Abstract
Inherited metabolic disorders are a large group of rare disorders affecting normal biochemical pathways. Many metabolic disorders can present with symptoms affecting the eye, and eye disorders can evolve later in the natural history of an already diagnosed metabolic disorder. The ophthalmic involvement can be very varied affecting any part of the eye, including abnormalities of cornea, lens dislocation and cataracts, retina and the distal optic pathway, and extraocular muscles. Awareness of inherited metabolic disorders is important to facilitate early diagnosis and in some cases instigate early treatment if a patient presents with eye involvement suggestive of a metabolic disorder. Ophthalmological interventions are also an important component of the multisystem holistic approach to treating patients with metabolic disorders.
Keywords: cataracts, enzyme replacement therapy, galactosaemia, genetics, inherited metabolic disorder
Many inherited metabolic disorders (IMD) can have ophthalmological effects, including disorders of any part of the eye, extraocular muscles and visual pathways. Awareness of the potential ophthalmic effects of IMD is important for any clinician including neonatologists, paediatricians and ophthalmologists who may encounter patients whose initial presentation or subsequent disease-course may be with the eye-related feature of a specific IMD. This brief review aims to offer a summary of eye involvement in the different IMD to assist in the earlier recognition and diagnosis of IMD that can facilitate earlier treatment.
Brief introduction/ overview of principles of IMD
The IMD encompass a large group of several hundred individually rare genetic disorders that each affect a specific chemical reaction within the myriad normal biochemical pathways.1 The majority are caused by absence or deficiency of a specific enzyme that catalyses a step in the biochemical pathway, with subsequent accumulation of the reaction’s substrate which can be directly toxic (such as ammonia in the urea cycle defects), conversion of the substrate via an alternative pathway to a toxic substance (such as the production of succinylacetone in tyrosinaemia type 1), or deficiency of the reaction’s product (such as glucose in the glycogen storage disorder, resulting in hypoglycaemia). Some IMD are due to absence or malfunction of a trans-membrane transporter, and others are caused by dysfunction of whole organelles (such as the peroxisomal biogenesis defects). While some IMD are relatively benign and easy to manage, many
cause multisystem dysfunction and are associated with progressive deterioration over time and are life-limiting. IMD can present with clinical features affecting any or multiple organ systems, including the eye and visual pathways.
Some IMD have specific treatments available in the form of specialised dietetic management, vitamin or co-factor supplementation, recombinant enzyme replacement therapy, or in some situations haematopoietic stem cell or solid organ transplantation. Increasingly gene therapy approaches are also being applied to the treatment of IMDs. Early identification of an IMD can facilitate treatment where available, and important genetic counselling for the wider family.
The majority of IMD are inherited in an autosomal recessive manner, where both parents are unaffected heterozygous carriers of the disorder and the child is only affected as they have inherited both the maternal and paternal mutated allele. In this situation, the recurrence risk for any future children is 1:4 (25%). Some IMD are inherited as autosomal dominant traits, while a small number are X-linked. The mitochondrial disorders can be inherited as autosomal recessive, dominant, or X-linked conditions due to mutations in a nuclear gene, but also inherited matrilineally (i.e. just from the maternal line) if caused by a mutation in the small 16.5 kb ring of mitochondrial DNA containing 37 genes present in the mitochondria.2
Eye involvement in IMDs
In some situations, eye involvement can be the primary presenting feature of an IMD that can lead to a diagnosis, for example, the identification of neonatal cataracts can be the first sign of disorders of galactose metabolism, while an incidental finding of cornea verticallata (vortex/whorl keratopathy) at a routine optician check can lead to the diagnosis of the lysosomal storage disorder (LSD) Fabry disease. Detection of a corneal cherry red spot is also a ‘red flag’ for a potential LSD. Awareness of the potential for an IMD in such clinical situations is important in facilitating prompt diagnosis and treatment.
Many IMD have ophthalmic involvement as an expected complication or sequela, and these patients will be referred to ophthalmology services to facilitate appropriate monitoring and treatment, and in this situation it is important that knowledge of the natural history and expected evolution of the ophthalmological component of the IMD guides the clinical surveillance.
IMD can affect any part of the visual pathway, from cerebral visual cortex dysfunction in many IMD with progressive central nervous system degeneration, to optic neuropathy, retinopathy, lens opacification and cataracts, corneal clouding and various other keratopathies, and abnormalities of eye movements if the extraocular muscles are affected or if the central optic motor nuclei are dysfunctional. It is important that an IMD is considered in the differential diagnosis of the eye condition, alongside common acquired or other non-metabolic genetic causes.
General diagnostic approach
Identification of an IMD requires a low threshold of clinical suspicion. Within the clinical history, evidence of extra-ocular features should be sought to determine if the presenting eye signs or symptoms are in the context of a multisystem disease. The age of the child is important; a neonate with failure to thrive, recurrent vomiting, prolonged jaundice or other developmental concerns may have an IMD. The family history is important, especially other affected siblings or relatives, unexplained neonatal or childhood deaths, recurrent miscarriages. Enquiring about parental consanguinity is important as many of the IMD are inherited in an autosomal recessive manner, although these conditions still occur in children of non-consanguineous parents. Subsequent dietary preferences, abnormalities of normal childhood developmental progress (especially developmental regression, i.e. loss of developmental milestones previously achieved), detection of hepato/splenomegaly or dysmorphic features can also allude to an IMD. Some children will have been reviewed by a number of specialists addressing individual manifestations of an IMD without the diagnosis being made, for example, a child with a mucopolysaccharidosis (MPS) disorder may have seen ENT surgeons for recurrent otitis media or obstructive sleep apnoea, general surgeons for inguinal or umbilical hernia, and community paediatrician for developmental delay, as well as ophthalmology services due to the development of corneal clouding, before the diagnosis is reached. Some children may have imaging studies undertaken, such as brain magnetic resonance imaging (MRI), and these can also reveal diagnostic information regarding IMD if a specific pattern such as a leukodystrophy is identified.3
In planning specific investigations it is important to review any prior specific biochemical/ metabolic tests that have been undertaken, and to then ensure that key diagnostic samples are collected determined by the specific ophthalmological problem detected. Metabolic testing will include general biochemistry (e.g. renal and liver function tests, cholesterol, creatine kinase [CK], uric acid), and specific metabolic testing on blood and urine samples (such as plasma amino acids, urine organic acids, urine glycosaminoglycans). In many situations, the diagnosis is then confirmed with specific enzyme assays which are done in blood samples, or occasionally in tissues such as cultured skin fibroblasts, or muscle/liver biopsies.4 Increasingly molecular genetic testing has a first-line role in diagnostic pathways, as well as the more typical role in confirmation of a biochemical diagnosis. For example, a number of next generation sequencing panels for genes causing cataracts may be employed that include genes causing IMDs. Furthermore, whole genome sequencing initiatives (such as the UK 100,000 genome project) may identify a specific but unexpected IMD, and in this situation biochemical assays may be required to substantiate and evaluate the genomic variants to determine pathogenicity.
Early discussion with a metabolic specialist can help with planning key investigations, and in some instances with instigating pre-emptive treatment while investigation results are sought such as appropriate galactose/lactose-free feeds in a neonate at risk of galactosaemia.
Specific eye findings
Table 1 provides a summary of the metabolic differential diagnosis for each eye finding, together with specific extra-ocular features, key diagnostic tests and specific treatments, and key literature references.
Table 1.
Summary of metabolic differentials, extra-ocular features, diagnostic tests and treatments for specific ophthalmic findings.
