Abstract
Nasopharyngeal carcinoma (NPC) is a head and neck cancer with severe local invasion and early distant metastasis. SIRT6 serves as a critical modulator of the development and metastasis of multiple types of cancer; however, the roles and underlying mechanisms of SIRT6 in regulating NPC metastasis remain largely unknown. Here, the expression of SIRT6 in high metastatic 5-8F cells and low metastatic 6-10B cells was analyzed. SIRT6 expression was found to be negatively associated with the metastatic capability of NPC cells. Moreover, we identified that SIRT6 inhibited NPC cell metastasis through suppression of SNAIL expression. Mechanistically, we demonstrated that SIRT6 interacted with transcription factor p65 (NF-kB subunit) and deacetylated histone H3 lysine 9 (H3K9) and lysine 56 (H3K56) at the promoter of SNAIL, leading to reduced transcription of SNAIL. In summary, SIRT6 functions as a metastasis suppressor in NPC cells through epigenetic regulation of SNAIL gene expression.
Keywords: Nasopharyngeal carcinoma, SIRT6, SNAIL, p65, metastasis
Introduction
Nasopharyngeal carcinoma (NPC) is a common primary malignancy originating from epithelial cells in the nasopharynx. NPC is characterized by unbalanced geographic distribution and is highly prevalent in Southern China and Southeast Asia [1,2]. The incidence of NPC in the world was 1.2 per 100,000 persons in 2012 (1.7 per 100,000 in men and 0.7 per 100,000 in women), and about 25-30 per 100,000 persons in Southern China [3,4]. The etiology of NPC has not been fully understood, but it is believed to be multifactorial. The known etiologic factors of NPC include Epstein-Barr virus infection, genetic mutations, sex, age, race, alcohol and tobacco [2]. NPC is a highly malignant tumor which is prone to metastasis without obvious symptoms at an early stage. Clinically, distant metastasis of NPC still remains the most serious challenge in NPC treatment due to early metastasis and the easy recurrence of tumors [5]. Therefore, elucidating the molecular mechanisms of NPC metastasis may help to identify new biomarkers and drug targets for NPC treatment.
SIRT6 (Sirtuin6) mainly functions as an NAD+-dependent histone deacetylase and has been implicated in regulating various biologic functions, such as genomic stability, metabolism, inflammation, and cancer [6,7]. The effects of SIRT6 on these biologic processes are mainly achieved by removing acetyl groups on lysine residues from target proteins, for example, histones or transcription-related factors [7,8]. SIRT6 was shown to function as a tumor suppressor through both deacetylation-dependent and -independent activity in many types of cancer, including pancreatic, colorectal, breast, and lung cancer [9-13]. In contrast, SIRT6 was found to act as an oncogene in other types of cancer, e.g. melanoma and prostate cancer [14,15]. Recently, a study reported that the expression of SIRT6 was reduced in human NPC specimens, and SIRT6 induced apoptosis by inhibiting NF-kB signaling, suggesting that SIRT6 may play a role in modulating NPC development [16]. However, the exact molecular mechanism through which SIRT6 regulates NPC cell proliferation, migration, and invasion is still elusive.
Metastasis is a multistep event, including transformation, growth, angiogenesis, mobility, and invasion [17]. During the metastatic process, epithelial cells lose the apicobasal polarity and cell-cell adhesions, and then gain mesenchymal, fibroblast-like properties (epithelial-mesenchymal transition, EMT) [18]. SNAIL (encoded by Snail1 gene) is a key transcription factor that modulates cancer cell metastasis mainly through lowering the expression of E-cadherin, a key regulator of EMT [19]. The mechanisms controlling the expression of SNAIL are starting to be elucidated. NF-kB is shown to regulate SNAIL expression by directly binding to its minimal promoter [20,21]. Emerging data have shown that high SNAIL expression is associated with high metastatic potential in colorectal cancer, hepatocellular carcinoma, lung cancer, and NPC [22].
In our study, we showed that SIRT6 plays important roles in regulating NPC cell metastasis. Furthermore, we also demonstrated that SIRT6 interacts with the NF-kB subunit p65 and attenuated NF-kB regulated SNAIL expression by removing acetyl residues of histone H3K9 and H3K56 in the promoter regions of Snail1 gene. This study may be beneficial for the development of therapeutic targets for NPC.
Materials and methods
Cell culture
The NPC cell lines, 5-8F and 6-10B, were generously provided by Dr. Xiwen Xiong (Xinxiang Medical University, China). The NPC cell lines, 5-8F and 6-10B, were cultured in RPMI 1640 Medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Hyclone, USA) and 1% penicillin-streptomycin (Invitrogen, USA) in standard conditions (5% CO2, 37°C).
Quantitative real-time PCR
Total RNA was extracted with TRIzol reagent (Takara, Japan). 2 μg RNA was converted into cDNA with a cDNA Reverse Transcription Kit (Vazyme, China). Quantitative real-time PCR was analyzed using SYBR Green Master Mix (Vazyme, China) in an ABI StepOnePlus Real-Time PCR instrument (Applied Biosystems, USA).
Western blot analysis
NPC cell lysates were extracted using RIPA lysis buffer (Beyotime Biotechnology, China) plus protease cocktail (Roche, USA) and PMSF (Sigma, USA). Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes (Pall Corporation, USA). Immunoblots were hybridized overnight at 4°C with the following primary antibodies: anti-SIRT6, anti-SNAIL (Abcam, UK), anti-E-cadherin (Proteintech, USA), anti-β-Actin (Santa Cruz, USA). Then, immunoblots were incubated with HRP secondary antibody and the immunoreactive proteins were detected using ECL reagents (Sigma, USA).
