Abstract
Aim
Cardiotocography is used worldwide to evaluate fetal well‐being during pregnancy and labor. In past guidelines, the management plan was determined based on the assessment of the most severe waveform. There are no guidelines for evaluating the integrated recurrent decelerations; however, we believe their assessment to be essential for predicting the status of the fetus. The objective of this study was to propose an indicator for performing medical interventions during labor by creating a scoring system that reflects integrated recurrent decelerations.
Methods
In this retrospective cohort study, we included data for only full‐term single fetus births from vaginal deliveries. The score named the iPREFACE score (integrated score index to predict fetal acidemia by intrapartum fetal heart rate monitoring) was calculated using cardiotocography findings from continuing 30 min before delivery. We examined the iPREFACE score and fetal acidemia association and calculated the cut‐off iPREFACE scores for acidemia using receiver operating characteristic curves.
Results
The study included 469 delivery cases. Their iPREFACE scores exhibited a significant negative correlation with the umbilical artery blood pH (correlation coefficient; −0.43). The cut‐off iPREFACE scores for the umbilical artery blood with pH <7.20, <7.10 and <7.0 were 44, 46 and 67, respectively (the areas under the curve were 0.776, 0.962 and 0.996, respectively).
Conclusion
The iPREFACE score may predict fetal acidemia and could be used as an indicator for timely medical interventions during labor. Because assessments using a cardiotocography are quick and easy to perform, the iPREFACE score could be a valuable tool in clinical practice.
Keywords: cardiotocography, CTG, electronic fetal monitoring, hypoxic–ischemic encephalopathy, umbilical artery blood pH
Introduction
Cardiotocography (CTG) is widely used worldwide to evaluate fetal well‐being during pregnancy and labor. In 1958, Hon performed the first continuous measurement of fetal heart rate (FHR) during labor 1 and subsequent studies reported that FHR reflects the status of fetal hypoxemia and acidosis. 2 To interpret the CTG waveforms, the U.S. National Institute of Child Health and Human Development published a guideline in 1997, which defined baseline FHR, baseline FHR variability, acceleration and deceleration to standardize the fetal heart rate pattern. 3 Also, several countries have published guidelines on the interpretation of CTG waveforms to standardize the assessments of fetal well‐being and clinical responses. 4 , 5 , 6 , 7 , 8 Methods for evaluating fetal well‐being have been proposed by considering the baseline FHR, baseline FHR variability and deceleration waveforms. The British, Canadian, American and International Federation of Obstetrics and Gynecology guidelines use a 3‐tier classification and present responses for each. 4 , 5 , 6 , 7 , 8 However, Parer et al. proposed a 5‐tier classification in 2007, 9 which was reported to be significantly better than the 3‐tier classification in predicting fetal acidemia. 10 The Japanese guidelines were also developed based on the 5‐tier classification proposed by Parer et al., 11 and this classification was reported to correlate significantly with umbilical artery blood pH. 12
CTG is highly sensitive in predicting fetal cerebral hypoxia and acidemia, although its specificity is low. Even when many new‐borns do not exhibit cerebral hypoxia or acidemia, the CTG findings will often indicate such conditions. 13 In some past guidelines on the interpretation of the CTG waveforms, the management plan was determined based on the assessment of FHR baseline, FHR baseline variability, deceleration, in the most severe waveform. Because the fetal condition is considered to worsen gradually with recurrent uterine contractions, we speculate that it is essential to use the integrated recurrent decelerations for predicting the status of the fetus.
Our study aimed to propose an indicator for performing timely interventions during labor by constructing a scoring system that reflected integrated recurrent decelerations.
Methods
Study design
We conducted a two‐center (Toho University Omori Medical Center and Ageo Central General Hospital) retrospective cohort study of all women with full‐term vaginal delivery (gestational age 37–41 weeks') with a singleton, nonanomalous infants from September 2018 to March 2019. Data were obtained from cases in which CTG was continuously recorded using external monitors at least 30 min before delivery. We selected ‘30 min’ cut‐off time for scoring, which was determined because the median time required for second stage of labor is approximately 50 min for nulliparas and 20 min for multiparas. 14 We excluded cases in which CTG could not be continuously recorded at least 30 min before delivery or in which the CTG findings could not be evaluated accurately (e.g. the fetal heart rate waveform was frequently interrupted). Cesarean delivery was excluded because CTG findings cannot be obtained until just before delivery.