| Ophthalmic finding | Example metabolic differential | Extra ocular features | Diagnostic tests | Treatments | Key references |
|---|---|---|---|---|---|
| Eye Movements | |||||
| Oculogyric crisis | |||||
| Neurotransmitter defects | Aromatic L-amino acid decarboxylase (AADC) deficiency | Hypotonia and movement disorders (especially oculogyric crisis and dystonia), autonomic dysfunction, behavioural disorders | CSF neurotransmitters, AADC activity, DNA | Medication, gene therapy, |
[5,6] |
| Pyridoxamine 5 Phosphate oxidase (PNPO) deficiency | Neonatal seizures | CSF neurotransmitters, CSF pyridoxal phosphate, bloodspot PNPO enzyme activity, DNA | B6 supplement | [7,8] | |
| Tyrosine hydroxylase deficiency (recessive Segawa syndrome) | Severe motor development delay, central hypotonia/peripheral hypertonia, hypokinesia, oculogyric crises, ptosis, autonomic instability | CSF neurotransmitters, DNA | L-Dopa, mono amine oxidase inhibition | [9] | |
| Ptosis | Pompe disease (glycogen storage disease type II) | Hypotonia, respiratory insufficiency, hypertrophic cardiomyopathy (infantile) | Creatine kinase, Bloodspot Pompe enzymology, DNA | ERT | [10] |
| Mitochondrial disorders | Diverse multisystem | DNA | Symptomatic | [2,11] | |
| Smith Lemli Optiz syndrome (7-dehydrocholesterol reductase deficiency) | Multiple malformations, mental retardation | Cholesterol (low), 7-dehydrocholesterol (high), DNA | Cholesterol supplementation | [12] | |
| Ophthalmoplegia | Gaucher disease type III | Horizontal supranuclear gaze paralysis, hepatosplenomegaly, cytopaenias | Enzymology, chitotriosidase, DNA | ERT | [13,14] |
| Niemann Pick type C (NPC): | Vertical supranuclear gaze palsy: Paralysis of vertical saccades with sparing of smooth pursuits. Neurodevelopmental |
Oxysterols, DNA | Miglustat | [15–17] | |
| Early mitochondrial disorders | Leigh: motor regression, acute onset hypotonia, swallow dysfunction | Biochemical, DNA | Symptomatic | [2,18] | |
| Congenital disorder of glycosylation (PMM2-CDG/CDG1a) | Multisystem disease, dysmorphic features, protein losing enteropathy | Transferrin isoelectric focussing, enzymology, DNA | Symptomatic. (monosaccharides for specific disorders) | [19] | |
| Later onset mitochondrial disorders | Progressive external ophthalmoplegia. Kearn Sayre Syndrome: cardiac conduction defects, renal tubulopathy |
Biochemical, DNA | Symptomatic | [17] | |
| Glutaric aciduria type 1 | Movement disorders, macrocephaly | Urine organic acids, carnitine, DNA | Specific diet, carnitine | [17,20] | |
| Late onset GM2 gangliosidosis | Neurological decline | Urine oligosaccharides, enzymology, DNA | Symptomatic | [21] | |
| Late onset non-ketotic hyperglycinaemia (NKH) | Chorea/dystonia | CSF amino acids, plasma amino acids, DNA | Sodium benzoate, supportive | [22] | |
| Wilson disease | Neurology and hepatic presentation | Copper, caeruloplasmin, DNA | Chelation therapy | [23,24] | |
| Cornea | |||||
| Keratitis | Tyrosinaemia type II | Skin lesions in pressure areas, developmental delay, microcephaly, seizures. | Plasma amino acids, DNA, urine organic acids | Specific diet. (Aim tyrosine < 800 umol/L) | [25,26] |
| Fabry | Cornea verticallata (whorling), later corneal opacity. Acroparaesthesia, risk of hypertrophic cardiomyopathy, progressive renal impairment, stroke | Lyso-GB3, Enzymology, DNA | ERT, oral therapy. | [27] | |
| Corneal crystal deposits | Cystinosis | Early infantile nephropathy, end stage renal failure. Thyroid, gonadal failure. | Leucocyte cystine assay. DNA | Eyes: topical cysteamine. | [28–30] |
| Corneal clouding/ opacities | Mucopolysaccharidosis (types I, IV, VI) | Dysmorphic, HSM, dysostosis multiplex, developmental delay. Short stature. | Urine glycosaminoglycans, enzymology, DNA | ERT | [31,32] |
| I-cell disease | Early onset dysmorphism. | I-cell screen enzymology, DNA | Symptomatic | [33] | |
| Galactosialidosis | Dysmorphic, cherry red spot | Urine sialic acid, glycosaminoglycans, DNA | Symptomatic | [34,35] | |
| Alpha mannosidosis | Dysmorphic features. Frequent infections. Hepatosplenomegaly | Urine oligosaccharides, enzymology, DNA | BMT, ERT | [36,37] | |
| Tangier | Orange/yellow tonsils, splenomegaly, peripheral neuropathy. Adulthood corneal infiltration. |
Cholesterol, lipoproteins, DNA | Low fat diet | [38] | |
| Infantile free sialic acid storage disease | Hepatosplenomegaly, dysmorphic, cardiomyopathy | Urine sialic acid, DNA | Symptomatic | [39] | |
| Lens | |||||
| Ectopia lentis | Homocystinuria | Marfanoid habitus, thromboembolic events. | Plasma amino acids, DNA | Diet, medication | [40] |
| Sulfite oxidase | Severe neonatal seizure disorder, microcephaly, psychomotor retardation | Urine sulphocysteine, DNA | Symptomatic | [41] | |
| Cataract | |||||
| Congenital (at birth) | Cockayne | Cachectic dwarfism, microcephaly, cognitive impairment, pigmentary retinopathy, cataracts, sensorineural deafness | DNA repair studies, DNA | Symptomatic | [42,43] |
| Lowe syndrome | Renal Fanconi syndrome, arthropathy, growth impairment. | DNA | Symptomatic | [44] | |
| Peroxisomal biogenesis | Hepatopathy, hypotonia, developmental delay, ataxia, peripheral neuropathy | VLCFA, plasmalogens, DNA | Symptomatic | [45,46] | |
| Sorbitol dehydrogenase | Isolated lens SDH deficiency | DNA | Symptomatic | [47] | |
| Neonatal | Galactosaemias | Hepatopathy, renal tubular dysfunction | Enzymology, DNA | Dietary | [48–52] |
| Polyol pathway | TKFC deficiency | Urine polyols, DNA | Symptomatic | [53] | |
| Infancy | Alpha mannosidosis | Dysmorphic features. Frequent infections. HSM | Urine oligosaccharides, enzymology, DNA | BMT, ERT | [36,37] |
| Pyrroline-5-carboxylate synthase deficiency | Mental retardation, skin and joint laxity, peripheral neuropathy | Plasma amino acids (fasted), enzyme, DNA | Symptomatic | [54] | |
| Respiratory chain/ mitochondrial | For example FBXL4- related cataracts | See above | Symptomatic | [55] | |
| Sialidosis | Cherry red spot; myoclonus, dysmorphism | Urine sialic acid, enzymology, DNA | Symptomatic | [56] | |
| Childhood | Mevalonic aciduria (hyper IgD syndrome) | Period fever, inflammatory disorder | Urine organic acids, DNA | BMT | [57] |
| Wilson | Neurology and hepatic presentation | Copper, caeruloplasmin, DNA | Chelation therapy | [23,24] | |
| Later | Cerebrotendinous xanthomatosis (sterol 27-hydroxylase deficiency) | Infantile cholestatic jaundice, mental retardation, cataracts, later tendon xanthomata, progressive spasticity, tremor, ataxia | Plasma sterol profile, (cholestanol), DNA | Chenodeoxycholic acid | [58] |
| Fabry | Cornea verticallata (whorling), later corneal opacity. Acroparaesthesia, risk of hypertrophic cardiomyopathy, progressive renal impairment, stroke | Lyso-GB3, Enzymology, DNA | ERT, oral therapy. | [27] | |
| Homocystinuria | Marfanoid habitus, thromboembolic events. Severe form now detected by new born screening (eg, in United Kingdom) | Plasma amino acids, DNA | Diet, medication | [40] | |
| Ornithine aminotransferase deficiency (gyrate atrophy of choroid and retina) | Posterior subcapsular cataract, visual field defect, retinopathy | Plasma amino acids, urine amino acids, DNA | Diet, medication (pyridoxine) | [59,60] | |
| Tangier | Orange/yellow tonsils, splenomegaly, peripheral neuropathy. Adulthood corneal infiltration. |
Cholesterol, lipoproteins, DNA | Low fat diet | [38] | |
| Peroxisomal PEX7 | Late onset ataxia, cognitive impairment | VLCFA, phytanic acid, DNA | Symptomatic, diet | [61] | |
| Glycogen storage disease type 1 and 3 | Hypoglycaemia, cardiomyopathy, hepatomegaly | Enzymology, DNA | Diet | [62] | |
| Vici (defect in autophagy) | Multisystem disorder | DNA | Symptomatic | [63,64] | |
| Glaucoma | |||||
| Congenital disorders of glycosylation | See above | See above | See above | [65] | |
| Leber hereditary optic neuropathy | See above | See above | See above | [66] | |
| Mucopolysaccharidoses | See above | See above | See above | [67] | |
| Retina | |||||
| Retinitis pigmentosa | Congenital disorders of glycosylation | Multisystem disorder | Enzymology, DNA | Symptomatic | [19, 68–72] |
| Neuronal ceroid lipofuscinoses | Progressive visual failure, neurodegeneration, epilepsy/seizure disorder | Enzymology, DNA | ERT (CLN2) | [73,74] | |
| Cobalamin C defect | Early-onset multisystem disease/: neurological impairment, haematological features, pulmonary. Late-onset patients with slowly progressing neurological involvement. |
Plasma MMA, urine organic acids, DNA | Medication | [75,76] | |
