Abstract
Objective:
Cardiovascular disease remains a leading cause of mortality worldwide. Ceramide scores have been associated with adverse outcomes in patients with established coronary artery disease (CAD). The prognostic value of ceramide score has not been assessed in the general population. We tested the hypothesis that ceramide scores are associated with major adverse cardiac events (MACE) in a community-based cohort with average CAD burden at enrollment.
Approach and results:
In a prospective community-based cohort we performed passive follow-up using a record linkage system to ascertain the composite outcome of MACE, defined as acute myocardial infarction, coronary revascularization (bypass grafting or percutaneous intervention), stroke or death. Ceramides were analyzed as log transformed continuous variables, ratios or scores and quartiles with adjustment for confounders. We analyzed 1131 subjects, 52% females, mean age ± (SD) 64 ± 9 years. After a median follow-up of 13.3 years (Q1 12.7, Q3 14.4), 486 patients experienced a MACE: myocardial infarction (80), coronary artery bypass surgery (34), percutaneous coronary intervention (62), stroke (94) and all-cause death (362). Ceramide ratios were significantly associated with MACE independently of LDL-c and conventional CAD risk factors. Those in the highest quartile of ceramide score had nearly 1.5-fold risk of MACE, HR 1.47 (95% CI, 1.12–1.92). There was a dose-response association across quartiles of ceramide ratios and MACE.
Conclusions:
Elevated ceramide score is a robust predictor of CVD and MACE in the community. The risk conferred by the ceramide score has a dose-response behavior and is independent of conventional risk factors.
Graphical Abstract

Introduction
Cardiovascular disease (CVD) remains a leading cause of mortality worldwide with more than 17.8 million deaths per year 1. Accordingly, improved prevention of cardiovascular morbidity and mortality would have a significant impact on public health. Traditional lipid biomarkers such as high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and triglycerides (TG) have been extensively used to evaluate the risk for atherosclerotic CVD for several decades 2–5. HDL-c and LDL-c, along with other constituents of the Framingham score predict approximately 75% of the risk 6 and thus they are important decision making instruments for prevention and treatment 7. However, the need for additional predictive biomarkers is underlined by the very substantial residual risk that exists above the standard clinical and biochemical risk predictors 8.
Ceramides are main constituents of the sphingolipid cell signaling pathways, and their plasma levels have been associated with several metabolic and cardiovascular conditions 9. Clinical studies with ceramides initially focused mainly on patients with known coronary artery disease (CAD) to identify patients with residual risk for secondary prevention. For example, in a European cohort of patients with stable ischemic heart disease and acute coronary syndromes (ACS) ceramide ratio (d18:1/16:0)/(d18:1/24:0) improved prediction of cardiovascular events by 8.2% % 10. The Mayo Clinic has validated this approach in an angiography cohort and thus has implemented the ceramide score in clinical practice as the first lipidomic biomarker for residual atherosclerotic risk 11. Data on primary prevention and ceramides have also been reported but not specifically in a community cohort 12, 13. Moreover, to date, ceramide scores have not been explored for primary prevention. The current study assesses the ceramide score in the community in a cohort of subjects initially enrolled for assessment the Prevalence of Asymptomatic Left Ventricular Dysfunction (PAVD) 14. Our investigations focus on primary prevention and the role of ceramide as lipidomic biomarkers of atherosclerotic risk in a cohort recruited from the general population who, at the time of enrollment, had no clinically known atherosclerotic disease. We hypothesized that similar to prior studies with secondary prevention, ceramides would be predictive of cardiovascular adverse end-points in the general population.
Methods
All data and supporting materials have been provided with the published article. The authors declare that all supporting data are available within the article (and its online supplementary files).
We performed a passive follow-up of a prospective community-based cohort using a record linkage system to determine the composite outcome of major adverse cardiac events ( MACE), defined as acute myocardial infarction, coronary revascularization (coronary artery bypass grafting or percutaneous coronary intervention), stroke or death. Ceramides were analyzed as continuous variables, ratios and quartiles adjusting for potential confounders.
Patients and methods
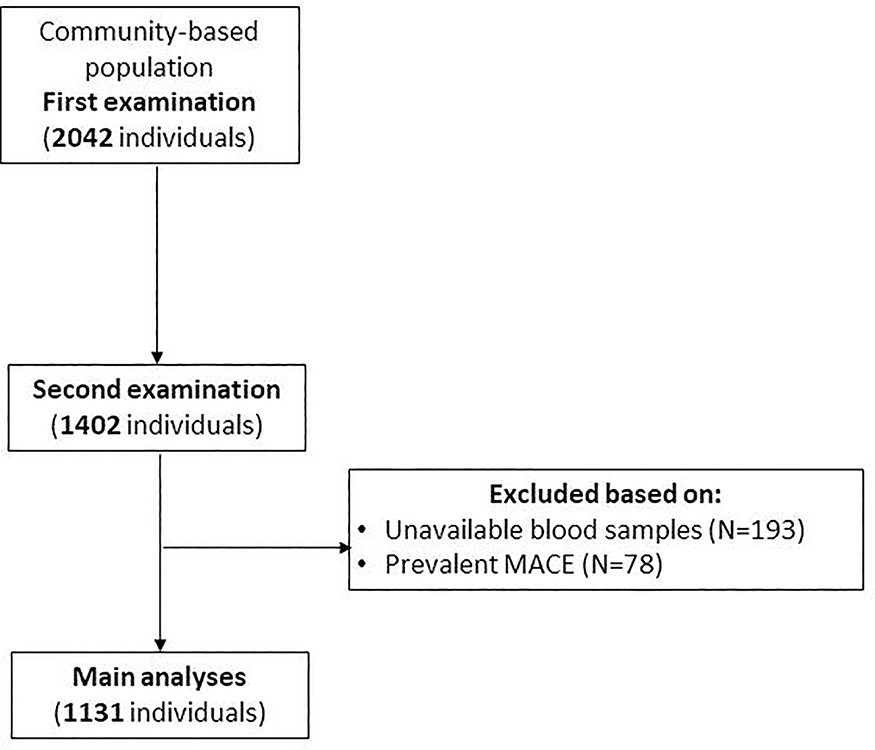
