Abstract
After a century, it's time to turn the page on understanding of lactate metabolism and appreciate that lactate shuttling is an important component of intermediary metabolism in vivo. Cell‐cell and intracellular lactate shuttles fulfil purposes of energy substrate production and distribution, as well as cell signalling under fully aerobic conditions. Recognition of lactate shuttling came first in studies of physical exercise where the roles of driver (producer) and recipient (consumer) cells and tissues were obvious. Moreover, the presence of lactate shuttling as part of postprandial glucose disposal and satiety signalling has been recognized. Mitochondrial respiration creates the physiological sink for lactate disposal in vivo. Repeated lactate exposure from regular exercise results in adaptive processes such as mitochondrial biogenesis and other healthful circulatory and neurological characteristics such as improved physical work capacity, metabolic flexibility, learning, and memory. The importance of lactate and lactate shuttling in healthful living is further emphasized when lactate signalling and shuttling are dysregulated as occurs in particular illnesses and injuries. Like a phoenix, lactate has risen to major importance in 21st century biology.

Keywords: exercise, fibre type, gene adaptation, gluconeogenesis, glycogenolysis, indirect pathway, lactate shuttle, lactate signalling, microbiome, muscle, postabsorptive metabolism, postprandial metabolism, satiety
Abstract figure legend: Roles of lactate in physiology and metabolism elaborated as: cell fuelling (the preferential use of lactate over other fuel energy substrates such as glucose, fatty and keto acids in cardiac and red skeletal muscle and brain for cognition); cell signalling (the mechanisms by which lactate affects metabolism via effects on cell redox, ROS production, lactylation and covalent binding); microbiome (the role of fermentation in health and disease and the possibility of a gut‐soma lactate shuttle); satiety (the role of lactate on the arcuate nucleus of the hypothalamus and gut‐hypothalamic signalling); adaptation (the role of lactate in expression of glycolytic and metabolic enzymes and insulin sensitivity, the latter by TGF‐β); reproduction (roles of lactate in sperm mitochondrial energetics, developing a favourable microenvironment for blastocysts and secretion of VEGF for uterine angiogenesis), and resuscitation (treatments of dehydration, acidosis, dengue, pancreatitis and hepatitis, myocardial infarction and wound healing).

Introduction
The story of lactate and its role in physiology and medicine may be a century old, but has changed dramatically in the last three decades (Brooks, 1986, 2002, 2018; Gladden, 2004). No longer conceived of as a dead‐end metabolite, a fatigue agent, or metabolic poison, in contemporary physiology, lactate is seen as a major metabolic intermediate that has wide ranging impacts in energy substrate utilization, cell signalling, and adaptation; simply, lactate is at the fulcrum of metabolic integration (Brooks, 1984, 1986, 2020 a). Now, rather than regarded as an oddity of exercise metabolism (Gladden, 2004; Chen et al. 2016; Hui et al. 2017; Brooks, 2018; Ferguson et al. 2018), the presence of lactate shuttling is recognized in fields as diverse as wound healing (Hunt et al. 2007), cancer biology (San‐Millan & Brooks, 2017), insulin secretion (Rutter et al. 2015), management of sepsis (Garcia‐Alvarez et al. 2014 a), learning and memory (Suzuki et al. 2011; El Hayek et al. 2019), and treatment of traumatic brain injury (TBI) (Brooks & Martin, 2014). Hence, in contemporary biology, the role of lactate in metabolism needs to be understood and viewed as a ‘phoenix risen’ (Brooks, 2002, 2018) in contrast to a remnant of (1988) early 20th century biology (Rabinowitz & Enerback, 2020).
As scholars we have our limitations and, as a consequence, in formal education we rely on tradition to a certain extent despite obvious evidence to the contrary. Commencing with work of early 20th century Nobel prize winners (Hill, 1914; Meyerhof, 1920), the role of lactate in metabolism has been misunderstood and perpetuated. Even as their cell and tissue cultures incubated in air often turned acidic overnight, textbook authors (e.g. Lehninger, 1970) perpetuated the myth of oxygen‐limited metabolism giving rise to lactate formation despite the fact that the partial pressure of oxygen in air over their mitochondrial preparations and culture dishes was 3–5 times higher than in vivo (Brooks et al. 2019). Hence, a reckoning of minds in biology is necessary to understand human and mammalian metabolism in a contemporary context.
Take for instance conflations of terms ‘glycolysis’ and ‘fermentation.’ In mammalian tissues, glycolysis (i.e. conversion of glucose and glycogen to lactate) is very different from aerobic fermentation of sugar to alcohol by yeast, or the production of swamp gases by anaerobic bacteria as occurs in the colon, at wound sites or in rotting (Brooks, 2018, 2020 c; Ferguson et al. 2018). Conflation of the terms perpetuates the myth of lactate poisoning and thus handicaps understanding of basic biology and its translation. Fortunately, textbook authors are noting a distinction between glycolysis as occurs in animals in vivo, and fermentation processes that occur in microbes giving rise to ethanol and foul gases (Urry et al. 2020).
Lactate shuttling: roles of driver and recipient cells
Like other metabolites, lactate flux between cells and tissue beds depends on concentration and hydrogen ion (pH) differences. Lactate exchanges between and among cells are facilitated by the presence of cell membrane lactate transport proteins termed monocarboxylate transporters (MCTs) (Garcia et al. 1994, 1995); MCTs are bidirectional symporters (Roth & Brooks, 1990a , b ; Brown & Brooks, 1994) sensitive to trans‐stimulation by lactate and hydrogen ion gradients. Hence, we have the concept of glycolytic lactate producing (driver) and lactate consuming (recipient) cells (Fig. 1). From first observations on dog gracilis muscle preparations that showed net lactate release at rest, an increased release when contractions started followed by net lactate uptake as contractions continued (Stainsby & Welch, 1966), the concepts of lactate driver and recipient cells could have been predicted. Subsequently, similar results of net lactate release from resting muscle, followed by increased release and switch to uptake during exercise were confirmed in human muscles (Stanley et al. 1985; Bergman et al. 1999 b). Again, from such results, the presence of lactate driver and recipient cells as part of lactate shuttling could be inferred. Not surprisingly, we now know not only that driver and recipient cells can switch roles depending on conditions, but that some cells can exchange lactate through the interstitium and vascular beds. For instance, during exercise, fast white fibres can provide oxidizable substrate to red, oxidative fibres in the same tissue bed (Baldwin et al. 1972; Hooker & Baldwin, 1979). Conversely, postprandial glucose uptake in red fibres can provide substrate to the body corpus as in the glucose paradox (Foster, 1984). Also, working muscle can fuel the beating heart (Gertz et al. 1981), brain (Suzuki et al. 2011; Glenn et al. 2015 a; Steinman et al. 2016) and provide gluconeogenic substrates to the splanchnic organs (Bergman et al. 2000; Gerich et al. 2001).
Figure 1. The concept of lactate shuttling between producer (driver) cells and tissues and consumer (recipient).

By this mechanism lactate has autocrine‐, paracrine‐ and endocrine‐like influences on metabolism that fulfil at least three purposes. Lactate is: (1) a major energy source; (2) the major gluconeogenic precursor; and (3) a signalling molecule. Revised from Brooks (2018).
Lactate exchanges among muscle, heart, liver and kidneys are obvious examples of lactate shuttling. However, are other organs involved? Some might argue that the integument is the largest organ in the body, and it is known to produce lactate under sympathetic stimulation (Johnson & Fusaro, 1972). Hence, is there a skin‐muscle lactate shuttle during exercise (Brooks, 2018)? Similarly, the gut, the only organ of fermentation in the body, produces lactate because of the presence of bacteria and dietary fibre in the colon may release lactate into the systemic circulation. Hence, we can speculate on the presence of a gut to soma lactate shuttle (Brooks, 2018). As there are no obvious venous drainage vessels to catheterize, other methods to detect lactate exchange in the integument and gut will need to be found to quantify their roles in lactate shuttling under various conditions.
Contributions from muscle and exercise physiology (the cell‐cell and intracellular lactate shuttles)
The cell‐cell lactate shuttle
As is often the case, it is difficult to identify a seminal moment of discovery or publication, but from this perspective, it was results on dog muscles made to contract in situ (Stainsby & Welch, 1966). At rest, those highly red, richly perfused muscles always released lactate. Then, at the onset of contractions, lactate release increased, but then switched to net uptake as contractions continued and oxygen consumption rose (vide supra). So, in a kernel, we have it, muscle lactate production under fully aerobic conditions followed by uptake as oxygen consumption rises to meet metabolic demand. Subsequently, using NADH fluoroscopy, investigators (Jöbsis & Stainsby, 1968) verified that working muscles were not oxygen limited. Later efforts to study working muscle lactate metabolism (Honig et al. 1992) using myoglobin cryomicroscopy again showed ‘aerobic glycolysis’ in working muscle. Importantly, Gladden and associates determined effects of exercise intensity, blood lactate concentration, O2 consumption and pH on lactate production and disposal using arterial‐venous differences ((a‐v)), blood flow and 14C‐lactate measurements on resting and contracting canine muscles in situ (Gladden, 1991; Gladden et al. 1994; Kelley et al. 2002). In their studies investigators showed lactate turnover (production and disposal) in resting and contracting, fully oxygenated and circulated canine muscle (for reviews see Gladden, 2004; Rogatzki et al. 2015).
The advent of radiotracers and their use in physiology and metabolism soon showed continuous lactate production under fully aerobic conditions in dogs (Depocas et al. 1969) and rats (Freminet et al. 1974; Donovan & Brooks, 1983), with disposal during exercise by oxidation and conversion to glucose (Brooks & Donovan, 1983). Moreover, in studies on rats recovering from exercise, the so called ‘oxygen debt’ (now referred to as EPOC: Gasser & Brooks, 1984) period showed oxidative disposal (4/5) predominating over gluconeogenesis and glyconeogenesis (1/5) (Brooks et al. 1973; Brooks & Gaesser, 1980; Gaesser & Brooks, 1980). Compared to the classic results of Meyerhof on isolated frog muscle preparations ex vivo (Meyerhof, 1920), results on rats in vivo upended the long‐held idea that after exercise 4/5 of the lactate produced during contractions was restored to muscle glycogen in situ.
Initial efforts to study lactate‐glucose interactions in humans involved (a‐v) and blood flow measurements across working muscle and splanchnic tissue beds (Ahlborg & Felig, 1982; Wahren & Ekberg, 2007). Such experiments are highly invasive and difficult to conduct and interpret. Nonetheless, the significance of the technologies employed and lessons learned from those difficult experiments showing inter‐organ metabolite exchanges in resting and exercising humans are noteworthy.
The advent of stable, non‐radioactive tracers and their use in physiology and metabolism soon replicated what was shown in mammalian models (Stanley et al. 1985, 1986; Mazzeo et al. 1986). Subsequently, combined use of (a‐v) differences and blood flow, as well as isotopic tracers, showed simultaneous lactate production and oxidative disposal within resting and working human skeletal muscles (Stanley et al. 1986; Bergman et al. 1999 b). More recently, the same technologies have been employed to show lactate turnover (production, net uptake and oxidative disposal) in the heart (Gertz et al. 1981, 1988; Bergman et al. 2009 b) and brain (Glenn et al. 2015 a).In their studies of muscle lactate metabolism Richardson and colleagues made ingenious use of repeated studies of muscle net balance ((a‐v)Δ and blood flow) and nuclear magnetic resonance (NMR) to determine intracellular from the myoglobin spectrum (Richardson et al. 1998).
Their results showed lactate production in, and release from, resting and exercising muscle when intracellular was significantly above the critical mitochondrial oxygen tension as previously determined (Wilson et al. 1988). Similarly, with their MRS experiments on rat heart preparations, Kreutzer and Jue demonstrated myocardial lactate formation above an intracellular that was well above the threshold for decreased ATP production (0.80 mmHg) (Kreutzer & Jue, 1995). Thus, while there is no singular value that would imply a threshold for increased lactate production in human muscle or rat heart, it is certain that lactate is formed under fully aerobic, normoxic conditions (Connett et al. 1990).
Complimentary to determinations of lactate disposal via oxidation were those determining lactate disposal via gluconeogenesis in resting postabsorptive humans. Hence, it is fair to state that while most lactate disposal is accomplished via oxidation, with a minority of lactate disposal accomplished via gluconeogenesis under postabsorptive resting conditions, lactate is the most important gluconeogenic precursor during rest and exercise (Stanley et al. 1988; Bergman et al. 2000; Gerich et al. 2001)
In sum, the above‐cited work shows continuous aerobic (not oxygen‐limited) lactate turnover (production and disposal) in humans and mammalian model systems. Further, the work shows two of three features of the lactate shuttle – lactate production in driver cells and disposal in recipient cells and tissues; signalling being a third feature of the lactate shuttle (Brooks, 2000, 2002, 2018). Hence, we now realize that lactate produced in working muscle is a major fuel energy source (Stanley et al. 1986; Bergman et al. 1999 b), but also that lactate release from working muscle fuels other organs such as the heart (Gertz et al. 1988; Bergman et al. 2009 b) and brain (Glenn et al. 2015 a) normally, but also in illness and following injury (Marik & Bellomo, 2013; Brooks & Martin, 2014; Garcia‐Alvarez et al. 2014 b). Moreover, lactate released from working muscles (Stanley et al. 1986; Bergman et al. 1999 b) and other driver cells such as the integument is the major gluconeogenic precursor (Stanley et al. 1988; Bergman et al. 2000) (Fig. 2).
