Abstract
Background.
Cortical demyelination is a relevant aspect of tissue damage in multiple sclerosis (MS). Microstructural changes may affect each layer in the cortex differently.
Objectives.
To evaluate the sensitivity of quantitative MRI (qMRI) measurements on cortical layers as clinically accessible biomarkers of grey matter (GM) pathology.
Methods.
45 participants with MS underwent 7T MRI of the brain. Magnetization prepared 2 rapid acquisition gradient echoes (MP2RAGE) was processed for T1-weighted images and a T1-map. Multi-echo gradient echo images were processed for quantitative susceptibility and R2* maps. Cortical GM volumes were segmented into four cortical layers and relaxometry metrics were calculated within and between these layers.
Results.
Significant correlations were found for disability scales and multilayer metrics, e.g. Expanded Disability Status Scale (EDSS) and peak height (PH) in the subpial (T1 ρ = −0.372, p < 0.050) and inner (R2*: ρ = −0.359, p < 0.050) cortical layers. Multivariate regression showed interdependency between atrophy and cortical metrics in some instances, but an independent relationship between cortical metrics and disability in others.
Conclusions.
Cortical layer 7T qMRI analyses reveal layer-specific relationships with disability in MS and allow emergence of clinically relevant associations that are hidden when analyzing the full cortex.
Keywords: Multi-layering, 7 Tesla, qMRI, Cortex, Multiple sclerosis
Introduction
Multiple sclerosis (MS) produces both inflammatory and degenerative pathology of the central nervous system (CNS) and usually leads to chronic disability.(1) MS is classically regarded as a white matter (WM) disease, but histopathological studies reveal that grey matter (GM) is also affected.(2) These studies show neuronal loss and demyelination in the cortical grey matter (cGM) and deep GM and that these changes are independent of WM lesions and are related to disability.(3)
Visualization and quantification of these changes to the cortex are necessary for a greater understanding of MS-related pathology. Quantitative MRI (qMRI) methods produce images and quantitative relaxometry maps that are more reproducible and more independent of acquisition details than weighted images, along with providing information that is a more direct correlate of in vivo histopathology.(4,5) The relationship between qMRI methods and histopathology have promise towards better understanding how MS affects varying depths of cortex – of importance due to the varying functions and levels of myelination seen between layers I – VI of human cortex. Nevertheless, applications of qMRI to the cortex at standard field strengths can be limited by the thin nature of the cortex, its gyrification, and partial volume averaging with adjacent WM and cerebrospinal fluid (CSF). These problems may be overcome using ultra-high field 7-tesla (7 T) acquisitions at high resolution, along with layer-based segmentation.(6)
The purpose of this study was to evaluate the sensitivity of qMRI measurements in multi-layered, segmented cortex from 7 T MRI as clinically-relevant biomarkers of cGM pathology in MS. Quantitative MRI measures in segmented cortical layers, along with the difference of these measures between layers, were compared to similar measures across the full cortical thickness on the hypothesis that analysis of segmented layers may reveal clinically relevant disability relationships hidden when summarizing qMRI values across the full cortical thickness.