The assessments of the FHR waveform levels in this study were performed using the 5‐tier classification as defined by the Japanese Society of Obstetrics and Gynecology (JSOG) (Figure 1). 12 This FHR waveform level was determined based on three factors: FHR baseline, FHR baseline variability and deceleration. CTG findings for continuing 30 min during the second stage of labor until delivery were used for scoring. All decelerations, except prolonged deceleration, were evaluated at the level of the 5‐tier classification and the sum of all numbers of these levels was calculated as the score. Prolonged deceleration was defined as a multiplication of the duration (minutes) by the number of the level on the 5‐tier classification. The durations (minutes) were expressed as whole numbers (Figure 2).
Figure 1.

Risk levels of the 5‐tier classification as defined by the Japanese Society of Obstetrics and Gynecology.
Figure 2.
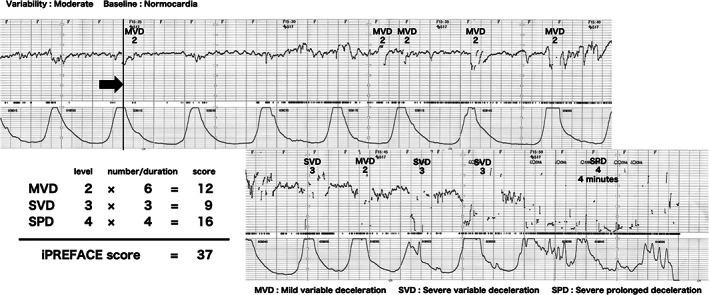
Example of scoring the iPREFACE score. iPREFACE, integrated score index to predict fetal acidemia by intrapartum fetal heart rate monitoring; MVD, mild variable deceleration; SVD, severe variable deceleration; SLD, severe late deceleration; SVD, severe prolonged deceleration.
We named this score the iPREFACE score (integrated score index to predict fetal acidemia by intrapartum fetal heart rate monitoring).
Umbilical cord blood was collected within a minute after delivery, and it was immediately measured with a blood gas apparatus located next to the delivery room.
Outcome
First, the validity of the iPREFACE score was assessed by examining its association with the postnatal umbilical artery blood pH.
Second, we calculated the cut‐off iPREFACE scores to predict the acidemia by using receiver operating characteristic (ROC) curves. The cut‐off values were calculated using the Youden index.
Third, to examine the inter‐rater reliability of the iPREFACE scores, the weighted kappa coefficient was calculated for two raters and the intraclass correlation coefficient (ICC) for three raters.
Statistical analyses
Statistical analyses were performed using SPSS Statistics software (ver. 25, SPSS Inc.). To examine the association between the iPREFACE scores and fetal acidemia, we first assessed normality using the Kolmogorov–Smirnov test, and the Pearson correlation coefficient, a parametric test, was used to determine normal distributions.
Ethical approval
This study protocol was approved by the Ethics Committee of Toho University Omori Medical Center (approval no. M18261) and Ageo Central General Hospital (approval no. 667). Informed consent was obtained in the form of opt‐out on the website. Information on the research was made public on the website of each institution, and the opportunity for the research subjects to refuse participation was guaranteed.
Results
There were 890 deliveries during the study period, of which 469 were included as subjects (Figure 3). Table 1 shows the patients' characteristics. The mean gestational age was 39.5 ± 1.1 weeks and vacuum extraction was used in 73 of the 469 deliveries (15.6%). The mean postnatal umbilical artery blood pH was 7.30 ± 0.1 (Table 1).
Figure 3.
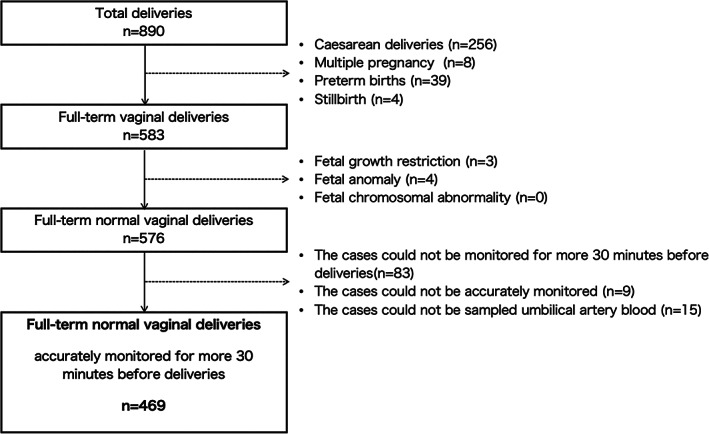
Study participants' selection flowchart.
