Introduction
Residual conduction gaps between the pulmonary veins (PVs) and atria during extensive PV isolation of atrial fibrillation (AF) are often mapped during sinus or a paced rhythm. It is sometimes difficult to identify the gaps during AF, especially if both PV fibrillation and AF simultaneously persist. Further, if the gaps are associated with epicardial conduction and are not on the previous PV isolation lines,1, 2, 3, 4 mapping of the gaps may be even more difficult. The high-resolution electroanatomic mapping system (Rhythmia; Boston Scientific, Marlborough, MA) is capable of rapidly and automatically creating high-resolution maps and has been reported to be useful for demonstrating the detailed mechanisms of atrial tachycardias (ATs) and identifying the critical regions of the ATs.5, 6, 7, 8 We present a case of the identification of a breakthrough from the right PVs (RPVs) to the right atrium (RA) during simultaneous persistent RPV fibrillation and organized AF by automated high-resolution mapping using the Rhythmia system, and the successful isolation of the RPVs by radiofrequency (RF) ablation at the breakthrough in the RA in a patient with an RPV reconnection after an extensive PV isolation of AF.
Key Teaching Points.
-
•
Residual conduction gaps between the pulmonary veins (PVs) and atria during extensive PV isolation of atrial fibrillation (AF) are sometimes difficult to map if both PV fibrillation and AF persist and the residual gaps are associated with epicardial conduction.
-
•
An epicardial connection from the right PVs (RPVs) to the right atrium was successfully mapped and ablated during simultaneous persistent RPV fibrillation and relatively organized AF using the automated high-resolution mapping system in a patient with an RPV reconnection after an extensive PV isolation of persistent AF.
-
•
The automated mapping method with modified beat acceptance criteria may be useful for identifying PV-to-atrium breakthroughs during simultaneous persistent PV fibrillation and relatively organized AF and eliminating the residual gaps with minimal additional radiofrequency application.
Case report
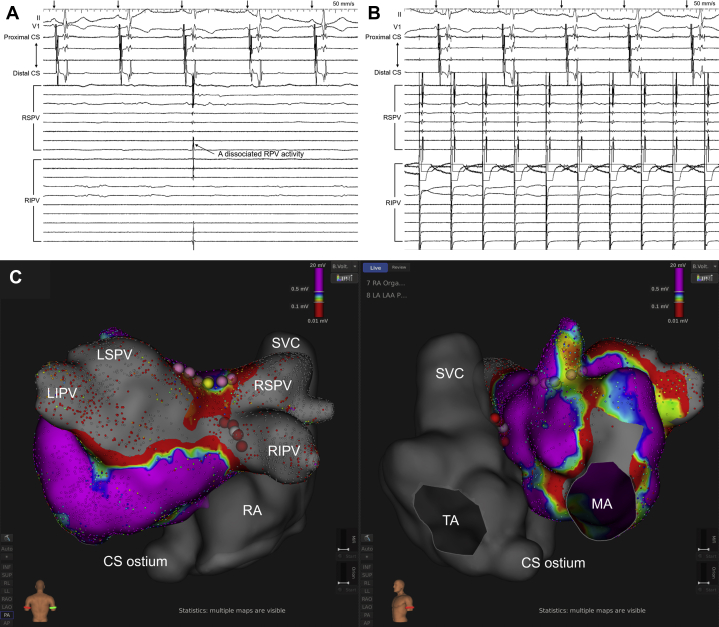
A 66-year-old man underwent a third RF catheter ablation of recurrent persistent AF. The first ablation procedure consisted of an extensive PV isolation and the second procedure consisted of a box isolation of the posterior left atrium (LA) with a reisolation of the RPVs. During the first ablation procedure, the RPVs were not isolated by the initial extensive PV isolation line. Finally, the RPV isolation was completed with touch-up ablation at the anterior carina between the superior and inferior RPVs, slightly inside the initial RPV isolation line. During the second ablation procedure, an RF application at the anterior region of the right inferior PV, which was close to the anterior carina, eliminated the residual conduction gap between the RPVs and atrium. During the third ablation procedure, conduction recovery of the RPVs and posterior LA was verified, and sinus rhythm could not be maintained despite repeat electrical cardioversion for the AF because RPV firing triggered the AF immediately after electrical cardioversion restored sinus rhythm. Disorganized PV electrograms were continuously recorded within the RPVs during the AF, while atrial electrograms recorded within the coronary sinus revealed an irregular cycle length (CL) and inconsistent activation sequence but partially revealed organized activation (Figure 1A). Therefore, to identify the breakthrough site from the RPVs to the atrium during the organized AF, activation maps in the LA and RA outside the previous RPV isolation line were created using a mini-basket mapping catheter (IntellaMap Orion; Boston Scientific). The following beat acceptance criteria normally used for mapping stable ATs were