Abstract
Evidence from both basic and clinical science suggests that neuropathic pain can induce cognitive dysfunction. However, these results are mainly based on a series of behavioral tests, there is a lack of quantitative variables to indicate cognitive impairment. Neuronal activity-regulated pentraxin (NPTX2) is a ubiquitously expressed, secreted protein in the nervous system. NPTX2 has been implicated to be involved in a variety of neuropathic diseases including Parkinson’s disease, ischemia, and Alzheimer’s disease. In a mouse model of chronic pain, NPTX2 is involved in the regulation of inflammatory responses. Here, we employ a variety of behavioral approaches to demonstrate that mice with chronic neuropathic pain have cognitive impairment and exhibit an increased anxiety response. The expression of NPTX2, but not NPTX1, was down-regulated in the hippocampus and cortex after chronic neuropathic pain exposure. The modulation effect of NPTX2 on cognitive function was also verified by behavioral tests using Nptx2 knock-out mice. Above all, we conclude that downregulation of NPTX2 induced by neuropathic pain may serve as an indicator of a progressive cognitive dysfunction during the induction and maintenance of spared nerve injury.
Keywords: cognitive dysfunction, neuropathic pain, NPTX1, NPTX2
Introduction
Neuropathic pain is a complex multidimensional insult with marked effects on many aspects of an individual’s daily life beyond physical functioning, especially cognitive components such as attentional capacity, processing speed, and psychomotor speed [1]. Existing therapeutics for pain are often less effective for neuropathic pain; moreover, effective pain relief with existing analgesics usually do not target improvement of pain-related cognitive dysfunction [2]. To this end, it is of immense importance to explore the mechanisms of chronic pain that induce cognitive disturbances.
Previous studies have demonstrated that chronic pain arising from peripheral nerve injury induces alterations in various areas of the brain including the hippocampus and cortex [3]. These alterations include changes in inflammatory mediators, neurotransmitters, and synaptic plasticity [4], which further burden cognitive impairment [5].
Neuronal Pentraxin 2 (NPTX2; also termed Narp and NP2) is a member of a family of ‘long’ Neuronal Pentraxins that also includes Neuronal Pentraxin 1 (NPTX1) and Neuronal Pentraxin Receptor. NPTX1 and NPTX2 are secreted, calcium-(Ca2+) dependent lectins, present at excitatory synapses where they bind the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-type glutamate receptors to form an extracellular synaptic scaffolding protein complex that contributes to synaptogenesis [6,7]. Previous studies have also shown that NPTX2 overexpression can increase excitatory synapse formation [8], while downregulation of NPTX2 decreases excitatory synapse formation [9]. Recently, NPTX2 has also been suggested as a cognitive biomarker, with patients with mild cognitive impairment and Alzheimer’s disease showing an obvious reduction in NPTX2 levels [10]. In a sciatic nerve transection (SNT) model, NPTX2 knock-out (KO) mice displayed an exaggerated microglia/macrophage response compared with wild-type mice [11] and also demonstrated cognitive inflexibility and addictive behavior, which are associated with abnormal reactivity to a novel stimulus [12]. All these pieces of evidence suggest that NPTX2 is associated with cognitive impairment.
To further understand the pattern of changes in NPTX1 and NPTX2 within the hippocampus and forebrain during the process of spared nerve injury (SNI)-induced cognitive dysfunction of mice, we investigated their expression levels using western blotting. First, we established the mouse SNI model and employed various behavioral methods to confirm neuropathic pain-induced cognitive impairment. Next, we explored the expression of NPTX1 and NPTX2 in the hippocampus and frontal lobe during SNI-induced cognitive impairment to observe the alteration of potential biomarkers during the development of neuropathic pain. Finally, we generated NPTX2 KO mice and conducted a battery of behavioral tests to test the hypothesis that NPTX2 modulates cognitive function and anxiety behavior.