Methods
Study Participants
Protocols were approved by the Institutional Review Boards at the University of Maryland School of Medicine, the Johns Hopkins University School of Medicine, and the Kennedy Krieger Institute. Written informed consent was obtained from all participants. Volunteers aged 18 – 65 with diagnoses of relapsing-remitting multiple sclerosis (RRMS), secondary progressive (SPMS) and primary progressive multiple sclerosis (PPMS) according to revised 2010 McDonald Criteria were recruited.(7) Participant study visits included demographic and clinical data collection and a neurological examination for calculation of the Expanded Disability Status Scale (EDSS) score,(8) timed 25-foot walk (T25FW), 9-hole peg test (9HPT). The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) battery was also obtained at each visit, which includes the California Verbal Learning Test (CVLT), Symbol Digit Modalities Test (SDMT) and Brief Visuospatial Memory Test (BVMT).(9)
MRI acquisition, processing and analysis
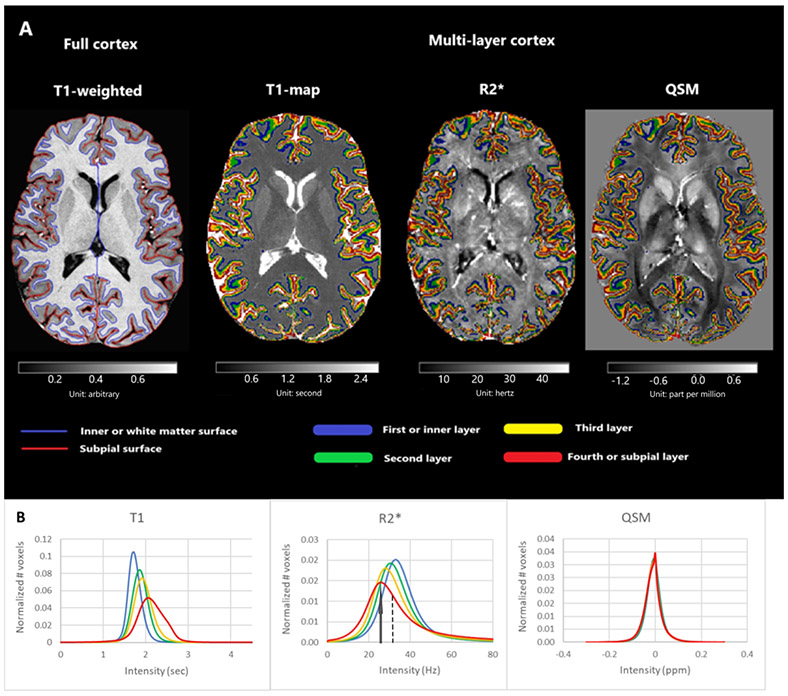
Detailed MRI acquisition parameters and details on processing and analysis can be found in the Supplement. In brief, participants underwent MRI in a 7.0 Tesla (7T) Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) with a volume transmit/32-channel receive head coil (Novamedical, Wilmington, MA, USA). Images underwent segmentation for various brain compartments, WM lesions, and cGM. The cGM volume was divided into four layers_(Figure 1 and 2) (10) and the related masks for each layer were applied to T1, R2* and relative susceptibility (χR) maps for the whole cohort and metrics were calculated.
Figure 1.
Cortical segmentation. (A) The image corresponds to an axial view from a MS patient for the MP2RAGE T1-weighted scan and the T1, R2* and χR maps. The boundaries of the full cortex (left) and our four layers segmentation (right) are shown after the application of the Volumetric Layering Module of CBS Tools. (B) Average multi-layer histograms across all patients of T1, R2* and χR. Details of the metrics are shown on the 4th layer of R2*: an arrow shows the peak height and a dashed line the median value in a positively skewed distribution. One-way ANOVA revealed significant differences in median T1 and R2* between layers, but not for χR.
Figure 2.
Coronal images with cortical multilayer overlay. (A) T1, (C) R2* and (E) quantitative susceptibility maps are shown with detailed multilayer segmentation overlay (B), (D) and (F). Cubic interpolation was used in the quantitative images and layers. The 4th (outer, subpial) layer can be seen in red, 3rd layer in yellow, 2nd in green and the 1st (inner) layer is in blue.
Statistical Analysis
The intensity distribution for T1 and R2* relaxation times and χR were calculated in Matlab and histograms were made for each subject, for the full cortex and each cortical layer. Distributions were normalized and divided into bins of width 0.25 percent units (p.u.). Smoothing by convolution with a Gaussian kernel was performed over the histograms and to correct the difference of tissue volume between subjects, each histogram was normalized by dividing it by the volume related to each layer or full cortex. Its arithmetic calculations were performed using FSL (Centre for Functional Magnetic Resonance Imaging of the Brain, University of Oxford, UK) and Matlab.
Spearman rank-order correlation coefficient was used to assess associations between T1, R2* and χR histogram metrics (median, skewness and peak height) along with their inter-layer differences, and disability scales: EDSS, T25FW, 9HPT, CVLT, SDMT and BVMT. Bonferroni-adjusted alpha (0.050/1) correction was used. Multivariable linear regressions were also performed to assess the influence of other factors (age, disease duration, volume layer fraction) on the relationship between qMRI metrics and disability as a dependent variable. Patients were divided into two groups according to its EDSS scale (EDSS ≤ median and EDSS > median) in order to perform histogram comparisons. One-way analysis of variance (ANOVA) was used to assess the significant difference of the cortical multi-layer metrics. All statistical analyses were performed with SPSS for Windows (v. 17, SPSS Inc., Chicago, IL).
