Abstract
Background/Objectives:
Pancreatic adenocarcinoma (PDAC) metastatic to the leptomeninges is a rare and lethal event. Leptomeningeal disease (LMD) research is limited in PDAC, and insights into clinical descriptors, possible disease predictors, and treatment strategies is necessitated.
Methods:
Memorial Sloan Kettering databases were queried with Institutional Review Board approval to identify patients with LMD and PDAC treated between January 2000 and June 2020. Medical record review was used to abstract clinical, genomic, pathologic, and radiographic data. Overall survival was calculated from date of PDAC diagnosis to date of death. Previously published literature on LMD from PDAC was reviewed.
Results:
Four patients with LMD from PDAC were identified, two males and two females. Age at diagnosis ranged from 57 to 68 years. All four patients had predominant lung metastasis and a relatively low burden of intra-abdominal disease. Somatic testing indicated alterations typical of PDAC and no PDAC defining pathogenic germline mutations were identified. An extended clinical course prior to LMD diagnosis was observed in all patients, ranging from 16 to 148 months. Upon diagnosis of LMD, three patients elected for supportive care and one patient received a limited course of craniospinal radiation. The median survival following diagnosis of LMD was 1.6 months (range 0.5–2.8 months).
Conclusions:
LMD from PDAC is a rare occurrence that may be more frequent in patients with lung metastasis and/or a more indolent clinical course. Following diagnosis of LMD, prognosis is poor, and survival is short. New treatment strategies for this manifestation of PDAC are needed.
Keywords: Cerebrospinal fluid, genomics, leptomeninges, pancreatic adenocarcinoma
Background
Pancreatic ductal adenocarcinoma (PDAC) is a challenging malignancy to treat, with an overall five-year survival rate of less than 10%1, 2. At least 50% of patients present with metastatic disease. Sites of metastasis commonly include the liver, lymph nodes, peritoneum, and lungs. In comparison, metastasis to the leptomeninges is very rare3. Leptomeningeal disease (LMD) is metastasis to the arachnoid and pia mater, and often is diagnosed concurrently with dural and/or parenchymal brain involvement 2. LMD is detected in 5–8% of patients with solid tumors, and typically occurs in patients with breast, lung, and melanoma malignancies4. In PDAC, metastasis to the brain or central nervous system (CNS) is estimated to occur in approximately 0.6% of patients5. However, autopsy reports indicate that the actual incidence of LMD is higher than clinically reported 6. Data suggests that LMD will become increasingly common as cancer patients continue to live longer7, 8.
Multi-agent systemic chemotherapies, such as FOLFIRINOX (5-fluorouracil, oxaliplatin, leucovorin, irinotecan), gemcitabine/nab-paclitaxel, and liposomal irinotecan/5-fluorouracil, have improved progression-free survival (PFS) and overall survival for PDAC patients9–11. In addition, targeted genetic therapies have shown to extend PFS in genomically defined subsets of patients12. As disease control continues to improve for PDAC patients, we hypothesize LMD may be more frequently observed.
Upon diagnosis of LMD, the median survival time of PDAC patients is a few short weeks to several months3, 8. LMD is commonly diagnosed with cerebrospinal fluid (CSF) cytology and/or contrast enhanced MR imaging 13. Indicators of possible LMD observed in CSF include increased opening pressure, elevated protein levels, and decreased glucose levels and positive cytology13, 14. LMD typically manifests in the form of headaches, mental status changes, confusion, seizures, diplopia, poor balance and weakness8, 15. The remarkably poor prognosis can be partially attributed to the common occurrence of LMD in the setting of systemic disease14. Additionally, the blood brain barrier reduces the efficacy of chemotherapeutic agents in permeating the metastasis6, 16. Although treatment guidelines are limited, common approaches include whole-brain and focal spinal radiation, continuation of systemic chemotherapy, and/or intrathecal chemotherapy (IT)2, 6. The aim of treatment is to maximize quality of life and preserve neurologic functioning2, 17.
The analysis herein aims to evaluate and characterize LMD in PDAC and identify possible predictive clinical descriptors for LMD. Specifically, this analysis focuses on LMD and not brain metastasis from PDAC18.
Methods
Upon Institutional Review and Privacy Board approval and oversight with waiver of consent, Memorial Sloan Kettering databases were queried to identify patients with PDAC metastatic to the leptomeninges treated between January 2000 and June 2020. The search was specified to identify patients with pathology reports and PDAC and radiology reports containing the terms “leptomeningeal” or “brain metastasis,” billing codes for neoplasms of the meninges or CNS (ICD: C70.1, C79.32, C79.40, C79.490), or pathology reports indicating malignant cells in the CSF. Medical records were retrospectively reviewed to abstract the clinical and pathologic characteristics of patients who met the inclusion criteria. Data abstracted included age and stage at presentation, sites of metastasis, treatment regimens and genomic profiles, including germline and somatic mutations. The features of leptomeningeal metastasis were further detailed with observed neurologic symptoms, methods of diagnosis (MRI, CT, and/or CSF cytology), treatment strategies and outcome data.