Materials and methods
Experimental animals
All protocols used in this study were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington DC, 1996) and approved by the Soochow University Animal Care Committee. Nine-week-old C57BL6 Narp −/− transgenic mice (Cyagen Biosciences, Suzhou, China) and wild-type littermates were housed in groups of five in cages (32 × 21 × 16 cm) on corn cob bedding at a constant temperature of 22–23 °C, with an alternating 12 h light/dark cycle (lights on at 07:00 h) and water and food available ad libitum. ARRIVE guidelines were followed as animals were involved in this study. All efforts were made to minimize suffering and reduce the number of animals used for the purposes of this study. A schematic diagram of the experimental timeline was shown in Fig. 1.
Fig. 1.

SNI mice showed nociceptive allodynia while maintained normal locomotor activity: (a) Schematic diagram of the experimental timeline. (b and c) Daily nociceptive behavior before (BL) and on day 3, 5, 7, 14 after SNI or sham surgery; SNI-induced mechanical allodynia was demonstrated as reduced mechanical withdrawal threshold (b), and heat hyperalgesia was manifested as reduced latency (c) (n = 6; P < 0.05 vs. sham group). At 14 days after surgery, the total travel distances (d; n = 6; P = 0.2151) and mean velocities (d; n = 6; P = 0.5888) were similar between SNI and sham mice in the open field test. BL, baseline; SNI, spared nerve injury.
Surgical procedures
Mice were anaesthetized with isoflurane (induced at 5% and maintained at 2%) inhalation in 100% oxygen, and then SNI was performed as previously described. Briefly, the sural, common peroneal, and tibial branches of the left sciatic nerve were exposed and subsequently transected. The sural nerve was left intact. In the sham group, the tibial and common peroneal nerves were exposed, but no ligation or axotomy was performed. Mice were allowed to recover for 2 weeks following surgery.
Behavioral tests
All behavioral experiments were carried out between 9 a.m. and 2 p.m. by investigators who were blinded to the treatment conditions. Animals were habituated to the testing environment for three consecutive days (30–60 minutes/d) before baseline testing.
Nociceptive behavior test
Paw withdrawal threshold
The mechanical pain threshold was assessed with von Frey filaments according to the ‘up-down’ method described by Chaplan et al. [13]. Mice were placed in a plastic cage with a wire-mesh bottom which allowed full access to the paws. An accommodation period was allowed until exploration and major grooming activities ceased. Nylon fibers of sequentially increasing stiffness (0.08–2 g; Stoelting Co., Wood Dale, Illinois, USA) were perpendicularly applied to the plantar surface of the left hind paw and kept in place for up to 5 seconds. If no response occurred, the next stiffer fiber was applied; if a response occurred, a less stiff fiber was applied. Stimuli were presented at intervals of 15–30 seconds allowing for the resolution of any behavioral response to the previous stimulus. Four more fibers were applied after the first withdrawal response occurred, allowing estimation of the mechanical withdrawal threshold.
Paw withdrawal latency
Thermal paw withdrawal latencies (PWLs) to radiant heat stimuli were measured with a plantar stimulator analgesia meter (Model 390 Analgesia Meter; IITC Inc./Life Science Instruments, Woodland Hills, California, USA). Briefly, radiant heat was applied from below to the middle of the plantar surface of the ipsilateral hind paw. The time between the starting of the beam and the foot lift was defined as the PWL. To avoid tissue damage, a cutoff time of 20 seconds was set. Each trial was repeated three times at a 10-minute interval to prevent sensitization.
Motor function evaluation
The mice were placed in the center of an open field apparatus (60 × 60 cm, 50 cm height), which was divided into a peripheral, center, and intermediate zones. Mice were put in the same place within the field and tested individually in a 5-minute session [14]. All activities were analyzed by the Ethovision XT (Noldus, Beijing, China) monitoring analysis software. The apparatus was cleaned with 75% alcohol after every test to eliminate the odor of the previously tested animal.
