) in NTG eyes remain unclear,3 damages due to low or fluctuating OBF and reperfusion injury in the optic nerve head (ONH) are widely accepted hypotheses.4
Optical coherence tomography angiography (OCTA) provides a fast and noninvasive assessment of microvascular perfusion. It can visualize the microvasculature of various retinal layers. The development and progression of glaucoma are both linked to the loss of retinal vessel density (VD), and recent OCTA studies have reported reduced VD in the optic disc and peripapillary retina of glaucomatous eyes.5,6 In addition, many studies have demonstrated high diagnostic values of OCTA in primary open-angle glaucoma (POAG) and NTG eyes, and VD in OCTA is also highly correlated with mean deviation (MD) and pattern standard deviation (PSD) values in the VF examination.7,8
Because VF testing measures visual function, it is theoretically more relevant than OCTA. However, VF testing requires cooperation and concentration from the patients, meaning it has lower repeatability and reproducibility. However, there is no established consensus on the proper region of VD measurement in OCTA to achieve the best correlation with the VF examination results anatomically. Hence, we investigated the characteristics of retinal VD in healthy and NTG eyes using commercially available OCTA. Furthermore, we determined the correlation between VD and the retinal nerve fiber layer (RNFL) thickness and functional VF defects for different peripapillary locations.
Method
This retrospective study was conducted on healthy individuals and individuals with NTG who visited the glaucoma clinic of Chang Gung Memorial Hospital, Keelung, Taiwan. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital. The study adheres to the Declaration of Helsinki.
Study Subjects
NTG eyes were included based on the following criteria of the European Glaucoma Society1: (1) patients older than 35 years, (2) normal intraocular pressure (IOP) less than 21 mm Hg without treatment, (3) ONH damage typical of glaucoma, (4) VF defects typical of glaucoma, (5) open anterior chamber angle in gonioscopy, and (6) no history of steroid use. The inclusion criteria for normal subjects were a normal-appearing ONH, intact neuroretinal rim and RNFL, normal standard automated perimetry (defined as a glaucoma hemifield test within normal limits and a PSD within 95% confidence interval limits), and IOP less than 21 mm Hg. Medical records and data pertaining to ophthalmologic examinations, including best-corrected visual acuity and central corneal thickness (CCT), slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, standard automated perimetry (Swedish interactive threshold algorithm standard 30–2 test; Humphrey field analyzer II; Carl Zeiss Meditec, Dublin, CA, USA), and OCTA measurements (Optovue Inc., Fremont, CA, USA), were reviewed. The severity of glaucomatous damage was classified as early stage (VF MD ≥ −6 dB), moderate stage (−12 dB ≤ VF MD < −6 dB), or severe stage (VF MD < −12 dB).9
Patients with a history of intraocular surgery, intraocular eye disease other than NTG or cataract, spherical equivalent (SE) error <−6.0 diopters (D) or >+3.0 D, axial length more than 26.5 mm, unreliable VF test results (i.e., false-positive errors >33% or fixation loss >33%), and OCTA signal strength index <50% were excluded from the study. If both eyes in a patient were diagnosed with NTG, only the right eye was included in the study.
Octa Measurements
RTVue-XR Avanti (Optovue Inc.) was used for automatically measuring the RNFL thickness. An 840 ± 10 nm laser emitted from the Avanti system scans ocular microstructures and can provide the data on the RNFL thickness and ganglion cell complex (GCC) thickness at a diameter of 3.45 mm around the center of the optic disc. A total of 775 such A-scans were obtained (Figs. 1c and 1g). The optic disc and peripapillary retina were analyzed using the Angio Disc protocol. The whole en face image VD was measured in the entire 4.5 × 4.5 mm image, and peripapillary VD was measured in the region defined as a 750-µm–wide elliptical annulus extending from the optic disc boundary (Fig. 1d).
