Abstract
A 30-year-old woman was diagnosed with T-lymphoblastic lymphoma (T-LBL) that harbored a clonal Epstein-Barr virus (EBV) genome. At relapse, axillary lymph node adenopathy, which was diagnosed as peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), was detected. Southern blot analyses of the T-cell receptor and EBV genome revealed that the T-LBL and PTCL-NOS were clonally identical. We previously showed that CD21 acted as an entry molecule that allowed EBV into the patient's T-LBL cells. Interestingly, the PTCL-NOS cells lacked CD21 expression. Our case suggests that EBV might infect immature CD21-positive T-cells, and CD21-negative PTCL-NOS might subsequently arise through phenotypic changes.
Keywords: Epstein-Barr virus, composite lymphoma, CD21, T-lymphoblastic lymphoma, peripheral T-cell lymphoma
Introduction
Epstein-Barr virus (EBV) contributes to the development of various human tumors (1). EBV primarily infects B-lymphocytes and epithelial cells and rarely infects T-cells (2). Thus, the incidence of EBV-related T-cell lymphoma is markedly lower than that of EBV-related B-cell lymphoma. In addition, most EBV-related lymphoid tumors exhibit a mature phenotype. Therefore, EBV-related T-lymphoblastic lymphoma (T-LBL), which is an immature lymphoid tumor, is rare.
The mechanism by which EBV causes carcinogenesis has not been fully elucidated. CD21 has been shown to be the main molecule responsible for EBV entry into cells (3). Although B-cells express CD21, mature T-cells do not (4). Therefore, how EBV infects T-cells and causes mature T-cell lymphoma is not fully understood (5).
Two distinct types of lymphoma occasionally exist in the same patient. These lymphomas can be composite lymphomas or transformed lymphomas. To increase our understanding of the pathogenesis of composite lymphomas, it is important to determine whether or not two lymphomas are clonally related. Clonally related lymphomas that occur in the same patient are suitable models for examining the multistep carcinogenic process responsible for malignant lymphoma.
In this study, we examined the clonality of two phenotypically distinct sets of tumor cells - T-LBL and peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) - that developed in the same patient. Based on our findings, we discuss a new tumorigenesis model for EBV-related T-cell lymphoma.
Case Report
A 30-year-old Japanese woman was admitted to our hospital with a mediastinal tumor. The clinical course of this case was described previously (6). In brief, the mediastinal tumor was diagnosed as a T-LBL without bone marrow involvement. The tumor cells were positive for CD3, CD4, CD8, terminal deoxynucleotidyl transferase (TdT), and EBV-encoded small RNAs (EBERs). The EBV viral load in the patient's serum was 2×105 copies/mL at the initial diagnosis. One month after allogeneic hematopoietic stem cell transplantation (HSCT) from a human leukocyte antigen-matched sibling was performed, pleural effusion appeared. The cells in the pleural effusion exhibited the same phenotype as those seen in the mediastinal tumor. The T-LBL cells were CD21-positive (6). At relapse, the EBV-DNA viral load in the patient's peripheral blood was 4×102 copies among 1×106 white blood cells, indicating that the EBV-DNA viral load in the peripheral blood had not markedly increased since the initial diagnosis.
