Abstract
Background:
Type Ⅲ and Ⅳ portal vein tumor thrombi (PVTT) cannot be removed through surgery, and no effective therapeutic procedure is available. Type Ⅲ/Ⅳ PVTT can be downstage to type I/II PVTT by using Radiotherapy, and can further be can be removed surgically. Thus, radiotherapy may be an effective treatment for type Ⅲ/Ⅳ PVTT. This study aims to evaluate the efficacy and toxicity of radiotherapy for type III-IV PVTT.
Methods:
This prospective study was conducted from August 1, 2017, to September 30, 2019, for patients with type Ⅲ and Ⅳ PVTT. Patients received radiotherapy with a target dose of 50Gy/25f or 59.5Gy/17 f. Advanced radiological technique such as image fusion technique for CT image and MRI image were utilized to produce more precise lesion localization, and limit the dose to organs at risk in order to get a better downstage rate and less adverse complications.
Results:
Nine (9) patients with type Ⅲ PVTT and 5 patients with type Ⅳ PVTT were included in this study. 12 patients received a radiotherapy dose of 50Gy/25f, 2 patients received 59.50Gy/17 f. After radiotherapy, 92.9% of patients with PVTT were successfully downstage to type II/I. In patients with primary hepatocellular carcinoma, 8 patients (accounting 88.9%) achieved down-stage. 5 patients with other types of tumors achieved downstage which accounts 100%. In addition, none of the 14 patients observed radiation hepatitis and radiation liver failure. And none of the patients developed gastrointestinal ulcers and thrombocytopenia.
Conclusion:
Radiotherapy is a suitable treatment measure for type Ⅲ and Ⅳ PVTT to get downstage and make the opportunity for surgery. Image fusion technology for precise lesion location such as CT-MRI image fusion, and strict dose limitation of organ at risk, contributed to the improvement of radiotherapy efficiency and the significant decrease in adverse complications.
Keywords: PVTT, radiotherapy, CTA-MRI fusion image
Background
The incidence of primary hepatocellular carcinoma (HCC) ranks the sixth in all kinds of tumors in the world. More than half of the new cases and deaths of liver cancer occur in China each year.1 Because of the atypical symptoms of liver cancer, 70% to 80% of patients have been in advanced stage at the time of diagnosis, and the incidence of venous tumor thrombosis is very high.2,3 Portal vein tumor thrombosis (PVTT) is the most common form of macrovascular invasion of advanced HCC and the incidence of PVTT is about 62.2% to 90.2%.4,5 And the prognosis of HCC patients is poor which has been demonstrated with a hazard ratio (HR) of death to 2 and the overall survival time (OS) is only 2-4 months with best support treatment.5,6
The classification method of PVTT is currently using Oriental hepatobiliary classification (Cheng ‘s classification),7 and Japanese VP classification.8 The Oriental hepatobiliary classification classify PVTT into I ∼ IV type according to the degree of development of the tumor thrombus and was used in this study. This classification is relayed on the growth rules and characteristics of tumor thrombi and the characteristics of portal vein anatomy and it can objectively reflect the order of severity of illness and the prognosis.7,9
Surgical resection is one of the treatments for liver cancer with portal vein thrombosis, and it is the most effective therapeutic among all treatments.10,11 Most PVTT developed from the primary tumor lesion as the base. The purpose of surgery is to remove both the primary tumor and the tumor thrombosis.12 In principle, surgery is suitable for only type I and II portal vein tumor thrombi. However, because of the extremely poor surgical efficacy for type Ⅲ and Ⅳ PVTT, it is not recommended to perform surgery for type Ⅲ/Ⅳ PVTT. Radiotherapy may be an effective therapeutic option for type Ⅲ and Ⅳ PVTT.13,14 Radiotherapy has the following advantages: Firstly, Type III/Ⅳ PVTT could be downstage to type I/II PVTT to obtain the possibility of surgery. Secondly, simultaneous irradiation of both tumor thrombi and primary tumors is helpful for local control of tumors. Thirdly, it is proved that radiotherapy can increase the survival rate of patients with advanced hepatocellular carcinoma and portal vein thrombosis.15,16 However, the radiation hepatitis and radiation liver failure were often observed in the patients of HCC and PVTT who received radiotherapy, since most of the patients with primary hepatocellular carcinoma (HCC) have underlying medical conditions such as hepatitis B/C or liver cirrhosis, and the liver function of most patients is abnormal. The poor liver function will get worse with the wrong target volume, or ignoring of respiratory exercise and so on.17,18
This study aims to utilize advanced radiological technique such as image fusion technique for CT image and MRI image to produce more precise lesion localization and better downstage effect, limit the dose to the organs at risk and decrease the adverse complications.