not available in the present case because of the irregularity of the atrial electrograms during the AF: (1) CL variation (±10 ms), (2) activation timing difference between 2 reference electrodes (±5 ms), (3) respiration gating, (4) catheter motion per beat (<1 mm), (5) catheter electrogram stability (0.25), and (6) catheter tracking quality (<3).6, 7, 8 Regarding the CL variation for mapping the AF, the mapping system initially exhibited an acceptance threshold of the CL of 246–266 ms using the autodetect function. However, the beats with a CL of 310–452 ms mostly had a consistent activation sequence, while those with a CL of 200–310 ms often had inconsistent activation sequences. Thus, we manually changed the CL variation from ±10 ms to ±70 ms and then the acceptance threshold of the CL from 246–266 ms to 312–452 ms in order to acquire only beats with a CL of >310 ms (Figure 1B). Consequently, a total of 8497 beats with a consistent activation sequence were automatically acquired. The activation maps during the organized AF exhibited a centrifugal pattern with the earliest activation site (EAS) on the RA septum (Figure 2, Online Video). After RF applications at the EAS, organized atrial electrograms changed to disorganized atrial electrograms and then the RPV fibrillation spontaneously terminated. Thereafter, sinus rhythm could be successfully maintained after the electrical cardioversion of the AF and bidirectional conduction block between the RPVs and both atria had already been completed (Figure 3A and 3B). Finally, additional RF applications for the conduction gap along the superior box isolation line completed the electrical isolation of the posterior LA (Figure 3C).
Figure 1.
A: Body surface and intracardiac electrograms during the atrial fibrillation (AF). The right pulmonary vein (PV) electrograms revealed disorganized activation and a shorter cycle length (CL) compared to the atrial electrograms recorded within the coronary sinus (CS), while the CS electrograms did not always reveal a stable CL and consistent activation sequence but revealed relatively organized activation. The numbers indicate the CLs measured in the CS. B: Automated atrial electrogram acquisition during the AF. During activation mapping, the beats that met all beat acceptance criteria were automatically acquired (green arrowheads), but those that did not meet any of the criteria were excluded (red arrowheads). The upper panels show whether each criterion was met or not: CL, CL variation (±70 ms); ΔR, activation timing difference between 2 reference electrodes (±5 ms); RSP, respiration gating; M, catheter motion per beat (<1 mm); S, catheter electrogram stability (0.25); TR, catheter tracking quality (<3). In the present case, the CL variation was manually set at ±70 ms, but the other parameters were the default values of the system. The bar became green if each criterion was met, and in contrast the bar became red if not. The middle panel shows the intracardiac electrograms recorded by the multielectrode catheter in the CS and the mini-basket catheter in the atrium. The numbers indicate the CLs measured by the reference electrode in the CS (CS 3-4). The lower panel shows the real-time curve of the CL, which fluctuated intensely between 210 and 430 ms. The green area in the lower panel indicates the acceptance threshold of the CL, and the upper and lower acceptance thresholds are 452 ms and 312 ms, respectively. If the CL was within the green area, the beat met the criterion of the CL variation, but if the CL was outside the green area, the beat did not. A4-5 to H4-5, mini-basket catheter recordings. CS1-2 to CS8-9, distal to proximal coronary sinus recordings. RIPV = right inferior PV electrograms recorded by a circular mapping catheter; RSPV = right superior PV electrograms recorded by the mini-basket catheter.
Figure 2.
