Abstract
Fine needle aspiration cytology (FNAC) is a valuable, safe and widely used method for preoperative diagnosis of salivary gland lesions. The diagnostic accuracy of FNAC is dependent on the quality and yield of the aspirate, as well as the experience and knowledge of the cytopathologist. 247 cases of FNAC of salivary gland lesions were performed in our 4-year retrospective study. FNAC diagnoses were divided into non-neoplastic lesions, benign and malignant neoplasms. Histopathologic confirmation was done in 101 cases. The cases with discrepancies between the FNAC and histopathologic results were reviewed to establish possible reasons for discordance. The measures of diagnostic validity of FNAC in diagnosing non-neoplastic, benign and malignant lesions were evaluated. Of the 247 FNAC samples, 135 cases were diagnosed as benign neoplasms, 15 as malignant neoplasms, and 97 as non-neoplastic lesions. Out of the 101 cases with histopathologic confirmation, discordant results between cytologic and histopathologic diagnosis were observed in 15 cases. Our study showed no false positive and 4 false negative results for cancer. Cystic presentation of a lesion was a common reason for diagnostic pitfall. Sensitivity of FNAC in various types of salivary gland lesions ranged from 75%-100%, specificity 81-100%, diagnostic accuracy 85-96%, PPV 31-100% and NPV 60-96%. FNAC is a highly sensitive and specific method for diagnosis of most salivary gland lesions. Despite the fact that histopathology remains the gold standard, preoperative FNAC should be considered for preliminary investigation. Due to the diagnostic pitfalls, FNAC should be used in conjunction with clinical information, physical examination, and radiologic findings to reach the right diagnosis.
Keywords: Salivary gland lesions, fine needle aspiration cytology, histopathology, cytopathology, diagnostic validity
Introduction
Histopathology is still the gold standard for establishing the final diagnosis and staging of salivary gland lesions [1]. However, fine needle aspiration cytology (FNAC) is a valuable, safe, and widely used method for preoperative diagnosis, that helps in the broad categorization of salivary gland lesions into inflammatory, non-neoplastic and neoplastic - benign versus malignant conditions. An accurate FNAC diagnosis has major therapeutic implications and guides the clinician in decision-making about patient management [2-4]. However, cytopathologic evaluation may be challenging and can be complicated by frequent pitfalls. The diagnostic validity of FNAC is dependent on the quality and yield of the aspirate, as well as the experience and knowledge of the cytopathologist. Diagnostic difficulties are increased by the fact that the salivary gland tumors are a heterogeneous group with extremely varied histopathologic features [5,6].
The main aim of our study was to examine the diagnostic reliability of FNAC in diagnosing non-neoplastic, benign and malignant salivary glands lesions by correlating the FNAC diagnosis with histopathology. Additionally, we have analysed the causes of diagnostic discrepancies in the salivary gland lesions by correlating the cytologic smears with the histopathologic findings of the resected specimens.
Materials and methods
This was a retrospective study done for a duration of 4 years from January 2016 to January 2020. During the study period 247 cases of FNAC of salivary gland lesions were performed. FNAC was performed at our pathology department using a 21-23 gauge fine needle. A minimum of two needle passes were made in each case. The specimens were expelled onto two or three slides, and thin smears were prepared between two slides and immediately fixed in 95% ethanol. The slides were generally stained with H&E (haematoxylin and eosin).
FNA diagnoses were divided into non-neoplastic lesions, benign neoplasms and malignant neoplasms. If it was possible, the diagnosis was precisely specified.
Following the cytologic diagnosis, 101 patients underwent appropriate surgical procedures and specimens were submitted for histopathologic examination. Patients who did not undergo surgical excision during the period of study were excluded.
The histologic diagnosis was considered as the gold standard for assessment of diagnostic reliability of FNAC. Cyto-histopathologic correlation was done. Where diagnoses differed between the FNAC and histopathology, the cases were reviewed in more detail to establish possible underlying reasons for this discordance.
This study was approved by the Ethics Committee of Medical University of Silesia in Katowice (PCN/0022/KB/26/20). An informed consent from all the included patients was taken. Anonymized and deidentified information was used for the analyses.