Patients and Methods
Patients
The eligibility criteria were: (1) age 20-60 years old, (2) pathologically confirmed HCC patients, (3)CT or MRI suggest that patients with primary hepatocellular carcinoma or other kinds of liver cancer with type III-IV portal vein tumor thrombus, (4) The patient has no other malignant tumors or distant metastases, (5) The tumor does not invade extrahepatic tissues or organs other than the portal vein, (6) The patient’s basic condition is stable before treatment, and the liver function is Child-pugh grade A or B, (7) No serious medical diseases, such as coronary heart disease, etc.
The exclusion criteria were: (1) The general condition of the patient is poor, with an EOCG score of 3-4, (2) The patients with other serious medical conditions, (3) The patient has refractory ascites or hepatic encephalopathy, (4) Patients with severe gastric esophageal varices or history of bleeding from rupture of gastric esophagus vein, (5) CT / MRI suggestive of liver metastatic tumor patients,(6)History of previous liver surgery and radiation.
Radiotherapy
Elekta Liniac (VersaHD, 2018) was used. Four dimensional CT simulation localization technology and Image guided radiotherapy (IGRT) were applied.
Image fusion
Image fusion software, MIM (MIM 6.7.5, Cleveland, USA), was utilized for the MRI-CT image fusion for delineation of gross target volume(GTV). The computed tomographic angiography (CTA) image of hepatic artery (25 seconds delay time) was used to outline the primary lesion of liver cancer. The computed tomographic venography (CTV) image (55-60 seconds delay time) of portal vein is used to outline PVTT. The enhanced MRI images included T1-weighted image and T2-weighted image, and the T2-weighted enhanced MRI image was selected to construct fusion images which were fused with CTV image. Meanwhile, the 4D-CT positioning method was adopted to eliminate the displacement and the error of radiotherapy target volume caused by respiratory motion.
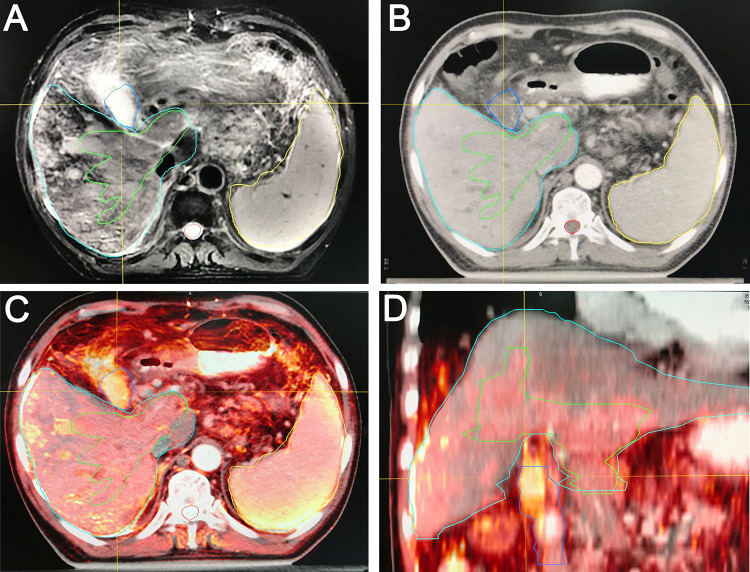
As shown in Figure 1, the T2-weighted MRI images (A), and the CT images (B) were accurately matched accurately into fusion images(C). GTV which including primary lesion and PVTT, and organs at risks were outlined in the fusion images.