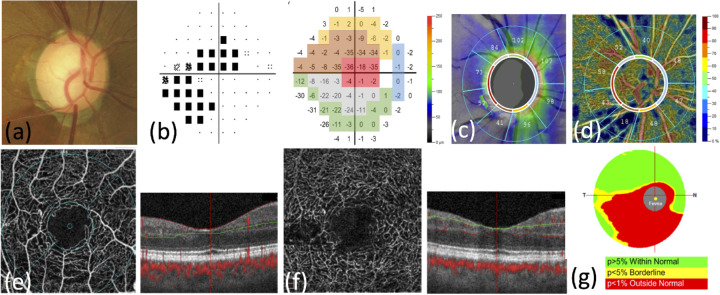
Figure 1.
Representative case of a normal-tension glaucoma eye. (a) The color photograph shows rim thinning at superotemporal and inferotemporal regions. (b) The 30–2 Swedish interactive threshold algorithm standard pattern deviation image obtained using the Humphrey visual field analyzer shows superior and inferior visual field defects with central involvement (visual field mean deviation: −8.94 dB). Scores in the pattern deviation map are grouped according to the Garway-Heath map. (c) The circumpapillary RNFL thickness map shows thinning at superior-temporal and inferior-temporal regions. (d) The OCTA vessel density image shows apparent microvascular reduction at inferior and superior regions inside and around the optic disc. (e) The superficial layer of the retina was measured in the 3.0 × 3.0 mm region centered on the fovea. The boundary of the superficial retinal layer extends from the internal limiting membrane to the inner plexiform layer. (f) The deep retinal layer measured in the 3.0 × 3.0 mm region centered on the fovea. The boundary of the deep retinal layer extends from inner to outer plexiform layers. (g) The GCC thickness map covering a 7.0 mm region shows thinning at the inferior area.
Images of the fovea microvasculature were produced using the Optovue OCTA device. Superficial and deep layers of the retina were observed. The boundary of the superficial retinal layer extends from the internal limiting membrane to the inner plexiform layer; the boundary of the deep retinal layer extends from the inner to outer plexiform layers (Figs. 1e and 1f). The OCTA scans a 3.0 × 3.0 mm region centered on the fovea. After the scan, motion artifacts are removed by applying an orthogonal registration algorithm.
Peripapillary VD and RNFL thickness at each Garway-Heath sector were measured (Figs. 1c and 1d). Furthermore, scores at each test spot of the VF pattern deviation map were recorded. We grouped the test points according to Garway-Heath map. The average of the scores at every test spots in each Garway-Heath sector was calculated for further statistical analysis (Fig. 1b).10
Statistical Analysis
Statistical analyses were performed using commercial software (SPSS, ver. 20.0; IBM Corp., Armonk, NY, USA). Independent t tests were conducted to compare continuous variables between healthy and NTG eyes. We took log on VD and RNFL thickness. Then Pearson correlation coefficients were calculated to evaluate the relationship between VD and VF pattern deviation values and between RNFL thickness and VF pattern deviation values. Dependent correlation coefficients calculated from the aforementioned parameters were compared using Fisher Z transformation. A P value < 0.05 was considered statistically significant.
Results
A total of 120 NTG eyes and 45 healthy eyes were recruited for initial evaluation. Of those, 46 NTG eyes and 21 healthy eyes were excluded due to the unacceptable quality of their OCTA images and VF examination results, and 74 NTG eyes (28 men, 46 women) and 24 healthy eyes (18 men, six women) were included in the final analysis. Table 1 summarizes the demographic and clinical characteristics of the participants. The proportion of early, moderate, and severe NTG was 45:16:13 (60.8%:21.6%:17.6%). However, no significant differences were observed in age, CCT, SE, or IOP between the two groups. The visual acuity (VA), VF MD, and PSD values were significantly lower in patients with NTG than in the healthy individuals. The VD in the whole disc image (4.5 × 4.5 mm), peripapillary retina, superficial macula and mean RNFL and GCC thicknesses were significantly lower in patients with NTG than in healthy individuals.
Table 1.
