Abstract
Spinal cord injury (SCI) is associated with damage to musculoskeletal tissues of the spine. Recent findings show that pain and inflammatory processes caused by musculoskeletal injury mediate plastic changes in the spinal cord. These changes could impede the adaptive plastic changes responsible for functional recovery. The underlying mechanism remains unclear, but may involve the microglia-BDNF-KCC2 pathway, which is implicated in sensitization of dorsal horn neurons in neuropathic pain and in the regulation of spinal excitability by step-training. In the present study, we examined the effects of step-training and lumbar muscle inflammation induced by complete Freund’s adjuvant (CFA) on treadmill locomotion in a mouse model of complete spinal transection. The impact on locomotor recovery of each of these interventions alone or in combination were examined in addition to changes in microglia and KCC2 expression in the dorsal and ventral horns of the sublesional spinal cord. Results show that step length and angular motion at the hip, knee and ankle joint during locomotion were decreased by CFA injection and improved by step-training. Moreover, CFA injection enhanced the expression of the microglial marker Iba1 in both ventral and dorsal horns, with or without step-training. However, this change was not associated with a modulation of KCC2 expression, suggesting that locomotor deficits induced by inflammation are independent of KCC2 expression in the sublesional spinal cord. These results indicate that musculoskeletal injury hinders locomotor recovery after SCI and that microglia is involved in this effect.
Keywords: spinal cord injury model, training, neuroplasticity, pain, nociception, disinhibition, chloride homeostasis
INTRODUCTION
Pain affects about 80% of spinal cord injured (SCI) patients (Siddall et al., 2003) and is present during rehabilitation, including during locomotor training. While maladaptive plasticity induced by pain may hinder adaptive plasticity associated with functional recovery, this link is not clearly established, including its impact on locomotor recovery (Dvorak et al., 2017).2 Such interaction between adequate/inadequate plastic changes have considerable implications for disability as fostering adaptive plasticity may highly impact recovery.
In animal models of SCI, accumulating evidence shows that nociception and inflammation impair locomotor recovery (Garraway et al., 2011; Jeffrey-Gauthier et al., 2017) by acting directly or indirectly (Jeffrey-Gauthier et al., 2018b) on locomotor spinal networks. Importantly, step-training contributes to locomotor recovery by inducing adaptive plastic changes within locomotor spinal networks (Alluin et al., 2015; de Leon et al., 1998; Ichiyama et al., 2008), suggesting that nociceptive processes and step-training interact to influence the same spinal pathways. Using flexion reflex conditioning in spinal rats, it was demonstrated that nociceptive electrical stimulation could prevent task-related training effects on reflex conditioning (Ferguson et al., 2006) by a glia-dependent mechanism (Grau et al., 2014; Huie et al., 2012a). Conversely, training could prevent the disruptive effect of nociceptive inputs on reflex conditioning (Crown and Grau, 2001) by a brain-derived neurotrophic factor (BDNF)-dependent mechanism (Gómez-Pinilla et al., 2007; Huie et al., 2012b).
In addition to the contribution of glia and BDNF to both nociceptive and recovery processes, the expression of the cation-chloride cotransporter type 2 (KCC2) is pivotal in chloride homeostasis responsible for GABA inhibitory transmission and is reduced after SCI (Boulenguez et al., 2010). Importantly, KCC2 expression is modulated by both step-training and pain-related processes oppositely. On the one hand, exercise increases KCC2 expression in lumbar motoneurons’ membrane and attenuates H-reflex disinhibition after SCI in rats (Côté et al., 2014), through a BDNF-dependent mechanism (Beverungen et al., 2019). On the other hand, persistent inflammation induced by CFA injection in the hind paw of rats enhances pain sensitivity by decreasing KCC2 expression in lumbar dorsal horns (Zhang et al., 2008). However, no study has investigated KCC2 expression when step-training and inflammation occur concurrently. Considering the high prevalence of pain in SCI patients, the interaction of pain and training and underlying contribution of glia- and KCC2-dependent processes should be clarified.
The objective of the present study was to investigate the involvement of microglia/KCC2 expression in the lumbar spinal cord in the impact of concurrent step-training and lumbar muscle inflammation on locomotor recovery after a complete mid-thoracic spinal transection in mice. Based on previous studies mentioned above, we hypothesize that inflammation hinders locomotor recovery by increasing microglial activity and downregulating KCC2, while step-training rescues recovery by influencing microglia and KCC2 oppositely.
MATERIALS AND METHODS
Animal care and ethics
This experiment was performed on 25 female CD1 mice (body weight: 25 g; Charles River Laboratories, Saint-Constant, QC, Canada). Animals arrived two weeks prior to testing to habituate to their environment, ambient temperature (26 °C) and cage mates (5 mice/cage). The laboratory staff provided the animals with a 12 – 12h light-dark cycle and constant access to food and water. All handling and procedures were carried out in accordance with Canadian Council on Animal Care guidelines. The protocol was approved by the UQTR Animal Care Committee and complies with guidelines from the Committee for Research and Ethical Issues of the International Association for the Study of Pain.
Surgical procedures
Surgical procedures were performed under anesthesia with isoflurane (2% mixed with medical O2, flow rate: 100 ml/min), preceded by the administration of the analgesic drug buprenorphine (0.1 mg/kg, s.c.) and followed by the administration of sterile saline solution (0.9 %, 1 ml, s.c.). Moreover, perioperative care initiated the day before surgery and terminated the day after surgery was provided by administering the anti-inflammatory drug carprofen (10 mg/kg, s.c., q.d.) and the analgesic drug buprenorphine (0.1 mg/kg, s.c., p.r.n.). After surgery, hydration was closely monitored and bladders were manually expressed twice a day. Access to water was facilitated and a bolus of warm saline (1 ml, s.c.) was provided when required. Spinal transections were performed as described previously (Leblond et al., 2003). Adequate body temperature was maintained by placing the animal on a heating pad while anesthetized. The skin overlying the spine from T5 to T9 was shaved and incised. The paraspinal muscles were separated from the spine on each side. The spinal cord was approached posteriorly by performing a double laminectomy of T7 and T8 vertebrae and removing the posterior aspect of the vertebral arches. Then, lidocaine droplets were applied on the spinal cord to avoid secondary damage just before complete transection with micro-scissors. The dural sac was also completely transected, causing a retraction of the rostral and caudal stumps and a clear discontinuation of the spinal cord. Absorbable hemostats (Surgicel, Ethicon, Somerville, NJ, USA) was applied to fill the cavity to promote hemostasis. Muscular and cutaneous tissues were sutured back in layers, after which anesthesia was discontinued.
Experimental interventions
Spinally transected mice were randomly assigned to one of the following groups: untrained (n=6), trained (n=6), untrained with CFA (n=7) and trained with CFA (n=6). Trained mice and trained mice with CFA were provided with daily step-training (6 days/week) consisting of 10 min session of tail pinching-triggered locomotion on the treadmill. This step-training regimen was initiated on day 2 post-transection and terminated at the end of the experiment on day 28 post-transection. Untrained mice with CFA and trained mice with CFA were administered 4 boluses of CFA (Sigma F5881, 4X25 μl of 0.5 mg/ml heat-killed Mycobacterium tuberculosis diluted 1:1 in warm saline 0.9 %) in left and right lumbar muscles at L1 and L5 segments. These injections were performed on isoflurane anesthetized animals on day 4 post-transection to avoid interaction with the anti-inflammatory drug carprofen given perioperative. One centimetre of skin overlying the L1-L5 spine was incised and injections were performed directly in exposed lumbar muscles. Injecting needles were secured in place for 10 min to ensure proper CFA diffusion in the muscles. Thereafter, skin was sutured and anesthesia discontinued. This procedure has been shown to cause an inflammatory response that persists over a month (Ambalavanar et al., 2006).
Assessment of locomotor recovery
Treadmill locomotion (Exer-3/6, Columbus Instruments, Columbus, OH, USA) with an implemented speed of 12 m/min was evaluated once before spinal transection (baseline) and once a week after (on day 2, 7, 14, 21 and 28 post-transection). Locomotor evaluations of the spinally transected mice or step-training sessions were performed while animals were provided with 1) a platform overhanging the treadmill on which they can rest their forelimbs, 2) balance from tail holding by the experimenter and 3) tail pinching. The latter has been experimentally recognized as a non-invasive stimulation that allows stepping generation in animals transected as adults (Meisel and Rakerd, 1982) and used to study locomotor recovery after spinal transection by our group (Jeffrey-Gauthier et al., 2018a; Leblond et al., 2003) and others (Sławińska et al., 2014). This amount was set individually for each animal to the lowest pressure that induced locomotion. Locomotor bout selection and analysis was performed by a second experimenter blind to the group affiliation of animals. The longest bout of consecutive stepping that mice could achieve in 5 min sessions was recorded by a high-speed camera (Proselica GC, Allied Vision Technologies, Irwin, PA, USA; 90 frames/s) facing the left side of the animal. Anatomical landmarks (iliac crest, great trochanter, knee, ankle, 5th MTP and 5th toe) were marked and digitalized to XY coordinates to document angular motion of the hip, knee, ankle and MTP throughout the locomotor bout. Right and left paw contacts and lifts were detected. They were used to divide step cycles into stance and swing phase and to document phases duration and step length at each cycle. In the absence of clear paws lifts, swing and stance were identified as forward and backward paw movements, respectively. Dragging during swing was calculated as the proportion of the swing phase being achieved with the paw in contact with the treadmill belt. Coordination between hind limbs was assessed with the evaluation of the homologous coupling, i.e., the phase relation between left and right step cycles. Homologous coupling value was scored between 0 and 1 with 0.5 consisting of out-of-phase left and right step cycles. Homologous coupling constancy over locomotor bout was also scored between 0 and 1 with 1 consisting of a constant phase coupling throughout the recording.
