Abstract
Background
Cytokine storm is the exaggerated immune response often observed in viral infections. It is also intimately linked with the progression of COVID-19 disease as well as associated complications and mortality. Therefore, targeting the cytokine storm might help in reducing COVID-19-associated health complications. The number of COVID-19 associated deaths (as of January 15, 2021; https://www.worldometers.info/coronavirus/) in the USA is high (1199/million) as compared to countries like India (110/million). Although the reason behind this is not clear, spices may have some role in explaining this difference. Spices and herbs are used in different traditional medicines, especially in countries such as India to treat various chronic diseases due to their potent antioxidant and anti-inflammatory properties.
Aim
To evaluate the literature available on the anti-inflammatory properties of spices which might prove beneficial in the prevention and treatment of COVID-19 associated cytokine storm.
Method
A detailed literature search has been conducted on PubMed for collecting information pertaining to the COVID-19; the history, origin, key structural features, and mechanism of infection of SARS-CoV-2; the repurposed drugs in use for the management of COVID-19, and the anti-inflammatory role of spices to combat COVID-19 associated cytokine storm.
Key findings
The literature search resulted in numerous in vitro, in vivo and clinical trials that have reported the potency of spices to exert anti-inflammatory effects by regulating crucial molecular targets for inflammation.
Significance
As spices are derived from Mother Nature and are inexpensive, they are relatively safer to consume. Therefore, their anti-inflammatory property can be exploited to combat the cytokine storm in COVID-19 patients. This review thus focuses on the current knowledge on the role of spices for the treatment of COVID-19 through suppression of inflammation-linked cytokine storm.
Keywords: SARS-CoV-2, Cytokine storm, COVID-19, Curcumin, Spices, Inflammation
Graphical abstract
1. Introduction
The ongoing novel coronavirus pandemic has taken a major toll on human lives worldwide. In December 2019, the first case of the ongoing pandemic of the novel coronavirus disease (COVID-19) was reported [1]. The epicenter of COVID-19 was identified as Wuhan, the capital of Hubei province, China. Initially, the outbreak was declared as a “Global Health Emergency” by the World Health Organization (WHO) on 30th January 2020 [2]. However, as COVID-19 spread rapidly across the globe affecting thousands of lives worldwide, the WHO finally declared it a Global pandemic on 11th March 2020 [3].
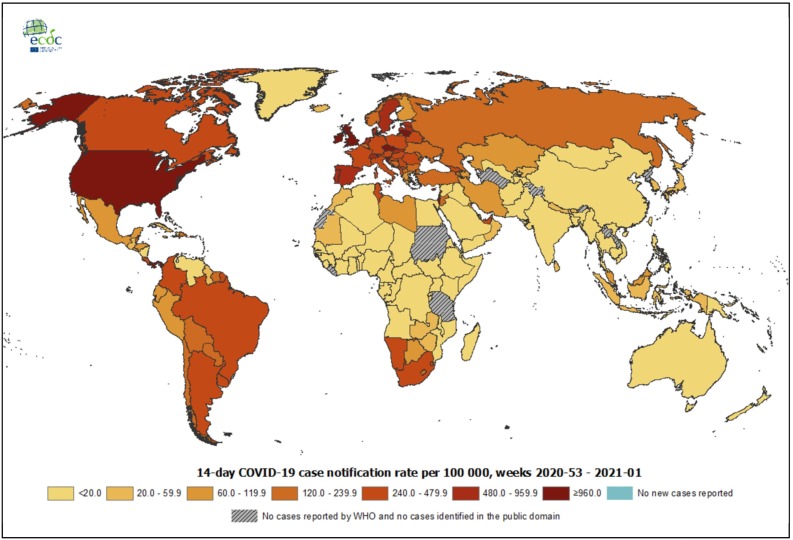
As per the weekly epidemiological update released by the WHO on 10th January 2021 at 10:00 CEST, the total number of cases worldwide were reported to be 88,387,352 with 1,919,204 deaths. In India, the total cases were reported to be 10,450,284 with 150,999 deaths [4]. The geographical distribution of the COVID-19 pandemic as per 14-day COVID-19 case notification rate per 100,000 population (as of 13th January 2021) has been illustrated in Fig. 1 [5].
Fig. 1.
The geographical distribution of the COVID-19 pandemic as per 14-day COVID-19 case notification rate per 100,000 population (as of 13th January 2021) [5].
The causative pathogen of COVID-19 has been identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a novel human coronavirus (HCoV) [6]. The SARS-CoV-2 is believed to have a zoonotic origin and bats are considered as their natural reservoir host. The transmission of the virus occurs from human-to-human mostly by direct and indirect contact with cough or sneeze droplets from an infected person and contaminated surfaces, respectively. The incubation period of the virus is approximately 2–14 days. Infected people may present symptoms such as fever, cough, breathlessness, etc. However, some may remain asymptomatic [7,8]. COVID-19 is usually mild; however, infected patients with comorbidities, such as hypertension, diabetes, cancer, immunodeficiency, etc. are more prone to poor prognosis. In such cases, the severity of disease progression might eventually result in pneumonia, acute respiratory distress syndrome (ARDS), and multi-organ failure which may ultimately cause death [7,9].
Although the exact pathophysiological mechanism of COVID-19 is poorly understood; clinical evidence has revealed that COVID-19 infected patients often show an elevated rise in the cytokine levels which is termed as “cytokine storm” or “cytokine release syndrome”. This abnormal level of cytokines is considered to be correlated with severe deterioration of health conditions in the infected patients [10]. Therefore, suppressing elevated inflammatory response produced during COVID-19 may prove crucial in preventing the severity of disease as well as associated health complications [11].
Various studies have reported that naturally occurring spices as well as their isolated active components target the inflammatory pathways and induce anti-inflammatory effects in many chronic diseases [[12], [13], [14]].
A recent study conducted on primary data of 163 countries worldwide in respect of total cases, deaths, and recoveries of COVID-19 revealed a close association of the total number of COVID-19 cases per million population tested and the gram of spice supplied per capita per day. This study further reported that the nation with an increased number of COVID-19 cases per million population corresponds to lower consumption of spices per capita, with some exceptions such as Luxembourg and Iceland [15]. As of 10th January 2021, the cumulative cases of COVID-19 in United States of America (USA) are 21,761,186 compared to 10,450,284 cases in India. Moreover, the cumulative cases and deaths per 100,000 population in the USA are reported to be 6574.3 and 110.5 (1.68%), respectively, as opposed to 757.3 cumulative cases and 10.9 deaths (1.439%) in India [4]. As of 20th January 12:07 PM IST, in India, Lakshwadeep (0.00019%), Dadar and Nagar Havelli; Daman and Diu (0.03%), and Mizoram (0.04%) have recorded low number of cases compared to the rest of the states, while Maharashtra recorded the highest number of cases (18.82%) out of the total number of cases (10,596,449). Lakshwadeep has not reported any COVID-19 associated death so far. Further, Dadar and Nagar Havelli; Daman and Diu (0.1%), Mizoram (0.2%), Arunachal Pradesh (0.3%), and Kerala (0.4%) have recorded a low fatality ratio. Interestingly, although Kerala has reported approximately 8% of the total cases, the fatality ratio is quite low (0.4%). This low fatality ratio in Kerala can be attributed to the efficient management of the disease, healthcare system, etc. [16,17]. Moreover, as spices and herbs are rich immunity boosters and are prevalently consumed in India and other Asian countries, it might be associated with faster recovery and lower per million population deaths. This was evinced by a study which reported that the intake of spicy food was associated with a 14% decrease in total mortality and thus suggested their inverse association [15,18]. This review thus focuses on the anti-inflammatory role of spices as potential therapeutic agents to combat the occurrence of “cytokine storm” in COVID-19.
2. Lockdown
The outbreak of COVID-19 was first reported in December in Wuhan and Hubei province. Several places were majorly affected by the COVID-19 which led to the imposition of lockdown. During this lockdown period, several activities such as travel via international flights as well as local transports, mass gatherings at public places such as schools, universities, etc., had been restricted. Moreover, people were not allowed to go outside for any work, except for certain essential activities for a limited period with strict guidelines of social distancing. Despite these enforcements and restrictions, the number of COVID-19 cases had increased significantly throughout the world [19,20]. Initially, India had reported fewer COVID-19 cases because it was able to enforce strict lockdown and social distancing. Moreover, the general public was strongly advised to wear a mask and wash or sanitize hands frequently with soap or sanitizers as a preventive measure throughout the country. The WHO had praised India's early implementation of nationwide lockdown as “tough and timely”. Later, when the restrictions were relaxed, the COVID-19 cases increased drastically [21,22]. Community transfer had been observed in various countries that had not implemented early lockdown. Previous studies reported that travel restrictions and isolation had played a pivotal role in the outbreaks of Ebola, SARS-CoV, and bubonic plague [[23], [24], [25]]. However, the implementation of lockdown alone is not the permanent solution to prevent the further spread of COVID-19 and this raised serious concerns [19].
Some studies also reported that South Korea and Sweden had not implemented stringent lockdown and restrictions on their countries. However, the rate of infections in these countries was observed to be lower than those which had implemented strict lockdown [26,27]. One of the important reasons behind fewer infection and death rates in South Korea was believed to be the rapid testing of COVID-19 [21]. Sweden and Denmark had also followed different strategies rather than stringent lockdown to mitigate the virus spread. The authorities in those countries had appealed for strong awareness and a personal sense of responsibility and further encouraged people to work from home. The patients with comorbidities, such as respiratory ailments, immune deficiency, hypertension, cancer, diabetes, diseases of the heart, liver, and kidneys were found to be more prone to the COVID-19 infection and death. Therefore, people with a high risk of COVID-19 due to comorbidities or age were strongly recommended to follow quarantine and self-isolation to ‘flatten the curve’. Moreover, the kindergartens, nurseries, schools, and colleges remained open during this period while following the norms of social distancing and awareness [9,[27], [28], [29], [30], [31]].
The USA has recorded the highest number of COVID-19 infections and deaths. Currently, India has the 2nd highest number of COVID-19 cases and the 3rd highest number of deaths. Brazil is 3rd and 2nd in terms of the number of cases and deaths, respectively. European countries (Russia, France, Spain, UK, Italy, and Germany) have also reported a significantly higher number of COVID-19 associated deaths; however, many people recovered and the number of active cases has declined lately [4]. A closer look has uncovered various reasons for the spread of community transfer of COVID-19 in these countries, such as frequent international travels as well as the late and casual implementation of social distancing [21,26]. The implementation of lockdown due to the COVID-19 pandemic has given birth to certain health-related issues, such as obesity, irregular sleeping behavior, anxiety, depression, etc. Moreover, it has also affected the economy very gravely throughout the world [[32], [33], [34]].
3. Human coronaviruses (HCoVs)
3.1. History
The human coronavirus (HCoV) was first characterized in the 1960s [35]. Tyrell and Bynoe from the Common Cold Unit, England, investigated samples from patients with the common cold and isolated a novel flu-like virus in the 1960s. These viruses were labeled as B814 and were reported as ether sensitive in nature. Initially, they were unable to culture B814 by utilizing the available standard culture techniques. However, in 1965, they were successful in growing B814 in organ cultures [[35], [36], [37], [38]]. In 1966, Hamre and Procknow from the University of Chicago isolated and reported the presence of a novel RNA virus associated with respiratory disease. This virus was labeled 229E and it exhibited ether sensitivity like the B814 virus [39].
The B814 and 229E viruses were characterized using electron microscopy by Almeida and Tyrell. These ether sensitive viruses were reportedly indistinguishable from one another as well as the avian infectious bronchitis virus (IBV) [40]. These novel viruses along with other morphologically identical animal viruses such as IBV were grouped into a new genus termed “Coronavirus” (Latin word “corona” meaning “crown”) in 1968. They were named after the characteristic fringe or crown-like rounded projections on their surface (resembling the solar corona) as observed under an electron microscope [35,41]. In 1975, the coronaviruses were clubbed under a novel family of viruses named “Coronaviridae” [42]. Apart from the aforementioned HCoVs, several other strains of HCoVs have been identified; some of these include the HCoV-OC43 (1967), SARS-CoV (2002–2003), HCoV-NL63 (2004), HCoV-HKU1 (2005), Middle East respiratory syndrome (MERS)-CoV (2012), and SARS-CoV-2 (2019) [[43], [44], [45]]. The HCoVs such as 229E, OC43, NL63, and HKU1 are known as endemic CoVs. They are commonly found in the human population and are known to cause mild respiratory infections [46]. However, HCOVs such as SARS-CoV, MERS-CoV, and SARS-CoV-2 are the deadlier viruses that have caused the global outbreak and infected thousands of people worldwide [44].
Towards the latter end of 2002, the emergence of an infectious virus was reported from the Guangdong province, China. This virus was reported to transmit from human-to-human and was later identified as the SARS-CoV in 2002–2003. The infected people mostly presented with symptoms such as fever, cough, myalgia, etc. Other symptoms included headache, dyspnea, hypoxia, vomiting, etc. [[45], [46], [47], [48], [49]]. In some cases, the occurrence of pneumonia and ARDS had also been reported [48].
In 2012, the the first case of MERS-CoV was reported from Saudi Arabia and the outbreak quickly spread across the Arabian Peninsula [50,51]. Several cases were also reported in Asia, Europe, and Africa [52]. The transmission of MERS-CoV reportedly occurs via human-to-human as well as dromedary camel-to-human. However, the cases of camel-to-human infection are comparatively less [53]. Infected people initially exhibit symptoms such as fever, headache, cough, myalgia, etc. However, the disease might progress in severe cases and cause pneumonia, ARDS, septic shock as well as multi-organ failure, which can be fatal. Besides, cases of asymptomatic MERS-CoV infection have also been reported [44].
The recently identified novel coronavirus SARS-CoV-2 belongs to the genera β-coronavirus of the Coronaviridae family [54]. It reportedly shares 96% and 79.6% sequence identity to the bat CoVRaTG13 and SARS-CoV, respectively [8].
3.2. Origin and structural features
The coronaviruses (CoVs) are single, positive-strand RNA viruses. Their genome is approximately 26–32 kilobases in size [44]. They belong to the coronaviridae family of the order nidovirales and are categorized into the genera – alpha (α), beta (β), gamma (γ), and delta (δ) coronavirus [54]. The α- and β-CoVs include both human and animal CoVs. The HCoVs such as 229E, NL63 belong to α-CoV while the OC43, HKU1, SARS-CoV, MERS-CoV, and SARS-CoV-2 belong to the β-CoVs. The γ- and δ-CoVs primarily consists of avian coronaviruses [45].
The viral genome of CoV encodes four important structural proteins. They are - the envelope (E), spike (S), membrane (M), and nucleocapsid (N) proteins [54]. The E, S, and M proteins are anchored in the lipid bilayer of the viral envelope [55]. The M protein is approximately 25–30 kDa and gives shape to the virus. The E protein is approximately 8–12 kDa and promotes viral release. Together, the M and E proteins are associated with the viral assembly. Furthermore, they also facilitate the maturation of viral envelopes [56]. The N protein is involved in the formation of the nucleocapsid. It binds with the viral genome and plays an essential role in viral packaging [55]. The S protein (class I fusion protein) is approximately 150 kDa. It is responsible for the characteristic spike-like protrusions on the virus. It comprises S1 and S2 subunits and undergoes cleavage by furin-like protease in the host. The S1 subunit contains a receptor-binding domain (RBD). It binds to the host receptor angiotensin-converting enzyme 2 (ACE2). The S2 subunit of the viral S protein then fuses with the cell membrane of the host, which facilitates viral entry into the host cells [[56], [57], [58]].
3.3. Mechanism of SARS-CoV-2 entry in cells
Till now, the mechanism of SARS-CoV-2 infection is not completely elucidated. Several studies are being conducted globally on SARS-CoV-2 to unravel the mechanism of infection and pathogenesis of the novel coronavirus. The β-CoVs - SARS-CoV and SARS-CoV-2 are substantially identical and are considered to infect humans similarly. The S protein contributes substantially to the attachment and fusion of the virus with the host cell. The RBD of the S1 subunit of the viral S protein binds to the host cell receptor which initiates the viral infection [[56], [57], [58]].
Studies have reported that SARS-CoV and SARS-CoV-2 utilize the same human ACE2 (hACE2) receptor to attach themselves to the host cells [8]. The ACE2 receptor is significantly expressed in the type II alveolar, oral mucosal, and nasal epithelial cells [[59], [60], [61]]. The respiratory airways, cornea, heart, kidneys, etc., also express the ACE2 receptor [59]. These organs are highly vulnerable and most affected in COVID-19 [62].
A recent study reported that SARS-CoV-2 has a greater affinity to the hACE2 receptor than SARS-CoV. They further stated that structural alterations in the ACE2-binding ridge of SARS-CoV-2 RBD are responsible for the high affinity towards the hACE2 receptor [63]. The enzyme furin cleaves the SARS-CoV-2 S protein at the S1/S2 site and exposes the S2 subunit, which mediates the fusion of viral and host membranes [58,64]. This cleavage is responsible for the pre-activation of the S protein which promotes the subsequent type II transmembrane serine protease (TMPRSS2)-dependent viral entry into host cells [64]. The TMPRSS2 is considered significant for the entry of SARS-CoV-2 in the host cell. A broader expression of TMPRSS2 is reported in the nasal cavity, lungs, colon, gall bladder, kidney, prostate, pancreas, heart, etc. Further, the nasal epithelial cells are enriched with TMPRSS2 as well as the ACE2 receptor [59]. The TMPRSS2 primes the ACE2 receptor-bound viral S protein leading to a conformational change [64,65]. This conformational change activates S protein and facilitates the viral entry into the host cells. Moreover, it also clears the ACE2 receptor [58].
A study reported that TMPRSS2 is expressed specifically in ACE2+ cell types. Further, the study also stated that the expression of proteases such as cathepsin B (Cat B) was observed in >70–90% of ACE2+ cells. Altogether, the findings implied that SARS-CoV-2 might also utilize alternative pathways for entry [59]. Similar findings were also reported in another in vitro study, which demonstrated that SARS-CoV-2 is dependent on both cathepsin B/L (CatB/L) and TMPRSS2 for priming and entry into the host cell. This study showed that inhibition of any one of these proteases leads to partial inhibition of viral entry, which suggested that in the absence of TMPRSS2, the virus may utilize CatB/L for its entry and vice-versa [66].
Following the entry, SARS-CoV-2 liberates its genomic material (mRNA) in the cytoplasm. It takes over the protein synthesis machinery in the host and translates the mRNA in the nucleus. Besides, it also utilizes the machinery to synthesize viral proteins and subsequently initiates viral replication [58] (Fig. 2 ).
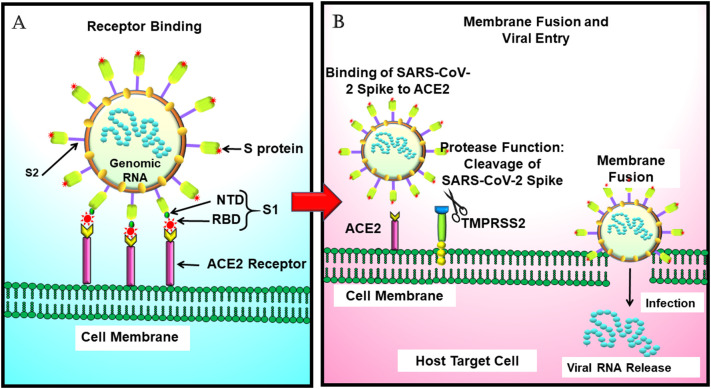
Fig. 2.
Mechanism of SARS-CoV-2 entry in cells. A. Binding of SARS-CoV-2 spike to the host ACE2 receptor. B. Cleavage of SARS-CoV-2 spike by TMPRSS2, membrane fusion, infection, and viral RNA release into the host cell.
Abbreviations: ACE2: Angiotensin converting enzyme-2, NTD: N-terminal domain, RBD: Receptor binding domain, S protein: Spike protein, SARS-CoV-2: Severe Acute Respiratory Coronavirus-2.
4. Cytokine storm in COVID-19
Accumulating pieces of evidence suggest that viral infection instigates an exaggerated or hyperactive immune response in the host leading to a “cytokine storm”. The novel coronavirus infection elicits a similar response in the host. This often involves the interplay of various chemokines, colony-stimulating factors, interferons (IFNs), interleukins (ILs) as well as tumor necrosis factor-α (TNF-α). The cytokine storm is correlated to the severity of the infection and often causes extensive damage or injury. Furthermore, it is also considered as a leading cause of ARDS, and multi-organ failure, which are closely associated with the severity and progression of COVID-19. Moreover, the cytokine storm and associated complications are the major cause of death in COVID-19 patients [10,67,68].
Several studies have investigated the clinical characteristics of the cytokine storm in COVID-19 patients [[69], [70], [71]]. In the extremely severe patients, elevated levels of IL-2 receptor (IL-2R), IL-6 as well as IL-10 were observed. Moreover, a gradual reduction in the absolute count of CD4+ T, CD8+ T, and B cells was also observed as the severity of the disease progressed. These findings suggested that there is a correlation between immune response and severity of COVID-19 progression [72,73].
A study conducted on forty three COVID-19 patients showed elevated IL-6 levels in severe cases and thus correlated to the severity of the disease [74]. A retrospective multicenter study investigated the deceased and discharged COVID-19 cases. The study reported that elevated IL-6 was observed in the deceased cases. Further, the cause of mortality in the deceased group was primarily due to respiratory failure (53%) [75]. The evaluation of the clinical features in deceased COVID-19 patients showed that a majority of deceased patients were associated with comorbidities, such as hypertension and cardiac anomalies. Further, the majority presented with complications, such as ARDS, respiratory failure, sepsis, acute cardiac injury, and heart failure. Moreover, the concentrations of the IL-2R, IL-6, IL-8, IL-10, and TNF-α were also found to be elevated [76].
A retrospective analysis of COVID-19 patients with pneumonia demonstrated an increased expression of serum IL-6. Furthermore, a decrease in the CD3, CD4, Natural Killer (NK), and CD8 cells were also observed [71]. Cytokine profiling of the peripheral blood samples obtained from severe patients revealed an increase in the levels of IL-6, IL-10, IL-2, and IFN-γ. Besides, it was also observed that the lymphocyte and T cell (especially CD8+ T) counts were substantially decreased while the neutrophil count was increased [70]. Another study also showed increased levels of IL-2, IL-7, IL-10, and TNF-α. Further, it reported similar trends for granulocyte colony-stimulating factor (GCSF), C-X-C motif chemokine 10 (CXCL10), monocyte chemoattractant protein (MCP)-1, and macrophage inflammatory protein (MIP)-1 α [69].
Transcriptomic profiling of cytokines in SARS-CoV-2 infected patients have revealed elevated levels of cytokines MCP-1, CXCL10, MIP-1α, and MIP-1β [77]. Increased expression of CXCL10, IL-6, IL-8, MCP-1, RANTES (regulated on activation, normal T cell expressed and secreted), and TNF-α was also observed in severe COVID-19 patients [78]. The diabetic COVID-19 patients showed substantially increased leukocyte and neutrophil count. Further, elevated level of IL-2R, IL-6, IL-8, and TNF-α were also observed [79].
Altogether, the aforementioned findings indicated a pivotal role of cytokine storm in COVID-19 patients. Therefore, targeting the cytokine storm might help in attenuating the severity of disease progression.
5. Molecular pathways linked to inflammation
Inflammation is a vital cellular process or an immune response to injury, tissue damage, or infection in the body which assists in upholding the tissue homeostasis under traumatic or stressed conditions and regulates the host defense mechanism against pathogens [80]. The key molecular mediators of inflammation include inflammatory cytokines such as TNF-α; chemokines; inflammatory enzymes such as cyclooxygenase (cox)-1, and -2; matrix metalloproteinase (MMP)-9, 5-lipooxygenase (5-LOX); transcription factors such as signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa-B (NF-κB); ILs, for example, IL-1, -6, and -8. The most important mediator of inflammation is the ubiquitous expression of NF-κB transcription factor which play an essential role in the modulation of a wide array of genes encoding cell adhesion molecules, cytokines, and its receptors which helps in triggering inflammation [14,[81], [82], [83], [84]]. NF-κB is a heterotrimer comprising of three subunits namely p65, p50, and an inhibitory subunit IκBα. It is mainly present in the cytoplasm, and upon activation by different inflammatory stimuli, various carcinogens, radiations (such as UV-light, γ-rays, and x-rays), several free radicals, and cytokines, etc. translocates into the nucleus. After translocation into the nucleus, the activated NF-κB can bind to different promoter regions of several genes and activate around 400 genes which play an important role in inflammation and various other chronic diseases [[85], [86], [87], [88]]. NF-κB activation can regulate the various hallmarks of cancer, such as cancer cell proliferation, survival, angiogenesis, invasion, migration, and metastasis. They also take part in instigating chemoresistance and radiation resistance. The expression of several inflammatory mediators, for instance, cox-2, inducible nitric oxide synthase (iNOS), TNF-α, and ILs are regulated through NF-κB [82,88,89]. TNF-α is the most potent pro-inflammatory cytokine discovered so far. Overexpression of this cytokine can ultimately lead to inflammation and various other chronic diseases, including cancer through the regulation of the NF-κB pathway [88,90,91]. Hence, the TNF-α blockers possess immense potential to control inflammation, and the overall global market for TNF-α blockers was valued at US$ 43.39 billion in 2017 and expected to reach US$ 181.13 billion by 2026 [81,90,92]. The macrophages mainly release a group of cytokines known as interleukins, for example, IL-1β, IL-6, and IL-8 which play crucial roles in inducing an inflammatory response. It is now well evinced that the augmentation of expression of iNOS, cox-2, and abnormal expression of IL-1, IL-6, IL-8 and TNF-α have been observed in case of oxidative stress that ultimately leads to inflammation [14,81,93]. IL-6 is an NF-κB-dependent cytokine that controls the activation of STAT3. STAT3, a transcriptional factor, is activated through Janus-activated kinase (JAK) 1, 2, and 3, which causes tyrosine phosphorylation, homodimerization, nuclear translocation of STAT3 where it binds to the DNA and is responsible for the induction of numerous inflammatory and immune responses. Moreover, several other transcription factors such as nuclear factor erythroid 2–related factor 2 (Nrf2), activator protein-1 (AP-1), nuclear factor of activated T cells (NFAT), and hypoxia-inducible factor-1α (HIF-1α) are also regulated through various inflammatory cytokines which play a pivotal role in controlling the cellular stress responses [14,[93], [94], [95]]. The mitogen-activated protein kinase (MAPK) pathway can act as a molecular target for the prevention and treatment of different inflammatory diseases. The MAPK family consists of mainly three types of stress-activated protein kinase pathways viz. extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK), which can regulate the IL-5 level and other cytokines during inflammation [14,96].
6. Available repurposed drugs against COVID-19
The molecular pathogenesis of SARS-CoV-2 is not completely known and studies are being conducted globally to investigate novel drugs and targets. However, at present, there are no available drugs to treat COVID-19. Although, many studies are currently ongoing to develop and test antiviral drugs and vaccines against the causative virus SARS-CoV-2 in preclinical and clinical settings; these vaccines or novel drugs might be unavailable until 2021 [45]. In the absence of novel antiviral drugs, “repurposed drugs” are often prescribed for the treatment of COVID-19 based on the symptoms [97]. Some of the repurposed drugs that are currently used or suggested against SARS-CoV-2 as well as COVID-19 associated cytokine storm and complications are mentioned in Table 1 .
Table 1.
Repurposed drugs against the novel coronavirus (SARS-CoV-2).
| Drug | Molecular target/mechanism of action | Company | Reference |
|---|---|---|---|
| Acalabrutinib | Can potentially exert antiviral and anti-inflammatory effects | – | [98] |
| Amphotericin B | Blocks the interaction of SARS-CoV-2 S-protein with hACE-2 receptor | – | [99] |
| Anakinra | IL-1 inhibitor; Neutralizes SARS-CoV-2 related hyperinflammation | Swedish Orphan Biovitrum | [97,100] |
| Arbidol | Inhibit membrane fusion; Prevents the viral entry | – | [101] |
| Atorvastatin | Attenuates NF-κB activation; Decreases hazard for death | – | [102] |
| Azithromycin | Mechanism unknown; commonly used as adjunct with hydroxychloroquine | – | [97] |
| Baricitinib | JAK1 and JAK2 inhibtor, Can potentially inhibit SARS-CoV-2 entry | – | [103] |
| Bemcentinib | Can potentially reduce viral infection and blocks SARS-CoV-2 spike protein | BerGenBio ASA, Norway | [98] |
| Bromhexine | Transmembrane protease serine inhibitor | – | [104] |
| Camostat mesilate | Inhibits serine protease | – | [66,105] |
| Chloroquine | Changes the pH of endosomes; Prevents viral entry, transport and post-entry events | – | [106] |
| Cefuroxime | Inhibits the viral RNA-dependent RNA Polymerase | – | [107] |
| Ciclesonide | Exerts antiviral and anti-inflammatory effects; Treated pneumonia and lung injury | – | [108] |
| Ciprofloxacin | Binds to SARS-CoV-2 Mpro; Inhibits viral replication | – | [109] |
| Clarithromycin | Exerts antiviral activity; Inhibits protein synthesis by binding to the 50S ribosomal subunit | – | [110] |
| Daclatasvir | Inhibits SARS-CoV-2 replication in vitro; Prevents the induction of pro-inflammatory cytokines | – | [111] |
| Darunavir/cobicistat | HIV protease inhibitor | – | [112] |
| Dasatinib | Inhibits SARS-CoV-2 3CL protease | – | [99] |
| Dexamethasone | Reduces inflammation, modulates immune system | – | [113] |
| Disulfiram | Inhibits 3CL protease | – | [114] |
| Doxycycline | Decreases pro-inflammatory cytokines like IL-6, TNF-α; Inhibits SARS-CoV-2 papain-like protease, MMPs; Protects against lung injury | – | [115] |
| Ergotamine | Blocks the interaction of SARS-CoV-2 S-protein with human ACE-2 receptor | – | [99] |
| Favipiravir | Inhibits the viral RNA-dependent RNA Polymerase | Toyama Chemical, Japan | [116,117] |
| Galidesivir | Binds to the viral RNA-dependent RNA polymerase | – | [118] |
| HCQ | Alters the pH of endosomes; prevents viral entry, transport and post-entry events | – | [97] |
| Imatinib | Suppresses the NF-κB signaling pathway; Stimulates PGE2; Decreases the release of TNF-α, IL-1β and IL-6 | – | [119] |
| Indomethacin | Blocks viral RNA synthesis | – | [120] |
| Interferron γ/β | Inhibits viral replication (SARS-CoV) | – | [121] |
| Ivermectin | Inhibits IMPα/β1-mediated nuclear import of viral proteins | – | [122] |
| Lactoferrin | Exerts immunomodulatory and anti-inflammatory effects; Reduces IL-6 and TNF-α; Inhibits viral entry by binding to the host cell surface HSPGs; Inhibits the SARS-CoV-2 invasion | – | [123] |
| Lopinavir/Ritonavir | HIV protease inhibitor | – | [97] |
| Losartan | Blocks AT1R | – | [124] |
| MEDI3506 | Can potentially treat respiratory failure caused by COVID, IL-33 inhibitor | – | [98] |
| Metformin | May induce activation of AMPK which may casuse phosphorylation of ACE2 receptor, thus interfering with viral entry; Inhibition of mTOR pathway and prevention of immune hyperactivation interference with viral endocytic cycle | – | [125,126] |
| Methylpred-nisolone | Inhibits inflammatory cascade | – | [127] |
| Moxifloxacin | Binds to SARS-CoV-2 Mpro; Inhibits viral replication | – | [109] |
| Nafamostat mesylate | Inhibits TMPRSS2; Prevents viral and host membrane fusion | – | [128] |
| Niclosamide | Inhibits viral replication (SARS-CoV, MERS-CoV) | – | [129] |
| Nitazoxanide | Supresses inflammation; Antiviral effects | – | [97,130] |
| Pirfenidone | Inhibits TNF-α | – | [131] |
| Povidone-Iodine | Exerts virucidal activity | – | [132] |
| Remdesivir | Inhibits the viral RNA-dependent RNA polymerase | Gilead Sciences, USA | [133] |
| Ribavarin | Binds to the viral RNA-dependent RNA polymerase | – | [118] |
| Rivaroxaban | Inhibits SARS-CoV-2 3CL protease | – | [99] |
| Sacubitril/Valsartan | Can potentially reduce pro-inflammatory, | – | [134] |
| Cytokines and neutrophil count; Increases lymphocyte count; reduces hs-CRP levels | |||
| Sarilumab | Blocks IL-6 | Regeneron Pharmaceuticals and Sanofi | [97,135] |
| Saquinavir | Inhibits SARS-CoV-2 3CL protease | – | [99] |
| Setrobuvir | Binds to the viral RNA-dependent RNA polymerase | – | [107] |
| Sildenafil | Inhibits SARS-CoV-2 3CL protease | – | [99] |
| Siltuximab | IL-6 blocker | – | [135] |
| Sirolimus | Modulates PI3K/Akt/mTOR pathway and inhibits MERS-CoV activity | – | [97] |
| Sofosbuvir | Binds to the viral RNA-dependent RNA polymerase | – | [118] |
| Tacrolimus | Inhibits replication of the | – | [136] |
| (FK506) | SARS-CoV, HCoV-NL63 and HCoV-229E | ||
| Tadalafil | Inhibits SARS-CoV-2 3CL protease | – | [99] |
| Telmisartan | Blocks AT1R | – | [124] |
| Tenofovir | Binds to the viral RNA-dependent RNA Polymerase | – | [118] |
| Thymosin α1 | Restores T cell exhaustion; Recovers the immune reconstitution via promoting thymus output | – | [137] |
| Tocilizumab | Inhibits IL-6 | Roche and Chugai Pharmaceutical | [97,135] |
| Vancomycin | Blocks interaction of the SARS-CoV-2 S-protein with hACE-2 receptor | – | [99] |
| Zilucoplan | C5 inhibitor; can potentially block the severe inflammatory response in COVID-19 | – | [98] |
| α-ketoamides | Binds to SARS-CoV-2 main protease (Mpro) | – | [138] |
Abbreviations:
ACE2: Angiotensin-converting enzyme-2, Akt: Protein kinase B, AMPK: AMP-activated protein kinase, AT1R: Angiotensin receptor 1, CoV: Coronavirus, COVID-19: Coronavirus disease-19, hACE-2: Human angiotensin-converting enzyme-2, HCoV: Human coronavirus, HCQ: Hydroxy-chloroquine, HIV: Human immunodeficiency virus, hs-CRP: High sensitivity C-reactive protein, HSPGs: Heparan sulfate proteoglycans, IL: Interleukin, IMPα/β1: Importin α/β1, JAK: Janus Kinase, MERS-CoV: Middle East respiratory syndrome coronavirus, MMP: Matrix metalloproteiniases, Mpro: Main protease, mTOR: Mammalian target of rapamycin, NF-κB: Nuclear factor kappa B, PGE2: Prostaglandin E2, PI3K: Phosphoinositide 3-kinases, SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2, TNF-α: Tumor necrosis factor-α, 3CL: 3C-like protease.