A literature review was also conducted to identify the current state of knowledge of PDAC metastatic to the leptomeninges.
Results
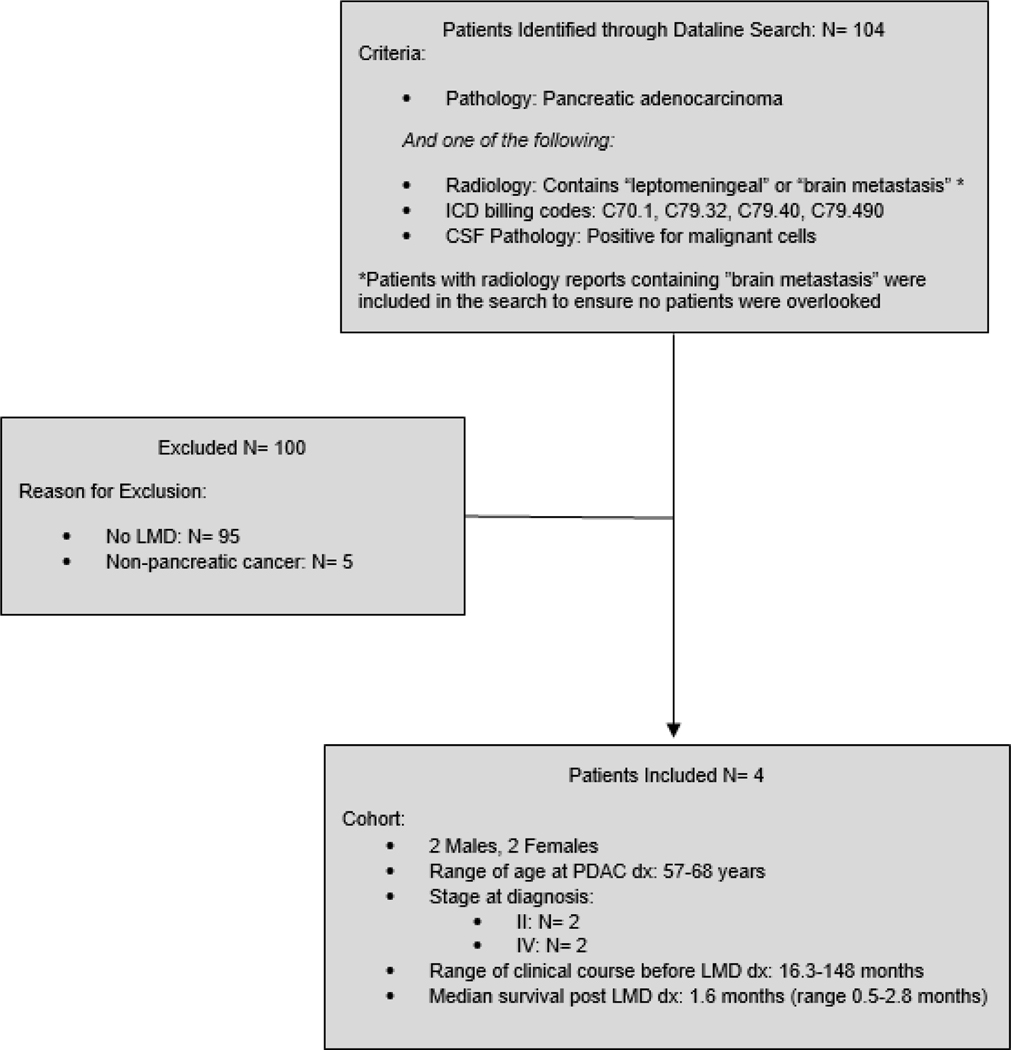
Our initial search identified 104 patients. Ninety-five patients were excluded for not having LMD and five patients were excluded for not having PDAC (Figure 1). Four patients ultimately met inclusion criteria (Table 1). Two patients were male and two were female. The median age at diagnosis of PDAC was 63 years (range 57–68 years). Two patients presented with PDAC with stage II disease and two presented with stage IV disease. The median age at diagnosis of LMD was 70 (range 58–76 years). All patients had predominant lung metastasis and no liver metastasis. All patients underwent MR imaging that confirmed the diagnosis of LMD. Three patients did not receive further active therapy and one patient received a limited course of craniospinal radiation. The median OS was 65 months (range 18–149 months) and the median survival post diagnosis of LMD was 1.6 months (range 0.5–2.8 months).
Figure 1:
Consort Diagram
Table 1.