The authors of the current manuscript will not make their data, analytic methods, and study materials available to other researchers. Study approval was issued by the Mayo Foundation and Olmsted Medical Center review board. Informed consent was obtained from all patients. Using the Rochester Epidemiology Project (REP), individuals older than 45 years were selected for participation in the study. As outlined in previous studies, this database is centered in Olmsted County, Minnesota 15, 16. The REP cohort is predominately white middle class. Further specifics of the population are described in past studies 17, 18. The REP has been successful in mapping out a variety of complex disease associations in the community settings. The REP framework was used to randomly select a fraction of 7% within each sex and age stratum of county residents 45 years of age or older. Of the 4203 selected residents, 2042 (47%) unrelated men and women were enrolled. Between the years 1997 and 2000, participants were recruited and data were passively collected from the REP, as previously described. After four years, all participants were invited to return for a second examination, and 1402 participated between the years 2001 and 2004. For this study, we first identified the 1402 individuals who were evaluated at the second examination. We excluded subjects who experienced MACE prior to the second examination (N=78) and those with unavailable samples for analysis (N=193). A total of 1131 subjects where included in the final analysis (Figure 1). Each of the enrolled participants underwent a physical examination; blood work that included kidney function, lipid testing, and neurohormonal biomarkers of cardiovascular damage/distress between September 1, 2001, and December 30, 2004. As previously described, measurements of body mass index (BMI); calculated as the weight in kilograms divided by the height in meters squared), blood pressure, height, and weight were obtained during the initial data collection period. All participants completed medical surveys and questionnaires following initial data collection. Method for ceramide testing and algorithm for ceramide score have been described previously 11, 19.
FIGURE 1. Cohort selection-.
a flow chart of patients included in our study. MACE major adverse cardiac events.
Statistical analyses
Participants were characterized by their demographics, clinical and laboratory data including ceramides. Ceramides along with other continuous variables including BMI, biomarkers and laboratory measurements were summarized by median and interquartile range. Age and blood pressure were approximately normally distributed and summarized by mean and standard deviation. Binary and multi-category variables were described by counts and percentages.
Combined [stroke/MI (myocardial infarction) and MACE] and individual [stroke, MI, CABG (coronary artery bypass grafting), PCI (percutaneous coronary intervention), and death)] endpoints were analyzed as time-to-event outcomes. The overall rate of events at 5 and 10 years were estimated via KM (Kaplan-Meier) method. To assess effects of ceramides on each outcome, Cox proportional hazards models were fit after log-transforming individual ceramides and their ratios to better approximate normality. Hazard ratios (HR), both unadjusted and adjusted for important covariates, were estimated and are presented with corresponding 95% confidence intervals (CI). Adjustment for traditional CAD risk factors was accomplished by fitting age, sex, BMI, hypertension, CAD, GFR (glomerular filtration rate), ApoB (apolipoprotein B), ApoA1 (apolipoprotein A1), LDL-C, and total cholesterol as covariates together with each ceramide. ASCVD (Atherosclerotic Cardiovascular Disease)-adjusted models included a categorical risk variable with cutoffs recommended by ASCVD guidelines. Survival C-statistics were calculated to assess discrimination ability and compared between models using bootstrapping with 1000 samples to estimate (with 95% CI) the improvement of each adjusted model’s c-statistic over that of the baseline model. The integrated discrimination index (IDI) and relative IDI at 5 years were also calculated as a way to assess improvement in discrimination. These estimates are reported with bootstrap 95% CIs and the bootstrap samples were used to test differences in IDI between models.
Several KM plots of survivorship from stroke/MI were produced. Each set of curves was accompanied by HR estimates obtained by fitting a Cox model with the relevant ceramide variable as a 4-level factor split by its quartiles as well as relevant adjustment variables. Both unadjusted and adjusted KM curves are shown, the adjusted curves were generated using a method of direct adjustment.
P-values < 0.05 were considered statistically significant. All analyses were conducted in SAS version 9.4 (Cary, NC).
Results
Baseline Characteristics
We included 1,131 subjects in our analysis. The main clinical and biochemical characteristics of the cohort are listed in Table 1. The mean age was 64 years old and 52.2% of the subjects were female. The majority of subjects were white (97.5%) with a slightly elevated BMI. Of the patients included, 40.8% carried a diagnosis of hypertension, 11.2% had diabetes and 6.6% were current cigarette smokers. A family history of CAD in first-degree relatives was present in 551 (59.1%) of patients with available information (n=933). The lipid profile at the time of enrollment showed a mean total cholesterol of 199 mg/dL, with a mean HDL-c 48 mg/dL, mean triglycerides of 128 mg/dL and a mean calculated LDL-c of 119 mg/dL. Of the subjects included 29% were on statin therapy at the time of inclusion. The mean fasting glucose was 96 mg/dL. The median ceramide score was 3. A score of 0–2 conferring low cardiovascular risk prediction was found in 42.9% of subjects; a Ceramide score of 3–6 suggesting moderate risk was found in 40.2% of cohort. Increased risk was identified in 13.8% of subjects as described by a ceramide score of 7–9, while 3% of patients were deemed at very high risk by a ceramide score 10–12 (Table 1).
Table 1.
