Abstract
The coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic as declared by World Health Organization (WHO). In the absence of an effective treatment, different drugs with unknown effectiveness, including antimalarial hydroxychloroquine (HCQ), with or without concurrent administration with azithromycin (AZM), have been tested for treating COVID-19 patients with developed pneumonia. However, the efficacy and safety of HCQ and/or AZM have been questioned by recent clinical reports. Direct effects of these drugs on the human heart remain very poorly defined. To better understand the mechanisms of action of HCQ +/− AZM, we employed bioengineered human ventricular cardiac tissue strip (hvCTS) and anisotropic sheet (hvCAS) assays, made with human pluripotent stem cell (hPSC)-derived ventricular cardiomyocytes (hvCMs), which have been designed for measuring cardiac contractility and electrophysiology, respectively. Our hvCTS experiments showed that AZM induced a dose-dependent negative inotropic effect which could be aggravated by HCQ; electrophysiologically, as revealed by the hvCAS platform, AZM prolonged action potentials and induced spiral wave formations. Collectively, our data were consistent with reported clinical risks of HCQ and AZM on QTc prolongation/ventricular arrhythmias and development of heart failure. In conclusion, our study exposed the risks of HCQ/AZM administration while providing mechanistic insights for their toxicity. Our bioengineered human cardiac tissue constructs therefore provide a useful platform for screening cardiac safety and efficacy when developing therapeutics against COVID-19.
1. Introduction
The coronavirus disease 2019 (COVID-19) outbreak induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread worldwide. As of November 2020, COVID-19 has infected more than fifty million people around the world and claimed the lives of over a million patients. In the setting of this pandemic, unproven drugs have been publicized to be effective in the treatment or prevention of COVID-19. Hydroxychloroquine (HCQ) or chloroquine, a medication for treating malaria, has been used alone or in combination with the antibiotic azithromycin (AZM) to treat COVID-19 patients who have developed pneumonia [1,2]. It was reported that the prescription of HCQ or chloroquine in the United States has been increased by 80-fold from March 2019 to March 2020 [3]. However, recent reports have shown no clinical benefits to support the usage of HCQ with or without AZM in the setting of COVID-19 infection [4,5]. In addition, it was reported that high dose of chloroquine would give rise to potential toxicities as shown by a recent randomized trial with 81 hospitalized patients with severe COVID-19, especially when taken together with AZM or oseltamivir [6]. In another small cohort study, 90 patients with COVID-19 pneumonia who received HCQ for the treatment of pneumonia associated with COVID-19 were found to be at high risk of QTc prolongation, and concurrent treatment with AZM was associated with even greater changes in QTc [7]. Despite these clinical observations, the cardiac effects in humans as well as the mechanistic basis for the reported arrhythmias remain very poorly defined.
Human cardiomyocytes (CMs) do not proliferate in culture and are difficult to obtain for practical reasons. With the advent of human pluripotent stem cells (hPSC), the derivation of CMs has been extensively used for insights into the development, physiology as well as pathophysiology of the human heart [[8], [9], [10], [11], [12], [13]]. Since the heart is a complex 3D organ, single-cell experiments often do not fully recapitulate cardiac physiological properties such as contractile force and conduction which are inherently multi-cellular in nature. As such, various 3D bioengineered tissue constructs have been developed using hPSC-CMs for modeling human-specific diseases and cardiotoxicity screening. In this study, hPSCs (HES2: human embryonic stem cell; ESI, NIH code ES02. And L-EdV: human induced pluripotent stem cell; provided by the University of Hong Kong) -were specified into human ventricular cardiomyocytes (hvCMs) as we have previously reported [14], followed by assembly into engineered tissues: human ventricular cardiac tissue strips (hvCTS) and anisotropic sheet (hvCAS) [9,15,16], which have been specifically designed and engineered for measuring contractility and electrophysiology, respectively, and used to investigate the cardiac effects of HCQ and AZM individually and in combination.