Figure 2. Illustration of lactate shuttling during exercise (the cell‐cell lactate shuttle).

Lactate released from muscles, skin and other driver cells provides energy for working muscles (Stanley et al. 1986; Bergman et al. 1999 b), heart (Gertz et al. 1981, 1988; Bergman et al. 2009 b) and brain (Glenn et al. 2015 a). Moreover, lactate released from working muscles (Stanley et al. 1986; Bergman et al. 1999 b) and other driver cells such as adipose tissue is the major gluconeogenic precursor (Stanley et al. 1988; Bergman et al. 2000) and brain fuel, even after injury when lactate supplementation may be efficacious (Brooks & Martin, 2014). Revised from Brooks (2018).
The intracellular lactate shuttle (ICLS)
The above‐cited studies using infused 13C‐lactate and observing 13CO2 excretion from resting and working muscles show simultaneous lactate uptake, production, and oxidation in vivo (Stanley et al. 1986; Bergman et al. 1999 b). However, those studies do not provide information on the intracellular path of lactate oxidation. Hence, studies on isolated mitochondria (Brooks et al. 1999 c, 1999 d; Dubouchaud et al. 2000; Hashimoto et al. 2006, 2008; Atlante et al. 2007; Passarella et al. 2008, 2014) and muscles (Park et al. 2015) were called for.
To understand mitochondrial lactate oxidation in vivo, one needs to appreciate that the cellular respiratory apparatus is composed not of discrete vesicles (i.e. mitochondria), but rather an extensive network, a mitochondrial reticulum (Bakeeva et al. 1978; Kirkwood et al. 1986, 1987) that extends from the sub‐sarcolemmal domain to deep within fibres, that represents an ‘energy highway’ (Glancy et al. 2015). To oxidize energy products of glycolysis, the reticulum contains both lactate (mMCT) (Brooks et al. 1999 c) and pyruvate (mPC) transporters (Bricker et al. 2012; Herzig et al. 2012). Importantly, because the product of glycolysis is lactate, not pyruvate (Rogatzki et al. 2015), mitochondrial uptake and oxidation far exceeds that of pyruvate. Hence, studies of muscle lactate oxidation led to discovery of the mitochondrial lactate oxidation complex (mLOC) in vivo.
In terms of functionality, the mLOC contains several essential components of lactate oxidation: an MCT, its membrane chaperone Basigin (BSG or CD147), lactate dehydrogenase (LDH), and cytochrome oxidase (COx) as seen in muscle (Hashimoto et al. 2006), liver (De Bari et al. 2004; Passarella et al. 2014), and brain (Hashimoto et al. 2008), and various model systems such as brain slices (Schurr, 2008), primary neuronal cultures (Atlante et al. 2007; Hashimoto et al. 2008), normal breast and transformed breast cancer cells (Hussien & Brooks, 2011), and tumours (Sonveaux et al. 2008). Colocalization of MCTs, LDH and COx has been seen in muscles (Hashimoto et al. 2005, 2007, 2013), brain (Hashimoto et al. 2008), lung (Johnson et al. 2012), and cancer cells (Hussien & Brooks, 2011).
Confirmation of the preference of mitochondrial lactate over pyruvate oxidation comes from studies of hyperpolarized lactate in muscles in situ (Park et al. 2015). So far as mitochondrial pyruvate transport and oxidation are concerned, it is known that the putative mPC colocalizes with mitochondrial MCT1 in L6 myocytes. In preliminary studies, colocalization analysis of mMCT1 and mPC1 using Imaris software revealed that both MCT1 and mPC are co‐localized to the mitochondria (r 2 = 0.8) (Brooks, 2020c ). However, because muscle lactate concentration exceeds that of pyruvate by one (10×) to two (200–400×) orders of magnitude in resting and exercising human muscles, respectively (Henderson et al. 2004), lactate oxidation is dominant in vivo.
As will be discussed in more detail later, an important feature of lactate shuttling is that it affects cell redox in both driver and recipient cells, and between cell compartments. At present there is discussion over whether the ICLS functions independently of or cooperatively with longer established malate‐aspartate and glycerol phosphate shuttles (Kane, 2014). Given redundancies in physiological control systems, it may be that cytosolic‐to‐mitochondrial shuttles work in parallel to manage the lactate load and balance cell redox during exercise and other conditions. Using CRISPR knockout technology in mouse or other mammalian models may provide missing data on the relative uses of specific intracellular redox shuttles under particular physiological circumstances (Kane, 2014).
Lactate shuttling as a basis for the lactate threshold in physical exercise
It has been half a century since introduction of the concept of oxygen‐limited metabolism producing an ‘anaerobic threshold’ (AT), that is, O2 limited metabolism during exercise of a particular intensity. The subject has been recently reviewed (Poole et al. 2021). With 1541 citations (Web of Science, 27th September, 2020) the paper of (Wasserman & McIlroy, 1964) is the eighth most cited paper of all time in the Journal of Applied Physiology. As recited in the recent review (Poole et al. 2021), in a letter from Karlman Wasserman to this author, the concept of an ‘anaerobic threshold’ was based on the assumption that ‘failure of the heart to transport O2 adequately would result in lactic acidosis’, an event that would trigger ventilatory compensation. Subsequently, concepts of anaerobic, lactate, ventilatory and other related thresholds (e.g. OBLA) are pervasive in the literature and have represented key concepts in basic and applied physiology, sports and pulmonary medicine. These thresholds are determined by turnpoints in the rise in blood lactate concentration, pulmonary minute ventilation, and heart rate during graded exercise. In a PubMed (National Library of Science) search on 20th October, 2020 the term ‘anaerobic threshold’ elicited 5902 citations. In their review, Poole et al. explain that while the basic tenant of the AT (i.e. oxygen‐limited metabolism) in exercise is no longer acceptable, use of the ‘gas exchange threshold’ (GET) in graded exercise possesses significant importance for cardio‐pulmonary and other types of evaluation in patients (Poole et al. 2021).
In sum, intracellular lactate disposal is accomplished by a transport mechanism for direct mitochondrial uptake and oxidation that involves a mLOC which probably works in parallel with malate‐aspartate, glycerol‐phosphate and other shuttles to balance cell redox and manage the lactate load from glycolysis under normal and stressful conditions (Brooks, 2020c ).
Contributions from studies of postprandial metabolism: the glucose paradox (indirect pathway of liver glycogen synthesis) and the postprandial lactate shuttle
The glucose paradox
Studies of postprandial glucose metabolism on rodent models and humans show what has been termed the ‘glucose paradox,’ or ‘indirect pathway of hepatic glycogen synthesis’ (Foster, 1984). This concept states that dietary glucose released into the hepatic portal vein initially bypasses the liver and goes to the periphery, where glycolysis converts glucose to lactate that is subsequently released into the central venous circulation and taken up from the arterial circulation by liver for glycogen synthesis. This, paradoxical, ‘indirect’ pathway of hepatic glycogen synthesis is to be contrasted with the ‘direct’ pathway in which dietary glucose from the gut is taken up and converted to liver glycogen on first circulatory pass (Fig. 3).
Figure 3. Illustration of lactate shuttling after consuming dietary carbohydrate: the postprandial period (the postprandial lactate shuttle).
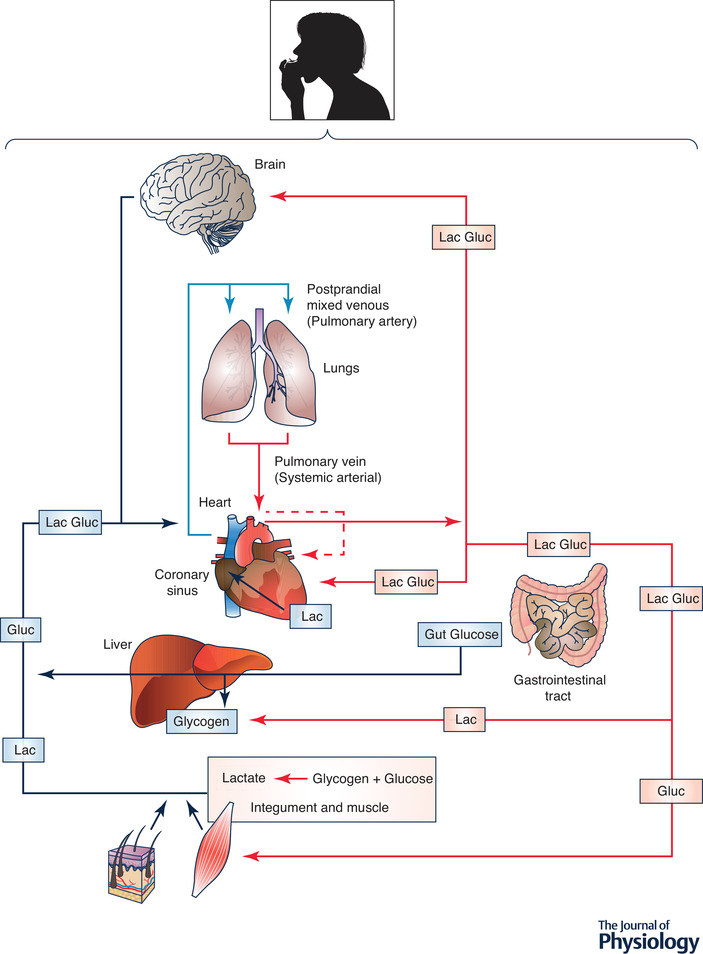
Depending on liver glycogen content some dietary glucose bypasses liver and enters the systemic circulation. From there glucose is taken up by non‐contracting muscles, particularly red and intermediate fibres (James et al. 1985, 1986). Glycolysis in these and other driver tissues results in lactate release into the central venous circulation and uptake by the recipient liver from the arterial circulation. Paradoxically, this circuitous, ‘indirect pathway,’ is preferred over glucose for hepatic glycogen synthesis (Foster, 1984). In contrast, glucose from dietary consumption or digestion or carbohydrate in the GI tract can be taken up by liver on the first circulatory pass for ‘direct’ glycogen synthesis. Again as indicated in Fig. 2, lactate released from driver cells and tissues supports cerebral metabolic needs (Glenn et al. 2015 a, b ) and functions such as glutamatergic signalling (Pellerin & Magistretti, 1994). Similarly, systemic lactate and fatty acids from digestion serve as an energy source for the heart (Bergman et al. 2009a,b). Figures 2 and 3 illustrate the extent of lactate shuttling under diverse conditions.
The initial concept developed from studies on lab animals has been replicated in human subjects showing both indirect and direct liver glycogen synthesis in healthy, postprandial humans. However, the balance of indirect and direct glucose conversion appears to be species related. Results of Gerich and colleagues in human subjects confirm that glycolysis is the main initial postprandial fate of glucose, accounting for ≈66% of overall disposal, while oxidation and storage each accounted for ≈45%. However, the majority of hepatic glycogen synthesis in postprandial humans (≈73%) was formed via the direct pathway (Woerle et al. 2003). In the near future it should be possible to better understand the issue of influences of diet and other factors on the balance of direct vs. indirect liver and muscle glycogen synthesis in humans using 13C‐tracers and magnetic resonance spectrometry (Stender et al. 2020).
While considered to be a homogenous ‘organ system,’ muscle is in fact a heterogeneous tissue containing different types of muscle fibres, and circulatory and connective tissue networks, all with different metabolic characteristics (Barnard et al. 1970, 1971). Postural muscles (e.g. soleus, erector spinae) are alternatively termed intermediate (i.e. pink, not red or white), or Type I fibres. In many species deep vastus and lateral gastric are bright red and termed red or Type IIA fibres. In contrast white, fast twitch fibres are termed Type IIX (in humans) or IIB (in rodents). Hence, results of the above‐cited studies on the glucose paradox are complimented by results of studies showing greater postprandial perfusion and glucose uptake in muscles containing predominantly oxidative Type I and IIA fibres than in muscles containing predominantly Type IIB/X fibres (James et al. 1985, 1986). Redundancy of terminology in science is confusing, but the glucose paradox can also be thought of as a postprandial lactate shuttle.
Phasic lactate shuttling: lessons from the heart
Tracer studies allow us to know that the heart produces lactate even as it acts as a lactate sink displaying net uptake from the arterial circulation (Gertz et al. 1988; Bergman et al. 2009 b). Assessing cardiac metabolism in humans is difficult at best and is complicated by the cardiac cycle. If it is true that even in a healthy heart systole interrupts coronary blood flow, especially in the endocardium, then the presence of a phasic intra‐cardiac lactate shuttle is indicated. Assuming a resting heart rate of 60 bpm, then systole followed by diastole occurs once per second. This could mean that there occurs 200 ms of stopped flow during the isometric phase of systole which is powered by glycolysis, followed by 800 ms of diastole for oxidative recovery and lactate clearance. Then heart rate increases during exercise, and time for the T‐P interval decreases, so also does the time for restoration of myocardial blood flow and intra‐cardiac oxidative metabolism decrease. Again, as with the hypothesis of gut lactate shuttling, the presence of an intra‐cardiac lactate shuttle would be difficult to prove because even coronary sinus sampling would be insufficient to determine phasic events. Hopefully in the future, hyperpolarized MRS (Park et al. 2015) or other technologies will allow for detection of lactate production, net uptake and oxidative disposal within a cardiac cycle.