| Long chain hydroxyacyl-CoA dehydrogenase deficiency | Cardiomyopathy, myopathy, acute liver dysfunction, polyneuropathy, sudden unexplained death in infancy. Acute fatty liver pregnancy (AFLP) or hypertension, elevated liver enzymes, and low platelet (HELLP) syndromes (both in mother of affected foetus) | Carnitine profile, DNA | Diet | [77,78] | |
| Ornithine aminotransferase deficiency (gyrate atrophy of choroid and retina) | Posterior subcapsular cataract, visual field defect, retinopathy | Plasma amino acids, urine amino acids, DNA | Diet, medication (pyridoxine) | [59,60] | |
| Vitamin E malabsorption | Vitamin E deficiency, ataxia. | Vitamin E, DNA (TTPA gene) | Parenteral vitamin E | [79] | |
| Abetalipoproteinaemia | Fat malabsorption, failure to thrive, dysmetria, cerebellar ataxia, peripheral neuropathy. | Cholesterol, lipoproteins, DNA | Diet, vitamins | [80] | |
| Pantothenate kinase (PKAN) | Dystonia, rigidity, choreoathetoid movements. | DNA (PANK2), MRI | Symptomatic | [81] | |
| Peroxisomal biogenesis | See above | See above | See above | ||
| Classic Refsum disease | Peripheral neuropathy, cerebellar ataxia, and elevated CSF protein peripheral neuropathy, cerebellar ataxia, and elevated protein levels in the cerebrospinal fluid (CSF) peripheral neuropathy, cerebellar ataxia, and elevated protein levels in the cerebrospinal fluid (CSF) | Phytanic acid, DNA | Diet | [82] | |
| Mitochondrial | Kearn Sayre Syndrome, NARP, other mtDNA deletions | See above | See above | [83,84] | |
| Aceruloplasminaemia | Adult-onset chorea, cerebellar ataxia, parkinsonism, diabetes mellitus | Caeruloplasmin, ferritin, DNA | Chelators | [85] | |
| Galactosialidosis | Coarse facies, skeletal/ vertebral changes, foam cells in the bone marrow, foetal hydrops | Urine glycosaminoglycans, urine oligosaccharides, enzymology, DNA | Symptomatic | [35] | |
| Cherry red spot | GM2 gangliosidosis | Tay Sachs, Sandhoff | Urine oligosaccharides, enzymology, DNA | Symptomatic | [21] |
| GM1 gangliosidosis | Neurodegenerative | Urine oligosaccharides, enzymology, DNA | Symptomatic | [21] | |
| Niemann Pick type A/B | HSM, lung disease, neurodegeneration | Enzymology, DNA | ERT | [86] | |
| Sialidosis | Cherry red spot; myoclonus, dysmorphism | Enzymology, urine sialic acid, DNA | Symptomatic | [87] | |
| Farber | Ceramidase deficiency | Fibroblast enzymology, DNA | BMT | [88] | |
| Metachromatic leukodystrophy, Krabbe | Neurodegeneration. (CRS is occasional feature) | Enzymology, DNA | Symptomatic, gene therapy | [89–91] | |
| Other retinal pathology | Sjogren Larsson syndrome | Crystalline maculopathy with macular degeneration. Ichthyosis, spastic diplegia, mental retardation | DNA | Symptomatic | [92] |
| Optic nerve | |||||
| Leber hereditary optic neuropathy and other mitochondrial | Diverse multisystem | Biochemistry, DNA | Symptomatic | [93–96,97] | |
| Optic atrophy | Biotinidase | alopecia, dermatitis, developmental delay, seizures, deafness | Biotinidase activity, urine organic acids, DNA | Biotin | [98–100] |
| Neuronal Ceroid lipofuscinoses (Batten-spectrum) | Progressive visual failure, neurodegeneration, epilepsy/seizure disorder | Enzymology, DNA | ERT (CLN2) | [73,74,101–103] | |
| Canavan | Macrocephaly, developmental delay, seizures, progressive spasticity. | MRI brain, MR spectroscopy, Urine organic acids, DNA | Symptomatic | [104] | |
| Krabbe | irritability, hypertonicity, bouts of hypothermia, mental regression, and possibly optic atrophy and seizures. | MRI brain, enzymology, DNA | Symptomatic (?BMT) | [89,90] | |
| Metachromatic leukodystrophy | Progressive neurodegeneration with loss of motor function | MRI brain, enzymology, DNA | Gene therapy | [91] | |
| Neurodegeneration with brain iron accumulation (NBIA): Infantile Neuroaxonal dystrophy (INAD) |
Severe early neurodevelopmental regression, myoclonic epilepsy severe progressive myoclonic epilepsy. | MRI, DNA | Symptomatic | [105,106] | |
| Pelizaeus Merzbacher disease | Hypotonia, global hypomyelination. | MRI, DNA | Symptomatic | [107] | |
| Peroxisomal biogenesis | See above | See above | See above | ||
| Ribose-5-phosphate isomerase | Developmental delay, ataxia, optic atrophy | Urine polyols, DNA | Symptomatic | [108] | |
| Sulfite oxidase | Severe neonatal seizure disorder, microcephaly, psychomotor retardation | Urine sulphocysteine, DNA | Symptomatic | [41] | |
| X-linked adrenoleukodystrophy | Adrenal insufficiency, progressive cerebral disease, or later spastic paraparesis. | Very long chain fatty acids, DNA | Adrenal replacement. BMT. Gene therapy | [109] | |
| 3 methylglutaconic aciduria type 3 (Costeff) | optic atrophy, ataxia, chorea and spastic paraparesis. | Urine organic acids, DNA | Symptomatic | [110–112] | |
BMT, bone marrow transplant; ERT, enzyme replacement therapy; lyso-GB3, globotriaosylsphingosine; MMA, methylmalonic acid; MRI, magnetic resonance imaging; VLCFA, very long chain fatty acids.
Table 2.
Summary of investigations.
| Urine | Blood | Other | |
|---|---|---|---|
| Lysosomal storage disorders | Glycosaminoglycans Sialic acids Oligosaccharides |
Enzyme assays Vacuolated lymphocytes and Electron microscopy of buffy coat DNA |
Histopathology |
| Mitochondrial disorders | Urine organic acids Renal tubular markers |
Lactate Plasma amino acids DNA (nuclear genes, mitochondrial (mtDNA)). |
Muscle biopsy (histopathology, respiratory chain enzymology |
| Peroxisomal disorders | Very long chain fatty acids Phytanate, pristanate Red cell plasmalogens DNA |
Skin fibroblasts studies | |
| General metabolic screening | Urine organic acids | Plasma amino acids Acylcarnitine profile DNA |
|
| Galactose/polyols | Urine polyols | Gal-1-put enzymology Red cell galactose-1-phosphate Epimerase/ Galactokinase enzymology DNA |
Eye movement disorders
Oculogyric crisis
Neonatal or early-onset oculogyric crisis may be due to a defect in neurotransmitter metabolism, and will usually be seen as part of a severe neurological phenotype with hypertonia and/or seizures; these can occur in disorders of vitamin B6 metabolism that are treatable with oral vitamin B6 supplementation. Diagnosis usually requires cerebrospinal fluid analysis of neurotransmitters together with molecular genetic testing. Treatment will depend on the specific diagnosis, and may include neurotransmitter replacement or specific blockade therapies.
Ophthalmoplegia
The differential diagnosis of new onset strabismus in early childhood is broad including simple refractive errors, space occupying lesions, but also including IMD. Strabismus is a feature of the commonest form of congenital disorder of glycosylation (CDG), phosphomannomutase 2 (PMM2-CDG) (previously known as CDG-Ia). These disorders impair the normal process of post-translational modification of proteins, and the abnormal sugar side chain (glycosylation) results in abnormal function of the mature protein. Ocular involvement in this multisystem disorder can also include nystagmus with or without visual impairment, and other subtypes of CDG also have eye involvement. Extraocular manifestations are expected including dysmorphic features, protein-losing enteropathy, pericardial effusions.15
Early-onset mitochondrial disorders including those presenting with Leigh and Leigh-like syndrome can present with new-onset squint together with other loss of previously acquired skills often after a minor febrile illness; imaging studies together with biochemical testing are the first steps in the diagnostic workup. Mitochondrial disorders can also present later in life with primary ocular motor defects, including progressive external ophthalmoplegia as either an isolated feature, or as part of a multisystem mitochondrial defect such as Kearn Sayre Syndrome. Importantly, some mitochondrial disorders such as autosomal recessive polymerase gamma (POLG)-related disease can present in early childhood with severe disease, where parents who are heterozygous carriers may be at risk of later onset autosomal dominant disease manifestations with particular mutations, warranting careful genetic counselling.
Some LSDs have specific eye movement abnormalities. Gaucher disease can be associated with a horizontal supranuclear gaze palsy that can be subtle and detected only with careful rotational movement assessments.13 In this situation, the detection of eye movement abnormalities can help differentiate patients with type III (chronic neuronopathic Gaucher disease) from the non-neuronopathic type 1 disease, guiding therapeutic approaches.