Novel object recognition test
The novel object recognition (NOR) test was performed in the same box used for the OFT as previously described. Prior to the test, all mice were handled for 6 days before habituation. On the first day, the mice were placed into the box for 10 minutes without any objects (S0 phase). Four hours later, two objects (A and B) were placed into the box and the mice were allowed to explore for 5 minutes (S1 phase). The time spent exploring each object was recorded. The test was performed in the same box after a retention interval of 24 h. In the test stage (S2 phase), one of the two objects used during exploration was replaced with a novel object (A and C). The mice were allowed to explore the objects freely for 5 minutes, and the time spent with each object was recorded. The recognition index (RI), calculated by (C − A)/(A + C), that is, a ratio of the difference between the time spent exploring the familiar object and the novel object over the total time spent exploring the two objects, was used to evaluate cognitive function.
Elevated plus-maze
Anxiety-related behavior was evaluated by the elevated-plus maze (EPM) as previously described. The EPM apparatus (made of Plexiglas, Beijing, China) consisted of four arms (two were open without walls and two were enclosed by 15.25 cm high walls) 30 cm long and 5 cm wide. The whole maze was elevated 40 cm above the floor. Mice were placed at the intersection of the open and closed arms, facing the open arm opposite to where the experiments, and were allowed to explore the maze for 5 minutes. The animal’s behavior was analyzed by a video tracking system (Stoelting Co.). All the arms were equally illuminated so that the animals did not perceive lighting differences.
Fear conditioning test
The trace conditioned fear test is based on previously described procedures [15]. On the conditioning day, mice were brought into a holding room and allowed to sit undisturbed in their home cages for 10 minutes. Mice were then placed in the testing chamber and allowed to explore for 3 minutes before the presentation of a tone (30 seconds, 80 dB, 3600 Hz). After a trace period of 30 seconds, a mild shock (2 seconds, 0.5 mA) was administered. The mice received five conditioning trials, each separated by a 200-second intertrial interval (ITI). The mice were removed from the conditioning chambers 1 minute after the last shock presentation and returned to their home cages. Chambers were cleaned with 30% isopropanol after each test. Trace memory testing was done on day 2. Mice were placed in a new context for the test. The tone test consisted of a 2-minute baseline period followed by three 30-second tone presentations. Each tone presentation was separated by a 220-second ITI. The chamber was cleaned with 70% ethanol instead of 30% isopropanol after each test. On the third day, contextual conditioning tests were conducted in a context identical to that of day 1. The freezing response (a defensive posture defined as the absence of motion except for that necessitated by breathing) was used as a measure of conditional fear [15]. The chamber was cleaned with 30% isopropanol after each test.
Western blot
Under deep anesthesia with pentobarbital (50 mg/kg, i.p.) and hypoxia with carbon dioxide, the bilateral frontal lobe and hippocampus of both sides were removed and immediately homogenized in ice-chilled lysis buffer. Samples were prepared as reported previously described [16]. Membranes were incubated with the following primary antibodies: goat anti-NPTX1 (Abcam, Cambridge, UK; 1:1000); rabbit anti-NPTX2 (Abcam; 1:1000); rabbit anti-GAPDH (HuaAn Biotechnology, Hangzhou, China; 1:2000). The membranes were then washed thrice in TBST and incubated for 2 h at room temperature with HRP-conjugated secondary antibodies (HuaAn Biotechnology, Hangzhou, China; 1:5000). Signals were detected by Image Quant Ai600 (General Electric Co., Pittsfield, Massachusetts, USA) using an enhanced chemiluminescence reagent (Thermo Fisher Scientific, Rockford, Illinois, USA). All western blot analyses were performed at least three times until we obtained consistent results.
Data analysis
Data were presented as mean ± SEM and analyzed with Graph-Pad Prism 7 (Graph Pad Software, San Diego, California, USA). Student’s t-test was used to compare the differences between two groups. Behavioral data were analyzed by two-way repeated-measures analysis of variance. Statistical significance was set at P < 0.05.
Results
Spared nerve injury mice showed nociceptive allodynia while normal locomotor activity was maintained
We first established a neuropathic-pain mouse model, and tested the mechanical and thermal pain threshold of mice to evaluate the success of the model. To evaluate nociceptive symptoms, the mechanical paw withdrawal thresholds (MPWT, Fig. 1b) and thermal PWLs (Fig. 1c) of the SNI group and the sham group were tested from day 0 to day 14 post-surgery. As expected, the MPWT and PWL decreased on day 3 and lasted until 14 days after surgery in SNI mice compared with that in the sham group. In the open field test, the distance traveled by (Fig. 1d; t = 1.324; df = 10; P = 0.2151, compared with the sham group) and velocity of (Fig. 1e; t = 0.5585; df = 10; P = 0.5888, compared with the sham group) SNI mice was similar to that of sham mice, suggesting that the surgery itself did not affect locomotor activity.
