Abstract
Background
The 2019 American Thoracic Society/Infectious Diseases Society of America guidelines for community-acquired pneumonia (CAP) revised recommendations for culturing and empiric broad-spectrum antibiotics. We simulated guideline adoption in Veterans Affairs (VA) inpatients.
Methods
For all VA acute hospitalizations for CAP from 2006–2016 nationwide, we compared observed with guideline-expected proportions of hospitalizations with initial blood and respiratory cultures obtained, empiric antibiotic therapy with activity against methicillin-resistant Staphylococcus aureus (anti-MRSA) or Pseudomonas aeruginosa (antipseudomonal), empiric “overcoverage” (receipt of anti-MRSA/antipseudomonal therapy without eventual detection of MRSA/P. aeruginosa on culture), and empiric “undercoverage” (lack of anti-MRSA/antipseudomonal therapy with eventual detection on culture).
Results
Of 115 036 CAP hospitalizations over 11 years, 17 877 (16%) were admitted to an intensive care unit (ICU). Guideline adoption would slightly increase respiratory culture (30% to 36%) and decrease blood culture proportions (93% to 36%) in hospital wards and increase both respiratory (40% to 100%) and blood (95% to 100%) cultures in ICUs. Adoption would decrease empiric selection of anti-MRSA (ward: 27% to 1%; ICU: 61% to 8%) and antipseudomonal (ward: 25% to 1%; ICU: 54% to 9%) therapies. This would correspond to greatly decreased MRSA overcoverage (ward: 27% to 1%; ICU: 56% to 8%), slightly increased MRSA undercoverage (ward: 0.6% to 1.3%; ICU: 0.5% to 3.3%), with similar findings for P. aeruginosa. For all comparisons, P < .001.
Conclusions
Adoption of the 2019 CAP guidelines in this population would substantially change culturing and empiric antibiotic selection practices, with a decrease in overcoverage and slight increase in undercoverage for MRSA and P. aeruginosa.
Keywords: pneumonia, guideline, empiric therapy
Across VA facilities nationwide, universal adoption of 2019 ATS/IDSA community-acquired pneumonia guidelines would substantially reduce blood culturing, empiric anti-MRSA, and antipseudomonal therapies and overcoverage for MRSA and Pseudomonas aeruginosa pneumonia but slightly increase respiratory cultures and undercoverage compared with previous practice.
Community-acquired pneumonia (CAP) is the most common infectious cause of death in the United States [1] and carries a significant and increasing economic burden [2]. Effective treatment requires timely administration of appropriate empiric antibiotic therapy [3], but the causative agent is rarely identified [4]. Without a reliable feedback mechanism to tailor empiric therapy decisions there remains much uncertainty with regard to what empiric therapy is appropriate. Recognizing that the concept of healthcare-associated pneumonia (HCAP) [5, 6] may have driven an increase in the use of empiric antibiotic regimens with activity against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa [7, 8] without observed improvements in decision accuracy [9, 10], the recently released 2019 American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) practice guidelines for CAP retracted the HCAP category and recommended new indications for empiric anti-MRSA or antipseudomonal therapy as well as blood and respiratory cultures [11].
The implications of these guidelines are unknown. The aim of this study was to estimate the impact of the new ATS/IDSA CAP guidelines on culturing and empiric antibiotic selection practices in a large national cohort of patients hospitalized for CAP. We conducted a thought experiment [12]—“How would practice change if the guidelines were universally adopted?”—by comparing observed versus guideline-expected practices.
METHODS
Study Design, Setting, and Participation
We conducted a retrospective cohort study of all immunocompetent patients hospitalized for CAP in the Veterans Affairs (VA) healthcare system from 1 January 2006 to 31 December 2016. We included adults (age ≥18 years) with hospitalization in acute inpatient wards following emergency department (ED) visits who underwent chest imaging and were diagnosed with CAP. We identified CAP diagnosis by a principal International Classification of Diseases, 9th or 10th revision (ICD-9/10), code for pneumonia or secondary ICD-9/10 code for pneumonia with a principal ICD-9/10 code for sepsis and respiratory failure [13, 14]. Patients were excluded if they had ED visits with chest imaging within the past 3 months, were not administered an antimicrobial within the first calendar day of hospitalization, or were immunocompromised (any of AIDS, solid-organ transplant, stem-cell transplant, or neutropenia) [15]. Data were accessed using the Veterans Informatics and Computing Infrastructure (VINCI) [16].
The study was reviewed and approved, and waivers of consent and authorization were granted, by the University of Utah Institutional Review Board and the Research and Development Committee of the VA Salt Lake City Health Care System. Study data were not de-identified.
Measurements
Baseline comorbidities were defined by the presence of an outpatient ICD-9/10 code within the past year prior to hospitalization [17]. Clinical risk factors for drug-resistant organisms including previous hospitalization within the prior 90 days, receipt of intravenous (IV; parenteral) antibiotics within the prior 90 days, and detection of a positive respiratory culture for MRSA or P. aeruginosa within the prior year were abstracted using a previously validated approach in VINCI [18]. We defined severe CAP as initial admission to an intensive care unit (ICU) rather than use severe CAP criteria due to the lack of unstructured chest imaging, and calculated the Pneumonia Severity Index score for context [19].