Baseline Demographics
| Overall | With Calculated ASCVD | ||||
|---|---|---|---|---|---|
| (N=1131) | [n miss] | (N=852) | [nmiss] | ||
| ASCVD, % | n (%) | [279] | |||
| Low ( < 7) | 339 (39.8%) | 339 (39.8%) | |||
| Mid ( ≥ 7, < 10) | 104 (12.2%) | 104 (12.2%) | |||
| High( ≥ 10) | 409 (48.0%) | 409 (48.0%) | |||
| Demographics | |||||
| Age, years | mean ± SD | 64 ± 9 | 63 ± 8 | ||
| Women | n (%) | 590 (52.2%) | 431 (50.6%) | ||
| Race: White | n (%) | 1103 (97.5%) | 835 (98.0%) | ||
| BMI, kg/m2 | median (IQR) | 28 (25, 31) | 28 (25, 31) | ||
| Hypertension | n (%) | 462 (40.8%) | 352 (41.3%) | ||
| Hypertension Drug | n (%) | 476 (42.1%) | 367 (43.1%) | ||
| Family history of premature coronary artery disease | N(%) | 551 (59.1%) | [198] | 417 (60.3%) | [160] |
| Ever smoked | n (%) | 522 (48.3%) | [50] | 419 (49.2%) | |
| Current smoker | n (%) | 71 (6.6%) | 53 (6.2%) | ||
| Statin at enrollment | n (%) | 310 (29.0%) | [63] | 238 (29.5%) | [46] |
| Calculated GFR (MDRD) | median (IQR) | 75 (67, 87) | [11] | 76 (69, 87) | [7] |
| Coronary Artery Disease | n (%) | 129 (11.4%) | 92 (10.8%) | ||
| Diabetes | n (%) | 109 (11.2%) | [155] | 93 (10.9%) | |
| Systolic BP, mm Hg | mean ± SD | 126 ± 19 | [2] | 124 ± 18 | |
| Biomarkers | median (IQR) | ||||
| ApoB, mg/dL | 105 (91, 120) | 105 (91, 120) | |||
| ApoA1, mg/dL | 157 (139, 180) | 156 (139, 179) | |||
| ApoB/A1 Ratio | 0.70 (0.50, 0.80) | 0.70 (0.60, 0.80) | |||
| Free Fatty Acids | 0.56 (0.39, 0.78) | [5] | 0.55 (0.38, 0.75) | [3] | |
| Lab data, mg/dL | median (IQR) | ||||
| Cholesterol (Total) | 199 (175, 222) | [5] | 197 (174, 221) | ||
| HDL (Cholesterol) | 48 (38, 59) | [5] | 47 (38, 59) | ||
| LDL (Cholesterol) | 119 (99.2, 141) | [22] | 118 (98.7, 140) | [12] | |
| CRP | 0.10 (0.10, 0.30) | [5] | 0.14 (0.07, 0.32) | ||
| Glucose | 96.0 (90.0, 104) | [5] | 96.0 (90.0, 104) | ||
| Triglyceride | 128 (96.0, 178) | [5] | 129 (96.0, 180) | ||
| Ceramides | median (IQR) | ||||
| Ceramide (16:0), μmol/L | 0.26 (0.23, 0.31) | [3] | 0.26 (0.22, 0.30) | [3] | |
| Ceramide (18:0), μmol/L | 0.09 (0.07, 0.12) | [16] | 0.09 (0.07, 0.12) | [14] | |
| Ceramide (24:1), μmol/L | 1.14 (0.94, 1.35) | [7] | 1.13 (0.92, 1.33) | [7] | |
| Ceramide (24:0), μmol/L | 3.56 (3.00, 4.33) | [3] | 3.50 (3.00, 4.40) | [3] | |
| Ceramide ratio (16:0)/(24:0) | 0.07 (0.06, 0.09) | [3] | 0.07 (0.06, 0.09) | [3] | |
| Ceramide ratio (18:0)/(24:0) | 0.03 (0.02, 0.03) | [16] | 0.03 (0.02, 0.03) | [14] | |
| Ceramide ratio (24:1)/(24:0) | 0.32 (0.26, 0.38) | [7] | 0.32 (0.26, 0.38) | [7] | |
| Ceramide Score (0–12) | 3 (1, 5) | [3] | 3 (1, 5) | [3] | |
| Ceramide Score Category | n (%) | [3] | [3] | ||
| 0–2 | 484 (42.9%) | 386 (45.5%) | |||
| 3–6 | 454 (40.2%) | 339 (39.9%) | |||
| 7–9 | 156 (13.8%) | 104 (12.2%) | |||
| 10–12 | 34 (3.0%) | 20 (2.4%) | |||
Apo apolipoprotein; ASCVD atherosclerotic cardiovascular disease; BMI body mass index; BP blood pressure; CRP C reactive protein; GFR glomerular filtration rate; HDL high density lipoprotein; LDL low density lipoprotein
Endpoints
MACE
Subjects were followed over a median of 13.3 (Q1 12.7, Q3 14.4) years. Overall, 486 patients developed MACE. KM-estimated rates of MACE were 13.6% (95% CI, 11.6–15.6%) and 29.2% (95% CI, 26.5–31.8%) at 5- and 10- years, respectively. By the end of the follow up, a personal history of CAD had developed in 11.4% of the cohort. Ceramide score had a HR of 1.16 (1.05–1.28, p=0.005) when adjusted for ASCVD, and 1.12 (1.02–1.23, p=0.018) when adjusted for traditional CAD risk factors.