For the present, perhaps the best evidence of a phasic, intra‐cardiac lactate shuttle may come from the lactate threshold/turn point field in which a steep rise in blood lactate occurs at 90% of maximal heart rate (Hofmann et al. 1994). Even in the healthy hearts of athletes with high heart rates, there simply may be inadequate time for intra‐myocardial lactate disposal plus a burden of systemic blood lactate clearance.
Can the splanchnic bed be a source of lactate?
Upper gut: the liver
Classically, the liver removes lactate from the circulation and converts it to glucose for release into the systemic circulation (Cori & Cori, 1946), or converts lactate to liver glycogen (Nilsson & Hultman, 1974). But, can the liver, or splanchnic bed as a whole contribute lactate to the systemic circulation? Evidence for splanchnic lactate production is sparse.
Hepatic release from dog liver under glucagon stimulation was not seen (Wasserman et al. 1984). However, after glucose ingestion in rats with indwelling portal vein catheters, a porto‐peripheral lactate gradient was present, reflecting the production of lactate in or by the intestine (Smadja et al. 1988). Unfortunately, because studies on humans would involve hepatic portal vein catheterization, an invasive procedure not known to be associated with any disease state, and hence probably ethically unjustified, we know of no report of hepatic or splanchnic net release during postprandial exercise. However, because the phenomenon of splanchnic lactate release has not been observed, it doesn't necessarily follow that the phenomenon fails to exist.
In addressing the issue of upper gastrointestinal (GI) lactate production in humans, consideration of the carbohydrate energy form may be helpful. Using combinations of glucose, fructose and lactate tracers to evaluate the use of oral carbohydrate energy sources in sports drinks investigators in the Tappy lab (Lecoultre et al. 2010; Theytaz et al. 2014) observed carbon atoms from an orally ingested fructose tracer appearing in the systemic circulation as labelled lactate. Hence, counter to classic thought, there is evidence for postprandial splanchnic lactate release in humans following the ingestion of one carbohydrate energy source, fructose.
In sum, like the event of physical exercise in which lactate plays a prominent role in energy substrate production and disposal, lactate also plays an important role in carbohydrate distribution and disposal after eating. Hence, whether after a meal when glycogen storage is prominent, or during post‐absorptive exercise when glycaemia is supported by hepatic glycogenolysis and gluconeogenesis, lactate plays important roles in carbohydrate (CHO) energy substrate distribution (Brooks, 2020b ). The issue of GI lactate production is addressed further in the section on the microbiome below.
Lower gut: the microbiome
The possibility of participation of the gut microbiome in lactate shuttling and a gut‐soma lactate shuttle has previously been suggested (Brooks, 2018, 2020 a), but we are in the early stages of discovery. Support for the idea is to be found in diverse sources. From epidemiology, we know that regular physical exercise is beneficial for reducing risks of many common cancers including those of the colon (Powell et al. 2018). Also, from nutrition science we know that pre‐ and pro‐biotic dietary components favourably affect gut fermentation and health (Hill et al. 2014) and data indicate relationships between microbiota and the prevalence of insulin resistance and metabolic syndrome (Vrieze et al. 2012). The concentration of lactate in human faeces is relatively low (< 5 mm) because of the presence of bacteria that convert lactate to butyrate (Duncan et al. 2004). More recently, we have learned that physical exercise and physical fitness encourage diversity of species including those that act to convert lactate to butyrate (Estaki et al. 2016, 2020). Recently also, the presence of ‘performance‐enhancing’ gut microbes, members of the genus Veillonella, in the stools of marathon runners (Scheiman et al. 2019) have been found. Unfortunately, those investigators were unaware of the importance of lactate shuttling during exercise, and did not consider a scenario in which the gut supplies lactate, a fermentation product that is exported via sodium‐mediated monocarboxylate transporters (sMCT) (Coady et al. 2004; Teramae et al. 2010), thus supporting athletes’ efforts by fuelling working muscles as opposed to clearing lactate from the circulation.
Lactate controls lipid metabolism: the role of lactate in metabolic flexibility and the crossover concept
Lactate from working muscle (Bergman et al. 1999 b) or other tissues such as the integument (Johnson & Fusaro, 1972) has short‐term effects on lipid metabolism by downregulating both lipolysis and mitochondrial free fatty acid entry and oxidation. In contrast, chronic exercise exposure, as with endurance training, improves capacities to maintain glycaemia and lipid oxidation by improving the ability to clear lactate by oxidation (Bergman et al. 1999 b) and gluconeogenesis (Bergman et al. 2000), as well as acting as a pseudo‐myokine (Takahashi et al. 2019) (vide infra). The role of lactate in affecting energy substrate partitioning in exercise and other conditions is imbedded in concepts of ‘crossover’ (Brooks & Mercier, 1994) and metabolic flexibility (Kelley et al. 1999; Goodpaster & Sparks, 2017; San‐Millan & Brooks, 2018). In the case of lactate and lipolysis in adipose tissue, during hard exercise inverse relationships between blood lactate and plasma free fatty acid concentration [FFA] in humans (Brooks & Mercier, 1994) and other mammals (Issekutz & Miller, 1962; Rodahl et al. 1964) have long been recognized. Also, lactate infusion into running dogs caused the circulating [FFA] to decline (Issekutz & Miller, 1962; Gold et al. 1963; Miller et al. 1964). In those investigations an effect of lactate on circulating [FFA] could be clearly observed, but whether the mechanisms involved hydrogen ions or lactate anions was not assessed.
The mechanism by which lactataemia suppresses circulating FFAs is now known to be due to suppression of adipose lipolysis by lactate binding to hydroxycarboxylic acid receptor 1 (HCAR‐1), formerly known as G‐protein coupled receptor 81 (GPR‐81), independently of changes in pH (Cai et al. 2008; Ge et al. 2008; Liu et al. 2009; Ahmed et al. 2010). Signalling effects of lactate on HCAR‐1 are further discussed below.
Lactate and pyruvate as inhibitors of mitochondrial β‐oxidation
When glycolysis is accelerated during muscle contraction, lactate (L) and pyruvate (P) concentrations rise and increase the L/P ratio, (Bergman et al. 1999 b; Henderson et al. 2004) reducing cytosolic redox (Brooks, 2020a ). At rest, the L/P ratio in muscle and venous effluent from a muscle bed approximates 10, but the ratio rises more than an order of magnitude (≥ 30×) during moderate intensity exercise (Henderson et al. 2004). By mass action the monocarboxylate pair floods into the mitochondrial reticulum (Saddik et al. 1993; Brooks et al. 1999 b; Passarella et al. 2008), giving rise to acetyl‐CoA and, thereby, malonyl‐CoA formation. The rise in malonyl‐CoA inhibits the entry of activated FFAs into the mitochondrial matrix by inhibiting carnitine‐palmitoyl transferase‐1 (CPT1) (McGarry et al. 1977; Saddik et al. 1993). As well, the effect of glycolysis driving an accumulation of acetyl‐CoA downregulates β‐ketothiolase, the terminal and rate‐limiting enzyme of the mitochondrial β‐oxidation pathway. Hence, by mass action, allosteric binding and effects on cell redox, lactate acts to prevent entry of activated fatty acids into the matrix of the mitochondrial reticulum, thus limiting β‐oxidation of fatty acid derivatives.
Lactate shuttling and metabolic signalling
The effects of increases in cell work and lactate production on redox, reactive oxygen species (ROS), allosteric binding, and histone lactylation have been recently reviewed (Brooks, 2020a ). Briefly, cell work leads to lactate production and changes in the cellular lactate/pyruvate ratio (Henderson et al. 2007) that accompanies changes in the cellular NAD+/NADH ratio and subsequent metabolic and regulatory effects in driver and recipient cells (vide supra). Importantly also, additional lactate signalling mechanisms have been revealed.
Cell redox
As described above, muscle contraction causes major changes in the L/P and NADH/NAD+ ratios. In driver cells that change allows glycolysis to proceed via providing NAD+ for GAPDH, but also by reducing pyruvate to lactate in the cytosol, which creates a driving force for mitochondrial substrate oxidation. In contrast, in recipient cells uptake of lactate raises the NADH/NAD+ ratio, thus downregulating glycolysis. This preferential use of lactate over endogenous cellular energy sources reduces oxidation of glucose in muscle (Miller et al. 2002 a, b ) and heart (Wisneski et al. 1987; Gertz et al. 1988; Bergman et al. 2009 a).
Moreover, aside from the major effects of muscle contraction and glycolysis on energy substrate partitioning, glycolysis leading to lactate production and resulting change in cytosolic NADH/NAD+ also effects the status of other redox couples such as the ratio of reduced to oxidized glutathione (GSH/GSSG) (Gohil et al. 1988; Viguie et al. 1993).
ROS
Lactate can raise cellular production of ROS via mitochondrial respiration (Powers et al. 1999; Passarella et al. 2008) and non‐enzymatically via lactate‐iron interactions that are capable of generating ROS (Wagner et al. 2004). Another way in which lactate can affect ROS production is via generation of hydrogen peroxide (H2O2) (Passarella et al. 2008). The latter mechanism involves generation of H2O2 via a flavine‐dependent lactate oxidase located in the mitochondrial intermembrane space (de Bari et al. 2010).
Sirtuins
Sirtuins are deacetylases regulated by the equilibrium between nicotinamide (NAM) and NAD+. Sirtuin activation is accomplished via changes in cell redox (i.e. the NAD+/NADH) through the concentration of NAM and the activity of the enzyme NAM phosphoribosyl transferase (Nampt). Many investigators are concerned with how the subtle changes in cell redox affect cell homeostasis as occur in apoptosis, inflammation, and other processes. However, more obvious are changes in cell redox with increments in cell work as occur in exercise. At present, data are lacking on the change in Nampt activity in working muscle or non‐working recipient tissues in which changes in NAD+ and NADH concentrations are likely to affect sirtuin regulation in vivo.
Hydroxycarboxylic acid receptor 1 (HCAR‐1)
The mechanism by which lactataemia suppresses circulating FFAs is now known to be due to suppression of adipose lipolysis (Ahmed et al. 2010). Independently of pH, lactate inhibits lipolysis in fat cells through activation of HCAR‐1 (vide supra). In studies of mouse, rat, and human adipocytes, HCAR‐1 appears to act as a lactate sensor with the inhibitory effect on lipolysis operating through cyclic AMP (cAMP) and cAMP response element binding (CREB) (Ahmed et al. 2010; Bergersen, 2015).
Transforming growth factor beta 2 (TGF‐β2)
The effects of lactate on inter‐organ signalling and dual or multiple effects of lactate signalling is illustrated by the recent discovery that transforming growth factor beta 2 (TGF‐β2) is secreted from adipose tissue in response to lactate released from working muscle (Takahashi et al. 2019). Because TGF‐β2 improved glucose tolerance in mice, the authors concluded that exercise training improves metabolic regulation through an inter‐organ (adipose to liver) communication via a ‘lactate‐TGF‐β2 signalling cycle.’ If validated on studies of human subjects, TGF‐β2 could be classified as an ‘adipokine,’ and lactate could be thought of as a ‘pseudo myokine’ and the value of enhancing lactate clearance during exercise by endurance training further emphasized (Donovan et al. 1983; Bergman et al. 1999 b; Messonnier et al. 2013). In this context both short‐ and long term‐effects are illustrated because lactate is known to suppress lipolysis in adipose via HCAR‐1 and CREB (vide supra).
Lactylation of histones
Regulation of gene expression by lactylation of 28 lysine residues on histones has been demonstrated (Zhang et al. 2019). The addition of lactate in addition to phosphate, methyl and acetyl tags to histones is yet another epigenetic way by which the genome and intermediary metabolism are regulated. As noted above, lactate release from driver cells into the circulation, as occurs in physical exercise and other stressful conditions, has the potential for epigenetic modifications in diverse cells around the body during and after physical exercise.
Lactate, endoplasmic reticulum Mg2± release and mitochondrial function
Recently, Daw et al. in the Madesh lab (Daw et al. 2020) showed that lactate is an activator of Mg2+ release from endoplasmic reticulum (ER). ER Mg2+ release facilitates mitochondrial Mg2+ (mMg2+) uptake, a process facilitated by magnesium lactate transporter 2 (Mrs2). Investigators observed that a surge in mitochondrial Mg2+ uptake promotes inflammation and organ failure by interfering with mitochondrial energetics. In contrast, suppression of the mMg2+ surge alleviates inflammation‐induced multi‐organ failure. Together, these findings reveal that lactate mobilizes intracellular Mg2+ and links the mMg2+ transport machinery with major metabolic feedback circuits and mitochondrial bioenergetics (Daw et al. 2020). Hence, on the basis of the work by Daw et al., it is apparent that, in addition to mass action, redox control, and ROS generation, lactate may affect mitochondrial energetics in other ways such as by Mg2+ release from endoplasmic reticulum.
The purported effects of lactate signalling HCAR‐1, TGF‐β2, Nampt, mMg2+ and lactylation of histones observed in rodent and cell models await validation in humans. However, for the present, it is certain that lactate both inhibits lipolysis and mitochondrial FFA oxidation in the short‐term and in the long‐term stimulates mitochondrial biogenesis and glucose tolerance and lipid oxidation in in vivo (Takahashi et al. 2019). Therefore, the new results are encouraging as the signalling patterns are consistent with what is known about the roles of lactate in physiology and metabolism.
