Abstract
Objectives
In Duchenne muscular dystrophy, quadriceps weakness is recognized as a key factor in gait deterioration. The objective of this work was three-fold: first, to document the strength of the quadriceps in corticosteroid-naïve DMD boys; second, to measure the effect of corticosteroids on quadriceps strength; and third, to evaluate the correlation between baseline quadriceps strength and the age when starting corticosteroids with the loss of ambulation.
Methods
Quadriceps muscle strength using hand-held dynamometry was measured in 12 ambulant DMD boys who had never taken corticosteroids and during corticosteroid treatment until the loss of ambulation.
Results
Baseline quadriceps muscle strength at 6 years of age was 28% that of normal children of the same age; it decreased to 15% at 8 years and to 6% at 10 years. The increase in quadriceps muscle strength obtained after 1 year of corticosteroid treatment had a strong direct correlation with the baseline strength (R = 0.96). With corticosteroid treatment, the age of ambulation loss showed a very strong direct relationship (R = 0.92) with baseline quadriceps muscle strength but only a very weak inverse relationship (R = -0.73) with the age of starting treatment. Age of loss of ambulation was 10.3 ± 0.5 vs 19.1 ± 4.7 (P < 0.05) in children with baseline quadriceps muscle strength less than or greater than 40 N, respectively.
Conclusions
Corticosteroid-naïve DMD boys have a quantifiable severe progressive quadriceps weakness. This long-term study, for the first time, shows that both of the positive effects obtained with CS treatment, i.e. increasing quadriceps strength and delaying the loss of ambulation, have a strong and direct correlation with baseline quadriceps muscle strength. As such, hand-held dynamometry may be a useful tool in the routine physical examination and during clinical trial assessment.
Key words: Duchenne muscular dystrophy, quadriceps muscle strength, hand-held dynamometry, corticosteroid treatment, prolongation of walking
Introduction
Sir William Richard Gowers (1845-1915) described and illustrated the peculiar maneuver that boys affected by the “pseudohypertrophic muscular paralysis”, now known as Duchenne Muscular Dystrophy (DMD), use to get up from the floor 1. Gowers observed that the boy’s “greatest defect is in the power of rising from the floor … he commonly has not sufficient power to extend the knees when the weight of the trunk is on the upper extremity of the femur … he therefore places his hands on his knees … when the knees are extended, the power of the extensors of the hip may be sufficient to raise the body into the upright position …” 1. This maneuver, known as Gowers’ sign, is adopted by the Duchenne boy to compensate for the quadriceps muscle weakness 2. Gowers also noticed “the difficulty in going upstairs is especially due to the weakness of the extensors of the knee” 1.
All four quadriceps are powerful extensors of the knee, and are therefore crucial in walking, running, jumping and squatting 3. When the quadriceps is weak, the patient will be unable to run and may have difficulty with stairs, because full extension is not attained in these cases and the knee tends to buckle into flexion 4.
A seminal work used hand-held dynamometry to quantify the peculiar weakness of knee extensors in corticosteroid-naïve Duchenne boys and its relationship with motor ability and time of loss of independent ambulation 5. During a 3-year sequential study, 61 DMD boys, aged 4.3 to 11.8 years, were reviewed every 3 to 4 months, and underwent a total of 360 assessments 5. The muscle strength of the knee extensors was very weak compared to that of normal peers, did not grow with age, and instead showed a progressive continual deterioration 5. Loss of independent ambulation occurred when knee extensors exerted less than 2.0 kg (19.6 Newton) 5. Another group subsequently confirmed both the knee extensor weakness and the declining trend with age in 27 corticosteroid-naïve DMD boys 6.
The age of loss of independent ambulation in DMD boys varies in a wide range (7 to 13 years) with a mean value of 9.5 7,8. The effect of corticosteroid (CS) treatment in term of prolongation of ambulation is also variable and could be related to dosage 9,10, age of administration 11-13, or other variables like residual muscle strength 14.