MI and stroke
Cox proportional analysis showed that ceramide score did not correlate strongly with myocardial infraction (MI) as an individual adverse end-point after adjustment for 10-year pooled cohort risk ASCVD score (HR 1.28, 95% CI 0.99–1.66, p=0.063). Ceramide score did however reach statistical significance as a predictor for stroke, with ASCVD-adjusted HR 1.32 (95% CI 1.04–1.66) and p=0.021 (Table 1 Supplement). Association of ceramide score with the combined stroke/MI outcome was significant as well, HR similar to that of the individual stroke outcome with an ASCVD-adjusted value of 1.31 (95% CI 1.09–1.57) and p=0.004 (Table 2). Kaplan Meier curves of survivorship from combined stroke and MI were divided according to ceramide ratio18:0/24:0, ceramide ratio 24:1/24:0, and ceramide score quartiles. These were plotted with and without adjustment for ASCVD. Corresponding Cox analyses showed statistically significant HRs for ceramide ratio 18:0/24:0, ceramide ratio 24:1/24:0, and ceramide score upper quartiles after adjustment for guideline-endorsed ASCVD risk categories, each referencing lowest quartiles. The HR (95% CI) estimates indicated decreased survival free of stroke and MI associated with elevated ceramides: 3.00 (1.17–7.68), 2.93 (1.52–5.67), and 2.63 (1.36–5.09) for the upper quartiles of ceramide ratio 18:0/24:0, ceramide ratio 24:1/24:0, and ceramide score, respectively (Figure 2). These indications remained true whether adjustment was made for decision cut points or risk categories as recommended by the ASCVD guidelines (Figure 2). Additionally there was a dose response behavior of the ceramide score distribution with MACE (Figure 3).
Table 2.
Combined stroke and myocardial infarction outcomes
| Outcome: Stroke/MI | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | |||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | C-stat (95% CI) | ||
| Baseline: ASCVD | Low (<7.5) | Ref | -- | -- | -- | 0.63 (0.59, 0.68) |
| Mid (7.5–10) | 2.24 (1.06, 4.76) | 0.036 | -- | -- | ||
| High (≥10) | 3.37 (2.07, 5.4!8) | < 0.001 | -- | -- | ||
| Cer (16:0) | 1.10 (0.91, 1.34) | 0.333 | 1.08 (0.89, 1.31) | 0.452 | 0.64 (0.59, 0.69) | |
| Log[Cer (18:0)] | 1.21 (1.00, 1.47) | 0.050 | 1.18 (0.97, 1.42) | 0.092 | 0.66 (0.61, 0.71) | |
| Log[Cer (24:0)] | 0.85 (0.71, 1.02) | 0.084 | 0.83 (0.69, 1.00) | 0.045 | 0.66 (0.60, 0.71) | |
| Cer (24:1) | 1.25 (1.06, 1.48) | 0.009 | 1.19 (1.00, 1.41) | 0.045 | 0.66 (0.61, 0.71) | |
| Log[Cer (16:0)/(24:0)] | 1.26 (1.05, 1.52) | 0.013 | 1.29 (1.07, 1.55) | 0.009 | 0.67 (0.62, 0.72) | |
| Log[Cer (18:0)/(24:0)] | 1.33 (1.10, 1.61) | 0.003 | 1.32 (1.09, 1.59) | 0.004 | 0.67 (0.62, 0.72) | |
| Log[Cer (24:1)/(24:0)] | 1.41 (1.17, 1.69) | < 0.001 | 1.37 (1.13, 1.64) | 0.001 | 0.68 (0.63, 0.72) | |
| Cer Score | 1.36 (1.14, 1.64) | < 0.001 | 1.31 (1.09, 1.57) | 0.004 | 0.67 (0.63, 0.72) | |
Models were adjusted for ASCVD with guideline-endorsed cutoffs
ASCVD atherosclerotic cardiovascular disease; Cer ceramides; MI myocardial infarction
FIGURE 2. Survival by Kaplan Meier curves of for ceramides (18:0)/(24:0) (2a), (24:1)/(24:0) (2b) and ceramide score (2c) by ASCVD cutoff point quartiles.
ASCVD atherosclerotic cardiovascular disease; Cer ceramides; MI myocardial infarction
FIGURE 3. Dose response curve of ceramide ratios and score with MACE.
Forrest plot of ceramide ratios and score by quartiles in relationship with MACE expressed as HR. MACE major adverse cardiac events events; HR hazard ratio
Mortality
At follow up there were 108 deaths among those with non-missing risk factors, and 58 for those with non-missing ASCVD score. When analyzed for mortality ceramide score did not show a statistically significant correlation (data not shown). Ceramide ratio16:0/24:0 was significant in the unadjusted and ASCVD-adjusted models, but lost significance when adjusted for conventional risk factors
Overall, the ceramide score predicted MACE and mortality in both the ASCVD-adjusted and unadjusted models with the adjusted HR (95% CI) of 1.16 (1.05, 1.28) (Table 3). When analyzed separately, ceramide score predicted stroke with HR (95% CI) of 1.32 (1.04–1.66) and MI with HR (95% CI) of 1.28 (0.99–1.66) in the ASCVD-adjusted model, however the score failed to predict individual outcomes such as MI, death, need for revascularization (coronary artery bypass grafting and percutaneous coronary intervention) (Table 1 Supplement).
Table 3.