Immunohistochemistry and microscopy
Anesthetized mice were perfused through the heart with PBS followed by paraformaldehyde (4 % in PBS). The perfused lumbar spinal cord was removed and immersed in sucrose (30 % in PBS) for cryoprotection. After 24 h, the spinal cord was removed from sucrose, put in OCT, then stored at −80 °C.
Frozen perfused tissue samples were cut in transversal sections of 25 μm from L2-L4 with a cryostat (Leica CM3050 S, Leica Biosystems, Concord, ON, Canada) and disposed on two sets of microscope slides for each mouse. Immunofluorostaining was achieved directly on these slides. Sections were covered with PBS-based incubation buffer (5 % donkey serum, 3 % bovine serum albumin – BSA, 0.1 % Triton X) with 0.9 % lysine for 1 h at room temperature (RT) to suppress non-specific binding. Slides from set #1 were incubated overnight at RT in incubation buffer with rabbit anti-KCC2 antibody (1:100, Millipore Catalog No. ab07-432) and goat anti-ChAT antibody (1:50, Millipore Catalog No. AB144P). Slides from set #2 were incubated overnight at RT in incubation buffer with rabbit anti-Iba1 (1:1000, Wako Catalog No. 019–19741), a marker specifically found on microglia. On the next day, the slides were washed 3x10 min in PBS, incubated in a dark chamber at RT for 2 h with a FITC-conjugated donkey anti-rabbit (1:400, Jackson Laboratories Catalog No. 711-095-152) and rhodamine red-conjugated donkey anti-goat (1:400, Jackson Laboratories Catalog No. 705-295-003) antibodies for set #1 and AF647-conjugated donkey anti-rabbit antibody (1:400, Jackson Laboratories Catalog No. 711-605-152) for set #2. Then, the slides were washed 3x10 min in PBS, dipped in distilled water and dried at RT in a dark chamber. At the end, the slides were covered with DAPI fluoromount (Southern Biotech, Birmingham, AL, USA) and protected with a cover slip.
For KCC2 visualization on motoneurons, images were acquired at 40X using a confocal laser scanning microscope (Leica TCS SP8, Leica Microsystems, Concord, ON, Canada). The motoneurons were identified as large choline acetyltransferase (ChAT)-expressing cells in the ventral horn with a soma diameter greater than 20 μm. KCC2 expression measurement was achieved on every motoneuron for which the soma was clearly visualized. Signal intensity was averaged from 3 regions of interest (ROI) evenly distributed on the plasma membrane and normalized to the signal intensity in the cytosol. In motoneurons selection and measurement were performed using LAS X software by a blind experimenter. A minimum of 13 motoneurons was examined per animal. For KCC2 evaluation in the dorsal horn and Iba1 evaluation in both ventral and dorsal horns, images were acquired at 20X using a fluorescent photonic microscope (Olympus BX51WI, Olympus Lifescience, Richmond Hill, ON, Canada). ROIs for KCC2 mean signal intensity and Iba1 area fraction evaluation were determined based on the mouse spinal cord anatomy described in the Allen Mouse Spinal Cord Atlas (© 2018 Allen Institute for Brain Science. Allen Mouse Spinal Cord Atlas. Available from: mousespinal.brain-map.org) and measured using ImageJ software (NIH, Bethesda, MD, USA) by a blind experimenter. A minimum of 3 sections were averaged per animal.
Data analysis and statistics
All results are expressed as mean ± SEM. For locomotor recovery data, the parameter values were averaged over the locomotor bout for each time point post-injury. Statistical analyses were conducted with Statistica v13.3 (TIBCO Software, Palo Alto, CA, USA). The significance threshold was set at p≤0.05. Differences in locomotor parameters (number of steps, angular motion of hind limb joints, step length, stance and swing duration, drag, homologous coupling value and homologous coupling constancy) were assessed with a mixed ANOVA, with one within-subject factor (number of days after transection, 5 levels) and two between-subject factors (CFA vs no CFA; training vs no training). Significant effects were decomposed with the Tukey HSD test. The effect of step-training and CFA on Iba1 area fraction and KCC2 signal intensity were assessed with a two-way ANOVA, with two between-subject factors (CFA vs no CFA; training vs no training).
RESULTS
Number of steps performed after a complete spinal transection
After spinal transection, the number of consecutive steps was decreased on post-transection day 2 (mean for all groups: 7.2 ± 1.5) compared with baseline (mean for all groups: 12.49 ± 1.77). During recovery, the number of steps varied over time (main effect: F4, 84 = 7.6, p < 0.001, ηp2 = 0. 27) but were not affected by CFA (main effect: F1, 84 = 2.7, p = 0.12, ηp2 = 0.11; Table 1) or training (main effect: F1, 84 = 0.2, p = 0.66, ηp2 = 0.01; Table 1). Moreover, there was no interaction between CFA and training (interaction: F1, 84 = 0.2, p = 0.68, ηp2 = 0.01). For the main effect of time, the Tukey HSD test revealed that the number of steps increased between consecutive time points, reaching significance between days 2 and 7 (p = 0.04), but not between days 7 and 14 (p = 0.23), between days 14 and 21 (p = 0.15) and between days 21 and 28 (p = 0.99).
Table 1.
Locomotor parameters before and after transection. Various locomotor parameters (number of steps, stance and swing durations, paw drag, homologous coupling and coupling constancy) were measured before and after transection. While they were affected by transection, they were not significantly affected by CFA or training (p’s > 0.05, see in text for more details).
