Abstract
Purpose
We aimed to identify pathogenic variants in two infertile sisters in a family with a thin zona pellucida (ZP) phenotype.
Methods
Whole-exome sequencing was performed in the two affected sisters, and Sanger sequencing was used to confirm the identified variants. The effects of the identified variant were further investigated in mouse oocytes and Chinese hamster ovary (CHO) cells.
Results
We identified a novel homozygous frameshift variant in ZP2 (c.1235_1236del, p.Q412Rfs*17) in the two affected individuals. Immunoblotting demonstrated that the variant produced a truncated ZP2 protein that was expressed at low levels in CHO cells. Immunofluorescence in mouse oocytes confirmed the decreased protein level of mutant ZP2, although the subcellular localization was not affected. In addition, immunoprecipitation showed that the pathogenic variant reduced the interaction between ZP2 and ZP3.
Conclusion
This study identified a novel pathogenic variant in ZP2 that produces a truncated ZP2 protein. The variant might disrupt the assembly of ZP2-ZP3 dimers, thus resulting in a thin ZP and female infertility.
Keywords: Female infertility, Zona pellucida, Novel variant, ZP2
Introduction
Infertility is estimated to affect 8–12% of reproductive-aged couples worldwide [1]. With the rapid development of assisted reproductive technology, in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) have helped millions of patients to conceive a child. However, there are also many couples undergoing recurrent IVF/ICSI failures. In the early 1990s, four infertile women were diagnosed with oocyte maturation abnormalities after repeated IVF attempts, and similar cases were subsequently reported [2]. Although many genetic factors have been found to be responsible for abnormal oocytes and embryos, the underlying genes or variants remain to be identified [3–5].
The zona pellucida (ZP) is a relatively thick extracellular matrix that surrounds mammalian oocytes. The mouse ZP is composed of three glycoproteins, namely ZP1, ZP2, and ZP3, while the human ZP consists of the four glycoproteins ZP1, ZP2, ZP3, and ZP4 [6]. These glycoproteins all have similar structural elements such as a N-terminal hydrophobic signal peptide, a ZP domain, a consensus furin cleavage site, a transmembrane-like domain, and a short cytoplasmic tail. In addition, ZP1 and ZP4 also have a trefoil domain, respectively [6]. A structural model suggested that ZP1 dimers might serve to cross-link ZP filaments constructed of ZP2-ZP3 dimers [7, 8]. The ZP is critically important during oogenesis, fertilization, and preimplantation development. In humans, ZP1, ZP3, and ZP4 bind to spermatozoa and induce acrosome reaction, while ZP2 binds to the acrosome region only in acrosome-reacted spermatozoa thus acting as a secondary sperm receptor [6]. ZP2 also plays a major role in the prevention of polyspermy in mice. Mice lacking ZP1 have early embryonic loss, and in these mice the matrix is more loosely organized than the ZP around normal oocytes thus leading to reduced fecundity [7]. The structural defects are even more severe in ZP2-null and ZP3-null mice, and female mice with these genotypes fail to form a visible ZP and are sterile [9].
In humans, pathogenic variants in ZP1 were reported to cause female infertility due to abnormal ZP formation, and either no oocytes or only ZP-free oocytes were retrieved from these patients [10–12]. The ZP1 mutant was suggested to abolish the interactions between ZP1 and ZP2/ZP3 [12]. Variants in ZP2 were identified in infertile females who experienced recurrent IVF failures [10, 13–15]. The reported ZP2 variants were predicted to produce truncated ZP2 proteins that cause intracellular sequestration of ZP2, partial retention of the three ZP proteins thus forming a thin ZP [15]. The thin ZP was defective for sperm binding and penetration and therefore led to failed IVF attempts [15]. Previous studies also indicated that ZP3 variants contribute to oocyte degradation and empty follicle syndrome [13, 16]. It was suggested that mutant ZP3 proteins might compete with wild-type ZP3 proteins for binding to the other three ZP proteins, thus impeding the assembly of heterodimeric mutants [13]. The reduced numbers of heterodimers formed by the variants might be insufficient for constructing a stable ZP [13].
In the present study, we identified a novel homozygous frameshift variant in ZP2 (c.1235_1236del, p.Q412Rfs*17) in two affected sisters who were diagnosed with primary infertility due to a thin ZP. We further investigated the effects of the variant in mouse oocytes and Chinese hamster ovary (CHO) cells. Our findings expand the mutational spectrum of ZP2 and provide a genetic marker for future diagnosis.
