Abstract
Substance abuse is a chronic, relapsing disorder characterized by compulsive drug use regardless of negative consequences. Incremental increases in pregabalin abuse have been observed in Saudi Arabia and throughout the world. In previous studies, the potential for pregabalin abuse with escalating doses of the drug (30, 60, 90, and 120 mg/kg) were investigated in male mice. Notably, researchers have argued that women may exhibit a greater tendency to consume drugs without a prescription to alleviate stress and depression. Moreover, female subjects are more prone to impulsivity in drug intake or abuse than their male counterparts. Therefore, in the present study, we compared the potential for pregabalin abuse between male and female mice using a conditioned place preference paradigm. Male and female BALB/c mice were divided into four groups based on the pregabalin dose administered (30, 60, 90, or 120 mg/kg, intraperitoneal). Preference scores were then calculated and compared between male and female mice in each dosage group. Interestingly, preference scores were significantly higher in female mice than in male mice at dosages of 30 and 120 mg/kg. These findings indicate that female mice may be more prone to pregabalin abuse and tolerance than male mice. These results might be helpful to the healthcare providers and policymakers to consider these sex differences in choosing therapeutic plans and consider alternatives to the misused prescription medications.
Keywords: Pregabalin, Addiction, Conditioned place preference, Sex differences, Drug abuse
Abbreviations: GABA, gamma-aminobutyric acid; CPP, conditioned place preference; ER, estradiol receptor; mGluR, metabotropic glutamate receptor
1. Introduction
Drug addiction is a major health problem worldwide. While previous reports have documented addiction to various substances of abuse such as amphetamine, heroin, alcohol, and cannabis in Saudi Arabia (Bassiony 2013), more recent studies have revealed a progressive increase in the abuse and misuse of prescription drugs (Maier and Schaub, 2015, Brady et al., 2016, Schifano et al., 2018). Among the most abused drugs in recent years is pregabalin—a gamma-aminobutyric acid (GABA) analog that binds to the alpha-2 delta protein in presynaptic voltage-dependent calcium channels (Carmeliet and Jain 2011). Pregabalin is used in the treatment of several diseases including generalized anxiety disorder, partial epilepsy, fibromyalgia, postherpetic neuralgia, and diabetic neuropathy (Boschen, 2011, Feltner et al., 2011, Pexton et al., 2011). Despite extensive research to suggest that misuse of pregabalin has increased considerably in the United States and Europe (Al-Husseini et al., 2018, Evoy et al., 2019), factors influencing pregabalin addiction remain to be fully elucidated.
Although addiction affects both men and women, several studies have reported sex-based differences in the ways in which it modulates various neurotransmitter systems in the brain (Lynch, 2006, Becker and Hu, 2008, Becker and Taylor, 2008, Becker et al., 2012, Bobzean et al., 2014). Accumulating evidence demonstrates that sex-related differences impact all phases of drug abuse, including initiation, escalation, addiction, and relapse following abstinence (Etten et al., 1999, Van Etten and Anthony, 2001, Lynch et al., 2002, Carroll et al., 2004). While these differences may vary among certain classes of misused drugs, they are largely similar for all drugs of abuse (Becker and Hu 2008).
Cumulative evidence suggests that women tend to abuse drugs at lower doses than men, although their risk of relapse is higher following abstinence (Becker and Hu, 2008, Ruda-Kucerova et al., 2015). Additional studies have reported that women are more likely than men to abuse cocaine, opioids, marijuana, and alcohol (Brady and Randall, 1999, Randall et al., 1999, Hernandez-Avila et al., 2004). Drug dependence also appears to be more difficult to reverse in women than in men (Lynch et al., 2002, Back et al., 2005, Breese et al., 2005, Carpenter et al., 2006). However, the overall percentage of women engaging in drug abuse is lower that reported for men (Becker and Hu 2008). Despite this trend, the number of women using and abusing both prescription and illegal drugs has increased in recent years (Becker and Hu 2008). Researchers have suggested that these phenomena may be due to the more profound effects of drug abuse/dependence among men than women, and that sex-based differences in drug abuse may reflect variations in opportunity rather than vulnerability (Etten et al., 1999, Van Etten and Anthony, 2001).