Spared nerve injury-induced cognitive deficits
To evaluate the effect of neuropathic pain on cognitive function, the NOR test was carried out. In the NOR test, mice remembered the objects they were exposed to during the training phase. In the testing phase, the mice would spend more time exploring a novel object, than the familiar one, which is expressed as a higher RI index for novel object [17]. SNI and sham mice spent similar time exploring object A and B (Fig. 2b; F1.2.2, 6.01 = 0.4267, P = 0.5740; P = 0.3305 sham A vs. B; P = 0.2133 SNI A vs. B; n = 6) during the training phase. In the testing phase, sham mice spent more time exploring the novel object, however, SNI mice showed no difference in object exploration (Fig. 2c; F1.765, 8.823 = 6.336, P = 0.0218; P = 0.0138 sham A vs. C; P = 0.1475 SNI A vs. C; n = 6). That is, SNI mice had a lower RI for a novel object than sham mice (Fig. 2d; t = 2.963, df = 10, P = 0.0142; n = 6).
Fig. 2.
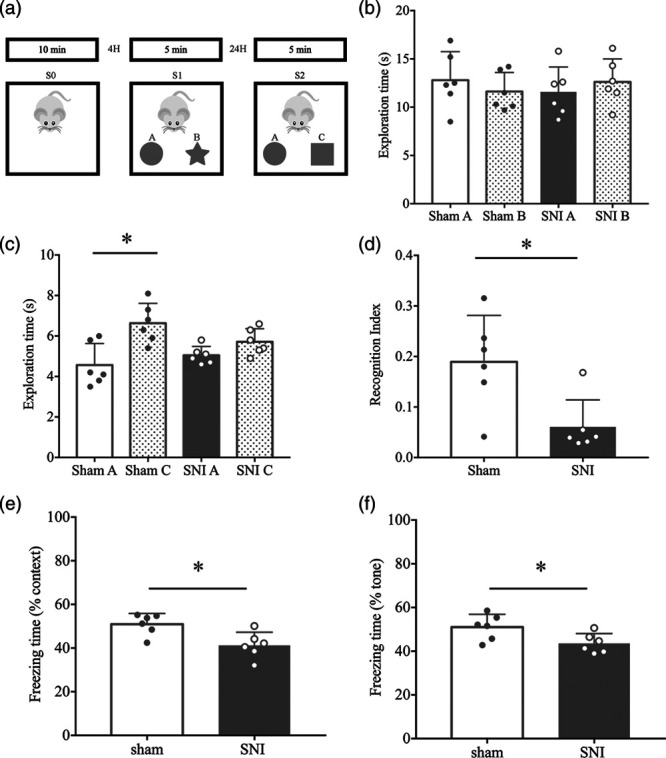
Chronic nociception impaired cognitive function in SNI mice in the novel object test: (a) the schematic presentation for the protocol of the novel object recognition test. Habituation, training, and test, represented by three phases: S0, S1, and S2. A, B, and C represent the different objects used during the test. In the training phase, A and B were placed in the box. In the test phase, A and C were placed in the box. (b) There were no significant differences in time spent on exploring both objects (A and B) during the training section (S1) between the sham and SNI group (n = 6; P = 0.3305 shamA vs. shamB; P = 0.2133 SNI A vs. SNI B). (c) The SNI mice demonstrated a significant reduction in exploration time of the novel object (object C) compared with the sham mice (n = 6; P = 0.0138 shamA vs. shamC; P = 0.1475 SNI A vs. SNI C). (d) Recognition index was lower in SNI mice compared with the sham group (n = 6; P = 0.0142 vs. sham group). Spared nerve surgery decreased the freezing time in the FCT context test (e: n = 6; P = 0.0116 vs. sham) and tone test (f: n = 6; P = 0.0340 vs. sham). SNI, spared nerve injury.