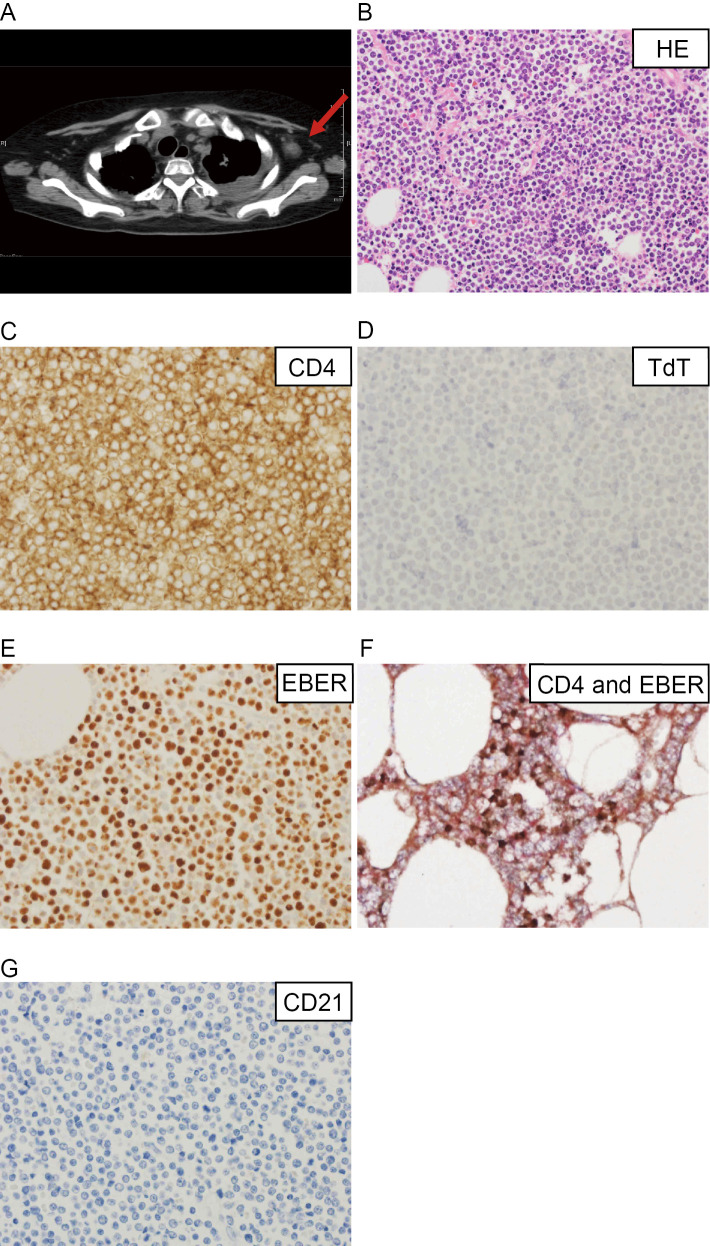
Three months after the first HSCT procedure, the patient underwent allogeneic HSCT again from the same donor along with high-dose chemotherapy because the tumor had regrown. At one month after the second HSCT procedure, the mediastinal tumor was still growing, and pneumonia had developed. Left-sided axillary lymphadenopathy was also seen (Fig. 1A). The patient ultimately died due to progressive respiratory failure. A postmortem examination of her axillary lymph nodes and lungs was performed. The necropsy specimen of the axillary lymph nodes showed small to medium-sized monotonous mature lymphoid cells, which had diffusely proliferated. These cells contained round nuclei with dispersed chromatin. The tumor cells in the peripheral lymph nodes were diagnosed as CD4-positive PTCL-NOS (Fig. 1B, C). They were negative for CD8 and TdT (Fig. 1D) (6) but were positive for EBV (Fig. 1E). They were also positive for CD3, CD5 and CD7 and negative for CD34. Double immunohistochemical staining showed that the CD4-positive and EBV-positive cells were identical (Fig. 1F). A lung biopsy also showed infiltrating lymphoma cells, suggesting that the lymphoma had caused the respiratory failure. The phenotype of the cells seen in the pleural effusion at the postmortem examination was the same as that of the T-LBL cells.
Figure 1.
Computed tomography (CT) and histopathological findings of the patient at relapse. A: CT image of the patient obtained at relapse. The red arrow indicates the left axillary lymph nodes resected during the postmortem examination. B-G: Histopathological findings of the peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), after it was resected from the axillary lymph nodes. B: Hematoxylin and Eosin staining (×400); C, D: Immunohistochemistry of CD4 (C) and TdT (D) (×600); E: In situ hybridization of Epstein-Barr virus (EBV)-encoded mRNA (×600). F: Double immunohistochemical staining of CD4 and EBER. The CD4- and EBER-positive cells were stained red and brown, respectively (×600). G: Immunohistochemistry of CD21 (×600).
Materials and Methods
Southern blot analyses
DNA was extracted from the tumor cells in the pleural effusion and left axillary lymph nodes using phenol chloroform extraction and ethanol precipitation. MD901, a diffuse large B-cell lymphoma cell line, was used as a negative control (7).