Plasmids and generation of stable cell lines
Human SIRT6 and p65 cDNA were cloned into pcDNA3 vector with FLAG or hemagglutinin (HA) tag. Plvx-puro and pLKO.1-puro lentiviral expression vectors were used for SIRT6/GFP overexpression and shSIRT6/shGFP lentivirus construction, respectively. Packaging of lentivirus was performed by transient transfection of HEK293T cells with a lentiviral expression construct and other packaging vectors. 72 hr after transfection, the lentiviral particles were collected and filtered. Subsequently, 5-8F and 6-10B cells were infected with SIRT6/GFP and shSIRT6/shGFP lentiviruses, respectively. After 2 weeks of puromycin (1 ug/ml, InvivoGen, USA) selection, puromycin-resistant cell clones were obtained and SIRT6 overexpression or knockdown was assessed by RT-PCR and western blot analysis. The shRNA sequence for SIRT6 knockdown is: 5’-GCTACGTTGACGAGGTCATGA-3’.
SiRNA transfection
To knock down SNAIL in 6-10B cells, siRNAs targeting SNAIL (siSNAIL) and control siRNAs (siSCR) were synthesized by GenePharma (Shanghai, China) and transfected into 6-10B cells by lipofectamine 3000 (Thermo Fisher Scientific, USA). After 48 hr, the knockdown efficiency was evaluated by western blot. The siRNA sequence of SNAIL is as follows: 5’-CCACAGAAATGGCCATGGGAAGGCCTC-3’.
Wound healing assay
The 5-8F and 6-10B cells were first seeded in 6-well tissue culture dishes and allowed to grow to near confluence. Then, the cells were starved in serum-free medium for 24 hrs. The cell monolayers were scratched with a 10 µl sterilized tip, followed by an additional incubation with serum-free medium for 48 hrs. Images were captured of cells migrating distance at the wound sites at time points of 0 hr and 48 hrs using an inverted microscope (Leica, Germany).
Cell invasion and migration assay
Cell invasion and migration assays were performed using Transwell inserts (Corning, USA) pre-coated with (invasion assay) or without (migration assay) Matrigel (BD Biosciences, USA). The cells were harvested, suspended in serum-free medium, and subsequently seeded into the upper chamber. Medium with 10% FBS was placed in the lower chamber. After 24 hr incubation, invaded or migrated cells were fixed with 4% paraformaldehyde (PFA, pH7.4), then stained with crystal violet (Sigma-Aldrich, USA), and counted using an inverted microscope (Leica, Germany).
Chromatin immunoprecipitation (ChIP) assay
6-10B cells were cultured up to reach 90% confluence and then fixed with 1% formaldehyde at RT for 15 min. The crosslinking reaction was quenched by the adding glycine to a final concentration of 125 mM. Chromatins were lysed and sonicated to an average size of ~250 bp. Immunoprecipitation was performed using anti-SIRT6 (Abcam, UK), anti-p65, anti-Histone H3, anti-Acetyl-H3K9 (CST, USA), anti-Acetyl-H3K56 (Millipore, USA), or negative control IgG at 4°C overnight. After the sheared chromatins underwent reverse-crosslinking, real-time quantitative PCR was employed on genes of interest.
Immunoprecipitation (IP)
pcDNA-SIRT6-FLAG, pcDNA-p65-HA, pcDNA-GFP-FLAG expression vectors were transfected into 6-10B cells by lipofectamine 3000 with different combinations. 48 hrs later, cells were lysed in RIPA buffer and incubated with anti-FLAG M2 affinity agarose gel (Sigma, USA) or anti-HA agarose (Pierce, USA) at 4°C overnight. Precipitates were washed 4 times with RIPA buffer, and then resuspended with 2× protein sample buffer. Proteins were released from agarose after heating for 5 min at 100°C and subsequently analyzed by western blot with antibodies as indicated.
Statistical analysis
Statistical analysis was performed using a SPSS software package (version 16.0; Chicago, IL, USA). Data derived from cell line experiments presented as mean ± SEM were extracted from no less than three independent experiments. The χ2 or Fisher exact tests were used for categorical variables. Statistical significance was determined by the two-tailed unpaired Student t-test, and a difference of P<0.05 was considered significant.
Results
SIRT6 expression is negatively associated with the metastatic capability of NPC cells
In order to investigate the regulatory mechanisms of SIRT6 in NPC metastasis, typical human nasopharyngeal cell lines 5-8F (high metastatic potential) and 6-10B (low metastatic potential) were selected for study. Both 5-8F and 6-10B cells were derived from the same precursor cell line SUNE-1 cells with different metastatic potential [23]. As shown in Figure 1A, qPCR analysis showed that SIRT6 expression was higher in 6-10B cells than that in 5-8F cells. Similarly, 6-10B cells expressed higher protein levels of SIRT6 than 5-8F cells (Figure 1B). Since SNAIL mediated E-cadherin repression is tightly associated with high metastatic potential in cancer cells, we observed that 5-8F cells had higher SNAIL expression but lower E-cadherin expression compared to 6-10B cells (Figure 1B).
Figure 1.