2. Hydroxychloroquine with or without azithromycin reduced hvCTS contractility
By casting 100 μl collagen and a cell mixture of 1.3 million hPSC-hvCMs and 0.13 million human fibroblasts into a custom-made PDMS bioreactor, contracting engineered hvCTS could be formed between the two force-sensing cantilever posts in a rectangular well. After 7–8 days in culture, forces generated by the hvCTS were measured at 37 °C using the post-tracking force measurement system (CTScreen by Novoheart, Irvine, CA) to record the displacements of the cantilever posts. During experiments, hvCTS was field-stimulated at 1.5 Hz. As shown in Fig. 1A and C, increasing concentrations of HCQ did not have a significant effect on developed force in hvCTS. By contrast, AZM reduced developed force in a concentration-dependent fashion, with 100 μM leading to a significant impairment by 47.8 ± 7.5% from baseline. The negative inotropic effect of AZM (100 μM) was exacerbated in the presence of HCQ (10 μM), further reducing developed force by 65.2 ± 2.9% (Fig. 1C). Similarly, there were significant decreases of normalized maximum +dF/dt and -dF/dt of AZM-alone and HCQ + AZM treated groups when compared to vehicle control group (Fig. 1D-E).Qualitatively similar results were obtained with hvCTS derived from another hPSC line (L-EdV; Fig. 1F-G).
Fig. 1.
(A) Representative tracings of hvCTS of vehicle (Control), hydroxychloroquine (HCQ), azithromycin (AZM) and the combined (HCQ + AZM) groups at 1.5 Hz pacing, normalized to baseline. (B) Bar graph showing force reduction of hvCTS by 100 μM AZM without (n = 5) and with (n = 5) 10 μM of HCQ. Concentration-response plot of developed force, maximum +dF/dt and maximum -dF/dt of (C-E) HES2-hvCTS (F-H) L-EdV-hvCTS for 1–10 μM HCQ (n = 3–7), 1–100 μM AZM (n = 3–5) and 1–100 μM AZM with 10 μM HCQ (n = 3–5) at 1.5 Hz pacing. B: Paired t-test between treated group and baseline; unpaired t-test between treated groups. C-H:: Two-way ANOVA test, followed by Holm-Sidak's multiple comparison to control group. All data are expressed as mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001.
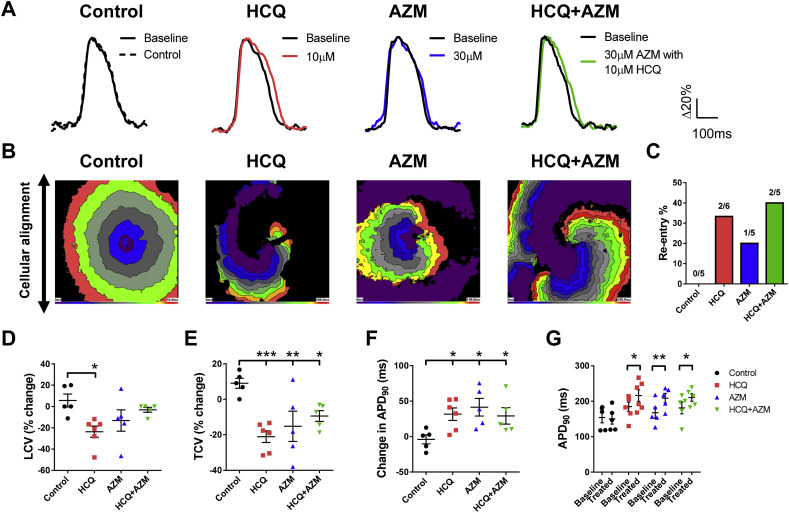
3. Hydroxychloroquine with or without azithromycin increased QTc interval and pro-arrhythmogenicity in hvCAS
We next examined the consequences of separate and combined effects of 10 μM HCQ and 30–100 μM AZM, on cardiac electrophysiology using our hvCAS assay, previously shown to successfully reveal pro-arrhythmic effects of failed Cardiac Arrhythmia Suppression Trial (CAST) drugs [11]. To fabricate hvCAS for high-resolution optical mapping, 0.25 million hPSC-hvCM per cm2 was seeded onto a Matrigel-coated, micro-grooved substrate fabricated from polystyrene shrink film, with a groove width of 15 μm, a groove depth of 5 μm, and an inter-groove distance of 5 μm. A high-resolution MiCAM03 optical mapping system (SciMedia) with a 1× lens setup was used to capture the action potential (AP) signal and conduction velocities of the test preparation in a 1 cm × 1 cm region of interest. hvCAS was paced by a unipolar point-stimulation electrode (Harvard Apparatus) and a programmable Master9 stimulator (AMPI). The temporal resolution was 5 ms. Data were analyzed with our in-house software. Upon the application of HCQ (10 μM), AZM (30 μM) or HCQ + AZM, prolongation in action potential duration of 90% repolarization (APD90) was always observed. APD90 of HCQ, AZM and HCQ+AZM increased by 37 ms, 39 ms and 17 ms, respectively (Fig. 2A, F, G). We also measured conduction velocities, in the longitudinal and the transverse axes relative to cell alignment, as the rate of action potential propagation between pairs of selected points on the hvCAS substrate. Analysis of isochronal maps showed that the conduction velocity (LCV) of HCQ group, and the transverse conduction velocity (TCV) of all treatment groups were significantly decreased (Fig. 2D, E) in comparison to vehicle control. Importantly, re-entry in the form of spiral wave formation was promoted in the presence of HCQ- or AZM-alone, or in combination (Fig. 2B, C). Collectively, these data were consistent with the findings that QTc interval and pro-arrhythmogenicity increases with HCQ +/− AZM.