MACE and Death adjusted for ASCVD
| Outcome: MACE/Death | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | |||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | C-stat (95% CI) | ||
| Baseline: ASCVD | Low (<7.5) | Ref | -- | -- | -- | 0.60 (0.57, 0.62) |
| Mid (7.5–10) | 1.44 (0.95, 2.17) | 0.087 | -- | -- | ||
| High (≥10) | 2.33 (1.84, 2.96) | < 0.001 | -- | -- | ||
| Cer (16:0) | 1.11 (0.99, 1.23) | 0.069 | 1.08 (0.97, 1.21) | 0.145 | 0.61 (0.57, 0.64) | |
| Log[Cer (18:0)] | 1.06 (0.95, 1.18) | 0.296 | 1.04 (0.93, 1.15) | 0.411 | 0.60 (0.57, 0.63) | |
| Log[Cer (24:0)] | 0.93 (0.84, 1.03) | 0.183 | 0.91 (0.82, 1.01) | 0.084 | 0.61 (0.58, 0.64) | |
| Cer (24:1) | 1.12 (1.01, 1.23) | 0.026 | 1.08 (0.97, 1.19) | 0.156 | 0.61 (0.58, 0.64) | |
| Log[Cer (16:0)/(24:0)] | 1.18 (1.06, 1.31) | 0.002 | 1.19 (1.07, 1.32) | 0.001 | 0.62 (0.59, 0.65) | |
| Log[Cer (18:0)/(24:0)] | 1.12 (1.01, 1.24) | 0.033 | 1.12 (1.01, 1.24) | 0.040 | 0.61 (0.58, 0.64) | |
| Log[Cer (24:1)/(24:0)] | 1.21 (1.09, 1.35) | < 0.001 | 1.19 (1.07, 1.32) | 0.001 | 0.62 (0.59, 0.65) | |
| Cer Score | 1.20 (1.08, 1.33) | < 0.001 | 1.16 (1.05, 1.28) | 0.005 | 0.62 (0.59, 0.65) | |
Models were adjusted for ASCVD with guideline-endorsed cutoffs
ASCVD atherosclerotic cardiovascular disease; Cer ceramides; MACE major adverse cardiac events
Additionally, our analyses identified ceramide ratio 24:1/24:0 as a marker of high risk in some patients otherwise classified low risk by the ASCVD score (Figure 4). Conversely, we show that some patients with higher risk by ASCVD score have low ceramide ratio 24:1/24:0, suggesting that these patients might not be at high risk (Figure 4).
FIGURE 4. Survival by Kaplan Meier curves of for ceramides (18:0)/(24:0) (4a), (24:1)/(24:0) (4b) and ceramide score (4c) by ASCVD risk categories.
ASCVD atherosclerotic cardiovascular disease; Cer ceramides; MI myocardial infarction
Performance of ceramide score in CV risk prediction
We assessed the ceramide score for CV risk prediction and found hazard ratio of 1.31 (1.09–1.57) for the stroke/MI outcome after adjustment for ASCVD. The model had a c-statistic of 0.67 (0.63–0.72) (Table 2 Supplement).
Performance of ceramide score in comparison with ASCVD score
When assessing the performance of ceramides in predicting events compared to the ASCVD score by use of the relative IDI (integrated discrimination improvement index), all ceramide ratios and the ceramide score showed statistically significant value added over ASCVD for stroke/MI (p≤0.03, Table 5) Ceramide score also improved discrimination over ASCVD for prediction of MACE/death with a relative IDI of 17.2% (1.0–53.5%) (Table 5).
Table 5.
Relative integrated discrimination improvement index for ACSVD and ceramide score
| Outcome: Stroke/MI | |||||
|---|---|---|---|---|---|
| C-stat increase from baseline model* | IDI+ | Relative IDI | |||
| with 95% CI | P-value | with 95% CI | P-value | with 95% CI | |
| Cer (16:0) | 0.010(−0.010, 0.030) | 0.320 | 0.001 (0.000, 0.005) | 0.109 | 7.2 (−0.5, 36.8) |
| Log[Cer (18:0)] | 0.026 (−0.003, 0.056) | 0.076 | 0.001 (−0.001, 0.007) | 0.261 | 7.3 (−4.9, 58.3) |
| Log[Cer (24:0)] | 0.026 (0.001, 0.050) | 0.043 | 0.004 (0.000, 0.014) | 0.044 | 26.1 (0.0, 105) |
| Cer (24:1) | 0.028 (0.003, 0.053) | 0.028 | 0.002 (0.000, 0.009) | 0.189 | 11.0 (−2.9, 69.7) |
| Log[Cer (16:0)/(24:0)] | 0.040 (0.014, 0.067) | 0.003 | 0.005 (0.000, 0.015) | 0.007 | 36.7 (1.5, 119) |
| Log[Cer (18:0)/(24:0)] | 0.042 (0.014, 0.014) | 0.004 | 0.004 (0.000, 0.016) | 0.034 | 31.5 (0.0, 127) |
| Log[Cer (24:1)/(24:0)] | 0.047 (0.018, 0.075) | 0.001 | 0.006 (0.001, 0.018) | 0.005 | 46.3 (2.4, 146) |
| Cer Score | 0.045 (0.016, 0.073) | 0.002 | 0.006 (0.001, 0.016) | 0.004 | 41.0 (2.4, 127) |
Baseline model c-stat = 0.63 (0.59, 0.68); Adjustment factor: ASCVD with guideline-endorsed cutoffs.
5 years follow-up.
ASCVD atherosclerotic cardiovascular disease; Cer ceramides; IDI integrated discrimination improvement; MACE major adverse cardiac events
Discussion
Our data provides several novel insights. This is the first study to date assessing the role of ceramide score for primary prevention of atherosclerotic CVD in a community cohort. We show that ceramide score is a robust approach to defining residual risk that can be used by providers in the general population without known CAD. These data augment the primary prevention trials previously reported in research populations 12, 13 and suggest a role for ceramide scores in the evaluation of the large number of patients in our communities.
Ceramides were first introduced clinically as biomarkers for atherosclerosis in the Ludwigshafen risk and cardiovascular health (LURIC) trial 20. In that study, ceramides 16:0, 18:0 and 24:1 were associated with CV risk independently of traditional lipid biomarkers HDL-c and LDL-c. Subsequently ceramides 16:0, 18:0 and 24:1 were found to be associated with negative cardiovascular outcomes in the FINRISK 2002, Corogene and Prevencion con Dieta Mediterranea (PREDIMED) trials 10, 13, 21.