Results
Analysis was performed on scans from forty-five patients with MS (33 RRMS, 4 SPMS and 8 PPMS). Median (range) age was 48.7 (22.6, 63.5) years and disease duration was 8.3 (0.7, 38.4) years. A full description of the clinical characteristics of the study group is seen in Table 1. Briefly, median EDSS score was 3.0 (1.0, 6.5), median T25FW was 4.6 (3.5, 90.9) seconds and median SDMT was 54.0 (35.0, 83.0).
Table 1:
Summary of clinical and imaging characteristics of patient cohort (n = 45).
| Characteristic | Value |
|---|---|
| RRMS (n, %) | 33 (73.3%) |
| SPMS (n, %) | 4 (8.9%) |
| PPMS (n, %) | 8 (17.8%) |
| Age (years) | 48.7 (22.6, 63.5) |
| Sex (F/M) | (28, 17) |
| Disease Duration (years) | 8.3 (0.7, 38.4) |
| Relapses within last year (n) | 0 (0, 3) |
| WM lesion volume (ml) | 3.33 (0.05, 21.16) |
| WMLF | 3.2e-3 (4.5e-5, 1.8e-2) |
| BPF | 0.85 (0.76, 0.89) |
| cGMF | 0.38 (0.32, 0.42) |
| L1F | 0.10 (0.09, 0.11) |
| L2F | 0.10 (0.09, 0.11) |
| L3F | 0.10 (0.08, 0.11) |
| L4F | 0.09 (0.07, 0.11) |
| Cortical thickness (mm) | 3.51 (0, 6) |
| EDSS | 3.0 (1.0, 6.5) |
| T25FW (sec) | 4.6 (3.5, 90.9) |
| 9HPT (sec) | 22.3 (16.1, 402.7) |
| CVLT score | 49.0 (28.0, 70.0) |
| SDMT score | 54.0 (35.0, 83.0) |
| BVMT score | 40.0 (1.0, 70.0) |
Median (range) values unless otherwise indicated.
Abbreviations: WM = white matter, RRMS = relapsing reemitting MS, SPMS = secondary progressive MS, PPMS = primary progressive MS, M = Male, F = Female, WMLF = white matter lesion fraction, BPF = brain parenchyma fraction, cGMF = cortical grey matter fraction, L1F = first layer or inner cortical layer fraction, L2F = second layer fraction, L3F = third layer fraction, L4F = fourth layer or subpial cortical layer fraction, EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test.
Histogram metrics for T1 and R2* maps, and to a lesser degree χR, showed significant inter-layer differences (Figure 1, Supplemental Table 1). Median T1 and R2* significantly varied between layers, whereas χR did not (p = 0.462). T1 relaxation time increased in each subsequent layer towards the subpial layer, whereas R2* increased towards the inner layer. Histogram peak height (PH) for T1 and R2*_significantly decreased towards the subpial layer, whereas χR significantly increased (Figure 1, Supplemental Table 1). Skewness also significantly varied between layers for T1 R2* and χR.
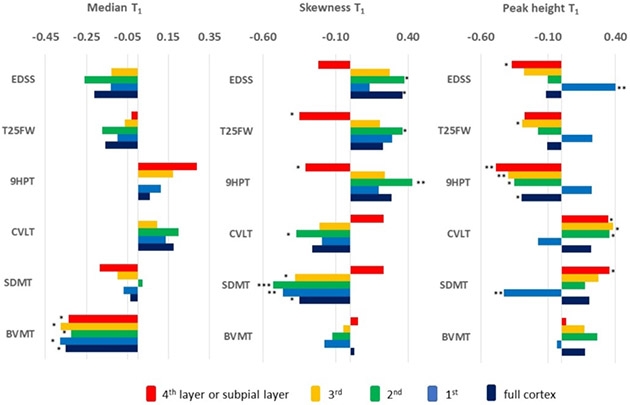
qMRI metrics measured across the full cortex only had mild relationships with disability. For example, skewness of T1 correlated with EDSS scores (ρ = 0.360, p < 0.050) and SDMT (ρ = −0.350, p < 0.050) and skewness of R2* correlated with SDMT scores (ρ = 0.366, p < 0.050) (Figure 3 and 4, Supplemental Table 2 and 3).