Among the available repurposed therapeutic drugs currently used against SARS-CoV-2, the most commonly prescribed drugs are antiviral. Several antiviral drugs previously tested against Ebola, human immunodeficiency virus (HIV), Influenza, etc., are currently being investigated to evaluate their efficacy and safety against SARS-CoV-2 [[139], [140], [141]]. These antiviral drugs are used commonly to treat infected patients, alone or in combination [142].
6.1. Arbidol
Arbidol (umifenovir) is an indole-derivative antiviral drug commonly used against influenza. Besides, it also suppresses viruses such as hepatitis B and C, chikungunya, reovirus, Hantaan [143]. Arbidol efficiently inhibits SARS-CoV-2 infection in Vero E6 cells by effectively blocking the entry as well as post-entry stages of the virus [141]. A study conducted molecular dynamics simulation and structure-based studies to analyze the exact target of arbidol on SARS-CoV-2 and determine the associated mechanism. The study revealed that arbidol binds to the S2 domain of S protein on SARS-CoV-2 and thereby interferes with the trimerization, which is essential for the adhesion and fusion with the host cell membrane [101]. Further, SARS-CoV-2 infected hospitalized patients treated with arbidol (0.4 g, three times per day for 9 days) exhibited improvement in the discharge rate as well as a reduction in the mortality rate as compared to the untreated patients [144]. However, a retrospective study stated that umifenovir does not improve the outcome of COVID-19 patients [145].
6.2. Chloroquine and hydroxychloroquine
Chloroquine is a well-known antimalarial drug. It is also used against lupus erythematosus and rheumatoid arthritis (RA). The hydroxylated form of chloroquine i.e., hydroxychloroquine (HCQ) exhibits similar properties [97]. Chloroquine is also reported to possess antiviral activity. Various studies have reported its antiviral activity against HIV-1, hepatitis A virus, and dengue virus. HCQ exerts antiviral activity against HIV-1 [139,146].
Chloroquine inhibits the viral infection by elevating the endosomal pH essential for viral-host cell fusion. It also interrupts the glycosylation of the SARS-CoV receptors [106]. Previously, chloroquine was also reported to exhibit effective antiviral activity against HCoV-OC43 in vivo [147]. It is also reported to be effective against the novel coronavirus infection in Vero E6 cells [106].
An in vitro study demonstrated that HCQ and azithromycin (AZM) exert synergistic effects against SARS-CoV-2 [148]. A retrospective study reported that early treatment of SARS-CoV-2 infected patients with HCQ and AZM resulted in low mortality rates [149]. Further, an open-label non-randomized clinical trial reported that HCQ caused a decline in the viral load in COVID-19 patients. Additionally, it was found that AZM further reinforced its effect but the mechanism by which the two drugs act in combination is unknown [150,151]. WHO has discontinued the HCQ arm for its Solidarity Trial as it induced little or no decrease in mortality [152]. HCQ has been associated with cardiac abnormalities like QT prolongation and AZM might enhance the risk [151].
6.3. Favipiravir
Favipiravir (T705) is an anti-influenza drug, manufactured by Toyama Chemical, Japan. It inhibits the viral RNA-dependent RNA polymerase [117]. An open-label, non-randomized, control study reported that the treatment of infected patients with a combination of favipiravir and IFN-α inhibited the disease progression and results in faster viral clearance as compared to lopinavir/ritonavir. Favipiravir was administered orally for 14 days (1600 mg twice on day 1 followed by 600 mg, twice, daily for the remaining days) while IFN-α was administered via aerosol inhalation (5,000,000 U twice, daily) [116].
6.4. Lopinavir/Ritonavir
Lopinavir a protease inhibitor prescribed for the treatment of HIV. The oral bioavailability of lopinavir is considerably poor; therefore it is often prescribed in combination with ritonavir which boosts its exposure. Ritonavir also inhibits the enzymes which metabolize lopinavir and further enhances its antiviral effect [97]. A recent in vitro study reported that lopinavir exhibits antiviral effect against HCoVs such as 229E, SARS-CoV, and MERS-CoV [153]. It is also reported to be effective against SARS-CoV-2 in vitro [154]. However, WHO recently discontinued the lopinavir/ritonavir arm for its WHO-led Solidarity Trial conducted for the treatment of COVID-19 hospitalized patients as the drug exerted little or no decrease in mortality [152].
6.5. Remdesivir
Remdesivir (GS-5734) is a nucleoside analog of adenosine, manufactured by Gilead Sciences. It is a broad-spectrum antiviral drug that inhibits the viral RNA-dependent polymerase and thus, interferes with viral replication [133,140]. It had been previously tested against the Ebola virus [155]. An in vitro study has also demonstrated its antiviral activity against viruses of the Paramyxoviridae, Pneumoviridae as well as Filoviridae families [156]. Remdesivir also shows promising results against SARS-CoV and MERS-CoV infection in human airway epithelial cells [157]. Therefore, various studies are conducted to investigate its efficacy against the novel coronavirus infection in both preclinical and clinical settings. An in vitro study demonstrated that remdesivir was efficacious against novel coronavirus infection [106]. The synergistic inhibitory effect of remdesivir with emetine (a protein synthesis inhibitor) against SARS-CoV-2 was also observed in vitro [154]. Furthermore, remdesivir also induced substantial clinical improvement, when administered during the early stages of SARS-CoV-2 infection, in the rhesus macaque model [158]. A study reported the compassionate use of remdesivir against COVID-19 in SARS-CoV-2 infected patients. 36 out of 53 patients intravenously treated with remdesivir (200 mg of on day 1 followed by 100 mg for 9 days) showed clinical improvement [159].
Antiviral drugs such as oseltamivir, lopinavir, ritonavir, ganciclovir have been used commonly to treat infected patients, alone or in combination or [142]. A cohort study on severe COVID-19 hospitalized patients revealed that the treatment with oseltamivir or ganciclovir lowered the risk of death [160].
6.6. Others
The aforementioned antiviral drugs are some of the most actively prescribed and studied repurposed drugs. However, apart from these, several other drugs are currently being tested in pre-clinical and clinical settings. Some of these drugs are antihelminthic, corticoids, immunomodulators, protease inhibitors, antifibrotic, anti-inflammatory, etc. These drugs have shown effectiveness either in the hospitalized COVID-19 patients or against SARS-CoV-2 in vitro/in vivo/in silico. Moreover, some of these drugs are hypothesized to target COVID-19 associated complications such as hyper inflammation (cytokine storm), pneumonia, ARDS, etc. [97].
Ivermectin and niclosamide are potent antihelminthic drugs [97]. A study demonstrated that ivermectin also shows antiviral activity against SARS-CoV-2 in vitro [122]. Previously, niclosamide had been shown effective against SARS-CoV, MERS-CoV, Ebola virus, rhinovirus, etc. Therefore, these broad-spectrum antihelminthic drugs might be quite promising in inhibiting SARS-CoV-2 [129].
Corticosteroids show anti-inflammatory effects and effectively inhibits the cytokine levels. Therefore, they might be used to combat the cytokine storm in COVID-19 patients. Dexamethasone is a synthetic corticosteroid that has shown promising results against COVID-19. A recent study investigated the short-term treatment with dexamethasone in SARS-CoV-2 patients with hypoxic respiratory failure. The study reported that dexamethasone was well-tolerated and may help in attenuation of the hyper-inflammatory phase [97,113]. Methylprednisolone is an anti-inflammatory and antifibrotic drug that efficiently inhibits the inflammatory response. Further, methylprednisolone might also be associated with an improved outcome as well as lung function in COVID-19 patients [127]. Although these corticosteroids show effective results, their usage for the treatment of COVID-19-associated pneumonia remains controversial. As their continuous administration can suppress the immune system, it is necessary to determine the appropriate dose as well as the rationale for its usage [97,127].
The IL-6 inhibitors tocilizumab, sarilumab, and siltuximab are hypothesized to be effective against COVID-19 patients. These IL-6 inhibitors are also tested against SARS-CoV-2 in both preclinical and clinical studies [97,135]. Early treatment with tocilizumab reportedly improves the clinical outcome and causes a decline in mortality in COVID-19 pneumonia patients [161]. A recent study investigated the effectiveness of tocilizumab for the treatment of mechanically ventilated COVID-19 patients. It was observed that tocilizumab was associated with a low death rate [162]. A study in severe COVID-19 pneumonia patients showed that treatment with sarilumab also exhibited promising results [163].
IL-1 promotes pro-inflammatory cytokines such as IL-6 and contributes significantly to cytokine storm. Anakinra, an IL-1 receptor antagonist, blocks both IL-1α and IL-β. It is therefore believed to be highly effective in combating COVID-19 associated cytokine storm [164]. The Ana-COVID study has shown that it reduces SARS-CoV-2 associated hyper inflammation [100].
Baricitinib is a JAK 1 and JAK 2 inhibitor [103]. A study showed that it improved the respiratory function in COVID-19 patients who failed to respond completely to sarilumab [165]. Further, baricitinib reportedly attenuated pneumonia and decreased the mortality rate in COVID-19 patients [103].
Bromhexine hydrochloride is a mucolytic cough suppressant that also acts as a TMPRSS2 inhibitor. As TMPRSS2 is responsible for the SARS-CoV-2 entry in the host cell, bromhexine might be effective in inhibiting the virus [104,[166], [167]]. Nafamostat mesylate inhibited MERS-CoV infection by acting as a TMPRSS2 inhibitor. A study reported that it also efficiently inhibited the SARS-CoV-2 infection in vitro. Further, it blocked the viral S protein-mediated fusion [128]. Camostat mesylate (also known as camostat mesilate) is a serine protease inhibitor. In vitro studies have shown that camostat mesylate can prevent SARS-CoV-2 infection by inhibiting the TMPRSS2 activity. Therefore, camostat mesylate might be beneficial in the treatment of COVID-19 patients [66,105].
Several studies have shown that the administration of the antidiabetic drug metformin in diabetic COVID-19 patients is associated with a decrease in mortality [[168], [169], [170], [171]]. Currently, a very few studies have been conducted on metformin in association with COVID-19 in vitro and therefore, the exact mechanism of action is unknown. However, a few possible mechanisms of action have been reported [125,126].
Darunavir is a potent HIV protease inhibitor [112]. Cobicistat is an effective booster that enhances the pharmacokinetics of the antiretroviral drugs and is hence often co-administered with darunavir [172]. A pilot study (NCT04252274) showed that darunavir/cobicistat in COVID-19 patients is well-tolerated. However, it did not show any significant improvement as compared to the control group [173]. The aforementioned findings aligned with another in vitro study which showed that darunavir/cobicistat is ineffective against SARS-CoV-2 in vitro [112].
Indomethacin, a cox inhibitor, has been reported to exhibit antviral effect against canine CoV (CCoV) as well as SARS-CoV. It inhibits viral RNA synthesis in vitro [120]. A recent study has shown that indomethacin is also effective against the SARS-CoV-2 in vitro as well as CCoV in vivo [174].
Doxycycline, an antibacterial drug, reduces the pro-inflammatory cytokines such as IL-6 and TNF-α. This anti-inflammatory property of doxycycline might repress cytokine storm and prove essential in preventing lung damage associated with COVID-19. Further, a computational study revealed that doxycycline might inhibit SARS-CoV-2 papain-like protease and thereby prevent the infection [115,175].
A retrospective study conducted on severe COVID-19 patients showed that the administration of thymosin-α1 supplement substantially decreased the mortality of severe COVID-19 patients. Further, it also induced the reversion of exhausted T cells [137].
Ciclesonide, an inhaled steroid, effectively inhibits SARS-CoV-2 infection by targeting the viral replication-transcription complex in vitro [176]. A study reported three cases where ciclesonide attenuated COVID-19 associated pneumonia [108].
Imatinib is an break point cluster (Bcr)-Abelson (Abl) tyrosine kinase inhibitor and a potent anticancer drug. It also modulates immune response and exerts anti-inflammatory effects. Further, it also exhibits antiviral effects against SARS-CoV and MERS-CoV. Therefore, it is believed that imatinib might also be effective against SARS-CoV-2 and exert an immunomodulatory effect against COVID-19 pneumonia [119,177]. Another drug, lactoferrin, a non-toxic glycoprotein, is found to have immunomodulatory and anti-inflammatory effects. Therefore, it has been proposed as an adjunct for the treatment of COVID-19 [123]. In addition, many other drugs have high potential in the treatment of COVID-19. For example, povidone-iodine also exhibits potent virucidal activity against SARS-CoV-2 [132]. The drug tacrolimus (FK506), which has been previously tested effective in inhibiting the replication of HCoVs such as SARS-CoV, NL63, and 229E, might also inhibit SARS-CoV-2 [136]. Pirfenidone, an antifibrotic drug, and inhibitor of TNF-α, which is used to treat idiopathic pulmonary fibrosis, might also be effective in combating cytokine storm in COVID-19 patients [131]. It also modulates the angiotensin II type 1 receptor/p38 MAPK/renin-angiotensin system (AT1R/p38 MAPK/RAS axis) [178]. Therefore, it has been hypothesized that pirfenidone might be useful in combating COVID-19 associated cytokine storm as well as lung fibrosis [131]. Another drug, sirolimus (rapamycin), a commonly used immunosuppressant and an inhibitor of mammalian target of rapamycin (mTOR) kinase, was also shown to suppress the MERS-CoV infection effectively [97,179]. Therefore, a Phase II clinical study has been initiated to investigate the effect of sirolimus on COVID-19 patients with pneumonia (NCT04341675) [180]. Another clinical trial, the ACCORD study (EudraCT 2020-001736-95), was initiated to investigate the efficacy of drugs such as bemcentinib and nebulized heparin (potential SARS-CoV-2 S protein blocker), MEDI3506 (anti-IL-33 monoclonal antibody), acalabrutinib (BTK inhibitor), and zilucoplan (complement C5 inhibitor) in COVID-19 patients and the results are awaited [98].
Besides, atorvastatin (an inhibitor of β-Hydroxy β-methylglutaryl-CoA or HMG-CoA reductase), chloroquine and clarithromycin (an antibiotic), losartan and telmisartan (AT1R inhibitor), sacubitril/valsartan (an angiotensin receptor-neprilysin inhibitor), IFN-β and IFN-γ, nitazoxanide (a thiazolide) and daclatasvir (anti-hepatitis C virus drug) have also found to have high potential in the management of COVID-19 [102,110,111,121,124,130,134,181]. For example, atorvastatin treatment was reported to reduce the mortality of COVID-19 patients [102]. Another study showed that the administration of chloroquine and clarithromycin improved the symptoms in a COVID-19 patient with pneumonia [110]. Further, losartan and telmisartan, which effectively block the AT1R, might be useful against SARS-CoV-2 [124]. Moreover, sacubitril/valsartan, which inhibits pro-inflammatory cytokines and suppresses the inflammation, was also found to have the potential for the treatment of COVID-19 patients [134,181]. Another study reported that a combination of IFN-β and IFN-γ inhibit SARS-CoV replication synergistically [121]. Further, daclatasvir was shown to inhibit viral replication of SARS-CoV-2 in vitro and suppress the production of pro-inflammatory cytokines [111]. Another study reported that nitazoxanide suppresses SARS-CoV, MERS-CoV, and influenza virus and inhibits inflammation in vitro [130].
Additionally, a number of in silico studies reported the potential of several compounds in the management of COVID-19. For example, an in silico study shows that disulfiram, rivaroxaban, saquinavir, tadalafil, sildenafil, dasatinib inhibit the SARS-CoV-2 3 C-like (3CL) protease enzyme, which is essential for the viral transcription and replication [99,114]. Further, it showed that ergotamine, amphotericin B, and vancomycin block interaction of SARS-CoV-2 S-protein with the ACE2 receptor in silico [99]. Molecular docking and simulation study also revealed that α-ketoamides, ciprofloxacin, and moxifloxacin bind to SARS-CoV-2 main protease (Mpro) in silico, which might be effective in inhibiting the SARS-CoV-2 infection [109,138]. The drugs setrobuvir and cefuroxime effectively bind to the SARS-CoV-2 RdRp in silico and hence can be used for treatment against the virus [107]. Similar findings were observed for ribavirin, sofosbuvir, galidesivir, and tenofovir [118].
Various studies have also suggested that the compounds derived from Mother Nature can also be effective in the treatment against COVID-19. A recent molecular docking study evaluated the binding potential of various phytochemicals to the non-structural protein 15 (Nsp15), which is associated with viral replication. The study reported that ajmalicine, alpha terpinyl acetate, curcumin, gingerol, novobiocin, piperine, rosmarinic acid, silymarin and aranotin, sarsasapogenin, and ursonic acid exhibited binding affinity with the Nsp15 protein. Therefore, these phytochemicals might effectively inhibit viral replication and their efficacy should be evaluated in pre-clinical as well as clinical studies [182]. Another study conducted molecular docking simulations between functional foods and SARS-CoV-2 Mpro. It reported that quercetrin exhibited inhibition against SARS-CoV-2 Mpro in silico [183]. Curcumin also demonstrated a high binding free energy for the enzymes Cat K, COVID-19 Mpro, and SARS-CoV 3CL protease [184].
The natural compounds such as andrographolide, berberine, curcumin, mangiferin, nimbin, piperine, thebaine, and withaferin A exhibited a binding affinity for the ACE2 receptor as well as SARS-CoV-2 S protein. Further, the compounds gallic acid, luteolin, naringenin, quercetin, resveratrol, and zingiberene showed an affinity to only the ACE2 receptor. These compounds might inhibit the attachment of the SARS-CoV-2 virus to the host cell [185] (Fig. 3 ).
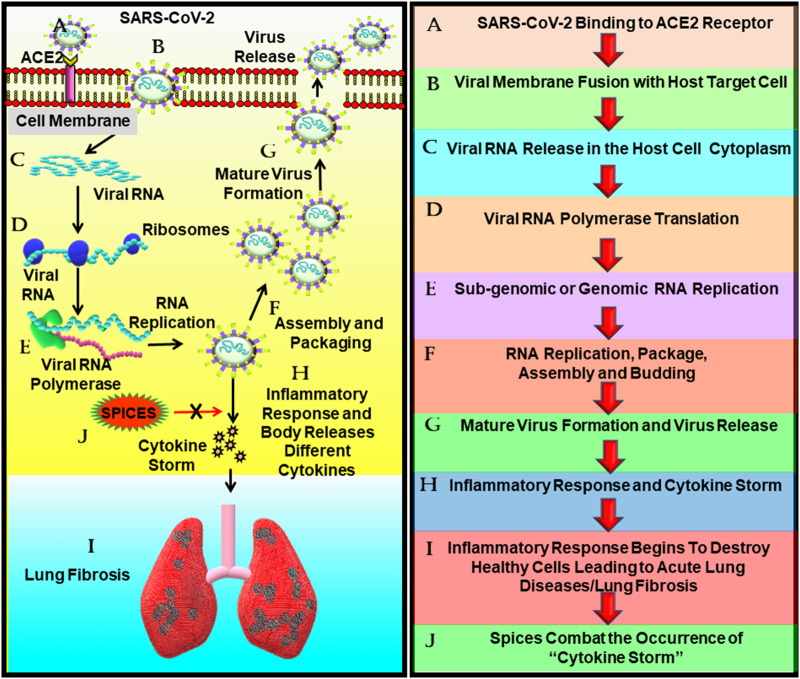
Fig. 3.
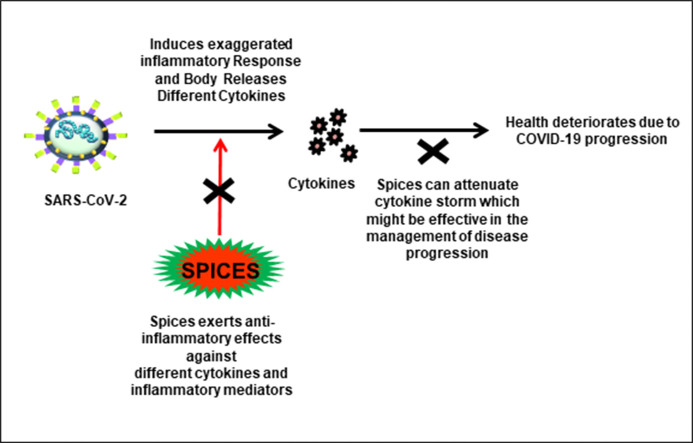
The potential of spices in suppressing SARS-CoV-2 cytokine storm-induced lung fibrosis. The binding of SARS-CoV-2 to the ACE2 receptor causes the fusion of the viral and the target host membrane which eventually leads to the release of the viral RNA in the cytoplasm of the host cell. Following its release, the virus utilizes the host machinery to synthesize viral proteins and subsequently initiates viral replication, followed by packaging and assembly of the viral particles. The mature virus thus formed and released ellicit aggravated inflammatory response or cytokine storm in the host, which causes ARDS or lung fibrosis in severe cases. As spices are potent anti-inflammatory agents, they might prove effective in combating the SARS-CoV-2 induced cytokine storm and thus might be beneficial in preventing cytokine storm-induced complications such as ARDS or lung fibrosis.
Abbreviations: ACE2: Angiotensin converting enzyme-2, SARS-CoV-2: Severe Acute Respiratory Coronavirus-2.
7. Anti-inflammatory role of spices
Mother Nature has bestowed us with various promising medicinal plants as well as plant-based products such as fruits, vegetables, herbs, and spices that are abundantly consumed [[186], [187], [188], [189], [190], [191], [192], [193], [194]]. Apart from their high nutritional value, these are also rich in therapeutic properties [189,193,[195], [196], [197], [198], [199], [200]].
Various medicinal plants have been a part of traditional medicine for ages and are consumed due to their health benefits as well as efficacy against a plethora of diseases [[201], [202], [203], [204], [205], [206], [207], [208], [209], [210], [211], [212], [213], [214], [215]].
The leaves of the plants which are utilized for culinary purposes and consumed fresh are known as herbs. Spices, on the other hand, are consumed as dried parts of a plant. It can be a bud, root, seed, bark, berries, or even stigma of a flower [14,186,216]. In addition to their usage in culinary purposes for imparting flavor and taste to food, they are also beneficial to health. Spices and herbs have been used in various traditional medicines since time immemorial. They effectively act against many diseases such as arthritis, asthma, cancer, diabetes, etc. [[217], [218], [219], [220]]. In severe cases of COVID-19, cytokine storm is commonly observed and is majorly responsible for the degradation of health conditions [10]. As spices and herbs exhibit potent anti-inflammatory activities, they could be used to combat the elevated levels in COVID-19 associated cases and boost immunity with minimal or no side-effects [14]. Some of the spices, as well as their anti-inflammatory roles, are mentioned below and in Table 2 (Fig. 4 ).
Table 2.
Anti-inflammatory role of spices.