Baseline Characteristics of Study Subjects
| Healthy Eyes | NTG Eyes | ||||
|---|---|---|---|---|---|
| N = 24 | SD | N = 74 | SD | P Valuea | |
| Laterality (OD/OS) | 15/9 | 43/31 | |||
| Gender (M/F) | 18/6 | 28/46 | |||
| Age(yrs) | 56.13 | 15.14 | 61.31 | 10.07 | 0.058 |
| CCT (µm) | 543.42 | 33.22 | 536.63 | 35.40 | 0.41 |
| SE (D) | −0.62 | 2.12 | −1.05 | 2.44 | 0.446 |
| VA (LogMAR) | 0.09 | 0.11 | 0.18 | 0.19 | 0.032 |
| IOP (mm Hg) | 15.47 | 1.73 | 15.16 | 2.42 | 0.563 |
| Visual field | |||||
| MD | −1.35 | 2.12 | −6.35 | 5.80 | <0.001 |
| PSD | 3.04 | 1.75 | 7.05 | 4.65 | <0.001 |
| Early defect (eyes) | 44 | ||||
| Moderate defect (eyes) | 17 | ||||
| Severe defect (eyes) | 13 | ||||
| OCTA | |||||
| VD in whole disc image | 48.41 | 3.03 | 43.9 | 6.14 | 0.001 |
| VD in peripapillary area | 50.73 | 3.31 | 45.46 | 7.87 | 0.002 |
| Superficial macula VD | |||||
| Whole macula image | 44.82 | 4.07 | 41.95 | 5.84 | 0.028 |
| Parafovea image | 47.70 | 4.39 | 44.73 | 6.34 | 0.036 |
| Deep macula VD | |||||
| Whole macula image) | 50.20 | 4.14 | 48.35 | 4.53 | 0.079 |
| Parafovea image) | 52.43 | 4.07 | 50.40 | 4.55 | 0.055 |
| OCT | |||||
| RNFL thickness (µm) | 99.75 | 6.78 | 85.34 | 15.22 | <0.001 |
| GCC thickness (µm) | 95.71 | 6.92 | 84.95 | 11.42 | <0.001 |
BCVA, best-corrected visual acuity; SD, standard deviation.
Independent sample t test.
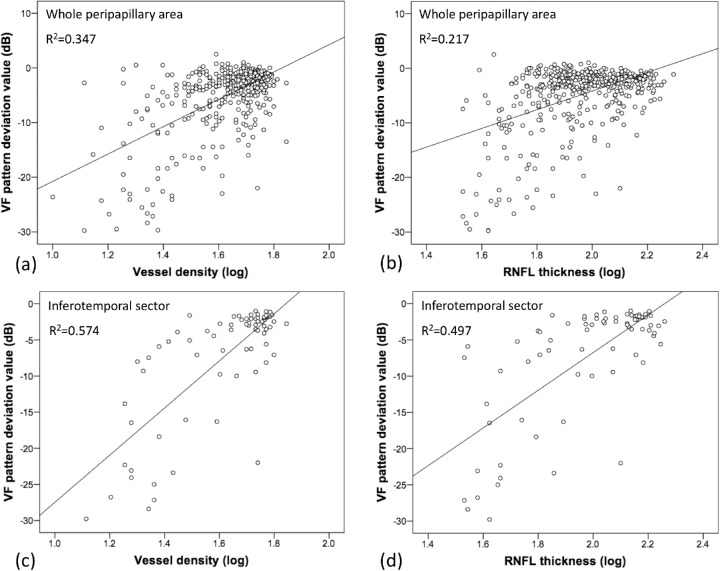
After taking log on VD and RNFL thickness, Pearson correlation coefficients between the VD/RNFL thickness and VF pattern deviation scores for each anatomical location are summarized in Table 2. Both VD and RNFL thickness were correlated with VF pattern deviation scores for the whole peripapillary area (r = 0.589, R2 = 0.347 for VD and VF; r = 0.466, R2 = 0.217 for RNFL and VF). According to the Garway-Heath sector analysis, the RNFL thickness was weakly to moderately correlated with VF pattern deviation scores in superotemporal, superonasal, inferotemporal, inferonasal and nasal sectors (r = 0.262∼0.705, R2 = 0.069∼0.497), whereas VD was weakly to moderately correlated with VF pattern deviation scores in superotemporal, superonasal, inferotemporal, inferonasal, and temporal sectors (r = 0.359∼0.757, R2 = 0.129∼0.574). The coefficients of VD and VF were higher than those of the RNFL thickness and VF in the whole peripapillary area and superotemporal, superonasal, inferotemporal, inferonasal, and temporal sectors. By comparing the dependent correlation coefficients using the Fisher Z transformation, we found that in the whole peripapillary area and inferotemporal region, correlations between VD and VF were significantly higher than those between the RNFL thickness and VF (Fig. 2).