Figure 3. Magnitude of correlations for quantitative T1 map values and disability scales in full cortex and individual layers.
Spearman rank-order correlation coefficients are represented as bar graphs. P values were corrected for multiple comparisons. *** P < 0.001; ** P < 0.010; * P < 0.050.
EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test. 4th = fourth layer or subpial cortical layer, 3rd = third layer, 2nd = second layer, 1st = first layer or inner cortical layer.
Unit for median: second, skewness and peak height: no unit.
Figure 4. Magnitude of correlations for quantitative R2* map values and disability scales in full cortex and individual layers.
Spearman rank-order correlation coefficients are represented as bar graphs. P values were corrected for multiple comparisons. *** P < 0.001; ** P < 0.010; * P < 0.050. EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test. 4th = fourth layer or subpial cortical layer, 3rd = third layer, 2nd = second layer, 1st = first layer or inner cortical layer.
Unit for median: hertz, skewness and peak height: no unit.
Segmented cortical layer analyses revealed significant correlations with multiple disability scores and T1 and R2* in individual layers (Figure 3 and 4, Supplemental Table 2 and 3) many of which were not observed in full cortex analyses. For example, PH of T1 correlated with EDSS (ρ = −0.372, p < 0.050) and SDMT (ρ = 0.355, p < 0.050) in the outermost layer (4th layer). Skewness of 4th layer R2* correlated with EDSS (ρ = −0.361, p < 0.050) and SDMT (ρ = 0.367, p < 0.050), whereas the full cortex did not. The ability of segmented layer analyses to reveal relationships with disability not seen in full cortex measurements did not appear to extend to χR (Figure 5, Supplemental Table 4). Multivariate regression was performed to determine if these relationships were independent of age, disease duration, and layer-specific atrophy (Supplemental Table 5). Although some relationships were no longer present, significant relationships remained between SDMT scores and T1 metrics in multiple layers and R2* in each of the four layers and T25FW scores.
Figure 5. Magnitude of correlations for quantitative χR map values and disability scales in full cortex and individual layers.
Spearman rank-order correlation coefficients are represented as bar graphs. P values were corrected for multiple comparisons. *** P < 0.001; ** P < 0.010; * P < 0.050.
EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test. 4th = fourth layer or subpial cortical layer, 3rd = third layer, 2nd = second layer, 1st = first layer or inner cortical layer.
Unit for median: parts per million, skewness and peak height: no unit.
Participants were stratified into those with low (≤ median) EDSS and high (> median) EDSS, with comparative histograms shown in Figure 9. These distributions show that the negative correlation between 4th layer T1 PH and skew with EDSS is due to participants with higher levels of disability having less voxels clustered around the median with a shift of those to a higher T1 value. Similarly, correlations between EDSS and R2* distributions are likely due to more heterogeneity of voxel intensity values (thus less clustered around the mean).
Figure 9.
Average multi-layer and full cortex T1, R2* and susceptibility histograms for patients with low (blue) EDSS (≤ median) and high (red) EDSS (> median).
The difference in qMRI values between consecutive layers were also calculated, and difference values were evaluated for correlations with disability. These analyses revealed significant correlations for all quantitative maps (Figure 6, 7 and 8, Supplemental Table 6, 7 and 8). The difference between inner layers correlated with disability to a greater degree than a difference between middle layers.
Figure 6. Magnitude of correlations between interlayer differences in quantitative T1 map values and disability scales.
Spearman rank-order correlation coefficients are represented as bar graphs. P values were corrected for multiple comparisons. *** P < 0.001; ** P < 0.010; * P < 0.050.
EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test. 4th = fourth layer or subpial cortical layer, 3rd = third layer, 2nd = second layer, 1st = first layer or inner cortical layer.
Unit for median: second, skewness and peak height: no unit.