Summary of PDAC Patients with Leptomeningeal Disease
| 61, Female | 68, Male | 57, Female | 65, Male | |
|---|---|---|---|---|
| Year of PDAC Dx | 2006 | 2009 | 2018 | 2018 |
| Stage at Diagnosis | II, II (Two primaries) | II | IV | IV |
| Surgery | Distal pancreatectomy Whipple/completion pancreatectomy | Distal pancreatectomy | No | No |
| Lines of Treatment Before LMD Dx | 3 | 8 | 4 | 2 |
| Year of LMD Dx | 2018 | 2018 | 2020 | 2020 |
| LMD in Spine | No | No | Yes | No |
| Other sites of Met. | Lung, lymph nodes, soft tissue, bone, brain | Lung, lymph nodes, adrenal, bone, brain | Lung | Lung, lymph nodes, peritoneum |
| Neurologic Symptoms at LMD Dx | Nausea, headache, confusion, vision loss, facial twitching | Nausea, headache, confusion, change in mental status | Nausea, headache, dizziness | Nausea, headache, neck stiffness, diplopia, dizziness |
| MRI/CT | MRI: Positive CT: Negative |
MRI: Positive CT: Negative |
MRI: Positive | MRI: Positive |
| MRI Impression | Leptomeningeal disease predominantly in the posterior fossa including about the cerebral aqueduct and in the right frontal operculum | Slightly increased size of lesions suspicious for metastases, again representing either parenchymal metastases or leptomeningeal deposits with parenchymal invasion | Superficial enhancing foci in the posterior aspect of the cervical cord, representing leptomeningeal disease | Leptomeningeal enhancement consistent with leptomeningeal carcinomatosis |
| Lumbar Puncture Analysis | None | Cytology: Suspicious for carcinoma Glucose: 92 mg/dL Protein: 56 mg/dL |
Cytology: Non-malignant Glucose: 60 mg/dL Protein: 42 mg/dL |
Cytology: Malignant cells Glucose: 106 mg/dL, 102 mg/dL Protein: 34 mg/dL, 21 mg/dL |
| Germline Mutations | MUTYH | - | - | - |
| Somatic Mutations | PDAC Primary: KRAS G12D, ATM | Lung: KRAS G12D, TP53, DNMT3A, NTRK1, PTPRT, POLE Adrenal: KRAS G12D, TP53, CARD11, DNMT3A, NTRK1, PTPRT, STAT3 |
CSF: KRAS G12R, TP53, MYC, CDKN2B, CDKN2Ap, SMAD4 | Insufficient tissue |
| Tumor Mutation Burden (TMB) and Microsatellite Instable (MSI) Sensor Score | PDAC Primary: TMB 2 mt/Mb, MSI Sensor Score 0.11 | Lung: TMB 4.9 mt/Mb, MSI Sensor Score 0 Adrenal: TMB 6.1 mt/Mb, MSI Sensor Score 0 |
CSF: TMB: 2.6 mt/Mb, MSI Sensor Score 0.39 | Insufficient tissue |
| Overall Survival | 12.4 years | 9.2 years | 1.5 years | 1.6 years |
| Survival following LMD Diagnosis | 0.5 months | 2.8 months | 2.1 months | 1.1 months |
Case Summaries
Patient 1
In 2006, a 61-year-old female underwent a pancreaticoduodenectomy for AJCC stage IIA PDAC (T3N0M0). The patient received adjuvant gemcitabine, capecitabine, and docetaxel and was disease free for five years. In 2012, the patient underwent a distal pancreatectomy and splenectomy, and pathology revealed a second primary PDAC. Lung metastasis was soon identified, and the patient received gemcitabine/nab-paclitaxel for 3.5 years. Somatic testing of her primary tumor demonstrated a KRAS and ATM mutation, with a tumor mutation burden (TMB) of 2 mutations per megabase (mt/Mb) and a microsatellite instable (MSI) sensor score of 0.11 (microsatellite stable). Germline testing identified an MUTYH mutation. Six years after the second PDAC primary was diagnosed, the patient reported confusion, vision loss, and headaches. An MRI confirmed brain metastasis and LMD. Over the next three weeks, the patient experienced seizures, agitation and confusion. She elected for palliative care and transferred to hospice, passing away three weeks after her diagnosis of LMD. Table 1 summarizes the data for all four patients.
Patient 2
In 2009, a 68-year-old male underwent a distal pancreatectomy and splenectomy and received adjuvant gemcitabine for a body PDAC, AJCC stage IIA (T3N0M0). One year later, the patient was treated with chemoradiation for locally recurrent disease. Following three years of disease-free survival, a solitary lung nodule was resected. Somatic testing of the metastasis revealed KRAS, TP53, DNMT3A, NTRK1, PTPRT, and POLE mutations, with a TMB of 4.9 mt/Mb and an MSI sensor score of 0 (microsatellite stable). He initiated FOLFIRINOX two years later in the setting of disease progression in the lungs, lymph nodes, and adrenal glands. Somatic testing of an adrenal metastasis revealed KRAS, TP53, CARD11, DNMT3A, NTRK1, PTPRT, and STAT3 mutations, with a TMB of 6.1 mt/Mb and an MSI sensor score of 0. After a favorable therapy response, the patient received combination CTLA-4 and CCR4 inhibitors on a clinical trial with transient benefit. Nine years into his disease course, the patient experienced clinical decline, fever, nausea, and headache. A CT scan demonstrated lesions in the brain and an MRI confirmed LMD. Cerebrospinal fluid (CSF) cytology demonstrated cells suspicious for carcinoma. Laboratory analysis indicated elevated protein levels of 56 mg/dL (reference range 21–38 mg/dL) and elevated glucose levels of 92 mg/dL (reference range 38–82 mg/dL). Given further clinical decline, supportive care was pursued, and the patient died three months after his LMD diagnosis.