| Day | Untrained | Trained | Untrained CFA | Trained CFA | |
|---|---|---|---|---|---|
| Mean ± sem | Mean ± sem | Mean ± sem | Mean ± sem | ||
| Nbr of steps | Intact | 13.67 ± 1.91 | 14.42 ± 2.10 | 12.29 ± 1.50 | 9,58 ± 1.58 |
| 2 | 8.83 ± 0.87 | 7.25 ± 2.20 | 6.57 ± 1.07 | 6.00 ± 1.64 | |
| 7 | 11.75 ± 1.62 | 14.00 ± 2.49 | 8.36 ± 2.03 | 10.42 ± 1.93 | |
| 14 | 13.92 ± 2.72 | 13.67 ± 2.77 | 15.21 ± 1.73 | 13.17 ± 1.75 | |
| 21 | 12.50 ± 1.56 | 16.58 ± 2.82 | 11.00 ± 1.61 | 9.75 ± 0.68 | |
| 28 | 12.83 ± 1.72 | 13.00 ± 1.49 | 12.21 ± 3.49 | 14.17 ± 1.55 | |
| Stance (ms) | Intact | 90.31 ± 33.16 | 131.33 ± 37.68 | 109.96 ± 33.62 | 123.41 ± 34.71 |
| 2 | 109.82 ± 52.83 | 423.22 ± 217.26 | 153.13 ± 49.52 | 450.53 ± 348.66 | |
| 7 | 135.93 ± 51.63 | 161.98 ± 40.68 | 209.72 ± 86.72 | 102.82 ± 37.43 | |
| 14 | 140.48 ± 50.18 | 163.45 ± 38.93 | 146.56 ± 38.82 | 200.51 ± 50.98 | |
| 21 | 140.43 ± 54.61 | 170.41 ± 38.60 | 222.55 ± 64.75 | 172.08 ± 40.09 | |
| 28 | 133.63 ± 54.05 | 191.66 ± 41.49 | 164.97 ± 45.96 | 170.50 ± 40.96 | |
| Swing (ms) | Intact | 39.86 ± 14.74 | 53.54 ± 15.29 | 45.24 ± 13.75 | 53.38 ± 14.89 |
| 2 | 61.96 ± 21.57 | 109.61 ± 27.54 | 91.03 ± 28.24 | 110.14 ± 27.17 | |
| 7 | 80.01 ± 26.68 | 78.15 ± 14.63 | 77.62 ± 16.33 | 165.06 ± 44.94 | |
| 14 | 54.98 ± 15.53 | 84.65 ± 15.88 | 64.84 ± 13.30 | 81.44 ± 15.72 | |
| 21 | 60.83 ± 17.06 | 80.20 ± 14.69 | 62.08 ± 12.00 | 79.68 ± 14.38 | |
| 28 | 64.14 ± 17.80 | 74.27 ± 12.99 | 60.07 ± 11.16 | 85.10 ± 15.44 | |
| Drag (% swing) | Intact | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 2 | 99.75 ± 0.12 | 99.04 ± 0.36 | 99.09 ± 0.57 | 95.88 ± 1.65 | |
| 7 | 95.23 ± 1.63 | 92.95 ± 1.82 | 98.81 ± 0.48 | 94.37 ± 2.19 | |
| 14 | 79.74 ± 5.43 | 78.34 ± 4.44 | 88.66 ± 2.61 | 85.09 ± 2.49 | |
| 21 | 71.00 ± 3.30 | 63.29 ± 3.77 | 79.46 ± 3.07 | 58.74 ± 5.85 | |
| 28 | 58.41 ± 6.21 | 55.26 ± 2.72 | 63.08 ± 2.85 | 64.23 ± 3.86 | |
| Homologous coupling | Intact | 0.48 ± 0.01 | 0.48 ± 0.00 | 0.47 ± 0.01 | 0.46 ± 0.01 |
| 2 | 0.43 ± 0.02 | 0.35 ± 0.02 | 0.32 ± 0.03 | 0.34 ± 0.03 | |
| 7 | 0.41 ± 0.01 | 0.43 ± 0.01 | 0.42 ± 0.01 | 0.44 ± 0.01 | |
| 14 | 0.46 ± 0.01 | 0.41 ± 0.00 | 0.41 ± 0.02 | 0.41 ± 0.01 | |
| 21 | 0.41 ± 0.01 | 0.45 ± 0.01 | 0.41 ± 0.01 | 0.45 ± 0.01 | |
| 28 | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.41 ± 0.01 | 0.43 ± 0.01 | |
| Coupling constancy | Intact | 0.92 ± 0.01 | 0.89 ± 0.02 | 0.90 ± 0.02 | 0.91 ± 0.02 |
| 2 | 0.65 ± 0.04 | 0.61 ± 0.04 | 0.63 ± 0.05 | 0.69 ± 0.04 | |
| 7 | 0.75 ± 0.03 | 0.72 ± 0.03 | 0.65 ± 0.04 | 0.65 ± 0.04 | |
| 14 | 0.70 ± 0.03 | 0.70 ± 0.04 | 0.71 ± 0.02 | 0.75 ± 0.02 | |
| 21 | 0.88 ± 0.01 | 0.71 ± 0.03 | 0.77 ± 0.02 | 0.82 ± 0.02 | |
| 28 | 0.80 ± 0.03 | 0.87 ± 0.02 | 0.82 ± 0.03 | 0.88 ± 0.01 |
Joint kinematics
Hind limb joint angles were evaluated throughout locomotor bouts over time within the animal (see Figure 1A for individual examples of 750 ms sequence of locomotion). Intra-limb coordination was visualized for each animal by plotting hip, knee and ankle angle changes across step cycles in a relative joint angle plot (right; Figure 1A–E). Individual examples of the hind limb joints variations over a locomotor sample of 750 ms are displayed in stick diagrams for each group (left; Figure 1A–D). A relative joint angle plot and stick diagram are depicted from an intact mouse to provide an appreciation of normal locomotion (Figure 1E). Relative joint angle variations during normal locomotion involve a multi-joint biphasic pattern of alternation between flexion and extension that is lost in untrained mice, untrained CFA mice and trained CFA mice. Trained mice show a partial recovery of the multi-joint biphasic pattern of flexion and extension.
Figure 1:
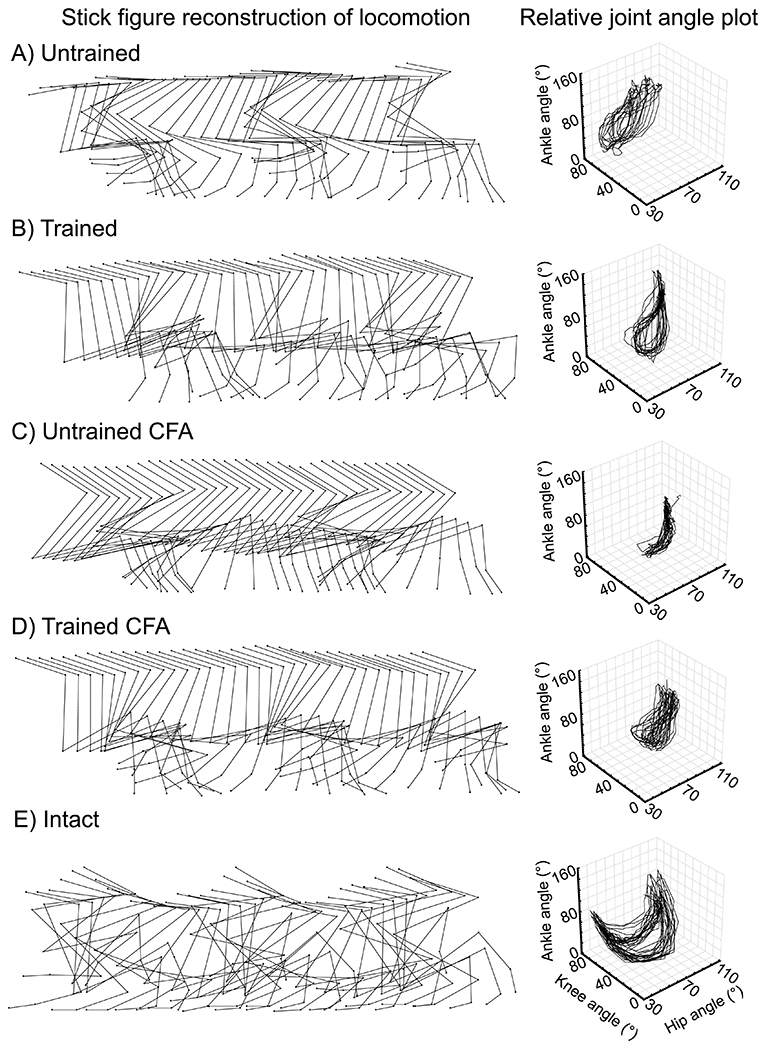
Locomotor kinematics overview.
(A) Stick figure displays individual examples of angular excursions of hind limb joints during a 750-ms recording of treadmill locomotion for each group on day 28 post-transection and in an intact mouse. (B) Relative joint angle plots of ankle excursion in function of hip and knee excursions during locomotion show decreased hind limb coordination and an abnormal locomotor kinematic pattern on day 28 compared to the intact state. These plots illustrate the decreased angular excursions in untrained CFA mice compared to untrained and trained mice and compared to trained CFA mice.
Hip angular excursion was measured between maximal flexion and extension angles for each step cycle and averaged over locomotor bouts (Figure 2A). For all groups combined, the mean hip excursion was reduced by spinal transection from 61.4 ± 5.5° (baseline) to 19.2 ± 4.9° (post-transection day 2). During recovery, excursion increased over time (main effect: F4, 84 = 14.5, p < 0.001, ηp2 = 0.41) and was significantly impacted by CFA (main effect: F1, 84 = 6.2, p = 0.02, ηp2 = 0.23), but not by training (main effect: F1, 84 = 2.9, p = 0.10, ηp2 = 0.12), without interaction between CFA and training (interaction: F1, 84 = 2.3, p = 0.15, ηp2 = 0.1). For all time points combined, hip angular excursion was decreased in CFA-injected mice (untrained CFA mice and trained CFA mice combined) compared with non-injected mice (untrained mice and trained mice combined; mean difference ± 95% CI = −6.55 ± 5.15). For the main effect of time, the Tukey HSD test revealed that day-to-day increase in hip excursion did not reach significance between days 2 and 7 (p=0.16), between days 7 and 14 (p = 0.09), between days 14 and 21 (p = 0.96) and between days 21 and 28 (p = 0.79).
Figure 2.

Hind limb joint kinematics variations over time and across groups
Hip (A), knee (B) and ankle (C) angular excursion measured between maximal flexion and maximal extension angles and MTP extension angle (D) were evaluated at each step and averaged over locomotor bout for each animal. Group median (black squares), interquartile (coloured bars) and extreme values (coloured lines) are shown on each time points (intact, day 2, day 7, day 14, day 21 and day 28). Open circles show outliers. Dashed lines over median values on each time point were added to improve visualization of joint excursion recovery for each group. After initial drops in hind limb joints excursion following spinal transection, partial recovery of hip, knee and ankle excursion and MTP extension during locomotion were observed in all groups. Regardless of training, mice that received CFA showed decreased hip, knee and ankle excursions (red brackets) compared to mice that did not received CFA. Moreover, regardless of CFA, mice that were trained showed increased knee and ankle excursion (blue brackets) compared untrained mice. * p ≤ 0.05.