Figure 1.
The CTV-MRI fusion images. A, T2 MRI image; B, CT image; C, MRI-CT fusion image (Transverse); D, MRI-CT fusion image (Coronal).
Radiotherapy planning
Intensity Modulated Radiation Therapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT) were used in this study to get better dose distribution. Principal radiotherapy planning system, Pinnacle 3, (Philips Radiation Oncology Systems, Fitchburg, MA, USA) was utilized to get best dose distribution of the target volumes. The clinical treatment volume (CTV) was defined by the GTV plus a 0.5-cm margin. The planning Target Volume (PTV) was expanded with a certain margin based on CTV, which is determined by the movement of the inner target area, the positioning error and set-up error.
Dose selection of liver cancer
If the volume enclosed by 50% isodose line, is less than 25% of residual liver volume, 60 Gy will be selected. If the volume enclosed by 50% isodose line, is between 25% to 49% of residual liver volume then 45-54 Gy will be selected. 30-45 Gy will be selected if the volume is between 50% to 75% of residual liver volume. And the radiotherapy will be prohibited if this volume is larger than 75% of residual liver volume.
The dose limitation of the organs at risk (OAR)
Firstly, if radiotherapy is conventional fractionation:
When liver function is Child-Pugh A, the following criteria should be met: the mean dose of residual liver should <23 Gy, The volume of residual liver (liver-GTV) of which Dmean <15 Gy should >700ml, V55 < 1 ml, V50 < 3ml, V45 < 5ml, V40 < 10ml;
When liver function is Child-Pugh B, the mean dose of residual liver should <6 Gy;
Secondly, if radiotherapy is stereotactic radiotherapy, liver function should be Child-Pugh A, and the mean dose of residual liver should <15 Gy.
Thirdly, the dose limitation of both stomach and duodenum must meet: Dmax < 52 Gy, V40 Gy < 10 ml; V45 Gy < 5 ml; V50 Gy < 3 ml; V55 Gy < 1 ml.19-23
Support Treatment
Before the start of radiotherapy, all patients were required to have liver function test (LFT). All patients with liver function of Child B level were treated with liver protection treatment including the albumin supplementation, and transaminase reduction treatment, and helped convert their liver function from Child B level to Child A level. In addition, all patients underwent gastroscopy examination before the radiotherapy. If the patient was found with peptic erosion or ulcer in stomach or duodenal, they first receive treatment for peptic erosion and ulcer. Radiotherapy was executed once the ulcer has healed. If patient was found with hepatitis B or C, antiviral therapy was given to inhibit the virus replication of hepatitis at the same time during the radiotherapy; Meanwhile, Sorafeni was also taken during the radiotherapy for hepatocellular carcinoma (HCC) if the liver function meets the requirement. All patients were given liver protection treatment during and after the radiotherapy;
Observation Index and Statistical Analysis
All patients performed CT and MRI examination to evaluate the primary lesion and PVTT 1 month and then every 3 months after the radiotherapy. Examination including blood routine and liver function, enhanced CT or MRI were also performed regularly after the radiotherapy. PVTT downgrade rate, incidence of adverse complications (Radiation hepatitis and Radiation liver failure, thrombocytopenia, etc) were analyzed. All statistical analysis was done with SPSS software (version 21.0).
Results
Patients Characteristics
From August 1, 2017, to September 30, 2019, 14 patients with type Ⅲ and Ⅳ PVTT had received the radiotherapy. All patients finished the treatment and none patients were excluded. Among them, liver cancer and pancreatic cancer are the most common pathological types, accounting 64.3% and 21.5% separately. Type Ⅳ PVTT, accounting for 35.7% of all patients, others were type Ⅲ PVTT, accounting for 64.3%. Moreover, 85.7% patients had received a dose of 50Gy/25f, and 2 patients received radiotherapy dose of 59.50Gy/17 f. Most of our research patients were with hepatitis B/C and/or hepatic cirrhosis simultaneously, accounting for 64.3% in all patients. The detailed characteristics of patients are presented in table 1.
