Fusarium head blight (FHB) caused by the fungal pathogen Fusarium graminearum is an economically important disease of wheat and other small grain cereal crops worldwide, and limited effective control strategies are available. A better understanding of the regulation mechanisms of F. graminearum development, deoxynivalenol (DON) biosynthesis, and pathogenicity is therefore important for the development of effective control management of this disease.
KEYWORDS: Fusarium graminearum, golgin, RUD3, cis-Golgi, intracellular protein trafficking, virulence, FgRud3
ABSTRACT
Golgins are coiled-coil proteins that play prominent roles in maintaining the structure and function of the Golgi complex. However, the role of golgin proteins in phytopathogenic fungi remains poorly understood. In this study, we functionally characterized the Fusarium graminearum golgin protein RUD3, a homolog of ScRUD3/GMAP-210 in Saccharomyces cerevisiae and mammalian cells. Cellular localization observation revealed that RUD3 is located in the cis-Golgi. Deletion of RUD3 caused defects in vegetative growth, ascospore discharge, deoxynivalenol (DON) production, and virulence. Moreover, the Δrud3 mutant showed reduced expression of tri genes and impairment of the formation of toxisomes, both of which play essential roles in DON biosynthesis. We further used green fluorescent protein (GFP)-tagged SNARE protein SEC22 (SEC22-GFP) as a tool to study the transport between the endoplasmic reticulum (ER) and Golgi and observed that SEC22-GFP was retained in the cis-Golgi in the Δrud3 mutant. RUD3 contains the coiled coil (CC), GRAB-associated 2 (GA2), GRIP-related Arf binding (GRAB), and GRAB-associated 1 (GA1) domains, which except for GA1, are indispensable for normal localization and function of RUD3, whereas only CC is essential for normal RUD3-RUD3 interaction. Together, these results demonstrate how the golgin protein RUD3 mediates retrograde trafficking in the ER-to-Golgi pathway and is necessary for growth, ascospore discharge, DON biosynthesis, and pathogenicity in F. graminearum.
IMPORTANCE Fusarium head blight (FHB) caused by the fungal pathogen Fusarium graminearum is an economically important disease of wheat and other small grain cereal crops worldwide, and limited effective control strategies are available. A better understanding of the regulation mechanisms of F. graminearum development, deoxynivalenol (DON) biosynthesis, and pathogenicity is therefore important for the development of effective control management of this disease. Golgins are attached via their extreme carboxy terminus to the Golgi membrane and are involved in vesicle trafficking and organelle maintenance in eukaryotic cells. In this study, we systematically characterized a highly conserved Golgin protein, RUD3, and found that it is required for vegetative growth, ascospore discharge, DON production, and pathogenicity in F. graminearum. Our findings provide a comprehensive characterization of the golgin family protein RUD3 in plant-pathogenic fungus, which could help to identify a new potential target for effective control of this devastating disease.
INTRODUCTION
Fusarium graminearum is a pathogen that causes Fusarium head blight (FHB) in wheat (1, 2). In addition to reducing wheat yield and quality, F. graminearum produces the trichothecene mycotoxin deoxynivalenol (DON), which inhibits protein synthesis in eukaryotic organisms and is harmful to humans and other animals (3–6). DON is also an important virulence factor during plant infection. Previous studies have shown that DON biosynthesis is regulated by enzymes encoded by the tri genes, including tri1, tri4, tri5, tri6, and tri10 (7, 8). When DON is induced during plant infection, levels of mRNA expression in tri genes increase. TRI proteins are localized on the smooth endoplasmic reticulum (ER), where they manufacture DON-toxisome (here referred to as toxisome), which is involved in DON biosynthesis (9, 10). In addition to the tri genes, studies have shown that vesicle transport regulators are indispensable for DON biosynthesis (11–16).
The secretory pathway is a basic physiological process in eukaryotes for cell growth and differentiation. Partial regulators of the secretory pathway are involved in the pathogenesis of F. graminearum. For example, the SNARE protein ScSEC22 regulates the early secretory pathway, and its ortholog, SEC22, is reportedly required for vegetative growth, DON biosynthesis, and pathogenesis in F. graminearum (17). The guanine nucleotide exchange factor (GEF) ScSEC2 regulates the late secretory pathway by activating the small GTPase ScSEC4 in yeast, as well as its orthologs SEC2A, SEC2B, and RAB8, which also regulate growth, DON production, and pathogenesis in F. graminearum (13). The Golgi apparatus lies at the center of the secretory pathway, where it acts as a sorting hub for cargo proteins (18). Many proteins have been implicated in secretory trafficking and Golgi organization. The cytoplasmic face of the Golgi complex is decorated with large coiled-coil proteins called golgins, which are very conservative in eukaryotes and attached via their extreme carboxy terminus to the Golgi membrane. The main functions of golgins are to regulate vesicular traffic at the cis- and trans-Golgi in yeast and mammalian cells (19–21). However, the roles of golgins in filamentous fungi have not been thoroughly documented.
The yeast protein ScRUD3 and its mammalian ortholog GMAP-210 are golgin proteins localized to the cis-Golgi (22–26). In yeast, ScRUD3 is localized to cis-Golgi via the interaction of its C-terminal GRIP-related Arf binding (GRAB) domain with the Golgi GTPase ScARF1. Furthermore, ScRUD3 was identified as a suppressor of temperature-sensitive mutations in ScUSO1 (the yeast homologue of the golgin p115), ScSEC34 (a subunit of the conserved oligomeric Golgi [COG] complex), and ScRIC1 (guanine exchange factor subunit of small GTPase ScYPT6) (22, 23, 27). As these proteins regulate vesicle transport between different organelles, ScRUD3 is speculated to be involved in these transport processes. In mammalian cells, GMAP-210, studies have shown that a lack of GMAP-210 in tissue culture cells leads to Golgi ribbon fragmentation (27, 28) and anterograde and retrograde trafficking defective in the early secretory pathway (25, 29). GMAP-210 also has a partial functional overlap GM130 (25), another golgin protein that binds the Golgi-localized Rab protein RAB1, a component of coat protein complex II (COPII) vesicles (30). However, the fungi do not contain an obvious homologue of GM130. Moreover, the physiological importance of GMAP-210 is indicated by the fact that mutations in human GMAP-210 cause the neonatal lethal skeletal dysplasia achondrogenesis type 1A, and in mice GMAP-210 is responsible for cartilage and bone deposition (31). However, the function of ScRUD3/GMAP-210 in plant-pathogenic fungi has not been clearly defined.
Vesicular trafficking plays a key role in the pathogenesis of plant-pathogenic fungi, but there are many regulatory mechanisms that are not well understood, and little is known about golgins in F. graminearum pathogenesis in plants. Here, we characterized an F. graminearum golgin protein, RUD3, and demonstrated its important functions in retrograde trafficking of SEC22 between the ER and Golgi. In addition, deletion of RUD3 caused defective vegetative growth, ascospore discharge, DON production, and pathogenicity. Our results indicate that the golgin RUD3 is a pivotal regulator of various developmental and infection processes in F. graminearum.
RESULTS
Identification of the RUD3 protein in F. graminearum.
The golgin protein RUD3 (FGSG_ 04393) in F. graminearum is the homologous protein of Saccharomyces cerevisiae ScRUD3 (YOR216C). Phylogenetic analysis revealed closely related homologs of RUD3 among filamentous fungi (see Fig. S1A in the supplemental material). The amino acid sequence of RUD3 showed 87% identity to Fusarium oxysporum, 61% to Neurospora crassa, 57% to Magnaporthe oryzae, 27% to S. cerevisiae, and 28% to Candida albicans (Fig. S1B). These results suggest that RUD3 is conserved among eukaryotes.
RUD3 is involved in vegetative growth and conidiation.
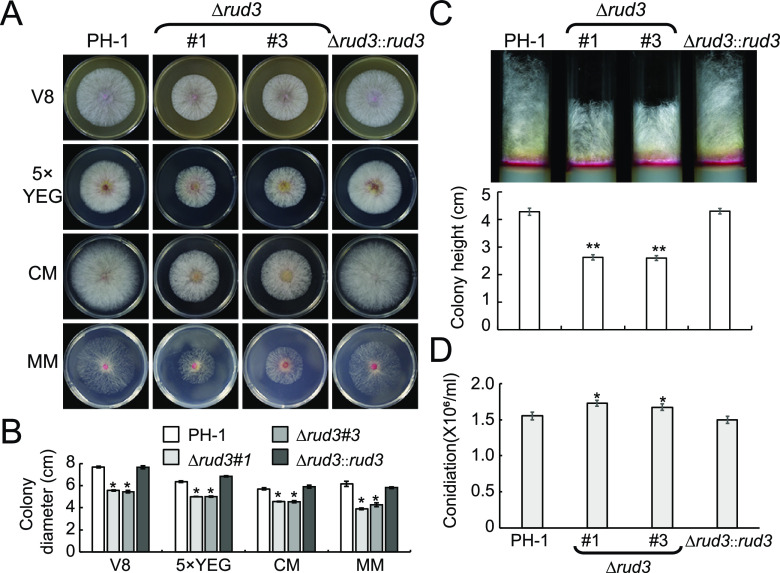
To characterize the cellular function of RUD3 in F. graminearum, we generated the Δrud3 mutant using the homologous recombination strategy (Fig. S2A). The deletion of rud3 was further confirmed by Southern blot analysis (Fig. S2B). When strain PH-1, Δrud3 mutants, and the Δrud3::rud3 complementary strain were each grown on V8 juice agar plates at 25°C for 3 days, the Δrud3 (no. 1 and no. 3) mutants showed significantly reduced growth rates (Fig. 1A and B). To extend our analysis, we examined whether this growth defect was medium-dependent. Compared with the strains PH-1 and Δrud3::rud3, the growth rates of Δrud3 (no. 1 and no. 3) mutants were reduced significantly on 5 × yeast extract-glucose (YEG), complete medium (CM), and minimal medium (MM) (Fig. 1A and B). We further examined the heights of aerial hyphae in the Δrud3 mutant and found that those of the Δrud3 (no. 1 and no. 3) mutants were drastically reduced compared to those of PH-1 and Δrud3::rud3 (Fig. 1C). To explore the role of RUD3 in asexual development, we examined whether RUD3 is required for conidial production. Surprisingly, we found that conidial production was slightly increased in the Δrud3 mutant compared to PH-1 and Δrud3::rud3, indicating that RUD3 negatively regulates conidial production (Fig. 1D).
FIG 1.
RUD3 plays an important role in vegetative growth but is not required for conidial production. (A) Colony morphology of the wild-type (WT) PH-1 and rud3 deletion mutants (Δrud3 no. 1 and no. 3) and complemented strain (Δrud3::rud3) grown on V8, 5 × yeast extract-glucose (YEG) medium, complete medium (CM), and minimal medium (MM) agar at 25°C for 3 days. (B) Statistical analysis of colony diameters of the indicated strains. Each graph represents the average of three experiments. *, P < 0.05. (C) Aerial hyphae of the indicated strains in test tubes cultured with potato dextrose agar (PDA) medium at 25°C for 3 days. Statistical analysis of the aerial hyphae of the indicated strains. **, P < 0.01. (D) Conidia produced by PH-1, Δrud3 (no. 1 and no. 3), and Δrud3::rud3 strains in 50 ml carboxymethyl cellulose (CMC) medium were counted after 5 days of incubation at 25°C. Error bars represent the standard deviation (SD). *, P < 0.05.