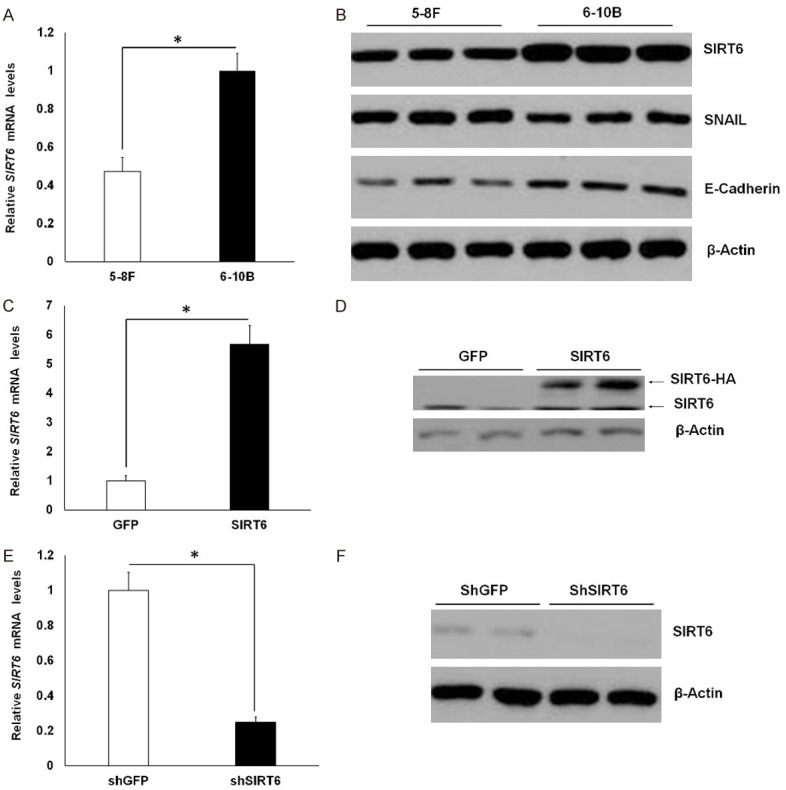
Comparison of SIRT6 expression in NPC cell lines. (A) qPCR analysis of SIRT6 expression in 5-8F and 6-10B cells. (B) Western blot analysis of SIRT6, SNAIL and E-cadherin protein levels in 5-8F and 6-10B cells. (C and D) qPCR (C) and western blot (D) analysis of SIRT6 expression in 5-8F-GFP and 5-8F-SIRT6 cells. (E and F) qPCR (E) and western blot (F) analysis of SIRT6 expression in 6-10B-shGFP and 6-10B-shSIRT6 cells. Data are shown as mean ± SEM (n=3-4 per group). *, P<0.05 by t test. For gel source data, see Supplementary Figure 1.
To better assess the impact of SIRT6 on NPC cell invasiveness, we used lentiviral vectors to overexpress HA-tagged SIRT6 in 5-8F cells (low SIRT6 expression) or to knockdown SIRT6 in 6-10B cells (High SIRT6 expression). As shown in Figure 1C and 1D, lentivirus-mediated SIRT6-HA expression in 5-8F-SIRT6 cells was around 5 times higher than endogenous SIRT6 expression in 5-8F-GFP cells. In contrast, SIRT6 expression was efficiently silenced in 6-10B-shSIRT6 cells compared to 6-10B-shGFP cells (Figure 1E and 1F).
The expression levels of SIRT6 influenced the metastatic capacity of NPC cell lines
First, we tested the effects of increased SIRT6 expression in 5-8F cells. Indeed, the invasive and migratory abilities of 5-8F-SIRT6 cells were significantly impaired, as determined by delayed scratch wound closure in wound-healing assay, and decreased invasion capacity in an invasion assay (with Matrigel) as well as reduced migration capacity in a transwell migration assay (Figure 2A-D). Next, we examined whether SIRT6 downregulation in 6-10B cells could promote metastasis or not. The scratch wound healing assay showed that reduced SIRT6 expression markedly enhanced wound closure in 6-10B-shSIRT6 cells compared with 6-10B-shGFP cells (Figure 2E and 2F). In addition, Matrigel invasion assay and transwell migration assay suggested that SIRT6 inhibition significantly upregulated the metastatic capacity of 6-10B cells (Figure 2G and 2H). Collectively, these data suggest that SIRT6 negatively regulates the metastasis of NPC cells.
Figure 2.
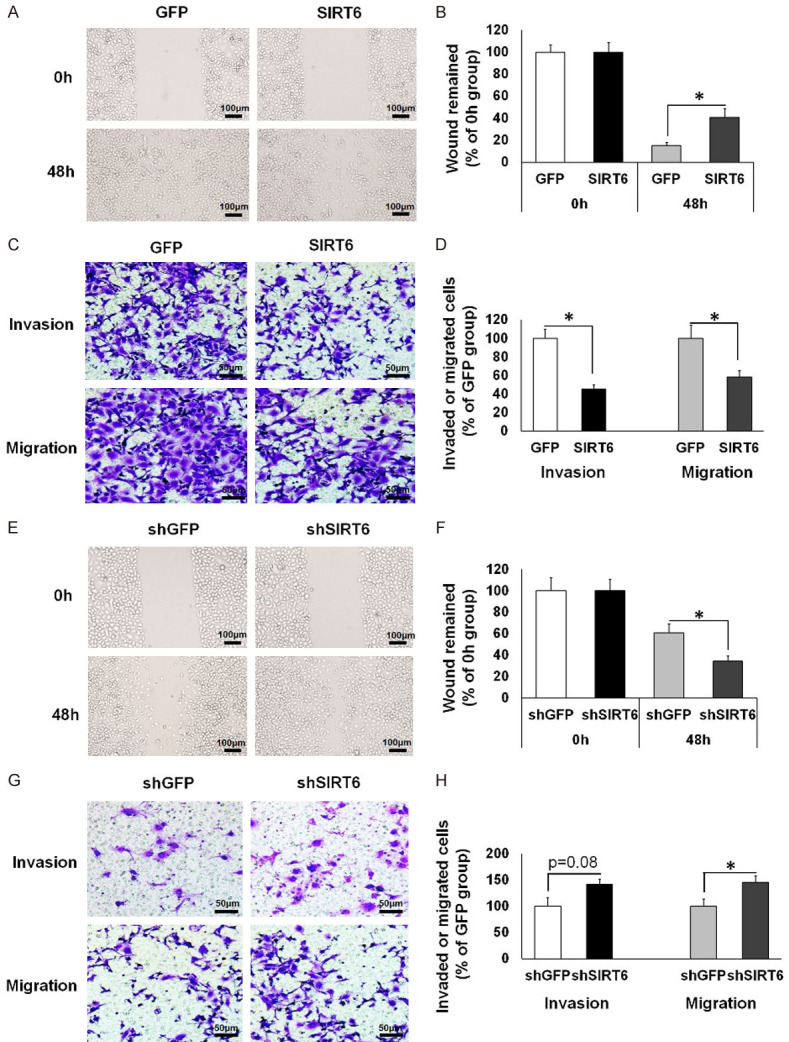
SIRT6 suppresses the migration and invasion of NPC cells. (A-D) In 5-8F cells that stably overexpressed with GFP or SIRT6, cell motility was analyzed using a wound healing assay (Magnification: 40×; Scale bars: 100 µm) (A) and the percentage of wound closure was quantified (B); migration and invasion abilities were evaluated by migration assay and Matrigel invasion assay, respectively (Magnification: 100×; Scale bars: 50 µm) (C); the