| Spices | Active compound/form of use | Disease | In vitro/in vivo | Model | Mechanism | References |
|---|---|---|---|---|---|---|
|
Asafetida (Ferula asafetida L.) |
–A | Liver cancer | In vitro | HepG2, SK-Hep1 | ↓ NF-κB, ↓ TGF-β1, ↑ caspase-3, ↑ TNF-α | [221] |
| –B | Breast cancer | In vivo | BALB/c mice | ↓ LOX | [222] | |
| – | Breast cancer | In vivo | SD rats | ↓ cyt-P450, ↓ cyt b5, ↑ catalase, ↑ GSH, ↑ GST, | [223] | |
| ↑ SOD, ↓ TBARS, ↑ DT-diaphorase | ||||||
| –B | – | In vivo | Albino mice | ↓ LOX | [224] | |
| Basil (Ocimum sp.) |
–C | Asthma | In vivo | Wistar rats | ↓ IL-4, ↓ IgE, ↓ PLA2, ↓ TP, ↑ IFN-γ/IL-4 ratio | [225] |
| –C | Gastric ulcer | In vivo | Swiss albino | ↓ TBARS, ↓ NO, ↓ H2O2, ↑ GSH, ↑ GPx, ↑ GST, | [226] | |
| CD1 mice | ↑ catalase, ↑ GR, ↓ TNF-α, ↓ IL-6, ↑ PGE2, ↑ IL-4 | |||||
|
Bay leaves (Laurus nobilis) |
–C | In vitro | BMDMs | ↓ p-IκB, ↓ p-STAT3, ↓ pro‐IL‐1β, ↓ procaspase‐1, | [227] | |
| ↓ IL‐1β, ↓ caspase‐1, ↓ NLRP3 inflammasome, | ||||||
| ↓ NF‐κB signaling, ↓ mRNA expression of IL‐6, | ||||||
| TNF‐α, and iNOS | ||||||
| –C | ALI | In vivo | C57BL/6 mice | ↓ MPO activity, ↓ IL‐1β, ↓ IL‐6, ↓ TNF‐α | [227] | |
| 1,8‐Cineole | – | In vitro | BMDMs | ↓ IL‐1β, ↓ caspase‐1, ↓ Activation of NF‐κB and | [227] | |
| STAT3, ↓ mRNA expression of IL‐6, TNF‐α, and | ||||||
| iNOS | ||||||
|
Black cumin (Nigella sativa) |
–C | Lung inflammation | In vivo | Wistar rats | ↓ TGF-β1, ↓ IFN-γ, ↓ PGE2, ↑ IL-4, ↑ catalase, | [228] |
| ↑ SOD, ↓ MDA, ↑ thiol | ||||||
| –A | Low-grade inflammation | In vitro | THP-1 cells | ↓ IL-1β, ↓ MCP-1, ↓ gene expression of DNMT3A and HDAC1 | [229] | |
| –C | Diabetes | In vivo | Wistar rats | ↓ mRNA expression of VCAM-1 and LOX-1, | [230] | |
| ↑ mRNA expression of eNOS | ||||||
| –C | – | In vivo | Wistar rats | ↓ MDA, ↓ NO, ↓ IL-6, ↑ thiol, ↑ SOD, ↑ catalase, | [231] | |
| ↓ AST, ↓ ALT, ↓ ALP, ↑ serum protein, ↑ albumin | ||||||
| –A | Allergic asthma | In vivo | Wistar rats | ↓ IL-4, ↓ NO | [232] | |
| TQ | AD | In vivo | SD rats | ↓ TLR2, ↓ TLR4, ↓ TNF-α, ↓ MyD88, ↓ IL-1β, | [233] | |
| ↓ IRF-3, ↓ NF-κB | ||||||
|
Black Pepper (Piper nigrum) |
–C | Asthma | In vivo | BALB/c mice | ↓ IL-1β, ↓ TNF-α, ↓ IL-4, ↓ RORγt, ↓IgE, | [234] |
| ↓ IL-17A | ||||||
| –C | AR | In vivo | BALB/c mice | ↓ E-cadherin, ↑ HO-1, ↑ Nrf2 | [235] | |
| –C | AR | In vivo | BALB/c mice | ↓ p-YSTAT3, ↓ IL-6, ↓ TNF-α, ↓ NF-κB p65, | [236] | |
| ↓ IL-1β | ||||||
| Pipernigramides | Edema | In vitro | RAW 264.7 | ↓ TNF-α, ↓ IL-1β, ↓ IL-6, ↓ PGE2, ↓ p-IKKβ | [237] | |
| Pipernigramides | Edema | In vivo | ICR mice | ↓ NO, ↓ neutrophil infiltration | [237] | |
| Piperine | Lung metastasis | In vivo | C57BL/6 mice | ↓ tumor nodule formation, ↑ survival rate, ↓ SA, | [238] | |
| ↓ GGT | ||||||
| Piperine | Bacterial sepsis | In vitro | J774A.1, BMDM | ↓ IL-1β, ↓ HMGB1, ↓ p-AMPK | [239] | |
| Piperine | Bacterial sepsis | In vivo | C57BL/6 mice | ↓ IL-1β release | [239] | |
| Piperine | AP | In vitro | PAC | ↓ MPO, ↓ TNF-α, ↓ IL-1β, ↓ IL-6 | [240] | |
| Piperine | AP | In vivo | C57BL/6 mice | ↓ p-ERK1/2, ↓ p-p38, ↓ p-JNK | [240] | |
| Piperine | LN | In vitro | HK-2 cells | ↓ p-AMPK, ↓ IL-1β, ↓ HMGB1, ↓ pro-caspase-1 | [241] | |
| Piperine | LN | In vivo | BALB/c mice | ↓ NLRP3 inflammasome activitation | [241] | |
| Chabamide | Inflammation | In vitro | RAW264.7 | ↑ HO-1, ↑ Nrf2, ↓ iNOS | [242] | |
|
Capsicum (Capsicum annum L.) |
–C | – | In vivo | Wistar rats | ↓ ALT, ↓ AST, ↓ ALP, ↓ TNF-α, ↓ IL-6, ↓ LPO, | [243] |
| ↓ Cu-Zn-SOD, ↓ GPx, ↑ CAT, ↑ Mn-SOD, ↑ GR, | ||||||
| ↓ VLDL, ↑ HDL | ||||||
| –C | Asthma | In vivo | BALB/c mice | ↓ IL-4, ↓ IL-5, ↓ IL-13, ↓ nuclear NF-κB p65 | [244] | |
| Capsaicin | – | In vitro | THP-1 | ↓ IL-1β, ↓ IL-6, ↓ TNF-α, ↑ LXRα expression | [245] | |
| Capsaicin | SGI | In vitro | HSG | ↓ IL-6, ↓ TNF-α | [246] | |
|
Cardamom (Elettaria cardamomum) |
–C | Cardiotoxicity | In vivo | Albino rats | ↓ NO, ↓ MDA, ↓ NF-κB, ↓ capase-3, ↑ VEGF, | [247] |
| ↑ catalase, ↑ SOD, ↑ GPx | ||||||
|
Celery seeds (Apium graveolans) |
–A, –C | Hyperuricemia | In vivo | BALB/c mice | ↓ ROS ↑ SOD, ↑ GPx | [248] |
| –C | Gouty arthritis | In vivo | Wistar rats | ↓ IL‑1β, ↓ IL‑6, ↓ TNF-α, ↑ IL‑10 | [248] | |
| –A | Gouty arthritis | In vivo | Wistar rats | ↓ IL‑1β, ↓ TNF-α, ↑ IL‑10 | [248] | |
| Sedanolide | Liver cancer | In vitro | J5 cells | ↓ PI3K-I, ↓ mTOR, ↓ Akt, ↑ PI3K-III, ↑ LC3-II, | [249] | |
| ↑ nuclear p53, ↑ DRAM, ↓ cytosolic p53, ↓ TIGAR, | ||||||
| ↑ Beclin-1 | ||||||
| –C | AD | In vivo | Hairless mice | ↓ IL-4, ↓ TNF-α, ↓ IFN-γ, ↓ IL-6, ↓ TSLP, ↓ IL-31 | [250] | |
| –C | AD | In vitro | RAW264.7 | ↓ NO, ↓ IgE | [250] | |
| Luteolin | AD | In vitro | RAW264.7 | ↓ NO | [250] | |
| –C | – | In vitro | RAW264.7 | ↓ IL-6, ↓ TNF-α, ↓ NF-κB | [251] | |
|
Cinnamon (Cinnamom sp.) |
TCA | OA | In vitro | SW1353, HPC | ↓ mRNA expression of MMP-1, -3 and -13, | [252] |
| ↓ mRNA expression of ADAMTS-4 and -5, | ||||||
| ↑ p-IκBα, ↓ NF-κB, ↓ IκBα, ↓ p-JNK 1/2, ↓ p-p38 | ||||||
| TCA | – | In vitro | RAW 264.7 | ↓ NO, ↓ iNOS | [253] | |
| TCA | Neuroinflammation | In vitro | BV2 | ↓ NO, ↓ iNOS, ↓ cox-2, ↓ IL-1β, ↓ IκBα, ↓ NF-κB | [254] | |
| –A | Skin disease | In vitro | HDF3CGF system | ↓ MCP-1, ↓ MIG, ↓ IP‐10, ↓ IL-8, ↓ VCAM-1, ↓ M-CSF, ↓ PAI-1, ↓ ICAM-1, ↓ EFGR, ↓ MMP-1, ↓ TIMP-1, ↓ TIMP-2 | [255] | |
| –C | Inflammation | In vitro | Murine macrophage | ↓ mRNA expression of TNF-α, ↓ p-p38, ↓ IκBα degradation, ↓ p-ERK 1/2, ↓ p-JNK | [256] | |
| –C | Inflammation | In vivo | BALB/c mice | ↓ TNF-α, ↓ IL-6 | [256] | |
| –C | Inflammation | In vitro | Splenocytes | ↑ IL-2, ↓ IL-4, ↓ IFN-γ, ↓ p-ERK1/2, ↓ p-p38, | [257] | |
| ↓ p-STAT4, ↓ p-JNK | ||||||
| –C | Inflammation | In vivo | BALB/c mice | ↓ IFN-γ | [257] | |
| BCA, HCA | – | In vitro | Murine splenocytes | ↓ IFN-γ, ↓ IL-2Rα, ↓ IgM | [258] | |
| BCA, HCA | – | In vivo | BALB/c mice | ↓ AFC response | [258] | |
|
Coriander (Coriandrum sativum) |
–C | Inflammation | In vitro | RAW264.7 | ↓ pro-IL-1β, ↓ PGE2, ↓ p-MAPK, ↓ NF-κB p65, ↓ cox-2, ↓ NO, ↓ iNOS |
[259] |
| –C | CD | In vivo | ICR mice | ↓ IL-1, ↓ IL-4, ↓ IL-13, ↓ TNF-α, ↓ IFN-γ, ↓ IgE, | [260] | |
| ↑ GSH, ↑ HO-1 | ||||||
| –C | Arthritis | In vivo | Wistar rat | ↓ IL-1β, ↓ IL-6, ↓ TNF-R1 | [261] | |
|
Cumin (Cuminum cyminum) |
–E | Hypertension | In vivo | SD rats | ↓ mRNA expression of IL-6, Bax, and TNF-α, | [262] |
| ↑ mRNA of expression TRX1, TRXR1, eNOS, | ||||||
| and Bcl-2 | ||||||
| –E | Gastric ulcer | In vivo | SD rats | ↓ TNF-α, ↓ MDA, ↑ GSH, ↑ catalase, ↑ ATPase activity | [263] | |
|
Curry leaves (Murraya koenigii) |
–C | Pancreatic Inflammation | In vitro | RAW 264.7 | ↑ GSH, ↓ MDA, ↓ IL‐1β, ↓ IL‐6, ↓ TNF-α | [264] |
| –C | Pancreatic Inflammation | In vivo | Swiss albino mice | ↓ MDA, ↑ GSH, ↓ IL‐1β, ↓ IL‐6, ↓ TNF-α, ↑ Nrf2, ↓ NFκB p65 activity, ↓ cox-2, ↓ ICAM-1 | [264] | |
| –C | Breast cancer | In vivo | 4T1-inoculated | ↓ NF-κB, ↓ IL-6, ↓ IL-1β, ↓ IL-10, ↓ iNOS, | [265] | |
| BALB/c mice | ↓ ICAM, ↓ c-myc | |||||
| Mahanimbine | – | In vivo | Swiss albino | ↓ TNF-α, ↓ IL‐1β, ↓ IL‐6 | [266] | |
| Girinimbine | Peritonitis | In vivo | ICR mice | ↓ TNF-α, ↓ IL-1β | [267] | |
|
Fenugreek (Trigonella foenum-graecum L.) |
–E | IPF | In vivo | SD rats | ↑ mRNA expression of Nrf2 and Bcl-2, ↓ mRNA expression of HO-1, TNF- α, IL-1β, IL-6, TGF-β, IL-8, NF-κB, Smad-3, collagen-1, ET-1, Bax and caspase-3 | [268] |
| –E | Testicular damage | In vivo | Wistar rats | ↓ NF-κB p65, ↓ iNOS | [269] | |
| –E | Diabetes | In vivo | Wistar rats | ↓ TNF-α, ↓ IL-1β, ↓ VEGF, ↓ PKC-β | [270] | |
| –D | RA | In vivo | SD rats | ↓ TNF-α, ↓ IL-6, ↓ cox, ↓ 5-LOX | [271] | |
| Trigonelline | AD | In vivo | Wistar rats | ↓ TNF-α, ↓ IL-6, ↓ cox-2, ↓ GFAP, ↓ MDA, ↓ LDH, ↓ protein carbonyl, ↑ SOD, ↑ GSH | [272] | |
|
Garcinia (Garcinia indica) |
Garcinol | Colitis | In vivo | ICR mice | ↓ iNOS, ↓ cox-2, ↓ PCNA | [273] |
| Garcinol | Colon cancer | In vivo | ICR mice | ↓ β-catenin, ↓ VEGF, ↓ cyclin D1, ↓ p-ERK1/2, ↓ p-Akt, ↓ p-p70S6K (Ser371 and Thr389), ↓ p-PI3K | [273] | |
| Garcinol | HNSCC | In vitro | CAL27 | ↓ p-STAT3, ↓ p-c-Src, ↓ p-JAK1, ↓ p-JAK2, ↓ NF-κB, ↓ TAK1, ↓ IKK, ↓ cyclin D1, ↓ Bcl-2, ↓ Bcl-xL, ↓ Mcl-1, ↓ survivin | [274] | |
| Garcinol | HNSCC | In vivo | Athymic nu/nu mice | ↓ Tumor growth | [274] | |
|
Garlic (Allium sativum) |
–C | Asthma | In vivo | BALB/c mice | ↓ IgE, ↓ IgG1, ↑ IgG2a, ↓ IL-13, ↓ IL-5, ↓ IL-4, ↑ IL-12, ↑ IFN-γ, ↓ TNF-α, ↓ IL-1β, ↓ IL-6 | [275] |
| –C | – | In vitro | A549 | ↓ IL-6/PI3K/Akt/NF-κB pathway | [275] | |
| DADS | AP | In vivo | Swiss albino mice | ↓ H2S, ↓ CSE, ↓ MPO, ↓ IκB degradation, ↓ TNF-α, ↓ mRNA expression of PPTA and NK1R | [276] | |
| DATS | RA | In vitro | RA-FLS | ↓ IL-8, ↓ IL-1β, ↓ p-p65, ↓ p-NF-κB, ↓ p-IκBα, ↑ IκBα, ↓ c-myc, ↓ β-catenin | [277] | |
| DATS | RA | In vivo | DBA/1J mice | ↓ IL-6, ↓ IL-1β, ↓ TNF-α | [277] | |
| SAC | PF | In vivo | C57BL/6 mice | ↓ α-SMA, ↓ p-Akt, ↓ p-p65, ↓ mRNA expression of α-SMA, TNF-α, iNOS, IL-6, IL-12p35, and TGF-β | [278] | |
| SAMC | ALI | In vivo | BALB/c mice | ↓ MPO, ↓ TNF-α, ↓ IL-1β, ↓ IL-6, ↓ NF-κB activation ↓ cox-2, ↓ p-NF-κB p65, ↑ Nrf-2, ↑ HO-1, ↑ GSH, ↑ NQO1, ↑ SOD, ↓ MDA | [279] | |
|
Ginger (Zingiber officinale) |
6-Shogaol | Oral carcinogenesis | In vivo | Syrian hamsters | ↓ c-jun, ↓ c-fos, ↓ AP-1, ↓ iNOS, ↓ TNF-α, ↓ IL-6, ↓ IL-1, ↓ cox-2, ↓ PCNA, ↓ cyclin D1, ↓ Ki-67 | [280] |
| 6-gingerol | Steatohepatitis | In vitro | HepG2 | ↓ MCP-1, ↓ TNF-α, ↓ IL-6 | [281] | |
| 6-gingerol | Steatohepatitis | In vivo | C57BL/6 mice | ↓ MCP-1, ↓ TNF-α, ↓ IL-6, ↓ IκBα degradation, ↓ NF-κB | [281] | |
|
Ginseng (Panax sp.) |
Isofraxidin | ALI | In vivo | ICR mice | ↓TNF-α, ↓ IL-1β, ↓ IL-6, ↓ MIP-2, ↓ p-PI3K, ↓ p-Akt | [282] |
| –C | Lung injury | In vivo | BALB/c mice | ↓ p38 MAPKs | [283] | |
| PNS | HAND | In vivo | SD rats | ↓ Bax/Bcl-2 ratio, ↓ caspase-3, 8, and -9 | [284] | |
| PNS | Colitis | In vivo | SD rats | ↓ M1 macrophages, ↓ PI3K/Akt, ↑ M2 macrophages | [285] | |
| NR | Renal injury | In vivo | Wistar rats | ↓ TNFα, ↓ TGF-ß1, ↓ INF-γ, ↓ IL-6, ↑ IL-10 | [286] | |
| GRg3 | RA | In vivo | C57BL/6 mice | ↑ CD4+CD25+Foxp3+Treg cells, ↓ TNF-α, ↓ IL-6, ↑ IL-10 | [287] | |
| GRg1 | Lung injury | In vivo | SD rats | ↓ TNF-α, ↓ α-SMA, ↓ Collagen I, ↓ TGF-β1, ↓ IL-6, ↓ TGF-βR1, ↓ p-Smad3 | [288] | |
| GRg1 | Lung injury | In vitro | MRC5 | ↓ IL-6, ↓ TNF-α, ↓-SMA, ↓ TGF-β1/Smad3 | [288] | |
| Ginsenoside Rd | Ischemic stroke | In vivo | SD rats | ↓ NF-κB activity, ↓ IκB, ↓ MMP-9 | [289] | |
|
Long Pepper (Piper longum) |
PL | Leukemia | In vitro | Leukemia | ↓ p-p65, ↓ cox-2, ↓ NF-κB activation | [290] |
| PL | LN | In vitro | Splenocytes | ↓ IL-6, ↓ IL-17, ↓ IL-23, ↓ TNF-α, ↓ p-STAT3, ↓ p-JAK1 | [291] | |
| PL | LN | In vivo | MRL-Fas(lpr) mice | ↓ IL-6, ↓ IL-17, ↓ IL-23, ↓ TNF-α | [291] | |
| PL | RA | In vivo | DBA/1 mice | ↓ TNF‑α, ↓ IL-1β, ↓ IL‑23, ↓ IL‑17 | [292] | |
| PL | Asthma | In vivo | C57BL/6 mice | ↓ TNF-α, ↓ IL-6, ↓ ICAM-1, ↓ IgE, ↓ MMP-9, ↓ IL-4, ↓ IL-5, ↓ IL-13 | [293] | |
| PL | Asthma | In vitro | Beas-2B | ↓ IL-6, ↓ IL-1β, ↓ ICAM-1, ↓ MCP-1 | [293] | |
| PL | – | In vitro | HUVEC | ↓ TNF-α, ↓ IL-6, ↓ NF-κB activation, ↓ p-p38, ↓ ICAM-1, ↓ VCAM-1, ↓ E-Selectin | [294] | |
| PL | Neuro-inflammation | In vitro | BV2 | ↓ PGE2, ↓ NO, ↓ iNOS, ↓ cox-2, ↓ TNF-α, ↓ IL-6, ↑ IL-10 | [295] | |
| PA | – | In vitro | PBMC | ↓ IL-1β, ↓ TNF-α, ↓ IFN-γ, ↓IL-2 | [296] | |
| PA | – | In vivo | BALB/c mice | ↓ IFN-γ, ↓ IL-2 | [296] | |
| PL | AD | In vitro | Astrocytes | ↓ cox-2, ↓ iNOS, ↓ NF-κB | [297] | |
| PL | AD | In vivo | ICR mice | ↓ NF-κB, ↓ β-, γ-secretases | [297] | |
| –C | – | In vitro | HUVEC | ↓ ICAM-1, ↓ VCAM-1, ↓ NF-κB activation | [298] | |
| PL | Atherosclerosis | In vitro | VSMC | ↓ NF-κB p65, ↓ p-Akt, ↓ p-ERK1/2, ↓ p-PLC-γ1 | [299] | |
| PL | Atherosclerosis | In vivo | ApoE KO mice | ↓ NF-κB p65 | [299] | |
| PL | – | In vitro | HUVEC | ↓ TACE, ↓ p-p38, ↓ p-JNK, ↓ p-ERK1/2 | [300] | |
| PL | COPD | In vivo | BALB/c mice | ↑ AnxA1, ↓ cox-2, ↓ NF-κB, | [301] | |
|
Mint (Mentha sp.) |
–C | – | In vitro | RAW 246.7 | ↓ MDA, ↓ NO | [302] |
| –C | – | In vivo | SD rats | ↓ MDA, ↓ NO, ↓ cox-2, ↓ MAPK signaling | [302] | |
| –C | – | In vitro | MH-S | ↓ NO, ↓ TNF‐α, ↓ IL-1α, ↓ ROS, ↓ p38, ↓ JNK1/2, ↓ p-JNK1/2 | [303] | |
|
Mustard seeds (Sinapis alba) |
–C | Ear edema | In vivo | BALB/c mice | ↓ MPO activity, ↓ TNF-α, ↓ ILβ, ↓ IL-6, ↓ mRNA expression of TNF-α and IL-6 | [304] |
| –E | Psoriasis-like inflammation | In vivo | BALB/c mice | ↓ NLRP3, ↓ ASC, ↓ IL-1β, ↓ caspase-1 and 11, ↓ IL-18 | [305] | |
| –E | Psoriasis | In vivo | BALB/c mice | ↑ CD4+/CD8+ T cells ratio, ↑ CD4+ T cells, ↑ GPx, | [306] | |
| ↓ NF-κB p65, ↓ IFN-α, ↓ IL-17, ↓ IL-22, ↑ SOD, | ||||||
| ↑ catalase, ↓ MDA, ↓ iNOS | ||||||
|
Nutmeg (Myristica fragrans) |
–C | Cortical injury | In vivo | Wistar rats | ↓ TNF-α, ↓ IL-1β, ↓ iNOS, ↑ HO-1, ↑ Bcl-2, ↓ Bax | [307] |
| Macelignan | Asthma | In vivo | C57BL/6J mice, OT-II mice | ↓ IL-4 | [308] | |
| Macelignan | Type I allergy | In vitro | RBL-2 H3 | ↓ PGE2, ↓ mRNA expression of cox-2, 5-LOX, | [309] | |
| TNF-α, IL-4, and IL-13, ↑ GSH | ||||||
| Macelignan | Renal I/R injury | In vivo | SD rats | ↓ IL-6, ↓ TNF-α, ↓ IFN-γ, ↑ catalase, ↑ SOD, ↓ MDA, ↓ Bax, ↓ caspase-3, ↑ Bcl-2 | [310] | |
| Myrislignan | Inflammation | In vitro | RAW 264.7 | ↓ iNOS, ↓ IL-6, ↓ TNF-α, ↓ NF-κB activation, | [311] | |
| ↓ cox-2 | ||||||
| Myristicin | Inflammation | In vitro | RAW 264.7 | ↓ NO, ↓ IL-6, ↓ IL-10, ↓ IP-10, ↓ GM-CSF, ↓ LIF, | [312] | |
| ↓ MCP-1, ↓ MCP-3, ↓ MIP-1α | ||||||
|
Onion (Allium cepa) |
–C | IBD | In vivo | BALB/c mice | ↓ p-ERK1/2, ↓ p-p38MAPK, ↓ p-Akt, ↓ mTOR, ↓ caspase-3, and -8, ↓ cox-2, ↓ cyt-c, ↓ Bcl-xL, ↓ IFN-γ, ↓ TIMP-1, ↓ MCP-1, ↓ MCP-5, ↓ MIG, ↓ MIP-1α, ↓ MIP-2, ↓ ACE-2, ↓ Bcl-2, |
[313] |
| –C | APH | In vivo | Wistar rats | ↓ IL-6, ↓ IL-8, ↓ TNF-α | [314] | |
| Quercetin | Atherosclerosis | In vitro | VSMC | ↓ IL-1β, ↓ IL-18, ↓IL-6, ↓TNF-α, ↑ HO-1, ↑ Nrf-2, | [315] | |
| ↑ SOD1, ↑ SOD2 | ||||||
| Quercetin | Atherosclerosis | In vivo | C57BL/6 mice | ↑ SOD, ↑ HO-1, ↑ Nrf-2, ↓ MDA, ↓ p-NF-kB, | [315] | |
| ↓ TNF-α, ↓ IL-1β, ↓ ROS | ||||||
| QG, QE | Inflammation, Hyperlipidemia | In vitro | THP-1 | ↓ TNF-α, ↓ IL-6, ↓ cox-2, ↓ PGE2 | [316] | |
| QE | Inflammation, Hyperlipidemia | In vivo | Wistar rats | ↓ IL-6 | [316] | |
|
Rosmary (Rosmarinus officinalis L.) |
Rosmarinic acid | Asthma | In vivo | Wistar rats | ↓ IL-4, ↓ IgE, ↓ IFN-γ, ↓ PLA2, ↓ TP | [317] |
| Rosmarinic acid | Asthma | In vivo | BALB/c mice | ↓ IL-4, ↓ IL-13, ↓ p-JNK/JNK ratio, ↓ p-p38/p38 ratio,↓ p-IκBα, ↓ mRNA expression of Ym2, CCR3 CCL11, AMCase, E-selectin | [318] | |
|
Saffron (Crocus sativus) |
Crocin | Inflammation | In vitro | H9c2 | ↓ TNF-α, ↓ PGE2, ↓ IL-1β, ↓ IL-6, ↓ mRNA expression of TNF-α, cox-2, IL-1β, IL-6, NO, iNOS | [319] |
| Crocin | Osteoporosis | In vivo | Wistar rats | ↓ IL-6, ↓ TNF-α, ↓ TRAP, ↓ CTXI, ↑ osteocalcin, | [320] | |
| ↑ ALP | ||||||
| Safranal | Colitis | In vitro | RAW264.7, | ↓ NO, ↓ iNOS, ↓ cox-2, ↓ IL-6, ↓ TNF-α, ↓ p-ERK, | [321] | |
| BMDM | ↓ p-p38, ↓ p-JNK | |||||
| Safranal | Colitis | In vivo | BALB/c mice | ↓ IL-6, ↓ TNF-α, ↓ p-ERK, ↓ p-JNK, ↓ p-IκBα | [321] | |
| ↓ p-p38 | ||||||
| Safranal | AD | In vivo | Wistar rats | ↓ MDA, ↑ SOD, ↓ NF-κB, ↓ TNF-α, ↓ IL-6, | [322] | |
| ↓ IL-1β, ↓ GFAP, ↓ MPO, ↓ ROS | ||||||
| Safranal | Gastric ulcer | In vivo | Wistar rats | ↑ SOD, ↑ TAC, ↓ MDA, ↓ TNF-α, ↓ caspase-3 | [323] | |
|
Sesame (Sesamum indicum) |
Sesamol | Asthma | In vivo | BALB/c mice | ↑ GSH, ↓ MDA, ↓ ICAM-1 ↓ IL-4, ↓ IL-5, ↓ IL-13, ↓ IL-16, ↓ CCL11, ↓ CCL24, ↓ TNF-α | [324] |
| Sesamol | Asthma | In vitro | BEAS-2B cells | ↓ CCL11, ↓ CCL24, ↓ CCL5, ↓ MCP-1, ↓ IL-6, | [324] | |
| ↓ IL-8 | ||||||
| Sesamin | ALI | In vivo | BALB/c mice | ↓ MPO, ↓ TNF-α, ↓ IL-6, ↓ IL-1β, ↓ TLR4, ↓ NF-κB activation | [325] | |
| Sesamin | Kidney injury | In vivo | C57BL/6 mice | ↑ GSH, ↓ MDA, ↑ SOD, ↑ Nrf2, ↑ catalase, ↓ IL-6, | [326] | |
| ↓ NF-κB, ↓ TLR4, ↓ cox-2, ↓ TNF-α | ||||||
| Sesamin | Neuroinflammation | In vivo | CD-1 mice | ↓ iNOS, ↓ cox-2, ↓ TNF-α, ↓ IL-1β | [327] | |
| –A | Asthma | In vivo | BALB/c mice | ↓ IL-1β, ↓ IL-6, ↓ NO, ↓ iNOS, ↓ IgE | [328] | |
|
Star anise (Illicium verum) |
–C | Atherosclerosis | In vitro | HASMC | ↓ TNF-α, ↓ IL-1β, ↓ NF-κB, ↓ cox, ↓ E-selectin, ↓ ICAM-1, ↓ VCAM-1 |
[329] |
| –C | Atherosclerosis | In vivo | C57BL/6 mice | ↓ TNF-α, ↓ IL-1β, ↓ NF-κB, ↓ cox, ↓ E-selectin, | [329] | |
| ↓ ICAM-1, ↓ VCAM-1, ↓ iNOS | ||||||
| –C | – | In vitro | HaCaT | ↓ IFN-γ Rα, ↓ ICAM-1, ↑ SOCS1, ↓ p-JAK2, | [330] | |
| ↓ p-STAT1 | ||||||
| Anethole | ALI | In vivo | BALB/c mice | ↓ MMP-9, ↓TNF-α, ↓ NO, ↑ IκBα, ↓ NF-κB p65 | [331] | |
| –C, AET | Asthma | In vitro | Splenocyte | ↓ IL-4, ↑ IFNγ | [332] | |
| –C, AET | Asthma | In vivo | BALB/c mice | ↓ IgE, ↓ IL-4, ↓ IL-5, ↓ IL-13, ↑ mRNA expression of Foxp3,↓ mRNA expression of IL-5, and IL-13 | [332] | |
|
Tamarind (Tamarindus Indica) |
–C | Pulmonary inflammation and fibrosis | In vivo | Wistar rats | ↓ ROS, ↓ LPO, ↓ PCC, ↓ NF-κB, ↓ p38α MAPK, ↓ NOX4, ↓ cox-2, ↑ HO-1, ↑ SOD2, ↑ catalase, ↑ GST, ↑ GSH, ↑ GPx |
[333] |
| –C | Arthritis | In vivo | Wistar rats | ↓ IL-1β, ↓ IL-6, ↓ IL-23, ↓ TNF-α, ↓ cox-2, ↓ MMP | [334] | |
| Xyloglucan | Ulcerative colitis | In vivo | C57Bl6 mice | ↓ IL-1β, ↓ IL-6, ↓ TLR4, ↓ NF-κB | [335] | |
|
Turmeric (Curcuma longa) |
Curcumin | PIVP | In vitro | A549, BMMF | ↓ IL6, ↓ TNF-α, ↓ MCP-1, ↓ NF-κB, ↑ IκBα | [336] |
| Curcumin | PIVP | In vivo | BALB/c mice | ↑ HO-1 | [336] | |
| Curcumin | Cystic fibrosis | In vitro | 16HBE14o | ↑ CFTR, ↓ cox-2, ↓ PGE2, ↓ IL-8 | [337] | |
| Curcumin | Cystic fibrosis | In vivo | SD rats | ↑ CFTR, ↓ cox-2, ↓ PGE2, ↓ IL-8 | [337] | |
| Curcumin | Diabetes | In vivo | SD rats | ↓ NF-κB, ↓ TNF-α, ↓ IL-1β, ↓ IL-6 ↓ NO, ↓ PGE2, | [338] | |
| ↓ cox-2 | ||||||
| Curcumin | ALI | In vivo | SD rats | ↓ TNF-α, ↓ IL-8, ↓ MIF | [339] | |
| Curcumin | Asthma | In vivo | BALB/c mice | ↓ NICD1, ↓ Notch 1/2 receptors | [340] | |
| Curcumin | Cerebral I/R injury | In vivo | SD rats | ↓ IL-1β, ↓ IL-8, ↑ p-JAK2, ↑ p-STAT3 | [341] | |
| ATM | Psoriasis | In vivo | BALB/c mice | ↓ NF-κB, ↓ cox-2, ↓ p-p38 MAPK, ↓ TNF-α, | [342] | |
| ↓ IL-6, ↓ mRNA synthesis of IL-17, -22, and -23 | ||||||
| MTrPP | Ulcer | In vivo | Wistar rats | ↓ TNF-α, ↓ IL-8, ↓ NF-κB, ↓ p-p38, ↓ MMP-9, | [343] | |
| ↓ cox-1 and -2 |
A : Oil, B: Resin, C : Extract, D : Mucilage, E : Seed.
Abbreviations: AA: Arachidonic acid, Ach: Acetylcholine, AChE: Acetylcholinesterase, AD: Alzheimer's disease, ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs, AET: Trans-Anethole, AFC: Antibody forming cell, Akt: Protein kinase B, ALI: Acute lung injury, ALP: Alkaline phosphatase, ALT: Alanine aminotrans-ferase, AMCase: Acidic mammalian chitinase, AMPK: Adenosine monophosphate-activated protein kinase, AP: Acute pancreatitis, AP-1: Activator protein-1, APH: Atypical prostatic hyperplasia, AR: Allergic rhinitis, AST: Aspartate aminotransferase, ATM: Aromatic-turmerone, ATPase: Adenosine triphosphatase, Bax: B-cell lymphoma 2 (Bcl-2)-associated X protein, BCA: 2′-benzoxy-cinnamaldehyde, Bcl-2: B-cell lymphoma 2, CCL: C-C Motif Chemokine Ligand, CCR3: C-C chemokine receptor type 3, CD: Contact dermatitis, CHLS: total cholesterol, CFTR: Cystic fibrosis transmembrane conductance regulator, COPD: Chronic obstructive pulmonary disease, cox-2: cyclooxygenase-2, CSE: Cystathionine-γ-lyase, CTXI: Collagen cross-linking carboxyterminal telopeptide, type I, DADS: Diallyl disulfide, DATS: Diallyl trisulfide, DNMT3A: DNA methyltransferase 3A, DRAM: Damage-regulated autophagy modulator, EFGR: Epidermal growth factor receptor, eNOS: endothelial nitric oxide, EPCR: Endothelial protein C receptor, ERK: Extracellular signal-regulated kinase, ET-1: endothelin-1, Foxp3: Forkhead Box Protein 3, GFAP: Glial fibrillary acidic protein, GGT: Gamma-glutamyl transpeptidase, GM-CSF: Granulocyte macrophage colony-stimulating factor, GPx: glutathione peroxidase, GR: Glutathione reductase, GRg1: Ginsenoside Rg1, GRg3: Ginsenoside Rg3, GSH: Glutathione, GSK3β: glycogen synthase kinase 3β, GST: Glutathione S-transferase, G-6P-D: Glucose-6-phosphate dehydrogenase, HAND: Human immunodeficiency virus, HAND: (HIV)- associated neurocognitive disorders, HCA: 2′-hydroxycinnamaldehyde, HDAC1: Histone Deacetylase 1, HDL: High-density lipoprotein, HO-1: Heme oxygenase-1, HMGB1: High mobility group box-1 protein, HNSCC: Squamous cell carcinoma of the head and neck, H2O2: Hydrogen peroxide, H2S: Hydrogen sulfide, IBD: Inflammatory bowel disease, ICAM-1- intercellular cell adhesion molecule-1, IFN: Interferon, IFN-γRα: IFN-γ receptor α, IKK: Inhibitor of M kinase, IL: Interleukin, iNOS: Inducible nitric oxide synthase, IPF: Idiopathic pulmonary fibrosis, IP-10: Interferon-inducible protein 10, IRF-3: Interferon regulatory factor 3, I/R: ischemia-reperfusion, JAK: Janus kinase 2: JNK: c-Jun N-terminal kinase, LC3: Light chain 3, LDH: Lactate dehydrogenase, LDL: Low density lipoprotein, LIF: leukemia inhibitory factor, LN: Lupus nephritis, LOX: Lipoxygenase, LPO: Lipid peroxidation, LXRα: Liver X receptor α, MAPK: Mitogen-activated protein kinase, MDA: Malondialdehyde, MCP: Monocyte chemoattractant protein, M-CSF: Macrophage colony-stimulating factor, MIF: Migration inhibitory factor, MIG: Monokine induced by gamma, MIP: Macrophage inflammatory protein, MMP: matrix metalloproteinases, MPO: Myeloperoxidase, mTOR: mammalian target of rapamycin, MTrPP: Modified pectin polysaccharide from turmeric, MyD88: Myeloid differential factor 88, NAFLD: Non-alcoholic fatty liver disease, NF-κB p65: Nuclear factor-κB p65, NICD: Notch intracellular domain, NK1R: Neurokinin-1-receptor, NLRP3: Nucleotide oligomerization domain (NOD)-like receptor protein 3, NO: Nitric oxide, NOX4: Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4, NR: Notoginsenoside R1, Nrf2: Nuclear factor erythroid 2-related factor 2, OA: Osteoarthritis, OSCC: Oral squamous cell carcinoma, p: phosphorylated, PA: Piperinic acid, PAI-1: Plasminogen activator inhibitor-1, PCC: Protein carbonyl content, PCNA: Proliferating cell nuclear antigen, PF: Pulmonary fibrosis, PGE2: prostaglandin E2, PIVP: Primary influenza viral pneumonia, PI3K: Phosphoinositide 3-kinases, PKC-β: Protein kinase C-β, PL: Piperlongumine, PLA2: Phospholipase 2, PNS: Panax notoginseng saponins, PPTA: Preprotachykinin A, p-YSTAT3: phosphorylated signal transducer and activator of transcription 3, QE: Quercetin-3-O-glucoside, QG: Quercetin-3-O-glucoside, RA: Rheumatoid arthritis, RA-FLS: Rheumatoid arthritis synovial fibroblast, ROS: Reactive oxygen species, RORγt: Retinoic acid-related orphan receptor-γt, SA: serum sialic acid, SAC: S-allyl-L-cysteine, SAMC: S-allyl-mercapto cysteine, SD: Sprague- Dawley, SGI: Salivary gland inflammation, Smad-3: small mothers against decapentaplegic homolog 3, SMC: S-allyl-mercapto cysteine, SOCS1: Suppressor of cytokine signaling 1, SOD: Superoxide dismutase, STAT: Signal transducer and activator of transcription, STZ: Streptozotocin, TACE: Tumor necrosis factor alpha converting enzyme, TBARS: Thiobarbituric acid reactive substances, TAC: Total anti-oxidant capacity, TAK1: TGF-β-activated kinase 1, TCA: Trans-cinnamaldehyde, TG: Triglyceride, TGF-β: Transforming growth factor-β, TIGAR: Tp53 induced glycolysis and apoptosis regulator, TIMP-1: Tissue inhibitor of metalloproteinase, TLRs: Toll-like receptors, TNF-α: Tumor necrosis factor-α, TP: Total protein, TQ: Thymoquinone, TRAP: Tartrate-resistant acid phosphatase, TRX1: Thioredoxin 1, TRXR1: Thioredoxin reductase 1, TSLP: Thymic stromal lymphopoietin, VCAM-1: Vascular cell adhesion protein 1, VEGF: Vascular endothelial growth factor, VLDL: Very low density lipoprotein, α-SMA: α-smooth muscle actin, ↓: Downregulation/Inhibition, ↑: Upregulation/Activation.
Fig. 4.
Spices regulates the expression of different inflammatory cytokines.
Several studies documented the role of spices in the modulation of different inflammatory proteins.
Abbreviations: GCSF: Granulocyte colony-stimulating factor, GM-CSF: Granulocyte-macrophage colony-stimulating factor, IFN: Interferon,; IL-Interleukin, M-CSF: Macrophage colony-stimulating factor, TNF-α: Tumor necrosis factor-α
7.1. Asafetida (Ferula asafetida Linn.)
It is derived from the roots of the F. asafetida (Umbelliferae family). Several studies have reported that asafetida exhibits protective effects against cancer, obesity, hepatotoxicity, etc. [344]. It has also been extensively used in traditional medicine for ages for the treatment of whooping cough, asthma, bronchitis, etc. [345]. The essential oil extracted from F. asafetida induces apoptosis and cytotoxicity against hepatocellularcarcinoma. It modulated the NF-κB and transforming growth factor (TGF)-β pathway by downregulating the expression of NF-κB1 and TGF-β1. Furthermore, it enhanced the production of caspase-3 and TNF-α [221]. Asafetida exhibits potent antitumor effects against breast cancer in BALB/c nude mice [222]. It also enhanced the activities of antioxidant enzymes such as catalase, glutathione (GSH), glutathione-s-transferase (GST), superoxide dismutase (SOD) in N-methyl-N-nitrosourea (MNU)-induced breast cancer in Sprague-Dawley rats [223]. The activity of LOX was also decreased by asafoetida [222,224].
7.2. Basil (Ocimum sp.)
Basil is a popular spice consumed all over the world [346]. It belongs to the Lamiaceae family [347]. Studies have shown that basil possesses medicinal properties such as gastroprotective, antioxidant, antimicrobial, etc. [226,348]. An in vivo study has also demonstrated the cardioprotective effects of basil leaves against isoproterenol-induced myocardial infarction in rats [349]. Hydro-ethanolic extract of basil leaf attenuated airway inflammation and exerted immunoprotective effects against asthma in the ovalbumin-sensitized rat model. It decreased the levels of IL-4, IgE, phospholipase A2 (PLA2), and increased the IFN-γ/IL-4 ratio in the bronchoalveolar lavage fluid (BALF) of the rats [225]. Furthermore, the hexane extract of basil (whole plant) modulated the expression of inflammatory markers such as TNF-α, IL-6, Prostaglandin E2 (PGE2), and IL-4. It also exerted anti-inflammatory as well as gastroprotective effects against an aspirin-induced gastric ulcer in vivo [226].
7.3. Bay leaf (Laurus nobilis)
Bay leaves belong to the family Lauraceae and are commonly used in cuisine for flavoring [227,350]. The administration of L. nobilis leaf extract attenuated the lipopolysaccharide (LPS)-induced mRNA expression of IL-6, TNF-α, and iNOS. It also inhibited the phosphorylation of IκB and STAT3 in vitro. Further, it inhibited the nucleotide oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome activation via the suppression of apoptosis-associated speck-like protein containing a CARD (ASC) oligomerization. In the BALF of the acute lung injury (ALI) model, the extract reduced the activity of the myeloperoxidase (MPO) enzyme as well as the levels of cytokines such as IL-1β, IL-6, and TNF-α. This suggests that bay leaf extract exerts ameliorative effects against ALI [227]. 1,8-Cineole is a bioactive component of L. nobilis. Similar to the leaf extract, 1,8-Cineole also downregulated the elevated LPS-induced mRNA expression of IL-6, TNF-α, and iNOS. It also inhibited the activation of NF-κB and STAT3 [227].