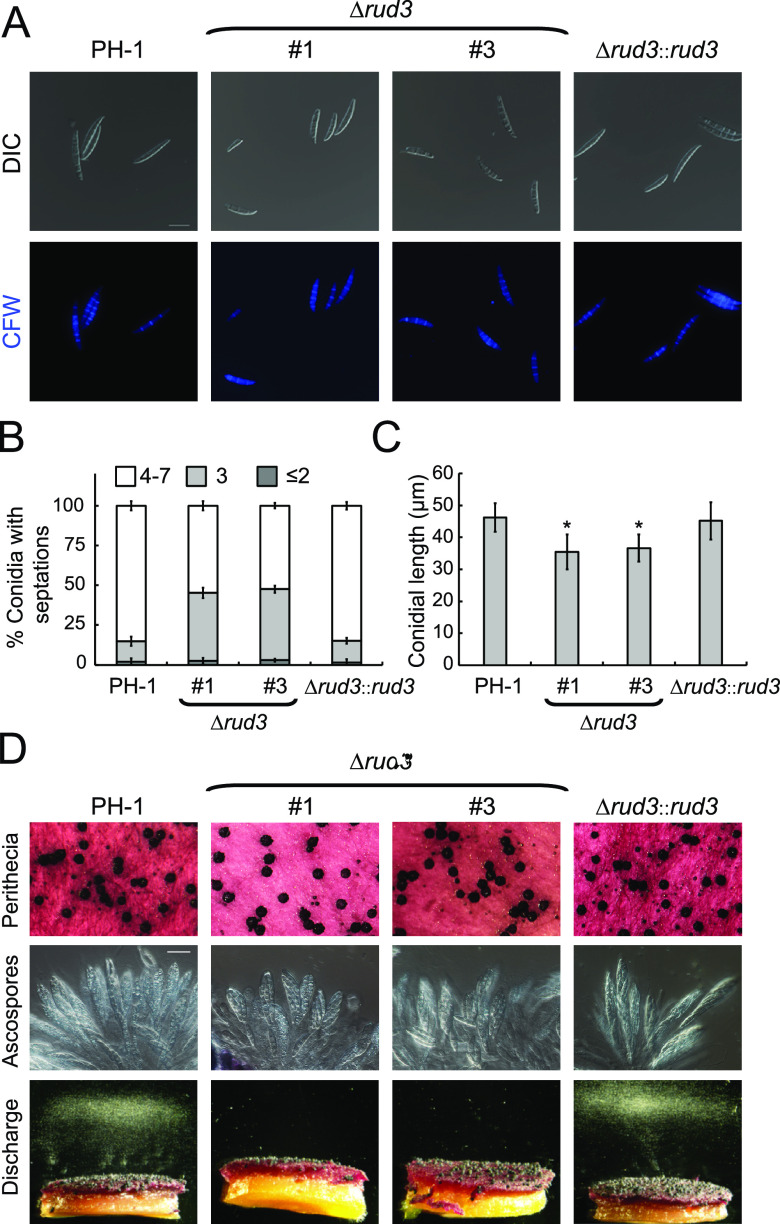
To examine conidial morphology further, conidia of each strain were stained with calcofluor white. The results showed that most conidia produced by the PH-1 (85.1%) and Δrud3::rud3 (84.9%) strains had a normal number of septa (four to seven). However, in the Δrud3 mutants, more than 45% of conidia had only two or three septa (Fig. 2A and B). We also found that conidia harvested from the Δrud3 mutants were shorter than those of the PH-1 and Δrud3::rud3 strains (Fig. 2C). These results suggest that RUD3 plays an essential role in hyphal growth and conidiation.
FIG 2.
RUD3 is essential for conidium morphology and ascospore discharge. (A) Conidia of the indicated strains cultured in CMC medium for 5 days. The septa were stained with calcofluor white and visualized by fluorescence microscopy. Bars, 20 μm. (B) Percentages of spores in panel A in each septa number category (≤2, 3, or 4 to 7). At least 300 spores were counted in at least three fields for each strain. Error bars represent the SD. (C) Statistical analysis of conidial length. Error bars represent the SD. *, P < 0.05. (D) Perithecia, ascospore formation, and ascospore discharge of the indicated strains on carrot agar plates, photographed at 14 days postinoculation (dpi). Bars, 20 μm.
RUD3 is required for ascospore discharge in F. graminearum.
In the FHB disease cycle, the sexual reproduction of F. graminearum plays an essential role in plant infection (32, 33). Therefore, we examined whether RUD3 is required for sexual reproduction on carrot agar plates. The results showed that PH-1, Δrud3 mutants, and Δrud3::rud3 produced abundant perithecia and normal ascospores 2 weeks postfertilization (Fig. 2D). When assayed for ascospore release, numerous ascospores were discharged from perithecia of PH-1 and Δrud3::rud3 (Fig. 2D). In contrast, no ascospores were discharged from the perithecia of the Δrud3 mutants (Fig. 2D). These results imply suggest RUD3 plays a crucial role in ascospore discharge in F. graminearum.
RUD3 is required for virulence and DON production.
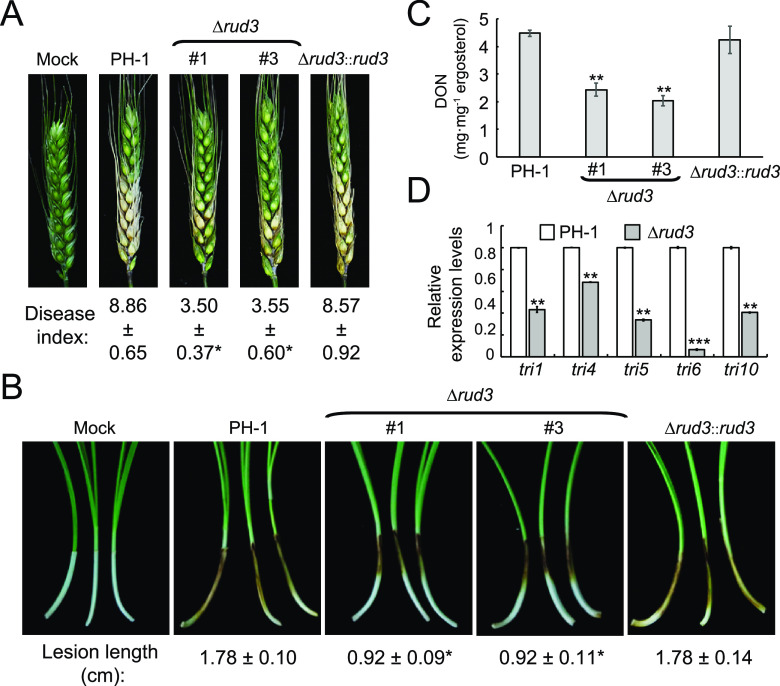
To determine whether RUD3 is essential for plant infection, the virulence of all strains was evaluated on flowering wheat heads by point inoculation. At 14 days postinoculation (dpi), wheat heads inoculated with PH-1 and Δrud3::rud3 showed severe head blight symptoms, with average disease index (diseased spikelets per head) values of approximately 8.86 and 8.57, respectively. In contrast, wheat heads inoculated with the Δrud3 mutant showed only mild symptoms, with an average disease index of <4 (Fig. 3A). The infection capability of the Δrud3 mutant was significantly lower than those of PH-1 and Δrud3::rud3. To extend our analysis, we conducted a droplet inoculation infection assay of wheat coleoptiles. The results were consistent with those following flowering wheat head inoculation (Fig. 3B). These results suggest that RUD3 is important for plant infection in F. graminearum.
FIG 3.
FgRud3 is required for full virulence of Fusarium graminearum. (A) Flowering wheat heads were inoculated with conidial suspensions of PH-1, Δrud3 (no. 1 and no. 3), and Δrud3::rud3 strains and checked at 14 dpi. The disease index was determined by the number of symptom spikelets per wheat head. Error bars represent the SD. *, P < 0.05. (B) Wheat coleoptiles were inoculated with 2 μl conidium suspension (2 × 105 spores/ml) and examined at 10 dpi. Error bars represent the SD. *, P < 0.05. (C) Deoxynivalenol (DON) production in wheat seeds infected with the indicated strains after 20 days of incubation. Error bars represent the SD. **, P < 0.01. (D) Relative expression levels of tri1, tri4, tri5, tri6, and tri10 in PH-1 and the Δrud3 mutant. The GAPDH gene was used as an internal control. Error bars represent the SD. **, P < 0.01; ***, P < 0.001.
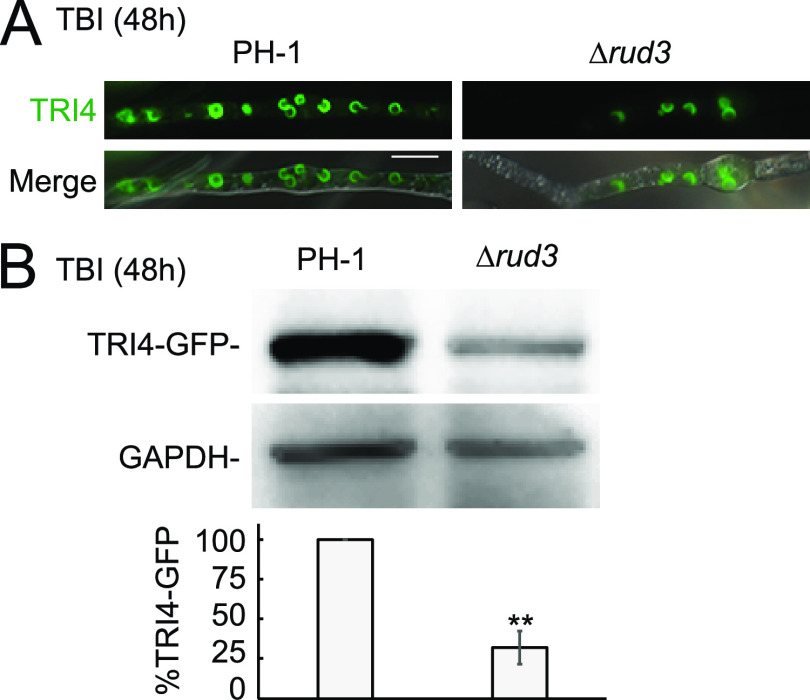
DON is a characterized virulence factor that contributes to pathogenicity in F. graminearum (34–36). Therefore, we examined the capability of DON production in the PH-1, Δrud3 mutant, and Δrud3::rud3 strains. The results showed that DON production was less than 2.5 mg · mg−1 of ergosterol in the Δrud3 mutant, which is around 55% of that produced by PH-1 or Δrud3::rud3 (Fig. 3C). Since DON production decreased in the Δrud3 mutant, we used quantitative real-time PCR (qRT-PCR) to determine the transcription levels of the DON biosynthesis-related genes tri1, tri4, tri5, tri6, and tri10. The expression levels of these genes were significantly reduced in the Δrud3 mutant (Fig. 3D), indicating that RUD3 is involved in expression modulation in these genes. DON biosynthesis is dependent on toxisome formation in the ER (10, 37). Therefore, we visualized the localization of TRI4-green fluorescent protein (GFP) in the PH-1, Δrud3 mutant, and Δrud3::rud3 strains to monitor toxisome formation directly. Under toxin-inducing conditions, we monitored toxisome formation at 24, 48, and 72 h. After 24 h of incubation, the TRI4-GFP signals had not accumulated in a crescent-shaped structure in the PH-1 and Δrud3 mutant strains (Fig. S3), whereas after 48 and 72 h of incubation, most of the TRI4-GFP signals had accumulated in a crescent-shaped structure in PH-1 hyphae, and few hyphae contained TRI4-GFP crescent-shaped structures in the Δrud3 mutant (Fig. 4A and Fig. S3). Western blot analysis was performed to detect the TRI4-GFP protein after 48 h of incubation in the wild-type (WT) and the Δrud3 mutant. The results showed that TRI4-GFP protein levels in the Δrud3 mutant were only 30% of that of the WT (Fig. 4B), indicating that RUD3 participates in toxisome formation. Moreover, after incubation in trichothecene biosynthesis induction (TBI) cultures, swollen hyphae or bulbous structures were observed in PH-1 and the Δrud3 mutant, but it is interesting that the swollen hyphae and bulbous structures in the Δrud3 mutant were bigger than those in wild types (Fig. S3). These results showed that RUD3 is involved in pathogenicity and DON production.