Figure 7. Magnitude of correlations between interlayer differences in quantitative R2* map values and disability scales.
Spearman rank-order correlation coefficients are represented as bar graphs. P values were corrected for multiple comparisons. *** P < 0.001; ** P < 0.010; * P < 0.050.
EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test. 4th = fourth layer or subpial cortical layer, 3rd = third layer, 2nd = second layer, 1st = first layer or inner cortical layer.
Unit for median: hertz, skewness and peak height: no unit.
Figure 8. Magnitude of correlations between interlayer differences in quantitative χR map values and disability scales.
Spearman rank-order correlation coefficients are represented as bar graphs. P values were corrected for multiple comparisons. *** P < 0.001; ** P < 0.010; * P < 0.050.
EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test. 4th = fourth layer or subpial cortical layer, 3rd = third layer, 2nd = second layer, 1st = first layer or inner cortical layer.
Unit for median: parts per million, skewness and peak height: no unit.
Analysis of cortical tissue volumes and thickness (Table 2) revealed significant correlations with EDSS for the full cortex (ρ = −0.494, p = 0.001) and each segmented cortical layer (e.g., ρ = −0.503, p < 0.001, for the subpial layer), but lack of such a relationship for brain parenchyma fraction (ρ = −0.228, p = 0.113).
Table 2.
Correlation of normalized volumes and cortical thickness with disability scales.
| BPF | WMLF | cGMF | L1F | L2F | L3F | L4F | Cortical thickness | |
|---|---|---|---|---|---|---|---|---|
| EDSS | −0.228 | 0.197 | −0.494** | −0.398** | −0.487** | −0.469** | −0.503*** | −0.446** |
| T25FW | −0.005 | 0.121 | −0.447** | −0.306* | −0.415** | −0.410** | −0.470** | −0.380* |
| 9HPT | −0.373* | 0.409** | −0.490** | −0.432** | −0.467** | −0.468** | −0.471** | −0.510*** |
| CVLT | 0.108 | −0.155 | 0.400** | 0.300* | 0.329* | 0.362* | 0.423** | 0.444** |
| SDMT | 0.306* | −0.399** | 0.409** | 0.385** | 0.400** | 0.391** | 0.359* | 0.394** |
| BVMT | 0.123 | −0.195 | −0.068 | −0.092 | −0.086 | −0.072 | −0.067 | 0.013 |
All volumes were normalized to intracranial volume and are thus reported as fractions. Spearman rank-order correlation coefficients. P values were corrected for multiple comparisons.
P < 0.001
P < 0.010
P < 0.050.
EDSS = Expanded Disability Status Scale, T25FW = timed 25-foot walk, 9HPT = 9-Hole Peg Test, CVLT = California Verbal Learning Test, SDMT = Symbol Digit Modalities Test, BVMT = Brief Visuospatial Memory Test. BPF = brain parenchyma fraction, WMLF = white matter lesion fraction, cGMF = cortical grey matter fraction, L1F = first layer or inner cortical layer fraction, L2F = second layer fraction, L3F = third layer fraction, L4F = fourth layer or subpial cortical layer fraction.
Discussion
These results suggest that stratification of cGM into layers and measurement of layer-specific qMRI may be more sensitive to pathology responsible for disability in MS than measurement of qMRI indices across the entire cortex. This supports the integration of cortical layer segmentation into future studies of cGM in MS and reinforces the importance of the use of 7 T imaging for the study of cGM – given the imaging resolution needed for reliable segmentation of cortex into individual layers.