Fig. 2.
(A) Representative action potential tracings and (B) isochronal maps of hvCAS with vehicle (Control), 10 μM hydroxychloroquine (HCQ), 30 μM azithromycin (AZM) or the combined (HCQ + AZM) treatment. (C-E) Comparisons of re-entry percentage measured by number of observed re-entry phenomena divided by number of total experiments, % change in longitudinal (LCV) and transverse (TCV) conduction velocities of hvCAS (n = 5–6). (F-G) The change in APD90 and the comparison of APD90 at baseline or treated condition of hvCAS with different treatments (n = 5–6). D-F: One-way ANOVA test, followed by Holm-Sidak's multiple comparison to control. G: Two-way ANOVA test, followed by Holm-Sidak's multiple comparison to baseline. All data were expressed as mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001.
4. Discussion
In this study, we report the reduction of contractile force in the presence of 10-100 μM AZM in a dose-dependent manner with or without 10 μM HCQ (Fig. 1C-D). Furthermore, the combined application of 30 μM AZM and 10 μM HCQ prolonged AP duration and slowed conduction, leading to the promotion of spiral wave formation (Fig. 2C-F). The concentrations of AZM and HCQ tested were based on published basic and clinical studies. In various clinical reports, the maximum dosages of HCQ and AZM for anti-SARS-CoV-2 range from 200 to 1200 mg per day and 500 mg per day, respectively [2,4,[17], [18], [19]]. It has been reported that healthy subjects who received 500 mg/day AZM for 3 days would have maximum blood and plasma concentrations of 49.7 nM and 56.6 nM, respectively. However, AZM is known to accumulate in leukocytes and pulmonary tissue, resulting in up 200-1000-fold increase in the effective concentration compared to the plasma [20]. Indeed, cellular accumulation of AZM has also been detected in the liver, spleen and heart [21]. For HCQ, the serum level in COVID-19 patients who receive 600 mg/day is 1.1–3.2 μM [2], but its blood concentration can be 3.2–3.7-fold higher [22]. Therefore, we have made every effort to match the drug conditions tested in our in vitro engineered tissues to the clinical situation.
Overall, our observations of compromised contractility and increased arrhythmogenicity by HCQ and AZM in human engineered cardiac tissue models were consistent with clinical reports. The action potential duration was significantly prolonged in hvCAS treated with 30 μM of AZM with or without 10 μM of HCQ, which was physiologically relevant to our observation in increased arrhymogenic risk in the treated group as the prolongation of the QT interval by as low as 20 ms is considered a high risk for Torsade de Pointes under the FDA guideline. Interestingly, the arrhythmogenic risk as shown by hvCAS treated with HCQ or AZM alone was similar to that of HCQ + AZM, although the combined treatment synergistically led to a stronger reduction of contractile force and HCQ alone did not show any significant changes (Figs. 1C and 2C). It is likely that HCQ and AZM act on multiple ion channels and pumps whose effects can counterbalance each other. For instance, HCQ has been reported to inhibit the hyperpolarization-activated c-AMP-modulated pacemaker current in an in vitro guinea pig cardiomyocyte model [23], but it is generally accepted that the inotropic action of HCQ/chloroquine is unspecified due to the mixed action of ion channels in cardiomyocytes and complicated by non-cardiac toxicity [24]. Further detailed patch-clamping studies for investigating the mechanistic and functional effects of HCQ and AZM on different ion channels, pumps and signaling transduction molecules in human cardiomyocytes are warranted. When studying the cardiac effects of HCQ and AZM as well as other therapeutic regimes for COVID-19, it will be imperative to consider both the contractile and electrophysiological properties.