Observed Practice
For each hospitalization, we determined whether initial respiratory or blood cultures were obtained within 24 hours before or after the time of ED presentation [20]. We extracted all antibiotics recorded in the barcode administration record within 6 hours prior to and 24 hours after initial ED presentation [18]. We classified “standard” therapy as the combination of a B-lactam plus macrolide or tetracycline, or as monotherapy with a respiratory fluoroquinolone (moxifloxacin and levofloxacin); anti-MRSA therapy as any regimen that included vancomycin or linezolid; antipseudomonal therapy as any regimen that included piperacillin-tazobactam, cefepime, imipenem, meropenem, ticarcillin-clavulanate, ceftazidime, or an aminoglycoside.
Guideline-Expected Practice
We then examined the practice patterns that would have occurred if the 2019 CAP guidelines were applied at the time of hospitalization, as summarized in Table 1. For the purposes of these analyses we assumed 100% guideline adoption. We treated recommendations for culturing and empiric antibiotic selection as independent, since physicians tend to adopt testing and treatment guidelines differently. In a secondary analysis we treated recommendations as dependent, which changed guideline-expected culturing to be contingent upon guideline-expected empiric antibiotic therapy instead of observed antibiotic practice.
Table 1.
Summary of Guideline-Expected Culturing and Empiric Antibiotic Inpatient Practice for Community-Acquired Pneumonia
| Recommended Practice | Nonsevere CAP (Ward) | Severe CAP (ICU) |
|---|---|---|
| Culturing | ||
| Obtain respiratory culturesa | No recommendation for/against, except for any of: | Routine |
| a. Empiric treatment for MRSA or PAb | ||
| b. Prior respiratory isolation of MRSA or PA | ||
| c. Clinical risk factors for resistant organismsc | ||
| Obtain blood cultures | Suggest against routinely obtaining, except for any of: | Routine |
| a. Empiric treatment for MRSA or PAb | ||
| b. Prior respiratory isolation of MRSA or PA | ||
| c. Clinical risk factors for resistant organismsc | ||
| Empiric antibiotic selection | ||
| Standard | Routine | Routine |
| Add anti-MRSA | Prior respiratory isolation of MRSA | Prior respiratory isolation of MRSA or clinical risk factors for resistant organismsc |
| Add anti-PA | Prior respiratory isolation of PA | Prior respiratory isolation of PA or clinical risk factors for resistant organismsc |
| Add anti-MRSA and anti-PA | Prior respiratory isolation of MRSA and PA | Prior respiratory isolation of MRSA and PA or clinical risk factors for resistant organismsb |
Adapted from 2019 American Thoracic Society/Infectious Diseases Society of America guidelines for community-acquired pneumonia [11]. Abbreviations: CAP, community-acquired pneumonia; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa.
aRespiratory culture indicates Gram stain and culture of sputum, tracheal aspirate, bronchoalveolar lavage specimens, and pleural fluid.
bEmpiric treatment with anti-MRSA or anti-PA therapy selected for some other reason is listed as an indication for obtaining cultures.
cClinical risk factors for resistant organisms include the combination of hospitalization within preceding 90 days and receipt of intravenous antibiotics (not necessarily during the prior hospitalization) within preceding 90 days.
For patients admitted to a hospital ward (non-ICU setting), we defined guideline-expected respiratory and blood cultures as present if there were anti-MRSA or antipseudomonal therapies initiated empirically on index hospitalization (observed empiric antibiotic use), prior respiratory isolation of MRSA or P. aeruginosa within 1 year, or clinical risk factors for resistant organisms including a history of hospitalization and receipt of IV antibiotics within 90 days. Guideline-expected blood and respiratory cultures were present in all ICU patients since ICU admission was used as a proxy for severe CAP. Guideline-expected anti-MRSA therapy was present if there was a history of MRSA isolation on respiratory culture within 1 year or ICU admission with clinical risk factors for resistant organisms. Guideline-expected antipseudomonal therapy was defined similarly. Guideline-expected “standard” therapy alone was present if there were no indications for anti-MRSA or antipseudomonal therapy. We additionally performed a secondary analysis using isolation from any culture source (not including MRSA nasal polymerase chain reaction [PCR]) in the definition of guideline-expected antibiotic selection.