FIG 4.
RUD3 is involved in toxisome formation. (A) PH-1 and Δrud3 mutant strains expressing TRI4-GFP were incubated in TBI for 2 days at 25°C and visualized using live-cell fluorescence microscopy. Scale bars, 10 μm. (B) TRI4-GFP protein was determined in the hypha lysates using immunoblot analysis with anti-GFP antibodies with GAPDH as a loading control. The band intensities of blots from three independent experiments were quantified using ImageJ software (National Institutes of Health). Each graph represents the average of three experiments. **, P < 0.01.
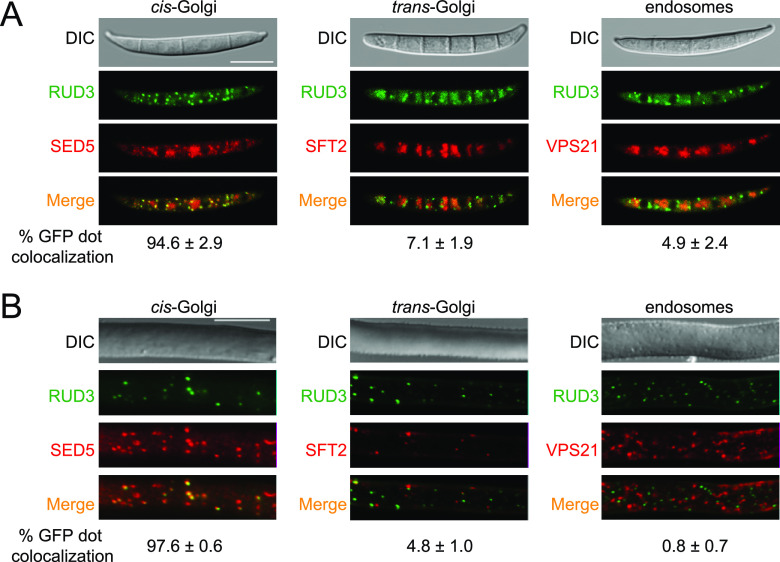
RUD3 is mainly localized in the cis-Golgi.
In yeast and mammalian cells, ScRud3/GMAP-210 is a golgin. Since golgins are reportedly attached to the Golgi membrane, to detect the localization of RUD3 in F. graminearum, we created a PH-1 strain expressing RUD3-GFP together with the cis-Golgi marker mCherry-SED5, trans-Golgi marker mCherry-SFT2, or early endosome marker tdTomato-VPS21. The results showed that in conidia, 94.6% of RUD3-GFP dots were colocalized with mCherry-SED5, 7.1% of RUD3-GFP dots were colocalized with mCherry-SFT2, and 4.9% of RUD3-GFP dots were colocalized with tdTomato-VPS21 (Fig. 5A). The location of RUD3-GFP in the hyphae was consistent with that in conidia (Fig. 5B). These results indicate that RUD3 is mainly localized in the cis-Golgi apparatus.
FIG 5.
RUD3 localizes to the cis-Golgi. (A and B) PH-1 coexpressed RUD3-GFP with mCherry-SED5, mCherry-SFT2, or tdTomato-VPS21 constructs. Conidia in panel A were harvested from 3-day-old CMC cultures. The hyphae in panel B were germinated in liquid CM for 24 h. The conidia and hyphae were visualized using a confocal microscope. Bars, 10 μm.
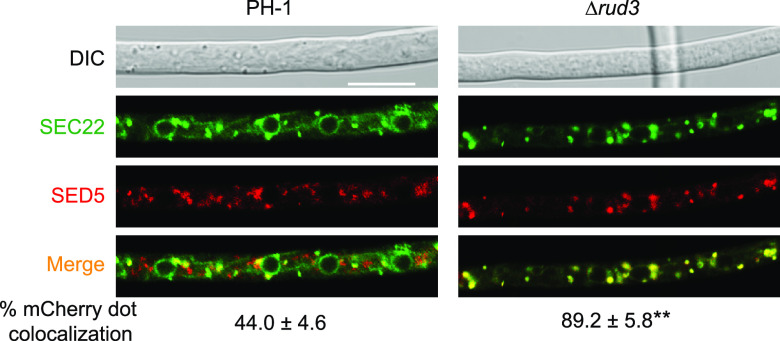
RUD3 affects SEC22 retrograde trafficking.
In eukaryotes, golgins regulate vesicle transport between different organelles. Therefore, we tested the effect of RUD3 on vesicle transport. Previous studies have shown that the SNARE protein ScSEC22, a v-SNARE in both COPII and COPI vesicles, is involved in both anterograde ER-to-Golgi trafficking and retrograde trafficking back to the ER (38–42), and SEC22 is reportedly required for pathogenicity in F. graminearum (17). First, we visualized the localization of SEC22 in PH-1 and the Δrud3 mutant. In the WT, SEC22-GFP cycled between the ER and Golgi and was mainly located in the ER. Our results showed that in PH-1, only 44% of cis-Golgi marker mCherry-SED5 dots were colocalized with GFP dots (Fig. 6). In the Δrud3 mutant, 89.2% of mCherry dots were colocalized with GFP dots (Fig. 6), suggesting that SEC22 can be transported to the cis-Golgi apparatus but is mainly trapped within the cis-Golgi apparatus and cannot return to the ER. Next, we tested whether RUD3 is required for the late secretory pathway (transport from trans-Golgi to the plasma membrane [PM]). In yeast, ScSNC1 is a v-SNARE that plays a role in fusion of trans-Golgi vesicles with the PM. For multiple rounds of vesicle fusion, ScSNC1 recycles back from the PM to the Golgi via endosomes (43). The ScSNC1 mutant with two amino acid substitutions (V40A and M43A), called ScSNC1-PEM protein, interferes with endocytosis and causes accumulation on the PM (44). Thus, GFP-ScSNC1-PEM is a marker for probing the late secretory transport. In F. graminearum, the function of the ortholog SNC1 is reportedly consistent with that of yeast (12). Therefore, we fused a GFP tag to the N terminus of SNC1-PEM (mutant V42A and M45A) and examined its cellular localization in the PH-1 and Δrud3 mutant strains. In PH-1 and Δrud3 hyphae, the GFP-SNC1-PEM accumulated on the PM (Fig. S4), demonstrating that RUD3 is not involved in the late secretory pathway. Third, we explored whether RUD3 plays a role in the endocytosis pathway by staining the hyphae of the PH-1 and Δrud3 mutant strains with FM4-64. FM4-64 was transported from the PM to the vacuole normally in both the WT and the Δrud3 mutant, indicating that RUD3 is not required for the endocytosis pathway (Fig. S5). These results support a role for RUD3 in retrograde trafficking from the cis-Golgi to the ER, which is consistent with GMAP-210 in mammalian cells (25).
FIG 6.
Multiple SEC22-GFP dots were trapped in the cis-Golgi apparatus of the Δrud3 mutant. WT and Δrud3 mutant strains coexpressing SEC22-GFP with mCherry-SED5 were treated as described in Materials and Methods. The percentage of mCherry-SED5 puncta-colocalized SEC22-GFP dots was quantified. At least 1,000 mCherry dots were counted in at least 3 fields for each strain. **, P < 0.01.
RUD3 is essential for various stress responses.
Previous studies have shown that a vesicular transport defect causes changes in responses to environmental stimuli (15, 17, 45, 46). Therefore, we examined the response of the mutant to various stresses such as oxidative, osmotic, and salt stress. First, the PH-1, Δrud3 mutant, and Δrud3::rud3 strains were cultured on CM plates with osmotic (NaCl, KCl, and sorbitol) stresses. The results showed that the inhibition rates of the Δrud3 mutant were lower than those of the WT and the complemented strain (Fig. 7A and D), suggesting that the Δrud3 mutant is more tolerant to osmoregulation stress. Next, we investigated the effect of the Δrud3 mutant on oxidative stress resistance (5 and 10 mM H2O2) and observed significantly decreased oxidative stress sensitivity; even under a stress of 5 mM H2O2, the Δrud3 mutant grew better than on CM plates (Fig. 7B and E). Third, to determine the effect of RUD3 on cell wall integrity, these strains were cultured on CM plates with cell wall stressors (SDS and CR). We found that the Δrud3 mutant expressed increased resistance to cell wall stressors (Fig. 7C and F), suggesting that the cell wall integrity of the Δrud3 mutant had increased.
FIG 7.
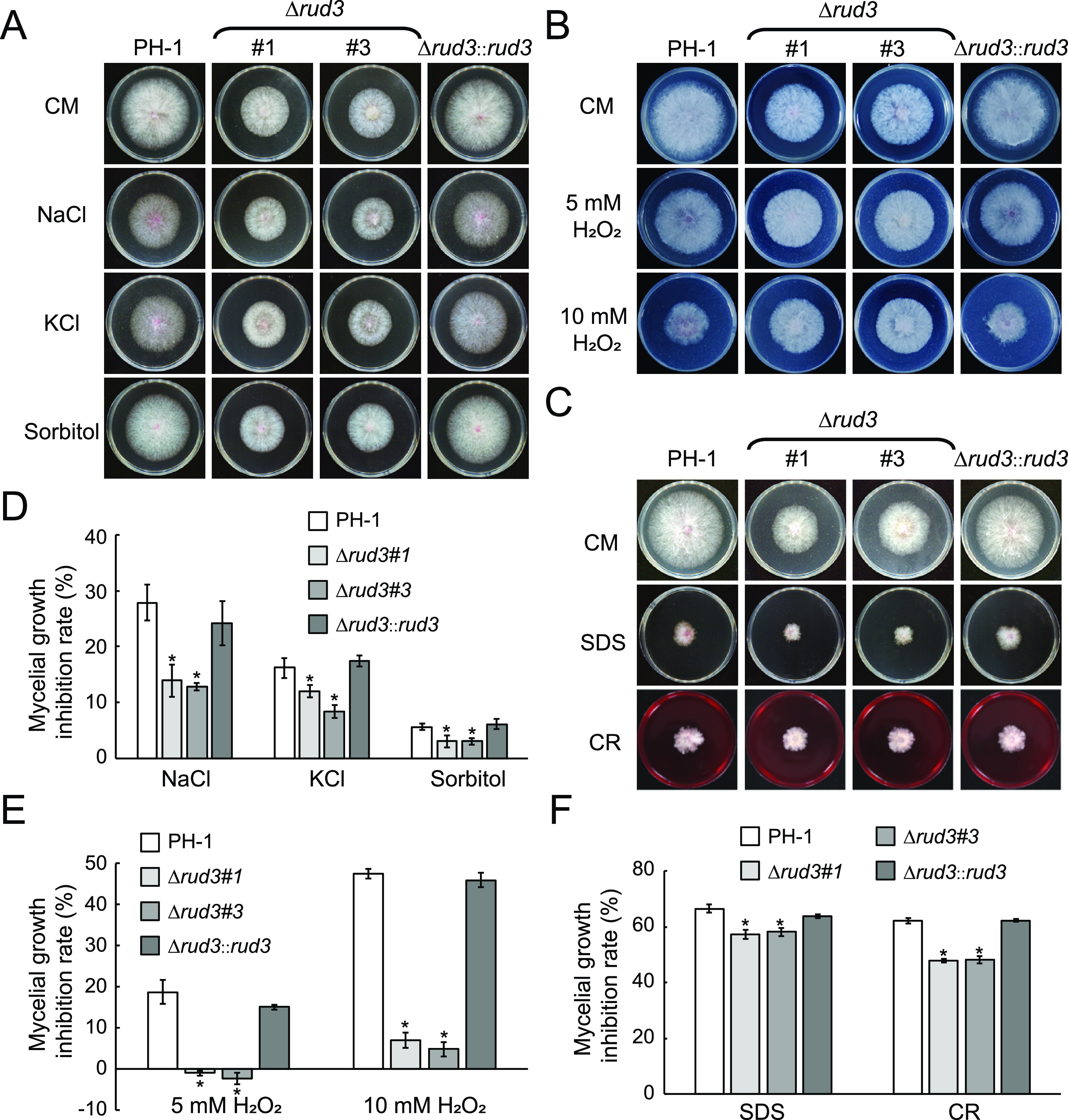
Vegetative growth of the PH-1, Δrud3, and Δrud3::rud3 strains under different stress conditions. (A) The PH-1, Δrud3, and Δrud3::rud3 strains were inoculated on CM containing NaCl, KCl, or sorbitol. (B) The indicated strains were inoculated on CM with 5 mM and 10 mM H2O2. (C) Cultures of all strains were grown on CM with sodium dodecyl sulfate (SDS) and Congo red (CR). (D to F). Statistical analysis of inhibition based on colony diameters after 3 days of incubation. Means and SDs were calculated from three replicates. *, P < 0.05.