percentage of invaded or migrated cells (% of GFP group) was quantified (D). (E-H) In control (shGFP) or stable SIRT6 knockdown (shSIRT6) 6-10B cells, cell motility was measured using a wound healing assay (Magnification: 40×; Scale bars: 100 µm) (E) and the percentage of wound closure was quantified (F); invasion and migration abilities were measured by Matrigel invasion assay and migration assay, respectively (Magnification: 100×; Scale bars: 50 µm) (G); the percentage of invaded or migrated cells (% of shGFP group) was quantified (H). Data are shown as mean ± SEM (n=4 for each group). *, P<0.05 by t test.
SIRT6 inhibits NPC cell metastasis through suppression of SNAIL expression
To further confirm the hypothesis that SIRT6 negatively regulates SNAIL expression in NPC cells, we tested the mRNA and protein levels of SNAIL in 5-8F-GFP/SIRT6 and 6-10B-shGFP/shSIRT6 cells. Overexpression of SIRT6 in 5-8F-SIRT6 cells remarkably reduced SNAIL expression, as followed by enhanced E-cadherin production (Figure 3A and 3B). In contrast, knockdown of SIRT6 in 6-10B-shSIRT6 cells increased the expression of SNAIL, thereby resulting in a reduction of E-cadherin abundance (Figure 3A and 3B). These data clearly demonstrate that SIRT6 negatively modulates SNAIL transcriptional expression in NPC cells. Next, we investigated whether SIRT6 regulated NPC cell metastasis through inhibiting SNAIL expression. SNAIL was knocked down by introducing SNAIL siRNA into 6-10B-shGFP or 6-10B-shSIRT6 cells (Figure 3C). The invasion and transwell assays showed that SNAIL knockdown significantly repressed invasion and migration, which were induced by SIRT6 knockdown in 6-10B cells (Figure 3D-F).
Figure 3.
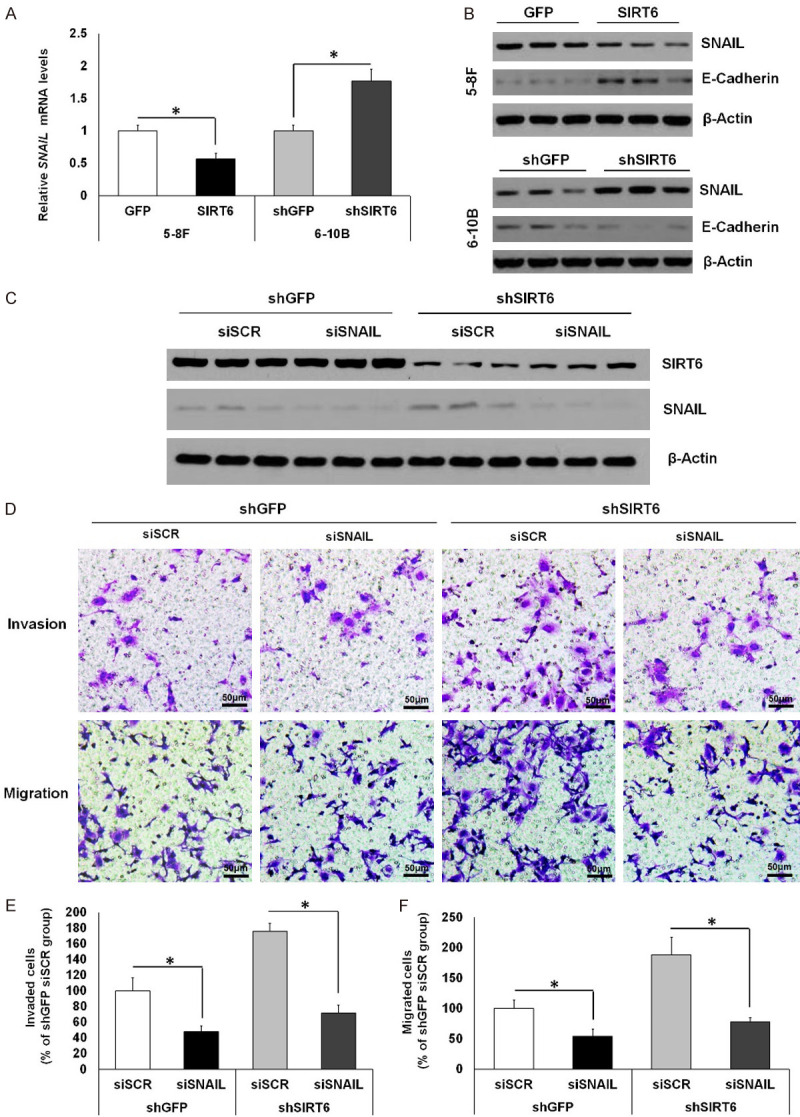
SIRT6 inhibits NPC cell metastasis by suppression of SNAIL expression. (A) qPCR analysis of SNAIL expression in 5-8F and 6-10B cells. (B) Western blot analysis of SNAIL and E-cadherin protein levels in stable 5-8F cell lines with GFP or SIRT6 overexpression and stable 6-10B cell lines with control or SIRT6 knockdown. (C-F) In control (shGFP) and stable SIRT6 knockdown (shSIRT6) 6-10B cells transfected with siSCR or siSNAIL, SIRT6 and SNAIL protein levels were analyzed by western blot (C); invasion and migration abilities were measured by Matrigel invasion assay and migration assay, respectively (Magnification: 100×; Scale bars: 50 µm) (D); the percentage of invaded (E) or migrated (F) cells (% of shGFP siSCR group) was quantified. Data are shown as mean ± SEM (n=3-4 per group). *, P<0.05 by t test. For gel source data, see Supplementary Figure 2.