Although it is now recognized that the treatment goal in children with DMD is to keep them ambulant as long as possible, aiming to preserve clinically important function and postpone spinal deformities and muscle contractures 10, and that quadriceps insufficiency is the key factor in gait deterioration 15, no study has yet specifically evaluated the effect of corticosteroid treatment on knee extensors.
The objective of this work was three-fold: first, to document the strength of the quadriceps in corticosteroid-naïve DMD boys; second, to measure the effect of corticosteroids on quadriceps strength; and third, to evaluate the correlation between baseline quadriceps strength and the age when starting corticosteroids with the loss of ambulation.
Materials and methods
Patients
We included in the study the Duchenne boys who were able to walk and had never received corticosteroid treatment and who subsequently began it and were followed until the loss of ambulation.
All patients had a clinical diagnosis confirmed by genetic investigation and in many of them also by the absence of dystrophin in the muscle biopsy.
Corticosteroid treatment
The corticosteroid treatment was approved by the Ethical Committee of the Istituto Ortopedico Rizzoli 11. In our study regimens, dosing and corticosteroids varied with time 11,12. At the start of the treatment, and for the first 2-4 weeks, the regimen was daily (prednisone 0.75 mg/kg or deflazacort 0.90 mg/kg), and then on alternate days. The alternate day dose was prednisone 1.25 mg/kg (50 mg maximum) or deflazacort 1.5 mg/kg (60 mg maximum). During periods of stability corticosteroid dosage was not increased with weight. However, after the age of 12-14 years, if the patient showed more weakness or fatigue, prednisone/deflazacort was given for 1-3 months at 0.75/0.90 mg/kg daily with a ceiling dose of 50/60 mg.
Hand-held dynamometry
To test knee extension, the subject was seated with the hip and knee flexed at 90°, and the foot dorsiflexed at 90°. The examiner sat in front of the subject and the dynamometer was placed on the anterior surface of the distal tibia just proximal to the ankle joint. The patient performed each movement three times with a 30-s pause between each. The highest score obtained on the dominant side was used for further analysis. If a patient complained of discomfort, additional padding was available to place on the applicator. Maximum voluntary isometric contraction of quadriceps was measured until 1997 using the Hammersmith myometer (Myometer, Penny and Giles Transducers Ltd, Dorset, U.K.) 5, and then with the Citec dynamometer (CT 3001, Citec, C.I.T. Technics BV, Groningen, The Netherlands) 16. The reliability and validity of both has been proven earlier 17,18.
Statistical analysis
To measure the strength of the linear association between two variables, we used linear regression with 95% confidence intervals and Wilcoxon two-tailed grade tests for paired samples, while the differences between the groups were evaluated using two-tailed Student’s t-tests. To test the differences between regression lines, we used two-tailed tests. Parametric variables are shown as mean ± SD. P values < 0.05 were considered statistically significant. Analyses were performed using IBM SPSS statistics 25.
Results
We assessed for eligibility 50 consecutive DMD boys evaluated from January 1994 to December 2018. Twenty-six were excluded: 19 were wheelchair-bound and 7 on CS were still ambulant. Corticosteroid treatment was proposed to the parents of 24 children: the parents of 20 children accepted and 4 refused the intervention. The remaining 20 children were allocated to intervention. Eight were excluded from the analysis: 3 were lost to follow-up, and 5 were on CS but still ambulant. The 12 patients who were corticosteroid-naïve and whose parents allowed corticosteroid treatment and were followed up to the time of loss of ambulation were therefore included in the study (see Table I for the genotype). The first 5 patients started corticosteroids treatment at a young age (< 4 years), between March 1996 and January 1997 11,12.
Table I.