The influence of sex on drug abuse has been investigated in both humans and animal models for many years (Cailhol and Mormede, 1999, Etten et al., 1999, Lynch and Carroll, 1999). Recently, we observed pregabalin preference in male mice utilizing the conditioned place preference (CPP) paradigm (Althobaiti et al., 2019). The conditioned place preference (CPP) paradigm is widely used as a tool to explore the reinforcing effects of many conditioning stimuli including drugs of abuse (Tzschentke 2007). This paradigm has been used to explore different pharmacological and genetical manipulation and its role in animal behaviors (McBride et al., 1999, Sakoori and Murphy, 2004, Müller et al., 2007, Brown et al., 2008). The conditioning process includes a series of repetitive sessions in the drug paired chamber in order to build an association between the rewarding/reinforcing stimuli and the chamber cues (Cunningham et al., 2006, Tzschentke, 2007, Aguilar et al., 2009). There are some advantages using the CPP over other models, such as CPP paradigm does not require surgical procedure which minimizes the amount of stress and discomfort in animals (Carr et al., 1989). However, to the best of our knowledge, no published studies have directly compared pregabalin preference between male and female mice using the CPP paradigm. Therefore, in the present study, we investigated the potential impact of sex differences on pregabalin-seeking behavior in BALB/c mice.
2. Materials and methods
2.1. Animals
Male and female BALB/c mice weighing 25–30 g were purchased from King Fahd Medical Research Center (Saudi Arabia). The mice were placed under a 12-h light/dark cycle in a temperature and humidity controlled enviroment. Mice were allowed ad libitum access to food and water throughout the experiments. Mice were acclimated to the environment for 7 days prior to any experiments. The experimental procedures of the present study were approved by the ethical committee of our institution, in accordance with the guidelines issued by the Institutional Animal Care and Use Committee of the National Institutes of Health.
2.2. Drugs and animal dosing
Pregabalin was obtained from Jamjoom Pharmaceuticals (Jeddah, Saudi Arabia), following which it was diluted in 0.9% NaCl. Mice were randomly divided into four groups, each of which included six males and six females. Each group received a fixed dose of pregabalin, as follows: group 1 (30 mg/kg, intraperitoneal (i.p.)), group 2 (60 mg/kg, i.p.), group 3 (90 mg/kg, i.p.), and group 4 (120 mg/kg, i.p.). Animals were injected with pregabalin one day and vehicle the next during the conditioning phase, resulting in four days of pregabalin treatment and four days of vehicle treatment. Following the conditioning phase, we investigated place preferences among mice in each group (Fig. 1A). The doses of 30, 60, 90 and 120 mg/kg/day, which are small as compared to the LD50 of pregabalin in mice (>5000 mg/kg), were selected based on our previous work and others (Andrews et al., 2001, Althobaiti et al., 2019). In these previous studies, the dose of 30 mg/kg did not induce any rewarding effects in male mice, so in our previous work, we have increased the dose to 60, 90, and 120 mg/kg (escalating doses) (Althobaiti et al., 2019). We chose the 8-day treatment as we did in our previous studies. In addition, the estrous cycle length in most laboratory mice is 4–5 days and the experiment performed here was 12 days long, so mice likely went through 2–3 estrous cycles during this period.
Fig. 1.
(A) Experimental design of the conditioned place preference (CPP) paradigm. (B) Baseline preference scores for all female and male mice prior to any treatments. No significant differences in preference scores were observed between female and male mice at baseline. (Note: All values are presented as the mean ± standard error of the mean (S.E.M.) (n = 6 mice/group).
2.3. CPP paradigm
The CPP device consisted of two conditioning chambers of identical size (35 cm × 35 cm × 50 cm) and one start box (10 cm × 15 cm × 10 cm) located outside of the CPP apparatus, as previously described (Althobaiti et al., 2019). The inside walls of chamber 1 were white with horizontal black lines, the walls were textured, and the floor was perforated with round holes. The inside walls of chamber 2 were black with vertical white lines, the walls were smooth, and the floor was perforated with rectangular holes. The habituation phase (days 1 to 3) and condititioning phase (days 4 to 11) were performed as previously described (Althobaiti et al., 2019).
On day 12 (post-test), mice were permitted to enter the conditioning chambers freely for 30 min, and the time spent in each chamber was recorded using the ANY-maze video tracking system (Stoelting Co; IL, USA). Preference scores were then calculated based on the time spent in each chamber during the pre- and post-tests, as follows: Preference score = time spent in pregabalin-paired chamber – time spent in saline-paired chamber.
2.4. Statistical analysis
Unpaired t-tests were used to compare preference scores between males and females. GraphPad Prism was for all analyses, and the level of statistical significance was set to p < 0.05.
3. Results
Unpaired Samples t-tests were used to compare preference scores between male and female mice during the pre-test, as shown in Fig. 1B. There were no significant differences in preference scores between female (M = 5.267) and male mice (M = 6.779) at this stage (t(46) = 0.01993, p = 0.9842).