Statistical Analysis
Our primary analysis was a cross-sectional comparison of culturing and empiric antibiotic selection between observed and guideline-expected practices in subgroups initially admitted to the hospital ward and to the ICU. We reported proportions, by facility, of hospitalizations in which each practice was found. We used Cochran-Mantel-Haenszel tests [21] with stratification by facility to assess for statistically significant differences in observed versus guideline-expected proportions for each practice. We estimated the impact of guideline-expected culturing practices on MRSA and P. aeruginosa case detection rate using 2 × 2 contingency tables. Calculating the number of additional cases identified by additional guideline-expected cultures was not possible in this study. We assessed the performance of clinicians to match empiric anti-MRSA or antipseudomonal therapy to detection on initial cultures (“bug–drug matching”) using 2 × 2 contingency tables, similar to previous work [10]. We calculated sensitivity, specificity, and the diagnostic odds ratio as performance parameters. We defined empiric “undercoverage” as false negatives: the proportion of hospitalizations with MRSA/P. aeruginosa detected that did not receive anti-MRSA/antipseudomonal therapy. We defined empiric “overcoverage” as false positives: the proportion of hospitalizations with anti-MRSA/antipseudomonal therapy in which MRSA/P. aeruginosa was not detected on culture. In a secondary analysis, we assessed bug–drug matching in the subset of hospitalizations with cultures obtained, thus excluding those in which MRSA and P. aeruginosa could not be ruled out by negative culture.
We used appropriate summary measures to describe the populations admitted to the hospital ward and ICU. We used a 2-tailed P < .01 as the a priori level for significance. All statistical analyses were conducted using Stata version 15 (StataCorp, College Station, TX) and figures were constructed using R version 3.5.3 (R Core Team, 2019) [22] with the ggplot2 package [23].
RESULTS
Across 114 VA hospitals over 11 years (1 January 2006–31 December 2016) there were 115 036 hospitalizations for CAP meeting inclusion criteria, of whom 17 877 (16%) were initially admitted to an ICU. The cohort was predominantly elderly (median age, 70; interquartile range, 63–82 years) and male (97%) with a high burden of chronic disease (Table 2). There was a low prevalence of prior respiratory isolation of MRSA (ward: 1%; ICU: 1%) or P. aeruginosa (ward: 1%; ICU: 2%). Clinical risk factors for resistant organisms were present in 5% of those admitted to a hospital ward and 8% of those admitted to an ICU.
Table 2.
Characteristics of Hospitalizations for Community-Acquired Pneumonia
| Characteristics | Hospital Ward (n = 97 159) | ICU (n = 17 877) |
|---|---|---|
| Comorbidities | ||
| Age, median (IQR), years | 71 (63–82) | 69 (62–80) |
| Male sex, n (%) | 93 859 (97) | 17 302 (97) |
| Pulmonary disease, n (%) | 43 455 (45) | 8658 (48) |
| Congestive heart failure, n (%) | 17 856 (18) | 4246 (24) |
| Coronary artery disease, n (%) | 41 609 (43) | 8254 (46) |
| Renal disease, n (%) | 14 947 (15) | 3114 (17) |
| Liver disease, n (%) | 1973 (2) | 573 (3) |
| Cancer, n (%) | 22 141 (23) | 3965 (22) |
| Drug-resistant pneumonia risk factors, n (%) | ||
| Drug-resistant organism risk | 5155 (5) | 1504 (8) |
| Hospitalization within past 90 days | 11 024 (11) | 2780 (16) |
| IV antibiotics within past 90 days | 7314 (8) | 1879 (11) |
| Prior MRSA respiratory isolation | 910 (1) | 259 (1) |
| Prior MRSA isolation any source | 2649 (3) | 756 (4) |
| Prior Pseudomonas aeruginosa respiratory isolation | 1237 (1) | 396 (2) |
| Prior P. aeruginosa isolation, any source | 2862 (3) | 883 (5) |
| Pneumonia severity factors | ||
| PSI risk class, n (%) | ||
| I—low risk of death | 2499 (3) | 149 (1) |
| II | 16 096 (17) | 1392 (8) |
| III | 24 925 (26) | 2868 (16) |
| IV | 42 641 (44) | 8132 (45) |
| V—high risk of death | 10 998 (11) | 5336 (30) |
| PSI risk score, median (IQR) | 93 (75–113) | 112 (90–134) |
| Detection of resistant organisms by culture, n (%) | ||
| MRSA | 1714 (2) | 743 (4) |
| P. aeruginosa | 1897 (2) | 749 (4) |
Prior isolations included any positive culture within the VA system for a given patient within 1 year prior to the index hospitalization. Abbreviations: CAP, community-acquired pneumonia; ICU, intensive care unit; IQR, interquartile range; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; PSI, Pneumonia Severity Index [19].