Detailed examination of the cGM is necessary in MS, as cortical pathology is the likely substrate of clinical symptoms and cognitive impairment in this condition (11-13) Our most robust findings were for multiple cortical layer T1 metrics and the SDMT score – a validated measure of cognitive impairment in MS.(14) Our results also found clinically relevant T1 abnormalities in the outer (subpial) layer. T1 is affected by myelin content, axonal density and by gliosis.(15) This is in accordance with previous measurements of magnetization transfer ratio (MTR) imaging in which the largest change in MTR in MS subjects was in the superficial cortical layer.(16)
Studies of microscopic myeloarchitecture carried out in parallel to classical cytoarchitectonic investigations confirm a six-layer pattern of myelin density corresponding to the known cellular layers of the cortex.(17) Although these patterns vary depending on anatomic location, inner cortical layers (V, VI) generally contain a higher density of myelinated fibers (interneurons and axonal projections to non-cortical regions), whereas outer layers (I, II) generally contain a higher density of dendritic connections and less myelin.(18) Since anatomic cortical layers have varying architecture, degrees of myelination, and cellular contents, it should be expected that individual layers may be affected in MS differently. Proximity to MS-related inflammation in the meninges may result in neuronal, oligodendrocyte, and astrocyte loss in layers close to the pial surface, which may be reflected in our findings of altered T1 and R2* in the outermost layer and its relationship with disability.(19) Conversely, given proximity to WM and higher levels of intracortical myelination, inner cortical layers may be directly affected by demyelination independently or in the setting of leukocortical lesions.(20) The varying sources of pathology leading to inner and outer layer relaxometry alterations in MS are likely responsible for the opposing directions for correlation coefficients in inner versus outer layers with disability scales seen in this study. This difference was especially notable for T1 relaxometry metrics. The inner layer showed a higher PH for the more disabled group of patients, whereas a lower PH in the subpial layer (Figure 9). This might be the result of the different impact of leukocortical and subpial lesions. In the inner layer, leukocortical lesions are like WM lesions, being relatively uniform. The related abnormalities might have similar T1 values, thus clustering around a peak. In the outer layer, where subpial lesions tend to be more diffuse, the damage related to subpial demyelination may result in T1 values shifted towards higher values reflecting a gradient of abnormality in T1. Given opposing signs for correlations seen between inner and outer layers, the counterbalance of these changes likely explains why in such situations metrics full cortex metrics did not significantly correlate with disability. In this sense, our data suggests that averaging of relaxometry metrics across the full cortex in MS may lead to pseudo-normalization and masking of clinically relevant pathology due to differences in how the surface and inner layers are affected in MS.
In addition to the differences in how MS affects the underlying histopathologic substrate of cortical layers, the associations noted in this study may also represent the varying function of cortical layers. The relationships seen between inner layer metrics and disability scales (i.e. T1 and R2* PH with EDSS and T25FW) may be due to the importance of the interruption of cortico-thalamic, cortico-brainstem, and callosal projections in anatomic layers V/VI in the physiologic functions measured by these scales. Similarly, the relationships between outer layer metrics and disability scales (i.e. T1 PH with 9HPT and CVLT and R2* skewness with EDSS, T25FW, 9HPT, and SDMT) may represent the importance of dendritic connections in anatomic layers I/II towards the functions measured by these scales.
These data may be used on conjunction with other MRI metrics to better explain MS-related pathology. As expected, our results also show a significant relationship between cortical GM atrophy, including layer-specific atrophy, and disability, and these relationships far exceeded any relationship between overall brain atrophy or WM lesions and disability. We also performed a multivariable linear regression to evaluate if our findings were entirely contingent on this layer-specific atrophy (Supplemental Table 5). In this analysis, some relationships seen in univariate correlation between qMRI metrics and disability remained, some did not, and new relationships not previously seen emerged. This mixed result likely indicates that in some instances the relationship between qMRI metrics within a particular layer are intertwined with the processes that cause atrophy, whereas in other cases the qMRI indices are independent of atrophy. More importantly, the revelation of new relationships shows that in some cases both atrophic and other pathologic processes (i.e. demyelination) are both necessary to explain disability – justifying evaluation of both atrophy and qMRI metrics in MRI research.
The difference between layers for χR showed an ability to discriminate levels of disability, despite a lack of sensitivity for individual layer assessment. Similar differences were not seen for R2*. On the mesoscopic scale, the intracortical variation of iron concentration is responsible for much of the changes of MR signal phase reflecting the changes of χR.(21) Our χR results may reflect such sensitivity to iron mapping because different cortical layers have different iron content.(22) In addition, the lipid molecules composing myelin have an overall diamagnetic susceptibility and thus are counteracting the paramagnetic contribution of iron. It seems plausible that different levels of demyelination between the inner layers contributed to the higher sensitivity of differences in χR Although in general more correlations were seen between disability measures and T1 and R2* metrics, the differential sensitivity of T1, R2*, and QSM to disability in this study and myelin and iron in others likely suggest the need for continued use of multi-modal analyses.