Table 1.
Baseline characteristics
| Characteristic | Value | |||
|---|---|---|---|---|
| Maternal age(years) | 32.0 ± 5.22 | |||
| Parity (n) | ||||
| 0 | 280 | |||
| 1 | 135 | |||
| 2 | 44 | |||
| ≧3 | 10 | |||
| Gestational week | 39.5 ± 1.11 | |||
| Vacuum delivery (%) | 15.6 (73) | |||
| Male (%) | 53.3% (250) | |||
| Birthweight (g) | 3064 ± 362 | |||
| Apgar score | ||||
| 1 min | 8 (8–9) | |||
| 5 min | 9 (9–10) | |||
| Umbilical artery blood acid–base analysis | ||||
| pH | 7.30 ± 0.07 | |||
| PCO2 (mmHg) | 47.6 ± 9.28 | |||
| PO2 (mmHg) | 20.6 ± 9.05 | |||
| HCO3− (mmol/L) | 23.2 ± 5.42 | |||
| BE (mEq/L) | −2.50 ± 3.09 | |||
Data are expressed as mean ± SD, median (25–75%) or n (%).
Of 469 cases, 39 cases were with umbilical artery blood with pH <7.2, five cases were pH <7.1 and three cases were pH <7.0. All three cases with umbilical artery blood with pH <7.0 had mixed acidemia, and two of them were admitted to the NICU. All of them had normal development at 1‐year of age.
Pearson's correlation coefficient showed a significant negative correlation between the iPREFACE scores of the 469 cases and their postnatal umbilical artery pH (correlation coefficient and coefficient of determination, −0.43 and −0.19, respectively) (Figure 4).
Figure 4.

Regression line between iPREFACE score and umbilical artery blood gas pH. iPREFACE, integrated score index to predict fetal acidemia by intrapartum fetal heart rate monitoring, pH, a pH: the numerical measure of acidity and alkalinity of a solution.
The cut‐off iPREFACE score for the umbilical artery blood pH <7.20 using the Youden index of the ROC curve was 44 (sensitivity, 64.1%; specificity, 85.6%; positive predictive value [PPV], 81.7%; negative predictive value [NPV], 70.5%), and area under the curve (AUC) was 0.776. The cut‐off score for the umbilical artery blood pH being <7.10 was 46 (sensitivity, 100%; specificity, 87.6%; PPV, 89.0%; NPV, 100%), and the AUC was 0.962. The cut‐off score for umbilical artery blood pH <7.0 was 67 (sensitivity, 100%; specificity, 99.2%; PPV, 92.6%; NPV, 100%), and the AUC was 0.996 (Figure 5).
Figure 5.

ROC curve comparisons for acidemia. (a) pH < 7.2, (b) pH < 7.1, (c) pH < 7.0. iPREFACE, integrated score index to predict fetal acidemia by intrapartum fetal heart rate monitoring. AUC, area under the curve; ROC, receiver operating characteristic.
The weighted kappa coefficient between the two iPREFACE raters was 0.75 (95% confidence interval [CI], 0.504–0.996; P < 0.01), and the ICC among the three raters was 0.91 (95% CI, 0.804–0.960, P < 0.01).
Discussion
The following three points were clarified in this study: First, the iPREFACE score exhibits a significant correlation with the umbilical artery blood pH. Second, the iPREFACE score has a high NPV for fetal acidemia, suggesting that the iPREFACE score may reduce unnecessary interventions for delivery. Third, the iPREFACE score shows good inter‐rater reliability.
The iPREFACE score exhibits a significant correlation with umbilical artery blood pH. Although many guidelines for CTG use the FHR waveform of the most severe deceleration in their assessments 3 , 4 , 5 , 6 , 11 , 13 the iPREFACE score was based on the integrated recurrence of decelerations. The integrated repetition of decelerations could influence fetal acidemia status as the uterine contraction are repeated. Some guidelines consider the duration of decelerations, although they do not mention how many uterine contractions are repeated in that duration to influence the fetus and some do not consider the substantive duration of decelerations. 3 , 6 , 13 The iPREFACE score evaluates the substantive duration of decelerations by integrating the number of repeated decelerations. Furthermore, the iPREFACE score uses a 5 tier‐classification defined by the JSOG, so it reflects the three elements of FHR baseline, FHR baseline variability, and deceleration. We consider that these elements contribute to a significant correlation between the iPREFACE score and umbilical artery blood pH. Therefore, we believe that the concept of iPREFACE score may support these guidelines as a specific indicator of medical intervention in situations of repeated decelerations.