Patient 3
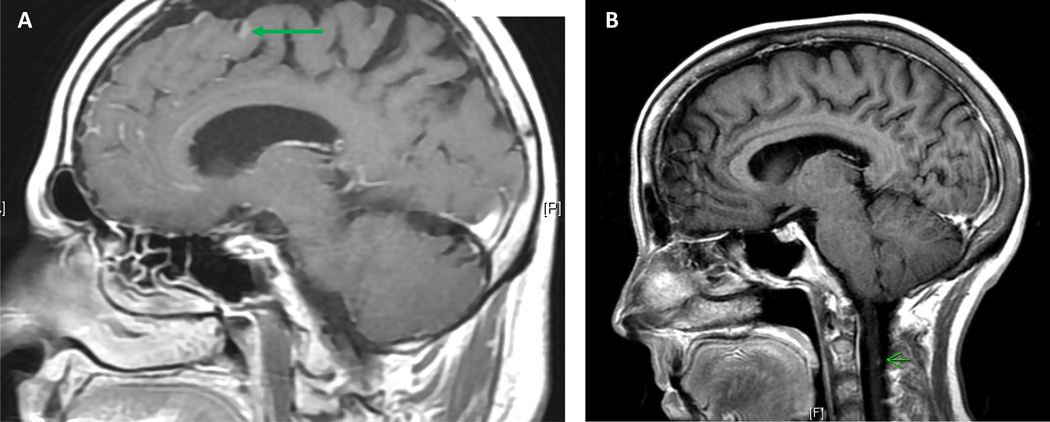
In 2018, a 57-year-old female was diagnosed with stage IV PDAC metastatic to the lungs and initiated FOLFIRINOX. Germline testing was negative for pathogenic mutations. With regression of lung metastasis and a stable pancreas tumor, she underwent definitive chemoradiation to the primary tumor. The patient subsequently received FOLFIRI and gemcitabine/nab-paclitaxel. Sixteen months after her diagnosis of PDAC, the patient developed headache, nausea, and dizziness. An MRI brain demonstrated LMD, also evident on spine MRI (Figure 2b). Cytology from lumbar puncture was negative for malignant cells, although somatic KRAS, TP53, and SMAD4 mutations were observed, felt to be consistent with the presence of malignant cells. The TMB of the sample was 2.6 mt/Mb and the MSI sensor score was 0.39. CSF analysis identified elevated protein levels of 42 mg/dL and normal glucose levels. The patient transitioned to hospice, where she died two months following her diagnosis of LMD.
Figure 2:
Leptomeningeal disease on MRI. Panel A demonstrates sulcal enhancement in the frontal lobe on sagittal T1-weighted post contrast imaging; Panel B demonstrates small cervical leptomeningeal nodule on sagittal T1-weighted post contrast imaging.
Patient 4
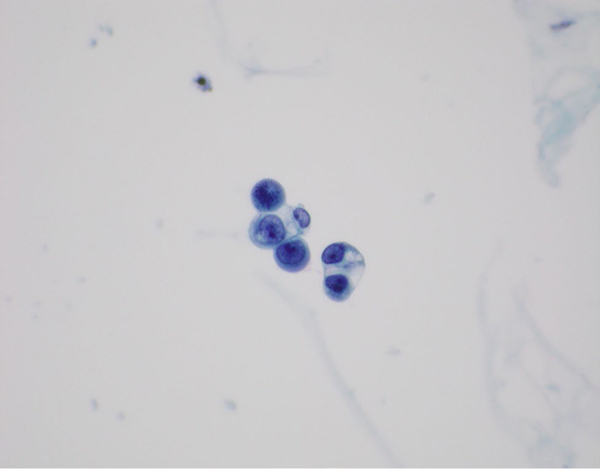
A 65-year-old male was diagnosed with PDAC metastatic to lungs and lymph nodes. He received gemcitabine/nab-paclitaxel and a PD-1 inhibitor with a partial response maintained for five months. The patient developed drug-related pneumonitis and therapy was subsequently switched to modified FOLFIRINOX. One year later, he developed nausea, headaches, weakness, diplopia and unsteadiness. An MRI identified LMD in the brain without spinal involvement (Figure 2a). The patient underwent two lumbar punctures, both of which were positive for malignant cells, the second performed to improve headaches (Figure 3). Further CSF examination identified normal protein levels of 34 mg/dL and elevated glucose levels of 102 mg/dL. The patient was enrolled on a clinical trial of proton craniospinal radiation for LMD, but experienced worsening gait, confusion, nausea, and headaches. In view of progressive clinical decline, radiation therapy was terminated, and the patient died one month after his LMD diagnosis.