Cognitive performance was also evaluated by a fear conditioning test wherein SNI mice exhibited a reduction in freezing time during the context test (Fig. 2e; t = 3.085; df = 10; P = 0.0116; n = 6) and tone test (Fig. 2f; t = 2.454; df = 10; P = 0.0340; n = 6) compared with the sham group. These results suggest that SNI could induce cognitive impairment without affecting locomotor activity.
Spared nerve injury-induced anxiety-like behavior
We also detected the relationship between neuropathic pain and anxiety-like behavior in the EPM test. When animals were tested in the EPM test, SNI mice were found to spend significantly more time in the closed arm compared with the sham group (% TSO; Fig. 3a; t = 3.366, df = 10, P = 0.0072; n = 6). Whereas a significant reduction in time spent in open arms (% FEO; Fig. 3b; t = 3.96, df = 10, P = 0027; n = 6) was observed in SNI mice compared to that in the sham mice. This suggests that SNI mice exhibit significantly more anxiety-like behavior compared with sham mice.
Fig. 3.

Anxiety-like behavior on the elevated-plus maze: (a) SNI mice spends more time in closed arms (n = 6; P = 0.0072 vs. sham). (b) SNI mice spend less time in open arms (n = 6; P = 0027 vs. sham). SNI, spared nerve injury.
Spared nerve injury had no effect on NPTX1 level in the hippocampal and cortex
Next, protein expression was detected in the bilateral hippocampus and cortex of the sham and SNI mice (Fig. 4a). The results showed that there was no statistical difference between the sham group and the SNI group with regard to the expression of NPTX1 in the cortex (Fig. 4b; t = 1.798, df = 16, P = 0.0910 sham vs. SNI left; t = 1.217, df = 16, P = 0.2412 sham vs. SNI right) and hippocampus (Fig. 4c; t = 0.3617, df = 24, P = 0.7207 sham vs. SNI, left; t = 0.07607, df = 24, P = 0.9400 sham vs. SNI, right) on the operative side or the nonoperative side.
Fig. 4.

Expression of NPTX1 in hippocampus and cortex of SNI and sham mice: (a) representative bands of western blot for NPTX1 and GAPDH in cortex and hippocampus of ipsilateral and contralateral of sham and SNI mice. (b) At protein levels, the expression of NPTX1 has no statistical difference in the cortex of sham and SNI mice, no matter which side the operation is on (n = 6; P = 0.0910 sham vs. SNI left; P = 0.2412 sham vs. SNI right). (c) The expression of NPTX1 has no statistical difference in the hippocampus of sham and SNI mice, no matter which side the operation is on (n = 6; P = 0.7207 sham vs. SNI left; P = 0.9400 sham vs. SNI right). SNI, spared nerve injury.
Hippocampal and cortex NPTX2 level was decreased after spared nerve injury
NPTX2 expression levels were also analyzed in the bilateral hippocampus and cortex of the sham group and SNI group (Fig. 5a). In comparison to the sham group, the expression of NPTX2 in the cortex (Fig. 5b; t = 4.688, df = 10, P = 0.0009 sham vs. SNI, left; t = 3.626, df = 10, P = 0.0046 sham vs. SNI, right) and hippocampus (Fig. 5c; t = 6.099, df = 10, P = 0.0001 sham vs. SNI, left; t = 4.852, df = 10, P = 0.0007 sham vs. SNI, right) of the SNI group was significantly decreased, that is, NPTX2 expression was generally decreased on both the surgical and the non-surgical side. Therefore, we speculate that once SNI causes cognitive impairment, there is a global reduction in NPTX2 levels.
Fig. 5.