Patients dystrophin gene mutations.
| Patient # | DMD mutation |
|---|---|
| 1 | dup ex 65-79 |
| 2 | del ex 10-44 |
| 3 | del ex 8-44 |
| 4 | del ex 20-25 |
| 5 | del ex 44 |
| 6 | del ex 51-62 |
| 7 | del ex 48-52 |
| 8 | c.10108C > T; p. Arg3370* |
| 9 | del ex 51-54 |
| 10 | del ex 3-17 |
| 11 | del ex 42-43 |
| 12 | c.1264G > T; p. Glu422* |
Baseline quadriceps muscle strength
The quadriceps strength measured in the 12 DMD children (Fig. 1) between the ages of 2 to 10 exactly reflected the range of values and the declining trend observed in the previous studies 5,6. DMD children were already much weaker than normal children at the age of 6, and their strength, unlike that of normal children 19, continued to decrease with age. In particular, the mean quadriceps strength of DMD children at 6 years was 28% that of normal children of the same age: it decreased to 15% at 8 years and to 6% at 10 years.
Figure 1.

Regression lines between age and quadriceps/knee extension (KE) muscle strength in normal boys (aged 6-11 years, green line) 19, in 61 corticosteroid-naïve DMD boys (aged 5-11 years, red line) 5, and in 12 DMD boys before starting CS (aged 2-10 years, blue line). The differences between the regression lines y = -6.679x + 84.57 by Scott et al. 5 and y = -6.686x + 81.65 of the 12 DMD boys were not significant (p = 0.65). The linear equation for normal boys19 aged 6-11 years was y = 21.543x + 16.55.
Corticosteroids effect on quadriceps strength
Corticosteroid treatment increased quadriceps strength (Figs.2-3A-B) in all but one patient (P6) in whom it was stabilized. Quadriceps muscle strength increased during the first months to a year of CS treatment (Figs. 2-3A) while the maximum increase in quadriceps strength (peak KE) was achieved at variable times between 1 and 7 years of treatment (Figs. 2-3B). There was a strong direct correlation (Fig. 3A-B) between the baseline KE and both the 1-year KE (R = 0.96) and the peak KE (R = 0.95). In the 12 boys, the increase in strength between baseline KE (44.5 ± 18 N) and peak KE (74.7 ± 48 N) was significant (p < 0.01).
Figure 2.

Linear trend of quadriceps strength for each of the 12 DMD boys from the age of initiation of corticosteroid treatment until the age of ambulation loss. The patients had 1-4 strength measurements each year and each line shows the maximum force value expressed during each year. The increase in knee extension muscle strength started in the first year of treatment in most patients and continued for 4-7 years in patients who at the beginning of the treatment had a force greater than 60 N (P1, P2, P4, P5). The 6 patients with baseline knee extension strength below 40 N (P3, P7, P9-P11) had a limited increase or only stabilization (P6) in KE muscle strength. For each of the 12 patients, the age of onset of CS and the age of loss of ambulation are shown in parentheses.
Figure 3.

Increase in KE muscle strength with CS treatment in 12 DMD boys. (A) Regression line between baseline KE (X) and 1-year KE (Y): R2 = 0.9281. This means that 92.8% of the variability in Y is explained by X. R = 0.9634. This means that there is a very strong direct relationship between X and Y. P-value = 4.879e-7. Y = -11.413 + 1.53X. (B) Regression line between baseline KE (X) and peak KE (Y): R2 = 0.9050. This means that 90.5% of the variability in Y is explained by X. R = 0.9513. This means that there is a very strong direct relationship between X and Y. P-value = 0.000001987. Y = -33.0743 + 2.4230X.
Age of starting CS treatment, quadriceps strength, and loss of ambulation
In these 12 boys, the correlation between the age of loss ambulation and the age of starting CS treatment (Fig. 4A) was weak and inverse (R = -0.73), while with the baseline quadriceps muscle strength (Fig. 4B) it was very strong and direct (R = 0.92). Note that the 6 children who lost ambulation before 12 years of age (Figure 2 and in Figure 4A from the left P3, P10, P9, P6, P7, P11) had started CS treatment between 3.8 and 9.5 years of age when their baseline KE (Fig. 4B) was below 40 N (23-39 N). In contrast, the 6 children who lost ambulation after 13 years of age (Figs. 2,4A) had started CS treatment between 2.4 and 5.2 years of age when their baseline KE (Fig. 4B) was 40 N or more (40-74 N). The mean age and IC95% of loss of ambulation was 10.3 (9.8-10.9) vs 19.1 (14.1-24.0) in children with baseline quadriceps muscle strength less than or greater than 40 N, respectively (p < 0.05).