SIRT6 interacts with p65 and deacetylates H3K9 and H3K56 at the promoter of Snail1
As shown in Figure 1A and 1B, SIRT6 expression was higher in 6-10B than 5-8F, so we used 6-10B cells for a mechanistic study. NF-kB signaling has been shown to modulate SNAIL expression through direct binding of transcription factor p65 to the promoter region of Snail1 [20,21]. To verify the molecular interactions between SIRT6 and p65 in NPC cells, we performed Co-IP analysis by co-transfection of the tagged constructs in 6-10B cells. As expected, SIRT6 interacted with p65 in 6-10B cells (Figure 4A). The interaction between SIRT6 and p65 suggested that SIRT6 might function as a co-repressor of the transcription factor p65 and then inhibit p65 mediated transcription of Snail1. To test this possibility, chromatin immunoprecipitation (ChIP) was performed in 6-10B cells to study whether the binding of SIRT6 and p65 occurred at the promoter region of Snail1. The promoter sequence (-1 to -500 from the transcription start site) of Snail1 is listed in Figure 4B. The sequences of primers used for ChIP analysis to examine the p65 or SIRT6 enrichment in region 1 or region 2 are also shown in Figure 4B. As expected, p65 was significantly enriched at the Snail1 gene promoter, especially in the region 2 (Figure 4C). Consistently, SIRT6 had highly enriched association with the region 2 of the Snail1 promoter (Figure 4D). Basically, SIRT6 inhibits gene transcription through deacetylating histone 3 at lysine 9 and/or 56 at the promoter region of target genes. In order to examine whether Sirt6 suppresses Snail1 gene expression through histone deacetylation, we performed a ChIP assay to detect the acetylation status of H3K9 and H3K56 in the region 2 of the Snail1 gene promoter in 6-10B cells. Indeed, we found the acetylation levels of H3K9 and H3K56 in the region 2 were significantly reduced in SIRT6 overexpressed 6-10B cells compared with GFP control cells (Figure 4E). These data indicate that SIRT6 functions as a histone deacetylase to suppress SNAIL expression through deacetylating histone H3K9 and H3K56 at the Snail1 gene promoter.
Figure 4.
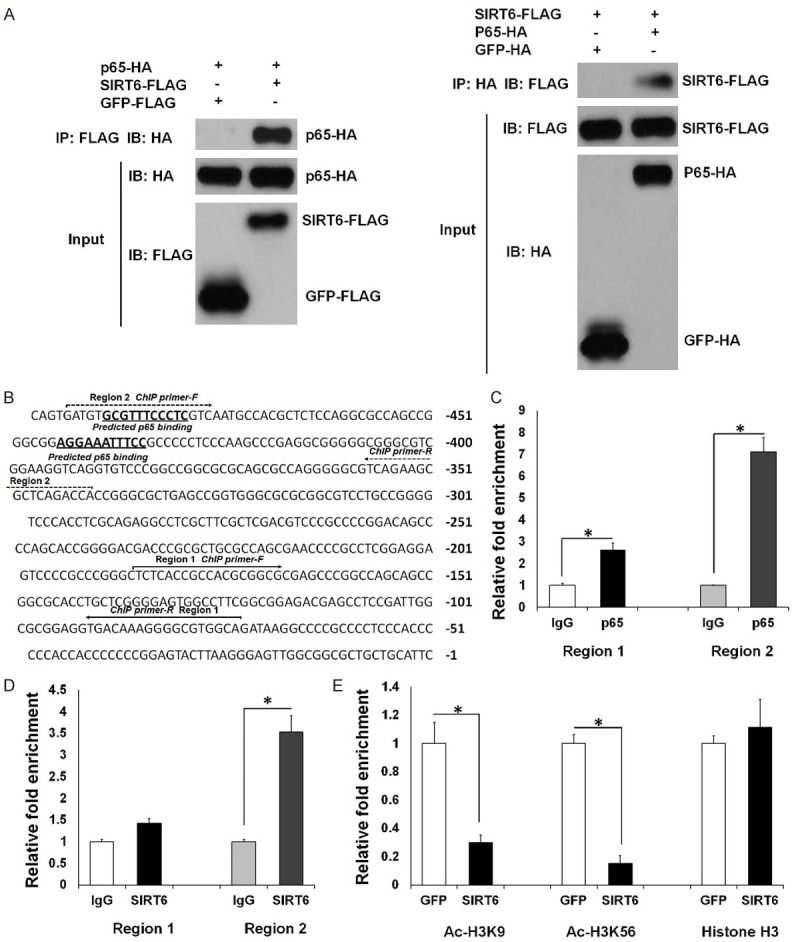
SIRT6 interacts with p65 and deacetylates H3K9 and H3K56 at the promoter of Snail1. (A) Co-IP assay to test the potential interaction between SIRT6 and p65 was performed by transfection of corresponding constructs into 6-10B cells. IP, immunoprecipitation; IB, immunoblot. (B) The sequence of the Snail1 promoter region (-500) is presented. The p65 DNA binding sites were predicted by program PROMO 3.0. (C and D) ChIP assay of the binding of p65 (C) and SIRT6 (D) to the promoter regions of the Snail1 gene in 6-10B cells using IgG as a negative control for chromatin pull-down. (E) The effect of SIRT6 overexpression on histone acetylation in the region 2 of Snail1 promoter was evaluated using ChIP in 6-10B cells transfected with GFP or SIRT6 expression constructs. Data are shown as mean ± SEM (n=3 for each group). *, P<0.05 by t test. For gel source data, see Supplementary Figure 3.