RUD3 interacts with itself in F. graminearum.
In eukaryotes, most golgins are often depicted as rod-shaped homodimers (47, 48). However, it is unclear whether the golgin protein ScRUD3/GMAP-210 forms a dimer. In this study, RUD3 exhibited interaction with itself in a bifluorescence interaction complementation (BiFC) assay (Fig. 8A). Consistent with this result, interactions between RUD3 and itself were further confirmed in yeast two hybrid (Y2H) (Fig. 8C). To determine whether the RUD3-RUD3 interaction occurs in the cis-Golgi, we tested the colocalization of the RUD3-RUD3 BiFC puncta with cis-Golgi marker SED5 tagged with mCherry. In all hyphae that showed most BiFC (YFP) and mCherry puncta, the fluorescence overlapped (Fig. 8A). This result supports most of RUD3-RUD3 interaction in the cis-Golgi.
FIG 8.
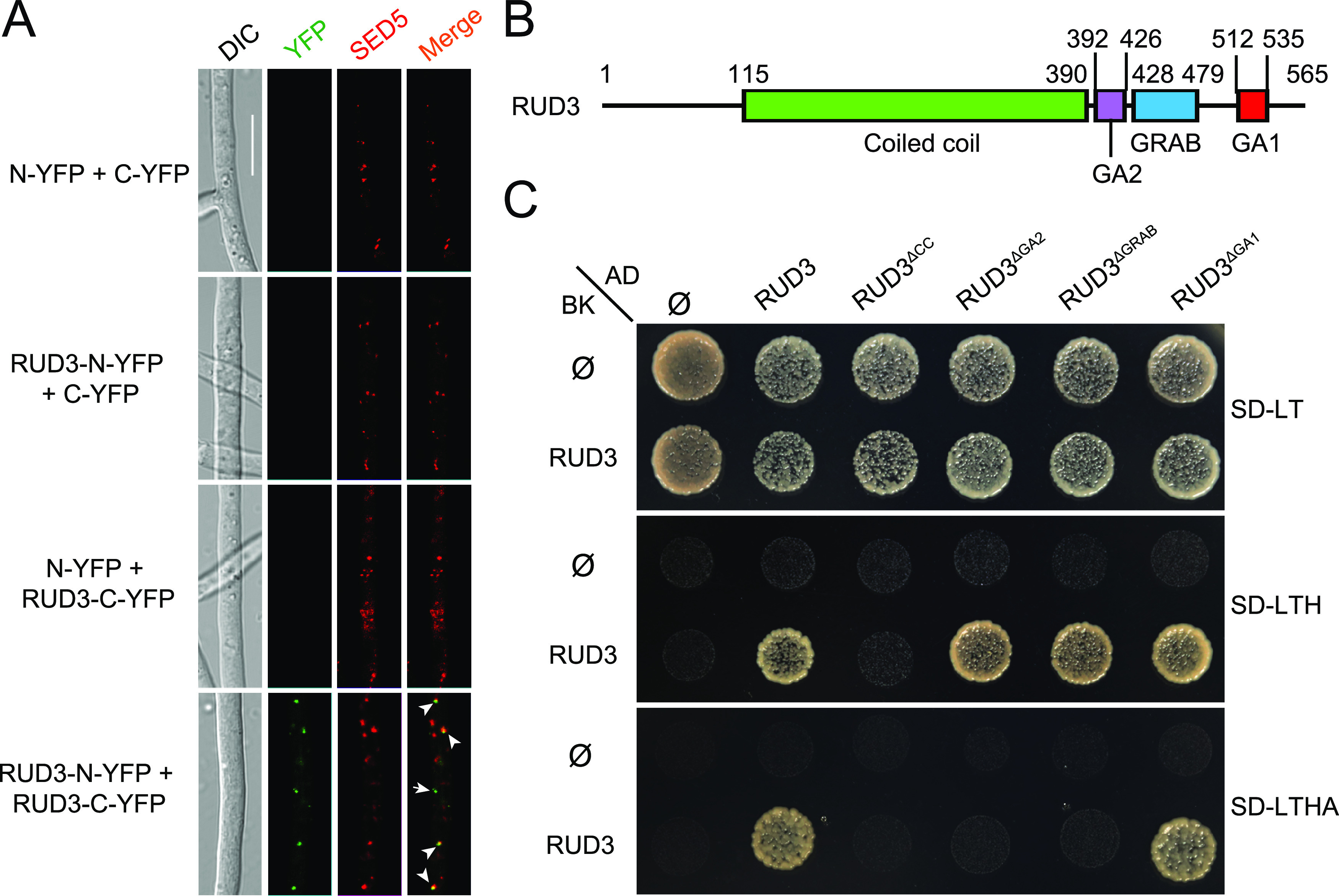
RUD3 interacts with itself through the coiled-coil (CC) domain. (A) RUD3-RUD3 interaction in SED5-marked cis-Golgi. YFP fluorescence was observed in hyphae coexpressing RUD3-N-YFP with RUD3-C-YFP; this strain also expressed mCherry-SED5. Overlap of YFP and mCherry fluorescence indicates that RUD3 self-interacts on the cis-Golgi (arrow). Arrowheads indicate the colocalization of green and red puncta. The arrow indicates green puncta that did not colocalize with red puncta. Bars, 10 μm. (B) The structure of RUD3. (C) RUD3 self-interacts via the CC domain, as shown in a Y2H assay. Prey and bait constructs were assayed for growth on SD-Leu-Trp, SD-Leu-Trp-His, and SD-Leu-Trp-His-Ade plates.
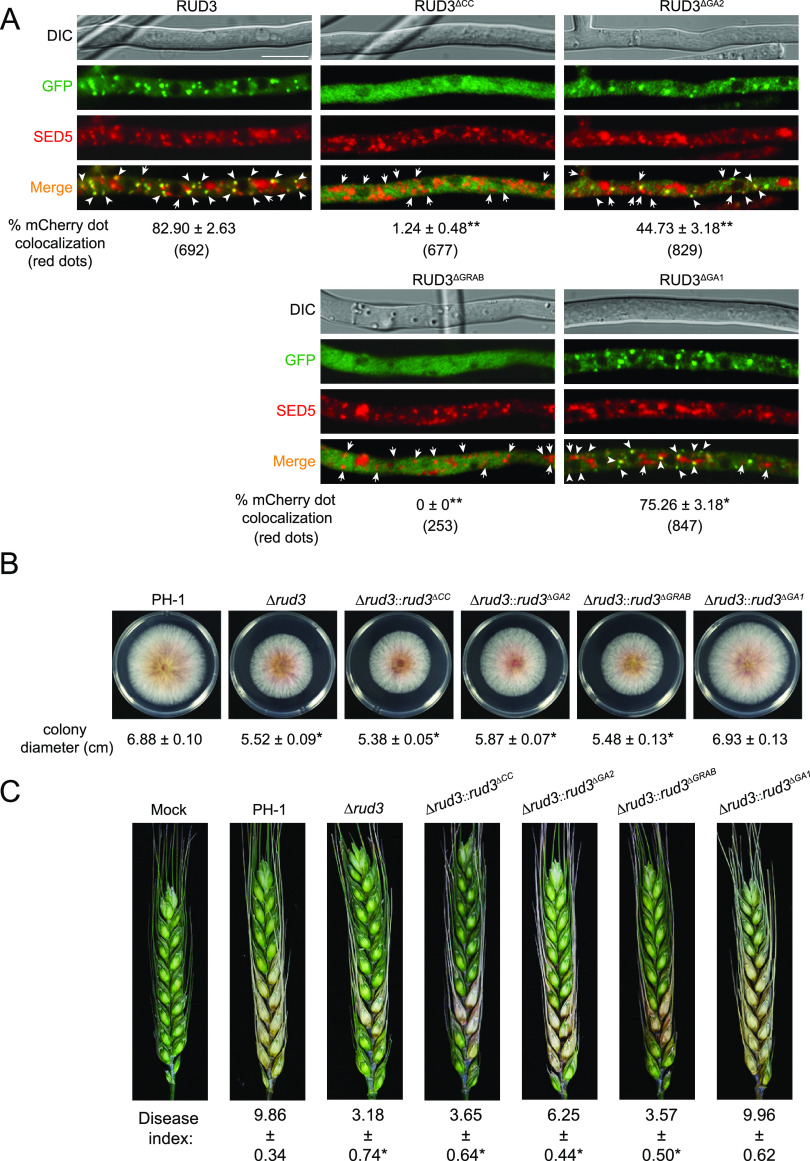
The domains of ScRUD3/GMAP-210 are highly conserved in eukaryotes. Sequence analysis has indicated that RUD3 contains four conserved domains, including the CC, GA2, GRAB, and GA1 domains (Fig. 8B). To screen for interacting domains, we constructed a series of RUD3 truncation mutants and tested self-interaction using Y2H assays. The results showed that RUD3 lacking the CC domain did not interact with RUD3, indicating that the CC domain is essential for RUD3-RUD3 interaction (Fig. 8C). When RUD3 lacked the GA2 or GRAB domain, the RUD3-RUD3 interaction became weak compared to that of the WT RUD3 (Fig. 8C). Thus, the GA1 domain was not required for RUD3 self-interaction (Fig. 8C).
Functional analysis of RUD3 domains.
The functions of different RUD3 domains in F. graminearum were also studied. The results showed that RUD3-GFP lacking the GRAB domain failed to locate to the cis-Golgi apparatus and remained diffused in the cytoplasm (Fig. 9A); the RUD3ΔCC-GFP signal also remained diffused in the cytoplasm (Fig. 9A), suggesting that RUD3 location to the cis-Golgi apparatus depends on the CC and GRAB domains. Consistent with the fluorescence phenotype, RUD3ΔCC-GFP and RUD3ΔGRAB-GFP with abnormal location failed to rescue the vegetative growth and pathogenesis defects of the Δrud3 mutant (Fig. 9B and C). As shown in Fig. 8, RUD3-RUD3 interaction is dependent on its CC domain; therefore, RUD3 self-interaction is important for its localization in the cis-Golgi, vegetative growth, and pathogenicity. When RUD3 lacked the GA1 or GA2 domain, the RUD3ΔGA1-GFP and RUD3ΔGA2-GFP puncta were colocalized with mCherry-SED5 (Fig. 9A), suggesting that RUD3 lacking the GA1 or GA2 domain is not involved in its normal location. Although RUD3ΔGA1-GFP and RUD3ΔGA2-GFP were located to the cis-Golgi apparatus, we found only 45% and 75% of mCherry puncta colocalized with RUD3ΔGA2-GFP and RUD3ΔGA1-GFP puncta, respectively (Fig. 9A). Compared with the 83% of mCherry puncta that colocalized with RUD3-GFP puncta, absence of the GA2 domain reduced the ability of RUD3 to locate to the cis-Golgi apparatus, and absence of the GA1 domain slightly reduced RUD3 location to the cis-Golgi apparatus. Further analysis revealed that RUD3ΔGA1 rescued the defective vegetative growth and pathogenesis of the Δrud3 mutant but that RUD3ΔGA2 failed to rescue these phenotype defects, indicating that the GA2 domain is essential for RUD3 function (Fig. 9B and C). These results indicate that the CC, GA2, and GRAB domains are important for proper RUD3 localization and full function.