Niemann Pick type C (NPC) is a cholesterol trafficking LSD; the age of presentation is very wide, but the typical eye movement defect is of a vertical supranuclear gaze palsy with paralysis of vertical saccades with sparing of smooth pursuits in the initial stages. This is seen in conjunction with developmental regression and splenomegaly in the early infantile forms, but may be an isolated feature initially in later onset disease. Treatment options include substrate reduction therapy with miglustat that can ameliorate the disease course.
Ptosis
Ptosis can be seen in a wide range of myopathies and myasthenic syndromes. Of the IMD, mitochondrial disorders can present with ptosis that is often worse with fatigue. Of the metabolic myopathies, Pompe disease (glycogen storage disease type II) may have progressive ptosis together with generalised motor and respiratory myopathy, and in infants also hypertrophic cardiomyopathy. Treatment with enzyme replacement therapy is available.
Corneal pathology
Keratopathy
Tyrosinaemia type II is a rare amino acid disorder that causes a painful keratitis, resulting in photophobia, lacrimation and burning pain. It is also associated with skin lesions particularly in pressure areas, and also neurodevelopmental delay with microcephaly and seizures in some patients. Slit lamp examination may reveal bilateral herpetic-like lesions with neovascularisation, which untreated can result in corneal scarring. Diagnosis is by identifying very high plasma tyrosine levels and excluding other subtypes of tyrosinaemia. Treatment with dietary measures to decrease the tyrosine level is effective.25,26
Fabry disease is an X-linked LSD that causes multisystem disease including progressive renal impairment, hypertrophic cardiomyopathy and strokes, although the phenotype is very variable. Females can be affected. Children present with early symptoms of painful acroparaesthesia. Patients may be detected incidentally in a pre-symptomatic stage by the finding of cornea verticallata (corneal whorling); at later stages corneal opacification manifests. Specific therapy is available with intravenous enzyme replacement, and oral disease modifying drugs, and so early diagnosis is important.27
Corneal clouding/opacification
Corneal clouding and opacification is a feature of several IMD, in particular some of the progressive LSD conditions,113 and these should be considered alongside other acquired causes of corneal opacification especially when bilateral.
Cystinosis is a LSD caused by a defect in the lysosomal cysteine exporter, and in its severe form causes an early infantile nephropathy with end stage renal failure. Eye involvement is usually at a later stage, and thyroid and gonadal pathology is also expected. There are milder forms isolated to ocular involvement. Cystine deposits accumulate within the stroma of the cornea, iris, lens and retina, and manifest as photophobia with visual defect. Systemic treatments as well as topical cysteamine are used.28–30
Other LSD that present with corneal clouding included the MPS (e.g. MPS I Hurler syndrome, MPS IV (Morquio), MPS VI (Maroteaux Lamy), but not the X-linked MPS II Hunter syndrome),31,32 sialic-acid-related LSD (galactosialidosis, sialic acid storage diseases), and other oligosaccharidoses such as alpha-mannosidosis. These are all associated with multisystem disease and progressive dysmorphic features; patients may have seen a number of specialists before a diagnosis is reached, emphasising the need to consider these disorders. In some attenuated forms of MPS, the ocular manifestations may be the initial presenting feature.114 Surgical intervention to address the corneal clouding may be necessary, as the response of the corneal clouding to intravenous enzyme replacement therapies is very variable; corneal transplant has been successfully applied in this situation with very important benefits for the patients.
Lipid-associated disorders can also present with corneal pathology. Tangier disease is a rare disorder affecting cholesterol efflux, associated with orange/yellow tonsils, splenomegaly, peripheral neuropathy and the later development of corneal infiltration. It is treated with a low fat diet.38 Corneal arcus is seen in patients with hypercholesterolaemia, and if this is seen in young patients may be a sign of the more severe forms of hypercholesterolaemia such as homozygous familial hypercholesterolaemia, where urgent treatment to avoid very early coronary artery morbidity and mortality is required.
Lens pathology
Ectopia lentis
Dislocation of the optic lens has a range of causes including Marfan syndrome, and the Weill-Marchesani syndromes115,116 while metabolic causes include homocystinuria, which is classically associated with downwards-dislocation where this may be the initial presenting feature, and the severe disorder sulfite oxidase deficiency that is associated with early onset seizures. Appropriate metabolic screening should be undertaken in patients with unexplained lens dislocation. While homocystinuria is now part of routine newborn screening in many countries including the United Kingdom, patients born prior to screening may still present symptomatically.
Cataract
The development of paediatric cataract always requires investigation to determine the aetiology. In many situations, this is a primary genetic disorder affecting the transcription factors such as Pax6, and c-Maf that have pivotal roles in the development of the lens and its polarity.117 A number of IMD can also present with cataracts, and the age that the cataract develops can help in the differential diagnosis.
Truly congenital cataract, that is, those present already at birth, may suggest conditions including Cockayne syndrome which is a DNA repair defect associated with severe short stature, retinitis pigmentosa (RP), sensorineural deafness and early mortality;42,43 Lowe syndrome, an X-linked disorder comprising cataracts, proximal renal tubular dysfunction and intellectual impairment due to mutations in the OCRL gene encoding OCRL-1 which is an inositol polyphosphate 5-phosphatase that has roles in endocytic trafficking;44 and isolated sorbitol dehydrogenase deficiency specifically affecting the lens.47
An important category of IMD causing cataracts are the peroxisomal disorders.45 Peroxisomes are intracellular organelles with a wide range of biochemical functions including beta-oxidation of very long chain fatty acids, alpha-oxidation of phytanic acid, and bile acid and plasmalogen synthesis. Peroxisomal defects include single enzyme defects (such as x-linked adrenoleukodystrophy) or defects affecting the biogenesis of the whole peroxisome (the Zellweger spectrum disorder [ZSD] peroxisomal biogenesis defects). Patients with ZSD can have congenital cataracts; there are numerous genes involved in peroxisomal biogenesis, and several have been associated with ocular disease including PEX2, PEX11B, PEX10, PEX12 and PEX16 (see reference Steinberg and colleagues45 for summary). These may even be detected antenatally.118 The severe forms of ZSD present in the neonatal period with severe hypotonia, enlarged fontanelle, hepatopathy and significantly shortened life expectancy, but those at the milder end of the spectrum can present much later. Cataract can be the presenting feature, and careful diagnostic work up is required, especially as the classical biochemical biomarker (very long chain fatty acids) may not show consistent abnormalities, such as in patients with PEX11B.46 Later onset cataracts in adulthood can also be due to peroxisomal biogenesis defects, for example, in PEX7-related late onset ataxia and cognitive impairment with cataracts.61 Biochemical testing for peroxisomal defects includes assays of the very long chain fatty acids and other products of peroxisomal function; many of the biogenesis genes are also incorporated into gene panels for investigation of cataracts.
Neonatal onset cataracts are a feature of the galactose metabolism disorders. Galactose is derived from the disaccharide lactose, the main carbohydrate in mammalian milk. Lactose is hydrolysed by intestinal lactase generating galactose and glucose which are then absorbed across the brush border by the SGLT1 co-transporter. Galactose is phosphorylated to galactose-1-phosphate by the galactokinase (GALK), and is then converted to uridine diphosphogalactose (UDPgalactose) by galactose-1-phosphate uridyltransferase (GALT or ‘Gal-1-put’). UDPgalactose is an important source of galactose for the generation of complex glycoconjugates such as glycoproteins and glycosaminoglycans. Galactose can also be converted to galactitol via aldose reductase; and UDPglucose can be interconverted to UDPgalactose by UDPgalactose-4-epimerase (GALE).