Statistical analysis
All analyses were performed using STATISTICA 13 software (StatSoft, USA). FNAC-based diagnoses were compared with diagnoses from the histologic examination of the specimens. The cytologic and histologic analysis was reported in terms of frequencies and percentages. Furthermore, measures of diagnostic validity of FNAC in terms of sensitivity, specificity, diagnostic accuracy (effectiveness), positive predictive value (PPV) and negative predictive value (NPV) were evaluated using formulas described by Parikh et al. [7]. Receiver operator characteristic (ROC) curves with area under the curve (AUC) were performed to determine diagnostic reliability of FNAC in various types of salivary gland lesions.
Results
FNAC results
Of all the FNAC undertaken, 132 (53.44%) were from female patients and 115 (45.55%) were from male patients. Patient age ranged from 9 to 93 years with mean age of 58 years. The incidence of salivary gland lesions was as high as 42.11% in the age group of 61 to 80 years, followed by 35.22% in the age group of 41 to 60 years, 12.96% in the age group of 21 to 40 years, 6.88% in the age group of 81 to 100 years and only 2.83% in the age group of 0 to 20 years. There was no sex predilection for any type of lesion. Of the 247 FNAC samples 135 (54.66%) cases were diagnosed as benign neoplasms, 15 (6.07%) as malignant neoplasms, and 97 (39.27%) as non-neoplastic lesions including 28 (11.34%) inflammatory lesions (Table 1). Parotid glands were the most commonly involved glands with an incidence of 90.69% (n=224), followed by the submandibular glands (n=23; 9.31%) with almost equal involvement over both sides (Table 2). Most of the parotid lesions were benign neoplasms (57.59%), while non-neoplastic lesions accounted for most (56.52%) of the submandibular gland lesions (Table 3). The most common benign neoplasm was Warthin tumor (papillary cystadenoma lymphomatosum) (n=63; 46.67%), followed by pleomorphic adenoma (n=62; 45.93%). Non-Hodgkin lymphoma (n=4; 26.67%) and mucoepidermoid carcinoma (n=2; 13.33%) were the most commonly encountered malignant neoplasms. Cysts (n=47; 48.45%) and sialadenitis (n=2; 24.74%) were the most common lesions among the non-neoplastic ones.
Table 1.
Diagnosis of salivary gland lesions on FNAC
| Lesion | Number of cases | % of cases |
|---|---|---|
| Benign neoplasms | 135 | 54.66 |
| Malignant neoplasms | 15 | 6.07 |
| Non-neoplastic including inflammatory lesions | 97 (including 28 inflammatory lesions) | 39.27 |
| Total | 247 | 100 |
Table 2.
Distribution of cases based on location
| Salivary gland | Number of cases | % of cases | Total |
|---|---|---|---|
| Parotid left | 109 | 89.34 | 224 (90.69%) |
| Parotid right | 115 | 92.00 | |
| Submandibular left | 13 | 10.66 | 23 (9.31%) |
| Submandibular right | 10 | 8.00 |
Table 3.
Distribution of salivary gland lesions based on location
| Parotid glands | Submandibular glands | |
|---|---|---|
| Non-neoplastic lesions | 84 (37.5%) | 13 (56.52%) |
| Benign neoplasms | 129 (57.59%) | 6 (26.09%) |
| Malignant neoplasms | 11 (4.91%) | 4 (17.39%) |
Histopathologic results
Histopathology was available in 101 (40.89%) cases. Of all patients, 52 (51.49%) were women and 49 (48.51%) were men. Patient age ranged from 18 to 91 years, with mean age of 54 years. Of all histopathologic results, 5 (2.02%) cases were non-neoplastic, 80 (32.39%) cases were benign neoplasms, and the other 16 (6.48%) cases were malignant neoplasms. Chronic sialadenitis (75%), pleomorphic adenoma (50%), and mucoepidermoid carcinoma (18.75%) were the most common non-neoplastic lesion, benign neoplasm, and malignant neoplasm diagnosed by histopathology, respectively.