Knee angular excursion was measured between maximal flexion and extension angles for each step cycle and averaged over locomotor bouts (Figure 2B). For all groups combined, the mean knee excursion was reduced by spinal transection from 53.9 ± 2.4° (baseline) to 23.2 ± 5.3° (post-transection day 2). During recovery, excursion varied over time (main effect: F4, 84 = 4.8, p = 0.002, ηp2 = 0.18) and was significantly impacted by CFA (main effect: F1, 84 = 4.7, p = 0.04, ηp2 = 0.18) and training (main effect: F1, 84 = 5.7, p = 0.03, ηp2 = 0.22), without interaction between CFA and training (interaction: F1, 84 = 0.04, p = 0.85, ηp2 = 0.002). For all time points combined, knee angular excursion was significantly decreased in CFA-injected mice (untrained CFA mice and trained CFA mice combined) compared to non-injected mice (untrained mice and trained mice combined; mean difference ± 95% CI = −5.55 ± 5). Moreover, knee angular excursion was increased in trained mice with and without CFA (trained mice and trained CFA mice combined) compared to untrained mice with and without CFA (untrained CFA mice ant untrained mice combined; mean difference ± 95% CI = 6.1 ± 5). For the main effect of time, the Tukey HSD test revealed that day-to-day variation in knee excursion reached significance between days 2 and 7 (p=0.02). However, it did not differ significantly between days 7 and 14 (p = 0.99), between days 14 and 21 (p = 0.08) and between days 21 and 28 (p = 0.23).
Ankle angular excursion was measured between maximal flexion and extension angles for each step cycle and averaged over locomotor bout (Figure 2C). For all groups combined, the mean ankle excursion was reduced by spinal transection from 66.1 ± 3.8° (baseline) to 31.4 ± 5.7° (post-transection day 2). During recovery, excursion increased over time (main effect: F4, 84 = 25.9, p < 0.001, ηp2 = 0.55) and was impacted by CFA (main effect: F1, 84 = 5.2, p = 0.03, ηp2 = 0.20) and training (main effect: F1, 84 = 5.8, p = 0.03, ηp2 = 0.22), without interaction between CFA and training (interaction: F1, 84 = 1.9, p = 0.19, ηp2 = 0.08). For all time points combined, ankle angular excursion was decreased in CFA-injected mice (untrained CFA mice and trained CFA mice combined) compared to non-injected mice (untrained mice and trained mice combined; mean difference ± 95% CI = −8.78 ± 7.5). Moreover, it was increased in trained mice with and without CFA (trained mice and trained CFA mice combined) compared to untrained mice with and without CFA (untrained mice and untrained CFA mice combined; mean difference ± 95% CI = −9.30 ± 7.5). For the main effect of time, the Tukey HSD test revealed that day-to-day increase in excursion reached significance between days 2 and 7 (p < 0.001), but not between days 7 and 14 (p = 0.64), days 14 and 21 (p = 0.41) and days 21 and 28 (p = 0.99).
MTP maximal extension angle was measured for each step and averaged over locomotor bout as an index of adequate contact of the paw on its plantar aspect. Stick diagram in Figure 1C shows an example of MTP extension impairment, which prevents proper plantar paw placement in this animal of the untrained CFA group. For all groups combined, the mean MTP extension was reduced by spinal transection from 249.7 ± 2.9° (baseline) to 164.9 ± 3.9° (post-transection day 2; Figure 2D). During recovery, MTP extension increased over time (main effect: F4, 84 = 39.1, p < 0.001, ηp2 = 0.65), without significant effect of CFA (main effect: F1, 84 = 2.79, p = 0.11, ηp2 = 0.12), training (main effect: F1, 84 = 2.45, p =0.13, ηp2 = 0.11) or interaction between CFA and training (interaction: F1, 84 = 1.6, p =0.2, ηp2 = 0.07). For the main effect of time, the Tukey HSD test revealed significant day-to-day increase between days 2 and 7 (p = 0.004) and days 7 and 14 (p = 0.002). Such increase did not reach significance between days 14 and 21 (p = 0.43) and between days 21 and 28 (p = 0.47).
Phase duration
Stance and swing duration were altered by spinal transection. For all groups combined, the mean stance duration was increased by spinal transection from 113.75 ± 34.79 ms (baseline) to 284.17 ± 179.26 ms (post-transection day 2). During recovery, stance duration decreased over time, but the effect of time did not reach significance (main effect: F4, 84 = 1.3, p = 0.28, ηp2 = 0.06; Table 1). Moreover, stance duration was not affected by CFA (main effect: F1, 84 = 0.1, p = 0.76, ηp2 = 0.01) or training (main effect: F1, 84 = 0.9, p = 0.37, ηp2 = 0.04). Moreover, there was no interaction between CFA and training (interaction: F1, 84 = 0.1, p = 0.73, ηp2 = 0.01).
For all groups combined, the mean swing duration was increased by spinal transection from 48.00 ± 14. 67 ms (baseline) to 93.19 ± 34.79 ms (post-transection day 2). During recovery, swing duration decreased over time (main effect: F4, 84 = 3.3, p = 0.01, ηp2 = 0.14; Table 1) but was not affected by CFA (main effect: F1, 84 = 0.3, p = 0.62, ηp2 = 0.01) or training (main effect: F1, 84 = 1.1, p = 0.30, ηp2 = 0.05). Moreover, there was no interaction between CFA and training (interaction: F1, 84 = 0.1, p = 0.81, ηp2 = 0.00). For the main effect of time, the Tukey HSD test revealed that day-to-day swing duration decreases was marginal between days 7 and 14 (p = 0.08), but not significant between days 2 and 7 (p = 0.97), between days 14 and 21 (p = 1.0) and between days 21 and 28 (p = 1.0).
Paw dragging on the treadmill belt during the swing phase is typically not observed in normal locomotion but very common after SCI, including transection. Various amounts of paw dragging can be deciphered from stick diagram of Figure 1. For all groups combined, paw dragging was absent pre-transection (0 ± 0 % of swing phase) and greatly increased on post-transection day 2 (98.44 ± 1.11 % of swing phase). During recovery, drag decreased over time (main effect: F4, 84 = 58.8, p < 0.001, ηp2 = 0.74; Table 1). Drag was not affected by CFA (main effect: F1, 84 = 0.8, p = 0.39, ηp2 = 0.04) or by training (main effect: F1, 84 = 1.4, p = 0.25, ηp2 = 0.06). Moreover, there was no interaction between CFA and training (interaction: F1, 84 = 0.2, p = 0.69, ηp2 = 0.02). For the main effect of time, the Tukey HSD test revealed that day-to-day drag decreases reached significance between days 7 and 14 (p = 0.001) and between days 14 and 21 (p < 0.001), but not between days 2 and 7 (p =0.85) and between days 21 and 28 (p = 0.09).
Step length
Step length was measured between consecutive paw contact and lift for each step and averaged over locomotor bout. For all groups combined, the mean step length was reduced by spinal transection from 38.3 ± 1.8 mm (baseline) to 6.6 ± 1.3 mm (post-transection day 2; Figure 3). During recovery, step length increased over time (main effect: F4, 84 = 49.6, p < 0.001, ηp2 = 0.70), but was not affected by training (main effect: F1, 84 = 3.4, p = 0.08, ηp2 = 0.14), or by CFA (main effect: F1, 84 = 1.4, p = 0.25, ηp2 = 0.06). Moreover, the impact of training was not affected by CFA (interaction: F1, 84 = 3.8, p = 0.07, ηp2 = 0.15). However, increases in step length were observed in trained CFA mice compared with untrained CFA mice (mean difference ± 95% CI = 5.51 ± 3.94). No differences in step length were observed when comparing trained mice with untrained mice (mean difference ± 95% CI = −0.14 ± 4.10), trained mice with untrained CFA mice (mean difference ± 95% CI = 4.39 ± 4.10), trained mice with trained CFA mice (mean difference ± 95% CI = 1.12 ± 5.51), trained CFA mice with untrained mice (mean difference ± 95% CI = 0.99 ± 5.51) and untrained CFA mice with untrained mice (mean difference ± 95% CI = −4.53 ± 4.10). For the main effect of time, the Tukey HSD test revealed that step length increases between consecutive time points reached significance between days 2 and 7 (p < 0.001) and between days 7 and 14 (p = 0.005) but not between days 14 and 21 (p = 0.57) and between days 21 and 28 (p = 0.63).
Figure 3.