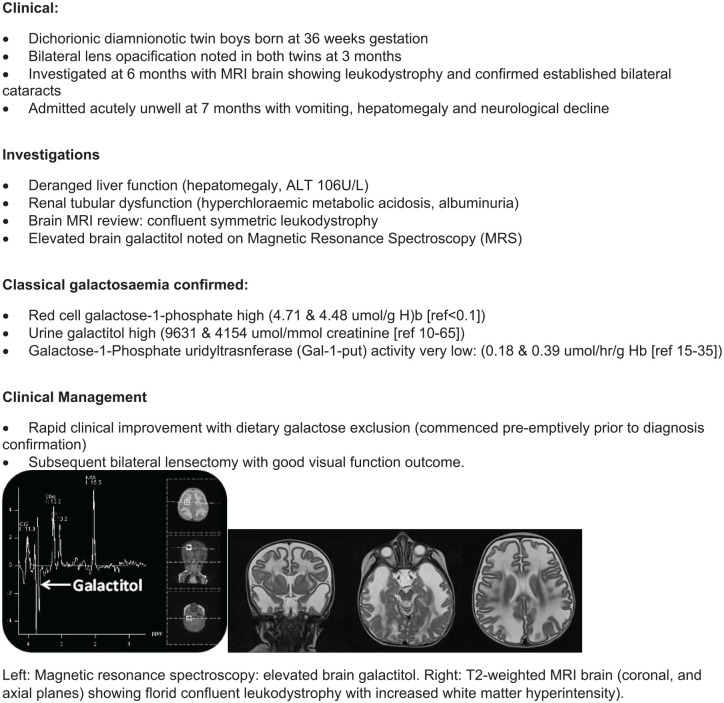
Disorders of galactose metabolism are associated with defects in any of these enzymes (GALT/Gal-1-put, GALK, epimerase/GALE). Cataract formation in all of the disorders occurs due to the accumulation of galactitol in the crystalline lens. Classical galactosaemia is caused by GALT deficiency, and is associated with very early oil drop cataract; this may resolve spontaneously if treatment with galactose-free diet is introduced promptly, but the cataract may mature and require surgical correction if left untreated (see Figure 1).48–50 Progressive liver dysfunction with jaundice and coagulopathy, renal and cerebral disease manifest in the neonatal period in children with classical galactosaemia, and urgent treatment is required to avert death. Epimerase (GALE) deficiency can present with severe neonatal disease, or can remain asymptomatic if there is residual enzyme activity; juvenile cataracts may be a presenting feature.51 In galactokinase deficiency cataracts are the only clinical feature seen, due to the osmotic effect of galactitol causing swelling of fibres within the lens. Liver disease is not seen, and treatment with galactose restriction can lead to resolution of the cataracts if they are not established.52
Figure 1.
Atypical presentation of classical galactosaemia [from JED clinical archive].
Testing for the galactosaemias includes erythrocyte enzyme assays, which are therefore not valid after a recent red cell blood transfusion. Erythrocyte galactose-1-phosphate levels are measured. In some situations (eg if a sick neonate has received a red cell transfusion) testing Gal-1-put activity in both parents can establish if they have heterozygous enzyme activity levels. Molecular genetic testing will help confirm the diagnosis. In all situations where galactosaemia is suspected, immediate treatment with dietary galactose restriction is mandatory until testing is completed to confirm or refute the diagnosis.
Next generation sequencing molecular genetic technologies have been employed in the investigation of children with idiopathic cataracts, for example, identifying a novel disorder also affecting the polyol pathways linked with galactitol accumulation.53
Cataracts appearing during infancy and childhood may be due to many different IMD. The LSD conditions including alpha mannosidosis, sialidosis and Fabry disease all are associated with cataracts, and would usually be seen in the context of multisystem disease. A recently described primary disorder of autophagy (the process of internal cellular housekeeping), Vici syndrome, also includes cataracts in its constellation of symptoms.63,64
Juvenile/adult onset cataracts are seen in cerebrotendinous xanthomatosis (CTX), associated with progressive neurological abnormalities and tendon xanthomata, and Wilson disease can also present with ‘sunflower cataracts’ as well as the classical Kaiser-Fleischer rings.23,24
Late (adult) onset cataracts may be seen in several IMD, including in gyrate atrophy of choroid and retina due to ornithine aminotransferase deficiency where posterior capsule cataracts are associated with visual field defects and retinopathy; affected patients may benefit from dietary measures and some oral medications have been trialled. These patients may have had biochemical abnormalities in the neonatal period that can include transient hyperammonaemia.59,60
Glaucoma
Raised intraocular pressure may be seen as a secondary feature in several IMD with other ocular pathologies, including in the congenital disorders of glycosylation,65 Leber’s hereditary optic neuropathy66 and the mucopolysaccharidoses.67
Retina
Retinitis pigmentosa
Most cases of RP with loss of retinal photoreceptor cells and pigmentary deposits are caused by primary genetic defects, either autosomal dominant or recessive.119,120 However, RP is seen as part of many different IMD.
Primary vitamin E malabsorption, or disorders with secondary vitamin E and A deficiency such as abetalipoproteinaemia, cause RP together with other neurological manifestations of vitamin E deficiency. The progression of eye disease in these conditions can be altered with high dose vitamin supplementation.
The Cobalamin C defect in the vitamin B12 metabolic pathway is associated with RP, together with multisystem disease including neurological, haematological and cardio-pulmonary features in early onset patients, or a milder phenotype with slower progressing neurological disease with visual loss, although the visual defect may progress despite treatment.75,76
Disorders of brain iron accumulation (neurodegeneration with brain iron accumulation [NBIA]) including infantile neuroaxonal dystrophy (INAD) and pantothenate kinase deficiency (PKAN) incorporate retinal pathology,105,106 with pigmentary retinopathy reported in 58% of early-onset PKAN patients and 15% of late-onset patients.81 Treatments are currently symptomatic only; the diagnosis is often suggested due to specific features on MRI neuroimaging showing iron accumulation in specific brain regions.
The neuronal ceroid lipofuscinoses (Batten-disease spectrum) conditions have a very important ocular component including RP and progressive optic atrophy, with visual loss being a hallmark of many of the subtypes associated with 13 different genes.73,74,101–103 Recently disease modifying treatment for one subtype (CLN2) has been licenced, and gene therapy approaches are being investigated for other subtypes.
Mitochondrial disorders can present with RP with visual failure as a primary feature, for example in neuropathy, ataxia with retinitis pigmentosa (NARP).83,84
Cherry red spot
The retinal ‘cherry red spot’ detected on fundoscopy describes the finding of a deep red circular area temporal to the optic disc at the centre of macula, surrounded by a pale ‘halo’. The pale halo is caused by accumulation of storage material within the retinal cells which lose their normal transparency, while the foveola which does not contain ganglion cells remains transparent and so the choroid vasculature remains visible and generates the ‘red spot’.121,122 It is seen as an acquired phenomenon if there is central retinal artery thrombosis, but in children is usually due to an IMD and its identification can help refine the differential diagnosis considerably in a child with multisystem disease. Specifically several LSDs are associated with the cherry red spot as these conditions result in accumulation of intracellular storage material. These disorders may also have hepatosplenomegaly as in GM1 gangliosidosis, galactosialidosis or Niemann Pick A disease, and dysmorphic facial features may also be evident. Prominent myoclonus or startle response is seen in GM2 gangliosidoses (Tay Sachs and Sandhoff variants). Diagnosis relies on both urinary studies identifying the storage material, and enzyme assays with confirmation with molecular genetic testing. As there is phenotypic overlap, a panel approach to both enzymology and molecular genetic testing is warranted.4 Treatment is mostly symptomatic, although disease modifying treatments are becoming available for some LSDs, and this together with the importance of genetic counselling for the family highlights the importance of prompt diagnosis.
Optic atrophy
Optic atrophy, i.e. degeneration of the retinal ganglion cells with optic nerve pallor due to deterioration of the optic nerve at any point in its course, can manifest in many IMD. Optic atrophy is an important feature in many of the mitochondrial disorders, including Leber hereditary optic neuropathy (LHON).97 The ocular features of mitochondrial disorders are very diverse, and include as described previously disorders of ocular motor function as well as visual function.93–96 The diagnosis of mitochondrial disease can be challenging, given the significant heterogeneity in clinical presentation, time course of disease evolution, and the complex genetics of mitochondrial function.2 Biotinidase deficiency is eminently treatable with simple oral biotin, which can prevent the progression of optic atrophy, and indeed is screened for in many countries as part of newborn screening.98–100
Optic atrophy can be seen in many of the lysosomal disorders including Krabbe and metachromatic leukodystrophy where there is progressive loss of cerebral nervous tissue, the peroxisomal biogenesis disorders and X-linked adrenoleukodystrophy.
Treatments
Treatment approaches to the different IMD are varied depending on the aetiology of the condition. Symptomatic management is the mainstay for many of the disorders. Some IMD have specific dietetic interventions (e.g. galactose restricted diet in the galactosaemias), while others respond to high dose vitamin or cofactor supplementation. For a number of the LSDs, enzyme replacement therapy is available. The impact of these therapies on the ophthalmic manifestations of the disease is very variable, in some situations (e.g. vitamin E replacement in primary vitamin E deficiency or abetalipoproteinaemia, or biotin replacement in biotinidase deficiency) this may prevent or reverse visual impairment, but in others the eye is relatively refractory to therapy.
Specific ophthalmological interventions may be required. For those with refractive error or visual impairment, appropriate provision of glasses or other visual aids will be required. Standard interventions for correction of strabismus may be undertaken, although understanding the natural history of each disorder is important in determining whether a therapy is appropriate. Management of cataract may require lensectomy, although in some situations instigation of appropriate dietetic management may facilitate resolution of the cataract if not established. For some conditions other surgical interventions including corneal grafting to correct corneal clouding has been beneficial, but appropriate investigation to determine retinal function should be undertaken in the surgical planning process as there may be concurrent optic atrophy or visual loss if there has been chronic uncorrected raised intraocular pressure. Certain disorders may lead to specific topical treatments such as cysteamine in cystinosis.