Table 1.
The Features of Patients with PVTT.
| Child-Pugh evaluate before radiotherapy | A | B |
| 9(64.3%) | 5(35.7%) | |
| Primary lesion site | liver cancer | Gallbladder cancer |
| 9(64.3%) | 1 (7.1%) | |
| Pancreatic cancer | Retroperitoneal paraganglioma | |
| 3 (21.5%) | 1 (7.1%) | |
| Type of PVTT before radiotherapy | III | IV |
| 9 (64.3%) | 5(35.7%) | |
| Combined hepatitis B/C cirrhosis | Yes | No |
| 9 (64.3%) | 5(35.7%) | |
| Dose of PTV | Dt: 50Gy/25f | Dt: 59.50Gy/17f |
| 12(85.7%) | 2(14.3%) | |
| Outcome of PVTT after radiotherapy | down staging (II) | shrink but not down staging |
| 13(92.9%) | 1 (7.1%) | |
| Abnormal liver function after radiotherapy | none | |
| Thrombocytopenia or peptic ulcer | none |
Treatment Response
All 14 patients with PVTT were observed to have down staged tumor or lesion shrunk, as shown in Table 2. Among these patients, 13 patients (92.9%) were successfully observed to have down staged from stage Ⅳ/Ⅲ to stage II. Only one patient was observed to have lesion shrunk but not down staged. Furthermore, one patient with PVTT from pancreatic cancer was found to have all PVTT lesions disappeared and down staged to type Ⅰ/0 stage successfully. All these indicated that radiotherapy is an effective treatment for patients with PVTT and could create opportunity for surgery or follow-up treatment. Meanwhile, among patients with primary hepatocellular carcinoma, 8 patients (accounts for 88.9%) were down staged. Five PVTT patients with other types of cancers were down staged (accounts for 100%). It indicates that both PVTT from hepatocellular carcinoma and PVTT from other types of tumors are all sensitive to radiotherapy. The reduction extent of PVTT was more than the reduction extent of primary hepatocellular carcinoma. It indicates that PVTT may be more sensitive to radiotherapy than the primary tumor.
Table 2.
The Major Features and Outcomes of Each Patients.
| Patients | Primary lesion | Type of PVTT before radiotherapy | Dose of PTV | PVTT after radiotherapy | Abnormal liver function after radiotherapy | Thrombocytopenia or peptic ulcer |
|---|---|---|---|---|---|---|
| 1 | Hepatocellular carcinoma | IV | Dt: 50Gy/25f | downstaging (II) | No | No |
| 2 | Hepatocellular carcinoma | III | Dt: 50Gy/25f | downstaging (II) | No | No |
| 3 | Hepatocellular carcinoma | IV | Dt: 50Gy/25f | shrink but not down staging | No | No |
| 4 | Hepatocellular carcinoma | IV | Dt: 50Gy/25f | downstaging (II) | No | No |
| 5 | Hepatocellular carcinoma | III | Dt: 50Gy/25f | downstaging (Ⅲ) | No | No |
| 6 | Hepatocellular carcinoma | III | Dt: 50Gy/25f | downstaging (II) | No | No |
| 7 | Hepatocellular carcinoma | IV | Dt: 50Gy/25f | downstaging (Ⅲ) | No | No |
| 8 | Hepatocellular carcinoma | IV | Dt: 50Gy/25f | downstaging (Ⅲ) | No | No |
| 9 | Hepatocellular carcinoma | III | Dt: 50Gy/25f | downstaging (II) | No | No |
| 10 | Gallbladder cancer | III | Dt: 50Gy/25f | downstaging (II) | No | No |
| 11 | Pancreatic cancer | III | Dt: 50Gy/25f | downstaging (II) | No | No |
| 12 | Pancreatic cancer | III | Dt: 59.50Gy/17f | downstaging (Ⅰ0) | No | No |
| 13 | Pancreatic cancer | III | Dt: 59.50Gy/17f | downstaging (II) | No | No |
| 14 | Retroperitoneal paraganglioma | III | Dt: 50Gy/25f | downstaging (II) | No | No |
Besides, It was also observed that type III and IV portal vein tumor thrombi, including tumor thrombus in trunk/branch of portal vein, superior mesenteric vein, inferior vena cava, right renal vein, or right atrial, might not show shrinkage in one month after the radiotherapy. They may show obvious shrinkage or even disappear in 2-3 months after the radiotherapy. Same for primary liver cancer lesions, they also shrunk obviously in 2-3 months after the radiotherapy.