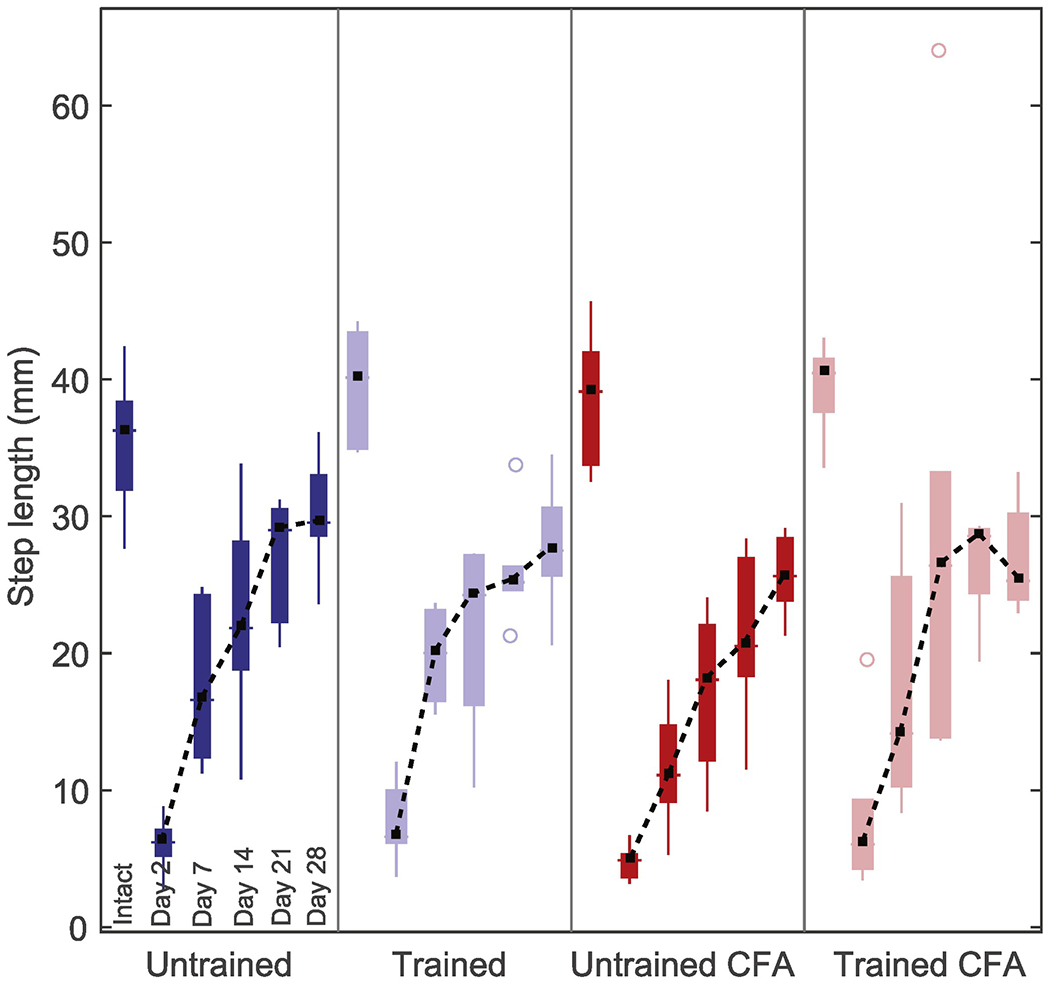
Variations in step length after spinal transection and during recovery period.
Step length averaged over locomotor bout was evaluated for each mouse before transection and on days 2, 7, 14, 21 and 28 after transection. Group median (black squares), interquartile (coloured bars) and extreme values (coloured lines) are shown for each time points. Outliers are shown as open circles. Dashed lines joining median values was added to improve visualization of recovery for each group. Step length decreased after transection, but partly recovered during the recovery period in all groups.
Coordination between hind limbs
Homologous coupling value provides the phase relation between left and right step cycles, with 0 and 1 consisting of in-phase movement and 0.5 consisting of out-of-phase alternation. For all groups combined, it was decreased by spinal transection from 0.47 ± 0.008 (baseline) to 0.36 ± 0.05 (post-transection day 2). During recovery, homologous coupling value increased over time (main effect: F4, 84 = 4.1, p = 0.004, ηp2 = 0.16; Table1) but was not affected by CFA (main effect: F1, 84 = 2.1, p = 0.16, ηp2 = 0.09) or training (main effect: F1, 84 = 0.02, p = 0.88, ηp2 = 0.001). Moreover, there was no interaction between CFA and training (interaction: F1, 84 = 1.1, p = 0.31, ηp2 = 0.05). For the main effect of time, the Tukey HSD test revealed that homologous coupling increases between consecutive time points reached significance between days 2 and 7 (p = 0.03), but not between days 7 and 14 (p = 1.00), between days 14 and 21 (p = 1.00) or between days 21 and 28 (p = 1.00).
For all groups combined, homologous coupling constancy throughout locomotor bout was greatly reduced by spinal transection from 0.90 ± 0.02 (baseline) to 0.65 ± 0.09 (post-transection day 2). During recovery, it increased over time (main effect: F4, 84 = 4.9, p = 0.001, ηp2 = 0.19; Table 1) but it was not affected by CFA (main effect: F1, 84 = 0.00, p = 0.99, ηp2 = 0.00) or training (main effect: F1, 84 = 0.05, p = 0.82, ηp2 = 0.003). Moreover, there was no interaction between CFA and training (interaction: F1, 84 = 1.8, p = 0.19, ηp2 = 0.08). For the main effect of time, the Tukey HSD test revealed that coupling constancy increases between consecutive time points did not reach significance between days 2 and 7 (p = 0.89), between days 7 and 14 (p = 0.99), between days 14 and 21 (p = 0.57) or between days 21 and 28 (p = 0.85).
IHC analyses
The expression of Iba1 and KCC2 was assessed in the dorsal and ventral horns in lumbar spinal cord (L2-L4, Figure 4A) to obtain histologic evidence of the central neuroinflammatory response to CFA injection and to evaluate whether step-training exerts its effect by upregulating KCC2. In dorsal horns, Iba1 expression was affected by CFA (main effect: F1, 21 = 5.4, p = 0.03, ηp2 = 0.21), but not by training (main effect: F1, 21 = 0.5, p = 0.50, ηp2 = 0.02). Moreover, there was no interaction between CFA and training (interaction: F1, 21 = 0.1, p = 0.77, ηp2 = 0.00). In ventral horns, Iba1 expression was impacted by CFA (main effect: F1, 21 = 5.6, p = 0.03, ηp2 = 0.21), but not by training (main effect: F1, 21 = 0.008, p = 0.93, ηp2 = 0.00). Moreover, there was no interaction between CFA and training (interaction: F1, 21 = 0.0, p = 0.96, ηp2 = 0.00). Regardless of training, Iba1 expression was decreased in CFA-injected mice (untrained CFA mice and trained CFA mice combined) compared to non-injected mice (untrained mice and trained mice combined) in both dorsal horns (mean difference ± 95% CI = 1.64 ± 1.38) and ventral horns (mean difference ± 95% CI = 1.78 ± 1.46). For KCC2, signal intensity was also assessed in dorsal horns and in the membrane of motoneurons in ventral horns, in which the membrane content was normalized to the cytosol content. In dorsal horns, KCC2 expression was unaffected by CFA (main effect: F1, 21 = 0.5, p = 0.47, ηp2 = 0.03) or by training (main effect: F1, 21 = 0.0, p = 0.98, ηp2 = 0.00; Figure 5), without interaction between CFA and training (interaction: F1, 21 = 2.8, p = 0.11, ηp2 = 0.12). Moreover, in motoneurons, KCC2 expression was unaffected by CFA (main effect: F1, 21 = 0.3, p = 0.62, ηp2 = 0.01) or by training (main effect: F1, 21 = 2.5, p = 0.13, ηp2 = 0.11; Figure 6), without interaction between CFA and training (interaction: F1, 21 = 2.3, p = 0.14, ηp2 = 0.10).
Figure 4.

CFA induces an increase in microglial inflammation in the lumbar spinal cord 28 days after a complete spinal cord transection in mice.
Iba1+ cells were labeled for microglia visualization in the L1-L2 spinal cord (A). Area fraction of stained tissue in dorsal (middle) and ventral horns (right) was evaluated (B). The area fraction median (black squares), 25–75% centiles (boxes) and extreme values (black lines) are shown. Regardless of step-training, Iba1 area fraction was enhanced in CFA-injected mice (both untrained CFA and trained CFA) compared to controls (both untrained and trained mice) in dorsal (p = 0.03) and ventral horns (p = 0.03).
Figure 5.

CFA and step-training fail to modulate KCC2 expression in the L2-L4 dorsal horns.
(A) Individual examples of KCC2 expression in the dorsal horn. (B) The signal intensity median (black squares), 25–75% centiles (boxes) and extreme values (bars) are shown. It did not differ between groups (p’s > 0.05).
Figure 6.

CFA and step-training fail to modulate KCC2 expression in lumbar motoneurons.