Expression of NPTX1 in hippocampus and cortex of SNI and sham mice: (a) representative bands of western blot for NPTX2 and GAPDH in cortex and hippocampus of ipsilateral and contralateral of the sham and SNI mice. (b) At protein levels, NPTX2 was dramatically reduced in the cortex of SNI mice compared with sham ones in both of the ipsilateral and contralateral (n = 6; P = 0.0009 sham vs. SNI left; P = 0.0046 sham vs. SNI right). (c) NPTX2 was dramatically reduced in the hippocampus of SNI mice compared with sham ones in both ipsilateral and contralateral (n = 6; P = 0.0001 sham vs. SNI left; P = 0.0.0007 sham vs. SNI right). SNI, spared nerve injury.
Increased cognitive dysfunction and anxiety responses in Nptx2 knock-out mice
Since peripheral nerve injury resulted in the impairment of cognitive function as well as a global decrease of NPTX2 in the hippocampus and cortex, we hypothesized that NPTX2 modulates cognitive function and anxiety-like behavior. To answer this, we subjected Nptx2 KO mice to a battery of behavioral tests. This battery included the NOR test, EPM, and fear conditioning test. In the NOR test, both KO mice and littermate controls spent similar time exploring objects A and B (Fig. 6c; t = 0.2807, df = 10, P = 0.7847 Ctrl A vs. B; t = 0.6851, df = 10, P = 0.5088 KO A vs. B; n = 6) in the training phase. In the testing phase, littermate controls spent more time exploring the novel object (Fig. 6c; t = 4.118, df = 10, P = 0.0021; n = 6), however, in terms of KO mice, no differences were observed (Fig. 6c;; t = 1.149, df = 10, P = 0.2771, n = 6). Nptx2 KO mice had a lower RI for a novel object than littermate controls (Fig. 6c; unpaired t-test; t = 6.745, df = 10, P < 0.0001; n = 6). In the fear condition test, KO mice showed less freezing time during the context test (Fig. 6d; unpaired t-test; t = 2.8, df = 10, P = 0.0188; n = 6) and tone test (Fig. 6d; unpaired t-test; t = 3.914, df = 10, P = 0.0029; n = 6) compared with the littermate controls. In the EPM test, we found that KO mice spent less time in the open arms (Fig. 6e; unpaired t-test, t = 2.615, df = 10, P = 0.0258) and more time in the closed arms (Fig. 6e; unpaired t-test, t = 3.278, df = 10, P = 0.0083) compared with the littermate controls. Overall, our results showed that Nptx2 deletion robustly increased cognition dysfunction and anxiety-like behaviors.
Fig. 6.

Effect of depletion of NPTX2 on cognitive dysfunction and anxiety-like behaviors. (a) NPTX2 genotyping. (b) The total distance traveled (L; unpaired t-test; t = 0.5977, P = 0.5634; n = 6) and mean velocities (R; unpaired t-test; t = 0.1538, P = 0.8808; n = 6) were similar between KO and littermate controls in the open field test. (c) No significant differences were observed in either KO or littermate controls in time spent exploring objects A and B during the training section (L; unpaired t-test; t = 0.2807, P = 0.7847 for Ctrl mice; t = 0.6851, P = 0.5088 for KO mice; n = 6); The KO mice demonstrated a significant reduction in exploration time of the novel object compared with the control mice (M; unpaired t-test; t = 4.118, P = 0.0021 for Ctrl mice; t = 1.149, P = 0.2771 for KO mice; n = 6). Recognition index of KO mice was lower than that of the control group (R; unpaired t-test; t = 6.754, p<0.0001; n = 6). (d) KO mice showed decreased freezing time in the fear conditioning context test (L; unpaired t-test; t = 2.8, P = 0.0188; n = 6) and tone test (R; unpaired t-test; t = 3.914, P = 0029; n = 6) compared with control mice. (e) KO mice spent more time in closed arms (L; unpaired t-test; t = 3.278, P = 0.0083; n = 6) and less time in the open arms (R; unpaired t-test; t = 2.615, P = 0.0258; n = 6) compared with control mice. KO, knock-out.
Discussion
In line with the published literature [18], we found that peripheral nerve injury resulted in the impairment of cognitive function. As for the expression of NPTX1, we found no statistical difference in its expression within the hippocampus and cortex during SNI-induced cognitive impairment. However, the expression of NPTX2 was significantly decreased in both the hippocampus and the frontal lobe, regardless of the side on which the operation was performed. In addition, behavioral experiments with Nptx2 KO mice showed that Nptx2 deletion could increase cognition dysfunction and anxiety-like behaviors.