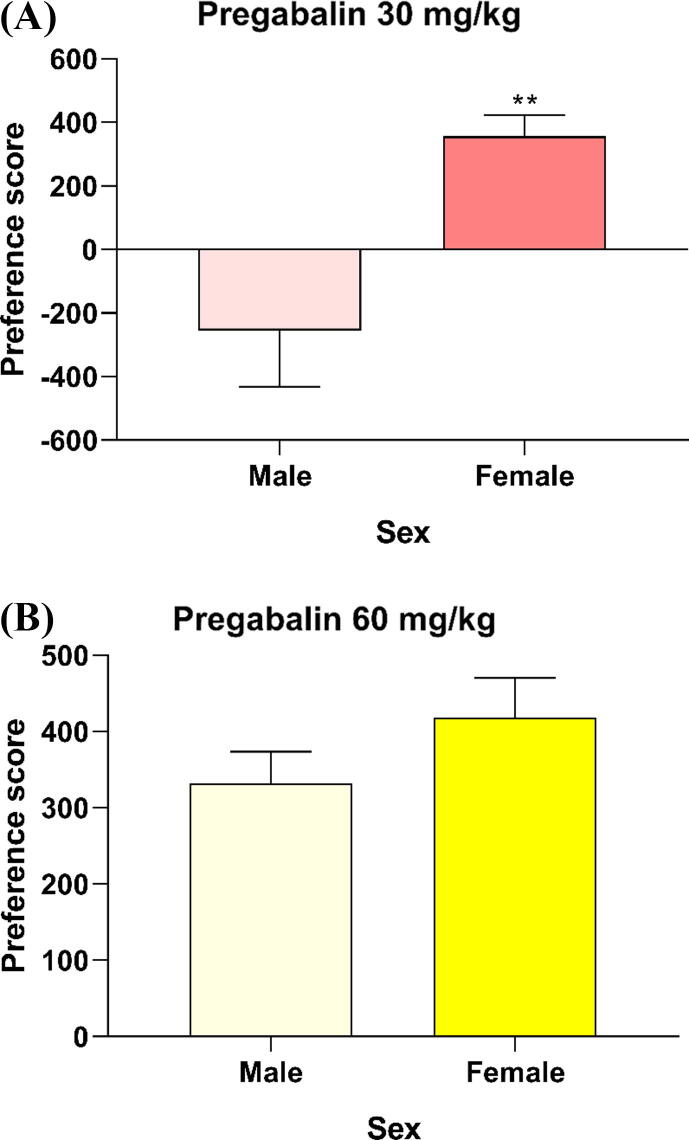
As shown in Fig. 2A, an unpaired Samples t-test revealed significant increases in preference scores among female mice in the 30 mg/kg group (M = 356.8), when compared to those observed for male mice (M = 254.4) (t(10) = 3.223, p = 0.0091). However, no significant differences in preference scores were observed between male (M = 331.8) and female mice (M = 418.4) following treatment with 60 mg/kg of pregabalin (t(10) = 1.302, p = 0.2221) (Fig. 2B). Similar results were observed when mice were treated with 90 mg/kg of pregabalin (females, M = 533.6; males, M = 572.8; t(10) = 0.1596, p = 0.8764) (Fig. 3A). Interestingly, preference scores were significantly higher in females (M = 874.3) than in males (M = 253.4) following administration of 120 mg/kg of pregabalin (t(10) = 3.203, p = 0.0094) (Fig. 3B).
Fig. 2.
Mice received intraperitoneal (i.p.) injections of pregabalin (30 mg/kg, 4 days) and vehicle (10 ml/kg, 4 days) for 8 days throughout the acquisition phase, as shown in panel (A). As shown in panel (B), mice in this group received intraperitoneal injections of pregabalin (60 mg/kg, 4 days) and vehicle (10 ml/kg, 4 days) for 8 days throughout the acquisition phase. Subsequently, place preference was assessed in all groups following the conditioning phase. (A) Preference scores were significantly higher in female mice than in male mice at 30 mg/kg. However, (B) no significant differences in preference score were observed between male and female mice at a pregabalin dose of 60 mg/kg. (Note: All values are presented as the mean ± standard error of the mean (S.E.M.) (n = 6 mice/group, **p < 0.01).
Fig. 3.
Mice received intraperitoneal (i.p.) injections of pregabalin (90 mg/kg, 4 days) and vehicle (10 ml/kg, 4 days) for 8 days throughout the acquisition phase, as shown in panel (A). As shown in panel (B), mice in this group received intraperitoneal injections of pregabalin (120 mg/kg, 4 days) and vehicle (10 ml/kg, 4 days) for 8 days throughout the acquisition phase. Subsequently, place preference was assessed in all groups following the conditioning phase. (A) No significant differences in preference score were observed between male and female mice at a pregabalin dose of 90 mg/kg. However, (B) preference scores were significantly higher in female mice than in male mice at 120 mg/kg. (Note: All values are presented as the mean ± standard error of the mean (S.E.M.) (n = 6 mice/group, **p < 0.01).