(A) Individual examples of KCC2 expression in the ventral horn. ChAT+ cells (red) were labeled for motoneurons visualization. (B) The KCC2 signal intensity in the membrane was averaged from 3 different ROIs and normalized to the cytosol signal intensity. (C) The signal intensity median (black squares), 25–75% centiles (boxes) and extreme values (bars) are shown. It did not differ between groups (p’s> 0.05).
DISCUSSION
Neurological trauma, including SCI, trigger nociceptive and inflammatory processes, which may persist during rehabilitation (Siddall et al., 2003). Recent evidence from animal models on reflex recovery suggests that activity-based therapy and pain-related processes, including inflammation, interact to influence spinal plasticity (Grau et al., 2014). Clinically, this implies that inflammation could hinder plastic changes responsible for locomotor recovery and that rehabilitation could attenuate the deleterious effects of inflammation and nociception. The results presented here in a mouse model of complete spinal cord transection are consistent with this idea and provide evidence that step-training protects against the detrimental effects of CFA injection on locomotor recovery. The main findings are 1) step-training partly improves locomotor deficits induced by CFA injection, 2) increases in microglial cell count in both dorsal and ventral horns could contribute to CFA-induced locomotor deficits and 3) KCC2 expression is not modulated by step-training or CFA injection in this model. Since concurrent effects of step-training and CFA injection on either Iba1 expression or KCC2 regulation was not confirmed, this suggests that the competitions between rehabilitation and inflammation on common mechanisms, if any, rely on processes independent of microglia or KCC2.
CFA injection in lumbar muscles and step-training interactions during locomotor recovery after a complete spinal transection
Animal models allowed examining the effects of training and nociception/inflammation on plastic changes mediating functional recovery after SCI. Recent findings indicate that peripheral inflammation and nociception impair locomotor recovery (Garraway et al., 2011; Jeffrey-Gauthier et al., 2017). Moreover, it has been observed that passive stretching of the hind limb of rats with a moderate contusion injury hinders locomotor recovery by activating nociceptive afferents (Keller et al., 2019; Keller et al., 2016). The present study shows that the influence of step-training and CFA-induced muscle inflammation is bidirectional and that step-training attenuates the CFA-induced locomotor deficits. Moreover, because the spinal transection model used prevent supraspinal structures from contributing to locomotor recovery, our results indicate that such interaction occurs spinally or peripherally.
Most locomotor parameters measured in this study significantly improved over time, indicating they are reliable markers of locomotor recovery. They also differed between group, suggesting an effect of both lumbar muscle inflammation and step-training on spinally mediated locomotion. Specifically, inflammation induced hind limbs kinematics deficits that were mitigated by step-training. In a series of experiments on rats with a complete spinal cord transection, Grau and colleagues demonstrated how nociceptive stimuli administered to the hind paw hindered adaptive plasticity in a task of flexion reflex conditioning (Grau et al., 1998; Grau et al., 2006). Moreover, they showed that preceding exposure to nociceptive stimuli by task-related training attenuates the negative influence of nociceptive inputs on reflex conditioning (Crown and Grau, 2001). They suggest that training protects against disruptive effect of nociceptive inputs by facilitating adaptive plasticity of the spinal circuitry by a BDNF-dependent mechanism (Huie et al., 2012b). Using a mouse model of complete spinal transection, our findings are consistent with their interpretation that training and inflammation interact either spinally or peripherally, but did not provide evidence of competition on a common mechanism.
CFA injection in lumbar muscles modulates microglia, with or without step-training
We previously showed that CFA injection in lumbar muscles of mice caused lumbar muscle inflammation evidenced 24 days after injection by leukocytic infiltrates (Jeffrey-Gauthier, Piché et al. 2017). Mononuclear infiltrates were also reported in response to CFA injection in lumbar muscles in spinally intact rats 12 days post-injection (Touj, Houle et al. 2017), with an increase in NFκB expression in the spinal cord measured by western blotting. Between 12 to 15 days post-injection, other groups reported similar findings when injecting CFA into other axial muscles (i.e. trapezius and masseter, Ambalavanar, Moritani et al. 2006, Kiyomoto, Shinoda et al. 2013) and appendicular muscles (i.e. triceps surae, Reinert, Kaske et al. 1998, Chacur, Lambertz et al. 2009). In the present study, inflammatory response to CFA is confirmed by an increase in microglial expression in the dorsal and ventral horns at L2-L4 that lasted several weeks after injection. Morphological changes and increased immunoreactivity to microglial markers were described in rats after CFA injection in the triceps surae (Chacur, Lambertz et al. 2009, Rosa, Freitas et al. 2017) and trapezius muscles (Kiyomoto, Shinoda et al. 2013). Microglia activation was also increased by intraplantar CFA injection in mice (Raghavendra, Tanga et al. 2004, Zhao, Zhang et al. 2015), and decreased together with central sensitization after glycyrrhizin treatment (Sun, Zeng et al. 2018). In rats, evidence of microglial involvement was further provided by the reduction of CFA-induced central sensitization by minocycline (Hoheisel, Chacur et al. 2019), a microglial inhibitor. These evidences provide strong support that microglia contribute to neuroinflammation after CFA injection. In the study of Chacur and colleagues, the involvement of microglia was evaluated in superficial and deep layers of the L5 dorsal horn. They showed that the microglia cell count was increased in both regions and was associated with a decrease in the withdrawal threshold in response to Von Frey filament stimulation (i.e. development of hypersensitivity). The inhibition of microglia activation by administration of minocycline and/or anti-TNFα antibody attenuated these changes (Chacur et al., 2009). However, the importance of microglia contribution to inflammation-related spinal processes including dorsal horn sensitization development is debated (Clark et al., 2007; Lin et al., 2007) and could be influenced by stimulus-dependent factors such as dosage and injection site. Overall, these neuroinflammatory processes could contribute to maladaptive plastic changes that hinder adaptive spinal plasticity. This is still speculative, nonetheless our data indicates that CFA injection changed the progression to locomotor recovery after spinal cord injury.
Our behavioral data suggest that step-training could facilitate locomotor recovery in mice whether they were administered CFA or not. However, step-training did not attenuate CFA-induced increase in microglial expression. This result supports the previous demonstration that training was not associated with decreased presence of Iba1+ cells despite decreased inflammatory gene expression (Shin et al., 2014). It also suggests that step-training attenuation of CFA-induced locomotor deficits most probably rely on a distinct mechanism. Non-linear relationship between microglial and spinal activity may still influence training-based plasticity underlying locomotor recovery. Indeed, previous evidence demonstrated that sublesional microglial activity is downregulated by early step-training (Detloff et al., 2014). In the contusion model, microglia are implicated in various outcomes contributing to functional recovery (David and Kroner, 2011; Donnelly and Popovich, 2008) including spared tissue volume (Watanabe et al., 1999) and dorsal horn activity both above-level (Carlton et al., 2009) and below-level of injury (Hains and Waxman, 2006). While the role of microglia in maladaptive plasticity leading to neuropathic pain has been extensively described (Detloff et al., 2008; Gwak and Hulsebosch, 2009; Gwak et al., 2009; Hains and Waxman, 2006; Hulsebosch et al., 2009; Zhao et al., 2007), its impact on other behavioral outcomes that rely on plasticity, including training-mediated locomotor recovery, is not known. Considering that models of complete spinal transection can evaluate the impact of plastic changes on functional recovery in isolation from other factors including spared tissue volume, such models are valuable and should be used to determine the influence of training on microglial activity and its role in SCI outcomes.
Locomotor performance and Iba1 expression changes mediated by inflammation and step-training are independent of KCC2 expression in both ventral and dorsal horns
Chloride homeostasis is essential for adequate GABAergic and glycinergic inhibitory transmission (Lu et al., 2008; Rivera et al., 2005) and is disrupted by a reduction in KCC2 expression after SCI (Boulenguez et al., 2010). Its decrease in ventral and dorsal horns causes disinhibition leading to motor (spasticity, hyperreflexia; Boulenguez et al., 2010; Modol et al., 2014) and sensory (neuropathic pain; Hasbargen et al., 2010; Lu et al., 2008) impairments, respectively. It is also reduced by inflammation through a microglia-dependent mechanism (Lin et al., 2017; Wu et al., 2009; Zhang et al., 2008), suggesting that the presence of inflammation during rehabilitation after SCI could further attenuate recovery by reducing KCC2 expression. Moreover, previous evidence showed that KCC2 expression in the lumbar spinal cord is upregulated by early exercise in motoneurons’ membrane after SCI (Côté et al., 2014) and in dorsal horns after a peripheral nerve injury (López-Ålvarez et al., 2015; Mòdol et al., 2014; Sánchez-Brualla et al., 2017). In both cases, KCC2 upregulation was associated with decreased hyperreflexia and dorsal horn sensitization. Altogether, these findings suggest that KCC2 expression is influenced by both inflammation and training.