Cyto-histopathologic correlation
Out of 101 cases, discordant results between cytologic and histopathologic diagnosis were observed in 15 cases (14.85%). Table 4 shows the proportion of concordant and discordant cytologic results in different types of lesions. Our study showed no false positive and 4 false negative results for cancer - one case of basal cell adenoma turned out to be adenoid cystic carcinoma on histology, one case diagnosed by histopathologic assessment as epithelial myoepithelial carcinoma was diagnosed as undetermined benign salivary gland neoplasm by FNAC; and two cases of Warthin tumor were diagnosed as squamous cell carcinoma and mucoepidermoid carcinoma on histology.
Table 4.
Concordance of cytologic results compared with histopathologic diagnoses
| Concordant results | Discordant results | |
|---|---|---|
| Non-neoplastic lesions | 5 (100%) | 0 (0%) |
| Benign neoplasms | 69 (86.25%) | 11 (13.75%) |
| Malignant neoplasms | 12 (75%) | 4 (25%) |
Cystic presentation of lesion was a common reason for diagnostic pitfall. Table 5 shows the details from discordant cytologic results.
Table 5.
Cases with discordance in cyto-histopathologic diagnosis
| Cytologic diagnosis | Histopathologic diagnosis | Number of disconcordant results | Predominant cytomorphologic characteristics |
|---|---|---|---|
| Cyst | Warthin tumor | 7 | Necrotic or proteinaceous debris |
| Sialadnitis | Warthin tumor | 1 | Rich lymphoid cells in a necrotic background |
| Cyst | Cystadenoma | 2 | Mucoid or proteinaceous material |
| Cyst | Basal cell adenoma | 1 | Proteinaceous material and macrophages with occasional cells without atypia |
| Warthin tumor | Mucoepidermoid carcinoma | 1 | Proteinaceous material and macrophages, oncocytic cells, occasional crushed lymphoid tangles |
| Basal cell adenoma | Adenoid cystic carcinoma | 1 | Nests of cells with hyperchromatic nuclei, no nucleoli and scant cytoplasm |
| Salivary gland benign neoplasm | Epithelial myoepithelial carcinoma | 1 | Monomorphic epithelial cells in nests and dispersion, numerous psammoma bodies |
| Warthin tumor | Squamous cell carcinoma | 1 | Proteinaceous material, lymphoid cells, oncocytic cells, occasional squamous cells without atypia |
Diagnostic reliability of FNAC of salivary gland lesions
Diagnostic reliability of FNAC was judged based on sensitivity, specificity, accuracy, PPV, and NPV. Sensitivity in various types of lesions ranged from 75%-100%, specificity ranged from 81-100%, diagnostic accuracy ranged from 85-96%, PPV ranged from 31-100% and NPV ranged from 60-96% (Table 6). All investigated values for all three types of lesions were comparable (75%-100%), except for NPV in benign neoplasms (60%) which was slightly lower and PPV in non-neoplastic lesions (31%) which was significantly lower than other values. Interestingly, sensitivity was higher in benign salivary gland neoplasms, but specificity, accuracy, PPV, and NPV were higher in the malignant neoplasms compared to the benign tumors. The AUC was 0.86 with malignancy as the end point, 0.82 with benign neoplasm as the end point, and 0.94 with non-neoplastic lesion as the end point (Table 7; Figure 1).
Table 6.
Comparison of diagnostic reliability of FNAC in various types of lesions
| Sensitivity | Specificity | Accuracy | PPV | NPV | |
|---|---|---|---|---|---|
| Non-neoplastic lesions | 100% | 88% | 89% | 31% | 89% |
| Benign neoplasms | 86% | 81% | 85% | 95% | 60% |
| Malignant neoplasms | 75% | 100% | 96% | 100% | 96% |
Table 7.
Receiver operator characteristic (ROC) curve analysis
| AUC | SE | AUC 95% CI | p | |
|---|---|---|---|---|
| Malignant neoplasms | 0.86 | 0.06 | 0.73-0.99 | 0.00 |
| Benign neoplasms | 0.82 | 0.05 | 0.71-0.93 | 0.00 |
| Non-neoplastic lesions | 0.94 | 0.02 | 0.90-0.99 | 0.00 |
AUC-area under the curve; SE-standard error; CI-confidence interval.
Figure 1.