4. Discussion
In this study, we investigated the potential impact of sex differences on pregabalin-seeking behavior in BALB/c mice. Preference scores for the pregabalin-paired chamber were significantly higher among female mice than among their male counterparts at doses of 30 and 120 mg/kg. Previous study reported that pregabalin induces place preference in a mouse model of drug addiction (Althobaiti et al., 2019). However, in most previous studies, the maximum dose used to examine the rewarding effects of pregabalin was 30 mg/kg—a small dose that may fail to induce any rewarding effects or place preference (Andrews et al., 2001, Rutten et al., 2011). For example, Andrews et al. reported that increasing pregabalin to 30 mg/kg did not induce any place preference in male rats (Andrews et al., 2001). Consistent with these findings, no changes in place preference among male mice at a pregabalin dose of 30 mg/kg have been detected in male mice in previous study. However, pregabalin doses of 60 and 90 mg/kg induced a significant place preference, while a dose of 120 mg/kg did not in male mice (Althobaiti et al., 2019). This finding indicates that the rewarding effects of pregabalin are dose-related, consistent with the findings of previous clinical studies (Drug Enforcement Administration, 2005, Lang et al., 2006, Chua et al., 2012, Chew et al., 2014). Interestingly, in the present study, preference scores for the pregabalin-paired chamber were significantly higher among female mice than among their male counterparts at doses of 30 and 120 mg/kg, although there were no significant differences between males and females at doses of 60 or 90 mg/kg. This finding may be explained by the remarkable increases in preference scores among male mice at the 60 and 90 mg/kg doses, consistent with recent findings (Althobaiti et al., 2019).
Numerous research groups have utilized animal models to investigate the influence of sex differences on drug abuse (Becker and Ramirez, 1981, Lynch and Carroll, 1999, Russo et al., 2003, Zakharova et al., 2009, Hilderbrand and Lasek, 2014). Different brain regions may play essential roles in the rewarding properties of drugs of abuse at different points throughout the menstrual cycle, with more stimulation occurring throughout the mid-follicular phase when levels of estrogen are highest (Dreher et al., 2007). Estrogen plays a crucial role in cocaine reward, whereas progesterone can block cocaine reward in rats and humans (Russo et al., 2008, Evans and Foltin, 2010). In ovariectomized rats, estradiol itself can express CPP (Frye and Rhodes 2006), increase cocaine CPP (Segarra et al., 2010, Twining et al., 2013), and enhance the sensitivity of the brain to reward (Galankin et al., 2010), whereas the estradiol antagonist tamoxifen can block the acquisition in healthy females (Lynch 2006). In addition, previous studies have demonstrated that the cocaine-seeking effect can be elicited in female mice at lower doses and with fewer conditioning sessions than required for males (Russo et al., 2003, Zakharova et al., 2009). Furthermore, female rats in estrus exhibit a preference for a more substantial amount of cocaine than that preferred by males or females in further phases of the estrus cycle (Lynch et al., 2000, Lynch, 2006). Moreover, individual reactions to cocaine and d-amphetamine are enhanced during the follicular phase (i.e., when progesterone and estradiol levels are low) when compared to those observed in the luteal phase (i.e., when progesterone levels are high and estradiol levels are moderate) (Evans and Foltin 2010). Given that the estrus cycle lasts 4 to 5 days in most laboratory mice (Byers et al., 2012), female mice in the present study likely completed 2 to 3 estrus cycles. As previously mentioned, ovarian hormones may affect pregabalin reward; however, we did not examine the estrus cycle of female mice throughout the experiments. The vaginal lavage process may affect pregabalin CPP results, as previous studies have suggested that it may be a reinforcing stimulus during the proestrus and estrus cycles, which may have in turn confounded our results (Romeo et al., 2000, Walker et al., 2002). Taken together, however, the present and previous findings suggest that females may be more sensitive to the addictive properties of pregabalin than males. Given that variations in levels of ovarian hormones can also influence the rewarding properties of pregabalin, the stage of the estrus cycle may also alter the experience of pregabalin-induced reward, which may in turn influence the extent of pregabalin use in females.