Figure 3:
Cytospin slide from cerebrospinal fluid specimen shows clusters of malignant epithelial cells with flocculent, vacuolated cytoplasm and a rare mitotic figure. (Papanicolaou stain, 600x)
Discussion
Leptomeningeal disease (LMD) in the setting of pancreatic adenocarcinoma (PDAC) is a rare occurrence that further complicates an already aggressive malignancy. Herein, we analyzed a series of patients to further detail clinical, genomic, and radiographic characteristics of PDAC patients with LMD.
Our literature search revealed that the majority of LMD research in PDAC consists of single case reports and these data are summarized in Table 2. These case reports include ten male and three female patients between the ages of 45 to 80 years at diagnosis of PDAC. Following a diagnosis of LMD, seven patients pursued whole brain radiation and/or chemotherapy. OS ranged from 1.5 months to 41 months and survival post LMD diagnosis ranged from 15 days to 12 months.
Table 2.
Case Reports of Leptomeningeal Disease in Pancreas Cancer
| Ref. (year) | Age, M/F | LMD Synchronous or Asynchronous | Other Sites of Met. | Neurologic Signs | Positive or Negative Imaging | CSF: Malignant or Atypical Cells | CSF: Other characteristics | Treatment for LMD | Overall Survival | Survival Post LMD Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Ikeda (2020)17 | 59, M | Asynchronous | Liver | Neck pain, back pain, dysarthria | CT: + | Atypical | Elevated protein, decreased glucose | Palliative | 3–4 months | 15 days |
| Ceccon (2020)16 | 51, M | Asynchronous | Kidney, spleen, lung | Headache, neck stiffness | MRI: + | Negative | Elevated protein, decreased glucose, mild pleocytosis | Gemcitabine/nab-paclitaxel | 35 months | 3 months |
| Johnson (2018)19 | 53, M | Asynchronous | Liver, brain | Headache, dysarthria | MRI: + | Not reported | Not reported | Whole-brain radiation, intrathecal topotecan, capecitabine, irinotecan, bevacizumab | 41 months | 12 months |
| Yoo (2015)3 | 80, M | Synchronous | Liver | Headache, seizure | MRI: + | Negative | Slightly elevated protein, slightly decreased glucose | Whole-brain radiation | Not reported | Not reported |
| Naqvi (2015)39 | 58, F | Asynchronous | Lymph nodes | Confusion, agitation | CT: + | Malignant | Not reported | Palliative | 2 months | 1 month |
| Hong (2014)8 | 72, F | Asynchronous | Liver, peritoneum | Ataxia, slurred speech, headache | MRI: + | Atypical | Elevated protein | Palliative | 8.5 months | 2.5 months |
| Madhurima (2013 40 | 45, F | Asynchronous | Peritoneum, lymph nodes | Headache, slurred speech, weakness, agitation | CT: + MRI: + | Malignant | Elevated protein | Palliative | Not reported | Not reported |
| Rao (2013)24 | 57, M | Synchronous | Lung, liver | Seizure, weakness, neck stiffness, photophobia | MRI: + | Atypical | Elevated protein | Whole-brain and spine radiation | Not reported | Not reported |
| Blows (2012) 41 | 72, M | Post-mortem | Liver, lymph nodes | Deafness, facial nerve palsy, bulbar nerve palsy, ataxia, consciousness | MRI: − | Negative | Elevated protein, decreased glucose | Palliative | Post-mortem report | Post-mortem report |
| Minchom (2010) 23 | 59, M | Synchronous | - | Leg flank pain, seizure | MRI: + | Malignant | Elevated protein, elevated white blood cell count | Intrathecal methotrexate and cytarabine, gemcitabine | 1.5 months | 1.5 months |
| Hirota (2008)20 | 64, M | Asynchronous | Vertebrae, peritoneum | Headache, vomiting | MRI: + | Not reported | Not reported | Whole-brain radiation | 41 months | 1 month |
| Grira (2007)22 | 55, M | Synchronous | Not reported | Bilateral vision loss, headaches, vomiting | CT: − | Malignant | Not reported | Palliative | 2 months | 2 months |
| Ferreira (2001)21 | 49, M | Asynchronous | Ribs, lymph nodes | Headache, vomiting, urinary retention, paraplegia | CT: − | Malignant | Elevated protein | Intrathecal methotrexate, cytarabine, thiotepa | 6–7 months | 1.5 months |
A notable characteristic of our case series is that all four patients had an OS of at least 18 months following their initial PDAC diagnosis, with a range of 18 to 149 months. Previous case reports have also noted longer clinical courses of three to four years in patients who developed LMD, supporting the hypothesis that PDAC patients with a longer OS are more likely to develop LMD16, 19, 20. However, it is important to note that LMD has also been reported in PDAC patients with shorter clinical courses and has been diagnosed synchronously3, 8, 17, 21–24.