7.4. Black cumin (Nigella sativa)
Black cumin belongs to the family Ranunculaceae. It is consumed as a spice or seasoning predominantly in the Middle Eastern as well as Mediterranean countries [351]. N. sativa plant is widely known for its medicinal properties such as antidiabetic, anti-inflammatory, hepatoprotective, hypotensive, etc. [352]. Cumin effectively ameliorated LPS-induced lung damage as well as inflammation. It also downregulated the expression of the inflammatory mediators such as IFN-γ, TGF-β1, and PGE2 but also upregulated the anti-inflammatory cytokine IL-4. It also exhibits potent antioxidant properties by enhancing the activity of SOD and catalase [228]. Besides, it attenuated low-grade inflammation in vitro by reducing the levels of IL-1β and MCP-1 [229]. It also increased the mRNA expression of endothelial nitric oxide synthase (eNOS) in a streptozotocin (STZ)-induced diabetic rat model. However, it decreased the mRNA expression of vascular cell adhesion molecule-1 (VCAM-1) and LOX-1 in this model [230]. N. sativa demonstrated renal and hepatoprotective effects in LPS-treated rats. Moreover, it also exhibited anti-inflammatory effects by decreasing the levels of cytokine IL-6 as well as NO [231]. Further, it also declined the levels of IL-4 and nitric oxide (NO) in an allergic asthma model in Wistar rats [232]. Thymoquinone (TQ) is one of the major active components of cumin seeds. Studies have shown that TQ exerted an anti-inflammatory effect on Alzheimer's disease (AD) by downregulating the expression of toll-like receptor (TLR)-2 and -4. This leads to a decrease in the production of their downstream effectors NF-κB and interferon regulatory factor 3 (IRF-3). TQ also significantly reduced the expression of the pro-inflammatory cytokines TNF-α and IL-1β [233].
7.5. Black pepper (Piper nigrum)
Black pepper from the Piperaceae family is a popular spice due to its dietary importance and beneficial agent against various chronic health ailments [353].
The administration of ethanol extracts of Piper nigrum in ovalbumin-induced asthma mice model decreased IL-4, IL-6, IL-1β, retinoic acid-related orphan receptor gamma t (RORγt), IL-17A, TNF-α, IgE, and increased the levels of IL-10, INF-γ. It decreased the state of fibrosis and inflammatory cells infiltration and also regulated the production of cytokines like Th1, Th2, and Th17. Further, the treatment of the extract blocked allergy through the prevention of degranulation of peritoneal mast cells [234]. Further, the effect of fruit extract from P. nigrum in allergic rhinitis ovalbumin-induced BALB/c mice model prevented allergic reactions by reducing antibodies, histamine release by mast cells. It has also decreased E-cadherin and was protective against nasal epithelial barrier impairment through increased Nrf2 leading to elevated heme oxygenase-1 (HO-1) level [235]. Similarly, the extract also decreased the expression of tyrosine phosphorylated STAT3 (p-YSTAT3), IL-6, IL-1β, TNF-α, and NF-κB p65 [236].
The ethanolic extract containing pipernigramides A-G decreased iNOS-induced NO and levels of IL-1β, IL-6, TNF-α, and PGE2 in RAW 264.7 cells. It also reduced the degradation of IκB and targets IKK-β that inhibits p65, which suppressed inflammation [237]. Further, the treatment of piperine in B16F-10-induced lung metastasis in C57BL/6 mice decreased collagen hydroxyproline in lungs, hexosamine level, uronic acid, serum sialic acid, and gamma-glutamyl transpeptidase, and increased the lifespan of treated animals [238].
Piperine was showed to be effective against bacterial sepsis by preventing pyroptosis through the reduction of IL-1β and adenosine monophosphate-activated protein kinase (AMPK) levels, both in vitro and in vivo [239]. Piperine also attenuated the condition of acute pancreatitis through the reduction of TNF-α, IL-1β, IL-6, p-ERK1/2, p-p38, p-JNK, and MAPKs expressions [240]. It improved lupus nephritis in HK-2 cells by inhibiting pyroptosis, NLRP3 inflammasome, HMGB1, caspase-1, and activation of AMPK. Similarly, piperine inhibited NLRP3 inflammasome and serum IL-1β in the BALB/c mice model of lupus nephritis [241]. The effect of chabamide from fruits of P. nigrum in LPS-stimulated RAW264.7 cells produced an anti-inflammatory effect via the Nrf2/HO-1 pathway through the reduction in iNOS and increased Nrf2 and its targets-NAD(P)H: quinone oxidoreductase 1 and γ-glutamyl cysteine synthetase [242].
7.6. Capsicum (Capsicum annum L.)
Capsicum belongs to the family Solanaceae. It exerts anti-inflammatory effects against ethanol-induced inflammation in rats by reducing the expression of pro-inflammatory cytokines TNF-α and IL-6. Additionally, it also exerts potent hepatoprotective effects by attenuating the elevated levels of malondialdehyde (MDA) in the liver, a marker for lipid peroxidation, as well as enzymes like alanine aminotransferase (ALT), aspartate transaminase (AST), which are the markers for liver damage. Capsicum also restores the abnormal levels of cytosolic Cu-Zn-SOD, mitochondrial Mn-SOD, as well as critical antioxidant enzymes [243]. The extract of capsicum also significantly elevated the expression of Th type 2 cytokines such as IL-4, IL-5, IL-13 in an ovalbumin-induced asthma mouse model [244].
Capsaicin is one of the major active components of capsicum. It is known to possess protective activities against asthma, cancer, diabetes, etc. [14]. It also ameliorated inflammation by reducing the levels of TNF-α, IL-6, and IL-1β. Enhanced expression of liver X receptor α (LXRα) is also regulated by capsaicin via the peroxisome proliferator-activated receptor γ (PPARγ) pathway [245]. Inflammation of the salivary glands is also attenuated by capsaicin. It markedly reduced the mRNA as well as protein expression of TNF-α and IL-6 in HSG cells [246].
7.7. Cardamom (Elettaria cardamomum)
Cardamom belongs to the Zingiberaceae family. It is commonly referred to as the “queen of spices” [354]. Several studies indicated that cardamom possesses different therapeutic activities such as chemopreventive, antidiabetic, anticancer, gastroprotective, etc. [354,355]. Treatment with cardamom extract ameliorated oxidative stress by enhancing the levels of antioxidant enzymes. It also attenuated inflammation by downregulating the expression of NF-κB and NO. Further, it also exerted protective effects against doxorubicin-induced cardiotoxicity in rats [247].
7.8. Celery seeds (Apium graveolens)
Celery seeds belong to the Apiaceae (Umbelliferae) family. It is consumed as a spice and herbal medicine [251]. Preclinical studies conducted on hyperuricemia mice model and monosodium urate-induced gouty arthritis rats reported that the aqueous and oil extracts of celery seeds exhibit anti-inflammatory and antioxidant properties. The administration of the extracts reduced the pro-inflammatory cytokines like IL-1β, TNF-α, and enhanced IL-10 levels [248]. Sedanolide, a compound isolated from celery seed essential oil, induced autophagy in human hepatocarcinoma cells, due to the reduced phosphatidylinositol-3-kinase (PI3K), mTOR, Protein kinase B (Akt) expression levels in those cells [249]. Another recent study shows that the hydrolyzed celery extract suppressed the inflammation in the chronic atopic dermatitis mice model. It effectively reduced the pro-inflammatory cytokines like IL-4, TNF-α, IFN-γ, IL-6, IL-31, and IgE as demonstrated from the preclinical experimental models [250]. The extract of celery seeds also exerts anti-inflammatory effects by downregulating IL-6, TNF-α, and NF-κB levels [251].
7.9. Cinnamon (Cinnamomum sp.)
The spice cinnamon is derived from the bark of plants belonging to the family Lauraceae and genus Cinnamomum [356]. It is a multipotential medicinal plant that possesses diverse properties such as antiseptic, antifungal, antiviral, anti-inflammatory, immunomodulatory, etc. [357].
Trans-cinnamaldehyde (TCA) is one of the components of Cinnamon. TCA inhibited NF-κB as well as the p38-JNK signaling pathway in the IL-1β-induced osteoarthritis model. Furthermore, it inhibited the activation of NF-κB as well as the degradation of IκB. It also slowed down the progression of osteoarthritis in vivo [252]. Suppression of NO due to a decrease in iNOS expression was also observed after TCA treatment in RAW 264.7 cells [253]. The administration of TCA also suppressed the production of NO in LPS-induced BV2 microglial cells. Furthermore, it also downregulated the expression of iNOS, cox-2, as well as IL-1β and inhibited NF-κB activation [254]. The essential oil extracted from cinnamon also possesses anti-inflammatory properties. A study showed that it downregulated inflammatory biomarkers, such as MCP-1, interferon gamma-induced protein 10 (IP-10), interferon-inducible T-cell alpha chemoattractant (I-TAC) monokine induced by gamma interferon (MIG) as well as IL-8 in a human skin disease model [255]. The water extract of cinnamon (CWE) substantially reduced the secretion of TNF-α, and IL-6 in LPS-induced in vivo model. In LPS-induced macrophages, the extract did not reduce the secretion of TNF-α; however, a substantial decrease in its mRNA expression was observed. Additionally, it also inhibited IκBα degradation as well as MAPK phosphorylation [256]. CWE also significantly decreased the levels of anti-CD3-induced IFN-γ in the serum of mice model. It also decreased the mRNA expression as well as the release of IFN-γ, and IL-4 in murine splenocytes. Additionally, CWE increased the secretion of IL-2 in splenocytes [257]. 2′-Hydroxycinnamaldehyde (HCA) and 2′-benzoxycinnamaldehyde (BCA) are the derivatives of cinnamaldehyde. A study showed that both HCA and BCA exhibit an inhibitory effect on the secretion of IgM as well as IFN-γ in murine splenocytes [258].
7.10. Coriander (Coriandrum sativum)
Coriander is an annual herb of the Apiaceae family [358]. It is commonly known as coriander, and its different parts are consumed worldwide. In addition to its culinary values, coriander is also often consumed as a traditional medicine against different ailments, such as diabetes, cancer, hypertension, etc. [359]. It also possesses different pharmacological activities such as hepatoprotective, antihelminthic, neuroprotective, antimicrobial, etc. [[359], [360], [361]].
Numerous studies have suggested that extracts prepared from different parts of coriander also induce potent anti-inflammatory effects in both in vitro as well as in vivo models [[259], [260], [261]]. A study demonstrated that the ethanolic extract of coriander leaves and stem downregulated the expression of inflammatory mediators, including pro-IL-1β, NO, and cox-2, as well as decreased the production of PGE2 and iNOS. It further inhibited NF-κB activation as well as MAPK signaling in LPS-induced RAW 264.7 cells [259]. It also substantially attenuated the 2,4-dinitrochlorobenzene-induced elevated expression of IL-1, IL-4, IL-13, TNF-α, IFN-γ as well as immunoglobulin E (IgE) in the contact dermatitis in vivo model [260]. The hydroalcoholic extract of coriander reduced the production of TNF-R1 protein as well as downregulated the levels of the cytokines IL-1β and IL-6 [261].
7.11. Cumin (Cuminum cyminum)
Cumin belongs to the Apiaceae family. It is commonly used for the treatment of diseases like diabetes, cancer, hypolipidemia, etc. [362]. In renal hypertension in vivo model, the aqueous extract of cumin seeds attenuated inflammation and oxidative stress and induced antihypertensive effects by modulating the gene expression of IL-6, TNF-α, thioredoxin 1 (TRX1), and eNOS [262]. The methanol extract of cumin also ameliorated inflammation and imparted protection against diabetes-associated gastric ulcer in vivo [263].
7.12. Curry leaves (Murraya koenigii)
Curry leaves belong to the Rutaceae family. They are are popularly used in Indian cuisine and have been a part of Indian traditional medicine for centuries due to its versatile medicinal properties [363]. A recent study showed the anti-inflammatory effect of the hydroalcoholic extract of curry leaves in vitro and in vivo. It reduced the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in LPS-induced macrophages. Similar effects were observed in the cerulein-induced acute pancreatitis in vivo model. Additionally, it also downregulated the activity of NFκB p65 as well as the expression of cox-2 and intercellular adhesion molecule-1 (ICAM-1) in vivo [264]. The aqueous extract reduced the levels of cytokines such as IL-6, IL-1β, and IL-10 in 4T1-inoculated mice. It also downregulated the expression of the inflammation regulator NF-κB [265]. Mahanimbine is a carbazole alkaloid found in curry leaves. It attenuated the elevated levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in high-fat diet (HFD)-induced mice, thereby exerting a potent anti-inflammatory effect [266]. Girinimbine, another carbazole alkaloid, is also a potent anti-inflammatory agent. It reduced the production of TNF-α, and IL-1β in carrageenan-induced peritonitis [267].
7.13. Fenugreek (Trigonella foenum-graecum L.)
Fenugreek belongs to the Fabaceae family [364]. It is rich in pharmacological and nutritional properties. It possesses different activities such as anticarcinogenic, anti-inflammatory, antidiabetic, etc. [365].
A study showed that standardized fenugreek seed extract-glycoside base (SFSE-G) exhibited potent anti-inflammatory effects by downregulating the expression of inflammatory mediators, including TNF-α, IL-1β, IL-6, IL-8 in a bleomycin-induced pulmonary fibrosis model. It also inhibited the mRNA expression of the fibrotic markers such as small mothers against decapentaplegic homolog 3 (Smad-3), collagen-I, endothelin-1 (ET-1), etc. eventually leading to amelioration of pulmonary fibrosis in vivo [268]. A study has demonstrated that the extract of fenugreek seeds suppressed cisplatin-induced expression of iNOS and NF-κB p65 in testicular tissues of Wistar rats [269]. It also reduced the expression of retinal inflammatory markers, such as TNF-α and IL-1β, in STZ-induced diabetic rat retina [270]. In RA, expression of the pro-inflammatory cytokines TNF-α, and IL-6 is significantly downregulated by fenugreek mucilage prepared from fenugreek seeds, thereby asserting its anti-inflammatory role [271]. The pretreatment of trigonelline derived from fenugreek exerts neuroprotective effects against amyloid β-induced AD in vivo. The mechanism is attributed to a decrease in hippocampal glial fibrillary acidic protein (GFAP) as well as pro-inflammatory cytokines [272].
7.14. Garcinia (Garcinia indica)
Garcinia is a spice which belongs to the family of Clusiaceae. Its fruit is commonly called kokum and it exhibits various pharmacological effects such as cardioprotective, hepatoprotective, antibacterial, antiulcer, antiarthritis, etc. [366]. Garcinol is a major component of Garcinia which possesses medicinal properties such as antiproliferative, antibacterial, anti-inflammatory, etc. [367]. A study showed that it significantly reduced the expression of inflammatory mediators like iNOS and cox-2 in a dextran sulfate sodium (DSS)-induced colitis mice model. Furthermore, it also prevented DSS/azoxymethane (AOM)-induced colon tumorigenesis by inhibiting the PI3K/Akt/p70S6K, as well as ERK signaling pathways [273]. Garcinol inhibited NF-κB activation in CAL27 cells suppressing TGF-β–activated kinase 1 (TAK1) and an inhibitor of IκB kinase (IKK). Further, it also inhibited the constitutive activation of STAT3 as well as its upstream kinases c-Src, JAK1, and JAK2. Moreover, it inhibited CAL27 xenograft tumors in nude mice [274].
7.15. Garlic (Allium sativum)
Garlic is a bulbous plant belonging to the family Liliaceae. It is commonly used as a spice and flavor additive in many cuisines across the globe. Apart from this, garlic has been used since ancient times in different cultures for its medicinal values [275].
An in vivo study has demonstrated that the aqueous extract of garlic exerts inhibitory effects against Dermatophagoides pteronyssinus (Der p)-induced allergic asthma. The study showed that aqueous extract of garlic substantially downregulated the levels of cytokines such as IL-13, IL-5, and IL-4 but upregulated the levels of IFN-γ and IL-12 in the BALF of the experimental mice. Moreover, it also decreased the levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 and thereby induced anti-inflammatory effects [275]. The treatment of Der p-stimulated A549 cells with aqueous garlic extract also inhibited inflammation by suppressing the IL-6/PI3K/Akt/NF-κB pathway [275].
Different bioactive components present in garlic are associated with anti-inflammatory as well as other pharmacological activities. Diallyl disulfide (DADS) is an organosulfur compound and is one of the key components of garlic. A study has shown that DADS ameliorated cerulein-induced acute pancreatitis in vivo by suppressing the substance P/neurokinin 1 receptor (SP/NK1R) signaling as well as the NF-кB pathway [276]. Another organosulfur compound diallyl trisulfide (DATS), inhibited the NF-κB and Wnt pathway in human fibroblast-like synoviocytes (FLS) obtained from RA patients. Moreover, key inflammatory mediators such as IL-1β, IL-6, and TNF-α are also downregulated by DATS in collagen-induced arthritis (CIA) mouse model [277]. Garlic, also another organosulfur compound namely S-allyl-L-cysteine (SAC) [368]. An in vivo study revealed that in a bleomycin-induced pulmonary fibrosis model, SAC reduced the mRNA expression of fibrosis markers such as α-smooth muscle actin (α-SMA), fibronectin, collagen-I, and –III. Additionally, it also downregulated the mRNA expression of markers associated with inflammatory responses, such as TNF-α, iNOS, IL-6, and IL-12p35. Furthermore, SAC also suppressed the Akt/NF-кB pathway in vivo [278]. The bioactive component S-allyl-mercapto cysteine (SAMC) found in garlic also exerts potent anti-inflammatory, antifibrotic, antioxidative, as well as antimetastatic effects [369,370]. A study demonstrated that SAMC attenuated inflammation by inducing inhibitory effects on pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 and suppressing the NF-кB pathway in LPS-induced ALI in mice model [279].
7.16. Ginger (Zingiber officinale)
Ginger is a widely consumed spice which belongs to the Zingiberaceae family. It is also known for its medicinal properties such as antitumor, anti-inflammatory, antioxidant, etc. [14,280,371]. Some of the active components of ginger are 6-shogaol, 6-gingerol, 6-paradol, and 6-gingerdiol. [14].
An in vivo study revealed that 6-shogaol inhibited 7,12-dimethylbenz[a]anthracene induced oral cancer by modulating the NF-κB pathway. It downregulated the levels of cytokines such as IL-1, IL-6, and TNF-α [280]. Another study demonstrated that 6-gingerol imparted protective effects against steatohepatitis in both in vitro and in vivo. In the HepG2 cells, gingerol reduced the enhanced levels of MCP-1, TNF-α, and IL-6. A similar effect of 6-gingerol was observed in the methionine and choline-deficient (MCD) diet-fed animal model. Besides, the upregulated NF-кB level was also attenuated by 6-gingerol [281].
7.17. Ginseng (Panax sp.)
Ginseng belongs to the Araliaceae family and is commonly used in herbal therapies. Both its extract and isolated active compounds were reported to possess extensive biological properties [372,373]. For instance, an active compound, isofraxidin (IFD), isolated from Siberian ginseng, exerted significant anti-inflammatory effects on a mouse model of ALI. The property of the compound was attributed to its ability to reduce the serum levels of various inflammatory cytokines such as TNF-α, IL-1β, IL-6, and MIP-2. Besides, IFD modulated various inflammatory factors linked to the PI3K/Akt and MAPK signaling pathways [282]. Black ginseng (BG) also displayed efficient activity against lung injury through modulation of Akt and reactive oxygen species (ROS)-induced p38-MAPK signaling [283]. Additionally, saponins isolated from Panax ginseng displayed a wide range of efficacy against various diseases. These saponins imparted protective roles against HIV-associated neurocognitive disorders (HAND) and DSS-induced colitis by regulating the levels of caspase-3, 8, 9, and PI3K/Akt signaling pathway respectively [284,285]. Further, notoginsenoside R1 (NR), isolated from Panax notoginseng, imparted protective effects against the rat model of renal ischemia-reperfusion (I/R) injury via downregulation of certain oxidative and inflammatory factors such as TNF-α, TGF-ß1, IFN-γ, and IL-6 [286]. Ginsenoside Rg3 (GRg3) induced anti-inflammatory effects and ameliorated RA in vivo via the regulation of CD4+CD25+Foxp3+Treg cells [287]. In vitro and in vivo studies reported that ginsenosides Rg1 suppressed the TGF-β1/Smad3 signaling pathway while Rg3 mitigated neutrophilic inflammation, migration, and the levels of PI3K, thereby exerting protective effect against chronic obstructive pulmonary disease (COPD) [288]. Another compound isolated from ginseng, ginsenoside Rd, prevented neuroinflammation in ischemic stroke by suppressing the NF-κB/MMP-9 signaling pathway [289].
7.18. Long pepper (Piper longum L.)
The powdered dry fruits of P. longum (family Piperaceae), are used in Ayurveda for the preparation of several medicines. The whole plant of this spice is known to yield many biologically important compounds, such as piperine, piperlongumine (PL), and piperinic acid and are used to treat numerous inflammation-mediated diseases like cancer, lupus nephritis, arthritis, and asthma due its ability to supress pro-inflammatory cytokines such as TNF-α, and IL-6 and induce the expression of anti-inflammatory cytokine IL-10 [[290], [291], [292], [293], [294], [295], [296]]. The compound PL is known to relieve the inflammation-associated neurotoxicity upon the activation of stress-inducing molecules like NO, PGE2, and cox-2 in the cultured LPS-stimulated BV2 cells. Further, it also ameliorated LPS-induced amyloidogenesis by inhibiting the NF-κB pathway [295,297]. Long pepper extracts and PL also prevented the cell-adhesion and migration of leukocytes to sites of inflammation by reducing the expression of cell adhesion molecules like ICAM-1, and VCAM-1 [293,294,298]. The anti-inflammatory activity of PL is also attributed to the downregulation of elevated expression levels of MAPK proteins like p38, ERK1/2, and JNK [294,299,300]. PL alleviated the overactivation of Th2 cytokines IL-4, IL-5, and IL-13 as well as the IgE levels in the ovalbumin-induced asthmatic lung tissue of mice. Besides, PL also prevented the airway remodeling in vivo via the inhibition of the NF-κB pathway [293]. Moreover, it attenuated systemic and pulmonary inflammation by inhibiting cox-2 and NF-κB [301]. In the collagen-induced arthritis mice model, PL suppressed the expression of TNF-α, IL-1β, IL-23, and IL-17 [292].
7.19. Mint (Mentha sp.)
Mint belongs to the genus Mentha of the Lamiaceae family. M. spicata, M. pulegium, and M. rotundifolia are some of the species belonging to the mint family. They are commonly consumed as herbal tea and spice. Moreover, mint is rich in antimicrobial, anti-inflammatory, neuroprotective, cardiovascular, and antitumor properties [374,375].
A study reported that M. spicata exerts ameliorative effects against acute and chronic inflammation in vivo [376]. In another study, M. arvensis was shown to reduce the levels of MDA and NO in LPS-induced RAW 246.7 cells. It also attenuated the elevated levels of NO, which was induced due to immobilization stress in the rat model. Further, it also inhibited the MAPK signaling and inflammatory mediator cox-2. These results suggested that M. arvensis might attenuate stress and associated inflammation [302]. The ethyl acetate extract of M. arvensis reduced the levels of the cytokines TNF-α, and IL-1α. It also modulated the LPS-activation of MAPK. Rosmarinic acid and L-menthone are some of the constituents of M. arvensis, which decreased the LPS- or H2O2-induced ROS levels in vitro [303].
7.20. Mustard (Sinapis alba)
White mustard belongs to the family of Brassicaceae [377]. These are widely consumed as a condiment. The dried mature seed of white mustard is also known as Sinapsis semen [304]. The extract of S. alba efficiently inhibited the mRNA as well as protein expression of inflammatory cytokines such as TNF-α, IL-6, and IL-1β in vivo. Further, it also suppressed the activity of MPO, which is a marker for inflammation [304]. Mustard seeds also ameliorated inflammation in psoriasis model. They repressed the expression of NLRP3 inflammasome. This further led to the inhibition of IL-1β and IL-18 induced inflammation [305]. Furthermore, it increased CD4+ T cell count and decreased the plasmacytoid dendritic cells (pDC) and macrophages. Low levels of pDC resulted in a decrease in the secretion of IFN-α. The expression of the NF-κB p65 subunit, as well as IL-17 and IL-22, were significantly inhibited which indicated that mustard seeds further abrogated inflammation. Moreover, the expression of the antioxidant enzymes such as GSH, glutathione peroxidase (GPx), and SOD were also enhanced by mustard seeds. This results in the inhibition of oxidative stress which was further evidenced by a decrease in the lipid peroxidation marker MDA as well as the reactive nitrogen species (RNS) generating iNOS [306].
7.21. Nutmeg (Myristica fragrans)
Nutmeg belongs to the Myristicaceae family and is a widely consumed spice [378,379]. Many studies have been conducted to evaluate the potential of its extract and isolated phytochemicals in the treatment and prevention of different diseases. Nutmeg extracts displayed significant antioxidant and anti-inflammatory activities and thus imparted protection against cortical liver injury in vivo [307,380]. Macelignan, an active compound of M. fragrans, was reported to exhibit anti-inflammatory effects in vivo in an asthmatic model by significantly reducing the expressions of IL-4 and Th2 cell-specific master transcription factor, GATA3 [308]. The compound was further reported to exert significant anti-allergic and anti-inflammatory effects via the modulation of p-Akt, p38-MAPK, and JNK. It also inhibited the expression of other inflammatory mediators such as cox-2, 5-LOX, IL-4, IL-13, and TNF-α [309]. Moreover, macelignan induced protective effect against renal I/R injury by imparting antioxidant activity as well as regulating the inflammatory and apoptotic mediators such as IL-6, TNF-α, IFN-γ and B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), caspase-3, respectively [310]. Myrislignan, a lignan found in M. fragrans, also ameliorated inflammation by inhibiting the activation of the NF-κB pathway as well as the expression of inflammatory mediators such as IL-6, TNF-α, and cox-2 [311]. Myristicin is an aromatic compound isolated from nutmeg. It suppressed the production of cytokines (such as IL-6, IL-10), chemokines (such as MCP-1, MCP-3, MIP-1α, MIP-1β) granulocyte-macrophage colony-stimulating factor (GMCSF), and leukemia inhibitory factor (LIF) [312].
7.22. Onion (Allium cepa)
Onion belongs to the Liliaceae family. It is consumed widely for its flavor and nutritional value. Moreover, it is also used in traditional medicine due to its variety of therapeutic properties such as anticancer, cardioprotective, antimicrobial, antifungal, and antiviral [381]. The extract of onion bulb suppressed DSS-induced colitis in a rat model by downregulating MAPK/Akt/mTOR signaling as well as the cox-2 levels. Moreover, pro-inflammatory cytokines and chemokines were also downregulated by the treatment with this extract [313]. The extract of red onion scales decreased the levels of IL-6, IL-8, and TNF-α in atypical prostatic hyperplasia in an in vivo model [314]. Quercetin, a major flavonoid found in onion, also possesses therapeutic properties [315]. A recent study demonstrated that it exerted protective effects against high fructose-induced atherosclerosis in mice. The protective effect was attributed to the suppression of ROS, by modulating the levels of SOD, HO-1, Nrf-2, H2O2, O2, and MDA. Quercetin also suppressed inflammation and apoptosis by regulating the NF-κB and PI3K/Akt pathway, respectively. In LPS-induced VMSCs, it down-regulated the cytokines such as IL-1β, IL-18, TNF-α, and IL-6. Additionally, it also improved LPS-induced atherosclerosis by inhibiting PI3K/Akt-regulated NF-κB [315].
In another study, quercetin-3-O-glucoside and eicosapentaenoic acid ester of quercetin-3-O-glucoside (QE) was shown to reduce the levels of TNF-α, IL-6, cox-2, PGE2 in vitro. The treatment of QE in hyperlipidemic rats also decreased the expression of LPS-induced IL-6 [316].
7.23. Rosemary (Rosmarinus officinalis L.)
Rosemary belongs to the Lamiaceae family. It possesses several therapeutic properties such as hepatoprotective, antifungal, antioxidant, antibacterial, etc. [382]. Rosmarinic acid is one of the major components of rosemary. It is a phenolic compound that possesses anti-inflammatory, hepatoprotective, and antihyperlipidemic properties [383,384]. A study showed that rosmarinic acid downregulated the enhanced levels of key inflammatory as well as immunological mediators such as IL-4, IgE, IFN-γ, and PLA2 induced by ovalbumin in asthmatic rats [317]. Another study revealed that it also modulated the MAPK and NF-κB signaling pathways in ovalbumin-induced asthma in a mice model [318].
7.24. Saffron (Crocus sativus)
Saffron belongs to the Iridaceae family. It is known to possess various therapeutic properties, such as anticonvulsant, antidepressant, anti-inflammatory, antitumor, etc. [385]. Crocin ameliorated LPS-induced sepsis as well as cardiotoxicity in H9c2 cells. It significantly downregulated the inflammatory mediators TNF-α, PGE2, IL-1β, and IL-6. It also decreased the mRNA expression of cox-2, iNOS, as well as NO [319]. Crocin induced osteoprotective effect by inhibiting inflammation in a metabolic syndrome (MetS)-induced osteoporosis model. It exhibited anti-inflammatory activity by reducing the levels of the pro-inflammatory cytokines IL-6 and TNF-α. Furthermore, it suppressed and elevated the levels of markers for bone resorption and bone formation, respectively, in (MetS)-induced osteoporosis model [320]. Safranal is another major component of saffron. A study showed that safranal inhibited MAPK and NF-κB pathways which led to a reduction in the expression of pro-inflammatory cytokines, such as IL-6 and TNF-α. It also inhibited the expression of iNOS and cox-2 in vitro. Furthermore, safranal exhibited similar anti-inflammatory responses in DSS-induced colitis mice [321]. Safranal also partially restored the levels of inflammatory mediators, such as IL-1β, IL-6, TNF-α, and NF-κB to normal levels, in the hippocampus of the amyloid β-induced AD model. It also attenuated cognitive deficits and exhibited antioxidant effects in the AD model [322]. In addition to the anti-inflammatory effect, safranal also exhibits potent antioxidant and gastroprotective effects [323].
7.25. Sesame (Sesamum indicum)
Sesame belongs to the Pedaliaceae family and is rich in medicinal properties such as anticancer, hepatoprotective, antihypertensive, etc. [386]. Sesamol is an active compound of sesame. A study reported that it decreased the eosinophil infiltration in lungs, Th2 cytokines, and MDA levels in asthmatic BALB/c mice, and BEAS-2B cells, which might attenuate inflammation in the lungs [324].
Sesamin is a lignan isolated from S. indicum [387]. The administration of sesamin attenuated LPS-induced ALI by reducing the expression of TNF-α, IL-6, and IL-1β. It also inhibited the TLR4 pathway and NF-κB activation [325]. It also ameliorated renal oxidative stress and inflammation by upregulating antioxidant enzymes such as SOD, GSH, and catalase, and downregulating inflammatory mediators such as TNF-α, IL-6, and cox-2, respectively [326]. Sesamin also attenuated the levels of iNOS, cox-2, TNF-α, and IL-1β in a stressed mice and thereby exerted antidepressant effects [327]. Similarly, sesame oil exerted a protective effect against the asthmatic mice model by reducing the levels of IL-1β, IL-6, IgE, and iNOS [328].
7.26. Star anise (Illicium verum)
The spice star anise belongs to the Illiciaceae family [388]. It possesses a variety of pharmacological properties such as anti-bacterial, anti-nociceptive, anthelmintic, antiviral, gastroprotective, etc. [389,390].
The extract of star anise exerted inhibitory effects on key inflammatory biomarkers such as TNF-α, IL-1β, NF-κB, and cox in an apolipoprotein E-knockout (ApoE−/−) mice. Similar findings were observed when TNF-α-stimulated-HASMC cells were treated with this extract [329]. Another study revealed that star anise extract exhibited potent anti-inflammatory effects by substantially reducing the expression of IFN-γ receptor α (IFN-γRα) as well as a suppressor of cytokine signaling 1 (SOCS1) protein in IFN-γ-induced human keratinocytes. Moreover, it also inhibited JAK/STAT signaling and decreased the production of ICAM-1 [330].
Anethole is one of the compounds responsible for the aroma and flavor of star anise. It exerts a potent anti-inflammatory effect by inhibiting the activation of NF-κB. It also substantially reduced the levels of pro-inflammatory mediators such as TNF-α, and IL-6 [331]. Trans-anethole is another major constituent found in star anise. It attenuated the elevated level of cytokines such as IL-4, IL-5, and IL-13, in the asthmatic in vivo model. The mRNA expression of forkhead box P3 (Foxp3), a transcription factor involved in the development and function of regulatory T cells, was also upregulated by trans-anethole. Besides, it also modulated the production of IL-4, and IFN-γ in vitro [332].
7.27. Tamarind (Tamarindus indica L.)
Tamarind (family Fabaceae) is one of the highly diverse and ethnopharmacologically valuable plant species known to man. A recent study showed that tamarind seed coat extract (TSCE) possesses antioxidant property that prevented oxidative stress-induced-erythrocyte loss. It is also known to prevent anemia, reduce lipid peroxidation, and regulated glutathione levels [391]. Besides, TSCE attenuates the pulmonary inflammation which is attributed to the reduced levels of inflammation-inducing NF-κB and cox-2 levels as well as oxidative stress-inducers such as nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase 4 (NOX4) and p38α MAPK. It also attenuated pulmonary fibrosis in vivo [333]. The seed extract also exerted antiarthritic effect in vivo by modulating the levels of anti-inflammatory mediators such as IL-10, TNF-α, IL-1β, IL-6, cox-2, and IL-23 [334]. Its fruit extract also possesses anti-inflammatory properties [392]. In another study mucoadhesive tamarind xyloglucan (TXG) was shown to exert anti-inflammatory effects against ulcerative colitis in vivo. TXG inhibited the levels of pro-inflammatory cytokines IL-1β, and IL-6 and further attenuated inflammation by inhibiting the TLR4/NF-κB signaling pathway [335].
7.28. Turmeric (Curcuma longa)
Turmeric from the Zingiberaceae family has been used since ancient times in various traditional medicines. It is profoundly rich in therapeutic activities such as anti-inflammatory, anticancer, antiatherosclerotic, antidepressant, antidiabetic, antiarthritic, etc. Its potent biological properties can be mostly attributed to one of its active components namely “Curcumin” [[393], [394], [395], [396], [397]]. Curcumin possess several pharmacological activities such as antiviral, antifungal, anticancer, etc., and is therefore rightfully proclaimed to be the “Golden nutraceutical” [14,394,398]. Moreover, it is highly efficacious against cancer (breast, cervical, colorectal, ovarian, pancreatic, prostate, etc.) RA, inflammatory bowel disease, psoriasis, neurological diseases, etc. [393,[399], [400], [401], [402], [403]].