A plethora of lactate shuttles and diverse roles in physiology and metabolism
Since recognition of the presence of lactate shuttling within and among various cells, tissues and organs such as muscle, heart and liver (Brooks, 1984, 1985, 1986, 2002, 2018), the concept has been extended to include other cells, tissues and organs such as brain (Pellerin et al. 1998; Liu et al. 2017), lung (Johnson et al. 2011, 2012), sperm (Storey & Kayne, 1977; Boussouar & Benahmed, 2004), adipose tissue (Cai et al. 2008; Liu et al. 2009; Ahmed et al. 2010), and peroxisomes (McClelland et al. 2003). The presences of multiple lactate shuttles with diverse functions in physiology has been recently summarized (Brooks, 2009), but for completeness the following are mentioned.
The astrocyte‐neuron lactate shuttle and brain functioning
Lactate shuttling between astrocytes and neurons linked to glutamatergic signalling was recognized by Pellerin, Magistretti and colleagues who used the term ‘astrocyte‐neuron lactate shuttle’ (ANLS) (Magistretti et al. 1999). Since its introduction and further exposition (Pellerin & Magistretti, 2012), the concept has remained controversial (Patel et al. 2014). However, subsequent studies show wide expression of brain MCTs (Pellerin et al. 2005) and support the presence of an ANLS.
While cerebral lactate flux may be related to glutamatergic signalling (Pellerin & Magistretti, 1994), there are other processes that are related to lactate shuttling. For instance, in health and after injury (Glenn et al. 2015 a), as well as during physical exercise (van Hall et al. 2009; Hashimoto et al. 2018), the brain takes up and oxidizes lactate from the systemic circulation. In such cases, the lactate shuttle concept holds true and explains the exchange between muscle lactate driver and brain recipient cells.
Characteristics of cellular lactate uptake such as stereo‐specificity, concentration and pH dependence, saturation, trans‐stimulation and inhibition by competitive and non‐competitive inhibitors were recognized early on (Watt et al. 1988; Roth & Brooks, 1990a , b ; Brown & Brooks, 1994). Since MCT isoforms were cloned and sequenced (Garcia et al. 1994, 1995; Price et al. 1998) and then studied in vitro, it has been appreciated that MCTs facilitate movement of monocarboxylates other than lactate (e.g. pyruvate, β‐hydroxybutyrate and acetoacetate) across biological membranes. However, for at least two sets of reasons, these other monocarboxylates are of lesser physiological importance in terms of carbon‐energy transfer. First, other monocarboxylates do not compete as well as lactate for exchange by MCTs because of Michaelis‐Menten kinetic properties (Roth & Brooks, 1990a , b ). Secondly, physiological levels of lactate exchange competitors are far less abundant (Zinker et al. 1990; Bergman et al. 1999 b; Henderson et al. 2004). Importantly, from a physiological standpoint, MCTs do not create the conditions for lactate exchange; rather, MCTs facilitate carbon exchange between loci of driver processes such as glycolysis and glycogenolysis and recipient loci where processes such as mitochondrial respiration and gluconeogenesis are responsible for lactate consumption (Brooks, 1984, 2002). Hence, it is appropriate to recognize the importance of MCTs in cerebral lactate shuttling in cognition, learning, memory and other executive functions (Holloway et al. 2007; Suzuki et al. 2011; Steinman et al. 2016; Hashimoto et al. 2018; El Hayek et al. 2019).
Lactate and inflammation
Other than acknowledging its complexity, the role of lactate in promoting or suppressing inflammation is incompletely understood at present. As mentioned above, Daw et al. have provided evidence that lactate‐induced Mg2+ release from ER affects mitochondrial energetics and could be involved in cell death and organ systems failure (Daw et al. 2020). In contrast, others have provided evidence that ‐lactate containing solutions have efficacy as resuscitation fluids for use in a variety of conditions including metabolic acidosis (Brooks, 2018), acute pancreatitis (Wu et al. 2011; Hoque et al. 2014), hepatitis (Hoque et al. 2014), sepsis (Garcia‐Alvarez et al. 2014 b) and dengue fever (Somasetia et al. 2014). In terms of mechanism, Hoque and colleagues (Hoque et al. 2014) found that lactate binding to HCAR‐1 downregulates Toll like receptor induction of the pyrin domain‐containing protein 3 (NLRP3) inflammasome and production of IL1beta, via Arrestin beta 2 (ARRβ2) and HCAR‐1, was the mechanism by which lactate suppressed inflammation in patients with acute organ injury. Because of the above, the science and translation of lactate shuttling in inflammation needs further attention.
The peroxisomal lactate shuttle and β‐oxidation
Peroxisomes are cellular organelles found in virtually all eukaryotic cells that are involved in catabolism of substances such as very long chain (i.e. C22 and longer) fatty acids, branched‐chain fatty acids, d‐amino acids, polyamines, and reactive oxygen species such as hydrogen peroxide (H2O2) (Gladden, 2004). While it was known that β‐oxidation of very long‐chain fatty acids occurred in mammalian peroxisomes (Lazarow & De Duve, 1976), in the absence of enzyme systems such as exist in the mitochondrial matrix, it was not understood how β‐oxidation could occur in peroxisomes. Key findings were those of Baumgart et al. (1996), who showed the presence of peroxisomal LDH isoforms, and McClelland et al. (2003), who confirmed the presence of peroxisome LDH and further demonstrated that rat liver peroxisomal membranes contained MCT1 and MCT2. From there, it was possible to hypothesize that peroxisomal redox control necessary to support β‐oxidation involved a lactate‐pyruvate shuttle. Discovery of the peroxisomal lactate shuttle involving pyruvate‐lactate conversion linked to changes in the NADH/NAD+ redox couple emphasizes the critical importance of redox changes in all forms of lactate shuttles.
Spermatogenic and Sertoli‐germ cell lactate shuttles
It has been known for decades that mammalian spermatozoa use lactate as an aerobic energy source (Storey & Kayne, 1977). Noteworthy also is that in their seminal papers on the discovery of MCT1 and MCT2 Garcia et al. (1994, 1995) observed high expression of MCT1 in sperm heads as they entered the epididymis, followed by lower expression of MCT1 expression as sperm coursed through the epididymis. It was also noted that lactate stimulates respiration in ejaculated bovine sperm (Halangk et al. 1985), and further that lactate maintains bovine sperm motility when maintained ex vivo (Inskeep & Hammerstedt, 1985). By analogy, just as fast twitch‐glycolytic driver muscle fibres fuel adjacent slow twitch‐oxidative muscle fibres, Sertoli cells in testes secrete lactate to fuel sperm motility in vivo. In effect then, the Sertoli‐sperm cell relationship is the primal demonstration of cell‐cell lactate shuttling. In sperm, the mitochondrial reticulum is elaborate, large and spiral shaped, located at the midpiece (Storey & Kayne, 1977) at the base of the sperm head. The ability of sperm mitochondria to oxidize exogenously supplied lactate (Jones, 1997) is analogous to capacities for mitochondrial lactate oxidation in other tissues (Baba & Sharma, 1971; Kline et al. 1986; Brandt et al. 1987; Brooks et al. 1999 a; De Bari et al. 2004).
Not a few, but many observers
Readers may note that the contemporary literature on the role of lactate in exercise physiology and metabolism comes from only a few laboratories (Brooks, 2018; Ferguson et al. 2018), but such is not the case if a wider perspective is taken, when it is apparent that support for the biological roles of lactate come from diverse scientific and clinical fields in which solutions to many problems are addressed utilizing a variety of technologies. In addition to the above identified lactate shuttles, the importance of lactate in contemporary biology is illustrated in the following examples.
The glycogen shunt
Shulman and colleagues used proton (1H) and 13C magnetic resonance spectroscopy (MRS) to explain the rapid rate of metabolic transitions in brain after stimulation; consequently, a glycogen shunt was proposed (Shulman et al. 2001). By this model, shunting a portion of glucose from the circulation into glycogen with subsequent glycogenolysis provides for lactate production by two parallel pathways, traditional glycolysis from glucose and glycolysis from glycogen. Although ATP energy from the glycogen shunt is energetically less efficient than glycolysis from glucose, the shunt allows cell energy storage and glial energy to be provided in milliseconds for rapid neurotransmitter release when needed. Enlarging on their work on cerebral energy needs, Shulman and colleagues expanded the model to include the energetics of working skeletal muscle (Shulman & Rothman, 2001) The hypothesis is a forceful reminder of the immediate role of glycogen over glucose in supplying energy via glycolysis, leading to lactate production in rodents (Brooks & Donovan, 1983; Donovan et al. 1983) and humans (Bergman et al. 1999 a, b ). The idea of a glycogen shunt is also a reminder that everything, including glycogen, turns over during exercise (Azevedo et al. 1998) and that reduced glycogen depletion in exercising muscle is due, in part, to a reduction in turnover.
Muscle lactate disposal and energy status
Bertocci, Haller and colleagues have used MRS and 13C‐lactate tracers to evaluate the role of lactate in muscle energy transduction. By tracking the conversion of 13C‐lactate into glutamate in a rat muscle preparation, a TCA cycle intermediate in equilibrium with α‐ketoglutarate, it was confirmed that lactate is readily oxidized by skeletal muscle during rest and contraction, and directly enters the TCA cycle (Bertocci & Lujan, 1999). Also, using 31P MRS to study muscle metabolism in patients with McArdle disease, a knock‐out experiment of Nature involving phosphofructokinase (PFK) deficiency, lactate infusion was shown to improve muscle force output and energy status in terms of higher phosphocreatine (PCr) and lower adenosine diphosphate (ADP) and inorganic phosphate (Pi) levels (Bertocci et al. 1993). Thus, by means of exogenous lactate infusion investigators were able to bypass the enzymatic block at PFK. Subsequently, in studies using vascular infusion of [1‐13C]lactate and arterial‐venous difference measurements across resting and exercising legs of McArdle disease patients, investigators concluded that ‘lactate formation is mandatory for muscle energy generation during exercise.’ In part those results could have been predicted based on results of studies on electrically stimulated isolated rat muscles in which lactate addition to the incubation bath helped maintain cell membrane chemical‐electrical gradients and ward off fatigue (de Paoli et al. 2007).
Lactate metabolism in injuries and illnesses
This issue has been addressed in recent reviews (Brooks, 2018, 2020 a), but there appear to be both positive and negative aspects to lactate metabolism in illnesses and injuries (Table 1).
Table 1.
Potential for lactate treatment for illness and injury (from Brooks, 2020a )
| Resuscitation (fluid, electrolytes, energy) (Azevedo et al. 2007; Garcia‐Alvarez et al. 2014 a; Marik & Bellomo, 2016) |
| Acidosis (exogenous lactate infusion has an alkalotic effect) (Miller et al. 2005; Wu et al. 2011; Marik & Bellomo, 2016) |
| Regulation of glycaemia (lactate is the major gluconeogenesis (GNG) precursor) (Meyer et al. 1998, 2002; Gerich et al. 2001; Marik, 2009) |
| Traumatic brain injury (lactate is brain fuel and anti‐inflammatory) (Glenn et al. 2015 a) |
| Inflammation (via GPR81 binding down stream signalling lactate inhibit the inflammasome) (Hoque et al. 2014) |
| Acute pancreatitis and hepatitis (lactate is an energy substrate, a GNG precursor and anti‐inflammatory agent) (Hoque et al. 2014) |
| Myocardial infarction, cardiac surgery and acute heart failure (lactate is heart fuel) (Shapiro et al. 2005; Bergman et al. 2009 b) |
| Burns (lactate is an energy substrate, a GNG precursor and anti‐inflammatory agent) (Spitzer, 1979) |
| Sepsis (lactate incorporation in resuscitation fluids can support maintenance of blood pressure and circulation, and help deliver antibiotics, as well as being an energy substrate, a GNG precursor and have an anti‐inflammatory effect) (Garcia et al. 1995; Marik & Bellomo, 2016) |
| Dengue (lactate is an energy substrate, a GNG precursor and anti‐inflammatory agent) (Wu et al. 2011; Somasetia et al. 2014) |
| Cognition (lactate readily crosses the blood‐brain barrier, fuels neurons and stimulates secretion of brain‐derived neurotrophic factor (BDNF), improves executive function and memory) (Rice et al. 2002; Holloway et al. 2007; Hashimoto et al. 2018) |
| Wound healing (Hunt et al. 2007) and muscle regeneration after injury (Tsukamoto et al. 2018; Ohno et al. 2019). |
Positive effects – lactate or supplemental lactate treatment
Lactate treatment can be involved in resuscitation following environmental stresses (dehydration and acidosis), inflammation (hepatitis and pancreatitis), infectious (Dengue and sepsis) and non‐infectious diseases (hypoglycaemia) and traumatic conditions (brain injury, myocardial infarction, burns and wound healing).
Negative effects – overabundance of lactate production and accumulation
Cancer
Seminal studies have shown that lactate production and accumulation occur in cancer cells incubated under fully aerobic condition (Warburg & Minami, 1923, 1927). Subsequently, accelerated glycolysis leading to lactate production in cancer cells has been implicated in many stages in carcinogenesis (San‐Millan & Brooks, 2017). More recently, blocking of MCTs in driver (glycolytic) and oxidative (recipient) tumour cells can minimize lactate exchange, in effect killing both cell types by pickling the drivers and starving the recipients, thus arresting tumour development (Semenza, 2008; Sonveaux et al. 2008). To date, however, the use of pharmacological agents to block MCTs in cancer is contraindicated because of the ubiquitous expression of MCTs in most cell types and negative consequences for the host. Additionally, blocking or deleting HCAR‐1 leads to a reduced transcription of cancer‐regulated genes, thus diminishing cancer cell proliferation in animal and cell culture models (Brown et al. 2020; Xie et al. 2020). At present there are no definitive data indicating that aberrant lactate metabolism causes cancer, but it has been observed that lactate accumulation is associated with upregulation of cancer promoting genes and downregulation of cancer suppressor genes (San‐Millan et al. 2020).