Receiver operator characteristic (ROC) curve analysis: (A) ROC curve with malignancy on surgical follow-up as end point, (B) ROC curve with benign neoplasm on surgical follow-up as the end point, (C) ROC curve with non-neoplastic lesion on surgical follow-up as the end point.
Discussion
FNAC has acquired an important place in the preoperative diagnosis of salivary gland lesions. Cytologic diagnosis can guide the clinician to the correct treatment strategy as to which patient requires further investigation, medical treatment, or excision [1,8].
We observed a slight surplus in the number of women than men in our study. The results of previous studies were variable. Some of the studies showed female preponderance where as others showed excess in males but sex differences were not significant [3,9-11]. No definite sex predilection was evident for either benign or malignant lesions, as in other studies [8].
Over 75% of our patients were between the ages of 41-80 years. The previously reported ages of patients were slightly lower [1,8,9]. The parotid gland was the most commonly studied gland in the present study, followed by the submandibular gland. Similar observations were made by previous studies [1,8,9,11]. We did not observe any lesions in the minor salivary glands in contrast to the above research. Our study reconfirms the increased incidence of benign neoplasms compared to malignant counterparts [1,8,9]. As high as 11.34% of our cases were diagnosed by FNAC as being inflammatory; however, some studies showed a higher percentage of these lesions [9]. Even though pleomorphic adenoma is the most common salivary gland tumor, the most common benign neoplasm diagnosed by FNAC in our study was Warthin tumor. Similarly to the results of Koirala et al. [8] and Kakoty et al. [1], mucoepidermoid carcinoma was the most common malignant neoplasm in our study.
Out of 101 cases, 15 cases had cyto-histologic disagreement in our study. The most common causes of diagnostic discrepancies described in the literature were sampling errors (mislabeled site, inadequate sample) and misinterpretation due to a relative lack of the so-called typical or classic cytomorphologic features [1,4]. Similar causes of diagnostic discrepancies were observed by us. Sampling error due to a cystic presentation of a lesion was the most common reason for pitfalls in our study. This cause of discrepancies was also reported in other studies [2,5,8]. Two cases diagnosed by histopathologic assessment as cystadenoma were diagnosed as cysts by FNAC. These mistakes resulted from sampling errors - material obtained from the major cystadenoma’s cavity revealed only a mucoid or proteinaceous background without epithelial cells. Seven Warthin tumors were also misdiagnosed by FNAC as cysts, due to inadequate aspirates associated with cystic change in these tumors. Aspirates from the cystic tumors revealed only necrotic or proteinaceous debris instead of the characteristic cytologic features of papillary cystadenoma lymphomatosum (oncocytic cells in cohesive, monolayered sheets and background lymphocytes) [2].
One case of Warthin tumor diagnosed as an inflammatory condition by cytology, showed rich lymphoid cells in the necrotic background and lacked the classic cytologic features of papillary cystadenoma lymphomatosum (as noted above). Similar erroneous diagnosis of chronic sialadenitis in a case of Warthin tumor has been described by Jo et al. [5].
We diagnosed one basal cell adenoma as a cyst by FNAC. This resulted from sparse specimen cellularity and lack of a representative sample (Figure 2). Lack of the so-called classic features of basal cell adenoma (tightly cohesive, 3-dimensional clusters and cords of basaloid epithelial cells with scant cytoplasm and uniform, bland nuclei) resulted in the diagnostic discrepancy [12]. Lack of a representative sample has previously been highlighted as a diagnostic problem in various types of salivary gland lesions. Thus multiple sampling is crucial to overcome problems of misdiagnosis due to selective sampling [9].
Figure 2.

Cytologic (A) and histologic (B) findings of a basal cell adenoma case misdiagnosed as cyst. The FNAC smear (A) shows proteinaceous material with macrophages (H&E, ×400), and histologically (B) a trabecular pattern of growth of basal cell adenoma is observed (H&E, ×100).