Further characterizing our cohort, all four patients had predominant lung metastasis and a relatively low burden of intra-abdominal disease. Notably, none of the patients had liver metastasis. Patient 1 and 2 were both noted to have lung metastasis at their first metastatic recurrence following surgical resection, a pattern suggesting relative biologic indolence25. While most previous case studies observed LMD in the setting of metastatic disease, LMD in the setting of lung metastasis was not as common, noted in two out of thirteen previous literature reports15, 22.
All patients in this cohort underwent germline genetic testing and only Patient 1 was identified to have a germline mutation (MUTYH), likely unrelated to her PDAC. Of the three patients with somatic testing, all three had a somatic KRAS (2 G12D, 1 G12R) mutation, and two had a TP53 mutation (Table 1). Although limited by the very small size of the cohort, these data do not support that PDAC patients with LMD are a genomically-defined subset. In contrast, lung cancer patients harboring an EGFR mutation are more likely to develop LMD. In an analysis of 5387 patients with lung cancer, 9.4% of patients with an EGFR mutation developed LMD in comparison to 1.7% of patients with wild-type EGFR26.
In the past, LMD was most frequently diagnosed with CSF cytology. However, the sensitivity of CSF cytology is limited, as only approximately 50% of initial specimens yield malignant cells in patients with LMD. With recent improvements in visualization and quality, MR imaging is a standard to definitively diagnose LMD13. Of the three patients with CSF cytology analysis, one patient had positive malignant cytology, one specimen was noted to have cells “suspicious for carcinoma”, and one specimen was negative, although somatic mutations in KRAS, TP53, and SMAD4 were observed in this patient’s CSF, consistent with the presence of malignant cells. Our study and previous findings underscore MR imaging as a critical diagnostic tool for detection of LMD and highlights the sensitivity limitations of CSF cytology.
Prior research suggests that rare cell capture technology used to detect circulating tumor cells (CTC) improves CSF cytology sensitivity in detecting LMD. In a study by Pentsova et al, the sensitivity of CSF cytology, MRI, and circulating tumor cell (CTC) cytology was evaluated in 95 patients with various solid tumors suspicious for LMD. Receiver operating characteristic analysis revealed that CTC-CSF had the highest sensitivity of 93% (95% CI, 84–100%), with a specificity of 95% (95% CI, 90–100%), positive predictive value 90% (95% CI, 79–100%), and negative predictive value of 97% (95% CI, 93–100%)27. In our study, Patient 4 was observed to have elevated CTC in his CSF. Increased use of CTC-CSF may aid in the early detection of LMD.
LMD is a prognostic indicator of a very poor outcome. In our cohort, all four patients had an OS of less than three months following LMD diagnosis, and three did not receive further therapy. In the past, IT has been preferred to X-ray-based photon craniospinal irradiation (CSI) due to toxicities. However, recent data suggests that patients without extra-CNS disease and high neurological functioning may experience greater benefit from x-ray-based photon CSI28, 29. Additionally, the control CSI toxicity has improved with Helical TomoTherapy (HT), which allows for homogenous dose distribution, in contrast to the field junctions required by traditional linear accelerators30–33. More recently, a phase I trial of CSI for solid tumor LMD using proton beam radiation demonstrated lower toxicity than reported with X-ray-based photon CSI and promising survival outcomes (median CNS PFS=7 months, OS=8 months)34. Although further investigation is needed, improved patient selection and advancements in radiation makes CSI a possible treatment option for LMD patients28, 29.
While many patients elect for palliative care, there are instances in which LMD from PDAC has been treated and briefly controlled. In the case study by Ceccon et al, the patient received gemcitabine/nab-paclitaxel following a diagnosis of LMD. The patient experienced clinical improvement for three months and CSF cytology demonstrated the elimination of tumor cells16. Previous research has shown that gemcitabine crosses the blood brain barrier, and therefore, could be effective in palliating LMD35, 36. In the case study by Johnson et al, a patient fared exceptionally well following a diagnosis of LMD, living for an additional nine months. The patient received whole-brain radiation therapy followed by multiple lines of therapy including, intrathecal topotecan, capecitabine and irinotecan (CAPIRI) and bevacizumab19.
The optimal therapeutic strategy for LMD in the setting of PDAC is unknown given its rarity. In lung and breast cancer, there is a greater depth of knowledge. LMD is more frequent and most patients are treated for LMD. In a retrospective review of 233 patients with breast cancer and LMD, Abouharb et al observed that only 10% of patients went directly to hospice37. Likewise, in an analysis of 171 patients with non-small-cell lung cancer, Nevel et al observed that 11% of patients transitioned straight to hospice post LMD diagnosis38.
Median OS of lung and breast cancer patients following diagnosis of LMD remains comparable to those with PDAC. In the cohort from Nevel et al, median OS from LMD diagnosis was 4.2 months (95% CI 3.6–4.9)38. In the cohort from Abouharb et al median OS from LMD diagnosis was 4.4 months for HER2+BC (95 % CI 2.8, 6.9), 3.7 months (95 % CI 2.4, 6.0) for HR+/HER2−BC, and 2.2 months (95 % CI 1.5, 3.0) for TNBC (p = 0.0002)37. The remarkably poor prognosis and need for better treatment options is shared by patients with LMD in the setting of various solid tumors, including breast and lung cancers.