Accumulating evidence has highlighted the modulatory impact of ovarian hormones on dopamine release in the central dopaminergic system (Chen et al., 2003). Estrogen is known to increase dopamine release in the nucleus accumbens (Thompson and Moss 1997). The classical estradiol receptors (ERs) act at the cell membrane in the brain and mediate rapid responses to estradiol (Becker and Hu 2008). In adult females, the influence of estradiol in the dorsal striatum has been reported to be mediated through ERs on medium spiny GABA neurons that lower GABA release, thus improving the potential for dopamine release (Mermelstein et al., 1996, Mermelstein, 2009, Schultz et al., 2009, Grove-Strawser et al., 2010). In female rats, estradiol rapidly down-regulates dopamine D2 receptor binding and regulates dopamine D1 receptor binding in the dorsal striatum, and the activation of D1 rather than D2 receptors is known to mediate rewarding effects (Lévesque et al., 1989, Bazzett and Becker, 1994, E Yoest et al., 2014). Calipari et al. noted that estradiol may increase dopamine release in the nucleus accumbens and basal ventral tegmental area, suggesting that dopamine neuron activity is enhanced in female mice during the estrus cycle, as well as an essential role of estradiol in the reward pathway (Calipari et al., 2017). Additional studies have reported that estradiol can block dopamine uptake, indicating that estradiol may be responsible for increases in dopamine release, dopamine neuron firing, and phosphorylation of dopamine transporters (Calipari et al., 2017). The Mermelstein laboratory has demonstrated that ERα and ERβ in the dorsal striatum can combine with caveolin protein in particular metabotropic glutamate receptors (mGluRs) in order to regulate dopamine release indirectly (Boulware et al., 2007, Mermelstein, 2009, Grove-Strawser et al., 2010, Meitzen and Mermelstein, 2011). Collectively, our data indicate that sex differences influence the expression of pregabalin preference in mice, thus mediating direct or indirect modifications of dopamine activity within the reward circuit. Further studies are required to investigate the neurochemical changes that occur in response to pregabalin, and to determine whether these changes differ between males and females (Becker and Ramirez, 1981, BeCker and Cha, 1989).
Researchers have argued that learning and memory processes provide the foundation for the development of CPP (Hsu et al., 2002, McIntyre et al., 2002). Ovarian hormones influence the activation of learning and memory processes, and ovarian hormone replacement or ovariectomy in female rats has been shown to impact the development of cocaine-induced CPP in learning and memory tasks (Farr et al., 1995, Gibbs, 2000, Johansson et al., 2002, Russo et al., 2008). Progesterone can inhibit avoidance conditioning, whereas allopregnanolone (an active progesterone metabolite) can inhibit learning in the Morris water maze test (Farr et al., 1995, Johansson et al., 2002). On the other hand, co-administration of progesterone and estrogen increases the ability to learn a spatial memory task in ovariectomized rats (Gibbs 2000). Several studies have provided evidence that hormone-dependent alterations in dopamine and serotonin activity play key roles in learning and memory processes (Fink et al., 1996, McEwen et al., 1997, Bowman et al., 1999, Heikkinen et al., 2002, Segarra et al., 2010). Therefore, ovarian hormones may influence storage and recall capabilities in relation to the rewarding effects in CPP paradigms via alterations in serotonergic/dopaminergic systems, which are directly connected to sex-based differences in drug abuse (Becker and Chartoff 2019). Despite evidence for the influence of sex differences on substance abuse behavior obtained from studies of neural systems development (Becker and Chartoff 2019), further studies are required to determine the molecular and genetic mechanisms that underlie sex differences in addictive behavior (Hilderbrand and Lasek 2014). Despite the reliability of the mouse as an animal model for genetic research, few such studies have been conducted (Hilderbrand and Lasek 2014).