Errors in the ability to transport or exclude lactate may have serious consequences. For instance, embryologic deletion of the lactate transporters (monocarboxylate transporters, MCTs) is lethal. For glucoregulation when blood lactate is elevated as in exercise or other conditions, MCTs must be excluded from insertion into pancreatic β‐cell plasma membranes (Pullen et al. 2010; Rutter et al. 2015). While MCTs are present on plasma and mitochondrial membranes of most cells and tissues, exclusion of MCTs from pancreatic β‐cell plasma membranes keeps extracellular lactate from entering and affecting intracellular redox, thus interfering with glucose sensing and insulin secretion (Bender et al. 2006). Noteworthy in this regard is that individuals experiencing failed suppression of pancreatic β‐cell MCT expression become hypoglycaemic during hard exercise leading to lactataemia because the combination of high insulin and increased glucose disposal through metabolism causes profound hypoglycaemia (Otonkoski et al. 2007).
Lactate shuttling into the future: unresolved issues and future directions
The more lactate shuttling is studied, the more is revealed about the expansive nature of lactate metabolism and its central role in physiology. Consider for instance several of the topics discussed above that have been studied separately, but seldom investigated in an integrated fashion. Seemingly, there is need for a more integrative approach to develop models of physiological organization. Seemingly also, we may learn from, or contribute to, research in other fields of science and medicine. Some examples follow.
Nutrition, digestion, muscle physiology and the microbiome
For instance, consider that the consumption of dietary CHO elicits neuroendocrine signals to splanchnic tissues and brain. In the upper GI tract, entry of glucose for CHO digestion is disposed of via direct and indirect pathways as described in the glucose paradox. Glucose released into the systemic circulation via the hepatic portal vein is taken up by red muscle where glycogen synthesis and glycolysis are stimulated. Subsequently, in the GI tract glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP) are released from enteroendocrine cells in response to nutrient ingestion and the presence of glucose and lactate. GLP‐1 receptors (GLP‐1Rs) on enteric neurons, including intrinsic afferent neurons, and extrinsic spinal and vagal sensory afferents, have potentially numerous effects including enhancement of insulin secretion and mediation of satiety signalling, via effects on the hypothalamic arcuate and paraventricular nuclei.
It goes without citation that short‐ and long‐term nutrition and blood glucose regulation is heavily tied to hunger and satiety signalling. The biochemistry behind appetite regulation is a complicated and active area of research (Gale et al. 2004; Murphy & Bloom, 2006). Suffice it to state that in the arcuate nucleus of the hypothalamus effects of the appetite stimulating (feeding) (neuropeptide y (NPY)) centre are balanced against those of the appetite inhibitory (satiation) [pro‐opio‐melanocortin (POMC)] centre. Hormones that inform the hypothalamic centres that regulate appetite include insulin, ghrelin, leptin, peptide YY (PYY), glucagon like peptide‐1 (GLP‐1) and others (Ghosal et al. 2013). Results of classic studies relating physical activity level and diet (Mayer et al. 1956), as well as practical experience, indicate that maximal exercise has an appetite inhibiting effect. For instance, few are hungry immediately after maximal exercise to exhaustion. The suppressive effect of lactate on appetite (Schmid et al. 2008; Schultes et al. 2012; McCarthy et al. 2020) is consistent with data showing that lactataemia suppresses ghrelin secretion (Islam et al. 2017; Vanderheyden et al. 2020). The ghrelin receptor (growth hormone secretagogue receptor (GHSR‐1α)) is a G‐protein coupled receptor expressed throughout both the stomach and GI tract. Recently, it was found that lactate, short chain fatty acids and other bacterial excretions in the GI tract are able to attenuate ghrelin mediated signalling through the GHSR‐1α (Torres‐Fuentes et al. 2019). Hence, in combination with lactate produced by gut microbiota, the high blood lactate of exercise can enter the bowel via sodium‐mediated monocarboxylate transporters (sMCT) and attenuate ghrelin receptor signalling, perhaps revealing how hard exercise attenuates hunger.
Continuing with the course of chyme down the GI tract, microbiota give rise to lactate that is either converted to butyrate, released into the systemic circulation, or excreted (vide supra). However, it is clear that more research is needed to better understand how upper and lower bowl processes are regulated and integrated in vivo. Consider for instance, the physiology of someone consuming a sports drink during exercise. Notwithstanding the organoleptic effects of cool beverage consumption (Jeukendrup & Chambers, 2010), we would benefit from the development of an overarching model of nutrient consumption and disposal during rest and exercise.
Metabolic flexibility and lactataemia
Lactataemia limits lipid oxidation by blocking lipolysis and oxidation of FFAs (vide supra). From the fields of obesity, diabetes and exercise physiology research come the concepts of metabolic flexibility (Kelley et al. 1999) and crossover (Brooks & Mercier, 1994). Metabolic flexibility describes the ability of individuals to switch between key fuel energy substrates in response to changing physiological conditions such as obesity and diabetes. The crossover concept additionally considers the effect of metabolic rate on energy substrate partitioning. With regard to an effect of lactataemia on energy substrate partitioning, Jones and colleagues observed that metabolically inflexible subjects had an increased fasting plasma lactate compared to aged matched controls (Jones et al. 2019). Similarly, San‐Millán and Brooks observed that diabetic and obese subjects showed increased circulating lactate and decreased lipid oxidation during rest and exercise compared to age matched lean and highly trained athletes (San‐Millan & Brooks, 2018). Further research is needed to determine the mechanisms by which lactataemia limits metabolic flexibility in sedentary, obese and older individuals. Elaboration of those mechanisms could lead to behavioural and pharmacological treatments for metabolic inflexibility in illnesses and ageing.
Epigenetic modifications by lactylation of histones and other potential mechanisms
Glis family zinc finger (Glis1) is a transcription factor known to be involved in cell reprogramming, such as transformation of somatic to pluripotent cells. In somatic cells Glis1 binds to glycolysis genes to open them and promote transcription (Li et al. 2020). This activates glycolysis, without affecting OXPHOS, thus increasing lactate and acetyl‐CoA levels that result in an increase in histone lactylation and acetylation. Further, histone lactylation in some (Pan Kla and H3K18la), but not all (H3K9me3 and H3K27me3) cultured tumour cells increased substantially after Glis1 overexpression and decreased after Glis1 knockdown. Taken together, the results offer the possibility that Glis1 modulates histone acetylation and lactylation during cell reprogramming. Given these hints in the literature, it may be worthwhile to learn from progress in regenerative medicine and cancer biology to expand our knowledge of the effects of diet and exercise on histone modulation in disease and ageing processes.
Fertilization in vivo and in vitro
It is recognized by workers in some fields of science (e.g. sepsis: Marik & Bellomo, 2016) that progress in understanding the biological roles of lactate is attributable to the contributions of exercise physiology summarized above and previously (Brooks, 2018; Ferguson et al. 2018). Clearly all can benefit from greater understanding, including those in the fields of in vitro fertilization (IVF) in which the classic antagonism between glycolytic and oxidative metabolism is on display. In describing lactate shuttles above, the relationship between lactate secreting Sertoli and sperm cells was described as the ‘primal demonstration’ of cell‐cell lactate shuttling. The ‘good thing, bad thing’ dichotomy of lactate carries over to the field of IVF, which is typically conducted on blastocysts in culture media exposed to air, in which the is 5× greater than in vivo. Therefore, the consequences of hyperoxic ROS generation are a major concern because of the potential effects of ROS on mitochondrial morphology and function in the embryos (Belli et al. 2020). Similarly, because a disrupted microenvironment can lead to problems in postnatal development (Feuer et al. 2017), high lactate and low pH have been matters of concern. Much like the Warburg effect of high rates of aerobic glycolysis in cancer cells and working muscles, mammalian blastocysts exhibit high capacities for aerobic glycolysis and lactate secretion that may create a favourable microenvironment for uterine implantation and invasion. Beyond providing an energy supply, glycolysis in blastocysts creates a microenvironment around the embryo to contribute to the disaggregation of uterine tissues to facilitate trophoblast invasion. Further, as in wound healing, it may be that lactate acts as a signalling molecule to elicit vascular endothelial growth factor (VEGF) recruitment from uterine cells to promote angiogenesis in the uterus. It has also been suggested that the region of high lactate created by the blastocyst modulates the activity of the local immune response, thereby promoting immune tolerance (Gardner, 2015). Moreover, lactate produced during delivery may reduce post‐partum uterine inflammation via the lactate receptor HCAR‐1 (Madaan et al. 2017). As discussed above, several sets of independent studies indicate a role of lactate in mitigating inflammation. Because of the role of inflammation in chronic diseases, it seems that focused efforts on the role of lactate in reducing inflammation may be of importance.
Summary
Time is overdue to turn the page on understanding lactate metabolism and consider lactate shuttling as an important component of intermediary metabolism in vivo. Lactate shuttling between producer (driver) and consumer (recipient) cells requires the presence of cell‐cell and intracellular lactate shuttles that fulfil at least three purposes; lactate is: (1) a major energy source, (2) the major gluconeogenic precursor and (3) a signalling molecule. There is little or no evidence for oxygen inadequacy giving rise to lactate production and accumulation in resting or exercising subjects, even in the hypoxia of high altitude (Brooks et al. 1991). Rather, there is abundant evidence that lactate production occurs in fully aerobic tissues and organs (Gertz et al. 1981; Stanley et al. 1985; Richardson et al. 1998; Park et al. 2015). Recognition for lactate shuttling came first in studies of physical exercise where roles of driver and recipient cells were obvious. However, simultaneously and independently the presence of lactate shuttling as part of postprandial glucose disposal was recognized in studies of lab animals (Foster, 1984) and humans (Woerle et al. 2003). Importantly, lactate (not pyruvate) enters the mitochondrial reticulum to support cell energy homeostasis by oxidative phosphorylation of ADP and creatine (Hashimoto et al. 2006). Hence, mitochondrial respiration creates the physiological sink for lactate disposal in vivo. As research progresses important facets of lactate shuttling are becoming recognized with regard to cell signalling and metabolic regulation (Brooks, 2020a ). In diverse tissues lactate acts by mass action, cell redox regulation, ROS generation, allosteric binding and lactylation of histones. By inhibiting lipolysis in adipose tissue, via HCAR‐1 binding and CREB activation, and muscle mitochondrial fatty acid uptake, via malonyl‐CoA and CPT1, lactate controls lipid oxidation and overall energy substrate partitioning. Repeated lactate exposure from regular exercise results in adaptive processes such as mitochondrial biogenesis and other healthful circulatory and neurological characteristics such as improved physical work capacity, metabolic flexibility (Brooks, 2018), memory and cognition (Suzuki et al. 2011; El Hayek et al. 2019). The importance of lactate and lactate shuttling in healthful living is further emphasized when lactate signalling and shuttling are dysregulated as occur in cancer (San‐Millan & Brooks, 2017) and other conditions such as following traumatic brain injury (Glenn et al. 2015 b), metabolic syndrome (Brooks, 2018; San‐Millan & Brooks, 2018), inappropriate insulin signalling (Rutter et al. 2015) and sepsis (Garcia‐Alvarez et al. 2014 a). While much has been done to determine energetic regulation in steady and transient states of metabolism, the importance of measuring the dynamics of metabolism beyond stagnant use of ‘metabolomics’, by assessing physiological, environmental and age‐related effects on the turnover rates of lactate, glucose, fatty and amino acids as well as structural and metabolic proteins and membranes (i.e. ‘fluxomics’), are research challenges for the new millennium.
Like a phoenix, lactate has again risen to major importance in 21st century biology.
Additional information
Competing interests
The authors have no competing interests.
Author contributions
All authors contributed to writing of the paper and approve of the final version. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Supported by NIH 1 R01 AG059715‐01, Pac‐12 Conference Grant No. 3‐02‐Brooks‐17 and the UCB Centre for Research and Education on Aging (CREA) to G.A.B. There are no other funding sources.
Acknowledgements
Michael Horning and Andrea Salvador Pascual are thanked for reading and commenting on the manuscript.
Biographies
George A. Brooks is Professor of Integrative Biology at the University of California, Berkeley and Docteur Honoris Causa de l'Université Montpellier. Dr Brooks has received Honor Awards from the American College of Sports Medicine and the Exercise and Environmental Physiology Section of the American Physiological Society. His research interests involve bioenergetics, mitochondrial morphology, energetics and biogenesis, and the regulation of energy substrate partitioning. His work on the lactate shuttle has influenced thinking in fields as diverse as brain and cancer metabolism. When not in the laboratory, his focus centres on interests of family and friends.

José Arevalo is a 3rd year PhD student at the University of California Berkeley investigating ageing mitochondrial fragmentation and what drives mitochondrial dysfunction with age progression in skeletal muscle. José was born in Guatemala and grew up in the Boyle Heights neighbourhood of East Los Angeles, California. José received his master's degree in kinesiology from California State University Fullerton. When not in the laboratory, his interests are football, rugby, trail running, and lifting weights.