To detect T-cell receptor (TCR) rearrangement, the Cβ2 fragment was used as a probe. A 934-bp fragment of TCR Cβ2 was generated using polymerase chain reaction (PCR) and the primers Cβ2F (GCTGTGTTTGAGCCATCAGA) and Cβ2R (GCAGAGACGGCGAAAGATAG). After being amplified, the fragment was inserted into the SmaI site of the pGEM/3Zf(+) vector. After being digested using BamHI, the Cβ2 fragment was labeled with digoxigenin (DIG) using T7 RNA polymerase and the DIG-RNA labeling kit (Roche, Mannheim, Germany), according to the manufacturer's instructions.
To detect the EBV genome, the EBV1 and EBV2 probes were used. The 895-bp EBV1 probe and 620-bp EBV2 probe were generated using previously reported PCR primers (8). The EBV1 and EBV2 probes recognize the 5' and 3' terminal repeat regions, respectively. These probes were labeled using DIG.
Genomic DNA from each set of tumor cells was digested using BamHI, HindIII, or EcoRI. After being subjected to electrophoresis in 0.8% agarose gel and 1x TAE buffer, the digested DNA was transferred to nylon membranes (Roche). Hybridization, washing, and detection were performed using the prepared TCR Cβ2 and EBV probes, according to the manufacturer's instructions (Roche).
PCR to detect TCR gene rearrangement
PCR to detect TCR clonality was performed at another laboratory (LSI Medience, Tokyo, Japan), according to the method outlined in the European BIOMED-2 collaborative study (9).
Immunohistochemistry
Immunohistochemistry was conducted with an antibody against CD21 (clone 1F8; DakoCytomation, Glostrup, Denmark). The EBV gene was detected in formalin-fixed paraffin-embedded sections by subjecting them to in situ hybridization using the fluorescein-conjugated EBV peptide nucleic acid probe kit (DakoCytomation), according to the manufacturer's instructions. The probe included with this kit is complementary to the two nuclear RNAs encoded by the EBV (EBERs).
Results
T-cell and EBV clonality
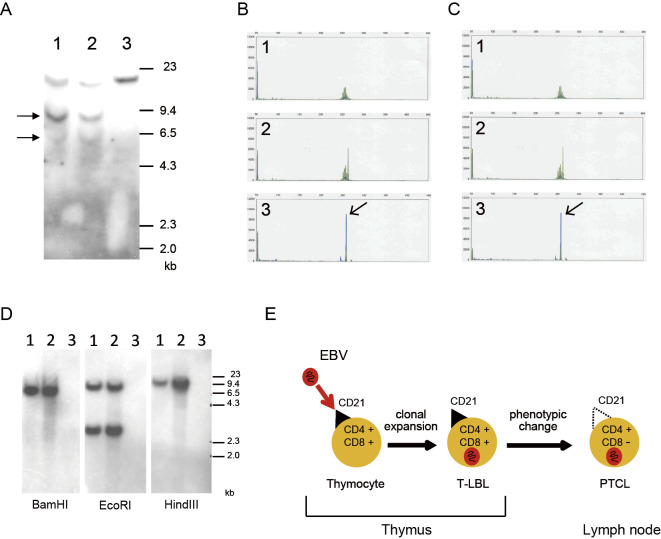
To assess whether or not the T-LBL and PTCL-NOS arose from the same T-cell origin, they were compared genetically. The cells obtained from the mediastinal tumor (at onset) and the axillary lymph nodes (at relapse) showed identical rearranged Cβ2 bands in the Southern blot analysis (Fig. 2A). The same rearranged bands were detected in the Southern blot analyses of the EcoRI- and HindIII-digested DNA (data not shown). PCR of TCR rearrangement also indicated that both sets of tumor cells were derived from the same T-cell clone (Fig. 2C, D). Furthermore, a Southern blot analysis of the EBV genome produced bands of the same size for both sets of tumor cells, indicating that they had both been infected by the same EBV (Fig. 2B). These results suggest that the tumor cells originated from the same T-cell clone that had been infected by the same EBV, although these cells exhibited different phenotypes in terms of their anatomical sites.
Figure 2.