5. Conclusion
In summary, our findings support the notion that sex differences play an essential role in producing pregabalin preference in mice. Ovarian hormones may represent the source of this variation, given that estradiol may influence the addictive properties of pregabalin in female mice. In addition, neuroendocrine mechanisms may mediate the influence of estrogen on the dopaminergic system to drive differences in pregabalin reward between male and female mice. Future studies should utilize ovariectomized BALB/c mice treated with estradiol to determine whether estradiol influences the expression of pregabalin CPP. Additional studies are required to identify potential interactions between estrogen and mesolimbic dopamine signaling, as well as their effects on pregabalin reward. Such studies will ultimately advance our understanding of sex-related differences in pregabalin abuse.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments and funding
This work was supported by Research group grant 1-439-6079 from the Deanship of Scientific Research at Taif University in Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aguilar M.A., Rodríguez-Arias M., Miñarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res. Rev. 2009;59(2):253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Al-Husseini A., Van Hout M.C., Wazaify M. Pregabalin misuse and abuse: a scoping review of extant literature. J. Drug Issues. 2018;48(3):356–376. [Google Scholar]
- Althobaiti Y.S., Almalki A., Alsaab H., Alsanie W., Gaber A., Alhadidi Q., Hardy A.M.G., Nasr A., Alzahrani O., Stary C.M. Pregabalin: potential for addiction and a possible glutamatergic mechanism. Sci. Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-51556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N., Loomis S., Blake R., Ferrigan L., Singh L., McKnight A.T. Effect of gabapentin-like compounds on development and maintenance of morphine-induced conditioned place preference. Psychopharmacology. 2001;157(4):381–387. doi: 10.1007/s002130100839. [DOI] [PubMed] [Google Scholar]
- Back S.E., Brady K.T., Jackson J.L., Salstrom S., Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180(1):169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Bassiony M. Substance use disorders in Saudi Arabia. J. Substance Use. 2013;18(6):450–466. [Google Scholar]
- Bazzett, T.J., Becker, J.B., 1994. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. [DOI] [PubMed]
- BeCker J.B., Cha J.-H. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav. Brain Res. 1989;35(2):117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker J.B., Chartoff E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 2019;44(1):166–183. doi: 10.1038/s41386-018-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Perry A.N., Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol. Sex Differences. 2012;3(1):14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.B., Ramirez V. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204(2):361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Becker, J.B., Taylor J.R., 2008. Sex differences in motivation. Becker, JB.; Berkley, K.; Geary, N: 177–199.
- Bobzean S.A., DeNobrega A.K., Perrotti L.I. Sex differences in the neurobiology of drug addiction. Exp. Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Boschen M.J. A meta-analysis of the efficacy of pregabalin in the treatment of generalized anxiety disorder. Can. J. Psychiatry. 2011;56(9):558–566. doi: 10.1177/070674371105600907. [DOI] [PubMed] [Google Scholar]
- Boulware M.I., Kordasiewicz H., Mermelstein P.G. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 2007;27(37):9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B.P., Vaughan S.R., Walker Q.D., Davis S.L., Little P.J., Scheffler N.M., Thomas B.F., Kuhn C.M. Effects of sex and gonadectomy on cocaine metabolism in the rat. J. Pharmacol. Exp. Ther. 1999;290(3):1316–1323. [PubMed] [Google Scholar]
- Brady K.T., McCauley J.L., Back S.E. Prescription opioid misuse, abuse, and treatment in the United States: an update. Am. J. Psychiatry. 2016;173(1):18–26. doi: 10.1176/appi.ajp.2015.15020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K.T., Randall C.L. Gender differences in substance use disorders. Psychiatr. Clin. North Am. 1999;22(2):241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Breese G.R., Chu K., Dayas C.V., Funk D., Knapp D.J., Koob G.F., Lê D.A., O’Dell L.E., Overstreet D.H., Roberts A.J. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol. Clin. Exp. Res. 2005;29(2):185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.E., Lee B.R., Sorg B.A. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn. Memory. 2008;15(12):857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers S.L., Wiles M.V., Dunn S.L., Taft R.A. Mouse estrous cycle identification tool and images. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S., Mormede P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999;842(1):200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Calipari E.S., Juarez B., Morel C., Walker D.M., Cahill M.E., Ribeiro E., Roman-Ortiz C., Ramakrishnan C., Deisseroth K., Han M.-H. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017;8(1):1–15. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Jain R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discovery. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- Carpenter M.J., Upadhyaya H.P., LaRowe S.D., Saladin M.E., Brady K.T. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob. Res. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Carr, G.D., Fibiger, H.C., Phillips, A.G., 1989. Conditioned place preference as a measure of drug reward. [DOI] [PubMed]