Materials and methods
Case report
The patients were recruited from the Shanghai Ji Ai Genetics and IVF Institute. The proband (family member II-2) was unable to conceive despite unprotected sexual intercourse. She had regular menstrual cycles and no dysmenorrhea, and infertility-related assessments did not reveal any abnormalities. She had two ICSI attempts but failed to establish a pregnancy. Her elder sister (family member II-1) shared similar clinical characteristics and underwent three unsuccessful IVF/ICSI treatment cycles. The study was approved by the Ethics Committee of the Medical College of Fudan University and the Reproductive Study Ethics Committee of Shanghai Ji Ai Genetics and IVF Institute. The patients provided written informed consent.
Genomic DNA extraction
Blood samples from the patient and her family members were isolated and preserved in EDTA tubes. Genomic DNA was then extracted from the peripheral blood using a HiPure Blood DNA Mini Kit (Magen). The DNA concentration and purity were measured with a NanoDrop 1000 spectrophotometer (Thermo Scientific).
Genetic studies
In order to identify potential pathogenic factors, exome capture and sequencing were carried out using the Agilent SureSelect Whole Exome Enrichment Kit and the Illumina HiSeq 3000 platform. Sequences were aligned to the human reference sequence (NCBI Genome build GRCh37) and annotated using the GRCh37, dbSNP (version 138), 1000 Genomes, and Exome Aggregation Consortium (ExAC, version 0.3.1) databases. Candidate variants were identified according to the following filtering criteria: (1) variants with an allele frequency below 1% in the ExAC database, (2) exonic or splice site variants, and (3) in silico prediction to be damaging. Candidate variants were subsequently confirmed by Sanger sequencing.
Expression vector and cRNA construction
Full-length coding sequences of ZP2 and ZP3 were amplified from the cDNA of abandoned human GV or MI oocytes and cloned into the pCMV6-Entry vector (Origene) by SfaAI and MluI dual-enzyme digestion (Thermo Scientific). A MYC-tag and HA-tag were fused to the N-termini of ZP2 and ZP3, respectively. Site-directed mutagenesis was performed to introduce the c.1235_1236del variant into the wild-type ZP2 vector using the KOD-Plus Mutagenesis Kit (Toyobo Life Science). Wild-type and mutant clones were further confirmed by Sanger sequencing. Wild type and mutant ZP2 constructs were linearized with the SacII restriction enzyme. The linearized products were purified and used as templates for in vitro transcription with the HiScribe T7 ARCA mRNA Kit (with tailing) (New England Biolabs). Wild-type and mutant ZP2 cRNAs were polyadenylated, purified and dissolved in RNase-free water.
Immunofluorescence
Wild-type and mutant ZP2 cRNAs were microinjected into GV oocytes obtained from ICR mice. After culturing for 12 h, the oocytes were fixed in 2% paraformaldehyde for 2 hours, permeabilized in 0.5% Triton X for 30 min, and then blocked in blocking buffer (3% BSA, 0.1% Tween 20, and 0.01% Triton X) for 1 h at room temperature. The oocytes were incubated with anti-MYC antibody (1:500 dilution, CST, cat no. 2276) at 4 °C overnight. The oocytes were then washed with washing buffer (0.1% Tween 20 and 0.01% Triton X) once and incubated with Alexa Fluor 594 secondary antibody (1:500, ThermoFisher, cat no. A-21125) for 1 h at 37 °C. Hoechst 33342 solution (1:700, BD, 561908) was used for DNA visualization. All images were captured on an EVOS M7000 Imaging System (Invitrogen).
Cell culture and transfection
CHO cells (Fudan IBS Cell Center) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution and incubated at 37 °C and 5% CO2 for 24 h. Wild-type and mutant ZP2 plasmids were transfected into CHO cells using the PolyJet in vitro DNA transfection reagent (SignaGen, cat no. SL100688).