Congregated evidence from various preclinical and clinical studies has shown the efficacy of curcumin in the prevention and treatment of diverse health ailments [[404], [405], [406], [407], [408], [409], [410], [411]]. Studies have shown that curcumin helps in regulating the cytokine storm both in vitro and in vivo in case of influenza virus A (IAV) infection thereby relieving chronic influenza, pneumonia, and lung injuries associated with these conditions. It attenuated the elevated levels of IL-6, MCP-1, TNF-α as well as inhibited NF-κB, besides inhibiting the replication of IAV. Curcumin also elevated HO-1 which ameliorated lung injuries [336]. An in vivo study on experimentally induced pulmonary inflammation in a rat model evidenced that curcumin significantly regulated the expressions of various inflammatory and fibrotic mediators, such as cystic fibrosis transmembrane conductance regulator (CFTR) and cox-2/PGE2/IL-8 [337]. Moreover, the anti-inflammatory and antioxidative potential of curcumin helped in relieving lung injury in diabetic rats through the inhibition of NF-κB, NO, PGE2, iNOS and cox-2 [338]. An in vivo study also evidenced the efficacy of curcumin against cecal ligature puncture (CLP)-induced lung injury through the suppression of the inflammatory mediators such as TNF-α, IL-8, and MIF [339]. The protective effects of curcumin against asthma were also reported where the compound significantly modulated the Notch1-GATA3 signaling pathway and thus inhibited the inflammation of the airways [340]. Curcumin imparted neuroprotective effects against cerebral I/R injury as well via downregulation of IL-1β and IL-8 besides triggering the JAK2/STAT3 signaling [341]. Apart from curcumin, modified polysaccharides and components of essential oils obtained from C. longa were also reported to have diverse biological activities. An important component of the essential oil is aromatic-turmerone (ATM), which is reported to have strong anti-inflammatory and antioxidant properties and thus proved to be useful in the treatment of psoriasis. It significantly inhibited the expressions of NF-κB, cox-2, p-p38 MAPK, TNF-α, and IL-6. ATM further suppressed the mRNA synthesis of IL-17, IL-22, and IL-23 [342]. Furthermore, the anti-inflammatory activity of a modified pectin polysaccharide from turmeric (MTrPP) contributed to its antiulcer activity and thus it aided in the treatment of LPS-induced ulcer in a rat model. MTrPP inhibited the release of inflammatory markers such as TNF-α, IL-8, NF-κB, MMP-9, cox-1, and cox-2. It also prevented the phosphorylation of p38, thus imparting protective effects [343].
8. Anti-inflammatory spices in clinical trials
Apart from the aforementioned preclinical studies, the anti-inflammatory activity of many of these spices has been evaluated in clinical trials and these were found to be very efficacious against many diseases. The administration of spices to the patients regulate cytokine storm and several other inflammatory mediators. This leads to an improvement of symptoms as well as treatment of various diseases. Some of the important clinical studies which show the modulation of inflammatory mediators by spices are summarized below and are mentioned in Table 3 .
Table 3.
Anti-inflammatory activity of spices against different diseases in clinical trials.
| Spice | Active compound | Health issues | Phase | Pts no. | Dosage ranges (mg)/day | Clinical outcome (mechanism) | References |
|---|---|---|---|---|---|---|---|
| Cardamom | – | Pre-diabetic subjects | – | 80 | 3000 | Significant anti-inflammatory and antioxidant effects (↓ hs-CRP, ↓ hs-CRP:IL-6 ratio) | [412] |
| – | T2D | – | 83 | 3000 | Improvement in clinical symptoms (↓ TG, ↑ Sirt1) | [413] | |
| – | NAFLD | – | 87 | 3000 | Improvement in clinical symptoms, safe to use (↑ Sirt1, ↑ irisin) | [414] | |
| Cinnamon | β-caryo phyllene | H. pylori infection | – | 66 | 126 | Improvement in clinical symptoms (↓ IL-1β) | [415] |
| Coriander | – | Erythema | – | 40 | 0.5%a | Moderate anti-inflammatory effect | [416] |
| Garlic | – | Obesity | – | 51 | 3600b | Significant anti-inflammatory effect (↓ TNF-α, ↓ IL-6, ↓ LDL) | [417] |
| – | OA | – | 80 | 1000 | Relieved pain (↓ Resistin) | [418] | |
| Ginger | – | RA | – | 70 | 1500 | Improvement in clinical symptoms (↓ gene expression of NFκB, RORγt and T-bet) | [419] |
| – | OA | – | 120 | 1000 | Significant anti-inflammatory effect (↓ IL-1β, ↓ TNF-α) | [420] | |
| – | TB | – | 69 | 3000 | Significant anti-inflammatory and antioxidant effect (↓ TNF-α) | [421] | |
| – | CRC | – | 20 | 2000 | Well-tolerated and safe (↑ LTB4) | [422] | |
| Ginseng | – | CHD | – | 24 | 1.35/kgc | Inhibition of gastrointestinal injury and inflammatory response (↓ IL-6, ↓ LPS, ↓ MDA) | [423] |
| – | RA | – | 84 | –d | Improved anti-inflammatory immunity, and analgesic effect (↓ CRP) | [424] | |
| Saffron | – | Asthma | – | 80 | 100 | Improvement in clinical symptoms, safe to use (↓TG, ↓ LDL cholesterol, ↓ basophil, ↓ eosinophil) | [425] |
| Crocin | MS | – | 40 | 30 | Significant anti-inflammatory and antioxidant effects (↓ TNF-α, ↓ IL-17) | [426] | |
| Sesame seed | – | OA | – | 50 | 40,000 | Significant anti-inflammatory effects, safe to use (↓ IL-6) | [427] |
| Turmeric | CU | MetS | – | 117 | 1000e | Significant anti-inflammatory and antioxidant effect, Safe to use (↓ hs-CRP) | [428] |
| CU | Obesity/hypertension | – | 90 | 900h | Significant anti-inflammatory effect, improvement in general health, safe to use (↓ CRP, ↓ TNF-α, ↓ IL-6, ↓ sVCAM-1) | [429] | |
| Curcumin | T2D | – | 118 | 1000e | Significant anti-inflammatory effects (↓ TNF-α) | [430] | |
| Curcumin | T2D | – | 44 | 1500 | Significant anti-inflammatory effect, reduced complications (↓ hs-CRP, ↓ TG) | [431] | |
| – | KOA | – | 160 | 500 | Significant anti-inflammatory and antioxidant effects, improvement in clinical symptoms (↓ IL-1β) | [432] | |
| – | OA | – | 42 | 1300–1950f | Significant anti-inflammatory effects, relieved pain, well-tolerated | [433] | |
| Curcumin | RA | – | 36 | 500–1000 | Significant anti-inflammatory and analgesic effects, relieved pain, safe to use, well-tolerated (↓ CRP) | [434] | |
| – | CKD | – | 16 | –g | Significant anti-inflammatory effects, safe to use, well-tolerated (↓ IL-6) | [435] | |
| Curcumin | Hemodialysis | – | 71 | 1500 | Significant anti-inflammatory effects, (↓ hs-CRP, ↓ IL-6, ↓ TNF-α) | [436] | |
| CU | SM-induced CPC | – | 89 | 1500j | Significant anti-inflammatory and pulmonoprotective effects, safe to use, well-tolerated (↓ IL-6, ↓ IL-8, ↓ TNF-α, ↓ TGF-β, ↓ hs-CRP,↓ CGRP, ↓ MCP-1) | [437] | |
| Curcumin | COPD | – | 39 | 180k | Significant anti-inflammatory effects, safe to use (↓ AT-LDL) | [438] | |
| Curcumin | Migraine | – | 74 | 80i | Significant neuromodulatory effects, relieved headache, safe to use (↓ cox-2/iNOS) | [439] |
a: Coriander oil; b: Aged Garlic Extract; c: Shen-fu injection (the major component=extract of Panax ginseng); d: Panax notoginseng Saponins; e: Combination with piperine (10 mg/day); f: Combination of H. procumbens, C. longa, and bromelain (AINAT); g: Herbal supplement composed of purified turmeric extract (824 mg), curcuminoids (95%), and Boswellia serrata extract (516 mg), 3-acetyl-11-keto-β-boswellic acid (10%); h: Combination of bisacurone (400 μg), turmeronol A (80 μg) and turmeronol B (20 μg); i: Combination with ω-3 fatty acids (5000 mg/day); j: Curcumin C3 Complex® capsules containing 500 mg curcuminoids plus 5 mg bioperine®; k: Theracurmin® capsules
Abbreviations: AGE: Aged garlic extract, AT-LDL: α1-antitrypsin-low-density lipoprotein, CGRP: Calcitonin gene related peptide; CHD: Congenital heart disease, CKD: Chronic kidney disease, COPD: Chronic obstructive pulmonary disease, cox-2: Cyclooxygenase-2, CP/CPPS: chronic prostatitis/chronic pelvic pain syndrome type III, CPM: Chronic pulmonary complications, CRC: Colorectal cancer, CU: Curcuminoids, CVD: Cardiovascular disease, H. pylori: Helicobacter pylori, hs-CRP: High-sensitivity C-reactive protein, IL: Interleukin, iNOS: Inducible nitric oxide synthase, KOA: Osteoarthritis of knee, LDL: Low density lipoprotein, LPS: Lipopolysaccharide, LTB4: Leukotriene B4, MCP-1: Monocyte chemotactic protein-1, MS: Multiple sclerosis, NAFLD: Non-alcoholic fatty liver disease, OA: Osteoarthritis, Pts: Patients, SM: Sulfur Mustard; RA: Rheumatoid Arthritis, Sirt1: Sirtuin-1, sVCAM-1: soluble vascular cell adhesion molecule-1, TB: Tuberculosis TG: Triglycerides, TGFβ: Transforming growth factor-β, TNF: Tumor necrosis factor, T2D: Type 2 diabetes, WEC: Water extract of C. longa L, ↓: Downregulation/Inhibition, ↑: Upregulation/Activation.
8.1. Cardamom
Clinical studies on cardamom have shown that this spice is effective against metabolic disorders, such as diabetes and non-alcoholic fatty liver disease (NAFLD). Randomized double-blind clinical trials evaluating the effects of cardamom on both pre-diabetic and diabetic subjects have reported significant clinical improvements. A dosage of 3 g cardamom supplement daily for 8 weeks regulated the levels of high-sensitivity C-reactive protein (hs-CRP): IL-6 ratio in pre-diabetic, obese subjects thus showing significant anti-inflammatory and antioxidant effects. Likewise, in the case of type 2 diabetes (T2D) patients, administration of the same dose of cardamom for 10 weeks regulated the serum levels of triglycerides, insulin, sirtuin-1 (SIRT1) besides improving the glycemic indices [412,413]. Moreover, the efficacy of this spice was also evaluated against NAFLD where it was found that treatment with 3 g of supplement for 3 months significantly improved the grade of fatty liver in overweight or obese individuals. It further improved the clinical symptoms of the disease by modulating the levels of glucose indices, lipids, and other biomarkers of the disease. Moreover, the administration of cardamom supplement did not show any side-effects and thus it was found to be safe [414].
8.2. Cinnamon
β-caryophyllene, one of the active compounds of cinnamon, was found to be effective against gastrointestinal disease caused due to Helicobacter pylori (H. pylori) infection. A randomized double-blind placebo-controlled study has reported that administration of 126 mg β-caryophyllene daily for 8 weeks showed significant anti-inflammatory properties by downregulating the level of IL-1β. It further improved conditions of nausea, epigastric pain, and dyspepsia associated with the disease thereby proving that it can stand as a potential therapy against inflammation and gastrointestinal ailments [415].
8.3. Coriander
A study evaluating the potency of essential oil from coriander has evidenced that topical administration of a lipolotion (containing 0.5% coriander oil) during UV exposure reduced the risk of UV-induced erythema to some extent. The treatment was found to show anti-inflammatory activity and was well-tolerated when applied on the skin of healthy volunteers [416].
8.4. Garlic
Clinical trials investigating the potency of garlic extract and its supplements against different diseases have reported that the spice showed significant anti-inflammatory and other beneficial properties. Supplementation of 3.6 g aged garlic extract (AGE) daily was found to diminish the serum levels of TNF-α and IL-6 in obese adults, thus lessening the risk of occurrence of multiple inflammatory chronic diseases associated with obesity [417]. Garlic extract supplementation also relieved pain and other clinical symptoms associated with knee osteoarthritis. Administration of 1 g of the supplement twice daily for 12 weeks significantly reduced the level of resistin, an inflammatory cytokine, thus displaying anti-inflammatory effects [418].
8.5. Ginger
Ginger powder supplementation was found to be effective against both RA and osteoarthritis as evidenced by several clinical studies. In the case of RA patients, on receiving 1.5 g ginger supplements daily for 12 weeks, an improvement in the clinical symptoms were observed. Ginger powder effectively regulated the levels of NF-κB, RORγt, T-box transcription factor TBX21 (T-bet) genes, thus leading to a decline in the severity of the disease. Similarly, the administration of ginger powder to osteoarthritis patients displayed potent anti-inflammatory effects by downregulating the inflammatory cytokines IL-1β and TNF-α [419,420]. A clinical trial of ginger extract on tuberculosis patients has conveyed that administration of 3 g of the supplement daily for 3 months reduced the blood levels of inflammatory cytokine TNF-α and other markers of the disease such as ferritin and MDA, thus displaying anti-inflammatory and antioxidant properties [421].
Furthermore, a pilot clinical study on the subjects at an increased risk of colorectal cancer (CRC) has shown that ginger extract was well tolerated by the subjects and did not show any adverse side effects. It regulated the levels of arachidonic acid (AA) and leukotriene B4 (LTB4) but did not show any change in the levels of eicosanoid [422].
8.6. Ginseng
Ginsenosides, the saponins extracted from ginseng, are found effective against many diseases. A clinical study investigating the potency of Panax notoginseng saponins (PNS) against RA, which displayed that the saponins, when administered for 28 days, led to significant improvement in clinical symptoms of the disease in terms of joint pain, swelling, tenderness, and stiffness. It also managed the dysregulated immune response and displayed significant anti-inflammatory and analgesic effects [424]. Further, the efficiency of ginsenosides therapy (in the form of shen-fu injection) was also evaluated against gastrointestinal mucosal injury which is associated with cardiopulmonary bypass in children suffering from congenital heart disease (CHD). It was found that administration of the intravenous injection before and during the bypass surgery resulted in a reduction of characteristic injury and post-surgery inflammation in patients [423].
8.7. Saffron
The anti-inflammatory property of saffron is evaluated by many studies and it was found to have protective effects against allergies such as asthma. Asthmatic patients, on receiving 100 mg/day saffron capsules for 8 weeks, showed improvement in clinical symptoms of the disease such as frequency of asthmatic attacks, waking up at night due to asthmatic symptoms, and limitation in the activity. The severity of the disease declined, and the supplement was well-tolerated by the patients without any adverse side-effects [425].
Crocin, an active component of saffron, was found to be an effective therapy against multiple sclerosis due to its efficient anti-inflammatory and antioxidant properties. It was found that the administration of two capsules of crocin (15 mg) for 28 days showed an improvement in the antioxidant status of the body. Additionally, a reduction in inflammatory mediators such as TNF-α, and IL-17 in the blood of the patients was also observed [426].
8.8. Sesame seed
Sesame seed therapy was found to improve the mediators of inflammation and oxidation in patients suffering from osteoarthritis of the knee. Consumption of 40 g of sesame seed daily for 2 months resulted in a decrease in the level of IL-6 in the serum of patients, thus proving that it can serve as a potential supplementary therapy in patients with osteoarthritis [427].
8.9. Turmeric
A handful of clinical studies have been performed to examine the potency of turmeric against various diseases. Curcumin and curcuminoids, the active components of turmeric, are found as effective therapies over the years. These are found to be very helpful in managing metabolic syndrome and disorders. For instance, a classic combination of curcuminoid-piperine in the ratio of 100:1 was administered daily for 8 weeks to patients with metabolic syndrome. It was found to regulate the levels of CRP, MDA, and SOD in patients thus imparting significant anti-inflammatory and antioxidant effects [428]. In the case of patients with obesity/hypertension, the hot water extract of C. longa L. (WEC) reduced the levels of CRP, TNF-α, IL-6, and soluble vascular cell adhesion molecule-1 (sVCAM-1), thereby amending chronic low-grade inflammation and overall health of patients [429]. Furthermore, in T2D patients, the uptake of curcuminoid-piperine has shown a decline in the level of TNF-α and an increased level of adiponectin. Furthermore, another study evaluating the effects of curcumin against T2D showed that the compound modulated the levels of hs-CRP and TG thereby imparting significant anti-inflammatory activity [430,431]. The compounds are further found to be effective against osteoarthritis and RA. In the case of osteoarthritis, C. longa L. (CL) extract showed significant anti-inflammatory effects via the downregulation of IL-1β and anti-oxidant effects via the downregulation of MDA and ROS. Thus, the administration of the extract was found to improve clinical symptoms of the disease such as pain and inflammation [432]. Another clinical study showed that the administration of a combination of H. procumbens, C. longa, and bromelain in the form of AINAT capsules showed similar improvements in terms of pain in osteoarthritis patients. Further, the capsules were well-tolerated and thus can be used as a safe and effective replacement for non-steroidal anti-inflammatory drugs (NSAIDs) [433]. Besides, the intake of 0.5–1 g of curcumin formulation daily for 90 days in two different treatment groups has shown significant anti-inflammatory and analgesic effects in patients suffering from RA. The treatment caused improvements in the levels of CRP and rheumatoid factor (RF) values without causing any adverse side-effects [434].
A clinical study evaluating the effects of a combination of turmeric extract and Boswellia serrata as a treatment of patients with chronic kidney disease (CKD) has reported that the supplement is well-tolerated by the patients and is safe to use. The treatment efficiently enhanced the inflammatory status of the patients via the downregulation of the inflammatory cytokine, IL-6 [435]. Moreover, regular intake of curcumin was found to impart anti-inflammatory effects in patients undergoing hemodialysis. The supplement helped in reducing the levels of hs-CRP, IL-6, and TNF-α in plasma without causing any side-effects [436].
Curcuminoids were also found to relieve chronic inflammation in patients suffering from chronic pulmonary complications (CPC) resulting from sulfur mustard (SM) intoxication. Treatment with curcuminoids supplement helped in modulating inflammatory responses by reducing the levels of IL-6, IL-8, TNF-α, TGF-β, hs-CRP, calcitonin gene-related peptide (CGRP), substance P, and MCP-1. The supplements were also well-tolerated and did not cause any adverse side-effects in the patients [437]. Also, in the case of chronic obstructive pulmonary disease (COPD), a curcumin supplement namely Theracurmin(®) was found to significantly amend the inflammatory status and levels of α1-antitrypsin-low-density lipoprotein (AT-LDL) in the blood of patients when taken for 24 weeks. This change might be useful in reducing the risk of any further cardiovascular events in COPD patients [438].
Furthermore, a nano-curcumin formulation in combination with omega-3 fatty acids was found to show neuroprotective effects against migraines. The therapy significantly reduced the levels of cox-2/iNOS which is associated with neuroinflammation and pain of the CNS. Hence, a decline in the rate and duration of repeated painful attacks and the severity of the disease was observed, making this a safe and useful therapy for the treatment and prevention of migraine [439].
9. Curcumin and COVID-19
Curcumin is a multipotential compound from C. longa that possesses diverse medicinal properties. It is a potent anti-inflammatory agent that is effective against numerous chronic diseases [14,[440], [441], [442], [443]].
Elevated cytokine levels or cytokine storm is considered as one of the critical process responsible for multi-organ failure and death in COVID-19 infected patients [[444], [445], [446]]. TNF-α also plays a pivotal role in pulmonary edema caused during COVID-19-associated lung diseases. Natural compounds like curcumin downregulate the levels of TNF-α [447].
Moreover, COVID-19 is also associated with the symptoms of pneumonia that causes severe respiratory distress. The treatment of the pneumonia model with curcumin was shown to decrease lung injury and inflammation through the modulation of HIF and NF-κB [448]. The treatment of curcumin in virus infected models has exhibited inhibition of cytokines, MMPs, and inflammatory cells. It also inhibited the fibrosis and expression of myofibroblasts in the lung tissues [449]. A study demonstrated the potency of a novel combination of vitamin C, curcumin and glycyrrhizic acid (VCG) against SARS-CoV-2 infection. System biology tools also found that VCG modulated various genes associated with immune and inflammatory responses via the regulation of crucial pathways such as NOD-like, Toll-like, PI3K/Akt, NF-κB, and MAPK signaling pathways [450]. TMPRSS2 is a crucial protease involved in the priming of ACE2 receptor-bound viral S protein and thus acts a therapeutic target for COVID-19 therapy [451]. Bromolein, a cysteine protease traditionally used for the treatment of arthritis, possesses potent immunomodulatory properties. Besides, studies have demonstrated that bromolein inhibits the expression of ACE-2 and TMPRSS2 in vitro. It has been hypothesized that the combination of curcumin and bromolein might exert a beneficial synergistic effect against SARS-CoV-2 infection [452,453]. Another study reported the potential immunomodulatory and antiviral efficacy of the combination of black pepper and curcumin extract along with multiactive ingredients such as pentatricontane, sitosterol, termerone, lupeol, amyrines, and vitamin D3 (EGYVIR). The administration of EGYVIR in SARS-CoV-2 infected cells decreased the levels of inflammatory mediators, such as TNF-α and IL-6, which might be effective in attenuating the virus-induced cytokine storm [454]. The combination of curcumin and zinc has also been hypothesized as an effective therapeutic strategy against COVID-19 [455,456].
A randomized clinical trial has been initiated to investigate the potency of the co-administration of curcumin and piperine on COVID-19 patients [457]. Another randomized clinical trial has been initiated to determine the efficacy of nano micelles containing curcumin on the levels of various serum cytokines, such as IFN-γ, IL-17, IL-4, and TGF-β in COVID-19 patients [458]. Nanonutraceuticals of vitamins, antioxidants, probiotics, etc. can modulate immune responses and therefore effectively strengthen the immune system of COVID-19 patients [459]. Nano-curcumin also significantly reduced the expression of serum IL-6 and IL-1β in COVID-19 patients [460]. Furthermore, nano-formulation of curcumin is also associated with faster recovery of COVID-19 patients [461]. It also exerted immunomodulatory effects and also decreased the levels of Th17 cell-related cytokines in the patients [462]. Therefore, curcumin might be useful as a traditional medicine to treat COVID-19-associated complications such as cytokine storm, ALI, ARDS, and pneumonia [463,464].
Apart from the anti-inflammatory effects, several studies have also reported that curcumin exhibits antiviral effects [[465], [466], [467]]. Curcumin was reported to inhibit the replication of the SARS-CoV in vitro. Therefore, the potency of curcumin as an antiviral agent might also be effective against the SARS-CoV-2 [465]. Numerous molecular docking studies have shown the efficacy of curcumin to target various key components of the novel SARS-CoV-2 in silico. A study reported that curcumin showed high-affinity binding towards the viral spike glycoprotein and ACE2 receptor [185]. Another study demonstrated its binding affinity towards the viral Nsp15 protein which is associated with replication. Therefore, curcumin might cause the inhibition of the viral replication by binding with Nsp15 [182]. Curcumin has also been reported as a potential inhibitor of Mpro protein of SARS-CoV-2 [[468], [469], [470], [471], [472]]. Moreover, it also inhibits the human cellular transmembrane serine proteinase [473]. Furthermore, curcumin and catechin also bind to the RBD of the viral S protein and inhibit the entry of SARS-CoV-2 in the host cell [474]. Multi-omics analyses have also demonstrated the potential of compounds such as curcumin, resveratrol, etc., as effective agents to inhibit SARS-CoV-2 viral infection [475].
Thus, several studies have hypothesized that the immunomodulatory and antiviral activities of curcumin might be beneficial in the treatment of SARS-CoV-2 and COVID-19 associated diseases [182,440,470,[476], [477], [478], [479], [480], [481]]. Curcumin possesses a huge potential as a drug against SARS-CoV-2 as well as COVID-19-associated diseases. Therefore, it should be further evaluated in the pre-clinical and clinical studies to determine its efficacy [479].
10. Discussion and conclusion
The COVID-19 pandemic has posed a great threat to healthcare across the globe. The causative pathogen is a novel coronavirus and there are currently no specific treatment strategies against it. Although several studies are going on to develop specific drugs targeted towards SARS-CoV-2, it might take some more time. Several vaccine development trials are also underway, but the end products will require some time to clear the safety studies. Moreover, a new variant of the SARS-CoV-2 had been reported for the first time on 14th December 2020 in the United Kingdom (UK). This variant of concern (VOC) had been labeled as the VOC 202012/01 and had been detected in over 50 countries in the UK. Other variants such as 501Y.V2 and B.1.1.28 were also reported from South Africa (18th December 2020) and Japan (9th January 2021), respectively. Therefore, considering the rapid evolution of this virus and urgency of the situation, one of the best options for management and treatment is repurposed drugs. These drugs are already approved for the treatment of some diseases in humans and are thus readily available in the market. In the paucity of SARS-CoV-2 specific drugs, these repurposed drugs with antiviral or inhibitory effects are prescribed to hospitalized patients [4,97]. Cytokine storm or hyper inflammation is commonly observed in severe COVID-19 patients. It is associated with the progression of the disease and poor clinical outcome [482]. Respiratory failure, ARDS, and pneumonia are some of the most common and fatal COVID-19 associated complications. Therefore, some repurposed drugs are known to exhibit potent anti-inflammatory effects and are thus used to combat COVID-19-associated inflammatory complications [76,97].
Some of the repurposed drugs show very promising results. However, most of them raise serious concerns as they are reported to have adverse side-effects [97]. Therefore, it is necessary to look for alternative medicines that may be effective against the novel virus and associated health complications which cause little or no side-effects.
Mother Nature has provided us with several natural compounds that have been used throughout the ages for the treatment of various diseases like cancer, asthma, diabetes, respiratory and cardiovascular disorder, etc. Spices are Nature's most potent anti-inflammatory and antioxidant agents. Besides enhancing the taste, flavor, aroma, and color of food and beverages but also imparts protection against various health ailments. These therapeutic properties of spices and culinary herbs are due to the presence of various bioactive components with significant biological activities. Besides, the consumption of spices has been proven to reduce inflammation and boost our immune response [14]. Therefore, in the absence of a drug or vaccine against SARS-CoV-2, spices might serve as an alternative treatment for infected patients. It can serve a dual purpose of both as a means of primary prevention, as well as to help mitigate the exaggerated immune response and cytokine storm. However, pre-clinical and clinical studies should be conducted to validate its efficacy in COVID-19 patients1 .
Credit authorship contribution statement
BBA and ABK contributed to the conceptualization of the study design. VR, DP, SG, HS, KB and KKT performed the bibliographic search and contributed to the writing of the manuscript. VR, DP, SG, and HS contributed to the tables. VR and DP contributed to the reference editing. KB contributed to the figures. BBA, ABK, UD, PG, and SCG contributed in the reviewing and proofreading of the manuscript.
Declaration of competing interest
The authors declare no conflict of interests related to this study.
Acknowledgements
This work was supported by the DBT-AIST International CENter for Translational and Environmental Research (DAICENTER) grant awarded to Professor Ajaikumar B. Kunnumakkara. The author Kishore Banik acknowledges the UGC New Delhi, India for providing him the fellowship.
Footnotes
NOTE: While this manuscript was in progress at least seven different vaccines (Pfizer-BioNTech, Moderna, AstraZeneca, Janssen, Novavax, and Sputnik Light from Russia, Covaxin from India) have been developed (https://absolutelymaybe.plos.org/2021/01/31/variants-3-new-covid-vaccines-and-contested-efficacy-claims-a-month-of-seismic-shifts) [483]. In addition, there are several drugs in the pipeline (https://www.goodrx.com/blog/coronavirus-treatments-on-the-way/) [484].