Genomic analyses of the T-cell receptor (TCR) and EBV. A: Southern blot analyses using a Cβ2 probe. The arrows indicate rearranged bands. DNA was digested with BamHI. Lane 1: peripheral lymph nodes, lane 2: pleural effusion, lane 3: MD901 (the negative control). B, C: TCR gene rearrangement of Vβ/Jβ1/Jβ2 in cells from the peripheral lymph nodes (B) or pleural effusion (C). The polymerase chain reaction was conducted using 23 Vβ primers and 9 Jβ primers, as described previously (9). The arrows indicate the clonal peaks. The same clonal peaks were seen in (B) and (C), indicating that these cells were identical. 1: negative control, 2: positive control, 3: patient’s sample. D: Southern blot analyses of the EBV genome. The EBV1 and EBV2 fragments were used as probes. DNA was digested with BamHI, EcoRI, and HindIII. Lane 1: peripheral lymph nodes, lane 2: pleural effusion, lane 3: MD901 (the negative control). E: Schematic diagram showing a putative mechanism that might have caused the EBV-induced T-cell malignancies seen in this patient. EBV-infected, CD4-positive, CD8-positive immature T-cells, which also expressed CD21, were present in the patient’s thymus. The cells developed into T-lymphoblastic lymphoma (T-LBL). After treatment, including allogeneic stem cell transplantation, CD4-positive, CD8-negative PTCL developed through phenotypic changes. The PTCL cells were negative for CD21, which was the molecule responsible for the entry of EBV into the T-LBL cells in this patient, but EBV had already infected the PTCL cells.
CD21 expression
The T-LBL cells were found to be CD21-positive at the initial diagnosis, as reported previously (6). The CD21 expression of the PTCL-NOS cells was examined in this study because we previously showed that the EBV infection of the T-LBL cells occurred through the CD21 molecule (6). Immunostaining revealed that the PTCL-NOS cells were CD21-negative (Fig. 1F). These results suggested that the PTCL-NOS cells did not express CD21, even though they had been infected by the same EBV as the T-LBL cells.
Discussion
EBV can cause various types of lymphoma, such as Burkitt's lymphoma, B-cell lymphoma in patients with immunosuppressive disease, nasal natural killer cell/T-cell lymphoma, and Hodgkin's lymphoma. Although EBV-related T-cell tumors are rare, the patient in this study suffered from simultaneous composite immature T-cell lymphoma and mature T-cell lymphoma. The clonal relationship between the T-LBL and PTCL-NOS cells was analyzed by performing Southern blot analyses of the TCR and EBV along with a PCR-based clonality study. Interestingly, the two sets of tumor cells exhibited different expression patterns of CD21, an EBV entry molecule, which might be important considering that EBV is found in EBV-related mature T-cell lymphoma.
In rare cases, different types of lymphoma exist simultaneously, and such cases are referred to as composite lymphoma. Composite lymphoma can involve combinations of Hodgkin's lymphoma and a non-Hodgkin's lymphoma, or two different non-Hodgkin's lymphomas (10,11). To our knowledge, although there have been two reports of composite lymphoma involving T-LBL and diffuse large B-cell lymphoma, composite lymphoma involving the combination of T-LBL and PTCL has not been reported (12,13). In addition, the concept of histological transformation has been discussed. Only two cases involving the transformation of T-LBL to PTCL have been reported, wherein the tumor cells originated from γδ T-cells (14). Our present findings indicate that cases of composite lymphoma involving T-LBL and PTCL-NOS derived from αβ T-cells can also occur. Composite lymphomas and histological transformation are suitable models for elucidating the steps of the lymphoma transformation process and examining the role of tumor heterogeneity in the pathology of lymphoma. Tumor heterogeneity contributes to therapy resistance, which is an important issue for the treatment of lymphoma (15,16). Thus, our case might provide new insight into the significance of tumor heterogeneity in T-cell lymphoma.
EBV is known to infect B-cells and epithelial cells through the CD21 molecule. However, the molecule responsible for the entry of EBV into T-cells has not been identified, as mature T-cells do not express CD21. Thus, the mechanism responsible for the development of EBV-related mature T-cell neoplasms has not been elucidated. Immature T-cells in the thymus can transiently express CD21 during the thymocyte differentiation stage (17). A previous study showed that immature CD21-expressing T-cells were susceptible to EBV infection through CD21 in vitro (17). The T-LBL cells in our case were also positive for CD21. The PTCL-NOS cells detected in the peripheral lymph nodes were negative for CD21, as is usually the case for mature T-cell lymphomas. However, we demonstrated that both lymphomas were clonally identical. Therefore, EBV was suggested to have infected the CD21-positive T-cells during the thymocyte differentiation stage, and the EBV-infected T-cells then subsequently developed into T-LBL and PTCL (Fig. 2E). Even though the PTCL cells were CD21-negative, some EBV-positive PTCL might arise from EBV-infected CD21-positive T-cells.