Western blotting
CHO cells were recovered 36 h after transfection and washed twice with PBS. Cells were then lysed in RIPA lysis buffer (Shanghai Wei Ao Biological Technology) supplemented with a protease inhibitor cocktail (B14001, Bimake) and centrifuged at 12,300×g at 4 °C for 25 min. Supernatants were collected and mixed with SDS-PAGE sample loading buffer, and the mixture was heated to 100 °C for 10 min. Proteins were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose filter membrane. After blocking with 5% nonfat dry milk in TBST for 2 h, the membrane was incubated with mouse anti-MYC antibody (1:3000 dilution, CST, cat no. 2276) at 4 °C overnight. Rabbit anti-vinculin (1:1,000 dilution, CST, cat no. 13901) was used as the internal control. The membrane was then rinsed three times with TBST, and HRP goat anti-rabbit IgG (1:5000 dilution, Abmart) and HRP goat anti-mouse IgG (1:5000 dilution, Abmart) secondary antibodies were added. After incubating at room temperature for 1 h, the membrane was washed another three times with TBST. ECL western blotting substrate detection reagents (180-501, Tanon) were mixed at a 1:1 ratio according to the manufacturer’s instructions and added to the membrane. The membrane was imaged with a Tanon 5200s Imaging Workstation.
Immunoprecipitation
To determine the effect of the ZP2 variant on the interaction between ZP2 and ZP3, wild-type and mutant ZP2 plasmids were separately co-transfected with the ZP3 vector into CHO cells. Cells were recovered 36 h after transfection and proteins were extracted with NP-40 lysis buffer (50 mM Tris, 150 mM NaCl, 0.5% NP-40 (pH 7.5), and 1% protease inhibitor cocktail (Bimake)). Anti-MYC beads (Bimake) were used for immunoprecipitation, and the immunoprecipitated proteins were separated by SDS-PAGE and evaluated by western blotting with antibodies against MYC (1:1,000 dilution, CST, cat no. 2276) and HA (1:1,000 dilution, CST, cat no. 3724).
Results
Clinical characteristics of the affected individuals
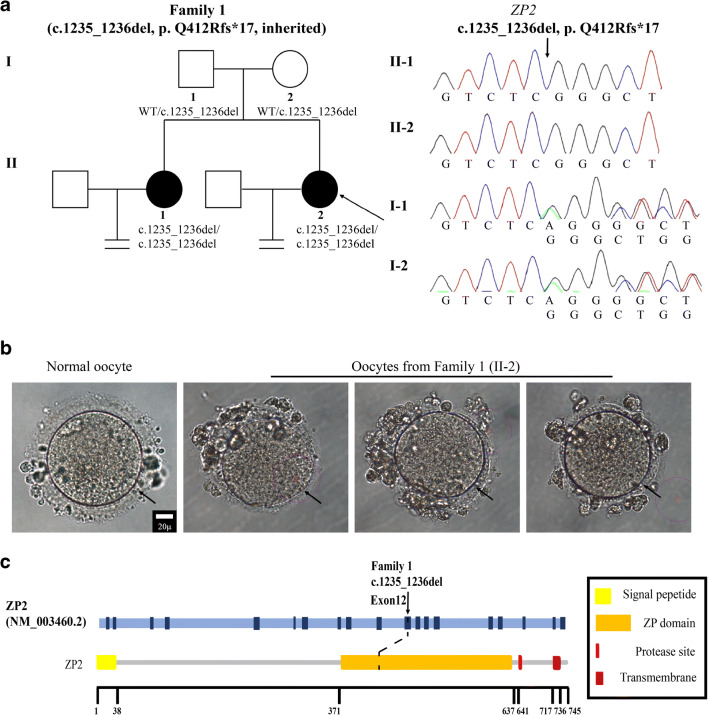
We recruited two affected sisters from a Chinese family (Fig. 1a). The proband (family member II-2) was a 34-year-old woman with a history of primary infertility for 6 years. Infertility-related assessments did not reveal any abnormalities, but she had two failed ICSI attempts (Table 1). In her first ICSI attempt, 9 oocytes were retrieved, including 1 MII oocyte, 4 immature oocytes, and 4 abnormal oocytes. All of the oocytes were surrounded by a relatively thin ZP (Fig. 1b). The only MII oocyte obtained was fertilized and underwent cleavage successfully. This embryo was viable on day 3 but failed to form a blastocyst on day 5. In the second ICSI attempt, 8 oocytes were retrieved, including 5 MII oocytes and 3 abnormal oocytes. All of the oocytes again exhibited a thin ZP (Fig. 1b). Only 2 MII oocytes were normally fertilized and formed 2PN embryos. The embryos were both viable on day 3, one of which was frozen while the other was further cultured but arrested on day 5. The proband had an older sister (family member II-1) who was also diagnosed with primary infertility. The older sister had three unsuccessful IVF/ICSI treatment cycles in which oocytes with a thin ZP were either not fertilized or failed to produce a viable embryo.
Fig. 1.