- Carroll M.E., Lynch W.J., Roth M.E., Morgan A.D., Cosgrove K.P. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Chen H.-H., Yang Y.-K., Yeh T., Cherng C., Hsu H., Hsiao S., Yu L. Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chin. J. Physiol. 2003;46(4):169–174. [PubMed] [Google Scholar]
- Chew M.L., Plotka A., Alvey C.W., Pitman V.W., Alebic-Kolbah T., Scavone J.M., Bockbrader H.N. Pharmacokinetics of pregabalin controlled-release in healthy volunteers: effect of food in five single-dose, randomized, clinical pharmacology studies. Clin. Drug Invest. 2014;34(9):617–626. doi: 10.1007/s40261-014-0211-4. [DOI] [PubMed] [Google Scholar]
- Chua Y., Ng K., Sharma A., Jafari J., Surguy S., Yazaki E., Knowles C., Aziz Q. Randomised clinical trial: pregabalin attenuates the development of acid-induced oesophageal hypersensitivity in healthy volunteers–a placebo-controlled study. Aliment. Pharmacol. Ther. 2012;35(3):319–326. doi: 10.1111/j.1365-2036.2011.04955.x. [DOI] [PubMed] [Google Scholar]
- Cunningham C.L., Gremel C.M., Groblewski P.A. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 2006;1(4):1662. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Dreher J.-C., Schmidt P.J., Kohn P., Furman D., Rubinow D., Berman K.F. Proceedings of the National Academy of Sciences. 2007. Menstrual cycle phase modulates reward-related neural function in women; pp. 2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration D.o.J. Schedules of controlled substances: placement of pregabalin into schedule V. Final rule. Fed. Reg. 2005;70(144):43633. [PubMed] [Google Scholar]
- E Yoest K., Cummings J.A., Becker J.B. Estradiol, dopamine and motivation. Central Nervous Syst. Agents Med. Chem. (Formerly Current Medicinal Chemistry-Central Nervous System Agents) 2014;14(2):83–89. doi: 10.2174/1871524914666141226103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etten M.L.V., Neumark Y.D., Anthony J.C. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94(9):1413–1419. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- Evans S.M., Foltin R.W. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm. Behav. 2010;58(1):13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evoy K.E., Covvey J.R., Peckham A.M., Ochs L., Hultgren K.E. Reports of gabapentin and pregabalin abuse, misuse, dependence, or overdose: An analysis of the Food And Drug Administration Adverse Events Reporting System (FAERS) Res. Soc. Admin. Pharmacy. 2019;15(8):953–958. doi: 10.1016/j.sapharm.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Farr S.A., Flood J.F., Scherrer J.F., Kaiser F.E., Taylor G.T., Morley J.E. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol. Behav. 1995;58(4):715–723. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- Feltner D.E., Liu-Dumaw M., Schweizer E., Bielski R. Efficacy of pregabalin in generalized social anxiety disorder: results of a double-blind, placebo-controlled, fixed-dose study. Int. Clin. Psychopharmacol. 2011;26(4):213–220. doi: 10.1097/YIC.0b013e32834519bd. [DOI] [PubMed] [Google Scholar]
- Fink G., Sumner B.E., Rosie R., Grace O., Quinn J.P. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell. Mol. Neurobiol. 1996;16(3):325–344. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C.A., Rhodes M.E. Administration of estrogen to ovariectomized rats promotes conditioned place preference and produces moderate levels of estrogen in the nucleus accumbens. Brain Res. 2006;1067(1):209–215. doi: 10.1016/j.brainres.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Galankin T., Shekunova E., Zvartau E. Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Horm. Behav. 2010;58(5):827–834. doi: 10.1016/j.yhbeh.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Gibbs R.B. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats☆. Neurobiol. Aging. 2000;21(1):107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D., Boulware M.I., Mermelstein P.G. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170(4):1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T., Puoliväli J., Liu L., Rissanen A., Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm. Behav. 2002;41(1):22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila C.A., Rounsaville B.J., Kranzler H.R. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hilderbrand E.R., Lasek A.W. Sex differences in cocaine conditioned place preference in C57BL/6J mice. NeuroReport. 2014;25(2):105. doi: 10.1097/WNR.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E.H., Schroeder J.P., Packard M.G. The amygdala mediates memory consolidation for an amphetamine conditioned place preference. Behav. Brain Res. 2002;129(1–2):93–100. doi: 10.1016/s0166-4328(01)00376-x. [DOI] [PubMed] [Google Scholar]
- Johansson I.-M., Birzniece V., Lindblad C., Olsson T., Bäckström T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934(2):125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Lang N., Sueske E., Hasan A., Paulus W., Tergau F. Pregabalin Exerts Oppositional Effects on Different Inhibitory Circuits in Human Motor Cortex: A Double-blind, Placebo-controlled Transcranial Magnetic Stimulation Study. Epilepsia. 2006;47(5):813–819. doi: 10.1111/j.1528-1167.2006.00544.x. [DOI] [PubMed] [Google Scholar]
- Lévesque D., Gagnon S., Di Paolo T. Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci. Lett. 1989;98(3):345–350. doi: 10.1016/0304-3940(89)90426-6. [DOI] [PubMed] [Google Scholar]