In contrast to our hypothesis, we did not observe any difference in KCC2 expression in dorsal horn and motoneurons between groups. This suggests that 1) CFA did not cause further downregulation in KCC2 expression occurring with the concurrent SCI and that 2) step-training did not cause further upregulation in KCC2 expression occurring with the spontaneous recovery. One limitation of this finding is that several mice could perform spontaneous locomotor movements in their cage, although not measured in this study, providing an opportunity to self-train. This may have contributed to decreased microglial activity, increased KCC2 and/or increased locomotor recovery. Mice have a remarkable ability to express locomotion after SCI, much better than rats (Leblond et al., 2003), which limits the effect of step-training on locomotor recovery (Battistuzzo et al., 2012; Battistuzzo et al., 2016). This is further supported by the fact that restoring KCC2 on motoneuronal membrane fails to improve locomotor recovery in mice (Chen et al., 2018), while it improves allodynia, neuropathic pain, spasticity and locomotion in rats (Boulenguez et al., 2010; Chen et al., 2018; Liabeuf et al., 2017; Sanchez-Brualla et al., 2017; Tashiro et al., 2015). Nevertheless, this should not be interpreted as a failure of step-training to foster adaptive plasticity in mice. It was recently evidenced that mice recovering from a left hemisection preserved some locomotor function on the side of the hemisection after a subsequent complete transection (Jeffrey-Gauthier et al., 2018a), indicating that locomotor spinal networks of mice can be modified by step-training similarly to what was described in cats (Barriere et al., 2008). However, differences in recovery between strains of mice suggest that unknown genetic factors may influence recovery (Basso et al., 2006). Such discrepancies between species and between strains indicate that findings from studies using mouse models of SCI should be interpreted with caution when compared with rats, cats and humans.
Conclusions
Lumbar muscle inflammation was previously used as a model of musculoskeletal injury that could impede with locomotor recovery after a spinal transection. The present results show that lumbar muscle inflammation is associated with enhanced microglial expression in sublesional spinal cord, which could be altering plastic changes responsible for locomotor recovery. In addition, results show that step-training attenuates the detrimental effect of inflammation, providing further evidence of the beneficial effects of training on inflammation during locomotor recovery. Considering the high prevalence of musculoskeletal injuries and acute or chronic pain associated with SCI, this may have significant implications for the field of rehabilitation.
Complete Freund’s adjuvant (CFA) was injected in lumbar muscles of spinally-transected mice;
Spinally mediated locomotion was impaired by CFA and improved by step-training;
CFA increased spinal level of microglial marker Iba1, with or without step-training;
CFA and step-training fail to modulate KCC2 expression in dorsal horn and motoneurons.
ACKNOWLEDGMENTS
We would like to thank our two blind experimenters, Yann Develle and Melodie Plourde for their help with kinematic analyses and image acquisitions, respectively.
Funding: This work was supported by the Natural sciences and engineering research council of Canada (NSERC – RGPIN-2014-05403) to Hugues Leblond and by a National Institute of Neurological Disorders and Stroke (RO1 NS083666) grant to Marie-Pascale Côté. Renaud Jeffrey-Gauthier was supported by scholarships from Fondation chiropratique du Quebec, the NSERC and the Fonds de recherche du Quebec en Santé (FRQS). The contribution of Mathieu Piché was supported by the FRQS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS DISCLOSE STATEMENT
No competing financial interests exist.
REFERENCES
- Alluin O, Delivet-Mongrain H, Rossignol S, 2015. Inducing hindlimb locomotor recovery in adult rat after complete thoracic spinal cord section using repeated treadmill training with perineal stimulation only. Journal of Neurophysiology 114, 1931–1946. 10.1152/jn.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanar R, Moritani M, Moutanni A, Gangula P, Yallampalli C, Dessem D, 2006. Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain 120, 53–68. 10.1016/j.pain.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Barrière G, Leblond H, Provencher J, Rossignol S, 2008. Prominent Role of the Spinal Central Pattern Generator in the Recovery of Locomotion after Partial Spinal Cord Injuries. Journal of Neuroscience 28, 3976–3987. https://10.1523/jneurosci.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG, 2006. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. Journal of Neurotrauma 23, 635–659. 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Battistuzzo CR, Callister RJ, Callister R, Galea MP, 2012. A Systematic Review of Exercise Training To Promote Locomotor Recovery in Animal Models of Spinal Cord Injury. Journal of Neurotrauma 29, 1600–1613. 10.1089/neu.2011.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistuzzo CR, Rank MM, Flynn JR, Morgan DL, Callister R, Callister RJ, Galea MP, 2016. Gait recovery following spinal cord injury in mice: Limited effect of treadmill training. Journal of Spinal Cord Medicine 39, 335–343. 10.1080/10790268.2015.1133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverungen H, Klaszky SC, Klaszky M, Côté M-P, 2019. Rehabilitation Decreases Spasticity by Restoring Chloride Homeostasis through the Brain-Derived Neurotrophic Factor–KCC2 Pathway after Spinal Cord Injury. Journal of Neurotrauma. 10.1089/neu.2019.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, 2010. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nature Medicine 16, 302 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE, 2009. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147, 265–276. 10.1038/nm.210710.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacur M, Lambertz D, Hoheisel U, Mense S, 2009. Role of spinal microglia in myositisinduced central sensitisation: An immunohistochemical and behavioural study in rats. European Journal of Pain 13, 915–923. 10.1016/j.ejpain.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Chen B, Li Y, Yu B, Zhang Z, Brommer B, Williams PR, Liu Y, Hegarty SV, Zhou S, Zhu J, 2018. Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell 174, 521–535. e513 10.1016/j.cell.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M, 2007. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. European Journal of Pain 11, 223–230. 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Côté M-P, Gandhi S, Zambrotta M, Houlé JD, 2014. Exercise Modulates Chloride Homeostasis after Spinal Cord Injury. Journal of Neuroscience 34, 8976–8987. 10.1523/jneurosci.0678-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Grau JW, 2001. Preserving and Restoring Behavioral Potential Within the Spinal Cord Using an Instrumental Training Paradigm. Journal of Neurophysiology 86, 845–855. 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- David S, Kroner A, 2011. Repertoire of microglial and macrophage responses after spinal cord injury. Nature Reviews Neuroscience 12, 388 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR, 1998. Locomotor Capacity Attributable to Step Training Versus Spontaneous Recovery After Spinalization in Adult Cats. Journal of Neurophysiology 79, 1329–1340. 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM, 2008. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Experimental Neurology 212, 337–347. 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houlé JD, 2014. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Experimental Neurology 255, 38–48. 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG, 2008. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Experimental Neurology 209, 378–388. 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak MF, Cheng CL, Fallah N, Santos A, Atkins D, Humphreys S, Rivers CS, White BAB, Ho C, Ahn H, Kwon BK, Christie S, Noonan VK, 2017. Spinal Cord Injury Clinical Registries: Improving Care across the SCI Care Continuum by Identifying Knowledge Gaps. Journal of Neurotrauma. 10.1089/neu.2016.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW, 2006. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience 141, 421–431. 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Turtle JD, Huie JR, Lee KH, Hook MA, Woller SA, Grau JW, 2011. Intermittent noxious stimulation following spinal cord contusion injury impairs locomotor recovery and reduces spinal brain-derived neurotrophic factor–tropomyosin-receptor kinase signaling in adult rats. Neuroscience 199, 86–102. 10.1016/j.neuroscience.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW, 2007. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience 148, 893–906. 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL, 1998. Instrumental learning within the spinal cord: I. Behavioral properties. Behavioral Neuroscience 112, 1366–1386. 10.1037/0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC, 2006. Instrumental Learning Within the Spinal Cord: Underlying Mechanisms and Implications for Recovery After Injury. Behavioral and Cognitive Neuroscience Reviews 5, 191–239. 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Huie JR, Lee KH, Hoy KC, Huang Y-J, Turtle JD, Strain MM, Baumbauer KM, Miranda RM, Hook MA, Ferguson AR, Garraway SM, 2014. Metaplasticity and Behavior: How Training and Inflammation Affect Plastic Potential within the Spinal Cord and Recovery after Injury. Frontiers in Neural Circuits 8 10.3389/fncir.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE, 2009. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience 161, 895–903. 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Unabia GC, Hulsebosch CE, 2009. Activation of p-38α MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Experimental Neurology 220, 154–161. 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG, 2006. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. Journal of Neuroscience 26, 4308–4317. 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbargen T, Ahmed MM, Miranpuri G, Li L, Kahle KT, Resnick D, Sun D, 2010. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Annals of the New York Academy of Sciences 1198, 168–172. 10.1111/j.1749-6632.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Chacur M, Treede R-D, Mense S, 2019. Action potentials and subthreshold potentials of dorsal horn neurons in a rat model of myositis: a study employing intracellular recordings in vivo. Journal of Neurophysiology 122, 632–643. 10.1152/jn.00338.2018. [DOI] [PubMed] [Google Scholar]