Discussion
Accumulating evidence has demonstrated that SIRT6 plays critical roles in various cancers, such as colon cancer, pancreatic cancer, and breast cancer [9,10,12,24]. SIRT6 inhibits survivin to modulate cancer initiation through AP-1 dependent signaling pathways in hepatocellular carcinoma [24]. Moreover, overexpression of SIRT6 induced apoptosis in many types of cancer cells, such as HT1080, Hela, HCA2 and MEF [25]. Collectively, these results suggest that SIRT6 functions as a tumor suppressor in cancer development. However, others have shown opposite outcomes of SIRT6 in regulating cancer metastasis. Bauer et al. demonstrated that SIRT6 increased the metastatic potential of pancreatic cancer cells by upregulating the expression of pro-inflammatory cytokines such as IL-8 and TNF in a Ca2+-dependent manner [26]. In non-small cell lung carcinoma (NSCLC), Bai et al. showed that SIRT6 enhanced migration and invasion of NSCLC cells via ERK1/2-MMP9 signaling [27]. So far, only one study has shown the effects of SIRT6 overexpression on NPC cells. In that study, SIRT6 was found to impair proliferation and induce apoptosis in NPC cells [16]. However, the exact mechanisms through which SIRT6 suppresses metastasis in NPC remain largely unknown.
In this study, a possible relationship between SIRT6 and the metastatic capability of NPC cells was investigated. Both of the 5-8F and 6-10B cell lines originated from the SUNE-1 NPC cell lines. Previous work has shown that the 5-8F cells are highly tumorigenic and metastatic while 6-10B cells just tumorigenic and less-metastatic. Western blot demonstrated that SIRT6 expression was lower in metastatic 5-8F cells, but higher in low metastatic 6-10B cells. Collectively, these findings underscore a possible important role of SIRT6 as a negative regulator controlling the metastatic capability of NPC. Further correlation analysis provided evidence that overexpression of SIRT6 in 5-8F cells impaired while knockdown of SIRT6 in 6-10B cells enhanced the metastatic ability of NPC cells.
SNAIL is zinc-finger transcription factor and promotes the EMT process via repression of E-cadherin expression [19]. SNAIL has been shown to promote tumor metastasis in many types of cancer, e.g. ovarian cancer, lung cancer, and breast cancer [22]. Many factors, such as BMI-1, EZH2, LMP1, and HOPX, regulate NPC cell metastasis through modulating SNAIL expression [3,28-30]. Moreover, a recent study demonstrated that SNAIL promoted NPC cell metastasis partly by lowering TEL2 expression [31]. Not surprisingly, a high level of SNAIL expression was associated with poor survival rate in NPC patients [32]. However, the regulatory mechanism of SNAIL expression in NPC is complex. Various signaling pathways, including TGF-β, Notch, MAPK, and NF-kB, activate SNAIL expression at transcriptional level, resulting in activation of EMT [22]. NF-kB enhances SNAIL transcription through direct binding of p65 to the promoter region [20,21]. Basically, SIRT6 acts as a transcriptional repressor of several transcription factors, including NF-kB/p65 [33]. SIRT6 deacetylates H3K9 at the promoter regions of NF-kB target genes and inhibits gene expression, thereby suppressing NF-kB signaling. Moreover, p65 heterozygosity partially rescued the premature lethality phenotype of Sirt6-/- mice, indicating that hyperactivation of NF-kB signaling contributes to the defects in SIRT6 knockout mice [33].
In our study, we verified that SIRT6 interacts with p65 and deacetylates H3K9 and H3K56 at the promoter of SNAIL. SIRT6 worked as a suppressor of p65 in modulating SNAIL transcriptional expression in NPC cells. Furthermore, our data suggest that downregulation of SNAIL is required for SIRT6-mediated inhibition of metastasis of NPC cells. We also found that the repressive activity of SIRT6 in E-cadherin expression was markedly prevented, when SNAIL was knocked down in NPC cells. These data suggest that the presence of SNAIL is required for the repressive function of SIRT6 on E-cadherin. Additionally, the repressive function of SIRT6 toward E-cadherin depends on its deacetylation activity.