Culturing and Empiric Antibiotic Practices
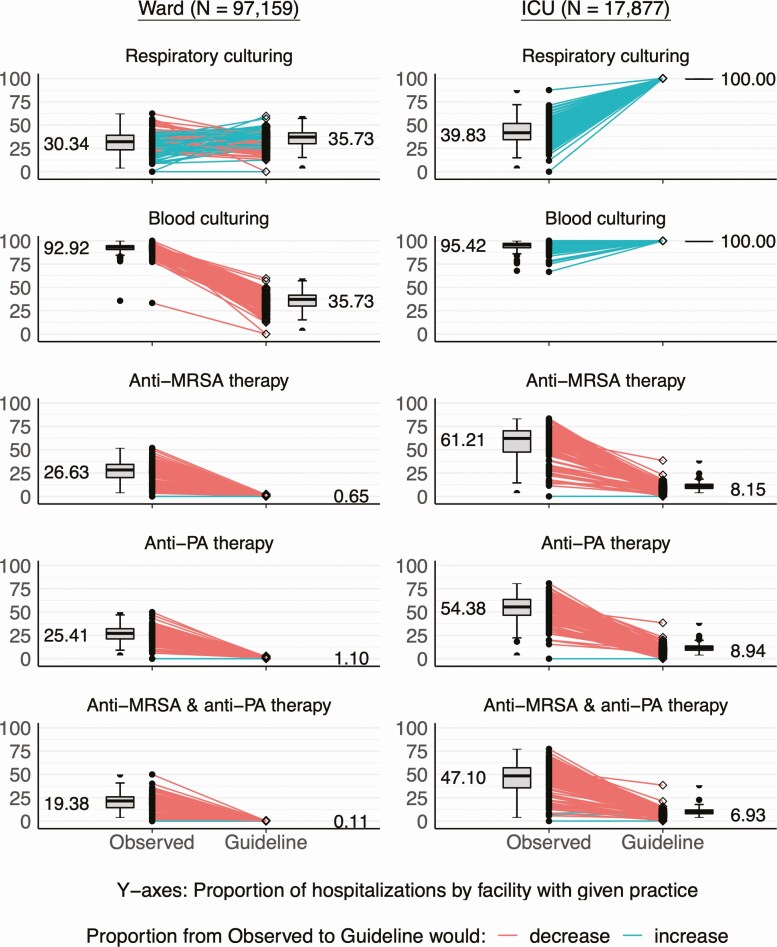
Among patients admitted to a hospital ward, adoption of new CAP guideline recommendations would result in a slight increase in the proportion with respiratory cultures (30% to 36%) and a substantial decrease in the proportion with blood cultures (93% to 36%) (Figure 1). Among patients admitted to the ICU (all severe CAP by our definition), both respiratory and blood culture proportions would increase (respiratory, 40% to 100%; blood, 95% to 100%). In the secondary analysis treating guideline-expected culturing as dependent on universal adoption of antibiotic recommendations (Supplementary Figure 1), guideline-expected respiratory and blood culture proportions on hospital wards would be 6%—substantially lower than the 36% seen in primary analysis (independent recommendations).
Figure 1.
Observed versus guideline-expected culturing and empiric antibiotic selection practices. Each plot depicts the proportion of hospitalizations with the indicated practice. Markers connected by colored lines represent proportions at the facility level under observed (closed dots, •) and guideline-expected (open diamonds, ◊) conditions. Adjacent boxplots depict the variability in these proportions, with median facility proportion labeled. For all observed-guideline proportion comparisons, P < .001 by Cochran-Mantel-Haenszel tests with stratification by facility. Abbreviations: ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa.
For admissions to hospital wards and ICUs guideline adoption would result in decreased proportions of both empiric anti-MRSA therapy (ward: 27% to 1%; ICU: 61% to 8%) and antipseudomonal therapy (ward: 25% to 1%; ICU: 54% to 9%) (Figure 1). Findings were similar, with slightly higher guideline-expected anti-MRSA and antipseudomonal therapy, in a secondary analysis using prior isolation of resistant organisms in any culture in the definition of guideline-expected antibiotic therapy (Supplementary Figure 2). Differences were not substantially different across study years (Supplementary Figure 3) (for all comparisons, P < .001).
Observed Versus Guideline Case Detection
Among patients admitted to hospital wards, guideline-expected practice would be expected to substantially shift which patients are cultured. Guideline adoption would add cultures to some (respiratory: 25%; blood: 2%), remove cultures from others (respiratory: 19%; blood: 57%), and leave the rest unchanged (respiratory: 57%; blood: 41%) (Figure 2). Overall, 860 cultures (0.7% of all cultures) that were positive for MRSA or P. aeruginosa would not have been obtained. This would correspond to missing 26% of MRSA and 36% of P. aeruginosa cases, including 45 cases of MRSA bacteremia, detected by observed culturing (0.8% of all ward admissions). It was not possible to assess how many additional cases may be identified by additional guideline-expected cultures.
Figure 2.
Reclassification of observed versus guideline-expected culturing and MRSA/PA case detection in hospital wards. (A, B) Contingency 2 × 2 tables between observed and guideline-expected respiratory and blood culturing. Insets show respective results for observed sent cultures. (C, D) Contingency 2 × 2 tables between observed and guideline-expected case detection of MRSA and PA by combined respiratory and blood culture results. Since observed-absent guideline-expected-present cultures are theoretical, the case detection from these is unknown and marked by “NA”. Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa.