Recent studies have shown that neuroinflammation is involved in the development of chronic neuropathic pain-induced cognitive dysfunction [19,20]. Supratentorial changes have been reported in association with pain modulation after spinal cord injury-induced hyperesthesia, including increased microglial activation, chemokine levels, and other chronic inflammatory changes associated with plasticity or electrophysiological alterations [21]. Using a mouse spinal cord contusion model, researchers demonstrated that increased numbers of reactive microglia and neuronal loss in the hippocampus and cerebral cortex were associated with the injury-induced cognitive changes and depression-like behavior [20]. In chronic pain models such as inflammatory pain and neuropathic pain, it was found that both wild-type and NPTX2 KO mice exhibited similar induction and recovery profiles. However, following SNT, NPTX2 knockout mice displayed an exaggerated microglia/macrophage response in the spinal dorsal horn compared with the wild-type mice [11]. The exaggerated microglia/macrophage response might also occur within the hippocampus in NPTX2 KO mice and thus aggravate symptoms of cognitive dysfunction.
NPTX2 is widely expressed in the hippocampus, cerebellum, cerebral cortex, and other regions of the brain, where it undergoes induction by synaptic activity and is present in pre- and postsynaptic compartments [9]. It is demonstrated that 90% of GluR1 clusters on aspiny neurons in rat postnatal hippocampal cultures are associated with NPTX2, but not on synapses that are GAD-positive. Overexpression of NPTX2 leads to co-localization and aggregation of AMPA receptor subunits in heterologous cells and spinal neurons. Conversely, deletion of NPTX2 results in a loss of excitatory inputs to fast-spiking parvalbumin-positive interneurons in the visual cortex and interferes with the timing and establishment of ocular dominance plasticity [8]. Mice with deletion of NPTX2 also demonstrate cognitive inflexibility [22] and miR-301b was reported to negatively regulate NPTX2 and is associated with cognitive impairment [23]. Furthermore, NPTX2 was reported to predict cognitive impairment [10] and is down-regulated significantly in Alzheimer’s disease [24]. NPTX2 is suggested as a promising synapse-derived disease progression biomarker in cognitive impairment-related diseases [10]. Here, down-regulated NPTX2 in the cortex and hippocampus may be an indicator of cognitive impairment and a regulator of anxiety in neuropathic pain.
In this study, the global decreased expression of NPTX2 might be regulated by the decreased expression of brain-derived neurotrophic factor (BDNF) in the hippocampus and cortex of neuropathic pain mice. Previous studies have confirmed that NPTX2 can be transcriptionally regulated by BDNF in a time-dependent manner. Furthermore, the regulation occurs through phosphorylation of the mitogen-activated protein kinase and phospholipase C gamma signaling pathways and consequent activation of cAMP-response element-binding protein to ultimately induce NPTX2 expression. Unlike the effect of BDNF on excitatory synaptic transmission in wild-type hippocampal neurons, BDNF dose not affect glutamatergic transmission in NPTX2 KO neurons, suggesting that NPTX2 is important for BDNF-mediated modulation of glutamatergic synapses [25]. Since NPTX2 is a secreted lectin that can be easily detected, we believe that it can be used as a biomarker to screen patients with neuropathy-induced cognitive dysfunction, promoting early intervention.
Conclusion
In summary, neuropathic pain generates an obvious cognitive impairment and is accompanied by a downregulation of NPTX2 in the cortex and hippocampus. NPTX2 has been shown to modulate neuroinflammation in a variety of chronic pain models and this inflammatory response was reported to play an important role in cognitive impairment induced by chronic pain. Here, NPTX2 may act as an indicator of neuropathic pain-induced cognitive dysfunction, as well as play a role in regulating the inflammatory response which contributes to the cognitive impairment induced by chronic pain.
Acknowledgements
We want to thank Editage for their help on the editing of this manuscript.
