Recently, there are several studies reporting that pre-existing chronic kidney disease (CKD) was related to the adverse outcomes of patients with coronavirus disease 2019 (COVID-19) [1, 2]. Moreover, a meta-analysis has indicated that CKD was associated with poor prognosis in patients with COVID-19 on the basis of unadjusted effect estimates [3]. To the best of our knowledge, several factors including age, gender and underlying diseases were reported to have effects on the clinical outcomes of COVID-19 patients [4]. Therefore, in this present meta-analysis, we aimed to investigate the relationship between CKD and the adverse outcomes in COVID-19 patients on the basis of adjusted effect estimates by performing a quantitative meta-analysis.
The electronic databases including PubMed, Web of Science, EMBASE and Chinese National Knowledge Infrastructure (CNKI) were searched by two independent authors to screen out eligible articles, using the keywords of “coronavirus disease 2019” OR “SARS-CoV-2” OR “2019 novel coronavirus” OR “2019-nCoV” OR “COVID-19” AND “chronic kidney disease” OR “chronic renal disease” (up to July 15th, 2020). The adverse outcomes were defined as severe illness, critical illness or death. Studies reporting the adjusted effect estimates (odds ratio (OR) or hazard ratio (HR)) on the association between CKD and adverse outcomes of COVID-19 patients were eligibly included. All analyses were performed by STATA 11.2. A fixed-effects model was used if I2 was < 50%. Otherwise, a random-effects model was applied. The stability of results was assessed by sensitivity analysis. Publication bias was evaluated by Begg’s test and Egger’s test.
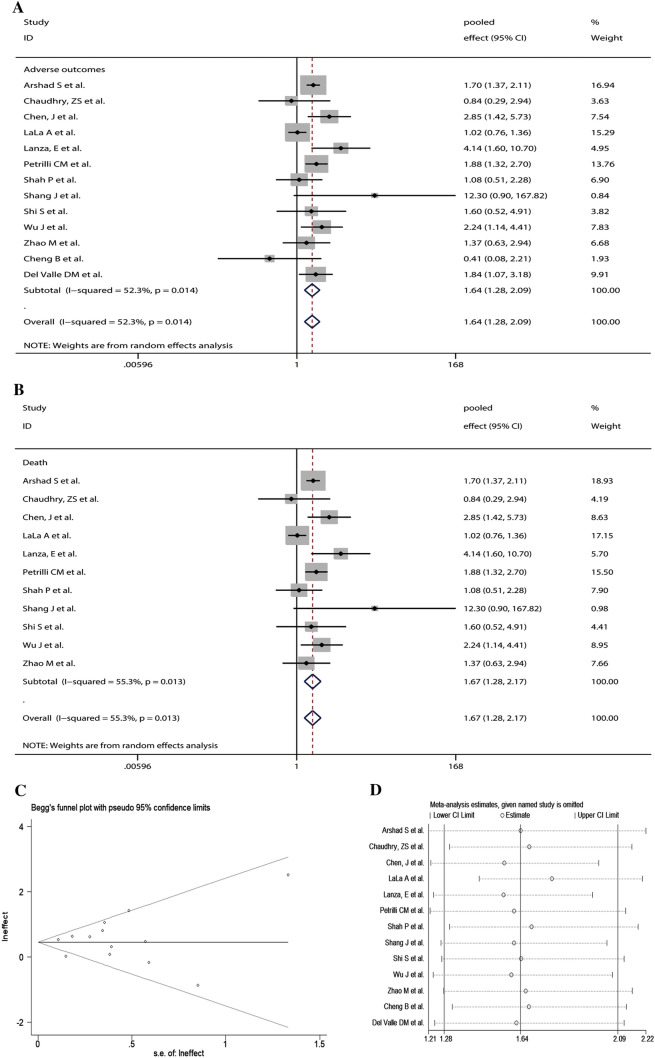
A total of 179 articles were identified. Finally, 13 articles with 12,999 patients were included in the study. The main characteristics of the included studies are shown in Table 1. Our results indicated that COVID-19 patients with a history of CKD had an increased risk for adverse outcomes (pooled effect = 1.64, 95% CI 1.28–2.09) (Fig. 1a). We also observed that CKD was significantly associated with an increased risk for COVID-19 death while adverse outcomes were only restricted to death (pooled effect = 1.67, 95% CI 1.28–2.17) (Fig. 1b). There was no publication bias (Begg’s test, P = 0.855 (Fig. 1c) and Egger’s test, P = 0.655). Sensitivity analysis exhibited that our results were stable (Fig. 1d).