5. Conclusion
In this study, we show that human engineered tissues, hvCTS and hvCAS, constructed from hvCMs predict the arrhythmogenic potential of administering HCQ alone or in combination with AZM. Re-entry is a substrate for ventricular arrhythmias and Torsades de Pointes which have been described in patients on HCQ [19]. Our data also showed that AZM can induce a dose-dependent negative inotropic effect which is exacerbated by the presence of HCQ. In COVID-19 patients with compromised cardiac function either at baseline or from sepsis, a further decrease in cardiac reserve may aggravate the patient's condition and increase the potential of life-threatening arrhythmias. Indeed, hPSC-hvCMs could be directly infected by the SARS-CoV-2 virus which apparently promoted cardiac cell death [25]. Collectively, our findings further delineate the risks of HCQ and AZM on QTc prolongation/ventricular arrhythmias and development of heart failure. HvCTS and hvCAS reliably recapitulate the clinical effects of HCQ and AZM, exposing the risks of their administration while providing mechanistic insights for their toxicity. Our bioengineered human cardiac tissue constructs therefore provide a useful platform for screening cardiac safety and efficacy when developing therapeutics against COVID-19.
Disclosures
K.D. Costa and R.A. Li hold equities in Novoheart whose value may potentially be affected by the publication of this manuscript. A.O.T. Wong, B. Gurung, W.S. Wong, S.Y. Mak, W.W. Tse, C.M. Li and D.K. Lieu declared no competing interests for this work. R.A. Li & R.J. Hajjar hold equities in Sardocor.
Acknowledgements
The authors thank Ms. Joey Chan, Ms. Yannes Leung, Mr. David Wong, Mr. Martin Chan and Dr. Virginia Kwan for their contributions to cell culture and cardiomyocyte differentiation. The authors also acknowledge Prof. Yiu-Fai Cheung and Dr. Wendy Keung for providing L-EdV-derived cardiomyocytes.
References
- 1.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Bull-Otterson L., Gray E.B., Budnitz D.S., Strosnider H.M., Schieber L.Z., Courtney J., Garcia M.C., Brooks J.T., Mac Kenzie W.R., Gundlapalli A.V. Hydroxychloroquine and chloroquine prescribing patterns by provider specialty following initial reports of potential benefit for COVID-19 treatment - United States, January-June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1210–1215. doi: 10.15585/mmwr.mm6935a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khuroo M.S. Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID-19). Facts, fiction and the hype: a critical appraisal. Int. J. Antimicrob. Agents. 2020;56:106101. doi: 10.1016/j.ijantimicag.2020.106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Jr., Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.-T., Monteiro W.M., Lacerda M.V.G., f.t.C.-. Team Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. (e208857-e208857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R.A., Keung W., Cashman T.J., Backeris P.C., Johnson B.V., Bardot E.S., Wong A.O.T., Chan P.K.W., Chan C.W.Y., Costa K.D. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials. 2018;163:116–127. doi: 10.1016/j.biomaterials.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keung W., Chan P.K.W., Backeris P.C., Lee E.K., Wong N., Wong A.O.T., Wong G.K.Y., Chan C.W.Y., Fermini B., Costa K.D., Li R.A. Human cardiac ventricular-like organoid chambers and tissue strips from pluripotent stem cells as a two-tiered assay for inotropic responses. Clin. Pharmacol. Ther. 2019;106:402–414. doi: 10.1002/cpt.1385. [DOI] [PubMed] [Google Scholar]
- 10.Wong A.O., Wong G., Shen M., Chow M.Z., Tse W.W., Gurung B., Mak S.Y., Lieu D.K., Costa K.D., Chan C.W., Martelli A., Nabhan J.F., Li R.A. Correlation between frataxin expression and contractility revealed by in vitro Friedreich's ataxia cardiac tissue models engineered from human pluripotent stem cells. Stem Cell Res Ther. 2019;10:203. doi: 10.1186/s13287-019-1305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shum A.M., Che H., Wong A.O., Zhang C., Wu H., Chan C.W., Costa K., Khine M., Kong C.W., Li R.A., Micropatterned Human A. Pluripotent stem cell-based ventricular cardiac anisotropic sheet for visualizing drug-induced arrhythmogenicity. Adv. Mater. 2017;29 doi: 10.1002/adma.201602448. [DOI] [PubMed] [Google Scholar]