Conclusion
This brief review has summarised some of the many IMD that may present initially, or subsequently, with ophthalmic involvement. Early diagnosis of an IMD may be made if this is considered in the differential diagnosis of the ophthalmic complaint, which may facilitate earlier treatment. Discussion with the metabolic clinical and biochemical services can ensure that appropriate diagnostics are undertaken.
Footnotes
Conflict of interest statement: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: James E. Davison  https://orcid.org/0000-0002-9009-5538
https://orcid.org/0000-0002-9009-5538
References
- 1. Saudubray J-M, Baumgartner M, Walter J. Inborn metabolic diseases: diagnosis and treatment. 6th ed. Berlin: Springer-Verlag, 2016. [Google Scholar]
- 2. Davison JE, Rahman S. Recognition, investigation and management of mitochondrial disease. Arch Dis Child 2017; 102: 1082–1090. [DOI] [PubMed] [Google Scholar]
- 3. van der Knaap MS, Schiffmann R, Mochel F, et al. Diagnosis, prognosis, and treatment of leukodystrophies. Lancet Neurol 2019; 18: 962–972. [DOI] [PubMed] [Google Scholar]
- 4. Davison J. How to use … white blood cell enzyme assays. Arch Dis Child Educ Pract Ed 2017; 102: 143–147. [DOI] [PubMed] [Google Scholar]
- 5. Wassenberg T, Molero-Luis M, Jeltsch K, et al. Consensus guideline for the diagnosis and treatment of aromatic l-amino acid decarboxylase (AADC) deficiency. Orphanet J Rare Dis 2017; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Himmelreich N, Montioli R, Bertoldi M, et al. Aromatic amino acid decarboxylase deficiency: molecular and metabolic basis and therapeutic outlook. Mol Genet Metab 2019; 127: 12–22. [DOI] [PubMed] [Google Scholar]
- 7. Mills PB, Surtees RAH, Champion MP, et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5’-phosphate oxidase. Hum Mol Genet 2005; 14: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 8. Guerriero RM, Patel AA, Walsh B, et al. Systemic manifestations in Pyridox(am)ine 5’-Phosphate oxidase deficiency. Pediatr Neurol 2017; 76: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furukawa Y, Kish S. Tyrosine hydroxylase deficiency. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1437/. [Google Scholar]
- 10. Yanovitch TL, Banugaria SG, Proia AD, et al. Clinical and histologic ocular findings in pompe disease. J Pediatr Ophthalmol Strabismus 2010; 47: 34–40. [DOI] [PubMed] [Google Scholar]
- 11. Grönlund MA, Honarvar AK, Andersson S, et al. Ophthalmological findings in children and young adults with genetically verified mitochondrial disease. Br J Ophthalmol 2010; 94: 121–127. [DOI] [PubMed] [Google Scholar]
- 12. Atchaneeyasakul LO, Linck LM, Connor WE, et al. Eye findings in 8 children and a spontaneously aborted fetus with RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet 1998; 80: 501–505. [DOI] [PubMed] [Google Scholar]
- 13. Harris CM, Taylor DS, Vellodi A. Ocular motor abnormalities in Gaucher disease. Neuropediatrics 1999; 30: 289–293. [DOI] [PubMed] [Google Scholar]
- 14. Eghbali A, Hassan S, Seehra G, et al. Ophthalmological findings in Gaucher disease. Mol Genet Metab 2019; 127: 23–27. [DOI] [PubMed] [Google Scholar]
- 15. Blundell J, Frisson S, Chakrapani A, et al. Oculomotor abnormalities in children with Niemann-Pick type C. Mol Genet Metab 2018; 123: 159–168. [DOI] [PubMed] [Google Scholar]
- 16. Vanier MT, Gissen P, Bauer P, et al. Diagnostic tests for Niemann-Pick disease type C (NP-C): a critical review. Mol Genet Metab 2016; 118: 244–254. [DOI] [PubMed] [Google Scholar]
- 17. Koens LH, Tijssen MAJ, Lange F, et al. Eye movement disorders and neurological symptoms in late-onset inborn errors of metabolism. Mov Disord 2018; 33: 1844–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Åkebrand R, Andersson S, Seyedi Honarvar AK, et al. Ophthalmological characteristics in children with Leigh syndrome – A long-term follow-up. Acta Ophthalmol 2016; 94: 609–617. [DOI] [PubMed] [Google Scholar]
- 19. Morava E, Wosik HN, Sykut-Cegielska J, et al. Ophthalmological abnormalities in children with congenital disorders of glycosylation type I. Br J Ophthalmol 2009; 93: 350–354. [DOI] [PubMed] [Google Scholar]
- 20. Bahr O, Mader I, Zschocke J, et al. Adult onset glutaric aciduria type I presenting with a leukoencephalopathy. Neurology 2002; 59: 1802–1804. [DOI] [PubMed] [Google Scholar]
- 21. Clarke JTR. The gangliosidoses. In: Mehta A, Winchester B. (eds) Lysosomal storage disorders: a practical guide. Oxford: John Wiley & Sons, 2012; 63–69. [Google Scholar]
- 22. Wiltshire EJ, Poplawski NK, Harrison JR, et al. Treatment of late-onset nonketotic hyperglycinaemia: effectiveness of imipramine and benzoate. J Inherit Metab Dis 2000; 23: 15–21. [DOI] [PubMed] [Google Scholar]
- 23. Ram J, Gupta A. Kayser-Fleischer ring and sunflower cataract in Wilson disease. JAMA Ophthalmol 2014; 132: 873. [DOI] [PubMed] [Google Scholar]
- 24. Goel S, Sahay P, Maharana PK, et al. Ocular manifestations of Wilson’s disease. BMJ Case Rep 2019; 12: e229662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabinowitz LG, Williams LR, Anderson CE, et al. Painful keratoderma and photophobia: hallmarks of tyrosinemia type II. J Pediatr 1995; 126: 266–269. [DOI] [PubMed] [Google Scholar]
- 26. Locatelli F, Puzenat E, Arnoux JB, et al. Richner-Hanhart syndrome (tyrosinemia type II). Cutis 2017; 100: E20–E22. [PubMed] [Google Scholar]
- 27. Mehta A, Ramaswami U. Fabry disease. In: Mehta A, Winchester B. (eds) Lysosomal storage disorders: a practical guide. Oxford: John Wiley & Sons, 2012: 58–62. [Google Scholar]
- 28. Dureau P, Broyer M, Dufier JL. Evolution of ocular manifestations in nephropathic cystinosis: a long-term study of a population treated with cysteamine. J Pediatr Ophthalmol Strabismus 2003; 40: 142–146. [DOI] [PubMed] [Google Scholar]
- 29. Gahl WA, Kuehl EM, Iwata F, et al. Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops. Mol Genet Metab 2000; 71: 100–120. [DOI] [PubMed] [Google Scholar]
- 30. Biswas S, Gaviria M, Malheiro L, et al. Latest clinical approaches in the ocular management of cystinosis: a review of current practice and opinion from the ophthalmology cystinosis forum. Ophthalmol Ther 2018; 7: 307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrari S, Ponzin D, Ashworth JL, et al. Diagnosis and management of ophthalmological features in patients with mucopolysaccharidosis. Br J Ophthalmol 2011; 95: 613–619. [DOI] [PubMed] [Google Scholar]
- 32. Tomatsu S, Pitz S, Hampel U. Ophthalmological findings in mucopolysaccharidoses. J Clin Med 2019; 8: 1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raas-Rothschild A, Pohl S, Braulke T. Multiple enzyme deficiencies. In: Mehta A, Winchester B. (eds) Lysosomal storage disorders: a practical guide. Oxford: John Wiley & Sons, 2012; 121–126. [Google Scholar]
- 34. Seyrantepe V, Hinek A, Peng J, et al. Enzymatic activity of lysosomal carboxypeptidase (cathepsin) A is required for proper elastic fiber formation and inactivation of endothelin-1. Circulation 2008; 117: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 35. D’Azzo A, Bonten EJ. Defect in protective protein/cathepsin A: galactosialidosis. In: Mehta A, Winchester B. (eds) Lysosomal storage diseases: A practical guide. Oxford: John Wiley & Sons, 2012; 115–120. [Google Scholar]
- 36. Matlach J, Zindel T, Amraoui Y, et al. Retinal and optic nerve degeneration in alpha-mannosidosis. Orphanet J Rare Dis 2018; 13: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ceccarini MR, Codini M, Conte C, et al. Alpha-mannosidosis: therapeutic strategies. Int J Mol Sci 2018; 19: 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brewer HB, Jr, Remaley AT, Neufeld EB, et al. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler Thromb Vasc Biol 2004; 24: 1755–1760. [DOI] [PubMed] [Google Scholar]
- 39. Adams D, Wasserstein M. Free Sialic acid storage disorders. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1470/. [Google Scholar]