This study is limited by its cross-sectional nature. It is unclear from our data if qMRI in segmented cortex would reveal changes over time indicative of MS pathology and/or responsible for disability worsening. Future work could look to follow a large cohort of MS subjects over time by this method. Further, our results could be influenced by partial volume averaging inherent to segmentation of the cortical layers. For example, the contribution of focal atrophy of the subpial cortex could explain the shift noted to higher T1 in the high EDSS group for the 4th layer (Figure 9) due to a greater CSF contribution to each voxel in this layer. Similar effects could occur at the inner layers due to WM lesions. In order to reduce this effect, our study utilized ultra-high field, high resolution images, and segmentation methods validated for more robust segmentation of the cortex based on such images.(23) Further, the segmentation was performed based on an equi-volume algorithm, which reduces partial volume effects in locations with a high curvature, and since it is based on volume, it should not be influenced by intensity characteristic changes induced by focal cortical lesions.(24) We also tried to address this possibility by re-evaluating correlations in a multi-variate model that included layer-specific atrophy as a co-variate. Although some of the correlations noted in univariate analysis were no longer significant, others remained, and new relationships emerged. We also have previously demonstrated the lack of a linear relationship between cortical depth and qMRI metric distribution, which would be expected if our results were due to partial volume averaging with CSF alone.(25) These data together suggest that our results cannot be explained by layer-specific atrophy and partial volume averaging alone, and more likely both atrophy and underlying tissue alterations (i.e. demyelination, oligodendrocyte loss, etc.) that lead to qMRI shifts represent parts of an interrelated disease process in the cortex causative of disability. Finally, the utilization of GRE-based parametric maps (R2* and QSM) translated to MP2RAGE space could have introduced noise to these measurements. The need to establish one space as native and registration of other parametric maps into this space is a necessary step in any multi-parametric neuroimaging study. Although there are drawbacks to this, previous work demonstrates that the impact on statistical analyses performed using translated qMRI maps is negligible.(26,27)
In conclusion, cortical multi-layer qMRI analyses at ultra-high field 7 T reveal layer-specific relationships with disability in MS and allow the rise of clinically relevant links that are hidden when analyzing the full cortex. We therefore suggest consideration of cortical layer stratification in future studies in MS, including those attempting to measure changes in myelination of the cortex and to monitor treatment response.
Supplementary Material
Acknowledgements
Thank you to the MRI technologists at the Kennedy Krieger Institute, including Terri Brawner, Kathleen Kahl, and Ivana Kusevic and to study nurses Julie Fiol and Kerry Naunton – all of whom were critical in collection of this data.
Funding
Data acquisition was funded by grants from NIH (1K23NS072366-01A1, PI: Harrison) and a grant from EMD-Serono (PI: Harrison). Analysis time was also supported by NIH 1R01NS104403-01 (PI: Harrison). Dr. Li is partly supported by NIBIB of NIH (P41EB015909, PI: van Zijl).
Footnotes
Declaration of conflicting interests
Dr. Harrison has received consulting fees from EMD-Serono, Genzyme, Biogen, and Genentech.
Drs. Lema Dopico, Choi, Hua, and Li have no financial disclosures.