Metastasis is a pathologic process that involves a complex interplay between intrinsic tumor cell properties as well as interactions between cancer cells and various microenvironments. Thus, the regulatory mechanism of metastasis is also complicated. Indeed, it has been shown that SIRT6 promotes epithelial-to-mesenchymal transition and metastasis in non-small cell lung cancer (NSCLC) [34] and hepatocellular carcinoma (HCC) [35]. However, in our current study, we report that SIRT6 suppresses NPC cell metastasis, which is contradictory to previous studies. Considering that the pathogenesis of metastasis is quite complicated and the multifaced functions of SIRT6, it is reasonable to believe SIRT6 could function as a metastasis inhibitor in different cancers. Consistent with our data, SIRT6 suppresses pancreatic ductal adenocarcinoma (PDAC) progression and metastasis [10]. Our study has some limitations listed as follows: (1) We just have used two cell lines to clarify the roles of SIRT6 in regulating NPC cell migration and metastasis; whether SIRT6 plays a similar role in other NPC cells is still unknown. (2) We only have done in vitro assays in this study; however, the in vivo role of SIRT6 in the controlling NPC metastasis still needs to be elucidated. Additional animal study is needed to clarify this issue. (3) SIRT6 is a histone deacetylase with multiple targets. We have shown the SNAIL is one of the targets of SIRT6 in NPC cells. However, it is also reasonable that SIRT6 may regulate NPC cell metastasis through other target genes or signaling pathways. Thus, identification of other SIRT6 targets is also important to further elucidate SIRT6 function in NPC.
In conclusion, our study showed that SIRT6 expression was negatively associated with the metastatic ability of NPC cells. Our results support a hypothesis that expression of SIRT6 in NPC may be important in the acquisition of an aggressive/poor prognostic phenotype. Furthermore, we showed that SIRT6 suppressed NPC cell invasion and migration though downregulating expression of SNAIL followed by increased E-cadherin expression. The repressive function of SIRT6 toward E-cadherin depends on histone deacetylation. Our research highlighted the importance of SIRT6 in regulating NPC metastasis and provide evidence that SIRT6 could serve as an attractive therapeutic target in NPC.
Acknowledgements
The study was supported by The Jiangsu Project of Invigorating Health Care through Science, Technology and Education Foundation (ZDXKB2016015); The Fundamental Research Funds for the Central Universities (YG2005005).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lin DC, Meng X, Hazawa M, Nagata Y, Varela AM, Xu L, Sato Y, Liu LZ, Ding LW, Sharma A, Goh BC, Lee SC, Petersson BF, Yu FG, Macary P, Oo MZ, Ha CS, Yang H, Ogawa S, Loh KS, Koeffler HP. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46:866–871. doi: 10.1038/ng.3006. [DOI] [PubMed] [Google Scholar]
- 2.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 3.Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH, Zhang HB, Liao YJ, Zheng F, Zhu W, Liu TH, Bian XW, Guan XY, Lin MC, Zeng MS, Zeng YX, Kung HF, Xie D. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene. 2012;31:583–594. doi: 10.1038/onc.2011.254. [DOI] [PubMed] [Google Scholar]
- 4.Salehiniya H MM, Mohammadian-Hafshejani A, Mahdavifar N. Nasopharyngeal cancer in the world: epidemiology, incidence, mortality and risk factors. World Cancer Res J. 2018;5:e1046. [Google Scholar]
- 5.Yu C, Chen L, Yie L, Wei L, Wen T, Liu Y, Chen H. Targeting FoxM1 inhibits proliferation, invasion and migration of nasopharyngeal carcinoma through the epithelialto-mesenchymal transition pathway. Oncol Rep. 2015;33:2402–2410. doi: 10.3892/or.2015.3834. [DOI] [PubMed] [Google Scholar]
- 6.Xiong X, Zhang C, Zhang Y, Fan R, Qian X, Dong XC. Fabp4-Cre-mediated Sirt6 deletion impairs adipose tissue function and metabolic homeostasis in mice. J Endocrinol. 2017;233:307–314. doi: 10.1530/JOE-17-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello M, Zullo A, Servillo L, Mancini FP, Borriello A, Giovane A, Della Ragione F, D’Onofrio N, Balestrieri ML. Multiple pathways of SIRT6 at the crossroads in the control of longevity, cancer, and cardiovascular diseases. Ageing Res Rev. 2017;35:301–311. doi: 10.1016/j.arr.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Xiong X, Wang G, Tao R, Wu P, Kono T, Li K, Ding WX, Tong X, Tersey SA, Harris RA, Mirmira RG, Evans-Molina C, Dong XC. Sirtuin 6 regulates glucose-stimulated insulin secretion in mouse pancreatic beta cells. Diabetologia. 2016;59:151–160. doi: 10.1007/s00125-015-3778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–1199. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kugel S, Sebastian C, Fitamant J, Ross KN, Saha SK, Jain E, Gladden A, Arora KS, Kato Y, Rivera MN, Ramaswamy S, Sadreyev RI, Goren A, Deshpande V, Bardeesy N, Mostoslavsky R. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell. 2016;165:1401–1415. doi: 10.1016/j.cell.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Z, Liu L, Liu Y, Li S. Sirtuin SIRT6 suppresses cell proliferation through inhibition of Twist1 expression in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7:4774–4781. [PMC free article] [PubMed] [Google Scholar]