R.W. contributes to data acquisition, data analysis, and interpretation; Y.M. contributes to data acquisition and data analysis; M.Z. contributes to data analysis; L.W. and J.Y. contribute to conception, design, and drafting the article.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Rongguo Wang and Yuanyuan Man contributed equally to the writing of this article.
References
- 1.Guida F, De Gregorio D, Palazzo E, Ricciardi F, Boccella S, Belardo C, et al. Behavioral, biochemical and electrophysiological changes in spared nerve injury model of neuropathic pain. Int J Mol Sci. 2020; 21:3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attridge N, Pickering J, Inglis M, Keogh E, Eccleston C. People in pain make poorer decisions. Pain. 2019; 160:1662–1669. [DOI] [PubMed] [Google Scholar]
- 3.Guida F, Luongo L, Marmo F, Romano R, Iannotta M, Napolitano F, et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol Brain. 2015; 8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis MF, Boyce-Rustay JM. Neuropathic pain: models and mechanisms. Curr Pharm Des. 2009; 15:1711–1716. [DOI] [PubMed] [Google Scholar]
- 5.Tyrtyshnaia A, Manzhulo I, Kipryushina Y, Ermolenko E. Neuroinflammation and adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment in aged mice. Int J Mol Med. 2019; 43:2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999; 23:309–323. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Wei M, Zhang C, Maxeiner S, Pak C, Calado Botelho S, et al. Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. J Neurosci. 2017; 37:1062–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, et al. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003; 39:513–528. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002; 22:4487–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galasko D, Xiao M, Xu D, Smirnov D, Salmon DP, Dewit N, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimers Dement (N Y). 2019; 5:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miskimon M, Han S, Lee JJ, Ringkamp M, Wilson MA, Petralia RS, et al. Selective expression of Narp in primary nociceptive neurons: role in microglia/macrophage activation following nerve injury. J Neuroimmunol. 2014; 274:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blouin AM, Han S, Pearce AM, Cheng K, Lee JJ, Johnson AW, et al. Role of medial prefrontal cortex Narp in the extinction of morphine conditioned place preference. Learn Mem. 2013; 20:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Jin X, You Z, Wang S, Lim G, Yang J, et al. Persistent nociception induces anxiety-like behavior in rodents: role of endogenous neuropeptide S. Pain. 2014; 155:1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiltgen BJ, Sanders MJ, Ferguson C, Homanics GE, Fanselow MS. Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABA(A) receptor. Learn Mem. 2005; 12:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Xu T, Ma X, Jiang W. Involvement of neuronal TGF-β activated kinase 1 in the development of tolerance to morphine-induced antinociception in rat spinal cord. Br J Pharmacol. 2015; 172:2892–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc. 2013; 8:2531–2537. [DOI] [PubMed] [Google Scholar]
- 18.Shiers S, Price TJ. Molecular, circuit, and anatomical changes in the prefrontal cortex in chronic pain. Pain. 2020; 161:1726–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong J, Zuo Z, Yan W, Liu W, Zheng Q, Liu X. Berberine ameliorates diabetic neuropathic pain in a rat model: involvement of oxidative stress, inflammation, and μ-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol. 2019; 392:1141–1149. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, et al. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci. 2014; 34:10989–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon EJ, Kim YK, Shin HI, Lee Y, Kim SE. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 2013; 1540:64–73. [DOI] [PubMed] [Google Scholar]
- 22.Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015; 85:1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang CZ, Zhang DF, Yang JT, Liu QH, Wang YR, Wang WS. Overexpression of microRNA-301b accelerates hippocampal microglia activation and cognitive impairment in mice with depressive-like behavior through the NF-κB signaling pathway. Cell Death Dis. 2019; 10:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao MF, Xu D, Craig MT, Pelkey KA, Chien CC, Shi Y, et al. NPTX2 and cognitive dysfunction in Alzheimer’s disease. Elife. 2017; 6:e23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariga A, Glaser J, Mathias L, Xu D, Xiao M, Worley P, et al. Definition of a bidirectional activity-dependent pathway involving BDNF and Narp. Cell Rep. 2015; 13:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]