Our study showed four false negative results for malignancy. Interestingly, we obtained no false positive result for cancer. This tendency to get more false negative than false positive reports was observed by other authors [9]. The first case of false negative result was adenoid cystic carcinoma which was misdiagnosed by FNAC as basal cell adenoma (Figure 3). The smears from solid adenoid cystic carcinoma show cell arrangements and nuclear features (uniform basaloid cells with scant cytoplasm and dark nuclei) virtually identical to those of basal cell adenoma [13]. The distinction between these lesions may sometimes be impossible without clinical correlation and/or a surgical resection specimen [6]. Another case diagnosed by histopathology as mucoepidermoid carcinoma was diagnosed as Warthin tumor by FNAC. Oncocytic cells and occasional crushed lymphoid tangles on a proteinaceous background were interpreted by us as components of papillary cystadenoma lymphomatosum (Figure 4). Histopathology of the resected tumor revealed focal oncocytic change and an inflammatory reaction to the extravasated mucin in mucoepidermoid carcinoma, hence our misinterpretation of the FNAC material. Additionally, relative lack of the typical features of mucoepidermoid carcinoma in the FNAC sample (three populations of cells - squamous cells with dense cytoplasm, glandular cells with more vacuolated cytoplasm and intermediate cells, with mucin in the background) made it difficult to make a proper diagnosis [14]. One case of epithelial myoepithelial carcinoma was diagnosed as undetermined benign salivary gland neoplasm by FNAC (Figure 5). The FNAC specimen of epithelial myoepithelial carcinoma is characteristically described in the literature as two cell populations: ductal epithelial cells and clear myoepithelial cells; however a biphasic pattern may not be evident since clear cells have fragile cytoplasm and often appear as naked nuclei [15]. Epithelial-myoepithelial carcinoma is a low grade neoplasm, so it may lack overt cytologic features of malignancy [16]. Lack of evident features of atypia and biphasic cell population in our smears resulted in a false negative result. One squamous cell carcinoma was misdiagnosed by FNAC as Warthin tumor in our study. The FNAC revealed lymphoid cells, oncocytic cells, and occasional squamous cells without evident features of atypia in a proteinaceous background. The smears lacked of typical features of squamous cell carcinoma - markedly atypical squamous cells seen against a background of tumor necrosis [17]. We have interpreted non-atypical squamous cells in our smears as squamous metaplasia in a Warthin tumor, which was diagnosed based on the presence of oncocytic and lymphoid cells.
Figure 3.

Adenoid cystic carcinoma case showing diagnostic discrepancies. A case, diagnosed as basal cell adenoma, shows in FNAC smear (A) nests of cells with hyperchromatic nuclei, no nucleoli, and scant cytoplasm (H&E, ×400). Histopathology (B) reveals tubular and cribriform architecture of adenoid cystic carcinoma with perineural invasion (arrow) (H&E, ×100).
Figure 4.

Diagnostic pitfalls in mucoepidermoid carcinoma case which was diagnosed as Warthin tumor by FNAC. Cytologic (A) findings reveal groups of oncocytic cells and crushed lymphoid tangles in the proteinaceous material (H&E, ×200), while histopathology (B) shows occasional oncocytic change and inflammatory reaction in mucoepidermoid carcinoma area (H&E, ×200).
Figure 5.
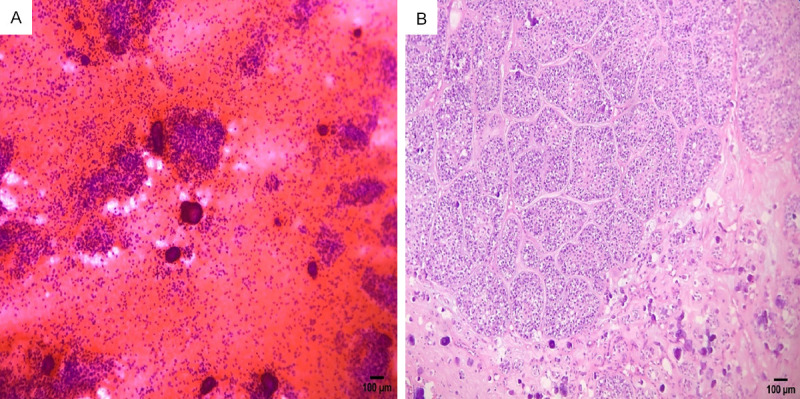
Cytologic (A) and histologic (B) findings of epithelial-myoepithelial carcinoma misdiagnosed as an undetermined benign neoplasm. The cytology smear (A) displays monomorphic epithelial cells without overt cytologic features of malignancy in nests and dispersion and psammoma bodies (H&E, ×200). Histopathology (B) shows islands of epithelial and myoepithelial cells with mild nuclear pleomorphism and numerous psammoma bodies (H&E, ×200).