FIG 9.
Functional characterization of RUD3 domains. (A) Subcellular localization of RUD3ΔCC-GFP, RUD3ΔGA2-GFP, RUD3ΔGRAB-GFP, and RUD3ΔGA1-GFP in F. graminearum. PH-1 strains coexpressing RUD3ΔCC-GFP, RUD3ΔGA2-GFP, RUD3ΔGRAB-GFP, and RUD3ΔGA1-GFP with mCherry-SED5 were treated as described in Materials and Methods. Arrowheads indicate the colocalization of red and green puncta. Arrows indicate red puncta that did not colocalize with green puncta. Scale bars, 10 μm. The percentage of mCherry dot-colocalized GFP dots was quantified. At least 500 mCherry dots were counted in at least 3 fields for each strain. *, P < 0.05; **, P < 0.01. (B) Colony morphology of indicated strains cultured on PDA for 3 days at 25°C. Statistical analysis of the colony diameters of the indicated strains. Each graph represents the average of three experiments. *, P < 0.05. (C) Infection assay of the domain deletion transformants. Flowering wheat heads were drop-inoculated with conidial suspensions of the indicated strains and examined at 14 dpi. The disease index was determined from the number of symptomatic spikelets per wheat head. At least 30 wheat heads infected with each strain were counted for each strain. *, P < 0.05.
DISCUSSION
Vesicular transport is a basic physiological process in eukaryotes that plays an important role in the growth and development of organisms, including plant-pathogenic fungi. The Golgi apparatus lies at the center of the eukaryotic secretory pathway. Golgins, which are located in the Golgi apparatus, play a crucial role in Golgi-associated vesicle transport (47, 49–52). Although golgins have been reported in yeast, mammalian cells, and Arabidopsis, their functions in filamentous fungi remain largely unknown. In this study, we identified and characterized the golgin protein RUD3, a homolog of the yeast ScRUD3 protein, in F. graminearum. Our findings demonstrate that RUD3 mediates retrograde trafficking between the ER and cis-Golgi, thereby controlling vegetative growth, ascospore discharge, and plant infection processes in F. graminearum.
Like ScRud3/GMAP-210, RUD3 is a coiled-coil protein located in the cis-Golgi (26, 28). In S. cerevisiae, genetic interactions occur between ScRUD3 and several vesicle-transport components and regulators, such as the small GTPase ScYPT6, the golgin ScUSO1, and the COG complex subunit ScSEC34 (22, 23); these proteins regulate vesicle transport between different organelles. However, no studies have reported the regulation of transport between organelles by ScRUD3. In this study, we found that RUD3 regulates the retrograde transport of SEC22 (Fig. 6), which cycles between the ER and Golgi. These results suggest that RUD3 is involved in transport from the Golgi to the ER; this phenotype is consistent with that of mammalian cells (25). However, GMAP-210 also regulates the transport of COPII vesicles from the ER to the Golgi apparatus in mammalian cells (25), which has not been observed in F. graminearum. This suggests that RUD3 is not required for the transport from the ER to the Golgi apparatus. Together, these results show that RUD3 is involved in ER-to-Golgi retrograde transport in F. graminearum.
Abnormal vesicular transport can lead to the decrease of pathogenicity of plant-pathogenic fungi. Previous studies showed that both early endocytosis regulators (PEP12 and ESCRT complex), late endocytosis components (RAB7 and MON1), and the late secretory regulators (SEC2A/B and RAB8) are necessary for pathogenicity in F. graminearum (11, 13, 16, 53). In this study, we also showed that inactivation of RUD3 that resulted in defect in retrograde transport of ER-to-Golgi (Fig. 6) also led to decreased virulence (Fig. 3). However, it is known that distinct regulators of transport between organelles are implicated in fungal virulence a different way, by specifically regulating different biological processes that largely affect fungal virulence, such as growth, sexual reproduction, ascospore discharge, DON biosynthesis, or secretion.
Absence of ScRUD3 does not affect yeast vegetative growth (22). Moreover, mutants lacking RUD3 displayed a reduced hyphal growth rate (Fig. 1), in contrast to the function of ScRUD3 in S. cerevisiae. Surprisingly, conidial production was slightly higher in the Δrud3 mutant than in PH-1; this phenotype differs from those partially lacking other vesicle transport regulators, such as YPT7, SEC2, SEC4, PEP12, etc. (11, 13, 16). However, the proportion of conidia with morphological defects produced by the Δrud3 mutant was higher than that of PH-1 (Fig. 2A to C).
In the FHB disease cycle, sexual reproduction and ascospores of F. graminearum play an essential role during plant infection (33). Ascospores are formed in the perithecium, which ejects individual ascospores into the air in succession, approximately 45 s apart (54, 55). Fungal mutants lacking RAB7 and MON1 (defective in late endocytosis) fail to produce perithecia (16), and mutants lacking SEC2A/B and RAB8 (defective in the late secretory stage) produce a few perithecia (13). In contrast, absence of PEP12 (defective in early endocytosis) does not affect sexual reproduction, but PEP12 plays a key role in ascospore discharge (11). Consistent with its role as an early endocytosis regulator, we found that RUD3 also plays an essential role in ascospore discharge but is not involved in the formation of ascospores and asci (Fig. 2D). This may be one of the reasons for the decreased virulence of the Δrud3 mutant.
DON is an important virulence factor in F. graminearum that is synthesized during plant infection (36). Until now, all vesicular transport regulators have been shown to affect not only the virulence but also DON production and tri gene expression. Compared with PH-1, DON production was also significantly lower in the Δrud3 mutant (Fig. 3D). Further analysis showed that RUD3 positively regulated expression of the trichothecene biosynthesis genes tri1, tri4, tri5, tri6, and tri10, which mediated DON biosynthesis (Fig. 3D). Previous studies showed that many F. graminearum vesicle-transport components and regulators, such as ESCRT complexes, type II phosphoinositide 4-kinase LSB6, the GTPase RAB7 and its guanine nucleotide exchange factor MON1, and the effector HOPS complex, positively regulate tri gene expression (14, 16, 53, 56, 57). DON biosynthesis also depends on toxisome formation on the ER. CAPA/B, which are involved in endocytosis, have been reported to be required for toxisome generation, whereas the SNARE protein SEC22 is required for DON but not involved in toxisome formation. In this study, we found that compared with PH-1, toxisome production decreased to about 30% in the Δrud3 mutant (Fig. 4 and Fig. S3). TRI5 has been reported to be required for toxisome formation (37), and tri5 expression decreased significantly in the Δrud3 mutant (Fig. 3D), which may explain the observed decrease in the number of toxisomes in the Δrud3 mutant. These results suggest that RUD3 modulates toxisome formation by regulating the expression of tri5 and then affecting DON biosynthesis, and this contributes significantly to the virulence of F. graminearum.
Under toxin-inducing conditions, toxisome formation is accompanied by swollen hyphae or bulbous structure formation (58). In mutants lacking the regulator of toxisome formation, such as PRK1, END3, and SYN8, the swollen hyphae or bulbous structures were reduced (10, 59). In contrast, in the absence of PDE2, negative regulation of DON biosynthesis, abundant swollen hyphae, or bulbous hyphal structures were detected (58). In contrast to these results, the deletion of RUD3 caused larger swollen hyphae or bulbous hyphae compared with the WT (Fig. S3), whereas DON biosynthesis and the number of toxisomes were reduced (Fig. 3C and 4 and Fig. S3). As the cell wall integrity of the Δrud3 mutant was changed (Fig. 7C and F), we speculated that the change of cell wall integrity causes a larger swollen hyphae or bulbous hyphae in Δrud3 mutant.
The results of this study show that RUD3 is located in the cis-Golgi, which is consistent with its orthologs in yeast and humans. Like ScRUD3 in yeast, RUD3 contains four conserved domains, CC, GA2, GRAB, and GA1 (Fig. 8B). Our result showed that the localization of RUD3 was altered in the absence of GRAB, CC, and GA2, but not the GA1 domain (Fig. 9A). Moreover, RUD3ΔGRAB, RUD3ΔCC, and RUD3ΔGA2, but not RUD3ΔGA1, failed to rescue the defects in vegetative growth and pathogenesis in the Δrud3 mutant (Fig. 9B and C). It is suggested that GRAB, CC, and GA2, but not the GA1 domain, are more important for the normal function of RUD3 in F. graminearum.
The GRAB and CC domains are highly conserved in eukaryotes. It was reported that ScRUD3/GMAP-210 recruitment to the cis-Golgi depends on the conserved COOH-terminal GRAB domain of both S. cerevisiae and mammalian cells (23). In yeast, the GRAB domain binds directly with the GTP-binding protein ScARF1 (23, 24, 50), which was previously shown to play several roles in Golgi function, including the regulation of clathrin vesicle formation on the trans-Golgi and COPI vesicle formation (60). Both the GRAB domain of ScRUD3/GMAP-210 COOH-terminal and small GTPase ScARF1 are very conserved. In this study, we found that the absence of the RUD3 GRAB domain prevented recruitment to the cis-Golgi (Fig. 9A) and that RUD3 is involved in COPI vesicle transport from the cis-Golgi to the ER (Fig. 6). Given that both RUD3 and ScARF1 participate in COPI vesicle transport, and the GRAB domain is highly conserved, we speculate that RUD3 recruitment to the cis-Golgi depends on the GRAB domain binding ARF1, which is similar with S. cerevisiae. The CC domain is the most important feature of golgins, which form a rod-shaped homodimer dependent on the CC domain. However, whether ScRUD3/GMAP-210 forms a homodimer has not been reported. Both Y2H and BiFC assays showed that RUD3 can self-interact in F. graminearum and that RUD3-RUD3 interaction depends on the CC domain (Fig. 8). RUD3 lacking the CC domain could not locate to the cis-Golgi (Fig. 9A). Therefore, we speculate that RUD3 is recruited to the cis-Golgi after forming a dimer. The GA2 motif exists in yeast and F. graminearum but not in mammalian cells. The GA2 domain is thought to affect ScRUD3 function on the Golgi but not localization (23). However, our results show that only 44.7% of cis-Golgi were colocalized with RUD3 lacking the GA2 motif (Fig. 9A). This percentage is far less than that of cis-Golgi colocalized with RUD3, indicating that the GA2 motif affects, but is not required for, RUD3 location.
Together, our results indicate that the golgin protein RUD3 is involved in vegetative growth, tri gene expression, DON production, ascopore discharge, stress response, and pathogenicity. RUD3 is involved in Golgi-to-ER retrograde transport as SEC22 trapped in cis-Golgi in the Δrud3 mutant. We also found that RUD3 contains the CC, GA2, GRAB, and GA1 domains and that these motifs, except GA1, are important for RUD3 targeting and function. Like most other golgins in yeast and mammalian cells, RUD3 self-interacts in a manner dependent on the CC domain in F. graminearum. Further studies should identify the set of interactive partners for RUD3 to elucidate the molecular mechanisms of golgin proteins in the physiological and pathological life cycle of F. graminearum.
MATERIALS AND METHODS
Strains and plasmids.
The F. graminearum strains used in this study are listed in Table S1. F. graminearum gene (rud3) knockout was produced using the split-marker approach (61). We amplified 1,020-bp upstream and 942-bp downstream flanking sequences from genomic DNA of F. graminearum PH-1 using the primer pairs RUD3-A-F/RUD3-A-R and RUD3-B-F/RUD3-B-R, respectively (Table 1).
TABLE 1.