- Lynch W.J. Sex differences in vulnerability to drug self-administration. Exp. Clin. Psychopharmacol. 2006;14(1):34. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Arizzi M.N., Carroll M.E. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152(2):132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Carroll M.E. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch W.J., Roth M.E., Carroll M.E. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Maier L.J., Schaub M.P. The use of prescription drugs and drugs of abuse for neuroenhancement in Europe. Eur. Psychol. 2015 [Google Scholar]
- McBride W.J., Murphy J.M., Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav. Brain Res. 1999;101(2):129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Alves S.E., Bulloch K., Weiland N.G. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48(5 Suppl 7):8S–15S. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- McIntyre C.K., Pal S.N., Marriott L.K., Gold P.E. Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J. Neurosci. 2002;22(3):1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J., Mermelstein P.G. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J. Chem. Neuroanat. 2011;42(4):236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein P.G. Membrane-localised oestrogen receptor α and β influence neuronal activity through activation of metabotropic glutamate receptors. J. Neuroendocrinol. 2009;21(4):257–262. doi: 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein P.G., Becker J.B., Surmeier D.J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16(2):595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C.P., Carey R.J., Huston J.P., Silva M.A.D.S. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog. Neurobiol. 2007;81(3):133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Pexton T., Moeller-Bertram T., Schilling J.M., Wallace M.S. Targeting voltage-gated calcium channels for the treatment of neuropathic pain: a review of drug development. Expert Opin. Invest. Drugs. 2011;20(9):1277–1284. doi: 10.1517/13543784.2011.600686. [DOI] [PubMed] [Google Scholar]
- Randall C.L., Roberts J.S., Del Boca F.K., Carroll K.M., Connors G.J., Mattson M.E. Telescoping of landmark events associated with drinking: a gender comparison. J. Stud. Alcohol. 1999;60(2):252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Romeo R.D., Diedrich S.L., Sisk C.L. Effects of gonadal steroids during pubertal development on androgen and estrogen receptor-α immunoreactivity in the hypothalamus and amygdala. J. Neurobiol. 2000;44(3):361–368. [PubMed] [Google Scholar]
- Ruda-Kucerova J., Amchova P., Babinska Z., Dusek L., Micale V., Sulcova A. Sex Differences in the Reinstatement of Methamphetamine Seeking after Forced Abstinence in Sprague-Dawley Rats. Front. Psychiatry. 2015;6(91) doi: 10.3389/fpsyt.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Jenab S., Fabian S.J., Festa E.D., Kemen L.M., Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970(1–2):214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Russo S.J., Sun W.L., Minerly A.C.E., Weierstall K., Nazarian A., Festa E.D., Niyomchai T., Akhavan A., Luine V., Jenab S. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Rutten K., De Vry J., Robens A., Tzschentke T.M., van der Kam E.L. Dissociation of rewarding, anti-aversive and anti-nociceptive effects of different classes of anti-nociceptives in the rat. Eur. J. Pain. 2011;15(3):299–305. doi: 10.1016/j.ejpain.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Sakoori K., Murphy N.P. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology. 2004;172(2):129–136. doi: 10.1007/s00213-003-1643-3. [DOI] [PubMed] [Google Scholar]
- Schifano F., Chiappini S., Corkery J.M., Guirguis A. Abuse of prescription drugs in the context of novel psychoactive substances (NPS): a systematic review. Brain Sci. 2018;8(4):73. doi: 10.3390/brainsci8040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K.N., Silke A., Hu M., Bennett A.L., Kennedy R.T., Musatov S., Toran-Allerand C.D., Kaplitt M.G., Young L.J., Becker J.B. Viral vector-mediated overexpression of estrogen receptor-α in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J. Neurosci. 2009;29(6):1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra A.C., Agosto-Rivera J.L., Febo M., Lugo-Escobar N., Menéndez-Delmestre R., Puig-Ramos A., Torres-Diaz Y.M. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm. Behav. 2010;58(1):33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T.L., Moss R.L. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci. Lett. 1997;229(3):145–148. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- Twining R.C., Tuscher J.J., Doncheck E.M., Frick K.M., Mueller D. 17β-estradiol is necessary for extinction of cocaine seeking in female rats. Learn. Memory. 2013;20(6):300–306. doi: 10.1101/lm.030304.113. [DOI] [PubMed] [Google Scholar]
- Tzschentke T.M. Review on CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van Etten M.L., Anthony J.C. Male-female differences in transitions from first drug opportunity to first use: searching for subgroup variation by age, race, region, and urban status. J. Women's Health Gender-based Med. 2001;10(8):797–804. doi: 10.1089/15246090152636550. [DOI] [PubMed] [Google Scholar]
- Walker Q.D., Nelson C.J., Smith D., Kuhn C.M. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol. Biochem. Behav. 2002;73(4):743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Zakharova E., Wade D., Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Behav. 2009;92(1):131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]