The key limitations of this study are the retrospective nature, single institution design and very small sample size accrued over an extended time period, although notably all four patients were diagnosed in recent years. Nonetheless, this analysis represents the largest series of PDAC patients with LMD.
Conclusion
LMD from PDAC is a rare diagnosis and is associated with relative longevity and may be more frequent in the setting of lung metastasis. Somatic driver oncogenic changes for patients with LMD appear typical of PDAC. Prognosis is poor and life expectancy is short following diagnosis of LMD. Further insight into therapeutic strategies for LMD from any solid organ malignancy is critically needed.
Acknowledgements
Funding
David M. Rubenstein Center for Pancreatic Cancer Research
NCI Cancer Center Support Grant: P30 CA008478 (PI Craig Thompson) supported the study PI (E.M O’Reilly) and the overall study conduct.
EOR Research Funding to MSK: Genentech/Roche, Celgene/BMS, BioNTech, BioAtla, AstraZeneca, Arcus.
EOR Consulting Role: Cytomx Therapeutics (DSMB), Rafael Therapeutics (DSMB), Sobi, Silenseed, Molecular Templates, Boehringer Ingelheim, BioNTech, Ipsen (+ spouse), Polaris (+ spouse), Merck (+ spouse), AstraZeneca (+ spouse), Bayer (spouse), Genentech/Roche (spouse), Celgene/BMS (spouse), Eisai (spouse).
Footnotes
Conflict of Interest/Disclosure
No other authors have disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Wang N, Bertalan MS, Brastianos PK: Letomeningeal metastasis from systemic cancer: Review and update on management. Cancer 2018; 124: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo IK, Lee HS, Kim CD, Chun HJ, Jeen YT, Keum B et al. : Rare case of pancreatic cancer with leptomeningeal carcinomatosis. World J Gastroenterol 2015; 21: 1020–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchesne P: Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol 2010; 11: 871–879. [DOI] [PubMed] [Google Scholar]
- 5.Park KS, Kim M, Park SH, Lee KW: Nervous system involvement by pancreatic cancer. J Neurooncol 2003; 63: 313–316. [DOI] [PubMed] [Google Scholar]
- 6.Mack F, Baumert BG, Schäfer N, Hattingen E, Scheffler B, Herrlinger U et al. : Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev 2016; 43: 83–91. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain MC: Neoplastic meningitis. Curr Neurol Neurosci Rep 2008; 8: 249–258. [DOI] [PubMed] [Google Scholar]
- 8.Hong CS, Kurt H, Elder JB: Asynchronous leptomeningeal carcinomatosis from pancreatic cancer: A case report and review of the literature. Clin J Gastroenterol 2014; 7: 434–440. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al. : Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y et al. : Folfirinox versus gemcitabine for metastatic pancreatic cancer. 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 11.Wang-Gillam A, Hubner RA, Siveke JT, Von Hoff DD, Belanger B, de Jong FA et al. : Napoli-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. European Journal of Cancer 2019; 108: 78–87. [DOI] [PubMed] [Google Scholar]
- 12.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ et al. : Maintenance olaparib for germline brca-mutated metastatic pancreatic cancer. 2019; 381: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM: Leptomeningeal metastases in the mri era. Neurology 2010; 74: 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F et al. : Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 1990; 9: 225–229. [DOI] [PubMed] [Google Scholar]
- 15.Pavlidis N: The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol 2004; 15 Suppl 4: iv285–291. [DOI] [PubMed] [Google Scholar]
- 16.Ceccon G, Wollring M, Brunn A, Deckert M, Waldschmidt D, Fink GR et al. : Leptomeningeal carcinomatosis in a patient with pancreatic cancer responding to nab-paclitaxel plus gemcitabine. Case Rep Oncol 2020; 13: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda Y, Yoshida M, Ishikawa K, Kubo T, Murase K, Takada K et al. : Pancreatic cancer with leptomeningeal carcinomatosis: Case report and literature review. Int Cancer Conf J 2020; 9: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan EJ, Lowery MA, Basturk O, Allen PJ, Yu KH, Tabar V et al. : Brain metastases in pancreatic ductal adenocarcinoma: Assessment of molecular genotype–phenotype features—an entity with an increasing incidence? Clinical Colorectal Cancer 2018; 17: e315–e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson WR, Theeler BJ, Van Echo D, Young P, Kwok M: Treatment of leptomeningeal carcinomatosis in a patient with metastatic pancreatic cancer: A case report and review of the literature. Case Rep Oncol 2018; 11: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirota M, Yagi Y, Yamashita K, Okamoto K, Sato T, Ichihara T: [a long survival case of unresectable pancreatic cancer by chemoradiotherapy with gemcitabine as key drug]. Gan To Kagaku Ryoho 2008; 35: 2413–2416. [PubMed] [Google Scholar]