References
- 1.Du Toit A. Outbreak of a novel coronavirus. Nat. Rev. Microbiol. 2020;18:123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Tropical Med. Int. Health. 2020;25:278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO COVID-19 Weekly epidemiological update. https://www.who.int/publications/m/item/weekly-epidemiological-update---12-january-2021 (2021, accessed 15 January 2021) [DOI] [PMC free article] [PubMed]
- 5.European Centre for Disease and Prevention Control COVID-19 situation update worldwide, as of week 1 2021. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (2021, accessed 19 January 2021)
- 6.Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9:231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhal T.A. Review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., Liu X.Q., Chen R.C., Tang C.L., Wang T., Ou C.Q., Li L., Chen P.Y., Sang L., Wang W., Li J.F., Li C.C., Ou L.M., Cheng B., Xiong S., Ni Z.Y., Xiang J., Hu Y., Liu L., Shan H., Lei C.L., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Cheng L.L., Ye F., Li S.Y., Zheng J.P., Zhang N.F., Zhong N.S., He J.X., China Medical Treatment Expert Group for COVID-19 Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J. Inf. Secur. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand P., Sundaram C., Jhurani S., Kunnumakkara A.B., Aggarwal B.B. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Khwairakpam A.D., Bordoloi D., Thakur K.K., Monisha J., Arfuso F., Sethi G., Mishra S., Kumar A.P., Kunnumakkara A.B. Possible use of Punica granatum (pomegranate) in cancer therapy. Pharmacol. Res. 2018;133:53–64. doi: 10.1016/j.phrs.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Kunnumakkara A.B., Sailo B.L., Banik K., Harsha C., Prasad S., Gupta S.C., Bharti A.C., Aggarwal B.B. Chronic diseases, inflammation, and spices: how are they linked? J. Transl. Med. 2018;16(14) doi: 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsayed Y., Khan N.A. Immunity-boosting spices and the novel coronavirus. ACS Chem. Neurosci. 2020;11:1696–1698. doi: 10.1021/acschemneuro.0c00239. [DOI] [PubMed] [Google Scholar]
- 16.COVID19 INDIA https://www.covid19india.org/ (2021, accessed 20 January 2021)
- 17.S J., Sreedharan S. Analysing the Covid-19 cases in Kerala: a visual exploratory data analysis approach. SN Compr. Clin. Med. 2020:1–12. doi: 10.1007/s42399-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv J., Qi L., Yu C., Yang L., Guo Y., Chen Y., Bian Z., Sun D., Du J., Ge P., Tang Z., Hou W., Li Y., Chen J., Chen Z., Li L., China Kadoorie Biobank Collaborative Group Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. 2015;351:h3942. doi: 10.1136/bmj.h3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Schubert J., Bania J., Khosrawipour T. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J. Travel. Med. 2020;27 doi: 10.1093/jtm/taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paital B., Das K., Parida S.K. Inter nation social lockdown versus medical care against COVID-19, a mild environmental insight with special reference to India. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Lancet India under COVID-19 lockdown. Lancet. 2020;395:1315. doi: 10.1016/S0140-6736(20)30938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowers K.W. Balancing individual and communal needs: plague and public health in early modern Seville. Bull. Hist. Med. 2007;81:335–358. doi: 10.1353/bhm.2007.0020. [DOI] [PubMed] [Google Scholar]
- 24.Peak C.M., Wesolowski A., Zu Erbach-Schoenberg E., Tatem A.J., Wetter E., Lu X., Power D., Weidman-Grunewald E., Ramos S., Moritz S., Buckee C.O., Bengtsson L. Population mobility reductions associated with travel restrictions during the Ebola epidemic in Sierra Leone: use of mobile phone data. Int. J. Epidemiol. 2018;47:1562–1570. doi: 10.1093/ije/dyy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camitz M., Liljeros F. The effect of travel restrictions on the spread of a moderately contagious disease. BMC Med. 2006;4(32) doi: 10.1186/1741-7015-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosal S., Bhattacharyya R., Majumder M. Impact of complete lockdown on total infection and death rates: a hierarchical cluster analysis. Diabetes Metab. Syndr. 2020;14:707–711. doi: 10.1016/j.dsx.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindeman R.J., Sund M., Löfgren J., Basso T., Søreide K. Preventing spread of SARS-CoV-2 and preparing for the COVID-19 outbreak in the surgical department: perspectives from two Scandinavian countries. J. Surg. Case Rep. 2020;2020 doi: 10.1093/jscr/rjaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kicska G., Litmanovich D.E., Ordovas K.G., Young P.M., Dennie C., Truong Q.A., Abbara S., Kirsch J. Statement from the North American Society for Cardiovascular Imaging on imaging strategies to reduce the scarcity of healthcare resources during the COVID-19 outbreak. Int. J. Cardiovasc. Imaging. 2020;36:1387–1393. doi: 10.1007/s10554-020-01861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Möhn N., Pul R., Kleinschnitz C., Prüss H., Witte T., Stangel M., Skripuletz T. Implications of COVID-19 outbreak on immune therapies in multiple sclerosis patients-lessons learned from SARS and MERS. Front. Immunol. 2020;11:1059. doi: 10.3389/fimmu.2020.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P.K.S.M., Cho S.G., Kumar N.S., Subramaniam M.D. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietrobelli A., Pecoraro L., Ferruzzi A., Heo M., Faith M., Zoller T., Antoniazzi F., Piacentini G., Fearnbach S.N., Heymsfield S.B. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity (Silver Spring) 2020;28:1382–1385. doi: 10.1002/oby.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reis R.F., de Melo Quintela B., de Oliveira Campos J., Gomes J.M., Rocha B.M., Lobosco M., Weber Dos Santos R. Characterization of the COVID-19 pandemic and the impact of uncertainties, mitigation strategies, and underreporting of cases in South Korea, Italy, and Brazil. Chaos Solitons Fractals. 2020;136 doi: 10.1016/j.chaos.2020.109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb L. COVID-19 lockdown: a perfect storm for older people's mental health. J. Psychiatr. Ment. Health Nurs. 2020 doi: 10.1111/jpm.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn J.S., McIntosh K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005;24:223–227. doi: 10.1097/01.inf.0000188166.17324.60. [DOI] [PubMed] [Google Scholar]
- 36.Kendall E.J., Bynoe M.L., Tyrrell D.A. Virus isolations from common colds occurring in a residential school. Br. Med. J. 1962;2:82–86. doi: 10.1136/bmj.2.5297.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyrrell D.A., Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. Br. Med. J. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyrrell D.A., Bynoe M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet. 1966;1:76–77. doi: 10.1016/s0140-6736(66)92364-6. [DOI] [PubMed] [Google Scholar]
- 39.Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 40.Almeida J.D., Tyrrell D.A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J. Gen. Virol. 1967;1:175–178. doi: 10.1099/0022-1317-1-2-175. [DOI] [PubMed] [Google Scholar]
- 41.Almeida J.D., Berry D.M., Cunningham C.H., Hamre D., Hofstad M.S., Mallucci L., McIntosh K., Tyrrell D.A.J. Coronaviruses. Nature. 1968;220:650. [Google Scholar]
- 42.Tyrrell D.A., Almeida J.D., Cunningham C.H., Dowdle W.R., Hofstad M.S., McIntosh K., Tajima M., Zakstelskaya L.Y., Easterday B.C., Kapikian A., Bingham R.W. Coronaviridae. Intervirology. 1975;5(76) doi: 10.1159/000149883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Hoek L. Human coronaviruses: what do they cause? Antivir. Ther. 2007;12:651–658. [PubMed] [Google Scholar]
- 44.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludwig S., Zarbock A. Coronaviruses and SARS-CoV-2: a brief overview. Anesth. Analg. 2020;131:93–96. doi: 10.1213/ANE.0000000000004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corman V.M., Lienau J., Witzenrath M. Coronaviren als Ursache respiratorischer Infektionen [Coronaviruses as the cause of respiratory infections] Internist (Berl) 2019;60:1136–1145. doi: 10.1007/s00108-019-00671-5. (German) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 48.Hui D.S., Wong P.C., Wang C. SARS: clinical features and diagnosis. Respirology. 2003;8:20–24. doi: 10.1046/j.1440-1843.2003.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Báez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 51.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(93) doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Killerby M.E., Biggs H.M., Midgley C.M., Gerber S.I., Watson J.T. Middle East respiratory syndrome coronavirus transmission. Emerg. Infect. Dis. 2020;26:191–198. doi: 10.3201/eid2602.190697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta Mol. basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashour H.M., Elkhatib W.F., Rahman M.M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Astuti I., Ysrafil Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab. Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(8) doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020 doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. (e8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang W., He J., Wu S. The definition and risks of cytokine release syndrome-like in 11 COVID-19-infected pneumonia critically ill patients: disease characteristics and retrospective analysis. Medrxiv. 2020 doi: 10.1093/infdis/jiaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., Liu W., Zhu Y., Lin Q., Mao L., Fang M., Zhang H., Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S., Jiang L., Li X., Lin F., Wang Y., Li B., Jiang T., An W., Liu S., Liu H., Xu P., Zhao L., Zhang L., Mu J., Wang H., Kang J., Li Y., Huang L., Zhu C., Zhao S., Lu J., Ji J., Zhao J. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Aggarwal B.B. Inflammation, a silent killer in cancer is not so silent. Curr. Opin. Pharmacol. 2009;9:347–350. doi: 10.1016/j.coph.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 82.Kawabata K., Tung N.H., Shoyama Y., Sugie S., Mori T., Tanaka T. Dietary crocin inhibits colitis and colitis-associated colorectal carcinogenesis in male ICR mice. Evid. Based Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/820415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aggarwal B.B., Kunnumakkara A.B., Harikumar K.B., Gupta S.R., Tharakan S.T., Koca C., Dey S., Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann. N. Y. Acad. Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta S.C., Kunnumakkara A.B., Aggarwal S., Aggarwal B.B. Inflammation, a double-edge sword for cancer and other age-related diseases. Front. Immunol. 2018;9:2160. doi: 10.3389/fimmu.2018.02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yadav V.R., Prasad S., Sung B., Kannappan R., Aggarwal B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins (Basel) 2010;2:2428–2466. doi: 10.3390/toxins2102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monisha J., Padmavathi G., Roy N.K., Deka A., Bordoloi D., Anip A., Kunnumakkara A.B. NF-κB blockers gifted by mother nature: prospectives in cancer cell chemosensitization. Curr. Pharm. Des. 2016;22:4173–4200. doi: 10.2174/1381612822666160609110231. [DOI] [PubMed] [Google Scholar]
- 87.Monisha J., Roy N.K., Bordoloi D., Kumar A., Golla R., Kotoky J., Padmavathi G., Kunnumakkara A.B. Nuclear factor kappa B: a potential target to persecute head and neck cancer. Curr. Drug Targets. 2017;18:232–253. doi: 10.2174/1389450117666160201112330. [DOI] [PubMed] [Google Scholar]
- 88.Kunnumakkara A.B., Shabnam B., Girisa S., Harsha C., Banik K., Devi T.B., Choudhury R., Sahu H., Parama D., Sailo B.L., Thakur K.K., Gupta S.C., Aggarwal B.B. Inflammation, NF-κB, and chronic diseases: how are they linked? Crit. Rev. Immunol. 2020;40:1–39. doi: 10.1615/CritRevImmunol.2020033210. [DOI] [PubMed] [Google Scholar]
- 89.Kunnumakkara A.B., Anand P., Aggarwal B.B. Drug Resistance in Cancer Cells. Springer; New York, NY: 2009. Nuclear factor-κB and chemoresistance: how intertwined are they? pp. 177–208. [Google Scholar]
- 90.Kunnumakkara A.B., Thakur K.K., Rana V., Bora B., Banik K., Khatoon E., Sailo B.L., Shabnam B., Girisa S., Gupta S.C., Aggarwal B.B. Upside and downside of tumor necrosis factor blockers for treatment of immune/inflammatory diseases. Crit. Rev. Immunol. 2019;39:439–479. doi: 10.1615/CritRevImmunol.2020033205. [DOI] [PubMed] [Google Scholar]
- 91.Sethi G., Sung B., Kunnumakkara A.B., Aggarwal B.B. Therapeutic Targets of the TNF Superfamily. Springer; New York: 2009. Targeting TNF for treatment of cancer and autoimmunity; pp. 37–51. [DOI] [PubMed] [Google Scholar]
- 92.CISION PR Newswire Global Tumor Necrosis Factor (TNF) Inhibitors Market 2018–2026: A $181.13 Billion Market Opportunity by 2026. https://www.prnewswire.com/news-releases/global-tumor-necrosis-factor-tnf-inhibitors-market-2018-2026-a-181-13-billion-market-opportunity-by-2026--300675249.html (2018, accessed 11 August 2020)
- 93.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pandurangan A.K., Ismail S., Saadatdoust Z., Esa N.M. Allicin alleviates dextran sodium sulfate- (DSS-) induced ulcerative colitis in BALB/c mice. Oxidative Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/605208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sung B., Prasad S., Yadav V.R., Aggarwal B.B. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer. 2012;64:173–197. doi: 10.1080/01635581.2012.630551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang Z., Xu Y., Wen X., Nie H., Hu T., Yang X., Chu X., Yang J., Deng X., He J. Rosmarinic acid attenuates airway inflammation and hyperresponsiveness in a murine model of asthma. Molecules. 2016;21:769. doi: 10.3390/molecules21060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu R., Wang L., Kuo H.D., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., Poiani G.J., Amorosa L., Brunetti L., Kong A.N. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilkinson T., Dixon R., Page C., Carroll M., Griffiths G., Ho L.P., De Soyza A., Felton T., Lewis K.E., Phekoo K., Chalmers J.D., Gordon A., McGarvey L., Doherty J., Read R.C., Shankar-Hari M., Martinez-Alier N., O'Kelly M., Duncan G., Walles R., Sykes J., Summers C., Singh D., ACCORD Collaborators ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:691. doi: 10.1186/s13063-020-04584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiao Z., Zhang H., Ji H.F., Chen Q. Computational view toward the inhibition of SARS-CoV-2 spike glycoprotein and the 3CL protease. Computation (Basel) 2020;8:53. doi: 10.3390/computation8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., Sacco E., Naccache J.M., Bézie Y., Laplanche S., Le Berre A., Le Pavec J., Salmeron S., Emmerich J., Mourad J.J., Chatellier G., Hayem G. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez-Nava G., Trelles-Garcia D.P., Yanez-Bello M.A., Chung C.W., Trelles-Garcia V.P., Friedman H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit. Care. 2020;24:429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cantini F., Niccoli L., Nannini C., Matarrese D., Natale M.E.D., Lotti P., Aquilini D., Landini G., Cimolato B., Pietro M.A.D., Trezzi M., Stobbione P., Frausini G., Navarra A., Nicastri E., Sotgiu G., Goletti D. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Inf. Secur. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maggio R., Corsini G.U. Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffmann M., Hofmann-Winkler H., Smith J.C., Krüger N., Sørensen L.K., Søgaard O.S., Hasselstrøm J.B., Winkler M., Hempel T., Raich L., Olsson S., Yamazoe T., Yamatsuta K., Mizuno H., Ludwig S., Noé F., Sheltzer J.M., Kjolby M., Pöhlmann S. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. bioRxiv. 2020 doi: 10.1016/j.ebiom.2021.103255. (2020.08.05.237651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iwabuchi K., Yoshie K., Kurakami Y., Takahashi K., Kato Y., Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J. Infect. Chemother. 2020;26:625–632. doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karampela I., Dalamaga M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch. Med. Res. 2020;51:741–742. doi: 10.1016/j.arcmed.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Millán-Oñate J., Millan W., Mendoza L.A., Sánchez C.G., Fernandez-Suarez H., Bonilla-Aldana D.K., Rodríguez-Morales A.J. Successful recovery of COVID-19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin. Ann. Clin. Microbiol. Antimicrob. 2020;19(16) doi: 10.1186/s12941-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sacramento C.Q., Fintelman-Rodrigues N., Temerozo J.R., Dias S.D., Ferreira A.C., Mattos M., Pão C.R., de Freitas C.S., Soares V.C., Bozza F.A., Bou-Habib D.C. The in vitro antiviral activity of the anti-hepatitis C virus (HCV) drugs daclatasvir and sofosbuvir against SARS-CoV-2. bioRxiv. 2020 doi: 10.1093/jac/dkab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M., Woodfall B., Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Selvaraj V., Dapaah-Afriyie K., Finn A., Flanigan T.P. Short-term dexamethasone in Sars-CoV-2 patients. R. I. Med. J. 2020;103:39–43. [PubMed] [Google Scholar]
- 114.Lobo-Galo N., Terrazas-López M., Martínez-Martínez A., Díaz-Sánchez Á.G. FDA-approved thiol-reacting drugs that potentially bind into the SARS-CoV-2 main protease, essential for viral replication. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1764393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Malek A.E., Granwehr B.P., Kontoyiannis D.P. Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing) 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernal-Bello D., Jaenes-Barrios B., Morales-Ortega A., Ruiz-Giardin J.M., García-Bermúdez V., Frutos-Pérez B., Farfán-Sedano A.I., de Ancos-Aracil C., Bermejo F., García-Gil M., Zapatero-Gaviria A., San Martín-López J.V. Imatinib might constitute a treatment option for lung involvement in COVID-19. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amici C., Di Caro A., Ciucci A., Chiappa L., Castilletti C., Martella V., Decaro N., Buonavoglia C., Capobianchi M.R., Santoro M.G. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. 2006;11:1021–1030. [PubMed] [Google Scholar]
- 121.Sainz B., Jr., Mossel E.C., Peters C.J., Garry R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) Virology. 2004;329:11–17. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang R., Ng T.B., Sun W.Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scheen A.J. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46:423–426. doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res. Clin. Pract. 2020;164 doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J., Zheng X., Huang Y., Shan H., Huang J. Successful use of methylprednisolone for treating severe COVID-19. J. Allergy Clin. Immunol. 2020;146:325–327. doi: 10.1016/j.jaci.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K., Matsuda Z., Kawaguchi Y., Kawaoka Y., Inoue J.I. The anticoagulant Nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu J., Shi P.Y., Li H., Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect. Dis. 2020;6:909–915. doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siddiqui A.J., Jahan S., Ashraf S.A., Alreshidi M., Ashraf M.S., Patel M., Snoussi M., Singh R., Adnan M. Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1802345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seifirad S. Pirfenidone: a novel hypothetical treatment for COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anderson D.E., Sivalingam V., Kang A.E.Z., Ananthanarayanan A., Arumugam H., Jenkins T.M., Hadjiat Y., Eggers M. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 2020;9:669–675. doi: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Acanfora D., Ciccone M.M., Scicchitano P., Acanfora C., Casucci G. Neprilysin inhibitor-angiotensin II receptor blocker combination (sacubitril/valsartan): rationale for adoption in SARS-CoV-2 patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020;6:135–136. doi: 10.1093/ehjcvp/pvaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Palanques-Pastor T., López-Briz E., Poveda Andrés J.L. Involvement of interleukin 6 in SARS-CoV-2 infection: siltuximab as a therapeutic option against COVID-19. Eur. J. Hosp. Pharm. 2020;27:297–298. doi: 10.1136/ejhpharm-2020-002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carbajo-Lozoya J., Müller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y., Pan Y., Hu Z., Wu M., Wang C., Feng Z., Mao C., Tan Y., Liu Y., Chen L., Li M., Wang G., Yuan Z., Diao B., Wu Y., Chen Y. Thymosin alpha 1 reduces the mortality of severe coronavirus disease 2019 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin. Infect. Dis. 2020;71:2150–2157. doi: 10.1093/cid/ciaa630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liang J., Pitsillou E., Karagiannis C., Darmawan K.K., Ng K., Hung A., Karagiannis T.C. Interaction of the prototypical α-ketoamide inhibitor with the SARS-CoV-2 main protease active site in silico: molecular dynamic simulations highlight the stability of the ligand-protein complex. Comput. Biol. Chem. 2020;87 doi: 10.1016/j.compbiolchem.2020.107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Chakraborty C. Probable molecular mechanism of Remdesivir for the treatment of COVID-19: need to know more. Arch. Med. Res. 2020;51:585–586. doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang X., Cao R., Zhang H., Liu J., Xu M., Hu H., Li Y., Zhao L., Li W., Sun X., Yang X., Shi Z., Deng F., Hu Z., Zhong W., Wang M. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6(28) doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020;26:917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Romanelli F., Smith K.M., Hoven A.D. Chloroquine and hydroxychloroquine as inhibitors of human immunodeficiency virus (HIV-1) activity. Curr. Pharm. Des. 2004;10:2643–2648. doi: 10.2174/1381612043383791. [DOI] [PubMed] [Google Scholar]
- 147.Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtz N., Rolain J.M., Colson P., La Scola B., Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Million M., Lagier J.C., Gautret P., Colson P., Fournier P.E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., Aubry C., Correard F., Giraud-Gatineau A., Roussel Y., Berenger C., Cassir N., Seng P., Zandotti C., Dhiver C., Ravaux I., Tomei C., Eldin C., Tissot-Dupont H., Honoré S., Stein A., Jacquier A., Deharo J.C., Chabrière E., Levasseur A., Fenollar F., Rolain J.M., Obadia Y., Brouqui P., Drancourt M., La Scola B., Parola P., Raoult D. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parra-Lara L.G., Martínez-Arboleda J.J., Rosso F. Azithromycin and SARS-CoV-2 infection: where we are now and where we are going. J. Glob. Antimicrob. Resis. 2020;22:680–684. doi: 10.1016/j.jgar.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.WHO WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 (2020, accessed 15 August, 2020)
- 153.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P., Huang X., Peiris M., Yen H.L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O., Mackman R.L., Hui H.C., Perron M., Ray A.S., Cihlar T., Nichol S.T., Spiropoulou C.F. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017;7 doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., van Doremalen N., Leighton I., Yinda C.K., Pérez-Pérez L., Okumura A., Lovaglio J., Hanley P.W., Saturday G., Bosio C.M., Anzick S., Barbian K., Cihlar T., Martens C., Scott D.P., Munster V.J., de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585:273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of Remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu J., Zhang S., Wu Z., Shang Y., Dong X., Li G., Zhang L., Chen Y., Ye X., Du H., Liu Y., Wang T., Huang S., Chen L., Wen Z., Qu J., Chen D. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann. Intensive Care. 2020;10(99) doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P., Cossi S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., Zhou N., Petty L.A., Baang J.H., Dillman N.O., Frame D., Gregg K.S., Kaul D.R., Nagel J., Patel T.S., Zhou S., Lauring A.S., Hanauer D.A., Martin E., Sharma P., Fung C.M., Pogue J.M. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gremese E., Cingolani A., Bosello S.L., Alivernini S., Tolusso B., Perniola S., Landi F., Pompili M., Murri R., Santoliquido A., Garcovich M., Sali M., De Pascale G., Gabrielli M., Biscetti F., Montalto M., Tosoni A., Gambassi G., Rapaccini G.L., Iaconelli A., Zileri Del Verme L., Petricca L., Fedele A.L., Lizzio M.M., Tamburrini E., Natalello G., Gigante L., Bruno D., Verardi L., Taddei E., Calabrese A., Lombardi F., Bernabei R., Cauda R., Franceschi F., Landolfi R., Richeldi L., Sanguinetti M., Fantoni M., Antonelli M., Gasbarrini A., GEMELLI AGAINST COVID-19 Group Sarilumab use in severe SARS-CoV-2 pneumonia. EClinicalMedicine. 2020;27:100553. doi: 10.1016/j.eclinm.2020.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dinarello C.A., Simon A., van der Meer J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cingolani A., Tummolo A.M., Montemurro G., Gremese E., Larosa L., Cipriani M.C., Pasciuto G., Liperoti R., Murri R., Pirronti T., Cauda R., Fantoni M., for COVID 2 Columbus Working Group Baricitinib as rescue therapy in a patient with COVID-19 with no complete response to sarilumab. Infection. 2020;48:767–771. doi: 10.1007/s15010-020-01476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li C.C., Wang X.J., Wang H.R. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov. Today. 2019;24:726–736. doi: 10.1016/j.drudis.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Publica. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh A.K., Singh R. Is metformin ahead in the race as a repurposed host-directed therapy for patients with diabetes and COVID-19? Diabetes Res. Clin. Pract. 2020;165 doi: 10.1016/j.diabres.2020.108268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., Liu C., Xiong M., Deng A., Zhang Y., Zheng L., Huang K. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 170.Bramante C., Ingraham N., Murray T., Marmor S., Hoversten S., Gronski J., McNeil C., Feng R., Guzman G., Abdelwahab N., King S., Meehan T., Benson B., Pendleton K., Vojta D., Tignanelli C.J. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with Covid-19. medRxiv. 2020 doi: 10.1016/S2666-7568(20)30033-7. (2020.06.19.20135095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luo P., Qiu L., Liu Y., Liu X.L., Zheng J.L., Xue H.Y., Liu W.H., Liu D., Li J. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Squillace N., Bozzi G., Colella E., Gori A., Bandera A. Darunavir-cobicistat-emtricitabine-tenofovir alafenamide: safety and efficacy of a protease inhibitor in the modern era. Drug Des. Devel. Ther. 2018;12:3635–3643. doi: 10.2147/DDDT.S147493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen J., Xia L., Liu L., Xu Q., Ling Y., Huang D., Huang W., Song S., Xu S., Shen Y., Lu H. Antiviral activity and safety of Darunavir/Cobicistat for the treatment of COVID-19. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu T., Gao X., Wu Z., Selinger D.W., Zhou Z. Indomethacin has a potent antiviral activity against SARS CoV-2 in vitro and canine coronavirus in vivo. bioRxiv. 2020 [Google Scholar]
- 175.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., Shimojima M., Fukushi S. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J. Virol. 2020 doi: 10.1128/JVI.01648-20. (JVI.01648-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Coleman C.M., Sisk J.M., Mingo R.M., Nelson E.A., White J.M., Frieman M.B. Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus fusion. J. Virol. 2016;90:8924–8933. doi: 10.1128/JVI.01429-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li C., Han R., Kang L., Wang J., Gao Y., Li Y., He J., Tian J. Pirfenidone controls the feedback loop of the AT1R/p38 MAPK/renin-angiotensin system axis by regulating liver X receptor-α in myocardial infarction-induced cardiac fibrosis. Sci. Rep. 2017;7 doi: 10.1038/srep40523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., Traynor D., Johnson R.F., Dyall J., Kuhn J.H., Olinger G.G., Hensley L.E., Jahrling P.B. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.ClinicalTrials.gov Sirolimus treatment in hospitalized patients with covid-19 pneumonia. https://clinicaltrials.gov/ct2/show/NCT04341675 (2020, accessed 11 November 2020)
- 181.Dargad R.R., Prajapati M.R., Dargad R.R., Parekh J.D. Sacubitril/valsartan: a novel angiotensin receptor-neprilysin inhibitor. Indian Heart J. 2018;70:102–110. doi: 10.1016/j.ihj.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kumar S., Kashyap P., Chowdhury S., Kumar S., Panwar A., Kumar A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang J., Zhang X., Omarini A.B., Li B. Virtual screening for functional foods against the main protease of SARS-CoV-2. J. Food Biochem. 2020 doi: 10.1111/jfbc.13481. [DOI] [PubMed] [Google Scholar]
- 184.Oso B.J., Adeoye A.O., Olaoye I.F. Pharmacoinformatics and hypothetical studies on allicin, curcumin, and gingerol as potential candidates against COVID-19-associated proteases. J. Biomol. Struct. Dyn. 2020:1–12. doi: 10.1080/07391102.2020.1813630. [DOI] [PubMed] [Google Scholar]
- 185.Maurya V.K., Kumar S., Prasad A.K., Bhatt M.L.B., Saxena S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease. 2020;31:179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kunnumakkara A.B., Koca C., Dey S., Gehlot P., Yodkeeree S., Danda D., Sung B., Aggarwal B.B. Traditional uses of spices: an overview. Molecular targets and therapeutic uses of spices: modern uses for ancient medicine. World Sci. 2009:1–24. [Google Scholar]
- 187.Monisha J., Padmavathi G., Bakliwal V., Katre N., Padikkala J., Kunnumakkara A.B. Cancer preventive and therapeutic properties of fruits and vegetables against commonly occurring cancers in humans. Anticancer properties of fruits and vegetables: a scientific review. World Sci. 2015:337–366. [Google Scholar]
- 188.Thomas D., Govindhan S., Baiju E.C., Padmavathi G., Kunnumakkara A.B., Padikkala J. Cyperus rotundus L. prevents non-steroidal anti-inflammatory drug-induced gastric mucosal damage by inhibiting oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2015;26:485–490. doi: 10.1515/jbcpp-2014-0093. [DOI] [PubMed] [Google Scholar]
- 189.Harsha C., Banik K., Bordoloi D., Kunnumakkara A.B. Antiulcer properties of fruits and vegetables: a mechanism based perspective. Food Chem. Toxicol. 2017;108:104–119. doi: 10.1016/j.fct.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 190.Gupta S.C., Prasad S., Tyagi A.K., Kunnumakkara A.B., Aggarwal B.B. Neem (Azadirachta indica): an indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20. doi: 10.1016/j.phymed.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 191.Khwairakpam A.D., Damayenti Y.D., Deka A., Monisha J., Roy N.K., Padmavathi G., Kunnumakkara A.B. Acorus calamus: a bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 2018;29:107–122. doi: 10.1515/jbcpp-2016-0132. [DOI] [PubMed] [Google Scholar]
- 192.Kunnumakkara A.B., Banik K., Bordoloi D., Harsha C., Sailo B.L., Padmavathi G., Roy N.K., Gupta S.C., Aggarwal B.B. Googling the guggul (commiphora and boswellia) for prevention of chronic diseases. Front. Pharmacol. 2018;9:686. doi: 10.3389/fphar.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Devi Khwairakpam A., Monisha J., Roy N.K., Bordoloi D., Padmavathi G., Banik K., Khatoon E., Kunnumakkara A.B. Vietnamese coriander inhibits cell proliferation, survival and migration via suppression of Akt/mTOR pathway in oral squamous cell carcinoma. J. Basic Clin. Physiol. Pharmacol. 2019;31 doi: 10.1515/jbcpp-2019-0162. (/j/jbcpp.2020.31.issue-3/jbcpp-2019-0162/jbcpp-2019-0162.xml) [DOI] [PubMed] [Google Scholar]
- 194.Banik K., Ranaware A.M., Harsha C., Nitesh T., Girisa S., Deshpande V., Fan L., Nalawade S.P., Sethi G., Kunnumakkara A.B. Piceatannol: a natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020;153 doi: 10.1016/j.phrs.2020.104635. [DOI] [PubMed] [Google Scholar]
- 195.Jagadeeshan S., Kunnumakkara A.B., Ramachandran I., Nair S.A. Anticancer activities of fruits and vegetables against gynecological cancers. Anticancer properties of fruits and vegetables: a scientific review. World Sci. 2015:131–159. [Google Scholar]
- 196.Choudhury B., Kandimalla R., Bharali R., Monisha J., Kunnumakara A.B., Kalita K., Kotoky J. Anticancer Activity of Garcinia morella on T-cell murine lymphoma via apoptotic induction. Front. Pharmacol. 2016;7(3) doi: 10.3389/fphar.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Roy N.K., Deka A., Bordoloi D., Mishra S., Kumar A.P., Sethi G., Kunnumakkara A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016;377:74–86. doi: 10.1016/j.canlet.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 198.Sailo B.L., Banik K., Padmavathi G., Javadi M., Bordoloi D., Kunnumakkara A.B. Tocotrienols: the promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018;130:259–272. doi: 10.1016/j.phrs.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 199.Babu B.H., Jayram H.N., Nair M.G., Ajaikumar K.B., Padikkala J. Free radical scavenging, antitumor and anticarcinogenic activity of gossypin. J. Exp. Clin. Cancer Res. 2003;22:581–589. [PubMed] [Google Scholar]