Adverse Effects
None of these patients had developed radiation hepatitis and radiation liver failure during or after the radiotherapy. Even patients with hepatitis and liver cirrhosis which accounted for 64.3% of all patients. Moreover, there were no patients developed gastrointestinal ulcers and thrombocytopenia during or 3 months after radiotherapy.
Discussion
liver cancer is a common malignant tumor, which is characterized with tumor thrombus formation even when the tumor size is small.24 Surgery is the main treatment measure for patients with liver cancer and type I/II PVTT. But it is difficult to apply surgery for the patients with type III/IV PVTT25 neither is TACE (Transhepatic arterial chemoembolization) suitable for the treatment of type III and IV PVTT.26
Studies revealed that radiotherapy can be applied in unresectable liver cancer, and appears to be an effective treatment for patients with liver cancer and PVTT.27 Several studies demonstrated that preoperative radiotherapy on the PVTT located in the main trunk or first branch of portal vein can improve the prognosis of patients with HCC with PVTT.15
In Asia, the main reason of the limitation of the radiotherapy application in liver cancer is the irradiation damage on the remaining liver tissue. In Asia, most of the patients with primary hepatocellular carcinoma (HCC) have underlying medical conditions such as hepatitis B/C or/and liver cirrhosis, and the liver function of most patients is abnormal. The precise implementation of radiotherapy is helpful to reduce the incidence of radiation hepatitis and radiation liver failure. All patients in this study were not observed with radiation hepatitis and radiation liver failure, even though most patients have viral hepatitis in this study. Thrombocytopenia and peptic ulcer of stomach and duodenum were not occurred in the process of radiotherapy in all patients. The good protection of organs at risks (OARs) including liver, stomach, and duodenum were achieved by decreasing the amount of radiation dose to OARs and by utilizing image fusion method, the fusion image of CT with MRI. A strict dose limitation of OAR and residual liver were also important in reducing the incidence of complication of radiotherapy, such as liver function failure and peptic ulcer.
Study had demonstrated that CT-scan was useful in the detection of lesions in portal vein in hepatocellular carcinoma and in evaluation of changes in hepatic parenchymal blood flow.28,29 Past research have recommended that the image of 25 seconds delay time and 55 seconds delay time were respectively used to outline the primary lesion of liver cancer and tumor thrombus in portal vein; Secondly, Because of the advantages of high resolution of soft tissue, multi-directional imaging, multi-parameters and multi-information, MRI has become an irreplaceable examination method in the diagnosis and treatment of liver cancer.30,31 Fusion MRI images with CT images could improve the accuracy of lesion localization and GTV delineation. In this study, MIM software, was used to fuse the enhanced MRI images with the CT images in this study. MIM is often used in automatic sketch function, but not widely used in image fusion of radiotherapy.32 Upon our best search in the literatures, this is the first study to fuse CT images with MRI images through MIM software in radiotherapy of patients with PVTT. The image fusion function of MIM were executed based on VoxAlign algorithm. The basic process were rigid registration and then automatic deformation registration. In this study, CT images and MRI images were matched precisely in most organs and tissues. In summary, this software can be an effective tool in image fusion as our results showed. In additional, 4D-CT scanning was adopted to eliminate the error of the radiotherapy target volume caused by respiratory motion in this study.