Studies using computer‐based analyses of CTG reported a correlation between the deceleration area in intrapartum FHR and neonatal acidemia. 15 , 16 The deceleration area is the value obtained by dividing the product of the duration of deceleration (measured in seconds) and the maximum depth below the fetal baseline heart rate by two. These studies indicated the importance of integrated recurrent decelerations for predicting the status of the fetus. In contrast, the iPREFACE score can be used to evaluate the fetal status during the ongoing progress of labor using intrapartum CTG in labor without a computer. We believe that one of the strengths of our scoring system is that it reflects the integrated recurrence of decelerations in the ongoing progress of labor.
The iPREFACE score also showed a high NPV for acidemia, which might reduce unnecessary interventions for delivery. Although Saling et al. defined fetal acidemia as fetal umbilical artery blood pH <7.2 in 1967, 17 most of such new‐borns show better prognoses even with acidemia of pH <7.2. Therefore, in recent years, pH <7.0 has been used to define pathological acidemia that would significantly affect neonatal prognosis, such as neonatal asphyxia. 18 Our results suggested that no intervention for delivery was required unless the score was >44 because the cut‐off scores for the umbilical artery with pH <7.2 and <7.1 are 44 (NPV 70.5%) and 46 (NPV 100%), respectively. However, careful observation may be needed taking into account the course of labor even if the score <44. Furthermore, careful management of delivery without cesarean section for medical intervention may be considered unless the score is >67 in a facility that allows pH >7.0. The iPREFACE score may help determine the timing of medical intervention in cases with recurrent decelerations of fetal heart rate, especially if level 3 or level 4 of the 5‐tier classification defined by JSOG recurs.
Finally, the iPREFACE score showed good inter‐rater reliability. Compared with the intra‐rater and inter‐rater reproducibility of the interpretations of the FHR waveform defined by the JSOG (weighted kappa coefficients 0.70–0.77 and 0.70, respectively), 19 the iPREFACE score exhibited higher reproducibility with a lower weighted kappa coefficient of 0.75 and an ICC of 0.91.
One of this study's strengths is that the iPREFACE score could be calculated simply by using only CTG without any special device such as a computer or a fetal electrocardiogram. Another strength is that the iPREFACE score that reflects integrated recurrent decelerations can be an objective indicator in determining the timing of medical intervention when decelerations that do not require urgent medical intervention appear. One of the limitations is that the subject is full‐term normal vaginal deliveries of healthy pregnant women with a single fetus. In deliveries involving fetal abnormalities or high levels of maternal risk, the presence of various confounding factors might have led to the formation of abnormal CTG waveforms. In such cases, the application of the iPREFACE score should be considered carefully based on the course of the delivery. This study was conducted to assess the effect of only uterine contraction on the fetus by excluding these confounding factors. Therefore, the prevalence of acidemia was very low in this study, with only three cases having a blood pH <7.0. With this in mind, we concluded that the scoring system is better at detecting negative (normal) than positive (acidemia). Therefore, we believe that this scoring system has the potential to reduce the number of unnecessary medical interventions. We conducted a retrospective study because we aimed to evaluate the association between the umbilical artery blood pH immediately after delivery and the iPREFACE score. Therefore, we are preparing a prospective intervention study for vaginal deliveries, including fetal growth restriction and emergency cesarean section, to confirm the usefulness of the iPREFACE score.
In conclusion, the findings of this study suggested that the iPREFACE score might become a candidate for predicting fetal acidemia and might be used as an indicator for reducing unnecessary interventions during delivery. Furthermore, because the assessments could be performed quickly and easily using only an ongoing intrapartum CTG device and without the requirement for any other special equipment, the iPREFACE score is likely to be useful in clinical practice and may support many guidelines for FHR monitoring.
Disclosure
All authors declare that there is no conflict of interest.