- 40. Morris AA, Kožich V, Santra S, et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis 2017; 40: 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Claerhout H, Witters P, Régal L, et al. Isolated sulfite oxidase deficiency. J Inherit Metab Dis 2018; 41: 101–108. [DOI] [PubMed] [Google Scholar]
- 42. Goyal R, Thompson D, Timms C, et al. Review of cases presenting with microcephaly and bilateral congenital cataract in a paediatric cataract clinic. Eye (Lond) 2008; 22: 273–281. [DOI] [PubMed] [Google Scholar]
- 43. Karikkineth AC, Scheibye-Knudsen M, Fivenson E, et al. Cockayne syndrome: clinical features, model systems and pathways. Ageing Res Rev 2017; 33: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bökenkamp A, Ludwig M. The oculocerebrorenal syndrome of Lowe: an update. Pediatr Nephrol 2016; 31: 2201–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steinberg SJ, Raymond GV, Braverman NE, et al. Zellweger spectrum disorder. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1448/. [Google Scholar]
- 46. Taylor RL, Handley MT, Waller S, et al. Novel PEX11B mutations extend the peroxisome biogenesis disorder 14B phenotypic spectrum and underscore congenital cataract as an early feature. Invest Ophthalmol Vis Sci 2017; 58: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vetter V, Shin YS. Lens sorbitol dehydrogenase deficiency in a patient with congenital cataract. Eur J Pediatr 1995; 154: 389–391. [DOI] [PubMed] [Google Scholar]
- 48. Berry GT. Classic galactosemia and clinical variant galactosemia. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1518/. [Google Scholar]
- 49. Chhapan RJ, Yerramneni R, Ramappa M. Diagnosing the oil drop: a case report and review of the literature. Indian J Ophthalmol 2019; 67: 1705–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beigi B, O’Keefe M, Bowell R, et al. Ophthalmic findings in classical galactosaemia–prospective study. Br J Ophthalmol 1993; 77: 162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Openo KK, Schulz JM, Vargas CA, et al. Epimerase-deficiency galactosemia is not a binary condition. Am J Hum Genet 2006; 78: 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bzduch V, Tomcikova D, Gerinec A, et al. Cataract and early nystagmus due to galactokinase deficiency. J Inherit Metab Dis 2017; 40: 749–750. [DOI] [PubMed] [Google Scholar]
- 53. Wortmann SB, Meunier B, Mestek-Boukhibar L, et al. Bi-allelic variants in TKFC encoding triokinase/FMN cyclase are associated with cataracts and multisystem disease. Am J Hum Genet 2020; 106: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marco-Marin C, Escamilla-Honrubia JM, Llácer JL, et al. Delta(1) -Pyrroline-5-carboxylate synthetase deficiency: an emergent multifaceted urea cycle-related disorder. J Inherit Metab Dis 2020. [DOI] [PubMed] [Google Scholar]
- 55. Almannai M, Dai H, El-Hattab AW, et al. FBXL4-related encephalomyopathic mitochondrial DNA depletion syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425540/. [Google Scholar]
- 56. Wang IH, Lin TY, Kao ST. Optical coherence tomography features in a case of Type I sialidosis. Taiwan J Ophthalmol 2017; 7: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilker SC, Dagnelie G, Goldberg MF. Retinitis pigmentosa and punctate cataracts in mevalonic aciduria. Retin Cases Brief Rep 2010; 4: 34–36. [DOI] [PubMed] [Google Scholar]
- 58. Degos B, Nadjar Y, del Mar Amador M, et al. Natural history of cerebrotendinous xanthomatosis: a paediatric disease diagnosed in adulthood. Orphanet J Rare Dis 2016; 11: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsang SH, Aycinena ARP, Sharma T. Inborn errors of metabolism: gyrate atrophy. Adv Exp Med Biol 2018; 1085: 183–185. [DOI] [PubMed] [Google Scholar]
- 60. Piozzi E, Alessi S, Santambrogio S, et al. Carbonic anhydrase inhibitor with topical NSAID therapy to manage cystoid macular edema in a case of gyrate atrophy. Eur J Ophthalmol 2017; 27: e179–e183. [DOI] [PubMed] [Google Scholar]
- 61. Nanetti L, Pensato V, Leoni V, et al. PEX7 mutations cause congenital cataract retinopathy and late-onset ataxia and cognitive impairment: report of two siblings and review of the literature. J Clin Neurol 2015; 11: 197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Allegrini D, Autelitano A, Fogagnolo P, et al. Lens opacities in glycogenoses type I and III. Can J Ophthalmol 2015; 50: 480–484. [DOI] [PubMed] [Google Scholar]
- 63. Byrne S, Dionisi-Vici C, Smith L, et al. Vici syndrome: a review. Orphanet J Rare Dis 2016; 11: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ebrahimi-Fakhari D, Saffari A, Wahlster L, et al. Congenital disorders of autophagy: an emerging novel class of inborn errors of neuro-metabolism. Brain 2016; 139: 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schweigert A, Areaux RG., Jr. Childhood glaucoma in association with congenital disorder of glycosylation caused by mutations in fucosyltransferase 8. J AAPOS 2019; 23: 351–352. [DOI] [PubMed] [Google Scholar]
- 66. Lin YH, Wang N-K, Yeung L, et al. Juvenile open-angle Glaucoma associated with Leber’s hereditary optic neuropathy: a case report and literature review. BMC Ophthalmol 2018; 18: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bruscolini A, Amorelli GM, Rama P, et al. Involvement of the anterior segment of the eye in patients with mucopolysaccharidoses: a review of reported cases and updates on the latest diagnostic instrumentation. Semin Ophthalmol 2017; 32: 707–714. [DOI] [PubMed] [Google Scholar]
- 68. Taylor RL, Arno G, Poulter JA, et al. Association of steroid 5alpha-reductase type 3 congenital disorder of glycosylation with early-onset retinal dystrophy. JAMA Ophthalmol 2017; 135: 339–347. [DOI] [PubMed] [Google Scholar]
- 69. Kousal B, Honzík T, Hansíková H, et al. Review of SRD5A3 disease-causing sequence variants and ocular findings in steroid 5alpha-reductase type 3 congenital disorder of glycosylation, and a detailed new case. Folia Biol (Praha) 2019; 65: 134–141. [DOI] [PubMed] [Google Scholar]
- 70. Esfandiari H, Mets MB, Kim KH, et al. Ocular abnormalities in a patient with congenital disorder of glycosylation type Ig. Ophthalmic Genet 2019; 40: 549–552. [DOI] [PubMed] [Google Scholar]
- 71. Thompson DA, Lyons RJ, Russell-Eggitt I, et al. Retinal characteristics of the congenital disorder of glycosylation PMM2-CDG. J Inherit Metab Dis 2013; 36: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 72. Morava E, Wevers RA, Cantagrel V, et al. A novel cerebello-ocular syndrome with abnormal glycosylation due to abnormalities in dolichol metabolism. Brain 2010; 133: 3210–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology 2012; 79: 183–191. [DOI] [PubMed] [Google Scholar]
- 74. Schulz A, Kohlschütter A, Mink J, et al. NCL diseases – clinical perspectives. Biochim Biophys Acta 2013; 1832: 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huemer M, Diodato D, Schwahn B, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis 2017; 40: 21–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martinelli D, Deodato F, Dionisi-Vici C. Cobalamin C defect: natural history, pathophysiology, and treatment. J Inherit Metab Dis 2011; 34: 127–135. [DOI] [PubMed] [Google Scholar]
- 77. Fahnehjelm KT, Holmström G, Ying L, et al. Ocular characteristics in 10 children with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: a cross-sectional study with long-term follow-up. Acta Ophthalmol 2008; 86: 329–337. [DOI] [PubMed] [Google Scholar]
- 78. Schrijver-Wieling I, van Rens GH, Wittebol-Post D, et al. Retinal dystrophy in long chain 3-hydroxy-acyl-coA dehydrogenase deficiency. Br J Ophthalmol 1997; 81: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Iwasa K, Shima K, Komai K, et al. Retinitis pigmentosa and macular degeneration in a patient with ataxia with isolated vitamin E deficiency with a novel c.717 del C mutation in the TTPA gene. J Neurol Sci 2014; 345: 228–230. [DOI] [PubMed] [Google Scholar]
- 80. Lee J, Hegele RA. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J Inherit Metab Dis 2014; 37: 333–339. [DOI] [PubMed] [Google Scholar]