Primers used in this study
| Primer name | Oligonucleotide sequence (5′–3′) | Remark |
|---|---|---|
| RUD3-A-F | AGGATCCACCTTGACGATGACT | For rud3 5′ flank sequence amplification |
| RUD3-A-R | TTGACCTCCACTAGCTCCAGCCAAGCCAGAGGTTCAATGTCGTGCAGAT | |
| RUD3-B-F | GAATAGAGTAGATGCCGACCGCGGGTTTGATGAATGCCGAGTATTGATGT | For rud3 3′ flank sequence amplification |
| RUD3-B-R | TACTTGCAGTTTGGTCCCGAT | |
| RUD3-T-F | TACACCCGCTGAGAAGAAGG | For rud3 gene probe amplification |
| RUD3-T-R | CTGAGTCATCGTCAAGGTGG | |
| RUD3-MF | AACCCGACCCAAGTGACGAA | For identification of rud3 deletion transformants |
| RUD3-MR | GCCTCTCGTTCCTGGCTCAT | |
| RUD3-K1F | TACGACGGTGAGAGGGAGAG | |
| RUD3-K1R | ATGTTGGCGACCTCGTATTGG | |
| RUD3-K2F | ACCTATTCTACCCAAGCATCCAA | |
| RUD3-K2R | AACACAAAGCCGCCTGC | |
| HYG/F | GGCTTGGCTGGAGCTAGTGGAGGTCAA | For hph N-terminal sequence amplification |
| HY/R | GTATTGACCGATTCCTTGCGGTCCGAA | |
| YG/F | GATGTAGGAGGGCGTGGATATGTCCT | For hph C-terminal sequence amplification |
| HYG/R | AACCCGCGGTCGGCATCTACTCTATTC | |
| RUD3-CF | ATCGTGGTTCTCATCACCATCACCATCACTCGAGGTTTTCGGAGTTAAAGCGGC | For Δrud3 complementation and subcellular localization |
| RUD3-CR | GGTGAACAGCTCCTCGCCCTTGCTCACCTCGAGACTCCTGGACTCTGGTTTTGTTG | |
| RUD3-GFP-F | ATCGTGGTTCTCATCACCATCACCATCACTCGAGGTTTTCGGAGTTAAAGCGGC | For RUD3-GFP fusion construct generation |
| RUD3-GFP-R | GGTGAACAGCTCCTCGCCCTTGCTCACCTCGAGACTCCTGGACTCTGGTTTTGTTG | |
| RUD3ΔCC-1F | GTGGTTCTCATCACCATCACCATCACTCGAGGTTTTCGGAGTTAAAGCGGCATAG | For rud3ΔCC domain deletion construct generation |
| RUD3ΔCC-2R | GAAGGTTTTTCTCCTTGACTTCCTTCTCATAAGGATCGTCGGTCTTTGCTTTTTCGC | |
| RUD3ΔCC-3F | TCCAGTGGATGGCGAAAAAGCAAAGACCGACGATCCTTATGAGAAGGAAGTCAAGGAG | |
| RUD3ΔCC-4R | CCGGTGAACAGCTCCTCGCCCTTGCTCACCTCGAGACTCCTGGACTCTGGTTTTG | |
| RUD3ΔGA2-1F | GTGGTTCTCATCACCATCACCATCACTCGAGGTTTTCGGAGTTAAAGCGGCATAG | For rud3ΔGA2 domain deletion fusion construct generation |
| RUD3ΔGA2-2R | AGGTGAACGAACCTGTCTACATTGTCCTCTGGCTTGGTAGGCGCAGTTCGCTCAAG | |
| RUD3ΔGA2-3F | GACTCTGACACAAGAGCTTGAGCGAACTGCGCCTACCAAGCCAGAGGACAATGTAG | |
| RUD3ΔGA2-4R | CCGGTGAACAGCTCCTCGCCCTTGCTCACCTCGAGACTCCTGGACTCTGGTTTTG | |
| RUD3ΔGRAB-1F | GTGGTTCTCATCACCATCACCATCACTCGAGGTTTTCGGAGTTAAAGCGGCATAG | For rud3ΔGRAB domain deletion fusion construct generation |
| RUD3ΔGRAB-2R | ATGGGTAGGCGCAAATTGTTAGAGGTGCCTGGACGGGTCTTCTTGAGATATCGCAATGC | |
| RUD3ΔGRAB-3F | ATCACTTGACCAAGGCATTGCGATATCTCAAGAAGACCCGTCCAGGCACCTCTAACAA | |
| RUD3ΔGRAB-4R | CCGGTGAACAGCTCCTCGCCCTTGCTCACCTCGAGACTCCTGGACTCTGGTTTTG | |
| RUD3ΔGA1-1F | CTCGCCTTCTCTCAACACCGACATTTTTGCGGATACAACTGCAGAGACACCAAGCAG | For rud3ΔGA1 domain deletion fusion construct generation |
| RUD3ΔGA1-2R | CTTGAGCTGCCCTTTCTGCTTGGTGTCTCTGCAGTTGTATCCGCAAAAATGTCGGTG | |
| GAPDH-F | CTTACTGCCTCCACCAACTG | For qRT-PCR analysis |
| GAPDH-R | TGACGTTGGAAGGAGCGAAG | |
| TRI1-QF | TTGAACACTACCTCGGTGCT | For tri1 qRT-PCR analysis |
| TRI1-QR | AGTTCGCGAGCATTCTTGAC | |
| TRI4-QF | CCTGGTCTGGTCACCATTCT | For tri4 qRT-PCR analysis |
| TRI4-QR | ATGGCCAGTGTCCTTGAAGT | |
| TRI5-QF | GAGTGTTTCATGCATGGCTACGTC | For tri5 qRT-PCR analysis |
| TRI5-QR | CTGAGCCTCCTTCACATCGTCC | |
| TRI6-QF | TGTCGCTACTCAGAATGCCCTCAG | For tri6 qRT-PCR analysis |
| TRI6-QR | CCACCCTGCTAAAGACCCTCAG | |
| TRI10-QF | GCGACAGGAGCAAGAACATAA | For tri10 qRT-PCR analysis |
| TRI10-QR | GGCGGCGTAAATCTGAGTG | |
| TRI4-GF | ATCGTGGTTCTCATCACCATCACCATCACTCGAGTCCCGACGATGTGGTTATAT | For TRI4-GFP fusion construct generation |
| TRI4-GR | GGTGAACAGCTCCTCGCCCTTGCTCACCTCGAGCAAAGCCTTGAGAACCTTGA | |
| AD-RUD3-1F | ATGGCCATGGAGGCCAGTGAATTCATGTCGTCCGCCGTTACAAC | For RUD3 yeast two-hybrid test |
| AD-RUD3-2R | TCGATGCCCACCCGGGTGGAATTCTTAACTCCTGGACTCTGGTTTTGTTG | |
| BD-RUD3-1F | CATATGGCCATGGAGGCCGAATTCATGTCGTCCGCCGTTACAAC | |
| BD-RUD3-2R | AGGTCGACGGATCCCCGGGAATTCTTAACTCCTGGACTCTGGTTTTGTTG | |
| pHZ65-RUD3-NYFP-1F | GTGGTTCTCATCACCATCACCATCACTCGAGGTTTTCGGAGTTAAAGCGGCATAG | For RUD3 BiFC fusion construct generation |
| pHZ65-RUD3-NYFP-2R | GCTCACCATCGTGGCGATGGAGCGCTCGAGACTCCTGGACTCTGGTTTTGTTG | |
| pHZ68-RUD3-CYFP-3F | GTGGTTCTCATCACCATCACCATCACTCGAGGTTTTCGGAGTTAAAGCGGCATAG | |
| pHZ68-RUD3-CYFP-4R | GTTCGGGATCTTGCAGGCCGGGCGCTCGAGACTCCTGGACTCTGGTTTTGTTG | |
| mCherry-SED5-1F | CTGTACAAGATGGCTGTCGCATCTATCCAA | For mCherry-SED5 fusion construct generation |
| mCherry-SED5-2R | GTGAGAGTAGACTCTCTCACCTTGG | |
| mCherry-SFT2-1F | CTGTACAAGATGGCTTCTTCTTCCTTCCGA | For mCherry-SFT2 fusion construct generation |
| mCherry-SFT2-2R | CCGCTCGAGCTACCCAGTCATCCAGGCTGTT | |
| tdTomato-VPS21-1F | TCATCACCATCACCATCACTCGAGCCTTCTTCTCGGCTTCTTTGCG | For tdTomato-VPS21 fusion construct generation |
| tdTomato-VPS21-2R | GACCTCCTCGCCCTTGCTCACCATTGTCGCGGGATCGCTCGGAGAT | |
| tdTomato-VPS21-3F | TACGGCATGGACGAGCTGTACAAGATGGCCGATTCCACCAACG | |
| tdTomato-VPS21-4R | GGAATGATGGGATCCAAGCTCGAGCTAGCAAGCGCAACTATCCTTGG | |
| SEC22-GFP-1F | ATCGTGGTTCTCATCACCATCACCATCACTCGAGAAAGTAAGTGGAGGAGTCATCAACC | For SEC22-GFP fusion construct generation |
| SEC22-GFP-2R | GGTGAACAGCTCCTCGCCCTTGCTCACCTCGAGAAAGAATCGCCAATACAGGAAAA |
RUD3 deletion mutants were generated using the split-marker approach. We amplified 1020-bp upstream and 942-bp downstream flanking sequences from genomic DNA of F. graminearum PH-1 using the primer pairs RUD3-A-F/RUD3-A-R and RUD3-B-F/RUD3-B-R, respectively (Table 1).
The hygromycin phosphotransferase (hph) gene was amplified using the primer pairs HYG-F/HY-R and YG-F/HYG-R using pCB1003 as a sample. rud3 gene-replacement constructs were generated by overlapping PCR. Then, the PCR products were transformed into protoplasts of the wild-type (WT) PH-1 with polyethylene glycol (PEG)-mediated transformation, and then resulting transformants were screened by PCR with RUD3-MF/R, RUD3-K1F/R, and RUD3-K2F/R and further confirmed by Southern blotting (Table 1).
To clone rud3 and sec22 into pFL2 vector harboring green fluorescent protein (GFP) using the yeast gap repair approach, the open reading frames (ORFs) (without stop codon) of rud3 and sec22 containing 1,500 bp of the 5′-promoter region were amplified from the PH-1 genome using oligonucleotides. The rud3ΔCC-GFP, rud3ΔGA2-GFP, rud3ΔGRAB-GFP, and rud3ΔGA1-GFP plasmids were constructed into pFL2 vector by overlap PCR (62) based on the plasmid pFL2-rud3-GFP. The mCherry-sed5, mCherry-sft2, or tdTomato-vps21 with 1,500 bp of the 5′-promoter region were cloned into pFL2 vector. Escherichia coli transformation was carried out by electroporation. Each plasmid was transformed into protoplasts of PH-1 or the corresponding mutant for generating fluorescence-labeled strains (63).
Phylogenetic analysis, sequence alignment, and domain architecture.
The golgin protein Rud3 in F. graminearum was identified by a BLAST search of the NCBI using the S. cerevisiae ScRUD3 as a query. The rud3 ORF is 1,904 nucleotides long and encodes 565 amino acids. The phylogenetic tree was generated with MEGA 7.0, and amino acid sequence comparison was performed using ClustalW. The identification of functional domains of RUD3 was based on the previously reported S. cerevisiae ScRUD3 (23).
Fungal strains and culture conditions.
The PH-1 and mutant strains were grown at 25°C on complete medium (CM), V8 medium, 5 × yeast extract-glucose (YEG) medium, and minimal medium (MM) and incubated at 25°C for 3 days (64). For the stress assay, strains were cultured on CM with different concentrations of NaCl, KCl, sorbitol, sodium dodecyl sulfate (SDS), Congo red (CR), and H2O2 and incubated at 25°C for 3 days (63).
Asexual reproduction, sexual reproduction, and ascospore discharge assays.