Performance of Empiric Therapy Selection (Bug–Drug Matching)
Compared with eventual microbiological detection of MRSA and P. aeruginosa pneumonia (both 2% in hospital wards, 4% in ICUs) on initial cultures, guideline-expected therapy would result in greatly decreased MRSA overcoverage (ward: 27% to 1%; ICU: 56% to 8%) and slightly increased MRSA undercoverage (ward: 0.6% to 1.3%; ICU: 0.5% to 3.3%), with similar findings for P. aeruginosa (Table 3). Guideline-expected use of anti-MRSA or antipseudomonal therapy was overall less sensitive, more specific, and more accurate (greater diagnostic odds ratios). When stratifying guideline-expected therapy by indication, a history of respiratory isolation was more accurate than healthcare exposure at predicting clinical infection. Findings were similar in sensitivity analyses excluding patients in whom cultures were not sent (Supplementary Table 1).
Table 3.
Performance Characteristics of Observed Versus Guideline-Expected Empiric Antibiotic Selection Against Microbiological Detection of Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Community-Acquired Pneumonia
| Comparison (95% CI) | |||||
|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | Diagnostic Odds Ratio | Undercoverage,a % | Overcoverage,b % | |
| Hospital ward (n = 97 159) | |||||
| Anti-MRSA | |||||
| Observed | 64 (62–66) | 72 (72–73) | 4.6 (4.3–4.9) | .6 (.6–.7) | 27.3 (27.0–27.6) |
| Guideline | 10 (09–12) | 99 (99–99) | 14.7 (12.4–17.3) | 1.6 (1.5–1.7) | .8 (.7–.8) |
| Anti-PA | |||||
| Observed | 52 (50–55) | 73 (73–74) | 3.0 (2.8–3.2) | .9 (.9–1.0) | 26.2 (25.9–26.4) |
| Guideline | 16 (15–18) | 99 (99–99) | 20.2 (17.7–22.9) | 1.6 (1.6–1.7) | 1.0 (.9–1.0) |
| ICU (n = 17 877) | |||||
| Anti-MRSA | |||||
| Observed | 87 (85–90) | 42 (41–43) | 5.0 (4.6–5.4) | .5 (.4–.6) | 55.5 (54.8–56.2) |
| Guidelinec | 21 (18–24) | 92 (92–92) | 3.1 (2.6–3.6) | 3.3 (3.0–3.5) | 7.7 (7.3–8.1) |
| Cx history | 7 (5–9) | 99 (99–99) | 9.1 (6.4–13.0) | 3.5 (3.3–3.8) | .7 (.6–.9) |
| Exposure | 13 (10–15) | 93 (93–93) | 1.9 (1.5–2.4) | 3.3 (3.1–3.6) | 6.8 (6.4–7.1) |
| Anti-PA | |||||
| Observed | 80 (77–83) | 46 (46–47) | 3.5 (3.2–3.8) | .8 (.7–1.0) | 51.4 (50.7–52.1) |
| Guidelinec | 30 (27–34) | 92 (91–92) | 4.7 (4.1–5.4) | 2.9 (2.7–3.2) | 8.1 (7.7–8.5) |
| Cx history | 14 (11–17) | 99 (99–99) | 15.9 (12.2–20.8) | 3.1 (2.9–3.4) | 1.0 (.8–1.1) |
| Exposure | 13 (10–16) | 93 (92–93) | 1.9 (1.5–2.4) | 3.0 (2.7–3.2) | 6.9 (6.5–7.2) |
Abbreviations: CAP, community-acquired pneumonia; CI, confidence interval; Cx, culture; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa.
aUndercoverage % = 100 × (false negatives/n) = proportion of all hospitalizations in which MRSA/PA was detected but did not receive empiric anti-MRSA/anti-PA therapy.
bOvercoverage % = 100 × (false positives/n) = proportion of all hospitalizations in which empiric anti-MRSA/anti-PA therapy was used but MRSA/PA was not detected.
cIn severe CAP (in the ICU), guideline anti-MRSA or anti-PA therapy is recommended if the patient has previously had a positive respiratory culture for MRSA/PA in the past year (Cx history) or has been exposed to either a hospitalization or intravenous antibiotics within the past 90 days (exposure). Nested rows for Cx history and Exposure indicate performance of guideline-recommended antibiotic selection stratified by those indications.
Discussion
In this observational cross-sectional analysis of more than 100 000 CAP hospitalizations in VA facilities nationwide during 2006–2016, we found that adoption of the new 2019 CAP guidelines would be expected to substantially change culturing and empiric antibiotic practices. Guidelines are expected to substantially reduce blood culturing and slightly increase respiratory culturing on hospital wards and increase all culturing in the ICU. In both hospital wards and ICUs, guideline adoption would be expected to substantially reduce the usage of empiric anti-MRSA and antipseudomonal therapies. This reduction in broad-spectrum therapy would result in large decreases (>20%) in overcoverage and small increases (<2%) in undercoverage for MRSA and P. aeruginosa. Practice patterns following guideline adoption could differ from what was anticipated in several ways.
First, the guideline authors anticipated that the new recommendations should decrease unnecessary blood cultures in patients without severe CAP and increase appropriate respiratory cultures. Our findings show that the expected effects are congruent with this intention. Presumably, culturing the “right” patients more frequently would increase the overall diagnostic yield [24–26]. However, we estimated that 370 (26%) of MRSA and 479 (36%) of P. aeruginosa cases would be “missed” by guideline-recommended culturing on hospital wards, including 45 (11%) of cases of MRSA bacteremia. We were unable to calculate how many additional cases might be identified by additional cultures.