- 200.Ajaikumar K.B., Asheef M., Babu B.H., Padikkala J. The inhibition of gastric mucosal injury by Punica granatum L.(pomegranate) methanolic extract. J. Ethnopharmacol. 2005;96:171–176. doi: 10.1016/j.jep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 201.Khanna D., Sethi G., Ahn K.S., Pandey M.K., Kunnumakkara A.B., Sung B., Aggarwal A., Aggarwal B.B. Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharmacol. 2007;7:344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 202.Kunnumakkara A.B., Nair A.S., Ahn K.S., Pandey M.K., Yi Z., Liu M., Aggarwal B.B. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappaB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood. 2007;109:5112–5121. doi: 10.1182/blood-2007-01-067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Padmavathi G., Roy N.K., Bordoloi D., Arfuso F., Mishra S., Sethi G., Bishayee A., Kunnumakkara A.B. Butein in health and disease: a comprehensive review. Phytomedicine. 2017;25:118–127. doi: 10.1016/j.phymed.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 204.Banik K., Harsha C., Bordoloi D., Lalduhsaki Sailo B., Sethi G., Leong H.C., Arfuso F., Mishra S., Wang L., Kumar A.P., Kunnumakkara A.B. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018;416:75–86. doi: 10.1016/j.canlet.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 205.Ranaware A.M., Banik K., Deshpande V., Padmavathi G., Roy N.K., Sethi G., Fan L., Kumar A.P., Kunnumakkara A.B. Magnolol: a neolignan from the Magnolia family for the prevention and treatment of cancer. Int. J. Mol. Sci. 2018;19:2362. doi: 10.3390/ijms19082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Banik K., Ranaware A.M., Deshpande V., Nalawade S.P., Padmavathi G., Bordoloi D., Sailo B.L., Shanmugam M.K., Fan L., Arfuso F., Sethi G., Kunnumakkara A.B. Honokiol for cancer therapeutics: a traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019;144:192–209. doi: 10.1016/j.phrs.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 207.Bordoloi D., Monisha J., Roy N.K., Padmavathi G., Banik K., Harsha C., Wang H., Kumar A.P., Arfuso F., Kunnumakkara A.B. An investigation on the therapeutic potential of butein, a tretrahydroxychalcone against human oral squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2019;20:3437–3446. doi: 10.31557/APJCP.2019.20.11.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Roy N.K., Parama D., Banik K., Bordoloi D., Devi A.K., Thakur K.K., Padmavathi G., Shakibaei M., Fan L., Sethi G., Kunnumakkara A.B. An update on pharmacological potential of boswellic acids against chronic diseases. Int. J. Mol. Sci. 2019;20:4101. doi: 10.3390/ijms20174101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Girisa S., Shabnam B., Monisha J., Fan L., Halim C.E., Arfuso F., Ahn K.S., Sethi G., Kunnumakkara A.B. Potential of zerumbone as an anti-cancer agent. Molecules. 2019;24:734. doi: 10.3390/molecules24040734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Singh Y.P., Girisa S., Banik K., Ghosh S., Swathi P., Deka M., Padmavathi G., Kotoky J., Sethi G., Fan L., Mao X., Halim C.E., Arfuso F., Kunnumakkara A.B. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J. Funct. Foods. 2019;53:248–258. [Google Scholar]
- 211.Henamayee S., Banik K., Sailo B.L., Shabnam B., Harsha C., Srilakshmi S., Vgm N., Baek S.H., Ahn K.S., Kunnumakkara A.B. Therapeutic emergence of rhein as a potential anticancer drug: a review of its molecular targets and anticancer properties. Molecules. 2020;25:2278. doi: 10.3390/molecules25102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Khatoon E., Banik K., Harsha C., Sailo B.L., Thakur K.K., Khwairakpam A.D., Vikkurthi R., Devi T.B., Gupta S.C., Kunnumakkara A.B. Phytochemicals in cancer cell chemosensitization: current knowledge and future perspectives. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.06.014. (S1044-579X(20)30150-4) [DOI] [PubMed] [Google Scholar]
- 213.Parama D., Boruah M., Yachna K., Rana V., Banik K., Harsha C., Thakur K.K., Dutta U., Arya A., Mao X., Ahn K.S., Kunnumakkara A.B. Diosgenin, a steroidal saponin, and its analogs: effective therapies against different chronic diseases. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118182. [DOI] [PubMed] [Google Scholar]
- 214.Tapsell L.C., Hemphill I., Cobiac L., Patch C.S., Sullivan D.R., Fenech M., Roodenrys S., Keogh J.B., Clifton P.M., Williams P.G., Fazio V.A., Inge K.E. Health benefits of herbs and spices: the past, the present, the future. Med. J. Aust. 2006;185:1–24. doi: 10.5694/j.1326-5377.2006.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 215.Buhrmann C., Shayan P., Banik K., Kunnumakkara A.B., Kubatka P., Koklesova L., Shakibaei M. Targeting NF-κB signaling by Calebin A, a compound of turmeric, in multicellular tumor microenvironment: potential role of apoptosis induction in CRC cells. Biomedicines. 2020;8:236. doi: 10.3390/biomedicines8080236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pandey M.K., Sandur S.K., Sung B., Sethi G., Kunnumakkara A.B., Aggarwal B.B. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaB-regulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue. J. Biol. Chem. 2007;282:17340–17350. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 217.Aggarwal B.B., Kunnumakkara A.B., Harikumar K.B., Tharakan S.T., Sung B., Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 218.Nilius B., Appendino G. Spices: the savory and beneficial science of pungency. Rev. Physiol. Biochem. Pharmacol. 2013;164:1–76. doi: 10.1007/112_2013_11. [DOI] [PubMed] [Google Scholar]
- 219.Opara E.I., Chohan M. Culinary herbs and spices: their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. Int. J. Mol. Sci. 2014;15:19183–19202. doi: 10.3390/ijms151019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Krishnaswamy K. Traditional Indian spices and their health significance. Asia Pac. J. Clin. Nutr. 2008;17:265–268. [PubMed] [Google Scholar]
- 221.Verma S., Khambhala P., Joshi S., Kothari V., Patel T., Seshadri S. Evaluating the role of dithiolane rich fraction of Ferula asafoetida (apiaceae) for its antiproliferative and apoptotic properties: in vitro studies. Exp. Oncol. 2019;41:90–94. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-2.12989. [DOI] [PubMed] [Google Scholar]
- 222.Bagheri S.M., Abdian-Asl A., Moghadam M.T., Yadegari M., Mirjalili A., Zare-Mohazabieh F., Momeni H. Antitumor effect of Ferula assa foetida oleo gum resin against breast cancer induced by 4T1 cells in BALB/c mice. J. Ayurveda Integr. Med. 2017;8:152–158. doi: 10.1016/j.jaim.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mallikarjuna G.U., Dhanalakshmi S., Raisuddin S., Rao A.R. Chemomodulatory influence of Ferula asafoetida on mammary epithelial differentiation, hepatic drug metabolizing enzymes, antioxidant profiles and N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Breast Cancer Res. Treat. 2003;81:1–10. doi: 10.1023/a:1025448620558. [DOI] [PubMed] [Google Scholar]
- 224.Bagheri S.M., Hedesh S.T., Mirjalili A., Dashti-R M.H. Evaluation of anti-inflammatory and some possible mechanisms of antinociceptive effect of Ferula assa foetida oleo gum resin. J. Evid. Based Complement. Altern. Med. 2016;21:271–276. doi: 10.1177/2156587215605903. [DOI] [PubMed] [Google Scholar]
- 225.Eftekhar N., Moghimi A., Mohammadian Roshan N., Saadat S., Boskabady M.H. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement. Altern. Med. 2019;19:349. doi: 10.1186/s12906-019-2765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Abd El-Ghffar E.A., Al-Sayed E., Shehata S.M., Eldahshan O.A., Efferth T. The protective role of Ocimum basilicum L. (basil) against aspirin-induced gastric ulcer in mice: impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct. 2018;9:4457–4468. doi: 10.1039/c8fo00538a. [DOI] [PubMed] [Google Scholar]
- 227.Lee E.H., Shin J.H., Kim S.S., Lee H., Yang S.R., Seo S.R. Laurus nobilis leaf extract controls inflammation by suppressing NLRP3 inflammasome activation. J. Cell. Physiol. 2019;234:6854–6864. doi: 10.1002/jcp.27434. [DOI] [PubMed] [Google Scholar]
- 228.Mokhtari-Zaer A., Norouzi F., Askari V.R., Khazdair M.R., Roshan N.M., Boskabady M., Hosseini M., Boskabady M.H. The protective effect of Nigella sativa extract on lung inflammation and oxidative stress induced by lipopolysaccharide in rats. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112653. [DOI] [PubMed] [Google Scholar]
- 229.Bordoni L., Fedeli D., Fiorini D., Gabbianelli R. Extra virgin olive oil and Nigella sativa oil produced in Central Italy: a comparison of the nutrigenomic effects of two Mediterranean oils in a low-grade inflammation model. Antioxidants (Basel) 2019;9:20. doi: 10.3390/antiox9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Abbasnezhad A., Niazmand S., Mahmoudabady M., Rezaee S.A., Soukhtanloo M., Mosallanejad R., Hayatdavoudi P. Nigella sativa L. seed regulated eNOS, VCAM-1 and LOX-1 genes expression and improved vasoreactivity in aorta of diabetic rat. J. Ethnopharmacol. 2019;228:142–147. doi: 10.1016/j.jep.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 231.Beheshti F., Norouzi F., Abareshi A., Khazaei M., Alikhani V., Moussavi S., Biglari G., Soukhtanloo M., Hosseini M. Nigella sativa prevented liver and renal tissue damage in lipopolysaccharide-treated rats. Saudi J. Kidney Dis. Transpl. 2018;29:554–566. doi: 10.4103/1319-2442.235184. [DOI] [PubMed] [Google Scholar]
- 232.Khaldi T., Chekchaki N., Boumendjel M., Taibi F., Abdellaoui M., Messarah M., Boumendjel A. Ameliorating effects of Nigella sativa oil on aggravation of inflammation, oxidative stress and cytotoxicity induced by smokeless tobacco extract in an allergic asthma model in Wistar rats. Allergol Immunopathol (Madr) 2018;46:472–481. doi: 10.1016/j.aller.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 233.Abulfadl Y.S., El-Maraghy N.N., Ahmed A.E., Nofal S., Abdel-Mottaleb Y., Badary O.A. Thymoquinone alleviates the experimentally induced Alzheimer's disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018;37:1092–1104. doi: 10.1177/0960327118755256. [DOI] [PubMed] [Google Scholar]
- 234.Bui T.T., Piao C.H., Song C.H., Shin H.S., Shon D.H., Chai O.H. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell. Immunol. 2017;322:64–73. doi: 10.1016/j.cellimm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 235.Bui T.T., Fan Y., Piao C.H., Van Nguyen T., Shin D.U., Jung S.Y., Hyeon E., Song C.H., Lee S.Y., Shin H.S., Chai O.H. Piper Nigrum extract improves OVA-induced nasal epithelial barrier dysfunction via activating Nrf2/HO-1 signaling. Cell. Immunol. 2020;351 doi: 10.1016/j.cellimm.2019.104035. [DOI] [PubMed] [Google Scholar]
- 236.Bui T.T., Piao C.H., Hyeon E., Fan Y., Van Nguyen T., Jung S.Y., Choi D.W., Lee S.Y., Shin H.S., Song C.H., Chai O.H. The protective role of Piper nigrum fruit extract in an ovalbumin-induced allergic rhinitis by targeting of NFκBp65 and STAT3 signalings. Biomed. Pharmacother. 2019;109:1915–1923. doi: 10.1016/j.biopha.2018.11.073. [DOI] [PubMed] [Google Scholar]
- 237.Pei H., Xue L., Tang M., Tang H., Kuang S., Wang L., Ma X., Cai X., Li Y., Zhao M., Peng A. Alkaloids from black pepper (Piper nigrum L.) exhibit anti-inflammatory activity in murine macrophages by inhibiting activation of NF-κB pathway. J. Agric. Food Chem. 2020;68:2406–2417. doi: 10.1021/acs.jafc.9b07754. [DOI] [PubMed] [Google Scholar]
- 238.Pradeep C.R., Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin. Exp. Metastasis. 2002;19:703–708. doi: 10.1023/a:1021398601388. [DOI] [PubMed] [Google Scholar]
- 239.Liang Y.D., Bai W.J., Li C.G., Xu L.H., Wei H.X., Pan H., He X.H., Ouyang D.Y. Piperine suppresses pyroptosis and interleukin-1β release upon ATP triggering and bacterial infection. Front. Pharmacol. 2016;7:390. doi: 10.3389/fphar.2016.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bae G.S., Kim M.S., Jeong J., Lee H.Y., Park K.C., Koo B.S., Kim B.J., Kim T.H., Lee S.H., Hwang S.Y., Shin Y.K., Song H.J., Park S.J. Piperine ameliorates the severity of cerulein-induced acute pancreatitis by inhibiting the activation of mitogen activated protein kinases. Biochem. Biophys. Res. Commun. 2011;410:382–388. doi: 10.1016/j.bbrc.2011.05.136. [DOI] [PubMed] [Google Scholar]
- 241.Peng X., Yang T., Liu G., Liu H., Peng Y., He L. Piperine ameliorated lupus nephritis by targeting AMPK-mediated activation of NLRP3 inflammasome. Int. Immunopharmacol. 2018;65:448–457. doi: 10.1016/j.intimp.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 242.Ngo Q.M., Tran P.T., Tran M.H., Kim J.A., Rho S.S., Lim C.H., Kim J.C., Woo M.H., Choi J.S., Lee J.H., Min B.S. Alkaloids from Piper nigrum exhibit antiinflammatory activity via activating the Nrf2/HO-1 pathway. Phytother. Res. 2017;31:663–670. doi: 10.1002/ptr.5780. [DOI] [PubMed] [Google Scholar]
- 243.Das M., Basu S., Banerjee B., Sen A., Jana K., Datta G. Hepatoprotective effects of green Capsicum annum against ethanol induced oxidative stress, inflammation and apoptosis in rats. J. Ethnopharmacol. 2018;227:69–81. doi: 10.1016/j.jep.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 244.Jang H.Y., Kim S.M., Yuk J.E., Kwon O.K., Oh S.R., Lee H.K., Jeong H., Ahn K.S. Capsicum annuum L. methanolic extract inhibits ovalbumin-induced airway inflammation and oxidative stress in a mouse model of asthma. J. Med. Food. 2011;14:1144–1151. doi: 10.1089/jmf.2011.1609. [DOI] [PubMed] [Google Scholar]
- 245.Tang J., Luo K., Li Y., Chen Q., Tang D., Wang D., Xiao J. Capsaicin attenuates LPS-induced inflammatory cytokine production by upregulation of LXRα. Int. Immunopharmacol. 2015;28:264–269. doi: 10.1016/j.intimp.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 246.Shin Y.H., Namkoong E., Choi S., Bae J.S., Jin M., Hwang S.M., Arote R., Choi S.Y., Park K. Capsaicin regulates the NF-κB pathway in salivary gland inflammation. J. Dent. Res. 2013;92:547–552. doi: 10.1177/0022034513487376. [DOI] [PubMed] [Google Scholar]
- 247.Abu Gazia M., El-Magd M.A. Ameliorative effect of cardamom aqueous extract on doxorubicin-induced cardiotoxicity in rats. Cells Tissues Organs. 2018;206:62–72. doi: 10.1159/000496109. [DOI] [PubMed] [Google Scholar]
- 248.Li S., Li L., Yan H., Jiang X., Hu W., Han N., Wang D. Anti gouty arthritis andanti hyperuricemia properties of celery seed extracts in rodent models. Mol. MedRep. 2019;20:4623–4633. doi: 10.3892/mmr.2019.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hsieh S.L., Chen C.T., Wang J.J., Kuo Y.H., Li C.C., Hsieh L.C., Wu C.C. Sedanolide inducesautophagy through the PI3K, p53 and NF-κB signaling pathways in human livercancer cells. Int. J. Oncol. 2015;47:2240–2246. doi: 10.3892/ijo.2015.3206. [DOI] [PubMed] [Google Scholar]
- 250.Che D.N., Cho B.O., Shin J.Y., Kang H.J., Kim J.S., Choi J., Jang S.I. Anti-atopic dermatitis effects of hydrolyzed celery extract in mice. J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13198. [DOI] [PubMed] [Google Scholar]
- 251.Si Y., Guo S., Fang Y., Qin S., Li F., Zhang Y., Jiao P., Zhang C., Gao L. Celery seed extract blocks peroxide injury in macrophages via Notch1/NF-κB pathway. Am. J. Chin. Med. 2015;433:443–455. doi: 10.1142/S0192415X15500287. [DOI] [PubMed] [Google Scholar]
- 252.Xia T., Gao R., Zhou G., Liu J., Li J., Shen J. Trans-cinnamaldehyde inhibits IL-1β-stimulated inflammation in chondrocytes by suppressing NF-κB and p38-JNK pathways and exerts chondrocyte protective effects in a rat model of osteoarthritis. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/4039472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lee H.S., Kim B.S., Kim M.K. Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J. Agric. Food Chem. 2002;50:7700–7703. doi: 10.1021/jf020751f. [DOI] [PubMed] [Google Scholar]
- 254.Fu Y., Yang P., Zhao Y., Zhang L., Zhang Z., Dong X., Wu Z., Xu Y., Chen Y. trans-Cinnamaldehyde inhibits microglial activation and improves neuronal survival against neuroinflammation in BV2 microglial cells with lipopolysaccharide stimulation. Evid. Based Complement. Alternat. Med. 2017;2017 doi: 10.1155/2017/4730878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Han X., Parker T.L. Antiinflammatory activity of cinnamon (Cinnamomum zeylanicum) bark essential oil in a human skin disease model. Phytother. Res. 2017;31:1034–1038. doi: 10.1002/ptr.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hong J.W., Yang G.E., Kim Y.B., Eom S.H., Lew J.H., Kang H. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complement. Altern. Med. 2012;12:237. doi: 10.1186/1472-6882-12-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lee B.J., Kim Y.J., Cho D.H., Sohn N.W., Kang H. Immunomodulatory effect of water extract of cinnamon on anti-CD3-induced cytokine responses and p38, JNK, ERK1/2, and STAT4 activation. Immunopharmacol. Immunotoxicol. 2011;33:714–722. doi: 10.3109/08923973.2011.564185. [DOI] [PubMed] [Google Scholar]
- 258.Koh W.S., Yoon S.Y., Kwon B.M., Jeong T.C., Nam K.S., Han M.Y. Cinnamaldehyde inhibits lymphocyte proliferation and modulates T-cell differentiation. Int. J. Immunopharmacol. 1998;20:643–660. doi: 10.1016/s0192-0561(98)00064-2. [DOI] [PubMed] [Google Scholar]
- 259.Wu T.T., Tsai C.W., Yao H.T., Lii C.K., Chen H.W., Wu Y.L., Chen P.Y., Liu K.L. Suppressive effects of extracts from the aerial part of Coriandrum sativum L. on LPS-induced inflammatory responses in murine RAW 264.7 macrophages. J. Sci. Food Agric. 2010;90:1846–1854. doi: 10.1002/jsfa.4023. [DOI] [PubMed] [Google Scholar]
- 260.Park G., Kim H.G., Lim S., Lee W., Sim Y., Oh M.S. Coriander alleviates 2,4-dinitrochlorobenzene-induced contact dermatitis-like skin lesions in mice. J. Med. Food. 2014;17:862–868. doi: 10.1089/jmf.2013.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nair V., Singh S., Gupta Y.K. Anti-granuloma activity of Coriandrum sativum in experimental models. J. Ayurveda Integr. Med. 2013;4:13–18. doi: 10.4103/0975-9476.109544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kalaivani P., Saranya R.B., Ramakrishnan G., Ranju V., Sathiya S., Gayathri V., Thiyagarajan L.K., Venkhatesh J.R., Babu C.S., Thanikachalam S. Cuminum cyminum, a dietary spice, attenuates hypertension via endothelial nitric oxide synthase and NO pathway in renovascular hypertensive rats. Clin. Exp. Hypertens. 2013;35:534–542. doi: 10.3109/10641963.2013.764887. [DOI] [PubMed] [Google Scholar]
- 263.Vador N., Jagtap A.G., Damle A. Vulnerability of gastric mucosa in diabetic rats, its pathogenesis and amelioration by Cuminum cyminum. Indian J. Pharm. Sci. 2012;74:387–396. doi: 10.4103/0250-474X.108413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Khurana A., Sikha M.S., Ramesh K., Venkatesh P., Godugu C. Modulation of cerulein-induced pancreatic inflammation by hydroalcoholic extract of curry leaf (Murraya koenigii) Phytother. Res. 2019;33:1510–1525. doi: 10.1002/ptr.6344. [DOI] [PubMed] [Google Scholar]
- 265.Yeap S.K., Abu N., Mohamad N.E., Beh B.K., Ho W.Y., Ebrahimi S., Yusof H.M., Ky H., Tan S.W., Alitheen N.B. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement. Altern. Med. 2015;15:306. doi: 10.1186/s12906-015-0832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jagtap S., Khare P., Mangal P., Kondepudi K.K., Bishnoi M., Bhutani K.K. Effect of mahanimbine, an alkaloid from curry leaves, on high-fat diet-induced adiposity, insulin resistance, and inflammatory alterations. Biofactors. 2017;43:220–231. doi: 10.1002/biof.1333. [DOI] [PubMed] [Google Scholar]
- 267.Iman V., Mohan S., Abdelwahab S.I., Karimian H., Nordin N., Fadaeinasab M., Noordin M.I., Noor S.M. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des. Devel. Ther. 2016;11:103–121. doi: 10.2147/DDDT.S115135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kandhare A.D., Bodhankar S.L., Mohan V., Thakurdesai P.A. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem. Biol. Interact. 2015;237:151–165. doi: 10.1016/j.cbi.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 269.Hamza A.A., Elwy H.M., Badawi A.M. Fenugreek seed extract attenuates cisplatin-induced testicular damage in Wistar rats. Andrologia. 2016;48:211–221. doi: 10.1111/and.12435. [DOI] [PubMed] [Google Scholar]
- 270.Gupta S.K., Kumar B., Nag T.C., Srinivasan B.P., Srivastava S., Gaur S., Saxena R. Effects of Trigonella foenum-graecum (L.) on retinal oxidative stress, and proinflammatory and angiogenic molecular biomarkers in streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2014;388:1–9. doi: 10.1007/s11010-013-1893-2. [DOI] [PubMed] [Google Scholar]
- 271.Sindhu G., Shyni G.L., Pushpan C.K., Nambisan B., Helen A. Evaluation of anti-arthritic potential of Trigonella foenum graecum L. (fenugreek) mucilage against rheumatoid arthritis. Prostaglandins Other Lipid Mediat. 2018;138:48–53. doi: 10.1016/j.prostaglandins.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 272.Fahanik-Babaei J., Baluchnejadmojarad T., Nikbakht F., Roghani M. Trigonelline protects hippocampus against intracerebral Aβ(1-40) as a model of Alzheimer's disease in the rat: insights into underlying mechanisms. Metab. Brain Dis. 2019;34:191–201. doi: 10.1007/s11011-018-0338-8. [DOI] [PubMed] [Google Scholar]
- 273.Tsai M.L., Chiou Y.S., Chiou L.Y., Ho C.T., Pan M.H. Garcinol suppresses inflammation-associated colon carcinogenesis in mice. Mol. Nutr. Food Res. 2014;58:1820–1829. doi: 10.1002/mnfr.201400149. [DOI] [PubMed] [Google Scholar]
- 274.Li F., Shanmugam M.K., Chen L., Chatterjee S., Basha J., Kumar A.P., Kundu T.K., Sethi G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. (Phila.) 2013;6:843–854. doi: 10.1158/1940-6207.CAPR-13-0070. [DOI] [PubMed] [Google Scholar]
- 275.Hsieh C.C., Liu K.F., Liu P.C., Ho Y.T., Li W.S., Peng W.H., Tsai J.C. Comparing the protection imparted by different fraction extracts of garlic (Allium sativum L.) against Der p-induced allergic airway iflammation in mice. Int. J. Mol. Sci. 2019;20:4879. doi: 10.3390/ijms20194879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mathan Kumar M., Tamizhselvi R. Protective effect of diallyl disulfide against cerulein-induced acute pancreatitis and associated lung injury in mice. Int. Immunopharmacol. 2020;80 doi: 10.1016/j.intimp.2019.106136. [DOI] [PubMed] [Google Scholar]
- 277.Liang J.J., Li H.R., Chen Y., Zhang C., Chen D.G., Liang Z.C., Shi Y.Q., Zhang L.L., Xin L., Zhao D.B. Diallyl trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-κB and Wnt pathways. Int. Immunopharmacol. 2019;71:132–138. doi: 10.1016/j.intimp.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 278.Nie Y., Yu K., Li B., Hu Y., Zhang H., Xin R., Xiong Y., Zhao P., Chai G. S-allyl-l-cysteine attenuates bleomycin-induced pulmonary fibrosis and inflammation via AKT/NF-κB signaling pathway in mice. J. Pharmacol. Sci. 2019;139:377–384. doi: 10.1016/j.jphs.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 279.Mo M., Li S., Dong Z., Li C., Sun Y., Li A., Zhao Z. S-allylmercaptocysteine ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammation and oxidative stress via nuclear factor kappa B and Keap1/Nrf2 pathways. Int. Immunopharmacol. 2020;81 doi: 10.1016/j.intimp.2020.106273. [DOI] [PubMed] [Google Scholar]
- 280.Annamalai G., Suresh K. [6]-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis. Biomed. Pharmacother. 2018;98:484–490. doi: 10.1016/j.biopha.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 281.Tzeng T.F., Liou S.S., Chang C.J., Liu I.M. 6-Gingerol protects against nutritional steatohepatitis by regulating key genes related to inflammation and lipid metabolism. Nutrients. 2015;7:999–1020. doi: 10.3390/nu7020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jin L., Ying Z.H., Yu C.H., Zhang H.H., Yu W.Y. Wu XN. Isofraxidin ameliorated influenza viral inflammation in rodents via inhibiting platelet aggregation. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106521. [DOI] [PubMed] [Google Scholar]
- 283.Lee W., Ku S.K., Kim J.E., Cho S.H., Song G.Y., Bae J.S. Inhibitory effects of black ginseng on particulate matter-induced pulmonary injury. Am. J. Chin. Med. 2019;47:1237–1251. doi: 10.1142/S0192415X19500630. [DOI] [PubMed] [Google Scholar]
- 284.Zhou Y.J., Chen J.M., Sapkota K., Long J.Y., Liao Y.J., Jiang J.J., Liang B.Y., Wei J.B., Zhou Y. Pananx notoginseng saponins attenuate CCL2-induced cognitive deficits in rats via anti-inflammation and anti-apoptosis effects that involve suppressing over-activation of NMDA receptors. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110139. [DOI] [PubMed] [Google Scholar]
- 285.Lu Q.G., Zeng L., Li X.H., Liu Y., Du X.F., Bai G.M., Yan X. Protective effects of panax notoginseng saponin on dextran sulfate sodium-induced colitis in rats through phosphoinositide-3-kinase protein kinase B signaling pathway inhibition. World J. Gastroenterol. 2020;26:1156–1171. doi: 10.3748/wjg.v26.i11.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fan C., Chen Q., Ren J., Yang X., Ru J., Zhang H., Yang X. Notoginsenoside R1 suppresses inflammatory signaling and rescues renal ischemia-reperfusion injury in experimental rats. Med. Sci. Monit. 2020;26 doi: 10.12659/MSM.920442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhang Y., Wang S., Song S., Yang X., Jin G. Ginsenoside Rg3 alleviates complete Freund's adjuvant-induced rheumatoid arthritis in mice by regulating CD4+CD25+Foxp3+Treg cells. J. Agric. Food Chem. 2020;68:4893–4902. doi: 10.1021/acs.jafc.0c01473. [DOI] [PubMed] [Google Scholar]
- 288.Guan S., Yu P., Cao J., Xi X., Zhang Q., Zhu C., Hu H., Gong X., Fan H. Ginsenoside Rg1 protects against cigarette smoke-induced airway remodeling by suppressing the TGF-β1/Smad3 signaling pathway. Am. J. Transl. Res. 2020;12:493–506. [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang X., Liu X., Hu G., Zhang G., Zhao G., Shi M. Ginsenoside Rd attenuates blood-brain barrier damage by suppressing proteasome-mediated signaling after transient forebrain ischemia. Neuroreport. 2020;31:466–472. doi: 10.1097/WNR.0000000000001426. [DOI] [PubMed] [Google Scholar]
- 290.Han J.G., Gupta S.C., Prasad S. Aggarwal BB. PL chemosensitizes tumor cells through interaction with cysteine 179 of IκBα kinase, leading to suppression of NF-κB-regulated gene products. Mol. Cancer Ther. 2014;13:2422–2435. doi: 10.1158/1535-7163.MCT-14-0171. [DOI] [PubMed] [Google Scholar]
- 291.Yao L., Chen H.P., Ma Q. Piperlongumine alleviates lupus nephritis in MRL-Fas(lpr) mice by regulating the frequency of Th17 and regulatory T cells. Immunol. Lett. 2014;161:76–80. doi: 10.1016/j.imlet.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 292.Sun J., Xu P., Du X., Zhang Q., Zhu Y. Piperlongumine attenuates collagen-induced arthritis via expansion of myeloid-derived suppressor cells and inhibition of the activation of fibroblast-like synoviocytes. Mol. Med. Rep. 2015;11:2689–2694. doi: 10.3892/mmr.2014.3001. [DOI] [PubMed] [Google Scholar]
- 293.Lu C., Zhang B., Xu T., Zhang W., Bai B., Xiao Z., Wu L., Liang G., Zhang Y., Dai Y. Piperlongumine reduces ovalbumin-induced asthma and airway inflammation by regulating nuclear factor-κB activation. Int. J. Mol. Med. 2019;44:1855–1865. doi: 10.3892/ijmm.2019.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ku S.K., Kim J.A., Bae J.S. Vascular barrier protective effects of piperlonguminine in vitro and in vivo. Inflamm. Res. 2014;63:369–379. doi: 10.1007/s00011-014-0708-6. [DOI] [PubMed] [Google Scholar]
- 295.Kim N., Do J., Bae J.S., Jin H.K., Kim J.H., Inn K.S., Oh M.S., Lee J.K. PL inhibits neuroinflammation via regulating NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. J. Pharmacol. Sci. 2018;137:195–201. doi: 10.1016/j.jphs.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 296.Devan P., Bani S., Suri K.A., Satti N.K., Qazi G.N. Immunomodulation exhibited by piperinic acid through suppression of proinflammatory cytokines. Int. Immunopharmacol. 2007;7:889–899. doi: 10.1016/j.intimp.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 297.Gu S.M., Lee H.P., Ham Y.W., Son D.J., Kim H.Y., Oh K.W., Han S.B., Yun J., Hong J.T.P.L. Improves lipopolysaccharide-induced amyloidogenesis by suppressing NF-KappaB pathway. NeuroMolecular Med. 2018;20:312–327. doi: 10.1007/s12017-018-8495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Singh N., Kumar S., Singh P., Raj H.G., Prasad A.K., Parmar V.S., Ghosh B. Piper longum Linn. extract inhibits TNF-alpha-induced expression of cell adhesion molecules by inhibiting NF-kappaB activation and microsomal lipid peroxidation. Phytomedicine. 2008;15:284–291. doi: 10.1016/j.phymed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 299.Son D.J., Kim S.Y., Han S.S., Kim C.W., Kumar S., Park B.S., Lee S.E., Yun Y.P., Jo H., Park Y.H. PL inhibits atherosclerotic plaque formation and vascular smooth muscle cell proliferation by suppressing PDGF receptor signaling. Biochem. Biophys. Res. Commun. 2012;427:349–354. doi: 10.1016/j.bbrc.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ku S.K., Kim J.A., Bae J.S. Piperlonguminine downregulates endothelial protein C receptor shedding in vitro and in vivo. Inflammation. 2014;37:435–442. doi: 10.1007/s10753-013-9756-2. [DOI] [PubMed] [Google Scholar]
- 301.Sant'Ana M., Souza H.R., Possebon L., Cornélio M.L., Riffo-Vasquez Y., Girol A.P., Oliani S.M. Effect of PL during exposure to cigarette smoke reduces inflammation and lung injury. Pulm. Pharmacol. Ther. 2020;61 doi: 10.1016/j.pupt.2020.101896. [DOI] [PubMed] [Google Scholar]
- 302.Tian W., Akanda M.R., Islam A., Yang H.D., Lee S.C., Lee J.H., Kim S.K., Choi Y.J., Im S.Y., Park B.Y. The anti-stress effect of Mentha arvensis in immobilized rats. Int. J. Mol. Sci. 2018;19:355. doi: 10.3390/ijms19020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yadav N., Chandra H. Modulation of alveolar macrophage innate response in proinflammatory-, pro-oxidant-, and infection-models by mint extract and chemical constituents: role of MAPKs. Immunobiology. 2018;223:49–56. doi: 10.1016/j.imbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 304.Xian Y.F., Hu Z., Ip S.P., Chen J.N., Su Z.R., Lai X.P., Lin Z.X. Comparison of the anti-inflammatory effects of Sinapis alba and Brassica juncea in mouse models of inflammation. Phytomedicine. 2018;50:196–204. doi: 10.1016/j.phymed.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 305.Hu J., Yang R., Wen C., Li H., Zhao H. Expression of NLRP3 inflammasome in BALB/c mice with imiquimod-induced psoriasis-like inflammation and therapeutic effect of mustard seed (Sinapis alba Linn) Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1394–1398. (Chinese) [PubMed] [Google Scholar]
- 306.Yang R., Zhou Q., Wen C., Hu J., Li H., Zhao M., Zhao H. Mustard seed (Sinapis alba Linn) attenuates imiquimod-induced psoriasiform inflammation of BALB/c mice. J. Dermatol. 2013;40:543–552. doi: 10.1111/1346-8138.12119. [DOI] [PubMed] [Google Scholar]
- 307.Al-Quraishy S., Dkhil M.A., Abdel-Gaber R., Zrieq R., Hafez T.A., Mubaraki M.A., Abdel Moneim A.E. Myristica fragrans seed extract reverses scopolamine-induced cortical injury via stimulation of HO-1 expression in male rats. Environ. Sci. Pollut. Res. Int. 2020;27:12395–12404. doi: 10.1007/s11356-020-07686-8. [DOI] [PubMed] [Google Scholar]
- 308.Shin K., Chung H.C., Kim D.U., Hwang J.K., Lee S.H. Macelignan attenuated allergic lung inflammation and airway hyper-responsiveness in murine experimental asthma. Life Sci. 2013;92:1093–1099. doi: 10.1016/j.lfs.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 309.Han Y.S., Kim M.S., Hwang J.K. Macelignan inhibits histamine release and inflammatory mediator production in activated rat basophilic leukemia mast cells. Inflammation. 2012;35:1723–1731. doi: 10.1007/s10753-012-9490-1. [DOI] [PubMed] [Google Scholar]
- 310.Long J., Qian K., Tan S., Liu J., Li J. Macelignan protects against renal ischemia-reperfusion injury via inhibition of inflammation and apoptosis of renal epithelial cells. Cell. Mol. Biol. (Noisy-le-grand) 2020;66:55–59. [PubMed] [Google Scholar]
- 311.Jin H., Zhu Z.G., Yu P.J., Wang G.F., Zhang J.Y., Li J.R., Ai R.T., Li Z.H., Tian Y.X., Zhang W.X., Wu S.G. Myrislignan attenuates lipopolysaccharide-induced inflammation reaction in murine macrophage cells through inhibition of NF-κB signalling pathway activation. Phytother. Res. 2012;26:1320–1326. doi: 10.1002/ptr.3707. [DOI] [PubMed] [Google Scholar]
- 312.Lee J.Y., Park W. Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules. 2011;16:7132–7142. doi: 10.3390/molecules16087132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Khajah M.A., Orabi K.Y., Hawai S., Sary H.G., El-Hashim A.Z. Onion bulb extract reduces colitis severity in mice via modulation of colonic inflammatory pathways and the apoptotic machinery. J. Ethnopharmacol. 2019;241 doi: 10.1016/j.jep.2019.112008. [DOI] [PubMed] [Google Scholar]
- 314.Elberry A.A., Mufti S., Al-Maghrabi J., Abdel Sattar E., Ghareib S.A., Mosli H.A., Gabr S.A. Immunomodulatory effect of red onion (Allium cepa Linn) scale extract on experimentally induced atypical prostatic hyperplasia in Wistar rats. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/640746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lu X.L., Zhao C.H., Yao X.L., Zhang H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017;85:658–671. doi: 10.1016/j.biopha.2016.11.077. [DOI] [PubMed] [Google Scholar]
- 316.Sekhon-Loodu S., Ziaullah Z., Rupasinghe H.P., Wang Y., Kulka M., Shahidi F. Novel quercetin-3-O-glucoside eicosapentaenoic acid ester ameliorates inflammation and hyperlipidemia. Inflammopharmacology. 2015;23:173–185. doi: 10.1007/s10787-015-0237-0. [DOI] [PubMed] [Google Scholar]
- 317.Shakeri F., Eftekhar N., Roshan N.M., Rezaee R., Moghimi A., Boskabady M.H. Rosmarinic acid affects immunological and inflammatory mediator levels and restores lung pathological features in asthmatic rats. Allergol Immunopathol (Madr) 2019;47:16–23. doi: 10.1016/j.aller.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 318.Liang Z., Nie H., Xu Y., Peng J., Zeng Y., Wei Y., Wen X., Qiu J., Zhong W., Deng X., He J. Therapeutic effects of rosmarinic acid on airway responses in a murine model of asthma. Int. Immunopharmacol. 2016;41:90–97. doi: 10.1016/j.intimp.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 319.Baradaran Rahim V., Khammar M.T., Rakhshandeh H., Samzadeh-Kermani A., Hosseini A., Askari V.R. Crocin protects cardiomyocytes against LPS-induced inflammation. Pharmacol. Rep. 2019;71:1228–1234. doi: 10.1016/j.pharep.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 320.Algandaby M.M. Crocin attenuates metabolic syndrome-induced osteoporosis in rats. J. Food Biochem. 2019;43 doi: 10.1111/jfbc.12895. [DOI] [PubMed] [Google Scholar]
- 321.Lertnimitphun P., Jiang Y., Kim N., Fu W., Zheng C., Tan H., Zhou H., Zhang X., Pei W., Lu Y., Xu H. Safranal alleviates dextran sulfate sodium-induced colitis and suppresses macrophage-mediated inflammation. Front. Pharmacol. 2019;10:1281. doi: 10.3389/fphar.2019.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Baluchnejadmojarad T., Mohamadi-Zarch S.M., Roghani M. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid β-induced rat model of Alzheimer's disease: underlying mechanisms. Metab. Brain Dis. 2019;34:1747–1759. doi: 10.1007/s11011-019-00481-6. [DOI] [PubMed] [Google Scholar]
- 323.Tamaddonfard E., Erfanparast A., Farshid A.A., Imani M., Mirzakhani N., Salighedar R., Tamaddonfard S. Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer. Life Sci. 2019;224:88–94. doi: 10.1016/j.lfs.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 324.Liou C.J., Chen Y.L., Yu M.C., Yeh K.W., Shen S.C., Huang W.C. Sesamol alleviates airway hyperresponsiveness and oxidative stress in asthmatic mice. Antioxidants (Basel) 2020;9:295. doi: 10.3390/antiox9040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Qiang L., Yuan J., Shouyin J., Yulin L., Libing J., Jian-An W. Sesamin attenuates lipopolysaccharide-induced acute lung injury by inhibition of TLR4 signaling pathways. Inflammation. 2016;39:467–472. doi: 10.1007/s10753-015-0270-6. [DOI] [PubMed] [Google Scholar]