Genotypic and phenotypic features of the patient. a Identification of the ZP2 variant. The pedigree of the patients (family 1) is shown in the left panel. Squares represent males and circles represent females. Solid circles indicate patients with primary infertility and abnormal oocyte phenotypes. The black arrow indicates the proband. Sanger sequencing confirmation is shown in the right panel. The variant displays a recessive inheritance pattern. b Phenotype of the oocytes from the proband (II-2). MII oocytes from the proband were photographed under a light microscope. The three oocytes all presented with a thin ZP, and a normal MII oocyte is shown for comparison. The black arrow points to the ZP. Scale bar = 20 μm. c Location of the variant in ZP2. The position of the variant is indicated in the gene structure and protein structure of ZP2
Table 1.
Oocyte and embryo characteristics of ICSI attempts for the proband (II-2 in family 1)
| Patient | Age (years) | Duration of infertility (years) | ICSI cycles | Number of retrieved oocytes | Phenotype | MII oocytes | Immature oocytes | Abnormal oocytes | Fertilized oocytes | Viable embryos on day 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Family 1 II-2 | 34 | 6 | 1st | 9 | Thin ZP | 1 | 4 | 4 | 1 | 1 |
| 2nd | 8 | Thin ZP | 5 | 0 | 3 | 2 | 2 |
Identification of the pathogenic variant in ZP2
Whole-exome sequencing was carried out, and candidate variants were identified according to the strict filtering criteria described above. A homozygous frameshift deletion variant in exon 12 of ZP2 (NM_003460.2, c.1235_1236del, p.Q412Rfs*17) was identified in the proband (Fig. 1a, Table 2), and the variant was verified by Sanger sequencing. Both of her parents carried a heterozygous ZP2 variant indicating a recessive inheritance pattern, and the same homozygous variant was also identified in her infertile sister (Fig. 1a). The corresponding altered amino acid was located in the ZP domain of ZP2 (Fig. 1c).
Table 2.
Overview of the ZP2 variant in family 1
| Gene | Genomic position (bp) | Exon | cDNA change | Protein change | Variant type | Genotype | Inheritance | gnomAD AFa |
|---|---|---|---|---|---|---|---|---|
| ZP2 | chr16:21213476 | 12 | c.1235_1236del | p.Q412Rfs*17 | Frameshift deletion | Homozygous | Autosomal recessive | 4 × 10-6 |
aAF: allele frequency in the gnomAD database
The p.Q412Rfs*17 variant reduced the protein level of ZP2 in mouse oocytes and CHO cells
To evaluate the effects of the identified variant on the protein level and distribution pattern of ZP2, wild-type and mutant ZP2 cRNAs were microinjected separately into ICR mouse GV oocytes. Immunofluorescence indicated that the mutation led to a decreased ZP2 protein level, although both wild-type and mutant ZP2 proteins were diffusely distributed in the ZP and cytoplasm (Fig. 2a). To further probe the effect of the variant on ZP2 protein level, wild-type and mutant ZP2 plasmids were transfected into CHO cells. Western blotting showed a decreased molecular mass and confirmed the reduced protein level of the mutant ZP2 protein (Fig. 2b). These results indicated that the variant produced a truncated ZP2 protein with a relatively low intracellular protein level.
Fig. 2.
Effects of the homozygous frameshift variant on ZP2. a Expression and location of the mutant protein in mouse oocytes. Oocytes microinjected with RNase-free water, wild-type ZP2, and mutant ZP2 cRNAs were immunolabeled with antibodies against MYC. Fluorescent signals of DNA (blue) and ZP2 (red) were imaged individually and merged using the EVOS M7000 Imaging System (Invitrogen). Scale bar = 20μm. b Expression of mutant protein in CHO cells. Left panel: effect of the variant on ZP2 protein levels by western blotting in CHO cells transfected with wild-type and mutant vectors. Right panel: bar graph of the relative expression levels of wild-type and mutant ZP2. Three independent experiments were performed, and the P value was calculated with t test. The data are shown as means and standard errors of the mean, *P < 0.05. c Effect of the mutant protein on the interaction between ZP2 and ZP3. Interactions between wild-type and mutant ZP2 with ZP3 were evaluated by immunoprecipitation with anti-MYC beads
Effect of the mutant p.Q412Rfs*17 on ZP2-ZP3 interactions
Upon fertilization, modifications of ZP2 and ZP3 result in changes to the physical and biological properties of ZP filaments, which are suggested to be constructed of ZP2-ZP3 dimers that are cross-linked by ZP1. In order to explore whether the ZP2 variant would affect the formation of ZP2-ZP3 dimers, we co-transfected wild-type ZP2 with ZP3 and mutant ZP2 with ZP3 separately into CHO cells to study the interactions between ZP2 and ZP3. Immunoprecipitation showed that mutant p.Q412Rfs*17 reduced the interaction between ZP2 and ZP3 (Fig. 2c) and might disrupt the formation of ZP2-ZP3 dimers.