Activation maps in the atria during the organized AF exhibited (A, B) a centrifugal pattern with the earliest activation on the right atrial septum and (C, D) conduction to the left atrium (LA) via the atrial septum (Online Video). The left and right panels show the LA and right atrium (RA) in the left anterior oblique view and the RA only in the left posterior oblique view, respectively. The gray tags are placed along the previous pulmonary vein isolation lines, and the white, light green, and light blue tags represent the earliest activation site (EAS) in the RA, LA site on the opposite side of the EAS in the RA, and EAS in the LA, respectively. The dark red and white arrows on the maps represent the activation wavefront and activation propagation, respectively. The numbers in the local electrograms indicate the intervals from the reference electrogram recorded in the coronary sinus (CS) to the local electrogram. The projection distance and confidence mask for the maps and online video were set at 2 mm and 0.03 mV, respectively. IVC = inferior vena cava; LAA = left atrial appendage; LSPV = left superior pulmonary vein; MA = mitral annulus; RSPV = right superior pulmonary vein; SVC = superior vena cave; TA = tricuspid annulus.
Figure 3.
A, B: Entrance block from the atria to the right pulmonary veins (RPVs) (A) and exit block from the RPVs to the atria (B) were verified during sinus rhythm and RPV pacing. The black arrows on the electrocardiograms indicate the P waves during sinus rhythm. C: Final radiofrequency (RF) ablation sites with the postablation left atrial voltage map in the posteroanterior (left panel) and left anterior oblique (right panel) views. The yellow and pink tags represent the conduction gap and RF ablation sites along the superior box isolation line, respectively. The white and red tags represent the earliest activation site during the organized atrial fibrillation and RF ablation sites on the right atrial septum, respectively. RPV = right pulmonary vein. The other abbreviations are as in Figures 1 and 2.
Discussion
This case report described an automated mapping method for detecting RPV-to-RA breakthroughs during simultaneous persistent RPV fibrillation and relatively organized AF using the Rhythmia system in a patient with an RPV reconnection after an extensive PV isolation of persistent AF. By modifying the beat acceptance criteria to automatically acquire only organized atrial electrograms and not acquire disorganized atrial electrograms, activation mapping in both atria during the organized AF could identify an RPV-to-atrium breakthrough site on the RA septum. Based on the mapping, an electrical reisolation of the RPVs was successfully completed by RF ablation at the breakthrough site during the AF.
Several strategies have been proposed for mapping conduction gaps between the PVs and atrium along the PV isolation lines.9, 10, 11, 12, 13 The previous mapping maneuvers using 3-dimensional electroanatomic systems can be performed during sinus or a paced rhythm. PV-to-atrium breakthroughs usually cannot be mapped if disorganized atrial electrograms are continuously recorded during persistent AF, whereas atrium-to-PV breakthroughs also cannot be mapped if disorganized PV electrograms are continuously recorded during persistent PV fibrillation. In the present case, disorganized RPV electrograms were continuously recorded, while relatively organized atrial electrograms were recorded in the atria. These findings appeared to be due to partial conduction block from the RPVs to the atria during the persistent RPV fibrillation, which was caused by the previous PV isolation procedures. Therefore, we speculated that the RPV-to-atrium breakthrough might be able to be mapped using the automated electrogram acquisition algorithm equipped with the Rhythmia system that automatically works in the background, however, the atrium-to-RPV breakthrough could not be mapped. During the AF, the beats with shorter CLs tended to have inconsistent activation sequences; on the other hand, those with longer CLs tended to have a consistent activation sequence. Based on the characteristics of AF, we modified the CL variation and acceptance threshold of the CL in the beat acceptance criteria and successfully performed automated mapping of the RPV-to-RA breakthrough during the PV fibrillation and AF.
During the activation mapping in this case, we initially created an LA map. The EAS in the LA activation map was identified on the LA septum, not along the previous RPV isolation line, which suggested that the breakthrough site from the RPVs to the atrium may be located in the RA.3 Thus, we subsequently created the RA map and noticed that the RPV-to-atrium breakthrough was located on the RA septum.