All these above measures contributed to the good treatment effect of PVTT in this study. In this present study, PVTT in all patients were downsized in various extent (objective response rate was 100%) and 92.9% patients were successfully down-staged. In addition, in the group of patients with primary hepatocellular carcinoma, 88.9% patients were down-staged. This created the opportunity for surgery. Zhang et al had reported objective response rate for radiotherapy of PVTT was 35.6% for patients with PVTT who had been underwood percutaneous transhepatic portal vein stenting and transarterial chemoembolization.33 Wei et al carried out a randomized, multicenter controlled study to explore the efficiency of 3D CRT for patients with resectable hepatocellular carcinoma with type I and II PVTT. They found 20.7% patients down staged successfully.34 Compared with other clinical trials, the efficiency of radiotherapy on PVTT in this study is better. This result suggests that radiotherapy for type III and IV PVTT is an effective treatment procedure.
This study also includes some patients with PVTT induced by tumors other than liver cancer, such as pancreatic cancer, gallbladder cancer and retroperitoneal paraganglioma. In this research, a phenomenon was observed that, remarkable shrinkage of portal vein tumor thrombi or primary liver tumor lesions were not observed in one month after the radiotherapy. The obvious shrinkage was observed at 2-3 months after the radiotherapy. This phenomenon may be caused by the fact that liver cancer, pancreatic cancer, and cholangiocarcinoma are all belong to the late response tissues to radiotherapy.
In conclusion, radiotherapy could be a suitable method to create an opportunity of surgery for patients with type Ⅲ and Ⅳ PVTT. Good treatment effect and low rate of adverse complications were observed in this study through CTV-MRI images fusion technique, strict dose limitation of OAR and 4D-CT scanning. But this study still has some limitations. Fewer patients were included in this research which may lead to the bias. And we will follow up the subsequent survival of these patients next.
Footnotes
Authors’ Contributions: JR conceived and supervised the study; JR designed experiments; JR, SHB, YYW, YLY, HJK, WM, JZZ, YG, and BNH performed experiments; HLM provided new tools and reagents; JR, HLM, XZZ, RL analyzed data; JR and SHB wrote the manuscript; JR and XZZ made manuscript revisions. All authors have read and approved the final version of this submission.
Ethics Approval: This study was approved by the Ethics Committees of Xi’an Jiaotong University. Informed consent was obtained from each patient.
Ethical Statement: Our study was approved by The First Affiliated Hospital of Xi’an Jiaotong University Ethics Committee (approval no. XJTU1AF2017LSK061). All patients provided written informed consent prior to enrollment in the study
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Natural Science Foundations of China (Juan Ren, 81772793/H1621; Juan Ren, 31201060/C0709; Juan Ren, 30973175/C1701; Juan Ren, 81172490/H1621); Scientific and Technological Research Foundation of Shaanxi Province (Juan Ren, 2020JM-368);
ORCID iD: Juan Ren, PhD  https://orcid.org/0000-0002-8360-8286
https://orcid.org/0000-0002-8360-8286
Reference
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Xiangji L, Weifeng T, Bin Y, et al. Surgery of hepatocellular carcinoma complicated with cancer thrombi in bile duct: efficacy for criteria for different therapy modalities. Langenbecks Arch Surg. 2009;394(6):1033–1039. [DOI] [PubMed] [Google Scholar]
- 3. Florman S, Weaver M, Primeaux P, et al. Aggressive resection of hepatocellular carcinoma with right atrial involvement. Am Surg. 2009;75(11):1104–1108. [PubMed] [Google Scholar]
- 4. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxi A, Camma C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274–1283. [DOI] [PubMed] [Google Scholar]
- 5. Chan SL, Mo FK, Johnson PJ, et al. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26(2):340–347. [DOI] [PubMed] [Google Scholar]
- 6. Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol. 2016;22(32):7289–7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi J, Lai EC, Li N, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18(1):74–80. [DOI] [PubMed] [Google Scholar]