Edited by: Ian Forsythe & Lykke Sylow
References
- Ahlborg G & Felig P (1982). Lactate and glucose exchange across the forearm, legs, and splanchnic bed during and after prolonged leg exercise. J Clin Invest 69, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J & Offermanns S (2010). An autocrine lactate loop mediates insulin‐dependent inhibition of lipolysis through GPR81. Cell Metab 11, 311–319. [DOI] [PubMed] [Google Scholar]
- Atlante A, de Bari L, Bobba A, Marra E & Passarella S (2007). Transport and metabolism of L‐lactate occur in mitochondria from cerebellar granule cells and are modified in cells undergoing low potassium dependent apoptosis. Biochim Biophys Acta 1767, 1285–1299. [DOI] [PubMed] [Google Scholar]
- Azevedo JL Jr, Linderman JK, Lehman SL & Brooks GA (1998). Training decreases muscle glycogen turnover during exercise. Eur J Appl Physiol Occup Physiol 78, 479–486. [DOI] [PubMed] [Google Scholar]
- Azevedo JL, Tietz E, Two‐Feathers T, Paull J & Chapman K (2007). Lactate, fructose and glucose oxidation profiles in sports drinks and the effect on exercise performance. PLoS One 2, e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba N & Sharma HM (1971). Histochemistry of lactic dehydrogenase in heart and pectoralis muscles of rat. J Cell Biol 51, 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeeva LE, Chentsov Yu S & Skulachev VP (1978). Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 501, 349–369. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA & Holloszy JO (1972). Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol 222, 373–378. [DOI] [PubMed] [Google Scholar]
- Barnard RJ, Edgerton VR, Furukawa T & Peter JB (1971). Histochemical, biochemical, and contractile properties of red, white, and intermediate fibers. Am J Physiol 220, 410–414. [DOI] [PubMed] [Google Scholar]
- Barnard RJ, Edgerton VR & Peter JB (1970). Effect of exercise on skeletal muscle. I. Biochemical and histochemical properties. J Appl Physiol 28, 762–766. [DOI] [PubMed] [Google Scholar]
- Baumgart E, Fahimi HD, Stich A & Volkl A (1996). L‐lactate dehydrogenase A4‐ and A3B isoforms are bona fide peroxisomal enzymes in rat liver. Evidence for involvement in intraperoxisomal NADH reoxidation. J Biol Chem 271, 3846–3855. [DOI] [PubMed] [Google Scholar]
- Belli M, Zhang L, Liu X, Donjacour A, Ruggeri E, Palmerini MG, Nottola SA, Macchiarelli G & Rinaudo P (2020). Oxygen concentration alters mitochondrial structure and function in in vitro fertilized preimplantation mouse embryos. Hum Reprod 35, 1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender K, Newsholme P, Brennan L & Maechler P (2006). The importance of redox shuttles to pancreatic beta‐cell energy metabolism and function. Biochem Soc Trans 34, 811–814. [DOI] [PubMed] [Google Scholar]
- Bergersen LH (2015). Lactate transport and signaling in the brain: potential therapeutic targets and roles in body‐brain interaction. J Cereb Blood Flow and Metab 35, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Butterfield GE, Wolfel EE, Lopaschuk GD, Casazza GA, Horning MA & Brooks GA (1999a). Muscle net glucose uptake and glucose kinetics after endurance training in men. Am J Physiol 277, E81‐92. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE & Brooks GA (2000). Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab 278, E244‐E251. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Tsvetkova T, Lowes B & Wolfel EE (2009a). Myocardial FFA metabolism during rest and atrial pacing in humans. Am J Physiol Endocrinol Metab 296, E358‐E366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Tsvetkova T, Lowes B & Wolfel EE (2009b). Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J Physiol 587, 2087–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA & Brooks GA (1999b). Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 87, 1684–1696. [DOI] [PubMed] [Google Scholar]
- Bertocci LA, Haller RG & Lewis SF (1993). Muscle metabolism during lactate infusion in human phosphofructokinase deficiency. J Appl Physiol 74, 1342–1347. [DOI] [PubMed] [Google Scholar]
- Bertocci LA & Lujan BF (1999). Incorporation and utilization of [3‐13C]lactate and [1,2‐13C]acetate by rat skeletal muscle. J Appl Physiol 86, 2077–2089. [DOI] [PubMed] [Google Scholar]
- Boussouar F & Benahmed M (2004). Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab 15, 345–350. [DOI] [PubMed] [Google Scholar]
- Brandt RB, Laux JE, Spainhour SE & Kline ES (1987). Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys 259, 412–422. [DOI] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, Gygi SP, Brivet M, Thummel CS & Rutter J (2012). A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA (1985). Lactate:glycolytic end product and oxidative substrate during sustained exercise in mammals — the “lactate shuttle”. In Circulation, Respiration, and Metabolism. Proceedings in Life Sciences. ed. Gilles R, Springer, Berlin, Heidelberg. [Google Scholar]
- Brooks GA (1985). Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc 17, 22–34. [PubMed] [Google Scholar]
- Brooks GA (1986). Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc 45, 2924–2929. [PubMed] [Google Scholar]
- Brooks GA (2000). Intra‐ and extra‐cellular lactate shuttles. Med Sci Sports Exerc 32, 790–799. [DOI] [PubMed] [Google Scholar]
- Brooks GA (2002). Lactate shuttles in nature. Biochem Soc Trans 30, 258–264. [DOI] [PubMed] [Google Scholar]
- Brooks GA (2009). Cell‐cell and intracellular lactate shuttles. J Physiol 587, 5591–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA (2018). The science and translation of lactate shuttle theory. Cell Metab 27, 757–785. [DOI] [PubMed] [Google Scholar]
- Brooks GA (2020a). Lactate as a fulcrum of metabolism. Redox Biol, 35, 101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA (2020b). The precious few grams of glucose during exercise. Int J Mol Sci 21, 5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA (2020c). The tortuous path of lactate shuttle discovery: from cinders and boards to the lab and ICU. J Sport Health Sci 9, 446‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Brauner KE & Cassens RG (1973). Glycogen synthesis and metabolism of lactic acid after exercise. Am J Physiol 224, 1162–1166. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP & Dubouchaud H (1999a). Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol (1985) 87, 1713–1718. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP & Dubouchaud H (1999b). Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol 87, 1713–1718. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP & Dubouchaud H (1999c). Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol 87, 1713–1718. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE & Reeves JT (1991). Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol 71, 333–341. [DOI] [PubMed] [Google Scholar]
- Brooks GA & Donovan CM (1983). Effect of endurance training on glucose kinetics during exercise. Am J Physio Endocrinol Metabl 244, E505–E512. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Dubouchaud H, Brown M, Sicurello JP & Butz CE (1999d). Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A 96, 1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Fahey TD & Baldwin KM (2019). The why of pulmonary ventilation. Exercise Physiology: human bioenergetics and its applications, 5th edn., vol. 1, pp. 243–256. Kindle Direct Publishing, Lexington, KY, USA. [Google Scholar]
- Brooks GA & Gaesser GA (1980). End points of lactate and glucose metabolism after exhausting exercise. J Appl Physiol Respir Environ Exerc Physiol 49, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Brooks GA & Martin NA (2014). Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front Neurosci 8, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA & Mercier J (1994). Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol 76, 2253–2261. [DOI] [PubMed] [Google Scholar]
- Brown MA & Brooks GA (1994). Trans‐stimulation of lactate transport from rat sarcolemmal membrane vesicles. Arch Biochem Biophys 313, 22–28. [DOI] [PubMed] [Google Scholar]
- Brown TP, Bhattacharjee P, Ramachandran S, Sivaprakasam S, Ristic B, Sikder MOF & Ganapathy V (2020). The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen‐presenting cells in the tumor microenvironment. Oncogene, 1–13. [DOI] [PubMed] [Google Scholar]
- Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, Wright SD, Taggart AK & Waters MG (2008). Role of GPR81 in lactate‐mediated reduction of adipose lipolysis. Biochem Biophys Res Commun 377, 987–991. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Mahieu NG, Huang X, Singh M, Crawford PA, Johnson SL, Gross RW, Schaefer J & Patti GJ (2016). Lactate metabolism is associated with mammalian mitochondria. Nat Chem Biol 12, 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, Markowitz SD, Romero MF & Lapointe JY (2004). The human tumour suppressor gene SLC5A8 expresses a Na+‐monocarboxylate cotransporter. J Physiol 557, 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connett RJ, Honig CR, Gayeski TE & Brooks GA (1990). Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol (1985) 68, 833–842. [DOI] [PubMed] [Google Scholar]
- Cori CF & Cori GT (1946). Carbohydrate metabolism. Annu Rev Biochem 15, 193–218. [DOI] [PubMed] [Google Scholar]
- Daw C.C., Ramachandran K, Enslow BT, Maity S, Bursic B, Novello MJ, Rubannelsonkumar CS, Mashal AH, Ravichandran J, Bakewell TM, Wanf W, Lj K, Madaris TR, Shannon CE, Norton L, Kandala S, Caplan J, Srikantan S, Stathopulos PB, Reeves WB & Madesh M (2020). Lactate elicits ER‐mitochondrial Mg2+ dynamics to integrate cellular metabolism. Cell 183, 474‐489.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari L, Atlante A, Valenti D & Passarella S (2004). Partial reconstruction of in vitro gluconeogenesis arising from mitochondrial L‐lactate uptake/metabolism and oxaloacetate export via novel L‐lactate translocators. Biochem J 380, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bari L, Valenti D, Atlante A & Passarella S (2010). L‐lactate generates hydrogen peroxide in purified rat liver mitochondria due to the putative L‐lactate oxidase localized in the intermembrane space. FEBS Lett 584, 2285–2290. [DOI] [PubMed] [Google Scholar]
- de Paoli FV, Overgaard K, Pedersen TH & Nielsen OB (2007). Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+ . J Physiol 581, 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depocas F, Minaire Y & Chatonnet J (1969). Rates of formation and oxidation of lactic acid in dogs at rest and during moderate exercise. Can J Physiol Pharmacol 47, 603–610. [DOI] [PubMed] [Google Scholar]
- Donovan CM & Brooks GA (1983). Endurance training affects lactate clearance, not lactate production. Am J Physiol Endocrinol Metab 244, E83–E92. [DOI] [PubMed] [Google Scholar]
- Donovan CM, Brooks GA, Donovan CM & Brooks GA (1983). Endurance training affects lactate clearance, not lactate production. Am J Physiol Endocrinol Metab 244, E83–E92. [DOI] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC & Brooks GA (2000). Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 278, E571–E579. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Louis P & Flint HJ (2004). Lactate‐utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol 70, 5810–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, El‐Ghandour R, Nasrallah P, Bilen M, Ibrahim P, Younes J, Abou Haidar E, Barmo N, Jabre V, Stephan JS & Sleiman SF (2019). Lactate mediates the effects of exercise on learning and memory through SIRT1‐dependent activation of hippocampal brain‐derived neurotrophic factor (BDNF). J Neurosci 39, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaki M, Morck DW, Quin C, Pither J, Barnett JA, Gill SK & Gibson DL (2020). Physical activity shapes the intestinal microbiome and immunity of healthy mice but has no protective effects against colitis in MUC2 −/− mice. mSystems 5. e00515‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, Ahmadi‐Vand Z, Marsden KR & Gibson DL (2016). Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z & Gladden LB (2018). Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 118, 691–728. [DOI] [PubMed] [Google Scholar]
- Feuer S, Liu X, Donjacour A, Simbulan R, Maltepe E & Rinaudo P (2017). Common and specific transcriptional signatures in mouse embryos and adult tissues induced by in vitro procedures. Reproduction 153, 107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DW (1984). Banting lecture 1984. From glycogen to ketones–and back. Diabetes 33, 1188–1199. [DOI] [PubMed] [Google Scholar]
- Freminet A, Bursaux E & Poyart CF (1974). Effect of elevated lactataemia on the rates of lactate turnover and oxidation in rats. Pflugers Arch 346, 75–86. [DOI] [PubMed] [Google Scholar]
- Gaesser GA & Brooks GA (1980). Glycogen repletion following continuous and intermittent exercise to exhaustion. J Appl Physiol Respir Environ Exerc Physiol 49, 722–728. [DOI] [PubMed] [Google Scholar]
- Gaesser GA & Brooks GA (1984). Metabolic bases of excess post‐exercise oxygen consumption: a review. Med Sci Sports Exerc 16, 29–43. [PubMed] [Google Scholar]
- Gale SM, Castracane VD & Mantzoros CS (2004). Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. J Nutr 134, 295–298. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK & Goldstein JL (1995). cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem 270, 1843–1849. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RG & Brown MS (1994). Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76, 865–873. [DOI] [PubMed] [Google Scholar]
- Garcia‐Alvarez M, Marik P & Bellomo R (2014a). Sepsis‐associated hyperlactatemia. Crit Care 18, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Alvarez M, Marik P & Bellomo R (2014b). Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol 2, 339–347. [DOI] [PubMed] [Google Scholar]
- Gardner DK (2015). Lactate production by the mammalian blastocyst: manipulating the microenvironment for uterine implantation and invasion? Bioessays 37, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Weiszmann J, Reagan JD, Gupte J, Baribault H, Gyuris T, Chen JL, Tian H & Li Y (2008). Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J Lipid Res 49, 797–803. [DOI] [PubMed] [Google Scholar]