As other authors emphasize, the pathologist should always be aware of cytologic pitfalls of salivary gland FNAC. Details of clinical history, physical examination, and radiologic features may help reach the appropriate diagnosis and reduce false interpretation [5,9].
According to the various studies, sensitivity of fine needle aspiration in various types of lesions ranged from 54% to 100%, specificity from 81.6% to 100%, accuracy from 80.2% to 97.6%, PPV from 66.7% to 91.6%, and NPV from 81.6% to 97.0% [1,8,9,18-23]. These findings were mostly concordant with ours; however, most authors evaluated diagnostic reliability of FNAC only for benign and malignant neoplasms, not taking into consideration non-neoplastic lesions.
The potential limitation of our study was the relatively small sample size of patients with histopathologic confirmations - out of 247 cases of FNAC of salivary gland lesions, only 101 patients underwent an appropriate surgical procedure.
Summarizing, FNAC is highly sensitive and specific method for diagnosis of most of the salivary gland lesions. Despite the fact that histopathologic examination remains the gold standard, preoperative FNAC should always be considered for preliminary investigation in the evaluation of salivary gland lesions. It not only helps in correct diagnosis but also avoids unnecessary surgery. Multiple sampling from different parts of the lesion enhances diagnostic accuracy. Due to the many diagnostic pitfalls, FNAC should always be used in conjunction with clinical information, physical examination, and radiologic findings to reach the right diagnosis.
Acknowledgements
This work was supported by the Medical University of Silesia, Katowice, Poland. The authors gratefully acknowledge financial support from the Medical University of Silesia, Katowice, Poland.
Disclosure of conflict of interest
None.
References
- 1.Kakoty S, Baruah TD, Babu CPG. FNAC and histopathological correlation of salivary gland lesions: an observational study. Int Surg J. 2017;4:2148–2152. [Google Scholar]
- 2.Parwani AV, Ali SZ. Diagnostic accuracy and pitfalls in fine-needle aspiration interpretation of Warthin tumor. Cancer. 2003;99:166–171. doi: 10.1002/cncr.11207. [DOI] [PubMed] [Google Scholar]
- 3.Mukundapai M, Sharma N, Patil A, Gopal C. Fine-needle aspiration cytology of salivary gland lesions: a revised classification based on “Milan system”-4 years experience of tertiary care cancer center of South India. J Cytol. 2020;37:12–17. doi: 10.4103/JOC.JOC_68_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schindler S, Nayar R, Dutra J, Bedrossian CW. Diagnostic challenges in aspiration cytology of the salivary glands. Semin Diagn Pathol. 2001;18:124–146. [PubMed] [Google Scholar]
- 5.Jo HJ, Ahn HJ, Jung S, Yoon HK. Diagnostic difficulties in fine needle aspiration of benign salivary glandular lesions. Korean J Pathol. 2012;46:569–575. doi: 10.4132/KoreanJPathol.2012.46.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David O, Blaney S, Hearp M. Parotid gland fine-needle aspiration cytology: an approach to differential diagnosis. Diagn Cytopathol. 2007;35:47–56. doi: 10.1002/dc.20581. [DOI] [PubMed] [Google Scholar]
- 7.Parikh R, Mathai A, Parikh S, Chandra Sekhar G, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008;56:45–50. doi: 10.4103/0301-4738.37595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koirala S, Sayami G, Pant AD. Correlation of FNAC and histopathology in diagnosis of salivary gland lesions. J Pathol Nepal. 2014;4:654–657. [Google Scholar]