- 8. Ikai I, Yamamoto Y, Yamamoto N, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12(1):65–75, ix. [DOI] [PubMed] [Google Scholar]
- 9. Peng SY, Wang XA, Huang CY, et al. Better surgical treatment method for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol. 2018;24(40):4527–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang L, Chen TH, Li C, et al. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB (Oxford). 2018;20(12):1119–1129. [DOI] [PubMed] [Google Scholar]
- 11. Zhang ZY, Dong KS, Zhang EL, Zhang LW, Chen XP, Dong HH. Resection might be a meaningful choice for hepatocellular carcinoma with portal vein thrombosis: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(50):e18362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang ZM, Lai EC, Zhang C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. 2015;20:8–16. [DOI] [PubMed] [Google Scholar]
- 13. Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–943. [DOI] [PubMed] [Google Scholar]
- 14. Cheng S, Chen M, Cai J, National Research Cooperative Group for D, Treatment of Hepatocellular Carcinoma with Tumor T. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget. 2017;8(5):8867–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamiyama T, Nakanishi K, Yokoo H, et al. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12(5):363–368. [DOI] [PubMed] [Google Scholar]
- 16. Lau WY, Zhou WP. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study: reply. World J Surg. 2014;38(5):1247–1248. [DOI] [PubMed] [Google Scholar]
- 17. Yu JI, Park HC. Radiotherapy as valid modality for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2016;22(30):6851–6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee DS, Seong J. Radiotherapeutic options for hepatocellular carcinoma with portal vein tumor thrombosis. Liver Cancer. 2014;3(1):18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng JC, Wu JK, Lee PC, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60(5):1502–1509. [DOI] [PubMed] [Google Scholar]
- 20. Culleton S, Jiang H, Haddad CR, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111(3):412–417. [DOI] [PubMed] [Google Scholar]
- 21. Velec M, Haddad CR, Craig T, et al. Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2017;97(5):939–946. [DOI] [PubMed] [Google Scholar]
- 22. Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810–821. [DOI] [PubMed] [Google Scholar]
- 23. Song JH, Son SH, Kay CS, Jang HS. Identification of biologically effective dose-volumetric parameters that predict radiation-induced hepatic toxicity in patients treated with helical tomotherapy for unresectable locally advanced hepatocellular carcinoma. Medicine (Baltimore). 2015;94(43):e1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ikai I, Arii S, Ichida T, et al. Report of the 16th follow-up survey of primary liver cancer. Hepatol Res. 2005;32(3):163–172. [DOI] [PubMed] [Google Scholar]
- 25. Liu PH, Huo TI, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis. 2018;38(3):242–251. [DOI] [PubMed] [Google Scholar]
- 26. Niu ZJ, Ma YL, Kang P, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29(4):2992–2997. [DOI] [PubMed] [Google Scholar]
- 27. Su F, Chen KH, Liang ZG, et al. Comparison of three-dimensional conformal radiotherapy and hepatic resection in hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med. 2018;7(9):4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dondelinger RF, Kurdziel JC. Computed tomographic arteriography (C.T.A.) of the liver. Bull Soc Sci Med Grand Duche Luxemb. 1988;125(1):27–34. [PubMed] [Google Scholar]
- 29. Kim HC, Kim TK, Sung KB, et al. CT during hepatic arteriography and portography: an illustrative review. Radiographics. 2002;22(5):1041–1051. [DOI] [PubMed] [Google Scholar]
- 30. Lincke T, Zech CJ. Liver metastases: detection and staging. Eur J Radiol. 2017;97:76–82. [DOI] [PubMed] [Google Scholar]
- 31. Pandey P, Lewis H, Pandey A, et al. Updates in hepatic oncology imaging. Surg Oncol. 2017;26(2):195–206. [DOI] [PubMed] [Google Scholar]
- 32. Delpon G, Escande A, Ruef T, et al. Comparison of automated atlas-based segmentation software for postoperative prostate cancer radiotherapy. Front Oncol. 2016, 6:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115(6):1245–1252. [DOI] [PubMed] [Google Scholar]
- 34. Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]