Acknowledgments
The author would like to thank all staff of the Department of Obstetrics and Gynecology, Toho University Omori Medical Center, and Department of Obstetrics and Gynecology, Ageo Central General Hospital.
References
- 1. Hon EH. The electronic evaluation of the fetal heart rate: Preliminary report. Am J Obstet Gynecol. 1996;175:747–8. [DOI] [PubMed] [Google Scholar]
- 2. Paul RH, Suidan AK, Yeh SY, Schifrin BS, Hon EH. Clinical fetal monitoring. VII. The evaluation and significance of intrapartum baseline FHR variability. Am J Obstet Gynecol. 1975;123:20–210. [PubMed] [Google Scholar]
- 3. Electronic fetal heart rate monitoring: Research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385–90. [PubMed] [Google Scholar]
- 4. Royal College of Obstetricians and Gynaecologists Clinical Effectiveness Support Unit . The Use of Electronic Fetal Monitoring: Evidence‐based Clinical Guideline No. 8. London: RCOG Press; 2001. [Google Scholar]
- 5. Liston R, Sawchuck D, Young D. Society of Obstetrics and Gynaecologists of Canada; British Columbia Perinatal Health Program. Fetal Health Surveillance: Antepartum and Intrapartum Consensus Guideline. J Obstet Gynaecol Canada. 2007;29:S3–56. [PubMed] [Google Scholar]
- 6. American College of Obstetricians and Gynecologists . ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: Nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192–202. [DOI] [PubMed] [Google Scholar]
- 7. Minakami H, Hiramatsu Y, Koresawa M, Fujii T, Hamada H, Iitsuka Y, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2011 edition. J Obstet Gynaecol Res. 2011;37:1174–97. [DOI] [PubMed] [Google Scholar]
- 8. Ayres‐De‐Campos D, Spong CY, Chandraharan E. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynecol Obstet. 2015;131:13–24. [DOI] [PubMed] [Google Scholar]
- 9. Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197:26.e1–6. [DOI] [PubMed] [Google Scholar]
- 10. Coletta J, Murphy E, Rubeo Z, Gyamfi‐Bannerman C. The 5‐tier system of assessing fetal heart rate tracings is superior to the 3‐tier system in identifying fetal acidemia. Am J Obstet Gynecol. 2012;206:226.e1–5. [DOI] [PubMed] [Google Scholar]
- 11. Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) . Guidelines for Obstetrical Practice in Japan 2020 Edition (in Japanese). Tokyo: Japan Society of Obstetrics and Gynecology; 2020. [Google Scholar]
- 12. Okai T, Ikeda T, Kawarabayashi T, Kozuma S, Sugawara J, Chisaka H, et al. Intrapartum management guidelines based on fetal heart rate pattern classification. J Obstet Gynaecol Res. 2010;36:925–8. [DOI] [PubMed] [Google Scholar]
- 13. Visser GH, Ayres‐De‐Campos D. FIGO consensus guidelines on intrapartum fetal monitoring: Adjunctive technologies. Int J Gynecol Obstet. 2015;131:25–9. [DOI] [PubMed] [Google Scholar]
- 14. Kilpatrick SJ, Laros RK Jr. Characteristics of normal labor. Obstet Gynecol. 1989;74:85–7. [PubMed] [Google Scholar]
- 15. Cahill AG, Tuuli MG, Stout MJ, López JD, Macones GA. A prospective cohort study of fetal heart rate monitoring: Deceleration area is predictive of fetal acidemia. Am J Obstet Gynecol. 2018;218:523.e1–523.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strachan BK, Sahota DS, Van Wijngaarden WJ, James DK, Chang AMZ. Computerised analysis of the fetal heart rate and relation to acidaemia at delivery. BJOG. 2001;108:848–52. [DOI] [PubMed] [Google Scholar]
- 17. Bretscher J, Saling E. pH values in the human fetus during labor. Am J Obstet Gynecol. 1967;97:906–11. [DOI] [PubMed] [Google Scholar]
- 18. Maclennan A. A template for defining a causal relation between acute intrapartum events and cerebral palsy: International consensus statement. BMJ. 1999;319:1054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi M, Nakai A, Sekiguchi A, Takeshita T. Fetal heart rate classification proposed by the perinatology committee of the Japan Society of Obstetrics and Gynecology – reproducibility and clinical usefulness. J Nippon Med Sch. 2012;79:60–8. [DOI] [PubMed] [Google Scholar]