Table 2.
Pearson Correlation Coefficients of VD/RNFL Thickness and VF Pattern Deviation Values Based on the Garway-Heath Map
| Location | N | r of RNFL&VF (95% CI)* | r of VD & VF (95% CI)* | P for Difference of r |
|---|---|---|---|---|
| All sectors | 444 | 0.466 (0.374 to 0.540)* | 0.589 (0.495 to 0.674)* | <0.001 |
| Superotemporal sector | 74 | 0.684 (0.513 to 0.795)* | 0.719 (0.519 to 0.844)* | 0.214 |
| Superonasal sector | 74 | 0.490 (0.311 to 0.618)* | 0.562 (0.343 to 0.744)* | 0.128 |
| Inferotemporal sector | 74 | 0.705 (0.558 to 0.816)* | 0.757 (0.611 to 0.860)* | 0.047 |
| Inferonasal sector | 74 | 0.487 (0.272 to 0.676)* | 0.556 (0.282 to 0.747)* | 0.112 |
| Temporal sector | 74 | 0.171 (‒0.084 to 0.404) | 0.359 (‒0.054 to 0.647)* | – |
| Nasal sector | 74 | 0.262 (‒0.138 to 0.547)* | 0.076 (‒0.214 to 0.344) | – |
P < .05.
N, number of spots in each sector; CI, confidence interval.
Figure 2.
Scatterplot between vessel density (VD) and VF pattern deviation values in the whole peripapillary area (a); between RNFL thickness and VF pattern deviation values in the whole peripapillary area (b); between VD and VF pattern deviation values in the inferotemporal sector (c); between RNFL thickness and VF pattern deviation values in the inferotemporal sector (d). The value of VD and RNFL thickness after taking log was used in the scatterplot.
In addition, this study included 44 NTG eyes with early VF defects (MD > −6.0 dB) and 30 eyes with moderate (MD −6.0to −12.0 dB) to severe VF defects (MD < −12.0 dB). The anatomical correlation between the aforementioned tests for NTG eyes in different stages is illustrated in Table 3. For early-stage NTG eyes, the RNFL thickness was not correlated with VF in most locations except for being weakly correlated with VF in the inferotemporal sector, whereas VD was correlated with VF in most peripapillary areas, including superotemporal, superonasal, inferotemporal, and inferonasal sectors (r = 0.350∼0.488, R2 = 0.11∼0.238). By contrast, for moderate- to severe-stage NTG eyes, both the RNFL thickness and VD were weakly to moderately correlated with VF, without statistical difference between the two examinations (r = 0.442∼0.673, R2 = 0.195∼0.453 for VD and VF; r = 0.382∼0.684, R2 = 0.146∼0.467 for RNFL and VF).
Table 3.