Conidiation was examined by incubating 3 to 5 mycelial plugs in 100 ml carboxymethyl cellulose (CMC) medium (0.5 g MgSO4 · 7H2O, 1 g KH2PO4, 1 g yeast extract, 2 g NaNO3, 15 g sodium carboxymethyl cellulose, and distilled H2O up to 1,000 ml) after 5 days of incubation in an incubator at 25°C (63). A hemocytometer was used to count the conidia. To determine the germination rate of conidia, freshly harvested conidia from 3-day-old CMC cultures were resuspended in yeast extract peptone dextrose (YEPD) medium (10 g yeast extract, 20 g peptone, 20 g glucose, and distilled H2O up to 1,000 ml) for 0 to 12 h and examined under an upright fluorescence microscope (Eclipse Ni-U; Nikon) with a ×40 object lens (64). The experiment was repeated at least three times.
Aerial hyphae of 7-day-old cultures on carrot agar plates (400 g carrot, 20 g agar, and distilled H2O up to 1,000 ml) were pressed down with 1 ml sterile 2.5% Tween 20 solution as previously described (65). Perithecium formation and ascospore production were examined after 7 days of incubation in an incubator at 25°C (65). This experiment was repeated at least three times.
For ascospore discharge, agar blocks (1 cm diameter) covered with perithecia were placed on the end of a glass slide and oriented, and the surface containing the perithecia was perpendicular to the surface of the glass slide. Then slides were placed on a platform in a transparent humidity chamber under 12 h darkness and 12 h light. Discharged spores were observed and photographed under a stereomicroscope (SMZ25; Nikon) with a ×1 object lens (65).
Fluorescence microscopy observations and quantifications.
Hyphae or conidia expressing fluorescently tagged proteins or stained by 1 μM FM4-64 (66) were examined with a Nikon fluorescence microscope (Eclipse Ni-U; Nikon) or with a confocal microscope (Zeiss LSM 880 NLO system) with a ×40 object lens as previously described (63). Conidia stained with calcofluor white (CFW) (Sigma-Aldrich, Shanghai Trading Co. Ltd.) were examined with a Nikon fluorescence microscope with a ×40 object lens (Eclipse Ni-U; Nikon) (63). More than 10 fields were visualized for each sample. The percentage of colocalized dots is based on red dots quantified from 10 fields from each experiment. Data are presented as the mean ± standard deviation of each variable from three independent experiments.
Immunoblot assay.
Immunoblotting was performed as previously described (67) and repeated at least three times. Blots were immunoblotted with mouse anti-GFP (Santa Cruz) or rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) (Cell Signaling Technology) antibodies, and enhanced chemiluminescence (ECL) (Millipore) was used as the substrate.
Y2H and bimolecular fluorescence complementation (BiFC) assays.
For Y2H assays, the full-length cDNAs of rud3, rud3ΔCC, rud3ΔGA2, rud3ΔGRAB, and rud3ΔGA1 were cloned into pGBKT7 or pGADT7 as bait or prey constructs, which were cotransformed into yeast strain AH109 as previously described (11). The prey and bait constructs were assayed for growth on SD-Leu-Trp, SD-Leu-Trp-His, and SD-Leu-Trp-His-Ade plates.
For BiFC assays, the rud3 gene was cloned into pHZ65 and pHZ68 (11) to generate the YFP-N and YFP-C fusion constructs, respectively. The resulting constructs were verified by sequencing analysis and cotransformed into the PH-1 protoplasts. The resulting transformants were screened for YFP signals under a confocal microscope (Zeiss LSM 880 NLO system) with a 40× object lens.
Wheat infection and DON production assays.
The infection assay was performed on flowering wheat inflorescences as described previously (63, 68). The interior of a wheat spike glume (Jimai 22) was inoculated at the heading and flowering stage with 10 μl conidium suspension (2 × 105 spores/ml in sterile distilled water). The disease index was determined and the sample was photographed at 14 days postinoculation (dpi).
To infect the wheat coleoptile, 2.5 μl conidial suspension (2 × 106 spores/ml in sterile distilled water) was applied, and symptoms were observed at 8 dpi (69). At least 20 plants were inoculated with each strain.
For DON production assays, three mycelial plugs of each strain were inoculated with 5 g autoclaved wheat kernels (70). After 20 days of incubation at 25°C, DON was extracted and concentrations were determined by high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS; AB Sciex, 5500) (71). Each experiment was repeated at least three times.
RNA extraction and quantitative real-time PCR analysis.
Total RNA was extracted from mycelia of each sample using TRIzol Reagent (Invitrogen), and 1 μg of each RNA sample was used for reverse transcription with a HiScript II 1st strand cDNA synthesis kit (+gDNA wiper) (Vazyme Biotech, Nanjing, China) (63). The expression levels of tri genes (tri1, tri4, tri5, tri6, and tri10) under the induction of TBI liquid medium were determined by quantitative real-time PCR with the primers listed in Table 1 (63). For each sample, the F. graminearum GAPDH gene was used as the internal control. The experiment was repeated three times independently.
Statistical analyses.
All experimental data were collected from three independent samples to ensure the repeatability of trends and relationships observed in culture. Each error bar represents the standard deviation (SD) of three replicate samples. Sample means were analyzed using Student’s t test. One-way analysis of variance (ANOVA), followed by Duncan’s multiple range test, was used to analyze differences in the means between groups.
Data availability.
The GenBank accession numbers of the sequences are as follows: RUD3 (XP_011321212.1) from F. graminearum, FoRUD3 (XP_018253221.1) from F. oxysporum, NcRUD3 (KHE80473.1) from N. crassa, MoRUD3 (XP_003715387.1) from M. oryzae, ScRUD3 (NP_014859.3) from S. cerevisiae, CaRUD3 (KGR13570.1) from C. albicans, GMAP-210(TRIP11) (NP_004230.2) from Homo sapiens, and GDAP1(GC3) (NP_191716.2) from Arabidopsis thaliana.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ida Bagus Andika for help editing the manuscript.
This work was supported by grants from the Natural Science Foundation of Shandong Province (ZR2020MC113 to S.Z., ZR2020MC120 to L.C., and ZR2020QC126 to L.Z.), National Natural Science Foundation of China (31772247 to H.D.) and Shandong Agricultural University Talent Introduction Funding (20171226 to H.D.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD. 2012. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Nicholson P. 2009. Community ecology of fungal pathogens causing wheat head blight. Annu Rev Phytopathol 47:83–103. doi: 10.1146/annurev-phyto-080508-081737. [DOI] [PubMed] [Google Scholar]
- 3.Pestka JJ. 2010. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84:663–679. doi: 10.1007/s00204-010-0579-8. [DOI] [PubMed] [Google Scholar]
- 4.Desjardins AE, Proctor RH. 2007. Molecular biology of Fusarium mycotoxins. Int J Food Microbiol 119:47–50. doi: 10.1016/j.ijfoodmicro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Audenaert K, Vanheule A, Hofte M, Haesaert G. 2013. Deoxynivalenol: a major player in the multifaceted response of Fusarium to its environment. Toxins (Basel) 6:1–19. doi: 10.3390/toxins6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van De Walle JV, Sergent T, Piront N, Toussaint O, Schneider YJ, Larondelle Y. 2010. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol Appl Pharmacol 245:291–298. doi: 10.1016/j.taap.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Brown DW, Dyer RB, McCormick SP, Kendra DF, Plattner RD. 2004. Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal Genet Biol 41:454–462. doi: 10.1016/j.fgb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Kistler HC, Ma Z. 2019. Fusarium graminearum trichothecene mycotoxins: biosynthesis, regulation, and management. Annu Rev Phytopathol 57:15–39. doi: 10.1146/annurev-phyto-082718-100318. [DOI] [PubMed] [Google Scholar]
- 9.Boenisch MJ, Broz KL, Purvine SO, Chrisler WB, Nicora CD, Connolly LR, Freitag M, Baker SE, Kistler HC. 2017. Structural reorganization of the fungal endoplasmic reticulum upon induction of mycotoxin biosynthesis. Sci Rep 7:44296. doi: 10.1038/srep44296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang G, Chen Y, Xu JR, Kistler HC, Ma Z. 2018. The fungal myosin I is essential for Fusarium toxisome formation. PLoS Pathog 14:e1006827. doi: 10.1371/journal.ppat.1006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li B, Dong X, Zhao R, Kou R, Zheng X, Zhang H. 2019. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. PLoS Pathog 15:e1007754. doi: 10.1371/journal.ppat.1007754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng W, Lin Y, Fang W, Zhao X, Lou Y, Wang G, Zheng H, Liang Q, Abubakar YS, Olsson S, Zhou J, Wang Z. 2018. The endosomal recycling of FgSnc1 by FgSnx41-FgSnx4 heterodimer is essential for polarized growth and pathogenicity in Fusarium graminearum. New Phytol 219:654–671. doi: 10.1111/nph.15178. [DOI] [PubMed] [Google Scholar]
- 13.Zheng H, Li L, Miao P, Wu C, Chen X, Yuan M, Fang T, Norvienyeku J, Li G, Zheng W, Wang Z, Zhou J. 2018. FgSec2A, a guanine nucleotide exchange factor of FgRab8, is important for polarized growth, pathogenicity and deoxynivalenol production in Fusarium graminearum. Environ Microbiol 20:3378–3392. doi: 10.1111/1462-2920.14373. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Dong X, Li X, Chen H, Zhang H, Zheng X, Zhang Z. 2018. A subunit of the HOPS endocytic tethering complex, FgVps41, is important for fungal development and plant infection in Fusarium graminearum. Environ Microbiol 20:1436–1451. doi: 10.1111/1462-2920.14050. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Li B, Fang Q, Li Y, Zheng X, Zhang Z. 2016. SNARE protein FgVam7 controls growth, asexual and sexual development, and plant infection in Fusarium graminearum. Mol Plant Pathol 17:108–119. doi: 10.1111/mpp.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Li B, Liu L, Chen H, Zhang H, Zheng X, Zhang Z. 2015. FgMon1, a guanine nucleotide exchange factor of FgRab7, is important for vacuole fusion, autophagy and plant infection in Fusarium graminearum. Sci Rep 5:18101. doi: 10.1038/srep18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adnan M, Fang W, Sun P, Zheng Y, Abubakar YS, Zhang J, Lou Y, Zheng W, Lu GD. 2020. R-SNARE FgSec22 is essential for growth, pathogenicity and DON production of Fusarium graminearum. Curr Genet 66:421–435. doi: 10.1007/s00294-019-01037-y. [DOI] [PubMed] [Google Scholar]
- 18.Rothman JE. 1981. The Golgi apparatus: two organelles in tandem. Science 213:1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- 19.Witkos TM, Lowe M. 2015. The golgin family of coiled-coil tethering proteins. Front Cell Dev Biol 3:86. doi: 10.3389/fcell.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert CE, Sztul E, Machamer CE. 2018. Commonly used trafficking blocks disrupt ARF1 activation and the localization and function of specific Golgi proteins. Mol Biol Cell 29:937–947. doi: 10.1091/mbc.E17-11-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillingham AK. 2018. At the ends of their tethers! How coiled-coil proteins capture vesicles at the Golgi. Biochem Soc Trans 46:43–50. doi: 10.1042/BST20170188. [DOI] [PubMed] [Google Scholar]