- 9.Singh A, Haritwal A, Murali B. Correlation between cytology and histopathology of the salivary gland. Australas Med J. 2011;4:66–71. doi: 10.4066/AMJ.2011.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei S, Layfield LJ, LiVolsi VA, Montone KT, Baloch ZW. Reporting of fine needle aspiration (FNA) specimens of salivary gland lesions: a comprehensive review. Diagn Cytopathol. 2017;45:820–827. doi: 10.1002/dc.23716. [DOI] [PubMed] [Google Scholar]
- 11.Gao N, Li Y, Li LJ, Wen YM. Clinical analysis of head and neck cancer cases in south west China 1953-2002. J Int Med Res. 2009;37:189–197. doi: 10.1177/147323000903700123. [DOI] [PubMed] [Google Scholar]
- 12.Cantley RL. Fine-needle aspiration cytology of cellular basaloid neoplasms of the salivary gland. Arch Pathol Lab Med. 2019;143:1338–1345. doi: 10.5858/arpa.2019-0327-RA. [DOI] [PubMed] [Google Scholar]
- 13.Pal S, Mondal PK, Sharma A, Sikder M. Fine needle aspiration cytology of basal cell adenoma of parotid simulating adenoid cystic carcinoma. J Cytol. 2018;35:55–57. doi: 10.4103/JOC.JOC_46_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasudevan G, Bishnu A, Singh BMK, Singh VK. Mucoepidermoid carcinoma of salivary gland: limitations and pitfalls on FNA. J Clin Diagn Res. 2017;11:ER04–ER06. doi: 10.7860/JCDR/2017/25341.9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aisagbonhi OA, Tulecke MA, Wilbur DC, Goldar-Najafi A, Iqbal S, Sadow PM, Faquin W. Fine-needle aspiration of epithelial-myoepithelial carcinoma of the parotid gland with prominent adenoid cystic carcinoma-like cribriform features: avoiding a diagnostic pitfall. Am J Clin Pathol. 2016;146:741–746. doi: 10.1093/ajcp/aqw128. [DOI] [PubMed] [Google Scholar]
- 16.Sehgal S, Goyal P, Singh S, Kumar A. Fine-needle aspiration cytology of myoepithelial carcinoma of salivary gland: diagnostic challenge to cytopathologist. J Cytol. 2013;30:207–210. doi: 10.4103/0970-9371.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maleki Z, Miller JA, Arab SE, Fadda G, Bo P, Wise O, Rossi ED, Jhala N, Ashish C, Ali SZ, Wang H. “Suspicious” salivary gland FNA: risk of malignancy and interinstitutional variability. Cancer Cytopathol. 2018;126:94–100. doi: 10.1002/cncy.21939. [DOI] [PubMed] [Google Scholar]
- 18.Akhter J, Hirachand S, Lakhey M. Role of FNAC in the diagnosis of salivary gland swellings. Kathmandu Univ Med J (KUMJ) 2008;6:204–208. [PubMed] [Google Scholar]
- 19.Singh Nanda KD, Mehta A, Nanda J. Fine-needle aspiration cytology: a reliable tool in the diagnosis of salivary gland lesions. J Oral Pathol Med. 2012;41:106–112. doi: 10.1111/j.1600-0714.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 20.Silva WP, Stramandinoli-Zanicotti RT, Schussel JL, Ramos GH, Ioshi SO, Sassi LM. Accuracy, sensitivity and specificity of fine needle aspiration biopsy for salivary gland tumors: a retrospective study from 2006 to 2011. Asian Pac J Cancer Prev. 2016;17:4973–4976. doi: 10.22034/APJCP.2016.17.11.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty A, Geethamani V. Role of fine-needle aspiration cytology in the diagnosis of major salivary gland tumors: a study with histological and clinical correlation. J Oral Maxillofac Pathol. 2016;20:224–229. doi: 10.4103/0973-029X.185899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar A, Sharma N, Sharma S. Fine needle aspiration cytology utility in salivary gland tumor diagnosis. Indian J Otolaryngol Head Neck Surg. 2017;69:147–154. doi: 10.1007/s12070-016-0982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gudmundsson JK, Ajan A, Abtahi J. The accuracy of fine-needle aspiration cytology for diagnosis of parotid gland masses: a clinicopathological study of 114 patients. J Appl Oral Sci. 2016;24:561–567. doi: 10.1590/1678-775720160214. [DOI] [PMC free article] [PubMed] [Google Scholar]