Pearson Correlation Coefficients of VD/RNFL Thickness and VF Pattern Deviation Values in Subgroup Analysis Based on the Garway-Heath Map
| Location | N | r of RNFL&VF (95% CI)* | r of VD & VF (95% CI)* | P for Difference of r |
|---|---|---|---|---|
| Early stage | ||||
| All sectors | 264 | 0.060 (‒0.076 to 0.189) | 0.322 (0.171 to 0.459)* | – |
| Superotemporal sector | 44 | 0.286 (‒0.088 to 0.591) | 0.348 (‒0.074 to 0.665)* | – |
| Superonasal sector | 44 | 0.266 (0.020 to 0.497) | 0.484 (0.208 to 0.725)* | – |
| Inferotemporal sector | 44 | 0.37 (‒0.047 to 0.653)* | 0.488 (0.137 to 0.768)* | 0.041 |
| Inferonasal sector | 44 | 0.198 (‒0.163 to 0.558) | 0.350 (‒0.043 to 0.694)* | – |
| Moderate-severe stage | ||||
| All sectors | 180 | 0.484 (0.364 to 0.595)* | 0.507 (0.369 to 0.625)* | 0.324 |
| Superotemporal sector | 30 | 0.684 (0.430 to 0.849)* | 0.673 (0.397 to 0.846)* | 0.441 |
| Superonasal sector | 30 | 0.467 (0.203 to 0.656)* | 0.446 (0.088 to 0.666)* | 0.401 |
| Inferotemporal sector | 30 | 0.651 (0.389 to 0.847)* | 0.661 (0.381 to 0.838)* | 0.429 |
| Inferonasal sector | 30 | 0.382 (0.089 to 0.626)* | 0.422 (0.033 to 0.732)* | 0.270 |
N, number of spots in each sector, CI, confidence interval.
P < .05.
Discussion
Knowledge about OBF is vital for identifying IOP-independent factors that influence NTG pathophysiology. Previous studies report reduced blood flow in the ONH of NTG eyes.11 In this study, we found that in the whole disc image and peripapillary retina, the values of VD were significantly lower in NTG eyes than in healthy eyes. This result was consistent with that of previous studies. Takeyama et al.12 report lower VD in the OCTA whole image and peripapillary area in NTG patients. Toshev et al.13 report lower global peripapillary VD in NTG and POAG eyes than in healthy eyes.
Furthermore, in this study, superficial macula VD was significantly reduced in NTG patients. Compared with our study, previous studies report reduced superficial macula VD in either POAG or NTG eyes.14–17 In addition, Shoji et al.18 find that superficial macula VD led to a faster loss in POAG eyes than in healthy eyes. Chao et al.19 and Shin et al.20 report that NTG groups have lower superficial and deep VD when compared with healthy eyes.
Optical coherence tomography and OCTA are independent of patient responses; they require less patient cooperation and therefore provide objective information on VD and retinal layer thickness, with high repeatability and reproducibility.21 Although VF testing is theoretically more relevant because it measures visual function, it requires cooperation and concentration from the patients and increases variability between the tests. Thus we determined corresponding VF patterns using VD or RNFL thickness values. Although the correlation was weak to moderate, both VD and RNFL thickness values were correlated with VF pattern deviation results in the global peripapillary retina, and VD had a better performance than RNFL thickness did. A similar comparative result was presented in the inferotemporal sector. In the inferotemporal sector, VD was moderately correlated with VF, and their correlation coefficient was significantly higher than that between the RNFL thickness and VF. In superotemporal, superonasal, and inferonasal sectors, the correlation coefficients between VD and VF were higher than that between the RNFL thickness and VF but did not reach statistical difference. Thus our study suggested that VD testing in the peripapillary area might aid in evaluating functional defects in patients with NTG, and its correlation with VF defect was better than that of the RNFL thickness. Moreover, we found that VD in the inferotemporal peripapillary area presented better correlation with VF results and discriminating power than the other sectors. Inferotemporal and superotemporal areas of ONH are more vulnerable to nerve damage in glaucoma eyes.22,23 Many previous studies demonstrate that VD or the blood flow index between healthy and glaucoma eyes are most discriminatory in inferotemporal and superotemporal sectors.5,20,24,25 Shin et al.20 find a higher AUC of VD for NTG diagnosis in the superior, inferior, and temporal peripapillary retina, but the diagnostic ability of VD is not superior to that of the RNFL thickness in all four quadrants. In POAG eyes, Akagi et al.5 reported significantly decreased VD on the surface mode in the superotemporal and inferotemporal peripapillary areas when comparing POAG patients with corresponding hemifield VF defects and healthy individuals.