- Huie JR, Baumbauer KM, Lee KH, Bresnahan JC, Beattie MS, Ferguson AR, Grau JW, 2012a. Glial Tumor Necrosis Factor Alpha (TNFα) Generates Metaplastic Inhibition of Spinal Learning. Plos One 7, e39751 10.1371/journal.pone.0039751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Garraway SM, Baumbauer KM, Hoy KC Jr, Beas BS, Montgomery KS, Bizon JL, Grau JW, 2012b. Brain-derived neurotrophic factor promotes adaptive plasticity within the spinal cord and mediates the beneficial effects of controllable stimulation. Neuroscience 200, 74–90. 10.1016/j.neuroscience.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM, 2009. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Research Reviews 60, 202–213. 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR, 2008. Step Training Reinforces Specific Spinal Locomotor Circuitry in Adult Spinal Rats. Journal of Neuroscience 28, 7370–7375. 10.1523/jneurosci.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey-Gauthier R, Josset N, Bretzner F, Leblond H, 2018a. Facilitation of Locomotor Spinal Networks Activity by Buspirone after a Complete Spinal Cord Lesion in Mice. Journal of Neurotrauma 35, 2208–2221. 10.1089/neu.2017.5476. [DOI] [PubMed] [Google Scholar]
- Jeffrey-Gauthier R, Piche M, Leblond H, 2018b. H-reflex disinhibition by lumbar muscle inflammation in a mouse model of spinal cord injury. Neuroscience Letters 690, 36–41. 10.1016/j.neulet.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Jeffrey-Gauthier R, Piché M, Leblond H, 2017. Lumbar muscle inflammation alters spinally mediated locomotor recovery induced by training in a mouse model of complete spinal cord injury. Neuroscience 359, 69–81. 10.1016/j.neuroscience.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Keller AV, Hainline C, Rees K, Krupp S, Prince D, Wood BD, Shum-Siu A, Burke DA, Petruska JC, Magnuson DSK, 2019. Nociceptor-dependent locomotor dysfunction after clinically-modeled hindlimb muscle stretching in adult rats with spinal cord injury. Experimental Neurology 318, 267–276. 10.1016/j.expneurol.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AVP, Wainwright G, Shum-Siu A, Prince D, Hoeper A, Martin E, Magnuson DSK, 2016. Disruption of Locomotion in Response to Hindlimb Muscle Stretch at Acute and Chronic Time Points after a Spinal Cord Injury in Rats. Journal of Neurotrauma 34, 661–670. 10.1089/neu.2015.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomoto M, Shinoda M, Okada-Ogawa A, Noma N, Shibuta K, Tsuboi Y, Sessle BJ, Imamura Y, Iwata K, 2013. Fractalkine Signaling in Microglia Contributes to Ectopic Orofacial Pain following Trapezius Muscle Inflammation. The Journal of Neuroscience 33, 7667–7680. 10.1523/JNEUROSCI.4968-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond H, L’Espérance M, Orsal D, Rossignol S, 2003. Treadmill Locomotion in the Intact and Spinal Mouse. Journal of Neuroscience 23, 11411–11419. 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liabeuf S, Stuhl-Gourmand L, Gackiere F, Mancuso R, Sanchez Brualla I, Marino P, Brocard F, Vinay L, 2017. Prochlorperazine increases KCC2 function and reduces spasticity after spinal cord injury. Journal of Neurotrauma 34, 3397–3406. 10.1089/neu.2017.5152. [DOI] [PubMed] [Google Scholar]
- Lin CR, Cheng JK, Wu CH, Chen KH, Liu CK, 2017. Epigenetic suppression of potassium-chloride co-transporter 2 expression in inflammatory pain induced by complete Freund’s adjuvant (CFA). European Journal of Pain 21, 309–321. 10.1002/ejp.925. [DOI] [PubMed] [Google Scholar]
- Lin T, Li K, Zhang F-Y, Zhang Z-K, Light AR, Fu K-Y, 2007. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. Journal of Neuroimmunology 192, 40–48. 10.1016/j.jneuroim.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Álvarez VM, Modol L, Navarro X, Cobianchi S, 2015. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain 156, 1812–1825. 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J, 2008. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with downregulation of the chloride transporter KCC2 in rat. Journal of Physiology 586, 5701–5715. 10.1113/jphysiol.2008.152348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Rakerd B, 1982. Induction of hindlimb stepping movements in rats spinally transected as adults or as neonates. Brain Research 240, 353–356. 10.1016/0006-8993(82)90235-9. [DOI] [PubMed] [Google Scholar]
- Mòdol L, Cobianchi S, Navarro X, 2014. Prevention of NKCC1 phosphorylation avoids downregulation of KCC2 in central sensory pathways and reduces neuropathic pain after peripheral nerve injury. Pain 155, 1577–1590. 10.1016/j.pain.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Modol L, Mancuso R, Ale A, Francos Quijorna I, Navarro X, 2014. Differential effects on KCC2 expression and spasticity of ALS and traumatic injuries to motoneurons. Frontiers in Cellular Neuroscience 8 10.3389/fncel.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA, 2004. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. European Journal of Neuroscience 20, 467–473. 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Reinert A, Kaske A, Mense S, 1998. Inflammation-induced increase in the density of neuropeptide-immunoreactive nerve ending in rat skeletal muscle. Experimental Brain Research 121, 174–180. 10.1007/s002210050449. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K, 2005. Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. Journal of Physiology 562 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AS, Freitas MF, Rocha IRC, Chacur M, 2017. Gabapentin decreases microglial cells and reverses bilateral hyperalgesia and allodynia in rats with chronic myositis. European Journal of Pharmacology 799, 111–117. 10.1016/j.ejphar.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Sánchez-Brualla I, Boulenguez P, Brocard C, Liabeuf S, Viallat-Lieutaud A, Navarro X, Udina E, Brocard F, 2017. Activation of 5-HT2A receptors restores KCC2 function and reduces neuropathic pain after spinal cord injury. Neuroscience. 10.1016/j.neuroscience.2017.08.033. [DOI] [PubMed] [Google Scholar]
- Shin HY, Kim H, Kwon MJ, Hwang DH, Lee K, Kim BG, 2014. Molecular and Cellular Changes in the Lumbar Spinal Cord following Thoracic Injury: Regulation by Treadmill Locomotor Training. Plos One 9, e88215 10.1371/journal.pone.0088215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ, 2003. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257. 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Sławińska U, Miazga K, Jordan LM, 2014. The role of serotonin in the control of locomotor movements and strategies for restoring locomotion after spinal cord injury. Acta Neurobiologiae Experimentalis 74, 172–187. [DOI] [PubMed] [Google Scholar]
- Sun X, Zeng H, Wang Q, Yu Q, Wu J, Feng Y, Deng P, Zhang H, 2018. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Experimental Cell Research 369,: 112–119. 10.1016/j.yexcr.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Tashiro S, Shinozaki M, Mukaino M, Renault-Mihara F, Toyama Y, Liu M, Nakamura M, Okano H, 2015. BDNF Induced by Treadmill Training Contributes to the Suppression of Spasticity and Allodynia After Spinal Cord Injury via Upregulation of KCC2. Neurorehabilitation and Neural Repair 29, 677–689. 10.1177/1545968314562110. [DOI] [PubMed] [Google Scholar]
- Touj S, Houle S, Ramla D, Jeffrey-Gauthier R, Hotta H, Bronchti G, Martinoli M-G, Piché M, 2017. Sympathetic regulation and anterior cingulate cortex volume are altered in a rat model of chronic back pain. Neuroscience 352, 9–18. 10.1016/j.neuroscience.2017.03.047. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamoto T, Abe Y, Saito N, Kumagai T, Kayama H, 1999. Differential activation of microglia after experimental spinal cord injury. Journal of Neurotrauma 16, 255–265. 10.1089/neu.1999.16.255. [DOI] [PubMed] [Google Scholar]
- Wu L-A, Huang J, Wang W, Wang W, Wang X-J, Wu S-X, 2009. Down-regulation of K+–C1– co-transporter 2 in mouse medullary dorsal horn contributes to the formalin-induced inflammatory orofacial pain. Neuroscience Letters 457, 36–40. 10.1016/j.neulet.2009.03.107. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu LY, Xu TL, 2008. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience 152, 502–510. 10.1016/j.neuroscience.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC, 2007. Extracellular Signal-Regulated Kinase-Regulated Microglia-Neuron Signaling by Prostaglandin E2 Contributes to Pain after Spinal Cord Injury. Journal of Neuroscience 27, 2357–2368. 10.1523/jneurosci.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X-H, Zhang T, Li Y-Q, 2015. The up-regulation of spinal Toll-like receptor 4 in rats with inflammatory pain induced by complete Freund’s adjuvant. Brain Research Bulletin 111, 97–103. 10.1016/j.brainresbull.2015.01.002. [DOI] [PubMed] [Google Scholar]