Figure 4.
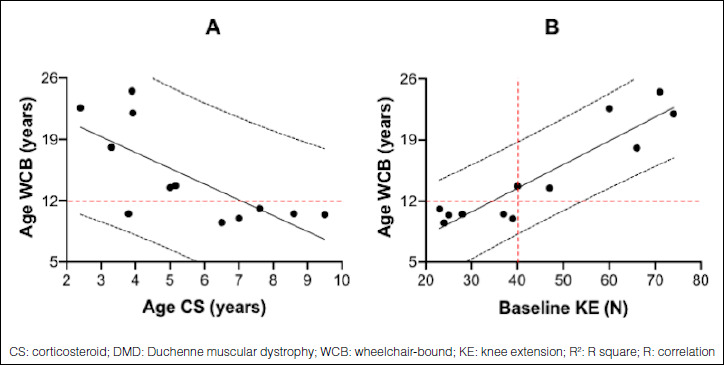
Regression lines in 12 CS treated DMD boys between (A) age of CS initiation (X) and age WCB (Y) and (B) baseline KE (X) and age wheelchair-bound (WCB) (Y). (A) R2 = 0.5347. This means that 53.5% of the variability in Y is explained by X. R = -0.7313. This means that there is a very weak inverse relationship between X and Y. P-value = 0.006884. Y = 24.736-1.80X. (B) R2 = 0.8489. This means that 84.9% of the variability in Y is explained by X. R = 0.9214. This means that there is a very strong direct relationship between X and Y. P-value = 0.00002072. Y = 2.6012 + 0.2719X.
The boy P3 who started CS at the age of 3.8 and lost ambulation at 10.5 years had a very low quadriceps strength value (37 N) at baseline and only a transient increase (44 N) after 2 months of corticosteroid treatment (Fig. 2). His cousin, with the same mutation and who was not treated with corticosteroids, ceased walking at 7.5 years 12. The boy P5 who started CS at the age of 3.3 when his baseline KE was 66 N reached a peak of 138 N at age 7 and lost ambulation at age 18.1 when his KE was still high (66 N) because a leg fracture with long immobilization.
Discussion
Our study documents the loss of quadriceps strength in 12 corticosteroid-naïve DMD children, confirming both the marked quadriceps weakness with respect to the controls 5,6,20,21 and its characteristic linear rate of decline with age 5,6,22.
For the first time, this very-long-term follow-up study documented a very strong direct relationship between quadriceps muscle strength at baseline and its increase after 1 year of CS treatment (R = 0.96); moreover, the correlation between baseline quadriceps muscle strength and the age of loss of ambulation was very strong and direct (R = 0.92), but only weak and inverse (R = -0.73) with the age of starting CS treatment.
Previously, a better effect of early CS treatment 11,12,23 had been shown compared to a later start, i.e. after 6 years of age 24. To reconcile the different positions, it is necessary to keep in mind two points:
DMD boys lose ambulation at the average age of 9.5 years, but with a large range (7-13 years) 7,8;
the mean strength of the quadriceps decreases with age, but the strength range per year is wide 5,6.
Since quadriceps strength is crucial for walking 3, reflects the overall strength of the child 5, and tends to decrease rapidly with age, it is to be expected that earlier treatment is better. However, this study demonstrates for the first time that the greater the basal strength of the quadriceps, the greater the increase in strength that is obtained following CS treatment. Above all, it is the basal strength of the quadriceps that best correlates with prolonging ambulation rather than the age of treatment initiation since DMD children of the same age have different quadriceps strength and the efficacy will be better in those with greater baseline quadriceps strength.