The mechanisms involving ceramide association with CVD are uncertain, but likely include their interdigitation with the lipid, atherosclerotic and inflammatory pathways 22. Structurally ceramides are important components of membrane stability; they also act as second messengers in membrane signaling pathways involved in inflammation 23. It is clear that ceramides accumulate in atherosclerotic plaques and stimulate the atherosclerosis process 24. In animal models of atherosclerosis, inhibition of ceramide synthesis is diminished in areas of atherosclerotic plaque 25 leading to speculation that enzymes in the ceramide synthesis pathways might serve as suitable targets for drug development 26.
Over time, it became clear that individual ceramides, ceramide ratios and scores bear important prognostic value in patients with known CAD 6, 10, 13, 21, 23. Distinct ceramides target different components of the lipidomic and inflammatory cascade and combining them into a score optimizes their prognostic potential and facilitates provider use and simplicity of interpretation. This rationale eventually led to the use of ceramide score to identify and risk stratify patients with established, residual or suspected ischemic heart disease (IHD) who might require more aggressive medical therapy. The ceramide score has worked extremely well in the secondary prevention setting 11. The present investigation is the first to date to deploy the clinically established ceramide score in the general population in the community. We now show that ceramide score as previously described, and recently implemented into clinical practice at our institution 11, is a reliable instrument that can be used for risk stratification of patients in the community for primary prevention purposes. The ceramide scores were nearly statistically significantly correlated with MI, and were significantly associated with stroke in the PAVD cohort even after adjustment for the ASCVD score which is the current US gold standard-, guideline-endorsed- instrument for assessing cardiovascular atherosclerotic risk in the general population 27. The lack of statistical significance for MI may reflect a lack of power for that outcome. However, it may also reflect the associations of the score with intermediate outcomes such as CABG and PCI as might be expected in a population closely surveilled by a facility with an aggressive outreach program such as the Mayo Clinic. The frequency of stroke and MI events may well have been even greater in the absence of such surveillance. We show that the ceramide score identifies subjects at cardiovascular risk, but does not define the specific event that will occur. It thus appears that the ceramide score is more of a marker of ASCVD than of a specific outcome as is the case with other lipidomic biomarkers. This finding is consistent with reports that the target of ASCVD cannot be predicted in a pre-hoc manner 7. An important finding of our study is a statistically significant ceramide score hazard ratio of 1.31 (1.09–1.57) for the stroke/MI outcome after controlling for ASCVD. Our model has a c-statistic of 0.67 (0.63–0.72) which is acceptable for a multifactorial condition such as CAD (Table 2 Supplement). Additionally, this model showed that ceramide score and ceramide ratios have statistically significant value when added over ASCVD for stroke/MI (p≤0.03, Table 5).Ceramide score improved discrimination over ASCVD for prediction of MACE/death with a relative IDI of 17.2% (1.0–53.5%) (Table 5). There is some criticism regarding the accuracy of ASCVD in predicting cardiovascular events 8, 28, 29. We show an increased risk of stroke and MI of subjects with elevated ceramide score in a dose-dependent manner, which maintained statistical significance even in the ASCVD-adjusted model with HR 2.63 (95% CI, 1.36–5.09) for the fourth quartile (Figure 2C). These results highlight the prognostic information provided by a simple, cost-effective serum biomarker in the general population for diagnosis, assessment and risk stratification of atherosclerotic CVD. Depending on the degree of risk and treatment targets, these data may also have important therapeutic implications for those patients who are at higher risk.
Another interesting finding of our analyses is the identification of ceramide 24:1/24:0 as a marker of high risk in patients otherwise deemed low risk by the ASCVD score (Figure 4). We further analyzed this category of patients and found no significant differences in regards to traditional risk factors for CAD except for age (data not shown). This finding might have important implications in clinical practice. It may be that these patients with higher ceramide ratio 24:1/24:0 and low ASCVD score are often missed and might benefit from more individualized, in depth testing and more aggressive treatment for primary prevention. Conversely, our analyses show that there is a particular category of patients with higher risk by ASCVD score and low ceramide ratio 24:1/24:0, suggesting that these patients might do as well with less aggressive medical intervention. This category of patients had no particular risk factor identified when compared to the rest of the cohort. This is in agreement with some suggestions that the ASCVD score might overestimate risk 29, questioning the accuracy of this scoring system, further underlying the importance of more precise and robust tools to assess CVD for primary prevention.
At the Mayo Clinic, the atherosclerotic ceramide score has been implemented for clinical use11 to risk stratify patients with known or suspected residual CAD and identify those at higher risk for more aggressive management. Based on the results of the current investigation we can extend the use of ceramide score for assessment of atherosclerotic CVD risk in the general population for primary prevention purposes. Previously two studies identified distinct ceramides as negative predictors of cardiovascular morbidity and mortality in the general population 12, 13; the specific ratio ceramide 18:1/18:0 was associated with incident MACE with a hazard ratio of 1.21, while C24:0/C16:0 was associated with CAD, HF (heart failure), and all-cause mortality at 6-year follow up. Our study demonstrates that ceramide score is a more robust, accurate and cost-effective instrument that can be used for risk stratification and primary prevention. This is particularly important for asymptomatic patients with no known personal history of CAD or risk factors, who are at intermediate risk due to the presence of other comorbidities, family history or the presence of other risk enhancers. Currently these patients are recommended additional risk stratification such as computer tomography coronary calcium (CAC) scoring 7. CAC scoring has been extensively used for asymptomatic patients at intermediate risk, however, the Agatston method which is widely used, has some limitations such as reproducibility, the presence of distinct calcium densities in particular patient populations and increase calcium density with statin treatment 30–32. Several studies are supportive of the fact that ceramide scores are reproducible 11 and modifiable with diet 21, aerobic exercise 33 and statin therapy 34, 35, conferring this lipidomic biomarker an attractive role not only in risk stratification, but also in assessing the response to lifestyle changes or pharmacologic interventions. Recently, Hilvo et al. have validated a new model of prediction involving ceramide score and 2 additional phospholipids with improved prediction accuracy 36. It would be useful to further assess such a combination approach of ceramide score and phospholipids in our community cohort as well, which will likely improve further the prediction accuracy.