- 12.Thirumurthi U, Shen J, Xia W, LaBaff AM, Wei Y, Li CW, Chang WC, Chen CH, Lin HK, Yu D, Hung MC. MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Sci Signal. 2014;7:ra71. doi: 10.1126/scisignal.2005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioris RM, Galie M, Ramadori G, Anderson JG, Charollais A, Konstantinidou G, Brenachot X, Aras E, Goga A, Ceglia N, Sebastian C, Martinvalet D, Mostoslavsky R, Baldi P, Coppari R. SIRT6 suppresses cancer stem-like capacity in tumors with PI3K activation independently of its deacetylase activity. Cell Rep. 2017;18:1858–1868. doi: 10.1016/j.celrep.2017.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Xie QR, Wang B, Shao J, Zhang T, Liu T, Huang G, Xia W. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein Cell. 2013;4:702–710. doi: 10.1007/s13238-013-3054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Peterson LM, Ndiaye MA, Singh CK, Chhabra G, Huang W, Ahmad N. SIRT6 histone deacetylase functions as a potential oncogene in human melanoma. Genes Cancer. 2017;8:701–712. doi: 10.18632/genesandcancer.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang L, Yi L, Li J, Yi S, Li S, Liu P, Yang X. SIRT6 overexpression induces apoptosis of nasopharyngeal carcinoma by inhibiting NF-kappaB signaling. Onco Targets Ther. 2018;11:7613–7624. doi: 10.2147/OTT.S179866. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Goossens S, Vandamme N, Van Vlierberghe P, Berx G. EMT transcription factors in cancer development re-evaluated: beyond EMT and MET. Biochim Biophys Acta Rev Cancer. 2017;1868:584–591. doi: 10.1016/j.bbcan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Dong C, Zhou BP. Epigenetic regulation of EMT: the Snail story. Curr Pharm Des. 2014;20:1698–1705. doi: 10.2174/13816128113199990512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, Baulida J, Franci C, Dedhar S, Larue L, Garcia de Herreros A. Regulation of snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 21.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 22.Kaufhold S, Bonavida B. Central role of Snail1 in the regulation of EMT and resistance in cancer: a target for therapeutic intervention. J Exp Clin Cancer Res. 2014;33:62. doi: 10.1186/s13046-014-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Huang W, Ren C, Wen Q, Liu W, Yang X, Wang L, Zhu B, Zeng L, Feng X, Zhang C, Chen H, Jia W, Zhang L, Xia X, Chen Y. Flotillin-2 promotes metastasis of nasopharyngeal carcinoma by activating NF-kappaB and PI3K/Akt3 signaling pathways. Sci Rep. 2015;5:11614. doi: 10.1038/srep11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min L, Ji Y, Bakiri L, Qiu Z, Cen J, Chen X, Chen L, Scheuch H, Zheng H, Qin L, Zatloukal K, Hui L, Wagner EF. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat Cell Biol. 2012;14:1203–1211. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- 25.Van Meter M, Mao Z, Gorbunova V, Seluanov A. SIRT6 overexpression induces massive apoptosis in cancer cells but not in normal cells. Cell Cycle. 2011;10:3153–3158. doi: 10.4161/cc.10.18.17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer I, Grozio A, Lasiglie D, Basile G, Sturla L, Magnone M, Sociali G, Soncini D, Caffa I, Poggi A, Zoppoli G, Cea M, Feldmann G, Mostoslavsky R, Ballestrero A, Patrone F, Bruzzone S, Nencioni A. The NAD+-dependent histone deacetylase SIRT6 promotes cytokine production and migration in pancreatic cancer cells by regulating Ca2+ responses. J Biol Chem. 2012;287:40924–40937. doi: 10.1074/jbc.M112.405837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai L, Lin G, Sun L, Liu Y, Huang X, Cao C, Guo Y, Xie C. Upregulation of SIRT6 predicts poor prognosis and promotes metastasis of non-small cell lung cancer via the ERK1/2/MMP9 pathway. Oncotarget. 2016;7:40377–40386. doi: 10.18632/oncotarget.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, Kong QL, Xu LH, Zhang X, Liu WL, Li MZ, Zhang L, Kang TB, Fu LW, Huang WL, Xia YF, Tsao SW, Li M, Band V, Band H, Shi QH, Zeng YX, Zeng MS. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119:3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horikawa T, Yoshizaki T, Kondo S, Furukawa M, Kaizaki Y, Pagano JS. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer. 2011;104:1160–1167. doi: 10.1038/bjc.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren X, Yang X, Cheng B, Chen X, Zhang T, He Q, Li B, Li Y, Tang X, Wen X, Zhong Q, Kang T, Zeng M, Liu N, Ma J. HOPX hypermethylation promotes metastasis via activating SNAIL transcription in nasopharyngeal carcinoma. Nat Commun. 2017;8:14053. doi: 10.1038/ncomms14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sang Y, Cheng C, Zeng YX, Kang T. Snail promotes metastasis of nasopharyngeal carcinoma partly by down-regulating TEL2. Cancer Commun (Lond) 2018;38:58. doi: 10.1186/s40880-018-0328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo WR, Li SY, Cai LM, Yao KT. High expression of nuclear Snail, but not cytoplasmic staining, predicts poor survival in nasopharyngeal carcinoma. Ann Surg Oncol. 2012;19:2971–2979. doi: 10.1245/s10434-012-2347-x. [DOI] [PubMed] [Google Scholar]
- 33.Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Huang J, Shen S, Ding Z, Luo Q, Chen Z, Lu S. SIRT6 drives epithelial-to-mesenchymal transition and metastasis in non-small cell lung cancer via snail-dependent transrepression of KLF4. J Exp Clin Cancer Res. 2018;37:323. doi: 10.1186/s13046-018-0984-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Han LL, Jia L, Wu F, Huang C. Sirtuin6 (SIRT6) promotes the EMT of hepatocellular carcinoma by stimulating autophagic degradation of E-cadherin. Mol Cancer Res. 2019;17:2267–2280. doi: 10.1158/1541-7786.MCR-19-0321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