Last but not least, our study demonstrated a higher correlation between VD and VF than that between the RNFL thickness and VF in early-stage NTG eyes. For moderate- and severe-stage NTG eyes, VD and RNFL both correlated with VF equally. No previous publication had compared correlation between OCTA parameters and VF defects in glaucomatous eyes at different stages. Our result indicated that vascular loss occurred earlier than RNLF loss did in NTG eyes, which was compatible with the widely accepted hypothesis that ocular vascular contributions are crucial in NTG eyes.2,4 The mechanisms underlying vascular dysfunction in NTG eyes remains unclear, but the proposed predisposing factors for glaucomatous optic neuropathy include vasospasm, oxidative stress, and endothelial dysfunction.26–28 Thus, for patients who cannot cooperate to properly complete the VF test, the VD pattern in OCTA is a good tool for disease detection in all NTG eyes, whereas RNFL thickness correlates with VF defect well only in moderate and severe NTG eyes.
Despite its achievements, our study has several limitations. First, the relatively small sample size and inclusion of participants of a single ethnicity might influence the results. Larger prospective studies are therefore required in the future to confirm its findings. Second, although few reports show a decrease in deep-macula VD in NTG or POAG patients,19,20 the result was not demonstrated in our study. Thus we cannot conclude this point. Third, although significant Pearson correlation between VD/RNFL and VF was demonstrated in this study, only weak to moderate correlation was achieved (R2 = 0.11∼0.574). However, the comparison of correlation coefficients for different exams and at different stages in this study were still valuable.
In conclusion, we observed VD reduction in the peripapillary retina and superficial macula area in NTG eyes. The microvascular dropout of VD in the peripapillary retina, especially in the inferotemporal sector, provided a better anatomic correlation with functional VF defects than the RNFL thickness, especially in early-stage NTG eyes.
Acknowledgments
We extend our deepest gratitude to Hsing-Fen Lin for offering us invaluable advice and informative suggestions along the way regarding statistical analysis.
Supported by one Grant (No. CMRPG2G0261) from the Chang Gung Medical Research Foundation, Taiwan.
Disclosure: Y.-H. Lin, None; S.-M. Huang, None; L. Yeung, None; W.-C. Ku, None; H.S.-L. Chen, None; C.-C. Lai, None; L.-H. Chuang, None
References
- 1. European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: Treatment principles and options Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 3 Treatment principles and options. Br J Ophthalmol. 2017; 101: 130–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaiser HJ, Schoetzau A, Stumpfig D, Flammer J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am J Ophthalmol. 1997; 123: 320–327. [DOI] [PubMed] [Google Scholar]
- 3. Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsai JC. Influencing ocular blood flow in glaucoma patients: the cardiovascular system and healthy lifestyle choices. Can J Ophthalmol. 2008; 43: 347–50. [DOI] [PubMed] [Google Scholar]
- 5. Akagi T, Iida Y, Nakanishi H, et al.. Microvascular density in glaucomatous eyes with hemifield visual field defects: an optical coherence tomography angiography study. Am J Ophthalmol. 2016; 168: 237–249. [DOI] [PubMed] [Google Scholar]
- 6. Liu L, Jia Y, Takusagawa HL, et al.. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015; 133: 1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Melkebeke L, Barbosa-Breda J, Huygens M, Stalmans I. Optical coherence tomography angiography in glaucoma: a review. Ophthalmic Res. 2018; 60: 1–13. [DOI] [PubMed] [Google Scholar]
- 8. Kurysheva NI, Maslova EV.. [Optical coherence tomography angiography in glaucoma diagnosis]. Vestn Oftalmol. 2016; 132: 98–102. [DOI] [PubMed] [Google Scholar]