This study was performed using a predominately rural white population located in Olmsted County, Minnesota, in the Midwest. Given the homogeneity and predominance of Caucasian population, it may be difficult to use this study to completely generalize the results to the entire US population. However, to date this is the first study assessing the use of ceramide score for primary prevention and to identify ceramide score as a robust serum biomarker that predicts cardiovascular morbidity in the community. Although ceramides have been studied for primary prevention previously 12, 13, none of these investigations assessed the ceramide score which is currently used clinically for secondary prevention 11, or compared these biomarkers with guideline-endorsed estimators of risk. In our cohort the ceramide score did not correlate with mortality likely due to the low number of events; additionally these patients were followed closely and risk factors were addressed aggressively. The c-statistic found when assessing the association between the ASCVD score and ceramide score is reasonable for a multifactorial condition such as CAD; additionally, there are studies suggesting the overestimation of CVD risk by ASCVD which may also play a role. Prior studies that led to the development of the ASCVD score were retrospective and were performed in subjects who were assessed and treated several decades ago per guidelines which are currently obsolete 37–39 while our subjects included in the current investigation were enrolled prospectively, followed and treated in conformity with more contemporary guidelines 16.
Conclusions
This current investigation is the first study to date examining the role of ceramide scores in a cohort of subjects from the community with average burden of CAD. This investigation identifies the ceramide risk score as a robust lipidomic biomarker that can be used for primary prevention, and can be applied particularly in patients at intermediate risk. This has important consequences for risk stratification and therapeutic intensity options, as well as a reasonable tool to assess response to intervention. Specific subgroups of ceramides may identify patients at higher risk who may be overlooked by the 10-year pooled cohort score and conversely, a particular category of high risk patients defined by the ASCVD score may be treated too aggressively, however further studies are warranted.
Supplementary Material
Table 4.
C-statistic for ceramide ratios and score
| Outcome: MACE/Death | |||||
|---|---|---|---|---|---|
| C-stat increase from baseline model* | IDI+ | Relative IDI | |||
| with 95% CI | P-value | with 95% CI | P-value | with 95% CI | |
| Cer (16:0) | 0.009 (−0.003, 0.021) | 0.132 | 0.001 (0.000, 0.004) | 0.106 | 5.0 (−0.3, 25.1) |
| Log[Cer (18:0)] | 0.005 (−0.006, 0.016) | 0.374 | 0.000 (0.000, 0.003) | 0.220 | 2.4 (−0.9, 16.1) |
| Log[Cer (24:0)] | 0.010 (−0.002, 0.023) | 0.115 | 0.002 (0.000, 0.007) | 0.051 | 9.3 (0.0, 38.6) |
| Cer (24:1) | 0.011 (−0.003, 0.024) | 0.128 | 0.000 (0.000, 0.003) | 0.291 | 2.2 (−1.7, 18.5) |
| Log[Cer (16:0)/(24:0)] | 0.023 (0.010, 0.037) | 0.001 | 0.004 (0.001, 0.011) | 0.001 | 24.9 (3.0, 68.0) |
| Log[Cer (18:0)/(24:0)] | 0.015 (0.001, 0.029) | 0.041 | 0.002 (0.000, 0.007) | 0.061 | 9.8 (−0.1, 42.4) |
| Log[Cer (24:1)/(24:0)] | 0.021 (0.008, 0.034) | 0.002 | 0.004 (0.000, 0.011) | 0.004 | 22.4 (1.7, 66.8) |
| Cer Score | 0.022 (0.006, 0.037) | 0.005 | 0.003 (0.000, 0.009) | 0.006 | 17.2 (1.0, 53.5) |
Baseline model c-stat = 0.60 (0.57, 0.62); Adjustment factor: ASCVD with guideline-endorsed cutoffs.
5 years follow-up.
Highlights.
Ceramide score:
Robust biomarker for atherosclerotic risk for primary prevention
Reproducible and modifiable with interventions: diet, aerobic exercise and lipid lowering agents
Assesses response to primary intervention and motivates patients
No radiation, cost effective
Results are easy to interpret by providers
Acknowledgments
Financial support: This work was supported in part by the Departments of Laboratory Medicine and Pathology, and Cardiovascular Medicine at the Mayo Clinic Rochester. Supported in part by the European Regional Development Fund – Project FNUSAICRC [no.CZ.1.05/1.1.00/02.0123], by project no. LQ1605 from the National Program of Sustainability II (MEYS CR), by project ICRC-ERA-Human Bridge [no. 316345] funded by the 7th Framework Program of the European Union, and by a grant by the Ministry of Health of the Czech Republic [NT13434-4/2012]. And by the National Institute of Health [NIH HL-R01-55502 (Dr Rodeheffer].
Abbreviations and Acronyms
- ACS
acute coronary syndromes
- Apo A1
apolipoprotein A1
- Apo B
apolipoprotein B
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CABG
coronary artery bypass graft
- CAC
Coronary calcium computer tomography
- CAD
coronary artery disease
- CVD
cardiovascular disease
- GFR
glomerular filtration rate
- HF
heart failure
- HR
hazard ratio
- HDL-c
high density lipoprotein cholesterol
- IDI
integrated discrimination index
- IHD
ischemic heart disease
- KM
Kaplan Meier
- LDL-c
low density lipoprotein cholesterol
- LURIC
Ludwigshafen risk and cardiovascular health
- MACE
major adverse outcomes
- MI
myocardial infarction
- PAVD
prevalence of asymptomatic left ventricular dysfunction
- PCI
percutaneous coronary intervention
- PREDIMED
prevencion con dieta mediterranea
- REP
Rochester epidemiologic project
- TG
triglycerides
Footnotes
Disclosures
Dr. Vlad Vasile. Nothing to disclose.