- 12.Li R.A. Cardiovascular regeneration. Stem Cell Res Ther. 2014;5:141. doi: 10.1186/scrt531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow M., Boheler K.R., Li R.A. Human pluripotent stem cell-derived cardiomyocytes for heart regeneration, drug discovery and disease modeling: from the genetic, epigenetic, and tissue modeling perspectives. Stem Cell Res Ther. 2013;4:97. doi: 10.1186/scrt308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng Z., Kong C.W., Ren L., Karakikes I., Geng L., He J., Chow M.Z., Mok C.F., Keung W., Chow H., Leung A.Y., Hajjar R.J., Li R.A., Chan C.W. A simple, cost-effective but highly efficient system for deriving ventricular cardiomyocytes from human pluripotent stem cells. Stem Cells Dev. 2014;23:1704–1716. doi: 10.1089/scd.2013.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbull I.C., Karakikes I., Serrao G.W., Backeris P., Lee J.J., Xie C., Senyei G., Gordon R.E., Li R.A., Akar F.G., Hajjar R.J., Hulot J.S., Costa K.D. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 2014;28:644–654. doi: 10.1096/fj.13-228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Chen A., Lieu D.K., Karakikes I., Chen G., Keung W., Chan C.W., Hajjar R.J., Costa K.D., Khine M., Li R.A. Effect of engineered anisotropy on the susceptibility of human pluripotent stem cell-derived ventricular cardiomyocytes to arrhythmias. Biomaterials. 2013;34:8878–8886. doi: 10.1016/j.biomaterials.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Mahevas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C., Fois E., Lepeule R., Szwebel T.A., Lescure F.X., Schlemmer F., Matignon M., Khellaf M., Crickx E., Terrier B., Morbieu C., Legendre P., Dang J., Schoindre Y., Pawlotsky J.M., Michel M., Perrodeau E., Carlier N., Roche N., de Lastours V., Ourghanlian C., Kerneis S., Menager P., Mouthon L., Audureau E., Ravaud P., Godeau B., Gallien S., Costedoat-Chalumeau N. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., Wu Y., Xiao W., Liu S., Chen E., Chen W., Wang X., Yang J., Lin J., Zhao Q., Yan Y., Xie Z., Li D., Yang Y., Liu L., Qu J., Ning G., Shi G., Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C., Knotts R., Bar-Cohen R., Kogan E., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M., Park D.S., Stefano C., Chinitz L.A., Jankelson L. QT interval prolongation and torsade De pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z., Prinsen J.K., Bersell K.R., Shen W., Yermalitskaya L., Sidorova T., Luis P.B., Hall L., Zhang W., Du L., Milne G., Tucker P., George A.L., Jr., Campbell C.M., Pickett R.A., Shaffer C.M., Chopra N., Yang T., Knollmann B.C., Roden D.M., Murray K.T. Azithromycin causes a novel proarrhythmic syndrome. Circ. Arrhythm. Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.115.003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araujo F.G., Shepard R.M., Remington J.S. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:519–524. doi: 10.1007/BF01963942. [DOI] [PubMed] [Google Scholar]
- 22.Morita S., Takahashi T., Yoshida Y., Yokota N. Population pharmacokinetics of hydroxychloroquine in Japanese patients with cutaneous or systemic lupus erythematosus. Ther. Drug Monit. 2016;38:259–267. doi: 10.1097/FTD.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 23.Capel R.A., Herring N., Kalla M., Yavari A., Mirams G.R., Douglas G., Bub G., Channon K., Paterson D.J., Terrar D.A., Burton R.A. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current if: novel electrophysiological insights and therapeutic potential. Heart Rhythm. 2015;12:2186–2194. doi: 10.1016/j.hrthm.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mubagwa K. Cardiac effects and toxicity of chloroquine: a short update. Int. J. Antimicrob. Agents. 2020;56:106057. doi: 10.1016/j.ijantimicag.2020.106057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma A., Garcia G., Wang Y., Plummer J.T., Morizono K., Arumugaswami V., Svendsen C.N. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. bioRxiv. 2020;1:100052. doi: 10.1016/j.xcrm.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]