Table 1.
Characteristics of the included studies
| Author | Country | Cases (n) | Age (years) | Male (%) | Study design | CKD (%) | Adjusted effect estimates (95% CI) | Confounders | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Arshad et al. (PMID: 32623082) | USA | 2541 | 64 (53–76) | 1243 (48.9) | R | 1099 (43.3) | HR 1.699 (1.370–2.108) | HCQ alone, AZM alone, HCQ + AZM, age, gender, race, BMI, lung comorbidity, immunodeficiency comorbidity, CVD, HTN, asthma, DM, percent O2 saturation, admitted to ICU, ventilator, given steroid, given tocilizumab | 6 |
| Chaudhry et al. (PMID: 32654332) | USA | 135 | NA | 73 (54.1) | R | 88 (65.2) | OR 0.84 (0.29–2.94) | Age, diabetes, HFH COVID-19 severity score, transplant status | 7 |
| Chen et al. (PMID: 32634830) | China | 3309 | 62 (49–69) | 1642 (49.6) | R | 57 (1.7) | OR 2.85 (1.42–5.73) | Gender, age, comorbidities, days from onset to clinics, days from onset to admission | 6 |
| Cheng et al. (PMID: 32622952) | China | 456 | 54.97 ± 18.59 | 211 (46.27) | R | 19 (4.16) | OR 0.415 (0.078–2.206) | Age, gender, comorbidities, neutrophil count, lymphocyte count, NLR, CRP, procalcitonin | 5 |
| Del Valle et al. (PMID: 32511562) | USA | 1268 | 63 (53–72) | 787 (60.1) | P | 167 (13.2) | HR 1.84 (1.07–3.18) | Cytokines, demographics, comorbidities, laboratory measurements | 5 |
| LaLa et al. (PMID: 32517963) | USA | 2736 | 48.9 ± 16.3 | 1630 (59.6) | R | 273 (10.0) | OR 1.02 (0.76–1.36) | Troponin strata, gender, age, race, CAD, diabetes, heart failure, HTN, atrial fibrillation, BMI, CURB-65 score, ACE-I or ARB use, statin use | 7 |
| Lanza et al. (PMID: 32591888) | Italy | 222 | 66.4 (53.8–75.8) | 334 (55) | R | 10 (4.5) | OR 4.14 (1.6–10.7) | Compromised lung volume, age, sex, smoke habit, CRP, heart disease, lung disease, cancer, diabetes, CURB-65a1, CURB-65a2, urea at admission, BMI | 6 |
| Petrilli et al. | Italy | 1603 | 58.0 ± 20.9 | 758 (47.3) | R | 69 (6) | OR 1.88 (1.32–2.7) | Age, cancer, CAD, diabetes, gender, heart failure, hyperlipidemia, HTN, BMI, pulmonary disease, race, tobacco use, CRP, creatinine, ferritin, lymphocyte count, procalcitonin, oxygen saturation on presentation, temperature | 7 |
| Shah et al. (PMID: 32620056) | USA | 552 | 63 (50–72) | 218(58.2) | R | 78 (14.9) | OR 1.08 (0.51–2.28) | Age, BMI, gender, race, comorbidities, tobacco smoking | 5 |
| Shang et al. (PMID: 32653423) | China | 584 | NA | 277 (47.4) | R | 8 (1.4) | HR 12.301 (0.902–167.823) | Age, sex, HTN, CVD, diabetes, chronic respiratory diseases, chronic liver diseases, acute kidney injury, acute liver injury, respiratory failure, acute cardiac injury | 8 |
| Shi et al. (PMID: 32391877) | China | 671 | 63 (50–72) | 322 (48.0) | R | 28 (4.2) | HR 1.6 (0.52–4.91) | Male, age, HTN, diabetes, coronary heart disease, chronic heart failure, cerebrovascular diseases, procalcitonin, CRP, CK-MB, MYO, cTnl, NT-proBNP | 8 |
| Wu et al. (PMID: 32503812) | China | 865 | 61 (50–69) | 825 (48.8) | P | 33 (2.0) | HR 2.24 (1.14–4.41) | Glu level, gender, age, diabetes, HTN, smoking history, insulin treatment, systemic glucocorticoids, COPD, cancer, admission white cell counts, admission lymphocyte counts, admission d-dimer, admission AST, admission ALT, admission creatinine | 7 |
| Zhao et al. (PMID: 32499448) | China | 1000 | 61 (46–70) | 466 (46.6) | R | 24 (2.4) | HR 1.365 (0.634–2.942) | Age | 8 |
All values are n (%), mean ± SD (standard deviation) or median (interquartile range, IQR); NA not available, P prospective, R retrospective, HR hazard ratio, OR odds ratio, CK-MB creatinine kinase-myocardial band, MYO myoglobin, cTnl cardiac troponin I, NT-proBNP N-terminal pro-B-type natriuretic peptide, AST aspartate aminotransferase, ALT alanine aminotransferase, CVD cardiovascular diseases, COPD chronic obstructive pulmonary diseases, CAD coronary artery disease, CRP C-reactive protein, Glu glucose, BMI body mass index, HCQ hydroxychloroquine, AZM azithromycin, ICU intensive care unit, HFH Henry Ford Hospital, NLR neutrophil count/lymphocyte count ratio, ACE angiotensin-converting enzyme, ARB angiotensin II receptor blocker, NOS Newcastle–Ottawa scale, CKD chronic kidney disease, HTN hypertension
Fig. 1.