Dr. Jeffrey Meeusen. Nothing to disclose.
Dr. Jose Medina Inojosa. Nothing to disclose.
Dr. Leslie Donato. Nothing to disclose.
Christopher Scott, M.S. Nothing to disclose.
Meredeith Hyun, M.D. Nothing to disclose.
Dr. Manlio Vinciguerra, Ph.D. Nothing to disclose.
Dr. Richard Rodeheffer. Nothing to disclose.
Dr. Francisco Lopez-Jimenez. Nothing to disclose.
Dr. Allan Jaffe. Presently or in the past has consulted for most of the major diagnostic companies but not the company who makes the assays used in this analysis.
References
- 1.Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawber TR, Kannel WB and McNamara PM. The Prediction of Coronary Heart Disease. Trans Assoc Life Insur Med Dir Am 1964;47:70–105. [PubMed] [Google Scholar]
- 3.Kannel WB, Dawber TR, Friedman GD, Glennon WE and McNamara PM. Risk Factors in Coronary Heart Disease. An Evaluation of Several Serum Lipids as Predictors of Coronary Heart Disease; the Framingham Study. Ann Intern Med 1964;61:888–99. [DOI] [PubMed] [Google Scholar]
- 4.Prospective Studies C, Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R and Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–39. [DOI] [PubMed] [Google Scholar]
- 5.Emerging Risk Factors C, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG and Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H and Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- 7.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFilippis AP, Young R and Blaha MJ. Calibration and Discrimination Among Multiple Cardiovascular Risk Scores in a Modern Multiethnic Cohort. Ann Intern Med 2015;163:68–9. [DOI] [PubMed] [Google Scholar]
- 9.Borodzicz S, Czarzasta K, Kuch M and Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis 2015;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB and Jaffe AS. Plasma Ceramides. Arterioscler Thromb Vasc Biol 2018;38:1933–1939. [DOI] [PubMed] [Google Scholar]
- 12.Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Volzke H, Felix SB, Jiang H, Sidhu R, Nauck M, Jiang X, Ory DS, Dorr M, Vasan RS and Schaffer JE. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havulinna AS, Sysi-Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V and Laaksonen R. Circulating Ceramides Predict Cardiovascular Outcomes in the Population-Based FINRISK 2002 Cohort. Arterioscler Thromb Vasc Biol 2016;36:2424–2430. [DOI] [PubMed] [Google Scholar]
- 14.Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC Jr. and Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–70. [DOI] [PubMed] [Google Scholar]
- 15.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM and Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR and Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR and Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–82. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–74. [DOI] [PubMed] [Google Scholar]
- 19.Kauhanen D, Sysi-Aho M, Koistinen KM, Laaksonen R, Sinisalo J and Ekroos K. Development and validation of a high-throughput LC-MS/MS assay for routine measurement of molecular ceramides. Anal Bioanal Chem 2016;408:3475–83. [DOI] [PubMed] [Google Scholar]
- 20.Sigruener A, Kleber ME, Heimerl S, Liebisch G, Schmitz G and Maerz W. Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS One. 2014;9:e85724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang DD, Toledo E, Hruby A, et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation. 2017;135:2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeusen JW, Donato LJ, Kopecky SL, Vasile VC, Jaffe AS and Laaksonen R. Ceramides improve atherosclerotic cardiovascular disease risk assessment beyond standard risk factors. Clin Chim Acta 2020;511:138–142. [DOI] [PubMed] [Google Scholar]
- 23.Kurz J, Parnham MJ, Geisslinger G and Schiffmann S. Ceramides as Novel Disease Biomarkers. Trends Mol Med 2019;25:20–32. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee SB, Dey S, Shi WY, Thomas K and Hutchins GM. Accumulation of glycosphingolipids in human atherosclerotic plaque and unaffected aorta tissues. Glycobiology. 1997;7:57–65. [DOI] [PubMed] [Google Scholar]
- 25.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK and Rekhter MD. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110:3465–71. [DOI] [PubMed] [Google Scholar]
- 26.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S and Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem 2005;280:10284–9. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFilippis AP and Blaha MJ. Predicted vs Observed Clinical Event Risk for Cardiovascular Disease. JAMA. 2015;314:2082. [DOI] [PubMed] [Google Scholar]
- 29.Nanna MG, Peterson ED, Wojdyla D and Navar AM. The Accuracy of Cardiovascular Pooled Cohort Risk Estimates in U.S. Older Adults. J Gen Intern Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R and Cainzos-Achirica M. Coronary Artery Calcium Scoring: Is It Time for a Change in Methodology? JACC Cardiovasc Imaging. 2017;10:923–937. [DOI] [PubMed] [Google Scholar]
- 31.Forbang NI, McClelland RL, Remigio-Baker RA, Allison MA, Sandfort V, Michos ED, Thomas I, Rifkin DE and Criqui MH. Associations of cardiovascular disease risk factors with abdominal aortic calcium volume and density: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2016;255:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leber AW, Knez A, White CW, Becker A, von Ziegler F, Muehling O, Becker C, Reiser M, Steinbeck G and Boekstegers P. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am J Cardiol 2003;91:714–8. [DOI] [PubMed] [Google Scholar]
- 33.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Kuo MS and Perreault L. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 2015;309:E398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng TW, Ooi EM, Watts GF, Chan DC, Weir JM, Meikle PJ and Barrett PH. Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. J Clin Endocrinol Metab 2014;99:E2335–40. [DOI] [PubMed] [Google Scholar]
- 35.Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R, Gouni-Berthold I, Berthold HK, Kleber ME, Laaksonen R and Marz W. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 2014;99:E45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilvo M, Meikle PJ, Pedersen ER, et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2020;41:371–380. [DOI] [PubMed] [Google Scholar]
- 37.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 38.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A and et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 39.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K and Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.