- 22.VanRheenen SM, Cao X, Sapperstein SK, Chiang EC, Lupashin VV, Barlowe C, Waters MG. 1999. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J Cell Biol 147:729–742. doi: 10.1083/jcb.147.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillingham AK, Tong AH, Boone C, Munro S. 2004. The GTPase Arf1p and the ER to Golgi cargo receptor Erv14p cooperate to recruit the golgin Rud3p to the cis-Golgi. J Cell Biol 167:281–292. doi: 10.1083/jcb.200407088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardenas J, Rivero S, Goud B, Bornens M, Rios RM. 2009. Golgi localisation of GMAP210 requires two distinct cis-membrane binding mechanisms. BMC Biol 7:56. doi: 10.1186/1741-7007-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roboti P, Sato K, Lowe M. 2015. The golgin GMAP-210 is required for efficient membrane trafficking in the early secretory pathway. J Cell Sci 128:1595–1606. doi: 10.1242/jcs.166710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DW. 2003. Characterization of Grp1p, a novel cis-Golgi matrix protein. Biochem Biophys Res Commun 303:370–378. doi: 10.1016/s0006-291x(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 27.Yadav S, Puri S, Linstedt AD. 2009. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell 20:1728–1736. doi: 10.1091/mbc.e08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rios RM, Sanchis A, Tassin AM, Fedriani C, Bornens M. 2004. GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell 118:323–335. doi: 10.1016/j.cell.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Friggi-Grelin F, Rabouille C, Therond P. 2006. The cis-Golgi Drosophila GMAP has a role in anterograde transport and Golgi organization in vivo, similar to its mammalian ortholog in tissue culture cells. Eur J Cell Biol 85:1155–1166. doi: 10.1016/j.ejcb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Weide T, Bayer M, Koster M, Siebrasse JP, Peters R, Barnekow A. 2001. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep 2:336–341. doi: 10.1093/embo-reports/kve065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits P, Bolton AD, Funari V, Hong M, Boyden ED, Lu L, Manning DK, Dwyer ND, Moran JL, Prysak M, Merriman B, Nelson SF, Bonafe L, Superti-Furga A, Ikegawa S, Krakow D, Cohn DH, Kirchhausen T, Warman ML, Beier DR. 2010. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N Engl J Med 362:206–216. doi: 10.1056/NEJMoa0900158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francl L, Shaner G, Bergstrom G, Gilbert J, Pedersen W, Dill-Macky R, Sweets L, Corwin B, Jin Y, Gallenberg D, Wiersma J. 1999. Daily inoculum levels of Gibberella zeae on wheat spikes. Plant Dis 83:662–666. doi: 10.1094/PDIS.1999.83.7.662. [DOI] [PubMed] [Google Scholar]
- 33.McMullen M, Jones R, Gallenberg D. 1997. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis 81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- 34.Proctor RH, Hohn TM, McCormick SP. 1995. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact 8:593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 35.Seong KY, Zhao X, Xu JR, Guldener U, Kistler HC. 2008. Conidial germination in the filamentous fungus Fusarium graminearum. Fungal Genet Biol 45:389–399. doi: 10.1016/j.fgb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Hallen-Adams HE, Wenner N, Kuldau GA, Trail F. 2011. Deoxynivalenol biosynthesis-related gene expression during wheat kernel colonization by Fusarium graminearum. Phytopathology 101:1091–1096. doi: 10.1094/PHYTO-01-11-0023. [DOI] [PubMed] [Google Scholar]
- 37.Flynn CM, Broz K, Jonkers W, Schmidt-Dannert C, Kistler HC. 2019. Expression of the Fusarium graminearum terpenome and involvement of the endoplasmic reticulum-derived toxisome. Fungal Genet Biol 124:78–87. doi: 10.1016/j.fgb.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballensiefen W, Ossipov D, Schmitt HD. 1998. Recycling of the yeast v-SNARE Sec22p involves COPI-proteins and the ER transmembrane proteins Ufe1p and Sec20p. J Cell Sci 111:1507–1520. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Barlowe C. 2002. Analysis of Sec22p in endoplasmic reticulum/Golgi transport reveals cellular redundancy in SNARE protein function. Mol Biol Cell 13:3314–3324. doi: 10.1091/mbc.e02-04-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frigerio G. 1998. The Saccharomyces cerevisiae early secretion mutant tip20 is synthetic lethal with mutants in yeast coatomer and the SNARE proteins Sec22p and Ufe1p. Yeast 14:633–646. doi:. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Flanagan JJ, Barlowe C. 2004. Sec22p export from the endoplasmic reticulum is independent of SNARE pairing. J Biol Chem 279:27225–27232. doi: 10.1074/jbc.M312122200. [DOI] [PubMed] [Google Scholar]
- 42.Andag U, Neumann T, Schmitt HD. 2001. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J Biol Chem 276:39150–39160. doi: 10.1074/jbc.M105833200. [DOI] [PubMed] [Google Scholar]
- 43.Protopopov V, Govindan B, Novick P, Gerst JE. 1993. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell 74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- 44.Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. 2000. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell 11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang G, Chen A, Dawood DH, Liang J, Chen Y, Ma Z. 2020. Capping proteins regulate fungal development, DON-toxisome formation and virulence in Fusarium graminearum. Mol Plant Pathol 21:173–187. doi: 10.1111/mpp.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Q, Chen A, Zheng W, Xu H, Shang W, Zheng H, Zhang D, Zhou J, Lu G, Li G, Wang Z. 2016. Endosomal sorting complexes required for transport-0 is essential for fungal development and pathogenicity in Fusarium graminearum. Environ Microbiol 18:3742–3757. doi: 10.1111/1462-2920.13296. [DOI] [PubMed] [Google Scholar]
- 47.Gillingham AK, Munro S. 2016. Finding the Golgi: golgin coiled-coil proteins show the way. Trends Cell Biol 26:399–408. doi: 10.1016/j.tcb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Anderson NS, Barlowe C. 2019. Conserved juxtamembrane domains in the yeast golgin Coy1 drive assembly of a megadalton-sized complex and mediate binding to tethering and SNARE proteins. J Biol Chem 294:9690–9705. doi: 10.1074/jbc.RA119.008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinka R, Gillingham AK, Kondylis V, Munro S. 2008. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol 183:607–615. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drin G, Morello V, Casella JF, Gounon P, Antonny B. 2008. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science 320:670–673. doi: 10.1126/science.1155821. [DOI] [PubMed] [Google Scholar]
- 51.Latijnhouwers M, Gillespie T, Boevink P, Kriechbaumer V, Hawes C, Carvalho CM. 2007. Localization and domain characterization of Arabidopsis golgin candidates. J Exp Bot 58:4373–4386. doi: 10.1093/jxb/erm304. [DOI] [PubMed] [Google Scholar]
- 52.Wong M, Gillingham AK, Munro S. 2017. The golgin coiled-coil proteins capture different types of transport carriers via distinct N-terminal motifs. BMC Biol 15:3. doi: 10.1186/s12915-016-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie Q, Chen A, Zhang Y, Yuan M, Xie W, Zhang C, Zheng W, Wang Z, Li G, Zhou J. 2019. Component interaction of ESCRT complexes is essential for endocytosis-dependent growth, reproduction, DON production and full virulence in Fusarium graminearum. Front Microbiol 10:180. doi: 10.3389/fmicb.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trail F, Gaffoor I, Vogel S. 2005. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fuarium graminearum). Fungal Genet Biol 42:528–533. doi: 10.1016/j.fgb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Trail F. 2007. Fungal cannons: explosive spore discharge in the Ascomycota. FEMS Microbiol Lett 276:12–18. doi: 10.1111/j.1574-6968.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 56.Xie Q, Chen A, Zhang Y, Zhang C, Hu Y, Luo Z, Wang B, Yun Y, Zhou J, Li G, Wang Z. 2019. ESCRT-III accessory proteins regulate fungal development and plant infection in Fusarium graminearum. Curr Genet 65:1041–1055. doi: 10.1007/s00294-019-00949-z. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Zhang L, Mei X, Wang C, Guo Z, Li L, Li B, Liang Y, Zou S, Dong H. 2020. The type II phosphoinositide 4-kinase FgLsb6 is important for the development and virulence of Fusarium graminearum. Fungal Genet Biol 144:103443. doi: 10.1016/j.fgb.2020.103443. [DOI] [PubMed] [Google Scholar]
- 58.Jiang C, Zhang C, Wu C, Sun P, Hou R, Liu H, Wang C, Xu JR. 2016. TRI6 and TRI10 play different roles in the regulation of deoxynivalenol (DON) production by cAMP signalling in Fusarium graminearum. Environ Microbiol 18:3689–3701. doi: 10.1111/1462-2920.13279. [DOI] [PubMed] [Google Scholar]
- 59.Adnan M, Islam W, Noman A, Hussain A, Anwar M, Khan MU, Akram W, Ashraf MF, Raza MF. 2020. Q-SNARE protein FgSyn8 plays important role in growth, DON production and pathogenicity of Fusarium graminearum. Microb Pathog 140:103948. doi: 10.1016/j.micpath.2019.103948. [DOI] [PubMed] [Google Scholar]
- 60.Nie Z, Hirsch DS, Randazzo PA. 2003. Arf and its many interactors. Curr Opin Cell Biol 15:396–404. doi: 10.1016/s0955-0674(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 61.Catlett NL, Lee B-N, Yoder OC, Turgeon BG. 2003. Split-marker recombination for efficient targeted deletion of Fungal genes. Fungal Genet Rep 50:9–11. doi: 10.4148/1941-4765.1150. [DOI] [Google Scholar]
- 62.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 63.Chong X, Wang C, Wang Y, Wang Y, Zhang L, Liang Y, Chen L, Zou S, Dong H. 2020. The dynamin-like GTPase FgSey1 plays a critical role in fungal development and virulence in Fusarium graminearum. Appl Environ Microbiol 86:e02720-19. doi: 10.1128/AEM.02720-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C, Wang Y, Wang Y, Wang Z, Zhang L, Liang Y, Chen L, Zou S, Dong H. 2020. The ADP-ribosylation factor-like small GTPase FgArl1 participates in growth, pathogenicity and DON production in Fusarium graminearum. Fungal Biol 124:969–980. doi: 10.1016/j.funbio.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Cavinder B, Sikhakolli U, Fellows KM, Trail F. 2012. Sexual development and ascospore discharge in Fusarium graminearum. J Vis Exp 61:5407. doi: 10.3791/3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer-Parton S, Parton RM, Hickey PC, Dijksterhuis J, Atkinson HA, Read ND. 2000. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J Microsc 198:246–259. doi: 10.1046/j.1365-2818.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 67.Zou S, Sun D, Liang Y. 2017. The roles of the SNARE protein Sed5 in autophagy in Saccharomyces cerevisiae. Mol Cells 40:643–654. doi: 10.14348/molcells.2017.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han X, Chen L, Li W, Zhang L, Zhang L, Zou S, Liang Y, Yu J, Dong H. 2020. Endocytic FgEde1 regulates virulence and autophagy in Fusarium graminearum. Fungal Genet Biol 141:103400. doi: 10.1016/j.fgb.2020.103400. [DOI] [PubMed] [Google Scholar]
- 69.Fan X, He F, Ding M, Geng C, Chen L, Zou S, Liang Y, Yu J, Dong H. 2019. Thioredoxin reductase is involved in development and pathogenicity in Fusarium graminearum. Front Microbiol 10:393. doi: 10.3389/fmicb.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yun Y, Liu Z, Zhang J, Shim WB, Chen Y, Ma Z. 2014. The MAPKK FgMkk1 of Fusarium graminearum regulates vegetative differentiation, multiple stress response, and virulence via the cell wall integrity and high-osmolarity glycerol signaling pathways. Environ Microbiol 16:2023–2037. doi: 10.1111/1462-2920.12334. [DOI] [PubMed] [Google Scholar]
- 71.Ding M, Zhu Q, Liang Y, Li J, Fan X, Yu X, He F, Xu H, Liang Y, Yu J. 2017. Differential roles of three FgPLD genes in regulating development and pathogenicity in Fusarium graminearum. Fungal Genet Biol 109:46–52. doi: 10.1016/j.fgb.2017.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GenBank accession numbers of the sequences are as follows: RUD3 (XP_011321212.1) from F. graminearum, FoRUD3 (XP_018253221.1) from F. oxysporum, NcRUD3 (KHE80473.1) from N. crassa, MoRUD3 (XP_003715387.1) from M. oryzae, ScRUD3 (NP_014859.3) from S. cerevisiae, CaRUD3 (KGR13570.1) from C. albicans, GMAP-210(TRIP11) (NP_004230.2) from Homo sapiens, and GDAP1(GC3) (NP_191716.2) from Arabidopsis thaliana.