It should be noted that strength, measured on MRC-based scores of 34 muscle groups, showed a significant improvement in corticosteroid-treated boys compared with placebo 25,27. In these studies, strength was already significantly greater at 10 days 26, reached a maximum by 3 months, and was maintained at 6 26,27 and 18 months 28. Knee extensor muscle strength, measured with an isokinetic dynamometer, was found higher in 9 DMD boys on corticosteroid compared to 6 corticosteroid-naive boys 29. So far, the only other study that has measured force using a hand-held myometer showed that high dose weekly oral prednisone improved bilateral knee extension and flexion in all 17 boys with antigravity quadriceps strength compared to untreated boys, even after 6 months 30.
The fact that corticosteroid treatment has shown evidence of clinical efficacy with an early effect on muscle strength supported by a subsequent effect on motor function should be taken into consideration in the design of clinical trials. Instead, most of the Duchenne trials have had ordinal scales of muscle strength (MRC) or motor function (Vignos’ lower limb score, the Brooke upper limb score) or the 6-minute-walk as the primary clinical endpoints of efficacy. However, it has been shown that the manual muscle test (MMT) and functional scales may take longer to demonstrate a trend than quantitative measures 31. Therefore, it is expected that any effective treatment in muscular dystrophy would first increase muscle strength and subsequently improve motor function 31. In addition, the MMT is known to be less reliable and sensitive compared to quantitative measurements; for example, by the time strength declined to MMT grade 4, isometrically measured strength was 40-50% of normal control 22, suggesting the use of a quantitative muscle test as an outcome measure in clinical trials in DMD to obtain maximum power and the greatest sensitivity 32. The six-minute walk has failed to show improvements in recent trials and its validity for DMD children was questioned on several aspects 33.
Conclusions
Corticosteroid-naïve DMD children have very weak quadriceps muscles that do not increase in strength but rather rapidly become weaker with age, causing the loss of ambulation. Corticosteroid treatment is effective in increasing quadriceps muscle strength and in prolonging ambulation. For the first time, this very long-term follow-up study showed that the increase in the strength of the quadriceps after one year of CS treatment is directly proportional to the initial strength of the muscle itself, and above all the best estimate of the age of ambulation loss is based on the strength of the quadriceps at the start of CS treatment and not on the age at which it starts. The quantitative measurement of quadriceps muscle strength is an easy-to-apply, non-invasive and inexpensive method and should be part of the clinical evaluation of the myopathic patient and included between clinical trial endpoints.
There may be some possible limitations in this long-term single center study. The first is the limited sample size particularly at certain age. The second limitation is that the results may be specific to the corticosteroid regimen utilized in this study. However, the fact that the best estimate of the age of ambulation loss is based on the strength of the quadriceps at the start of corticosteroid treatment is a new exciting finding that deserves to be confirmed in future larger studies.
Figures and tables
Acknowledgements
We thank the patients and their families for supporting this long-term study.