- 9. Susanna R Jr., Vessani RM. Staging glaucoma patient: why and how? Open Ophthalmol J. 2009; 3: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000; 107: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 11. Chiba N, Omodaka K, Yokoyama Y, et al.. Association between optic nerve blood flow and objective examinations in glaucoma patients with generalized enlargement disc type. Clin Ophthalmol. 2011; 5: 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeyama A, Ishida K, Anraku A, Ishida M, Tomita G. Comparison of optical coherence tomography angiography and laser speckle flowgraphy for the diagnosis of normal-tension glaucoma. J Ophthalmol. 2018; 2018: 1751857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toshev AP, Schuster AK, Ul Hassan SN, Pfeiffer N, Hoffmann EM. Optical coherence tomography angiography of optic disc in eyes with primary open-angle glaucoma and normal-tension glaucoma. J Glaucoma. 2019; 28: 243–251. [DOI] [PubMed] [Google Scholar]
- 14. Richter GM, Chang R, Situ B, et al.. Diagnostic performance of macular versus peripapillary vessel parameters by optical coherence tomography angiography for glaucoma. Transl Vis Sci Technol. 2018; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penteado RC, Zangwill LM, Daga FB, et al.. Optical coherence tomography angiography macular vascular density measurements and the central 10-2 visual field in glaucoma. J Glaucoma. 2018; 27: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manalastas PIC, Zangwill LM, Daga FB, et al.. The association between macula and ONH optical coherence tomography angiography (OCT-A) vessel densities in glaucoma, glaucoma suspect, and healthy eyes. J Glaucoma. 2018; 27: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onishi AC, Treister AD, Nesper PL, Fawzi AA, Anchala AR. Parafoveal vessel changes in primary open-angle glaucoma and normal-tension glaucoma using optical coherence tomography angiography. Clin Ophthalmol. 2019; 13: 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shoji T, Zangwill LM, Akagi T, et al.. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol. 2017; 182: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chao SC, Yang SJ, Chen HC, Sun CC, Liu CH, Lee CY. Early macular angiography among patients with glaucoma, ocular hypertension, and normal subjects. J Ophthalmol. 2019; 2019: 7419470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shin JW, Sung KR, Lee JY, Kwon J, Seong M. Optical coherence tomography angiography vessel density mapping at various retinal layers in healthy and normal tension glaucoma eyes. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 21. Shin JW, Lee J, Kwon J, Choi J, Kook MS. Regional vascular density-visual field sensitivity relationship in glaucoma according to disease severity. Br J Ophthalmol. 2017; 101: 1666–1672. [DOI] [PubMed] [Google Scholar]
- 22. Quigley HA, Addicks EM.. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981; 99: 137–143. [DOI] [PubMed] [Google Scholar]
- 23. Leung CK, Chan WM, Yung WH, et al.. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology. 2005; 112: 391–400. [DOI] [PubMed] [Google Scholar]
- 24. Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al.. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016; 57: OCT451–OCT459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rao HL, Pradhan ZS, Weinreb RN, et al.. Relationship of optic nerve structure and function to peripapillary vessel density measurements of optical coherence tomography angiography in glaucoma. J Glaucoma. 2017; 26: 548–554. [DOI] [PubMed] [Google Scholar]
- 26. Delaney Y, Walshe TE, O'Brien C. Vasospasm in glaucoma: clinical and laboratory aspects. Optom Vis Sci. 2006; 83: 406–414. [DOI] [PubMed] [Google Scholar]
- 27. Mozaffarieh M, Flammer J.. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013; 13: 43–49. [DOI] [PubMed] [Google Scholar]
- 28. Fan N, Wang P, Tang L, Liu X. Ocular blood flow and normal tension glaucoma. Biomed Res Int. 2015; 2015: 308505. [DOI] [PMC free article] [PubMed] [Google Scholar]