- Gerich JE, Meyer C, Woerle HJ & Stumvoll M (2001). Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24, 382–391. [DOI] [PubMed] [Google Scholar]
- Gertz EW, Wisneski JA, Neese R, Bristow JD, Searle GL & Hanlon JT (1981). Myocardial lactate metabolism: evidence of lactate release during net chemical extraction in man. Circulation 63, 1273–1279. [DOI] [PubMed] [Google Scholar]
- Gertz EW, Wisneski JA, Stanley WC & Neese RA (1988). Myocardial substrate utilization during exercise in humans. Dual carbon‐labeled carbohydrate isotope experiments. J Clin Invest 82, 2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Myers B & Herman JP (2013). Role of central glucagon‐like peptide‐1 in stress regulation. Physiol Behav 122, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden LB (1991). Net lactate uptake during progressive steady‐level contractions in canine skeletal muscle. J Appl Physiol 71, 514–520. [DOI] [PubMed] [Google Scholar]
- Gladden LB (2004). Lactate metabolism: a new paradigm for the third millennium. J Physiol 558, 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden LB, Crawford RE & Webster MJ (1994). Effect of lactate concentration and metabolic rate on net lactate uptake by canine skeletal muscle. Am J Physio Regul Integr Comp Physioll 266, R1095–R1101. [DOI] [PubMed] [Google Scholar]
- Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S & Balaban RS (2015). Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523, 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TC, Martin NA, Horning MA, McArthur DL, Hovda D, Vespa PM & Brooks GA (2015a). Lactate: brain fuel in human traumatic brain injury. A comparison to normal healthy control subjects. J Neurotrauma 32, 820–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TC, Martin NA, McArthur DL, Hovda DA, Vespa P, Johnson ML, Horning MA & Brooks GA (2015b). Endogenous nutritive support after traumatic brain injury: peripheral lactate production for glucose supply via gluconeogenesis. J Neurotrauma 32, 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil K, Viguie C, Stanley WC, Brooks GA & Packer L (1988). Blood glutathione oxidation during human exercise. J Appl Physiol (1985) 64, 115–119. [DOI] [PubMed] [Google Scholar]
- Gold M, Miller HI, Issekutz B Jr & Spitzer JJ (1963). Effect of exercise and lactic acid infusion on individual free fatty acids of plasma. Am J Physiol 205, 902–904. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH & Sparks LM (2017). Metabolic flexibility in health and disease. Cell Metab 25, 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halangk W, Bohnensack R, Frank K & Kunz W (1985). Effect of various substrates on mitochondrial and cellular energy state of intact spermatozoa. Biomed Biochim Acta 44, 411–420. [PubMed] [Google Scholar]
- Hashimoto T, Hussien R & Brooks GA (2006). Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab 290, E1237–E1244. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Cho HS, Kaufer D & Brooks GA (2008). Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One 3, e2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Oommen S, Gohil K & Brooks GA (2007). Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21, 2602–2612. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Masuda S, Taguchi S & Brooks GA (2005). Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol 567, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Sato K & Iemitsu M (2013). Exercise‐inducible factors to activate lipolysis in adipocytes. J Appl Physiol (1985) 115, 260–267. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tsukamoto H, Takenaka S, Olesen ND, Petersen LG, Sorensen H, Nielsen HB, Secher NH & Ogoh S (2018). Maintained exercise‐enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J 32, 1417–1427. [DOI] [PubMed] [Google Scholar]
- Henderson GC, Horning MA, Lehman SL, Wolfel EE, Bergman BC & Brooks GA (2004). Pyruvate shuttling during rest and exercise before and after endurance training in men. J Appl Physiol 97, 317–325. [DOI] [PubMed] [Google Scholar]
- Henderson GC, Horning MA, Wallis GA & Brooks GA (2007). Pyruvate metabolism in working human skeletal muscle. Am J Physiol Endocrinol Metab 292, E366. [DOI] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER & Martinou JC (2012). Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96. [DOI] [PubMed] [Google Scholar]
- Hill AV (1914). The oxidative removal of lactic acid. J Physiol 48, x‐xi. [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC & Sanders ME (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11, 506–514. [DOI] [PubMed] [Google Scholar]
- Hofmann P, Bunc V, Leitner H, Pokan R & Gaisl G (1994). Heart rate threshold related to lactate turn point and steady‐state exercise on a cycle ergometer. Eur J Appl Physiol Occup Physiol 69, 132–139. [DOI] [PubMed] [Google Scholar]
- Holloway R, Zhou Z, Harvey HB, Levasseur JE, Rice AC, Sun D, Hamm RJ & Bullock MR (2007). Effect of lactate therapy upon cognitive deficits after traumatic brain injury in the rat. Acta Neurochir (Wien) 149, 919–927; discussion 927. [DOI] [PubMed] [Google Scholar]
- Honig CR, Connett RJ & Gayeski TE (1992). O2 transport and its interaction with metabolism; a systems view of aerobic capacity. Med Sci Sports Exerc 24, 47–53. [PubMed] [Google Scholar]
- Hooker AM & Baldwin KM (1979). Substrate oxidation specificity in different types of mammalian muscle. Am J Physiol Cell Physiol 236, C66–C69. [DOI] [PubMed] [Google Scholar]
- Hoque R, Farooq A, Ghani A, Gorelick F & Mehal WZ (2014). Lactate reduces liver and pancreatic injury in Toll‐like receptor‐ and inflammasome‐mediated inflammation via GPR81‐mediated suppression of innate immunity. Gastroenterology 146, 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J, White E & Rabinowitz JD (2017). Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Aslam RS, Beckert S, Wagner S, Ghani QP, Hussain MZ, Roy S & Sen CK (2007). Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal 9, 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussien R & Brooks GA (2011). Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics 43, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep PB & Hammerstedt RH (1985). Endogenous metabolism by sperm in response to altered cellular ATP requirements. J Cell Physiol 123, 180–190. [DOI] [PubMed] [Google Scholar]
- Islam H, Townsend LK, McKie GL, Medeiros PJ, Gurd BJ & Hazell TJ (2017). Potential involvement of lactate and interleukin‐6 in the appetite‐regulatory hormonal response to an acute exercise bout. J Appl Physiol (1985) 123, 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz BJ, and & Miller H (1962). Plasma free fatty acids during exercise and the effect of lactic acid. Proc Soc Exp Biol Med 110, 237–239. [Google Scholar]
- James DE, Kraegen EW & Chisholm DJ (1985). Effects of exercise training on in vivo insulin action in individual tissues of the rat. J Clin Invest 76, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DE, Zorzano A, Boni‐Schnetzler M, Nemenoff RA, Powers A, Pilch PF & Ruderman NB (1986). Intrinsic differences of insulin receptor kinase activity in red and white muscle. J Biol Chem 261, 14939–14944. [PubMed] [Google Scholar]
- Jeukendrup AE & Chambers ES (2010). Oral carbohydrate sensing and exercise performance. Curr Opin Clin Nutr Metab Care 13, 447–451. [DOI] [PubMed] [Google Scholar]
- Jöbsis FF & Stainsby WN (1968). Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol 4, 292–300. [DOI] [PubMed] [Google Scholar]
- Johnson JA & Fusaro RM (1972). The role of the skin in carbohydrate metabolism. Adv Metab Disord 60, 1–55. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Emhoff CA, Horning MA & Brooks GA (2012). Transpulmonary lactate shuttle. Am J Physiol Regul Integr Comp Physiol 302, R143–R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Hussien R, Horning MA & Brooks GA (2011). Transpulmonary pyruvate kinetics. Am J Physiol Regul Integr Comp Physiol 301, R769–R774. [DOI] [PubMed] [Google Scholar]
- Jones AR (1997). Metabolism of lactate by mature boar spermatozoa. Reprod Fertil Dev 9, 227–232. [DOI] [PubMed] [Google Scholar]
- Jones TE, Pories WJ, Houmard JA, Tanner CJ, Zheng D, Zou K, Coen PM, Goodpaster BH, Kraus WE & Dohm GL (2019). Plasma lactate as a marker of metabolic health: Implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 166, 861–866, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane DA (2014). Lactate oxidation at the mitochondria: a lactate‐malate‐aspartate shuttle at work. Front Neurosci 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR & Simoneau JA (1999). Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277, E1130–E1141. [DOI] [PubMed] [Google Scholar]
- Kelley KM, Hamann JJ, Navarre C & Gladden LB (2002). Lactate metabolism in resting and contracting canine skeletal muscle with elevated lactate concentration. J Appl Physiol (1985) 93, 865–872. [DOI] [PubMed] [Google Scholar]
- Kirkwood SP, Munn EA & Brooks GA (1986). Mitochondrial reticulum in limb skeletal muscle. Am J Physiol Cell Physiol 251, C395–C402. [DOI] [PubMed] [Google Scholar]
- Kirkwood SP, Packer L & Brooks GA (1987). Effects of endurance training on a mitochondrial reticulum in limb skeletal muscle. Arch Biochem Biophys 255, 80–88. [DOI] [PubMed] [Google Scholar]
- Kline ES, Brandt RB, Laux JE, Spainhour SE, Higgins ES, Rogers KS, Tinsley SB & Waters MG (1986). Localization of L‐lactate dehydrogenase in mitochondria. Arch Biochem Biophys 246, 673–680. [DOI] [PubMed] [Google Scholar]
- Kreutzer U & Jue T (1995). Critical intracellular O2 in myocardium as determined by 1H nuclear magnetic resonance signal of myoglobin. Am J Physiol Heart Circ Physiol 268, H1675–H1681. [DOI] [PubMed] [Google Scholar]
- Lazarow PB & De Duve C (1976). A fatty acyl‐CoA oxidizing system in rat liver pEeroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A 73, 2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoultre V, Benoit R, Carrel G, Schutz Y, Millet GP, Tappy L & Schneiter P (2010). Fructose and glucose co‐ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose. Am J Clin Nutr 92, 1071–1079. [DOI] [PubMed] [Google Scholar]
- Lehninger AL (1970). Biochemistry, p. 326. Worth, New York. [Google Scholar]
- Li L, Chen K, Wang T, Wu Y, Xing G, Chen M, Hao Z, Zhang C, Zhang J, Ma B, Liu Z, Yuan H, Liu Z, Long Q, Zhou Y, Qi J, Zhao D, Gao M, Pei D, Nie J, Ye D, Pan G & Liu X (2020). Glis1 facilitates induction of pluripotency via an epigenome‐metabolome‐epigenome signalling cascade. Nat Metab 2, 882–892. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F & Lovenberg TW (2009). Lactate inhibits lipolysis in fat cells through activation of an orphan G‐protein‐coupled receptor, GPR81. J Biol Chem 284, 2811–2822. [DOI] [PubMed] [Google Scholar]
- McCarthy SF, Islam H & Hazell TJ (2020). The emerging role of lactate as a mediator of exercise‐induced appetite suppression. Am J Physiol Endocrinol Metab 319, E814–E819. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Khanna S, Gonzalez GF, Butz CE & Brooks GA (2003). Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem Biophys Res Commun 304, 130–135. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Mannaerts GP & Foster DW (1977). A possible role for malonyl‐CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest 60, 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, MacKenzie KR, Putluri N, Maletic‐Savatic M & Bellen HJ (2017). The glia‐neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab 26, 719–737.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaan A, Nadeau‐Vallee M, Rivera JC, Obari D, Hou X, Sierra EM, Girard S, Olson DM & Chemtob S (2017). Lactate produced during labor modulates uterine inflammation via GPR81 (HCA1). Am J Obstet Gynecol 216, 60.e1‐60.e17. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL & Shulman RG (1999). Energy on demand. Science 283, 496–497. [DOI] [PubMed] [Google Scholar]
- Marik P & Bellomo R (2013). Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Critical Care 1, 3–5. [Google Scholar]
- Marik P & Bellomo R (2016). A rational approach to fluid therapy in sepsis. Br J Anaesth 116, 339–349. [DOI] [PubMed] [Google Scholar]
- Marik PE (2009). Glycemic control in critically ill patients: what to do post NICE‐SUGAR? World J Gastrointest Surg 1, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J, Roy P & Mitra KP (1956). Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr 4, 169–175. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Brooks GA, Schoeller DA & Budinger TF (1986). Disposal of blood [1‐13C]lactate in humans during rest and exercise. J Appl Physiol 60, 232–241. [DOI] [PubMed] [Google Scholar]
- Messonnier AL, Emhoff CW, Fattor JA, Horning MA, C T.J. & Brooks GA (2013). Lactate kinetics at the lactate threshold in trained and untrained men. J Appl Physiol 1593‐1602, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Dostou J, Nadkarni V & Gerich J (1998). Effects of physiological hyperinsulinemia on systemic, renal, and hepatic substrate metabolism. Am J Physiol Renal Physiol 275, F915–F921. [DOI] [PubMed] [Google Scholar]
- Meyer C, Dostou JM, Welle SL & Gerich JE (2002). Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab 282, E419–E427. [DOI] [PubMed] [Google Scholar]
- Meyerhof O (1920). Die Energieumwandlungen im Muskel II. Das Schicksal der Milchsaure in der Erholungsperiode des Muskels. Pflügers Archiv ges Physiol Mensch Tiere 182‐182, 284–317. [Google Scholar]
- Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI & Brooks GA (2002a). Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol 544, 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Fattor JA, Jacobs KA, Horning MA, Suh SH, Navazio F & Brooks GA (2002b). Metabolic and cardiorespiratory responses to “the lactate clamp”. Am J Physiol Endocrinol Metab 283, E889–E898. [DOI] [PubMed] [Google Scholar]
- Miller BF, Lindinger MI, Fattor JA, Jacobs KA, Leblanc PJ, Duong M, Heigenhauser GJ & Brooks GA (2005). Hematological and acid‐base changes in men during prolonged exercise with and without sodium‐lactate infusion. J Appl Physiol 98, 856–865. [DOI] [PubMed] [Google Scholar]
- Miller HI, Issekutz B Jr, Rodahl K & Paul P (1964). Effect of lactic acid on plasma free fatty acids in pancreatectomized dogs. Am J Physiol 207, 1226–1230. [DOI] [PubMed] [Google Scholar]