Some residual conduction gaps after a PV isolation are associated with epicardial conduction between the PVs and atria.1, 2, 3, 4 In the present case, activation mapping outside the RPV isolation line during the AF exhibited an EAS on the RA septum. Further, RF applications at the EAS successfully completed an electrical isolation of the RPVs. These results suggested that the residual gap was associated with epicardial conduction between the RPVs and RA, which may have consisted of intercaval bundle fibers, as reported in the previous studies.2,3 During the first and second ablation procedures, the electrical connections between the RPVs and RA were not evaluated. However, the RPVs were electrically isolated by RF applications around the anterior carina inside the previous extensive RPV isolation line, which may suggest that the RPV-RA connection during the third procedure was likely to have been associated with the residual conduction gap after the first and second procedures.
Footnotes
Disclosures: No author has a real or perceived conflict of interest. Funding Sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.10.009.
Appendix. Supplementary data
References
- 1.Barrio-Lopez M.T., Sanchez-Quintana D., Garcia-Martinez J. Epicardial connections involving pulmonary veins: the prevalence, predictors, and implications for ablation outcome. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007544. [DOI] [PubMed] [Google Scholar]
- 2.Patel P.J., D'Souza B., Saha P., Chik W.W., Riley M.P., Garcia F.C. Electroanatomic mapping of the intercaval bundle in atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:1262–1267. doi: 10.1161/CIRCEP.114.001738. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K., Baba M., Shinoda Y. Epicardial connection between the right-sided pulmonary venous carina and the right atrium in patients with atrial fibrillation: A possible mechanism for preclusion of pulmonary vein isolation without carina ablation. Heart Rhythm. 2019;16:671–678. doi: 10.1016/j.hrthm.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Castellano N., Villacastin J., Salinas J. Epicardial connections between the pulmonary veins and left atrium: relevance for atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2011;22:149–159. doi: 10.1111/j.1540-8167.2010.01873.x. [DOI] [PubMed] [Google Scholar]
- 5.Anter E., McElderry T.H., Contreras-Valdes F.M. Evaluation of a novel high-resolution mapping technology for ablation of recurrent scar-related atrial tachycardias. Heart Rhythm. 2016;13:2048–2055. doi: 10.1016/j.hrthm.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita S., Takigawa M., Denis A. Pulmonary vein-gap re-entrant atrial tachycardia following atrial fibrillation ablation: an electrophysiological insight with high-resolution mapping. Europace. 2019;21:1039–1047. doi: 10.1093/europace/euz034. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura T., Martin R., Denis A. Characteristics of single-loop macroreentrant biatrial tachycardia diagnosed by ultrahigh-resolution mapping system. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005558. [DOI] [PubMed] [Google Scholar]
- 8.Luther V., Sikkel M., Bennett N. Visualizing localized reentry with ultra-high density mapping in iatrogenic atrial tachycardia: Beware pseudo-reentry. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004724. [DOI] [PubMed] [Google Scholar]
- 9.Martins R.P., Galand V., Behar N. Localization of residual conduction gaps after wide antral circumferential ablation of pulmonary veins. JACC Clin Electrophysiol. 2019;5:753–765. doi: 10.1016/j.jacep.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Eitel C., Hindricks G., Sommer P. Circumferential pulmonary vein isolation and linear left atrial ablation as a single-catheter technique to achieve bidirectional conduction block: the pace-and-ablate approach. Heart Rhythm. 2010;7:157–164. doi: 10.1016/j.hrthm.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Steven D., Reddy V.Y., Inada K. Loss of pace capture on the ablation line: a new marker for complete radiofrequency lesions to achieve pulmonary vein isolation. Heart Rhythm. 2010;7:323–330. doi: 10.1016/j.hrthm.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto K., Tsuchiya T., Yamaguchi T. A new method of a pulmonary vein map to identify a conduction gap on the pulmonary vein antrum ablation line. Circ J. 2011;75:2363–2371. doi: 10.1253/circj.cj-11-0198. [DOI] [PubMed] [Google Scholar]
- 13.Salas J., Castellanos E., Peinado R. Atrial mapping during pulmonary vein pacing: a novel maneuver to detect and close residual conduction gaps in an ablation line. J Interv Card Electrophysiol. 2016;47:299–307. doi: 10.1007/s10840-016-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.