Shifting culture practice, while unlikely to directly impact most (~99%) patients, could impact several important health-system-level activities via alterations in sampling [27]. With prevalence already low, missing cases of resistant CAP could compromise efforts at local risk factor identification and validation, which generally requires at least 30 cases per year [28]. Since isolation and resistance patterns in CAP are dependent on the source (respiratory vs blood) [29], local antibiograms could shift without actual changes in true organism prevalence. As a consequence of both of these processes the predictive utility of previously developed risk scores for resistant CAP (eg, DRIP (Drug Resistance In Pneumonia) score [30]) could also shift in unpredictable ways. This highlights the tension between the goals of reducing unnecessary culturing and improving surveillance.
We found that guideline adoption would match the stated intention of substantially decreasing the use of empiric anti-MRSA and antipseudomonal therapies, but perhaps more than anticipated. Since the introduction of the HCAP concept in 2005 [5] the use of anti-MRSA and antipseudomonal regimens has more than doubled [7, 10]. Our findings confirm a large, sustained increase in observed use of anti-MRSA and antipseudomonal therapies and suggest that the 2019 guidelines would accomplish the stated intent to curb this growth and then some. Use of anti-MRSA and antipseudomonal regimens could fall far below the pre-2005 levels. This implies a small but significant absolute increase (~2%) in the rate of empiric undercoverage with a large absolute decrease (26%) in overcoverage for MRSA/P. aeruginosa pneumonia.
Weighing the consequences of changes in undercoverage and overcoverage is complex, particularly if those patients who would experience undercoverage are sicker. Increased mortality associated with initial undercoverage for patients presenting with septic shock [31] and corresponding sepsis guideline recommendations [32, 33] support more frequent use of broad-spectrum antibiotics [31]. However, even patients admitted to ICUs may have just as great a risk of harm from overcoverage with these agents, including renal toxicity and secondary infection [34]. In pneumonia, anti-MRSA and antipseudomonal therapy has not been associated with improved outcomes in observational cohort studies [8, 10], or pre-post analyses of antimicrobial stewardship programs [35]. For the VA population, we previously failed to establish a benefit of empiric anti-MRSA therapy, even when used in those with elevated risk for MRSA pneumonia [36]. In settings of diagnostic uncertainty such as this, providers must weigh risks and benefits. The work of Kahneman and Tversky [37], and others has demonstrated that humans have difficulty thinking probabilistically and tend to overweight possible negative outcomes. Fear is a powerful, sometimes useful, component of clinical decision making [38, 39], and influences bedside application of clinical decision guidelines [40]. The magnitude of change in empiric antibiotic practices that we find the 2019 CAP guidelines suggest raises an important unanswered question: What is the optimal balance between overcoverage and undercoverage with broad-spectrum antibiotics that will maximize benefit and reduce harms, and to what degree, if any, should this vary across settings?
Limitations
This study has several important limitations to consider. As this was a “thought experiment,” we did not evaluate changes in health outcomes but only expected changes in clinical practice and explicitly ignored the variable clinical application of guideline recommendations that might be seen in a prospective interventional trial. We chose to simulate 100% (“complete”) guideline adoption to most closely examine the theoretical implications of the guideline statement. Our results provide food for thought rather than a forecast. Providers are also influenced by the Surviving Sepsis Guideline recommendations for blood cultures in all patients and timely initiation of broad-spectrum IV antibiotics [32, 33]. This may partially explain high observed proportions of combined anti-MRSA and antipseudomonal therapy (eg, “vanc-zosyn” [the combination of vancomycin and piperacillin-tazobactam]). We explicitly did not address impacts of MRSA nasal PCRs given their current variability in turnaround times. This study relies upon structured data readily available in the electronic health record in the VA patient population and there are many uncaptured factors, values, and preferences that are appropriately used in conjunction with guidelines at the bedside for clinical decision making. As in many studies on CAP, accurate selection of CAP cases is challenging. We relied on discharge diagnosis codes to select CAP cases, which may have included cases in which there was a different initial working diagnosis (ie, undifferentiated sepsis) that drove observed practice. Our study period (2006–2016), while relatively recent, may not represent practice patterns in more recent years (2017–2020). We explicitly ignored the guideline recommendation to develop “locally validated risk factors” to predict MRSA or P. aeruginosa CAP since validating such factors was not feasible in this dataset. It remains to be seen how well organizations will be able to identify and validate local risk factors in practice and future research will be needed to evaluate the impact of this strategy on clinical outcomes. Last, the VA population is unique in several ways, most notably in the predominance of older men, so our findings may not be generalizable to other populations or settings.
Our study has several notable strengths including a large nationwide sample size, well-validated measurements of patient characteristics, culturing, and antibiotic practices, and a high proportion of objectively documented risk factors for drug-resistant pneumonias due to the single-system nature of the VA.