- Murphy KG & Bloom SR (2006). Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859. [DOI] [PubMed] [Google Scholar]
- Nilsson LH & Hultman E (1974). Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest 33, 5–10. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Ando K, Ito T, Suda Y, Matsui Y, Oyama A, Kaneko H, Yokoyama S, Egawa T & Goto K (2019). Lactate stimulates a potential for hypertrophy and regeneration of mouse skeletal muscle. Nutrients 869, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otonkoski T, Jiao H, Kaminen‐Ahola N, Tapia‐Paez I, Ullah MS, Parton LE, Schuit F, Quintens R, Sipila I, Mayatepek E, Meissner T, Halestrap AP, Rutter GA & Kere J (2007). Physical exercise‐induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic beta cells. Am J Hum Genet 81, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Josan S, Mayer D, Hurd RE, Chung Y, Bendahan D, Spielman DM & Jue T (2015). Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J Exp Biol 218, 3308–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarella S, de Bari L, Valenti D, Pizzuto R, Paventi G & Atlante A (2008). Mitochondria and L‐lactate metabolism. FEBS Lett 582, 3569–3576. [DOI] [PubMed] [Google Scholar]
- Passarella S, Paventi G & Pizzuto R (2014). The mitochondrial L‐lactate dehydrogenase affair. Front Neurosci 8, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG & Behar KL (2014). Direct evidence for activity‐dependent glucose phosphorylation in neurons with implications for the astrocyte‐to‐neuron lactate shuttle. Proc Natl Acad Sci U S A 111, 5385–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bergersen LH, Halestrap AP & Pierre K (2005). Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res 79, 55–64. [DOI] [PubMed] [Google Scholar]
- Pellerin L & Magistretti PJ (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91, 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L & Magistretti PJ (2012). Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32, 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N & Magistretti PJ (1998). Evidence supporting the existence of an activity‐dependent astrocyte‐neuron lactate shuttle. Dev Neurosci 20, 291–299. [DOI] [PubMed] [Google Scholar]
- Poole DC, Rossiter HB, Brooks GA & Gladden LB (2021). The anaerobic threshold: 50+ years of controversy. J Physiol 599, 737–767. [DOI] [PubMed] [Google Scholar]
- Powell KE, King AC, Buchner DM, Campbell WW, DiPietro L, Erickson KI, Hillman CH, Jakicic JM, Janz KF, Katzmarzyk PT, Kraus WE, Macko RF, Marquez DX, McTiernan A, Pate RR, Pescatello LS & Whitt‐Glover MC (2018). The scientific foundation for the physical activity guidelines for Americans, 2nd edition. J Phys Act Health, 1–11. [DOI] [PubMed] [Google Scholar]
- Powers SK, Ji LL & Leeuwenburgh C (1999). Exercise training‐induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc 31, 987–997. [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN & Halestrap AP (1998). Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J 329 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen TJ, Khan AM, Barton G, Butcher SA, Sun G & Rutter GA (2010). Identification of genes selectively disallowed in the pancreatic islet. Islets 2, 89–95. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JD & Enerback S (2020). Lactate: the ugly duckling of energy metabolism. Nat Metab 2, 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AC, Zsoldos R, Chen T, Wilson MS, Alessandri B, Hamm RJ & Bullock MR (2002). Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res 928, 156–159. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Leigh JS & Wagner PD (1998). Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol 85, 627–634. [DOI] [PubMed] [Google Scholar]
- Rodahl K, Miller HI & Issekutz B Jr (1964). Plasma free fatty acids in exercise. J Appl Physiol 19, 489–492. [DOI] [PubMed] [Google Scholar]
- Rogatzki MJ, Ferguson BS, Goodwin ML & Gladden LB (2015). Lactate is always the end product of glycolysis. Front Neurosci 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DA & Brooks GA (1990a). Lactate and pyruvate transport is dominated by a pH gradient‐sensitive carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279, 386–394. [DOI] [PubMed] [Google Scholar]
- Roth DA & Brooks GA (1990b). Lactate transport is mediated by a membrane‐bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys 279, 377–385. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Pullen TJ, Hodson DJ & Martinez‐Sanchez A (2015). Pancreatic beta‐cell identity, glucose sensing and the control of insulin secretion. Biochem J 466, 203–218. [DOI] [PubMed] [Google Scholar]
- Saddik M, Gamble J, Witters LA & Lopaschuk GD (1993). Acetyl‐CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 268, 25836–25845. [PubMed] [Google Scholar]
- San‐Millan I & Brooks GA (2017). Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 38, 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San‐Millan I & Brooks GA (2018). Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less‐fit individuals. Sports Med 48, 467–479. [DOI] [PubMed] [Google Scholar]
- San‐Millan I, Julian CG, Matarazzo C & Brooks GA (2020). Is lactate an oncometabolite? evidence supporting a role for lactate in the regulation of transcriptional activity of cancer‐related genes in MCF7 breast cancer cells. Front Oncol ‐ Cancer Metab 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, Wibowo MC, Wurth RC, Punthambaker S, Tierney BT, Yang Z, Hattab MW, Avila‐Pacheco J, Clish CB, Lessard S, Church GM & Kostic AD (2019). Meta‐omics analysis of elite athletes identifies a performance‐enhancing microbe that functions via lactate metabolism. Nat Med 25, 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SM, Jauch‐Chara K, Hallschmid M, Oltmanns KM, Peters A, Born J & Schultes B (2008). Lactate overrides central nervous but not beta‐cell glucose sensing in humans. Metabolism 57, 1733–1739. [DOI] [PubMed] [Google Scholar]
- Schultes B, Schmid SM, Wilms B, Jauch‐Chara K, Oltmanns KM & Hallschmid M (2012). Lactate infusion during euglycemia but not hypoglycemia reduces subsequent food intake in healthy men. Appetite 58, 818–821. [DOI] [PubMed] [Google Scholar]
- Schurr A (2008). Lactate: a major and crucial player in normal function of both muscle and brain. J Physiol 586, 2665–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2008). Tumor metabolism: cancer cells give and take lactate. J Clin Invest 118, 3835–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE & Weiss JW (2005). Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med 45, 524–528. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F & Rothman DL (2001). Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci U S A 98, 6417–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG & Rothman DL (2001). The “glycogen shunt” in exercising muscle: a role for glycogen in muscle energetics and fatigue. Proc Natl Acad Sci U S A 98, 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadja C, Morin J, Ferre P & Girard J (1988). Metabolic fate of a gastric glucose load in unrestrained rats bearing a portal vein catheter. Am J Physiol Endocrinol Metab 254, E407–E413. [DOI] [PubMed] [Google Scholar]
- Somasetia DH, Setiati TE, Sjahrodji AM, Idjradinata PS, Setiabudi D, Roth H, Ichai C, Fontaine E & Leverve XM (2014). Early resuscitation of dengue shock syndrome in children with hyperosmolar sodium‐lactate: a randomized single‐blind clinical trial of efficacy and safety. Crit Care 18, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O & Dewhirst MW (2008). Targeting lactate‐fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118, 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer JJ (1979). Gluconeogenesis in the burned patient. J Trauma 19, 899–900. [PubMed] [Google Scholar]
- Stainsby WN & Welch HG (1966). Lactate metabolism of contracting dog skeletal muscle in situ. Am J Physiol 211, 177–183. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Gertz EW, Wisneski JA, Morris DL, Neese RA & Brooks GA (1985). Systemic lactate kinetics during graded exercise in man. Am J Physiol Endocrinol Metab 249, E595–E602. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL & Brooks GA (1986). Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol 60, 1116–1120. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Wisneski JA, Gertz EW, Neese RA & Brooks GA (1988). Glucose and lactate interrelations during moderate‐intensity exercise in humans. Metabolism 37, 850–858. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Gao V & Alberini CM (2016). The role of lactate‐mediated metabolic coupling between astrocytes and neurons in long‐term memory formation. Front Integr Neurosci 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stender S, Zaha VG, Malloy CR, Sudderth J, DeBerardinis RJ & Park JM (2020). Assessment of rapid hepatic glycogen synthesis in humans using dynamic (13)C magnetic resonance spectroscopy. Hepatol Commun 4, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey BT & Kayne FJ (1977). Energy metabolism of spermatozoa. VI. Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol Reprod 16, 549–556. [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ & Alberini CM (2011). Astrocyte‐neuron lactate transport is required for long‐term memory formation. Cell 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Pasquale N, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee MY, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P, Kalliokoski KK, Nielsen S, Pedersen BK, Kahn CR, Tseng YH & Goodyear LJ (2019). TGF‐beta2 is an exercise‐induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 1, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramae H, Yoshikawa T, Inoue R, Ushida K, Takebe K, Nio‐Kobayashi J & Iwanaga T (2010). The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed Res 31, 239–249. [DOI] [PubMed] [Google Scholar]
- Theytaz F, de Giorgi S, Hodson L, Stefanoni N, Rey V, Schneiter P, Giusti V & Tappy L (2014). Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients 6, 2632–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Fuentes C, Golubeva AV, Zhdanov AV, Wallace S, Arboleya S, Papkovsky DB, El Aidy S, Ross P, Roy BL, Stanton C, Dinan TG, Cryan JF & Schellekens H (2019). Short‐chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J 33, 13546–13559. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S, Shibasaki A, Naka A, Saito H & Iida K (2018). Lactate promotes myoblast differentiation and myotube hypertrophy via a pathway involving MyoD in vitro and enhances muscle regeneration in vivo. Int J Mol Sci 3649, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry LA, Cain ML, Wasserman SL, Minorsky PV, & Orr R (2020). Campbell biology. In Campbell Biology, 12 edn, pp. 164–170.Pearson. [Google Scholar]
- van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH & Nielsen HB (2009). Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Vanderheyden LW, McKie GL, Howe GJ & Hazell TJ (2020). Greater lactate accumulation following an acute bout of high‐intensity exercise in males suppresses acylated ghrelin and appetite postexercise. J Appl Physiol 128, 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguie CA, Frei B, Shigenaga MK, Ames BN, Packer L & Brooks GA (1993). Antioxidant status and indexes of oxidative stress during consecutive days of exercise. J Appl Physiol 75, 566–572. [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga‐Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB & Nieuwdorp M (2012). Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913‐916.e7. [DOI] [PubMed] [Google Scholar]
- Wagner S, Hussain MZ, Hunt TK, Bacic B & Becker HD (2004). Stimulation of fibroblast proliferation by lactate‐mediated oxidants. Wound Repair Regen 12, 368–373. [DOI] [PubMed] [Google Scholar]
- Wahren J & Ekberg K (2007). Splanchnic regulation of glucose production. Annu Rev Nutr 27, 329–345. [DOI] [PubMed] [Google Scholar]
- Warburg O & Minami S (1923). Versuche an überlebendem carcinom‐gewebe. J Mol Med 2, 776–777. [Google Scholar]
- Warburg O, Wind F & Negelein E (1927). The metabolism of tumors in the body. J Gen Physiol 8, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH, Lickley HL & Vranic M (1984). Interactions between glucagon and other counterregulatory hormones during normoglycemic and hypoglycemic exercise in dogs. J Clin Invest 74, 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman K & McIlroy MB (1964). Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol 14, 844–852. [DOI] [PubMed] [Google Scholar]
- Watt PW, MacLennan PA, Hundal HS, Kuret CM & Rennie MJ (1988). L(+)‐lactate transport in perfused rat skeletal muscle: kinetic characteristics and sensitivity to pH and transport inhibitors. Biochim Biophys Acta 944, 213–222. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Rumsey WL, Green TJ & Vanderkooi JM (1988). The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem 263, 2712–2718. [PubMed] [Google Scholar]
- Wisneski JA, Gertz EW, Neese RA & Mayr M (1987). Myocardial metabolism of free fatty acids. Studies with 14C‐labeled substrates in humans. J Clin Invest 79, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerle HJ, Meyer C, Dostou JM, Gosmanov NR, Islam N, Popa E, Wittlin SD, Welle SL & Gerich JE (2003). Pathways for glucose disposal after meal ingestion in humans. Am J Physiol Endocrinol Metab 284, E716–E725. [DOI] [PubMed] [Google Scholar]
- Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA & Conwell DL (2011). Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 9, 710–717.e1. [DOI] [PubMed] [Google Scholar]
- Xie Q, Zhu Z, He Y, Zhang Z, Zhang Y, Wang Y, Luo J, Peng T, Cheng F & Gao J (2020). A lactate‐induced Snail/STAT3 pathway drives GPR81 expression in lung cancer cells. Biochim Biophys Acta 1866, 165576. [DOI] [PubMed] [Google Scholar]
- Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez‐Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, Becker L & Zhao Y (2019). Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinker BA, Britz K & Brooks GA (1990). Effects of a 36‐hour fast on human endurance and substrate utilization. J Appl Physiol 69, 1849–1855. [DOI] [PubMed] [Google Scholar]