References
- 1.Gowers WR. A manual of disease of the nervous system. London: Churchill; 1886. [Google Scholar]
- 2.Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surg 2002;10:138-51. https://doi.org/10.5435/00124635-200203000-00009 10.5435/00124635-200203000-00009 [DOI] [PubMed] [Google Scholar]
- 3.Bryanton MA, Carey JP, Kennedy MD, et al. Quadriceps effort during squat exercise depends on hip extensor muscle strategy. Sports Biomech 2015;14:122-38. https://doi.org/10.1080/14763141.2015.1024716 10.1080/14763141.2015.1024716 [DOI] [PubMed] [Google Scholar]
- 4.Ganjwala D, Shah H. Management of the knee problems in spastic cerebral palsy. Indian J Orthop 2019;53:53-62. https://doi.org/10.4103/ortho.IJOrtho_339_17 10.4103/ortho.IJOrtho_339_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott OM, Hyde SA, Goddard C, et al. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle Nerve 1982;5:291-301. https://doi.org/10.1002/mus.880050405 10.1002/mus.880050405 [DOI] [PubMed] [Google Scholar]
- 6.Hyde SA, Steffensen BF, Floytrup I, et al. Longitudinal data analysis: an application to construction of a natural history profile of Duchenne muscular dystrophy. Neuromuscul Disord 2001;11:165-70. https://doi.org/10.1016/s0960-8966(00)00175-9 10.1016/s0960-8966(00)00175-9 [DOI] [PubMed] [Google Scholar]
- 7.Merlini L, Barile P, Bartone MT, et al. Storia naturale della distrofia muscolare di Duchenne. Editore AG, Ed. Ventilazione meccanica nelle miopatie. Bologna: 1989, pp. 111-27. [Google Scholar]
- 8.Emery AE. Clinical and molecular studies in Duchenne muscular dystrophy. Prog Clin Biol Res 1989;306:15-28. PMID: 2662210 [PubMed] [Google Scholar]
- 9.Manzur AY, Kuntzer T, Pike M, et al. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev 2008:CD003725 https://doi.org/10.1002/14651858.CD003725.pub3 10.1002/14651858.CD003725.pub3 [DOI] [PubMed] [Google Scholar]
- 10.Griggs RC, Herr BE, Reha A, et al. Corticosteroids in Duchenne muscular dystrophy: major variations in practice. Muscle Nerve 2013;48:27-31. https://doi.org/10.1002/mus.23831 10.1002/mus.23831 [DOI] [PubMed] [Google Scholar]
- 11.Merlini L, Cicognani A, Malaspina E, et al. Early prednisone treatment in Duchenne muscular dystrophy. Muscle Nerve 2003;27:222-7. https://doi.org/10.1002/mus.10319 10.1002/mus.10319 [DOI] [PubMed] [Google Scholar]
- 12.Merlini L, Gennari M, Malaspina E, et al. Early corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-up. Muscle Nerve 2012;45:796-802. https://doi.org/10.1002/mus.23272 10.1002/mus.23272 [DOI] [PubMed] [Google Scholar]
- 13.McDonald CM, Han JJ, Mah JK, et al. Corticosteroids and Duchenne muscular dystrophy: does earlier treatment really matter? Muscle Nerve 2012;45:777-9. https://doi.org/10.1002/mus.23304 10.1002/mus.23304 [DOI] [PubMed] [Google Scholar]
- 14.Buckon C, Sienko S, Bagley A, et al. Can quantitative muscle strength and functional motor ability differentiate the influence of age and corticosteroids in ambulatory boys with Duchenne muscular dystrophy? PLoS Curr 2016;8 https://doi.org/10.1371/currents.md.1ced64dff945f8958221fddcd4ee60b0 10.1371/currents.md.1ced64dff945f8958221fddcd4ee60b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland DH, Olshen R, Cooper L, et al. The pathomechanics of gait in Duchenne muscular dystrophy. Dev Med Child Neurol 1981;23:3-22. https://doi.org/10.1111/j.1469-8749.1981.tb08442.x 10.1111/j.1469-8749.1981.tb08442.x [DOI] [PubMed] [Google Scholar]
- 16.Merlini L, Mazzone ES, Solari A, et al. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve 2002;26:64-70. https://doi.org/10.1002/mus.10166 10.1002/mus.10166 [DOI] [PubMed] [Google Scholar]
- 17.Backman E, Odenrick P, Henriksson KG, et al. Isometric muscle force and anthropometric values in normal children aged between 3.5 and 15 years. Scand J Rehabil Med 1989;21:105-14. PMID 2749194 [PubMed] [Google Scholar]