References
- 1.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. The Lancet. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005; [DOI] [PubMed] [Google Scholar]
- 3.Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001; [DOI] [PubMed] [Google Scholar]
- 4.Weiskopf N, Suckling J, Williams G, Correia M. MM, Inkster B, Tait R, et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: A multi-center validation. Front Neurosci. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng X, Deistung A, Reichenbach JR. Quantitative susceptibility mapping (QSM) and R2* in the human brain at 3 T: Evaluation of intra-scanner repeatability. Z Med Phys. 2018; [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Adad J What can we learn from T2* maps of the cortex? NeuroImage. 2014. [DOI] [PubMed] [Google Scholar]
- 7.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. [DOI] [PubMed] [Google Scholar]
- 9.Beier M, Gromisch ES, Hughes AJ, Alschuler KN, Madathil R, Chiaravalloti N, et al. Proposed cut scores for tests of the Brief International Cognitive Assessment of Multiple Sclerosis (BICAMS). J Neurol Sci. 2017; [DOI] [PubMed] [Google Scholar]
- 10.Landman BA, Bogovic JA, Carass A, Chen M, Roy S, Shiee N, et al. System for integrated neuroimaging analysis and processing of structure. Neuroinformatics. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson F, Datta S, Garcia N, Rozario NL, Perez F, Cutter G, et al. Intracortical lesions by 3T magnetic resonance imaging and correlation with cognitive impairment in multiple sclerosis. Mult Scler J. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen AS, Kinkel RP, Madigan N, Tinelli E, Benner T, Mainero C. Contribution of cortical lesion subtypes at 7T MRI to physical and cognitive performance in MS. Neurology. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulou A, Müller-Lenke N, Naegelin Y, Kalt G, Bendfeldt K, Kuster P, et al. Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis. Mult Scler J. 2013; [DOI] [PubMed] [Google Scholar]
- 14.Rao SM, Martin AL, Huelin R, Wissinger E, Khankhel Z, Kim E, et al. Correlations between MRI and information processing speed in MS: A meta-analysis. Mult Scler Int. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brex PA, Parker GJM, Leary SM, Molyneux PD, Barker GJ, Davie CA, et al. Lesion heterogeneity in multiple sclerosis: A study of the relations between appearances on T1 weighted images, T1 relaxation times, and metabolite concentrations. J Neurol Neurosurg Psychiatry. 2000; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derakhshan M, Caramanos Z, Narayanan S, Arnold DL, Louis Collins D. Surface-based analysis reveals regions of reduced cortical magnetization transfer ratio in patients with multiple sclerosis: A proposed method for imaging subpial demyelination. Hum Brain Mapp. 2014;35(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieuwenhuys R, Broere CAJ, Cerliani L. A new myeloarchitectonic map of the human neocortex based on data from the Vogt–Vogt school. Brain Struct Funct. 2015; [DOI] [PubMed] [Google Scholar]
- 18.Lodato S, Arlotta P. Generating Neuronal Diversity in the Mammalian Cerebral Cortex. Annu Rev Cell Dev Biol. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010; [DOI] [PubMed] [Google Scholar]
- 20.Louapre C, Govindarajan ST, Giannì C, Madigan N, Nielsen AS, Sloane JA, et al. The association between intra- and juxta-cortical pathology and cognitive impairment in multiple sclerosis by quantitative T2* mapping at 7 T MRI. Neuroimage Clin. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Wen J, Cross AH, Yablonskiy DA. On the relationship between cellular and hemodynamic properties of the human brain cortex throughout adult lifespan. Neuroimage. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukunaga M, Li TQ, Van Gelderen P, De Zwart JA, Shmueli K, Yao B, et al. Layer-specific variation of iron content in cerebral cortex as a source of MRI contrast. Proc Natl Acad Sci U S A. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazin PL, Weiss M, Dinse J, Schäfer A, Trampel R, Turner R. A computational framework for ultra-high resolution cortical segmentation at 7 Tesla. NeuroImage. 2014. [DOI] [PubMed] [Google Scholar]
- 24.Waehnert MD, Dinse J, Weiss M, Streicher MN, Waehnert P, Geyer S, et al. Anatomically motivated modeling of cortical laminae. Neuroimage. 2014; [DOI] [PubMed] [Google Scholar]
- 25.Lema-Dopico A, Choi S, Harrison DM. Depth-dependent cortical distributions of quantitative 7 T MRI parameters are associated with disability in MS. In: ACTRIMS-ECTRIMS poster presentation. Washington, USA; 2020. [Google Scholar]
- 26.Hinoda T, Fushimi Y, Okada T, Fujimoto K, Liu C, Yamamoto A, et al. Quantitative Susceptibility Mapping at 3 T and 1.5 T: Evaluation of Consistency and Reproducibility. Invest Radiol. 2015; [DOI] [PubMed] [Google Scholar]
- 27.Deh K, Nguyen TD, Eskreis-Winkler S, Prince MR, Spincemaille P, Gauthier S, et al. Reproducibility of quantitative susceptibility mapping in the brain at two field strengths from two vendors. J Magn Reson Imaging. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.