Discussion
Here, we identified a novel homozygous frameshift variant in ZP2 (c.1235_1236del, p.Q412Rfs*17) in a Chinese family. The two affected sisters from the same family were diagnosed with primary infertility and experienced recurrent IVF/ICSI failures due to a thin ZP.
The ZP is a translucent extracellular matrix surrounding mammalian oocytes and is critically important during oogenesis, fertilization, and preimplantation development. In humans, ZP1, ZP3, and ZP4 bind to spermatozoa and induce acrosome reaction, while ZP2 binds to the acrosome region only in acrosome-reacted spermatozoa thus acting as a secondary sperm receptor [6]. ZP2 also plays a major role in the prevention of polyspermy in mice. Following the fusion of a sperm with an oocyte, ZP2 is suggested to be cleaved by the ovastacin metalloendoprotease that is released during exocytosis of the cortical granules [17], and this modification of ZP2 prevents gamete recognition and the formation of nonviable polyploid embryos. However, whether ZP2 has a similar role in preventing polyspermy in humans needs to be established in future studies.
Previous studies have shown that variants in ZP1, ZP2, and ZP3 lead to female infertility and recurrent IVF/ICSI failures due to abnormal ZP formation. Two homozygous variants in ZP2 (c.1695-2A>G and c.1691_1694dup) were identified in patients from two unrelated consanguineous families whose oocytes exhibited a thin ZP that prevented sperm binding and led to fertilization failure [15]. A homozygous variant in ZP2 (c.1115G > C) was also found in a consanguineous family. The majority of oocytes retrieved from the patient had no ZP, while a few had a thin ZP, and the oocytes either failed to develop into viable embryos or the poor-quality embryos were arrested after implantation [13]. Most recently, a compound-heterozygous mutation in ZP2 gene (c.860_861del and c.1924 C>T) was identified. Some of the retrieved oocytes from the patient were found to be ZP-free, and the others had an abnormal ZP with a thin matrix and an enlarged perivitelline space. Either no viable embryos were obtained or the embryos failed in implantation [10]. In our study, oocytes retrieved from the patient with the novel homozygous frameshift ZP2 variant (c.1235_1236del, p.Q412Rfs*17) were surrounded by a relatively thin ZP. These oocytes could be fertilized after ICSI treatment and underwent cleavage successfully, but they failed to form a blastocyst. Immunoblotting demonstrated that the variant produced low levels of a truncated ZP2 protein in CHO cells. Immunofluorescence in mouse oocytes confirmed the decreased protein level of mutant ZP2, although the subcellular localization was not affected.
All four human ZP glycoproteins share a common structural element called the ZP domain. The ZP domain consists of approximately 260 aa including eight conserved cysteine residues, and it is suggested to play an important role in the polymerization of human ZP proteins into a filamentous structure [6]. It is noteworthy that the variant (c.1235_1236del, p.Q412Rfs*17) in our study is situated in the ZP domain of ZP2. Consistent with this, immunoprecipitation showed a reduced interaction between the truncated ZP2 and wild-type ZP3, which was in accordance with previous studies [16, 18]. The variant in ZP2 impeded the interaction between ZP2 and ZP3, which might lead to disruption of the assembly of ZP2-ZP3 dimers and thus to abnormal ZP formation.
In conclusion, we identified a novel homozygous frameshift variant in ZP2 (c.1235_1236del, p.Q412Rfs*17). The variant produced low levels of truncated ZP2 proteins and thus impeded the interaction between ZP2 and ZP3. Our study further reveals the possible genetic pathogenesis of female infertility and expands the mutational spectrum of ZP2, thus providing a genetic basis for targeted treatment of female infertility in the future.
Acknowledgements
We would like to sincerely thank the patients and their families for their support and participation.