In conclusion, our case suggests that EBV can cause composite lymphoma involving T-LBL and PTCL. Although the molecule responsible for the entry of EBV into mature T-cells in T-cell lymphoma has not been clarified, this case suggests that EBV might infect T-cells that express CD21 during the immature stage, and CD21-negative PTCL might subsequently arise through phenotypic changes. Further investigation of the role of EBV in immature T-cell malignancies and the pathogenesis of EBV-related T-cell lymphoma is warranted.
Informed consent for the diagnostic and treatment procedures was obtained from the patient. An autopsy limited to the axillary lymph nodes and lungs was performed after receiving informed consent from the patient's family.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported by a grant from JSPS KAKENHI (19K17839) to HH.
References
- 1.Young LS, Yap LF, Murray PG. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer 16: 789-802, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Fox CP, Shannon-Lowe C, Rowe M. Deciphering the role of Epstein-Barr virus in the pathogenesis of T and NK cell lymphoproliferations. Herpesviridae 2: 8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutt-Fletcher LM. Epstein-Barr virus entry. J Virol 81: 7825-7832, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun M, Melchers I, Peter HH, Illges H. Human B and T lymphocytes have similar amounts of CD21 mRNA, but differ in surface expression of the CD21 glycoprotein. Int Immunol 10: 1197-1202, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Castillo JJ, Reagan JL, Bishop KD, Apor E. Viral lymphomagenesis: from pathophysiology to the rationale for novel therapies. Br J Haematol 165: 300-315, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Hosoi H, Imadome KI, Tamura S, et al. An Epstein-Barr virus susceptible immature T-cell line, WILL4, established from a patient with T-lymphoblastic lymphoma bearing CD21 and a clonal EBV genome. Leuk Res 55: 1-5, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Miki T, Kawamata N, Arai A, et al. Molecular cloning of the breakpoint for 3q27 translocation in B-cell lymphomas and leukemias. Blood 83: 217-222, 1994. [PubMed] [Google Scholar]
- 8.Langerak AW, Moreau E, van Gastel-Mol EJ, van der Burg M, van Dongen JJ. Detection of clonal EBV episomes in lymphoproliferations as a diagnostic tool. Leukemia 16: 1572-1573, 2002. [DOI] [PubMed] [Google Scholar]
- 9.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17: 2257-2317, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Kuppers R, Duhrsen U, Hansmann ML. Pathogenesis, diagnosis, and treatment of composite lymphomas. Lancet Oncol 15: e435-e446, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Suefuji N, Niino D, Arakawa F, et al. Clinicopathological analysis of a composite lymphoma containing both T- and B-cell lymphomas. Pathol Int 62: 690-698, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Niino D, Ohsaki K, Arakawa F, et al. Composite T lymphoblastic leukemia/lymphoma and diffuse large B-cell lymphoma: case report. Pathol Int 61: 363-368, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Guan C, Li J, Xu P, Ouyang J, Chen B. Composite primary breast diffuse large B-cell lymphoma and T lymphoblastic leukemia/lymphoma: report of a case and review of literature. Int J Clin Exp Pathol 8: 9629-9637, 2015. [PMC free article] [PubMed] [Google Scholar]
- 14.Markow M, Mirza AS, Perez L, et al. Transformation of T-cell acute lymphoblastic lymphoma to peripheral T-cell lymphoma: a report of two cases. Case Rep Hematol 2018: 9191582, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushton CK, Arthur SE, Alcaide M, et al. Genetic and evolutionary patterns of treatment resistance in relapsed B-cell lymphoma. Blood Adv 4: 2886-2898, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the Rosetta Stone of therapy resistance. Cancer Cell 37: 471-484, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer EM, Mouhoub A, Maillet F, et al. Expression of CD21 is developmentally regulated during thymic maturation of human T lymphocytes. Int Immunol 11: 1841-1849, 1999. [DOI] [PubMed] [Google Scholar]