Conclusions
Adoption of the 2019 ATS/IDSA CAP guidelines across the VA system would be expected to decrease blood culturing in non-ICU patients and substantially decrease the use of empiric broad-spectrum antibiotics. These changes would correspond to decreased rates of overcoverage and increased rates of undercoverage with overall slightly improved accuracy of empiric antibiotic selection for microbiological detection. These findings suggest that hospital administrators, antibiotic stewardship directors, and health systems should carefully consider how to implement the new guidelines depending on local priorities. Conducting simulations such as ours to estimate the degree of change called for by new recommendations could be a useful tool for future guideline development.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to acknowledge funding for this supplement from the Informatics, Decision-Enhancement and Analytic Sciences (IDEAS) Center and the VA Health Services Research and Development (HSR&D) Service.
Author contributions. Study concept and design: M. A. C., M. H. S., and B. E. J. Acquisition, analysis, or interpretation of data: M. A. C., C. H., M. N., J. Y., and V. S. Drafting of the manuscript: M. A. C., M. H. S., and B. E. J. Critical revision of the manuscript for important intellectual content: all authors.
Financial support. This work was supported by a contract from the Centers for Disease Control and Prevention (14FED1408985) and by career development grants from the Veterans’ Affairs Health Service Research and Development (grant number 150HX001240 to B. J. E. and grant number IK2HX001165 to M. M. J.).
Supplement sponsorshi p. This supplement is sponsored by the Informatics, Decision-Enhancement and Analytic Sciences (IDEAS) Center.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Matthew A Christensen, Department of Internal Medicine, University of Utah, Salt Lake City, Utah, USA.
McKenna Nevers, Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation, Veterans Affairs, Salt Lake City Health Care System, Salt Lake City, Utah, USA; Division of Epidemiology, University of Utah, Salt Lake City, Utah, USA.
Jian Ying, Division of Epidemiology, University of Utah, Salt Lake City, Utah, USA.
Candace Haroldsen, Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation, Veterans Affairs, Salt Lake City Health Care System, Salt Lake City, Utah, USA; Division of Epidemiology, University of Utah, Salt Lake City, Utah, USA.
Vanessa Stevens, Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation, Veterans Affairs, Salt Lake City Health Care System, Salt Lake City, Utah, USA; Division of Epidemiology, University of Utah, Salt Lake City, Utah, USA.
Makoto M Jones, Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation, Veterans Affairs, Salt Lake City Health Care System, Salt Lake City, Utah, USA; Division of Epidemiology, University of Utah, Salt Lake City, Utah, USA.
Peter M Yarbrough, Department of Internal Medicine, Veterans Affairs Salt Lake City Health Care System and University of Utah, Salt Lake City, Utah, USA.
Matthew Bidwell Goetz, Division of Infectious Disease, Veterans Affairs Greater Los Angeles Healthcare System and David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
Marcos I Restrepo, Division of Pulmonary and Critical Care, South Texas Veterans Health Care System and UT Health San Antonio, San Antonio, Texas, USA.
Karl Madaras-Kelly, Pharmacy Service, Veterans Affairs Boise Idaho and Idaho State University, Boise, Idaho, USA.
Matthew H Samore, Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation, Veterans Affairs, Salt Lake City Health Care System, Salt Lake City, Utah, USA.
Barbara Ellen Jones, Informatics, Decision-Enhancement, and Analytic Sciences (IDEAS) Center of Innovation, Veterans Affairs, Salt Lake City Health Care System, Salt Lake City, Utah, USA; Division of Pulmonary and Critical Care, University of Utah, Salt Lake City, Utah, USA.
References
- 1. Centers for Disease Control and Prevention. Underlying cause of death 1999–2015 on CDC WONDER online database. Released December, 2016. Available at: http://wonder.cdc.gov/ucd-icd10.html. Accessed 23 October 2017.
- 2. Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67:71–9. [DOI] [PubMed] [Google Scholar]
- 3. Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 2010; 38:1045–53. [DOI] [PubMed] [Google Scholar]
- 4. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
- 6. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berger A, Edelsberg J, Oster G, Huang X, Weber DJ. Patterns of initial antibiotic therapy for community-acquired pneumonia in U.S. hospitals, 2000 to 2009. Am J Med Sci 2014; 347:347–56. [DOI] [PubMed] [Google Scholar]
- 8. Webb BJ, Dangerfield BS, Pasha JS, Agrwal N, Vikram HR. Guideline-concordant antibiotic therapy and clinical outcomes in healthcare-associated pneumonia. Respir Med 2012; 106:1606–12. [DOI] [PubMed] [Google Scholar]
- 9. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014; 58:330–9. [DOI] [PubMed] [Google Scholar]
- 10. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006–2010. Clin Infect Dis 2015; 61:1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mach E. On thought experiments. In: McGuinness BF, ed. Knowledge and error: sketches on the psychology of enquiry. Netherlands: Springer Netherlands, 1976:134–47. [Google Scholar]
- 13. Lindenauer PK, Lagu T, Shieh M-S, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 2012; 307:1405–13. [DOI] [PubMed] [Google Scholar]
- 14. Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Marked reduction in 30-day mortality among elderly patients with community-acquired pneumonia. Am J Med 2011; 124:171–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Pasquale MF, Sotgiu G, Gramegna A, et al. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis 2019; 68:1482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Department of Veterans Affairs. VA Informatics and Computing Infrastructure (VINCI). Available at: http://hsrd.research.va.gov/ for_researchers/vinci. Accessed 18 May 2020.