Funding
This study was funded by the National Key Research and Development Program of China (2018YFC1003800, 2017YFC1001500, and 2016YFC1000600), the National Natural Science Foundation of China (81725006, 81822019, 81771581, 81971450, and 81971382), the project supported by the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), Project of the Shanghai Municipal Science and Technology Commission (19JC1411001), the Natural Science Foundation of Shanghai (19ZR1444500), the Shuguang Program of the Shanghai Education Development Foundation and the Shanghai Municipal Education Commission (18SG03), the Foundation of Shanghai Health and Family Planning Commission (20154Y0162), the Capacity Building Planning Program for Shanghai Women and Children’s Health Service, and the collaborative innovation center project construction for Shanghai Women and Children’s Health.
Declarations
Ethics approval
The study was approved by the Ethics Committee of the Medical College of Fudan University and the Reproductive Study Ethics Committee of Shanghai Ji Ai Genetics and IVF Institute.
Patient consent
Obtained.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Biaobang Chen and Congjian Xu contributed equally as corresponding authors.
Contributor Information
Biaobang Chen, Email: chenbiaobang@163.com.
Congjian Xu, Email: xucj@hotmail.com.
References
- 1.Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Rudak E, Dor J, Kimchi M, Goldman B, Levran D, Mashiach S. Anomalies of human oocytes from infertile women undergoing treatment by in vitro fertilization. Fertil Steril. 1990;54(2):292–296. doi: 10.1016/S0015-0282(16)53706-6. [DOI] [PubMed] [Google Scholar]
- 3.Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML, Jr, Cowan NJ, Wang L. Mutations in TUBB8 and Human Oocyte Meiotic Arrest. N Engl J Med. 2016;374(3):223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christou-Kent M, Kherraf ZE, Amiri-Yekta A, Le Blevec E, Karaouzene T, Conne B, et al. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med. 2018;10(5):e8515. [DOI] [PMC free article] [PubMed]
- 5.Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, Wu L, Lyu Q, Fu Y, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102(4):649–657. doi: 10.1016/j.ajhg.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta SK. The human egg’s zona pellucida. Curr Top Dev Biol. 2018;130:379–411. doi: 10.1016/bs.ctdb.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development. 1999;126(17):3847–3855. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- 8.Bokhove M, Jovine L. Structure of Zona Pellucida Module Proteins. Curr Top Dev Biol. 2018;130:413–442. doi: 10.1016/bs.ctdb.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development. 2001;128(7):1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- 10.Luo G, Zhu L, Liu Z, Yang X, Xi Q, Li Z, Duan J, Jin L, Zhang X. Novel mutations in ZP1 and ZP2 cause primary infertility due to empty follicle syndrome and abnormal zona pellucida. J Assist Reprod Genet. 2020;37(11):2853–2860. doi: 10.1007/s10815-020-01926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H-L, Lv C, Zhao Y-C, Li W, He X-M, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in Familial Infertility. N Engl J Med. 2014;370(13):1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Zhu X, Maqsood M, Li W, Tong X, Kong S, Wang F, Liu X, Wei Z, Zhang Z, Zhu F, Cao Y, Bao J. A novel homozygous nonsense ZP1 variant causes human female infertility associated with empty follicle syndrome (EFS) Mol Genet Genomic Med. 2020;8(7):e1269. doi: 10.1002/mgg3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, Mu J, Li B, Yan Z, Fu J, Wang W, Zhao L, Dong J, Sun X, Kuang Y, Sang Q, Wang L. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138(4):327–337. doi: 10.1007/s00439-019-01990-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Li K, Bai D, Yin J, Tang Y, Chi F, Zhang L, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kou X, Zhao Y, Wang H, Chen J, Teng X, Gao S. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet. 2017;136(8):975–985. doi: 10.1007/s00439-017-1822-7. [DOI] [PubMed] [Google Scholar]
- 15.Dai C, Hu L, Gong F, Tan Y, Cai S, Zhang S, Dai J, Lu C, Chen J, Chen Y, Lu G, du J, Lin G. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med. 2018;21(2):431–440. doi: 10.1038/s41436-018-0064-y. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet. 2017;101(3):459–465. doi: 10.1016/j.ajhg.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkart AD, Xiong B, Baibakov B. Jim¨¦nez-Movilla M, Dean J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J Cell Biol. 2012;197(1):37–44. doi: 10.1083/jcb.201112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Q, Zhao C, Zhang X, Zhang H, Lu Q, Wang C, Hu Y, Ling X, Zhang J, Huo R. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med. 2020;24(15):8557–8566. doi: 10.1111/jcmm.15482. [DOI] [PMC free article] [PubMed] [Google Scholar]