- 81. Chang X, Zhang J, Jiang Y, et al. Natural history and genotype-phenotype correlation of pantothenate kinase-associated neurodegeneration. CNS Neurosci Ther 2020; 26: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Skjeldal OH, Stokke O, Refsum S, et al. Clinical and biochemical heterogeneity in conditions with phytanic acid accumulation. J Neurol Sci 1987; 77: 87–96. [DOI] [PubMed] [Google Scholar]
- 83. Stendel C, Neuhofer C, Floride E, et al. Delineating MT-ATP6-associated disease: from isolated neuropathy to early onset neurodegeneration. Neurol Genet 2020; 6: e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thorburn DR, Rahman J, Rahman S. Mitochondrial DNA-associated Leigh syndrome and NARP. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1173/. [Google Scholar]
- 85. Marchi G, Busti F, Lira Zidanes A, et al. Aceruloplasminemia: a severe neurodegenerative disorder deserving an early diagnosis. Front Neurosci 2019; 13: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hollak CE, de Sonnaville ES, Cassiman D, et al. Acid sphingomyelinase (Asm) deficiency patients in The Netherlands and Belgium: disease spectrum and natural course in attenuated patients. Mol Genet Metab 2012; 107: 526–533. [DOI] [PubMed] [Google Scholar]
- 87. Franceschetti S, Canafoglia L. Sialidoses. Epileptic Disord 2016; 18: 89–93. [DOI] [PubMed] [Google Scholar]
- 88. Yu FPS, Sajdak BS, Sikora J, et al. Acid ceramidase deficiency in mice leads to severe ocular pathology and visual impairment. Am J Pathol 2019; 189: 320–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Duffner PK, Barczykowski A, Jalal K, et al. Early infantile Krabbe disease: results of the World-Wide Krabbe Registry. Pediatr Neurol 2011; 45: 141–148. [DOI] [PubMed] [Google Scholar]
- 90. Duffner PK, Barczykowski A, Kay DM, et al. Later onset phenotypes of Krabbe disease: results of the world-wide registry. Pediatr Neurol 2012; 46: 298–306. [DOI] [PubMed] [Google Scholar]
- 91. Gieselmann V, Wenger DA, Krageloh-Mann I. Metachromatic leukodystrophy and globoid cell leukodystrophy. In: Mehta A, Winchester B. (eds) Lysosomal storage diseases: a practical guide. Oxford: John Wiley & Sons, 2012; 70–79. [Google Scholar]
- 92. Fouzdar-Jain S, Suh DW, Rizzo WB. Sjogren-Larsson syndrome: a complex metabolic disease with a distinctive ocular phenotype. Ophthalmic Genet 2019; 40: 298–308. [DOI] [PubMed] [Google Scholar]
- 93. Kisilevsky E, Freund P, Margolin E. Mitochondrial disorders and the eye. Surv Ophthalmol 2020; 65: 294–311. [DOI] [PubMed] [Google Scholar]
- 94. Finsterer J, Zarrouk-Mahjoub S, Daruich A. The eye on mitochondrial disorders. J Child Neurol 2016; 31: 652–662. [DOI] [PubMed] [Google Scholar]
- 95. Yu-Wai-Man P, Newman NJ. Inherited eye-related disorders due to mitochondrial dysfunction. Hum Mol Genet 2017; 26: R12–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pilz YL, Bass SJ, Sherman J. A Review of mitochondrial optic neuropathies: from inherited to acquired forms. J Optom 2017; 10: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Theodorou-Kanakari A, Karampitianis S, Karageorgou V, et al. Current and emerging treatment modalities for Leber’s hereditary optic neuropathy: a review of the literature. Adv Ther 2018; 35: 1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gannavarapu S, Prasad C, DiRaimo J, et al. Biotinidase deficiency: spectrum of molecular, enzymatic and clinical information from newborn screening Ontario, Canada (2007-2014). Mol Genet Metab 2015; 116: 146–151. [DOI] [PubMed] [Google Scholar]
- 99. Kellom ER, Wolf B, Rice GM, et al. Reversal of vision loss in a 49-year-old man with progressive optic atrophy due to profound biotinidase deficiency. J Neuroophthalmol. Epub ahead of print 31 March 2020. DOI: 10.1097/WNO.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 100. Hayati AA, Wan-Hitam WH, Cheong MT, et al. Optic neuritis in a child with biotinidase deficiency: case report and literature review. Clin Ophthalmol 2012; 6: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mole SE, Anderson G, Band HA, et al. Clinical challenges and future therapeutic approaches for neuronal ceroid lipofuscinosis. Lancet Neurol 2019; 18: 107–116. [DOI] [PubMed] [Google Scholar]
- 102. Kleine Holthaus SM, Smith AJ, Mole SE, et al. Gene therapy approaches to treat the neurodegeneration and visual failure in neuronal ceroid lipofuscinoses. Adv Exp Med Biol 2018; 1074: 91–99. [DOI] [PubMed] [Google Scholar]
- 103. Chen FK, Zhang X, Eintracht J, et al. Clinical and molecular characterization of non-syndromic retinal dystrophy due to c.175G>A mutation in ceroid lipofuscinosis neuronal 3 (CLN3). Doc Ophthalmol 2019; 138: 55–70. [DOI] [PubMed] [Google Scholar]
- 104. Hoshino H, Kubota M. Canavan disease: clinical features and recent advances in research. Pediatr Int 2014; 56: 477–483. [DOI] [PubMed] [Google Scholar]
- 105. Gregory A, Hayflick S. Neurodegeneration with brain iron accumulation disorders overview. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK121988/. [Google Scholar]
- 106. Hayflick SJ, Kurian MA, Hogarth P. Neurodegeneration with brain iron accumulation. Handb Clin Neurol 2018; 147: 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nahhas N, Conant A, Orthmann-Murphy J, et al. Pelizaeus-Merzbacher-like disease 1. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews. Seattle, WA: University of Washington, Seattle University of Washington, 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470716/. [Google Scholar]
- 108. Kaur P, Wamelink MMC, van der Knaap MS, et al. Confirmation of a rare genetic leukoencephalopathy due to a novel bi-allelic variant in RPIA. Eur J Med Genet 2019; 62: 103708. [DOI] [PubMed] [Google Scholar]
- 109. Engelen M, Kemp S, de Visser M, et al. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis 2012; 7: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wortmann SB, Kluijtmans LA, Rodenburg RJ, et al. 3-Methylglutaconic aciduria–lessons from 50 genes and 977 patients. J Inherit Metab Dis 2013; 36: 913–921. [DOI] [PubMed] [Google Scholar]
- 111. Gaier ED, Sahai I, Wiggs JL, et al. Novel homozygous OPA3 mutation in an Afghani family with 3-methylglutaconic aciduria type III and optic atrophy. Ophthalmic Genet 2019; 40: 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yahalom G, Anikster Y, Huna-Baron R, et al. Costeff syndrome: clinical features and natural history. J Neurol 2014; 261: 2275–2282. [DOI] [PubMed] [Google Scholar]
- 113. Biswas J, Nandi K, Sridharan S, et al. Ocular manifestation of storage diseases. Curr Opin Ophthalmol 2008; 19: 507–511. [DOI] [PubMed] [Google Scholar]
- 114. Curran L, Davison J, Shaughnessy L, et al. Visual loss post Ross procedure in an adolescent with newly diagnosed Mucopolysaccharidosis Type II. Ann Thorac Surg 2019; 108: e297–e299. [DOI] [PubMed] [Google Scholar]
- 115. Sadiq MA, Vanderveen D. Genetics of ectopia lentis. Semin Ophthalmol 2013; 28: 313–320. [DOI] [PubMed] [Google Scholar]
- 116. Fuchs J, Rosenberg T. Congenital ectopia lentis. A Danish national survey. Acta Ophthalmol Scand 1998; 76: 20–26. [DOI] [PubMed] [Google Scholar]
- 117. Khokhar SK, Pillay G, Dhull C, et al. Pediatric cataract. Indian J Ophthalmol 2017; 65: 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shen O, Michaelson-Cohen R, Gross-Tsur V, et al. Prenatal observation of nystagmus, cataracts, and brain abnormalities in a case of Zellweger spectrum disorder syndrome. Prenat Diagn 2016; 36: 894–895. [DOI] [PubMed] [Google Scholar]
- 119. Tsang SH, Sharma T. Retinitis pigmentosa (non-syndromic). Adv Exp Med Biol 2018; 1085: 125–130. [DOI] [PubMed] [Google Scholar]
- 120. Tsang SH, Sharma T. Autosomal dominant retinitis pigmentosa. Adv Exp Med Biol 2018; 1085: 69–77. [DOI] [PubMed] [Google Scholar]
- 121. Tripathy K, Patel BC. Cherry red spot. Treasure Island, FL: StatPearls; 2020. [PubMed] [Google Scholar]
- 122. Suvarna JC, Hajela SA. Cherry-red spot. J Postgrad Med 2008; 54: 54–57. [DOI] [PubMed] [Google Scholar]