- 21.Ferreira Filho AF, Cardoso F, Di Leo A, Awada A, da Silva VD, Tovar RB et al. : Carcinomatous meningitis as a clinical manifestation of pancreatic carcinoma. Ann Oncol 2001; 12: 1757–1759. [DOI] [PubMed] [Google Scholar]
- 22.Grira MT, Ben Jemaa HM, Lammouchi TM, Benammou SA: Meningitis revealing pancreatic carcinoma. Neurosciences (Riyadh) 2007; 12: 256–258. [PubMed] [Google Scholar]
- 23.Minchom A, Chan S, Melia W, Shah R: An unusual case of pancreatic cancer with leptomeningeal infiltration. J Gastrointest Cancer 2010; 41: 107–109. [DOI] [PubMed] [Google Scholar]
- 24.Rao R, Sadashiv SK, Goday S, Monga D: An extremely rare case of pancreatic cancer presenting with leptomeningeal carcinomatosis and synchronous intraparenchymal brain metastasis. Gastrointest Cancer Res 2013; 6: 90–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin IH, Elias H, Chou JF, Capanu M, O’Reilly EM: Pancreatic adenocarcinoma: Insights into patterns of recurrence and disease behavior. BMC cancer 2018; 18: 769–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB et al. : Leptomeningeal metastases in patients with nsclc with egfr mutations. J Thorac Oncol 2016; 11: 1962–1969. [DOI] [PubMed] [Google Scholar]
- 27.Lin X, Fleisher M, Rosenblum M, Lin O, Boire A, Briggs S et al. : Cerebrospinal fluid circulating tumor cells: A novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro Oncol 2017; 19: 1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Shafie RA, Böhm K, Weber D, Lang K, Schlaich F, Adeberg S et al. : Outcome and prognostic factors following palliative craniospinal irradiation for leptomeningeal carcinomatosis. Cancer management and research 2019; 11: 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devecka M, Duma MN, Wilkens JJ, Kampfer S, Borm KJ, Münch S et al. : Craniospinal irradiation(csi) in patients with leptomeningeal metastases: Risk-benefit-profile and development of a prognostic score for decision making in the palliative setting. BMC cancer 2020; 20: 501–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugie C, Shibamoto Y, Ayakawa S, Mimura M, Komai K, Ishii M et al. : Craniospinal irradiation using helical tomotherapy: Evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat 2011; 10: 187–195. [DOI] [PubMed] [Google Scholar]
- 31.Schiopu SRI, Habl G, Häfner M, Katayama S, Herfarth K, Debus J et al. : Craniospinal irradiation using helical tomotherapy for central nervous system tumors. J Radiat Res 2017; 58: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baisden JM, Benedict SH, Sheng K, Read PW, Larner JM: Helical tomotherapy in the treatment of central nervous system metastasis. 2007; 22: 1. [DOI] [PubMed] [Google Scholar]
- 33.Hermann B, Hültenschmidt B, Sautter-Bihl M-L: Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlentherapie und Onkologie 2001; 177: 195–199. [DOI] [PubMed] [Google Scholar]
- 34.Yang TJ, Wijetunga NA, Yamada J, Wolden S, Mehallow M, Goldman DA et al. : Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigmond J, Honeywell RJ, Postma TJ, Dirven CM, de Lange SM, van der Born K et al. : Gemcitabine uptake in glioblastoma multiforme: Potential as a radiosensitizer. Ann Oncol 2009; 20: 182–187. [DOI] [PubMed] [Google Scholar]
- 36.Maraveyas A, Sgouros J, Upadhyay S, Abdel-Hamid AH, Holmes M, Lind M: Gemcitabine twice weekly as a radiosensitiser for the treatment of brain metastases in patients with carcinoma: A phase i study. British journal of cancer 2005; 92: 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abouharb S, Ensor J, Loghin ME, Katz R, Moulder SL, Esteva FJ et al. : Leptomeningeal disease and breast cancer: The importance of tumor subtype. Breast Cancer Res Treat 2014; 146: 477–486. [DOI] [PubMed] [Google Scholar]
- 38.Nevel KS, DiStefano N, Lin X, Skakodub A, Ogilvie SQ, Reiner AS et al. : A retrospective, quantitative assessment of disease burden in patients with leptomeningeal metastases from non-small-cell lung cancer. Neuro Oncol 2020; 22: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naqvi SA, Ahmed I: Carcinomatous meningitis: A rare complication of pancreatic adenocarcinoma. J Coll Physicians Surg Pak 2015; 25: 458–459. [PubMed] [Google Scholar]
- 40.Anne M, Ahmad N, Lee P, Aziz M, Lebowicz Y: An unusual presentation of isolated leptomeningeal disease in carcinoma of unknown primary with pancreatic features. J Investig Med High Impact Case Rep 2013; 1: 2324709613494830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blows SJ, Morgan R, Dhariwal U, Petts G, Roncaroli F: Pancreatic adenocarcinoma presenting with sudden onset bilateral deafness secondary to metastatic leptomeningeal infiltration. Age Ageing 2012; 41: 818–819. [DOI] [PubMed] [Google Scholar]