The pooled effects and 95% confidence interval (CI) of the relationship between chronic kidney disease (CKD) and adverse outcomes in patients with coronavirus disease 2019 (COVID-19) (a); The pooled effects and 95% CI of the relationship between CKD and death in patients with COVID-19 (b); Publication bias was assessed by Begg’s funnel plot (c); Sensitivity analysis of the relationship between CKD and adverse outcomes in patients with COVID-19 (d)
Previous meta-analyses have reported the association of CKD with poor outcomes including mortality among COVID-19 patients, but their findings were based on unadjusted effect estimates [5–10]. Our present study based on adjusted effect estimates indicated that pre-existing CKD was independently associated with an increased risk for adverse outcomes, especially for mortality. Thus, co-existing CKD patients should be taken care not to get COVID-19 infection. Of course, there are some limitations to this study. First, the patients in each article might be in different stages of the disease, which may have a certain impact on the overall effects. Second, supportive treatment and medications are not clear in the included studies, thus, the data could not be analyzed currently. Third, the included studies were mainly from China, USA and Italy. As a result, it should be cautious to extrapolate the inclusion to other regions. Fourth, the findings were based on the adjusted effect estimates, but the adjusted factors were not completely consistent. This issue should be addressed in the future studies. Taken together, our current study is needed to be verified by further well-designed studies with a large sample size.
Acknowledgement
We would like to thank Ying Wang, Hongjie Hou, Yang Li and Jian Wu (All are from the Department of Epidemiology, School of Public Health, Zhengzhou University) for their kind help in discussing manuscript revision and assessing the quality score of the included studies.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81973105). The funder has no role in data collection, data analysis, preparation for this manuscript and the decision to manuscript submission.
Compliance with ethical standards
Conflict of interest
All the authors declare that there is no potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cen Y, Chen X, Shen Y, Zhang XH, Lei Y, Xu C, Jiang WR, Xu HT, Chen Y, Zhu J, Zhang LL, Liu YH. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-centre observational study. Clin Microbiol Infect. 2020;S1198-1743X(1120):30341–30344. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, Xu J, Wu F, Duan L, Yin Z, Luo H, Xiong N, Xu M, Zeng T, Jin Y. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020 doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang X, Li S, Yu H, Wang P, Zhang Y, Chen Z, Li Y, Cheng L, Li W, Jia H, Ma X. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Földi M, Farkas N, Kiss S, Zádori N, Váncsa S, Szakó L, Dembrovszky F, Solymár M, Bartalis E, Szakács Z, Hartmann P, Pár G, Erőss B, Molnár Z, Hegyi P, Szentesi A, Group KS. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020 doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandy K, Salunke A, Pathak SK, Pandey A, Doctor C, Puj K, Sharma M, Jain A, Warikoo V. Coronavirus disease (COVID-19): a systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr. 2020;14(5):1017–1025. doi: 10.1016/j.dsx.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, Seidu S, Zaccardi F, Davies MJ, Khunti K. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS ONE. 2020;15(8):e0238215. doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, Wang F. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research. 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pranata R, Supriyadi R, Huang I, Permana H, Lim MA, Yonas E, Soetedjo NNM, Lukito AA. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insight Circul Respirat Pulmon Med. 2020;14:1179548420959165. doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020 doi: 10.3390/tropicalmed5020080. [DOI] [PMC free article] [PubMed] [Google Scholar]