- 18.McKay MJ, Baldwin JN, Ferreira P, et al. Normative reference values for strength and flexibility of 1,000 children and adults. Neurology 2017;88:36-43. https://doi.org/10.1212/WNL.0000000000003466 10.1212/WNL.0000000000003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beenakker EA, van der Hoeven JH, Fock JM, et al. Reference values of maximum isometric muscle force obtained in 270 children aged 4-16 years by hand-held dynamometry. Neuromuscul Disord 2001;11:441-6. https://doi.org/10.1016/s0960-8966(01)00193-6 10.1016/s0960-8966(01)00193-6 [DOI] [PubMed] [Google Scholar]
- 20.Lerario A, Bonfiglio S, Sormani M, et al. Quantitative muscle strength assessment in duchenne muscular dystrophy: longitudinal study and correlation with functional measures. BMC Neurol 2012;12:91 https://doi.org/10.1186/1471-2377-12-91 10.1186/1471-2377-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brussock CM, Haley SM, Munsat TL, et al. Measurement of isometric force in children with and without Duchenne’s muscular dystrophy. Phys Ther 1992;72:105-14. https://doi.org/10.1093/ptj/72.2.105 10.1093/ptj/72.2.105 [DOI] [PubMed] [Google Scholar]
- 22.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil 1995;74:S70-92. https://doi.org/10.1097/00002060-199509001-00003 10.1097/00002060-199509001-00003 [DOI] [PubMed] [Google Scholar]
- 23.Merlini L. A 19-year-old ambulant Duchenne patient with stunted growth on long-term corticosteroids. Neuromuscul Disord 2014;24:417-8. https://doi.org/10.1016/j.nmd.2014.02.006 10.1016/j.nmd.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 24.McDonald CM, Henricson EK, Abresch RT, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 2018;391:451-61. https://doi.org/10.1016/S0140-6736(17)32160-8 10.1016/S0140-6736(17)32160-8 [DOI] [PubMed] [Google Scholar]
- 25.Angelini C, Pegoraro E, Turella E, et al. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle Nerve 1994;17:386-91. https://doi.org/10.1002/mus.880170405 10.1002/mus.880170405 [DOI] [PubMed] [Google Scholar]
- 26.Griggs RC, Moxley RT, 3rd, Mendell JR, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol 1991;48:383-8. https://doi.org/10.1001/archneur.1991.00530160047012 10.1001/archneur.1991.00530160047012 [DOI] [PubMed] [Google Scholar]
- 27.Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med 1989;320:1592-7. https://doi.org/10.1056/NEJM198906153202405 10.1056/NEJM198906153202405 [DOI] [PubMed] [Google Scholar]
- 28.Griggs RC, Moxley RT, 3rd, Mendell JR, et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathioprine (12 months). Neurology 1993;43:520-7. https://doi.org/10.1212/wnl.43.3_part_1.520 10.1212/wnl.43.3_part_1.520 [DOI] [PubMed] [Google Scholar]
- 29.Arpan I, Willcocks RJ, Forbes SC, et al. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology 2014;83:974-80. https://doi.org/10.1212/WNL.0000000000000775 10.1212/WNL.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connolly AM, Schierbecker J, Renna R, et al. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord 2002;12:917-25. https://doi.org/10.1016/s0960-8966(02)00180-3 10.1016/s0960-8966(02)00180-3 [DOI] [PubMed] [Google Scholar]
- 31.Cook JD, Glass DS. Strength evaluation in neuromuscular disease. Neurol Clin 1987;5:101-23. PMID: 3550413 [PubMed] [Google Scholar]
- 32.Mayhew JE, Florence JM, Mayhew TP, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve 2007;35:36-42. https://doi.org/10.1002/mus.20654 10.1002/mus.20654 [DOI] [PubMed] [Google Scholar]
- 33.Hoffman EP, Connor EM. Orphan drug development in muscular dystrophy: update on two large clinical trials of dystrophin rescue therapies. Discov Med 2013;16:233-9. PMID 24229740 [PubMed] [Google Scholar]