- 17. Madaras-Kelly KJ, Burk M, Caplinger C, et al. ; Pneumonia Duration of Therapy Medication Utilization Evaluation Group . Total duration of antimicrobial therapy in veterans hospitalized with uncomplicated pneumonia: results of a national medication utilization evaluation. J Hosp Med 2016; 11:832–9. [DOI] [PubMed] [Google Scholar]
- 18. Jones BE, Haroldsen C, Madaras-Kelly K, et al. In data we trust? Comparison of electronic versus manual abstraction of antimicrobial prescribing quality metrics for hospitalized veterans with pneumonia. Med Care 2018; 56:626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 20. Jones BE, Brown KA, Jones MM, et al. Variation in empiric coverage versus detection of methicillin-resistant Staphylococcus aureus and pseudomonas aeruginosa in hospitalizations for community-onset pneumonia across 128 US Veterans Affairs medical centers. Infect Control Hosp Epidemiol 2017; 38:937–44. [DOI] [PubMed] [Google Scholar]
- 21. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Analyses involving multiple categorical predictors. In: Regression methods in biostatistics. Boston, MA: Springer US, 2012:50–2. Available at: http://link.springer.com/10.1007/978-1-4614-1353-0. [Google Scholar]
- 22. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available at: https://www.r-project.org/.
- 23. Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer, 2016. Available at: http://ggplot2.tidyverse.org. [Google Scholar]
- 24. Restrepo MI, Babu BL, Reyes LF, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence st udy of hospitalised patients. Eur Respir J 2018; 52: 1701190. doi: 10.1183/13993003.01190-2017. [DOI] [PubMed] [Google Scholar]
- 25. Aliberti S, Reyes LF, Faverio P, et al. Global initiative for meticillin-resistant Staphylococcus aureus pneumonia (GLIMP): an international, observational cohort study. Lancet Infect Dis 2016; 16:1364–76. [DOI] [PubMed] [Google Scholar]
- 26. Carugati M, Aliberti S, Sotgiu G, et al. Bacterial etiology of community-acquired pneumonia in immunocompetent hospitalized patients and appropriateness of empirical treatment recommendations: an international point-prevalence study. Eur J Clin Microbiol Infect Dis 2020; 39:1513–25. Available at: 10.1007/s10096-020-03870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones BE, Jones MM. Pneumonia and Electronic Health Records—A Window Into Disease, A Mirror of Our Behavior, or Just Another Streetlight? Clin Infect Dis 2020; 71: 1613–15. [DOI] [PubMed] [Google Scholar]
- 28. Hindler JF, Stelling J. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 2007; 44:867–73. [DOI] [PubMed] [Google Scholar]
- 29. Haessler S, Lindenauer PK, Zilberberg MD, et al. Blood cultures versus respiratory cultures: two different views of pneumonia. Clin Infect Dis 2019; Available at: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciz1049/5609008. Accessed 10 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Webb BJ, Dascomb K, Stenehjem E, et al. Derivation and multicenter validation of the drug resistance in pneumonia clinical prediction score. Antimicrob Agents Chemother 2016; 60:2652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136:1237–48. [DOI] [PubMed] [Google Scholar]
- 32. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45:486–552. [DOI] [PubMed] [Google Scholar]
- 33. Dellinger RP, Carlet JM, Masur H, et al. ; Surviving Sepsis Campaign Management Guidelines Committee . Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004; 32:858–73. [DOI] [PubMed] [Google Scholar]
- 34. Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med 2008; 36:S216–23. [DOI] [PubMed] [Google Scholar]
- 35. Kamata K, Suzuki H, Kanemoto K, et al. Clinical evaluation of the need for carbapenems to treat community-acquired and healthcare-associated pneumonia. J Infect Chemother 2015; 21:596–603. [DOI] [PubMed] [Google Scholar]
- 36. Jones BE, Ying J, Stevens V, et al. Empirical anti-MRSA vs standard antibiotic therapy and risk of 30-day mortality in patients hospitalized for pneumonia. JAMA Intern Med 2020; 84132:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kahneman D. Thinking fast and slow. New York: Farrar, Straus and Giroux, 2011. [Google Scholar]
- 38. Islam R, Weir CR, Jones M, Del Fiol G, Samore MH. Understanding complex clinical reasoning in infectious diseases for improving clinical decision support design. BMC Med Inform Decis Mak 2015; 15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kozlowski D, Hutchinson M, Hurley J, Rowley J, Sutherland J. The role of emotion in clinical decision making: an integrative literature review. BMC Med Educ 2017; 17:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Croskerry P. A universal model of diagnostic reasoning. Acad Med 2009; 84:1022–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.