- 326.Rousta A.M., Mirahmadi S.M., Shahmohammadi A., Nourabadi D., Khajevand-Khazaei M.R., Baluchnejadmojarad T., Roghani M. Protective effect of sesamin in lipopolysaccharide-induced mouse model of acute kidney injury via attenuation of oxidative stress, inflammation, and apoptosis. Immunopharmacol. Immunotoxicol. 2018;40:423–429. doi: 10.1080/08923973.2018.1523926. [DOI] [PubMed] [Google Scholar]
- 327.Zhao Y., Wang Q., Jia M., Fu S., Pan J., Chu C., Liu X., Liu X., Liu Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019;64:61–71. doi: 10.1016/j.jnutbio.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 328.Hsu D.Z., Liu C.T., Chu P.Y., Li Y.H., Periasamy S., Liu M.Y. Sesame oil attenuates ovalbumin-induced pulmonary edema and bronchial neutrophilic inflammation in mice. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/905670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Park S.H., Sung Y.Y., Nho K.J., Kim H.K. Protective activity ethanol extract of the fruits of Illicium verum against atherogenesis in apolipoprotein E knockout mice. BMC Complement. Altern. Med. 2015;15:232. doi: 10.1186/s12906-015-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sung Y.Y., Kim H.K. Illicium verum extract suppresses IFN-γ-induced ICAM-1 expression via blockade of JAK/STAT pathway in HaCaT human keratinocytes. J. Ethnopharmacol. 2013;149:626–632. doi: 10.1016/j.jep.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 331.Kang P., Kim K.Y., Lee H.S., Min S.S., Seol G.H. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013;93:955–961. [PubMed] [Google Scholar]
- 332.Sung Y.Y., Kim S.H., Kim D.S., Lee J.E., Kim H.K. Illicium verum extract and trans-anethole attenuate ovalbumin-induced airway inflammation via enhancement of Foxp3+ regulatory T cells and inhibition of Th2 cytokines in mice. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/7506808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ameeramja J., Perumal E. Possible modulatory effect of tamarind seed coat extract on fluoride-induced pulmonary inflammation and fibrosis in rats. Inflammation. 2018;41:886–895. doi: 10.1007/s10753-018-0743-5. [DOI] [PubMed] [Google Scholar]
- 334.Sundaram M.S., Hemshekhar M., Santhosh M.S., Paul M., Sunitha K., Thushara R.M., NaveenKumar S.K., Naveen S., Devaraja S., Rangappa K.S., Kemparaju K., Girish K.S. Tamarind seed (Tamarindus indica) extract ameliorates adjuvant-induced arthritis via regulating the mediators of cartilage/bone degeneration, inflammation and oxidative stress. Sci. Rep. 2015;5 doi: 10.1038/srep11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Periasamy S., Lin C.H., Nagarajan B., Sankaranarayanan N.V., Desai U.R., Liu M.Y. Mucoadhesive role of tamarind xyloglucan on inflammation attenuates ulcerative colitis. J. Funct. Foods. 2018;47:1–10. doi: 10.1016/j.jff.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Han S., Xu J., Guo X., Huang M. Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clin. Exp. Pharmacol. Physiol. 2018;45:84–93. doi: 10.1111/1440-1681.12848. [DOI] [PubMed] [Google Scholar]
- 337.Dong Z.W., Chen J., Ruan Y.C., Zhou T., Chen Y., Chen Y., Tsang L.L., Chan H.C., Peng Y.Z. CFTR-regulated MAPK/NF-κB signaling in pulmonary inflammation in thermal inhalation injury. Sci. Rep. 2015;5 doi: 10.1038/srep15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zhang F., Yang F., Zhao H., An Y. Curcumin alleviates lung injury in diabetic rats by inhibiting nuclear factor-κB pathway. Clin. Exp. Pharmacol. Physiol. 2015;42:956–963. doi: 10.1111/1440-1681.12438. [DOI] [PubMed] [Google Scholar]
- 339.Xiao X., Yang M., Sun D., Sun S. Curcumin protects against sepsis-induced acute lung injury in rats. J. Surg. Res. 2012;176:e31–e39. doi: 10.1016/j.jss.2011.11.1032. [DOI] [PubMed] [Google Scholar]
- 340.Chong L., Zhang W., Nie Y., Yu G., Liu L., Lin L., Wen S., Zhu L., Li C. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation. 2014;37:1476–1485. doi: 10.1007/s10753-014-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Li L., Li H., Li M. Curcumin protects against cerebral ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway in rats. Int. J. Clin. Exp. Med. 2015;8:14985–14991. [PMC free article] [PubMed] [Google Scholar]
- 342.Li Y.L., Du Z.Y., Li P.H., Yan L., Zhou W., Tang Y.D., Liu G.R., Fang Y.X., Zhang K., Dong C.Z., Chen H.X. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018;64:319–325. doi: 10.1016/j.intimp.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 343.Rajagopal H.M., Manjegowda S.B., Serkad C. Dharmesh SM. A modified pectic polysaccharide from turmeric (Curcuma longa) with antiulcer effects via anti-secretary, mucoprotective and IL-10 mediated anti-inflammatory mechanisms. Int. J. Biol. Macromol. 2018;118:864–880. doi: 10.1016/j.ijbiomac.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 344.Amalraj A., Gopi S. Biological activities and medicinal properties of asafoetida: a review. J. Tradit. Complement. Med. 2016;7:347–359. doi: 10.1016/j.jtcme.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mahendra P., Bisht S. Ferula asafoetida: traditional uses and pharmacological activity. Pharmacogn. Rev. 2012;6:141–146. doi: 10.4103/0973-7847.99948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sestili P., Ismail T., Calcabrini C., Guescini M., Catanzaro E., Turrini E., Layla A., Akhtar S., Fimognari C. The potential effects of Ocimum basilicum on health: a review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018;14:679–692. doi: 10.1080/17425255.2018.1484450. [DOI] [PubMed] [Google Scholar]
- 347.Mostafavi S., Asadi-Gharneh H.A., Miransari M. The phytochemical variability of fatty acids in basil seeds (Ocimum basilicum L.) affected by genotype and geographical differences. Food Chem. 2019;276:700–706. doi: 10.1016/j.foodchem.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 348.Eftekhar N., Moghimi A., Hossein Boskabady M., Kaveh M., Shakeri F. Ocimum basilicum affects tracheal responsiveness, lung inflammatory cells and oxidant-antioxidant biomarkers in sensitized rats. Drug Chem. Toxicol. 2019;42:286–294. doi: 10.1080/01480545.2018.1459672. [DOI] [PubMed] [Google Scholar]
- 349.Kavitha S., John F., Indira M. Amelioration of inflammation by phenolic rich methanolic extract of Ocimum sanctum Linn. leaves in isoproterenol induced myocardial infarction. Indian J. Exp. Biol. 2015;53:632–640. [PubMed] [Google Scholar]
- 350.Bower A., Marquez S., de Mejia E.G. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2016;56:2728–2746. doi: 10.1080/10408398.2013.805713. [DOI] [PubMed] [Google Scholar]
- 351.Eğilmez O.K., Kökten N., Kalcıoğlu M.T., Ekici A.I.D., Şerifler S., Yeşilada E. Investigation of the protective effect of Nigella sativa oil in cisplatin induced oral mucositis: an experimental study. Turk. Arch. Otorhinolaryngol. 2020;58:10–15. doi: 10.5152/tao.2020.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Amin B., Hosseinzadeh H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med. 2016;82:8–16. doi: 10.1055/s-0035-1557838. [DOI] [PubMed] [Google Scholar]
- 353.Butt M.S., Pasha I., Sultan M.T., Randhawa M.A., Saeed F., Ahmed W. Black pepper and health claims: a comprehensive treatise. Crit. Rev. Food Sci. Nutr. 2013;53:875–886. doi: 10.1080/10408398.2011.571799. [DOI] [PubMed] [Google Scholar]
- 354.Qiblawi S., Dhanarasu S., Faris M.A. Chemopreventive effect of cardamom (Elettaria cardamomum L.) against benzo(α)pyrene-induced forestomach papillomagenesis in Swiss albino mice. J. Environ. Pathol. Toxicol. Oncol. 2015;34:95–104. doi: 10.1615/jenvironpatholtoxicoloncol.2015010838. [DOI] [PubMed] [Google Scholar]
- 355.Ashokkumar K., Murugan M., Dhanya M.K., Warkentin T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton] - a critical review. J. Ethnopharmacol. 2020;246:112244. doi: 10.1016/j.jep.2019.112244. [DOI] [PubMed] [Google Scholar]
- 356.Hariri M., Ghiasvand R. Cinnamon and chronic diseases. Adv. Exp. Med. Biol. 2016;929:1–24. doi: 10.1007/978-3-319-41342-6_1. [DOI] [PubMed] [Google Scholar]
- 357.Kumar S., Kumari R., Mishra S. Pharmacological properties and their medicinal uses of Cinnamomum: a review. J. Pharm. Pharmacol. 2019;71:1735–1761. doi: 10.1111/jphp.13173. [DOI] [PubMed] [Google Scholar]
- 358.Heidari B., Sajjadi S.E., Minaiyan M. Effect of Coriandrum sativum hydroalcoholic extract and its essential oil on acetic acid-induced acute colitis in rats. Avicenna J. Phytomed. 2016;6:205–214. [PMC free article] [PubMed] [Google Scholar]
- 359.Prachayasittikul V., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Coriander (Coriandrum sativum): a promising functional food toward the well-being. Food Res. Int. 2018;105:305–323. doi: 10.1016/j.foodres.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 360.Eguale T., Tilahun G., Debella A., Feleke A., Makonnen E. In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J. Ethnopharmacol. 2007;110:428–433. doi: 10.1016/j.jep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 361.Pandey A., Bigoniya P., Raj V., Patel K.K. Pharmacological screening of Coriandrum sativum Linn. for hepatoprotective activity. J. Pharm. Bioallied Sci. 2011;3:435–441. doi: 10.4103/0975-7406.84462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Mnif S., Aifa S. Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem. Biodivers. 2015;12:733–742. doi: 10.1002/cbdv.201400305. [DOI] [PubMed] [Google Scholar]
- 363.Jain S., Buttar H.S., Chintameneni M., Kaur G. Prevention of cardiovascular diseases with anti-inflammatory and anti-oxidant nutraceuticals and herbal products: an overview of pre-clinical and clinical studies. Recent Patents Inflamm. Allergy Drug Discov. 2018;12:145–157. doi: 10.2174/1872213X12666180815144803. [DOI] [PubMed] [Google Scholar]
- 364.Nagulapalli Venkata K.C., Swaroop A., Bagchi D., Bishayee A. A small plant with big benefits: fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201600950. [DOI] [PubMed] [Google Scholar]
- 365.Ouzir M., El Bairi K., Amzazi S. Toxicological properties of fenugreek (Trigonella foenum graecum) Food Chem. Toxicol. 2016;96:145–154. doi: 10.1016/j.fct.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 366.Warriar P., Barve K., Prabhakar B. Anti-arthritic effect of garcinol enriched fraction against adjuvant induced arthritis. Recent Patents Inflamm. Allergy Drug Discov. 2019;13:49–56. doi: 10.2174/1872213X12666181120091528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Pandey M.K., Kunnumakkara A.B., Aggarwal B.B. Kokum (Garcinol). Molecular targets and therapeutic uses of spices: modern uses for ancient medicine. World Sci. 2009:281–309. [Google Scholar]
- 368.Xu X., Miao Y., Chen J.Y., Zhang Q., Wang J. Effective production of S-allyl-L-cysteine through a homogeneous reaction with activated endogenous γ-glutamyltranspeptidase in garlic (Allium sativum) J. Food Sci. Technol. 2015;52:1724–1729. doi: 10.1007/s13197-013-1138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lv Y., So K.F., Wong N.K., Xiao J. Anti-cancer activities of S-allylmercaptocysteine from aged garlic. Chin. J. Nat. Med. 2019;17:43–49. doi: 10.1016/S1875-5364(19)30008-1. [DOI] [PubMed] [Google Scholar]
- 370.Li C., Sun X., Li A., Mo M., Zhao Z. S-Allylmercaptocysteine attenuates bleomycin-induced pulmonary fibrosis in mice via suppressing TGF-β1/Smad and oxidative stress pathways. Int. Immunopharmacol. 2020;79 doi: 10.1016/j.intimp.2019.106110. [DOI] [PubMed] [Google Scholar]
- 371.Haniadka R., Saldanha E., Sunita V., Palatty P.L., Fayad R., Baliga M.S. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe) Food Funct. 2013;4:845–855. doi: 10.1039/c3fo30337c. [DOI] [PubMed] [Google Scholar]
- 372.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Shi Z.Y., Zeng J.Z., AST Wong. Chemical structures and pharmacological profiles of ginseng saponins. Molecules. 2019;24:2443. doi: 10.3390/molecules24132443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kunnumakkara A.B., Chung J.G., Koca C., Dey S. Mint and its constituents. Molecular targets and therapeutic uses of spices: modern uses for ancient medicine. World Sci. 2009:373–401. [Google Scholar]
- 375.Brahmi F., Hadj-Ahmed S., Zarrouk A., Bezine M., Nury T., Madani K., Chibane M., Vejux A., Andreoletti P., Boulekbache-Makhlouf L., Lizard G. Evidence of biological activity of Mentha species extracts on apoptotic and autophagic targets on murine RAW264.7 and human U937 monocytic cells. Pharm. Biol. 2017;55:286–293. doi: 10.1080/13880209.2016.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Arumugam P., Priya N.G., Subathra M., Ramesh A. Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environ. Toxicol. Pharmacol. 2008;26:92–95. doi: 10.1016/j.etap.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 377.Mitrović P.M., Stamenković O.S., Banković-Ilić I., Djalović I.G., Nježić Z.B., Farooq M., Siddique K.H.M., Veljković V.B. White mustard (Sinapis alba L.) oil in biodiesel production: a review. Front. Plant Sci. 2020;11:299. doi: 10.3389/fpls.2020.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Barceloux D.G. Nutmeg (Myristica fragrans Houtt.) Dis. Mon. 2009;55:373–379. doi: 10.1016/j.disamonth.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 379.Francis S.K., James B., Varughese S., Nair M.S. Phytochemical investigation on Myristica fragrans stem bark. Nat. Prod. Res. 2019;33:1204–1208. doi: 10.1080/14786419.2018.1457670. [DOI] [PubMed] [Google Scholar]
- 380.Yang X.N., Liu X.M., Fang J.H., Zhu X., Yang X.W., Xiao X.R., Huang J.F., Gonzalez F.J., Li F. PPARα mediates the hepatoprotective effects of nutmeg. J. Proteome Res. 2018;17:1887–1897. doi: 10.1021/acs.jproteome.7b00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Suleria H.A., Butt M.S., Anjum F.M., Saeed F., Khalid N. Onion: nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015;55:50–66. doi: 10.1080/10408398.2011.646364. [DOI] [PubMed] [Google Scholar]
- 382.Nieto G., Ros G., Castillo J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): a review. Medicines (Basel) 2018;5(98) doi: 10.3390/medicines5030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Rocha J., Eduardo-Figueira M., Barateiro A., Fernandes A., Brites D., Bronze R., Duarte C.M., Serra A.T., Pinto R., Freitas M., Fernandes E., Silva-Lima B., Mota-Filipe H., Sepodes B. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015;116:398–413. doi: 10.1111/bcpt.12335. [DOI] [PubMed] [Google Scholar]
- 384.Martínez-Rodríguez J.L., Gutiérrez-Hernández R., Reyes-Estrada C.A., Granados-López A.J., Pérez-Veyna O., Arcos-Ortega T., Hepatoprotective López J.A. Antihyperlipidemic and radical scavenging activity of hawthorn (Crataegus oxyacantha) and rosemary (Rosmarinus officinalis) on alcoholic liver disease. Altern. Ther. Health Med. 2019;25:54–63. [PubMed] [Google Scholar]
- 385.Moshiri M., Vahabzadeh M., Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents: a review. Drug Res. (Stuttg.) 2015;65:287–295. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- 386.Majdalawieh A.F., Massri M., Nasrallah G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum) Eur. J. Pharmacol. 2017;815:512–521. doi: 10.1016/j.ejphar.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 387.Bai X., Gou X., Cai P., Xu C., Cao L., Zhao Z., Huang M., Jin J. Sesamin enhances Nrf2-mediated protective defense against oxidative stress and inflammation in colitis via AKT and ERK activation. Oxidative Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/2432416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Domiciano T.P., Dalalio M.M., Silva E.L., Ritter A.M., Estevão-Silva C.F., Ramos F.S., Caparroz-Assef S.M., Cuman R.K., Bersani-Amado C.A. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn Schmiedeberg's Arch. Pharmacol. 2013;386:331–338. doi: 10.1007/s00210-012-0820-5. [DOI] [PubMed] [Google Scholar]
- 389.De M., De A.K., Sen P., Banerjee A.B. Antimicrobial properties of star anise (Illicium verum Hook f) Phytother. Res. 2002;16:94–95. doi: 10.1002/ptr.989. [DOI] [PubMed] [Google Scholar]
- 390.Patra J.K., Das G., Bose S., Banerjee S., Vishnuprasad C.N., Del Pilar Rodriguez-Torres M., Shin H.S. Star anise (Illicium verum): chemical compounds, antiviral properties, and clinical relevance. Phytother. Res. 2020;34:1248–1267. doi: 10.1002/ptr.6614. [DOI] [PubMed] [Google Scholar]
- 391.Kengaiah J., Nandish S.K.M., Ramachandraiah C., Chandramma, Shivaiah A., Vishalakshi G.J., Paul M., Santhosh M.S., Shankar R.L., Sannaningaiah D. Protective effect of tamarind seed coat ethanol extract on eryptosis induced by oxidative stress. Biochemistry (Mosc) 2020;85:119–129. doi: 10.1134/S0006297920010113. [DOI] [PubMed] [Google Scholar]
- 392.Chong U.R., Abdul-Rahman P.S., Abdul-Aziz A., Hashim O.H., Mat-Junit S. Effects of Tamarindus indica fruit pulp extract on abundance of HepG2 cell lysate proteins and their possible consequential impact on metabolism and inflammation. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/459017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 394.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Arablou T., Kolahdouz-Mohammadi R. Curcumin and endometriosis: review on potential roles and molecular mechanisms. Biomed. Pharmacother. 2018;97:91–97. doi: 10.1016/j.biopha.2017.10.119. [DOI] [PubMed] [Google Scholar]
- 396.Sandur S.K., Ichikawa H., Pandey M.K., Kunnumakkara A.B., Sung B., Sethi G., Aggarwal B.B. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic. Biol. Med. 2007;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Anand P., Thomas S.G., Kunnumakkara A.B., Sundaram C., Harikumar K.B., Sung B., Tharakan S.T., Misra K., Priyadarsini I.K., Rajasekharan K.N., Aggarwal B.B. Biological activities of curcumin and its analogues (congeners) made by man and mother nature. Biochem. Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 398.Kunnumakkara A.B., Guha S., Krishnan S., Diagaradjane P., Gelovani J., Aggarwal B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 399.Kunnumakkara A.B., Diagaradjane P., Anand P., Harikumar K.B., Deorukhkar A., Gelovani J., Guha S., Krishnan S., Aggarwal B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer. 2009;125:2187–2197. doi: 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- 400.Kunnumakkara A.B., Bordoloi D., Harsha C., Banik K., Gupta S.C., Aggarwal B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. (Lond.) 2017;131:1781–1799. doi: 10.1042/CS20160935. [DOI] [PubMed] [Google Scholar]
- 401.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 402.Lin Y.G., Kunnumakkara A.B., Nair A., Merritt W.M., Han L.Y., Armaiz-Pena G.N., Kamat A.A., Spannuth W.A., Gershenson D.M., Lutgendorf S.K., Aggarwal B.B., Sood A.K. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin. Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 403.Shabnam B., Harsha C., Thakur K.K., Khatoon E., Kunnumakkara A.B. The Chemistry and Bioactive Components of Turmeric. Royal Society of Chemistry; 2020. Curcumin: a potential molecule for the prevention and treatment of inflammatory diseases; pp. 150–171. [Google Scholar]
- 404.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Hong D.S., Camacho L.H., Ng C., Kurzrock R. Curcumin and pancreatic cancer: phase II clinical trial experience. J. Clin. Oncol. 2007;25:4599. [Google Scholar]
- 405.Kunnumakkara A.B., Anand P., Aggarwal B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 406.Kunnumakkara A.B., Guha S., Aggarwal B.B. Curcumin and colorectal cancer: add spice to your life. Curr. Colorectal Cancer Rep. 2009;5(5) [Google Scholar]
- 407.Bordoloi D., Roy N.K., Monisha J., Padmavathi G., Kunnumakkara A.B. Multi-targeted agents in cancer cell chemosensitization: what we learnt from curcumin thus far. Recent Pat. Anticancer Drug Discov. 2016;11:67–97. doi: 10.2174/1574892810666151020101706. [DOI] [PubMed] [Google Scholar]
- 408.Kunnumakkara A.B., Harsha C., Banik K., Vikkurthi R., Sailo B.L., Bordoloi D., Gupta S.C., Aggarwal B.B. Is curcumin bioavailability a problem in humans: lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019;15:705–733. doi: 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- 409.Bordoloi D, Kunnumakkara AB. The potential of curcumin: a multitargeting agent in cancer cell chemosensitization. Role of Nutraceuticals in Cancer Chemosensitization. Academic Press, pp. 31–60.
- 410.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Ng C.S., Badmaev V., Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 411.Kunnumakkara A.B., Diagaradjane P., Guha S., Deorukhkar A., Shentu S., Aggarwal B.B., Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 412.Kazemi S., Yaghooblou F., Siassi F., Rahimi Foroushani A., Ghavipour M., Koohdani F., Sotoudeh G. Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: a randomized double-blind clinical trial. J. Sci. Food Agric. 2017;97:5296–5301. doi: 10.1002/jsfa.8414. [DOI] [PubMed] [Google Scholar]
- 413.Aghasi M., Koohdani F., Qorbani M., Nasli-Esfahani E., Ghazi-Zahedi S., Khoshamal H., Keshavarz A., Sotoudeh G. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double-blind placebo controlled clinical trial. J. Sci. Food Agric. 2019;99:3933–3940. doi: 10.1002/jsfa.9617. [DOI] [PubMed] [Google Scholar]
- 414.Daneshi-Maskooni M., Keshavarz S.A., Qorbani M., Mansouri S., Alavian S.M., Badri-Fariman M., Jazayeri-Tehrani S.A., Sotoudeh G. Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: a double-blind randomized placebo-controlled clinical trial. BMC Complement. Altern. Med. 2019;19(59) doi: 10.1186/s12906-019-2465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Shim H.I., Song D.J., Shin C.M., Yoon H., Park Y.S., Kim N., Lee D.H. Inhibitory effects of β-caryophyllene on helicobacter pylori infection: a randomized double-blind, placebo-controlled study. Kor. J. Gastroenterol. 2019;74:199–204. doi: 10.4166/kjg.2019.74.4.199. [DOI] [PubMed] [Google Scholar]
- 416.Reuter J., Huyke C., Casetti F., Theek C., Frank U., Augustin M., Schempp C. Anti-inflammatory potential of a lipolotion containing coriander oil in the ultraviolet erythema test. J. Dtsch Dermatol. Ges. 2008;6:847–851. doi: 10.1111/j.1610-0387.2008.06704.x. [DOI] [PubMed] [Google Scholar]
- 417.Xu C., Mathews A.E., Rodrigues C., Eudy B.J., Rowe C.A., O'Donoughue A., Percival S.S. Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: a randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN. 2018;24:148–155. doi: 10.1016/j.clnesp.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 418.Dehghani S., Alipoor E., Salimzadeh A., Yaseri M., Hosseini M., Feinle-Bisset C., Hosseinzadeh-Attar M.J. The effect of a garlic supplement on the pro-inflammatory adipocytokines, resistin and tumor necrosis factor-alpha, and on pain severity, in overweight or obese women with knee osteoarthritis. Phytomedicine. 2018;48:70–75. doi: 10.1016/j.phymed.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 419.Aryaeian N., Shahram F., Mahmoudi M., Tavakoli H., Yousefi B., Arablou T., Jafari Karegar S. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active rheumatoid arthritis. Gene. 2019;698:179–185. doi: 10.1016/j.gene.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 420.Mozaffari-Khosravi H., Naderi Z., Dehghan A., Nadjarzadeh A., Fallah Huseini H. Effect of ginger supplementation on proinflammatory cytokines in older patients with osteoarthritis: outcomes of a randomized controlled clinical trial. J. Nutr. Gerontol. Geriatr. 2016;35:209–218. doi: 10.1080/21551197.2016.1206762. [DOI] [PubMed] [Google Scholar]
- 421.Kulkarni R.A., Deshpande A.R. Anti-inflammatory and antioxidant effect of ginger in tuberculosis. J. Complement Integr. Med. 2016;13:201–206. doi: 10.1515/jcim-2015-0032. [DOI] [PubMed] [Google Scholar]
- 422.Zick S.M., Turgeon D.K., Ren J., Ruffin M.T., Wright B.D., Sen A., Djuric Z., Brenner D.E. Pilot clinical study of the effects of ginger root extract on eicosanoids in colonic mucosa of subjects at increased risk for colorectal cancer. Mol. Carcinog. 2015;54:908–915. doi: 10.1002/mc.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zhang J.H., Wang J.P., Wang H.J. Clinical study on effect of total panax notoginseng saponins on immune related inner environment imbalance in rheumatoid arthritis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27:589–592. (Chinese) [PubMed] [Google Scholar]
- 424.Xia Z.Y., Liu X.Y., Zhan L.Y., He Y.H., Luo T., Xia Z. Ginsenosides compound (shen-fu) attenuates gastrointestinal injury and inhibits inflammatory response after cardiopulmonary bypass in patients with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2005;130:258–264. doi: 10.1016/j.jtcvs.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 425.Zilaee M., Hosseini S.A., Jafarirad S., Abolnezhadian F., Cheraghian B., Namjoyan F., Ghadiri A. An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: a double-blind, randomized placebo-controlled trial. Respir. Res. 2019;20(39) doi: 10.1186/s12931-019-0998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Ghiasian M., Khamisabadi F., Kheiripour N., Karami M., Haddadi R., Ghaleiha A., Taghvaei B., Oliaie S.S., Salehi M., Samadi P., Ranjbar A. Effects of crocin in reducing DNA damage, inflammation, and oxidative stress in multiple sclerosis patients: a double-blind, randomized, and placebo-controlled trial. J. Biochem. Mol. Toxicol. 2019;33 doi: 10.1002/jbt.22410. [DOI] [PubMed] [Google Scholar]
- 427.Khadem Haghighian M., Alipoor B., Malek Mahdavi A., Eftekhar Sadat B., Asghari Jafarabadi M., Moghaddam A. Effects of sesame seed supplementation on inflammatory factors and oxidative stress biomarkers in patients with knee osteoarthritis. Acta Med. Iran. 2015;53:207–213. [PubMed] [Google Scholar]
- 428.Panahi Y., Hosseini M.S., Khalili N., Naimi E., Majeed M., Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015;34:1101–1108. doi: 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 429.Uchio R., Muroyama K., Okuda-Hanafusa C., Kawasaki K., Yamamoto Y., Murosaki S. Hot water extract of Curcuma longa L. improves serum inflammatory markers and general health in subjects with overweight or prehypertension/mild hypertension: a randomized, double-blind, placebo-controlled trial. Nutrients. 2019;11:1822. doi: 10.3390/nu11081822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Panahi Y., Khalili N., Sahebi E., Namazi S., Atkin S.L., Majeed M., Sahebkar A. Curcuminoids plus piperine modulate adipokines in type 2 diabetes mellitus. Curr. Clin. Pharmacol. 2017;12:253–258. doi: 10.2174/1574884713666180104095641. [DOI] [PubMed] [Google Scholar]
- 431.Adibian M., Hodaei H., Nikpayam O., Sohrab G., Hekmatdoost A., Hedayati M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019;33:1374–1383. doi: 10.1002/ptr.6328. [DOI] [PubMed] [Google Scholar]
- 432.Srivastava S., Saksena A.K., Khattri S., Kumar S., Dagur R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology. 2016;24:377–388. doi: 10.1007/s10787-016-0289-9. [DOI] [PubMed] [Google Scholar]
- 433.Conrozier T., Mathieu P., Bonjean M., Marc J.F., Renevier J.L., Balblanc J.C. A complex of three natural anti-inflammatory agents provides relief of osteoarthritis pain. Altern. Ther. Health Med. 2014;20:32–37. [PubMed] [Google Scholar]
- 434.Amalraj A., Varma K., Jacob J., Divya C., Kunnumakkara A.B., Stohs S.J., Gopi S.A. Novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: a randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food. 2017;20:1022–1030. doi: 10.1089/jmf.2017.3930. [DOI] [PubMed] [Google Scholar]
- 435.Moreillon J.J., Bowden R.G., Deike E., Griggs J., Wilson R., Shelmadine B., Cooke M., Beaujean A. The use of an anti-inflammatory supplement in patients with chronic kidney disease. J. Complement. Integr. Med. 2013;10 doi: 10.1515/jcim-2012-0011. (/j/jcim.2013.10.issue-1/jcim-2012-0011/jcim-2012-0011.xml) [DOI] [PubMed] [Google Scholar]
- 436.Samadian F., Dalili N., Poor-Reza Gholi F., Fattah M., Malih N., Nafar M., Firoozan A., Ahmadpoor P., Samavat S., Ziaie S. Evaluation of Curcumin's effect on inflammation in hemodialysis patients. Clin. Nutr. ESPEN. 2017;22:19–23. doi: 10.1016/j.clnesp.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 437.Panahi Y., Ghanei M., Bashiri S., Hajihashemi A., Sahebkar A. Short-term curcuminoid supplementation for chronic pulmonary complications due to sulfur mustard intoxication: positive results of a randomized double-blind placebo-controlled trial. Drug Res. (Stuttg.) 2015;65:567–573. doi: 10.1055/s-0034-1389986. [DOI] [PubMed] [Google Scholar]
- 438.Funamoto M., Sunagawa Y., Katanasaka Y., Miyazaki Y., Imaizumi A., Kakeya H., Yamakage H., Satoh-Asahara N., Komiyama M., Wada H., Hasegawa K., Morimoto T. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:2029–2034. doi: 10.2147/COPD.S104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Abdolahi M., Jafarieh A., Sarraf P., Sedighiyan M., Yousefi A., Tafakhori A., Abdollahi H., Salehinia F., Djalali M. The neuromodulatory effects of ω-3 fatty acids and nano-curcumin on the COX-2/iNOS network in migraines: a clinical trial study from gene expression to clinical symptoms. Endocr Metab Immune Disord Drug Targets. 2019;19:874–884. doi: 10.2174/1871530319666190212170140. [DOI] [PubMed] [Google Scholar]
- 440.Babaei F., Nassiri-Asl M., Hosseinzadeh H. Curcumin (a constituent of turmeric): new treatment option against COVID-19. Food Sci. Nutr. 2020;8:5215–5227. doi: 10.1002/fsn3.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Saeedi-Boroujeni A., Mahmoudian-Sani M.R., Bahadoram M., Alghasi A. COVID-19: a case for inhibiting NLRP3 inflammasome, suppression of inflammation with curcumin? Basic Clin. Pharmacol. Toxicol. 2021;128:37–45. doi: 10.1111/bcpt.13503. [DOI] [PubMed] [Google Scholar]
- 442.Quiles J.L., Rivas-García L., Varela-López A., Llopis J., Battino M., Sánchez-González C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ. Res. 2020;191:110053. doi: 10.1016/j.envres.2020.110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Gupta H., Gupta M., Bhargava S. Potential use of turmeric in COVID-19. Clin. Exp. Dermatol. 2020;45:902–903. doi: 10.1111/ced.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Horowitz R.I., Freeman P.R. Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials. Med. Hypotheses. 2020;143:109851. doi: 10.1016/j.mehy.2020.109851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Hooper P.L. COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones. 2020;25:707–710. doi: 10.1007/s12192-020-01126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Stancioiu F., Papadakis G.Z., Kteniadakis S., Izotov B.N., Coleman M.D., Spandidos D.A., Tsatsakis A. A dissection of SARS‑CoV2 with clinical implications (review) Int. J. Mol. Med. 2020;46:489–508. doi: 10.3892/ijmm.2020.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Eisenhut M., Shin J.I. Pathways in the pathophysiology of coronavirus 19 Lung disease accessible to prevention and treatment. Front. Physiol. 2020;11:872. doi: 10.3389/fphys.2020.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.He Y.Q., Zhou C.C., Yu L.Y., Wang L., Deng J.L., Tao Y.L., Zhang F., Chen W.S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2020;105224 doi: 10.1016/j.phrs.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Lin C.Y., Yao C.A. Potential role of Nrf2 activators with dual antiviral and anti-inflammatory properties in the management of viral pneumonia. Infect Drug Resist. 2020;13:1735–1741. doi: 10.2147/IDR.S256773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., Du J. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 2020;12:1193. doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Zarubin A., Stepanov V., Markov A., Kolesnikov N., Marusin A., Khitrinskaya I., Swarovskaya M., Litvinov S., Ekomasova N., Dzhaubermezov M., Maksimova N., Sukhomyasova A., Shtygasheva O., Khusnutdinova E., Radzhabov M., Kharkov V. Structural variability, expression profile, and pharmacogenetic properties of TMPRSS2 gene as a potential target for COVID-19 therapy. Genes (Basel) 2020;12 doi: 10.3390/genes12010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Sagar S., Rathinavel A.K., Lutz W.E., Struble L.R., Khurana S., Schnaubelt A.T., Mishra N.K., Guda C., Broadhurst M.J., Reid S.P.M., Bayles K.W., Borgstahl G.E.O., Radhakrishnan P. Bromelain inhibits SARS-CoV-2 infection in VeroE6 cells. bioRxiv. 2020 doi: 10.1002/ctm2.281. 09.16.297366. [preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kritis P., Karampela I., Kokoris S., Dalamaga M. The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metabol. Open. 2020;8:100066. doi: 10.1016/j.metop.2020.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Roshdy W.H., Rashed H.A., Kandeil A., Mostafa A., Moatasim Y., Kutkat O., Abo Shama N.M., Gomaa M.R., El-Sayed I.H., El Guindy N.M., Naguib A., Kayali G., Ali M.A. EGYVIR: an immunomodulatory herbal extract with potent antiviral activity against SARS-CoV-2. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Roy A., Sarkar B., Celik C., Ghosh A., Basu U., Jana M., Jana A., Gencay A., Can Sezgin G., Ildiz N., Dam P., Mandal A.K., Ocsoy I. Can concomitant use of zinc and curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19? Phytother. Res. 2020;34:2425–2428. doi: 10.1002/ptr.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Celik C., Gencay A., Ocsoy I. Can food and food supplements be deployed in the fight against the COVID 19 pandemic? Biochim. Biophys. Acta Gen. Subj. 1865;2021:129801. doi: 10.1016/j.bbagen.2020.129801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Miryan M., Bagherniya M., Sahebkar A., Soleimani D., Rouhani M.H., Iraj B., Askari G. Effects of curcumin-piperine co-supplementation on clinical signs, duration, severity, and inflammatory factors in patients with COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:1027. doi: 10.1186/s13063-020-04924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Hassaniazad M., Inchehsablagh B.R., Kamali H., Tousi A., Eftekhar E., Jaafari M.R., Fathalipour M., Nikoofal-Sahlabadi S., Gouklani H., Alizade H., Nikpoor A.R. The clinical effect of Nano micelles containing curcumin as a therapeutic supplement in patients with COVID-19 and the immune responses balance changes following treatment: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:876. doi: 10.1186/s13063-020-04824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Jampilek J., Kralova K. Potential of nanonutraceuticals in increasing immunity. Nanomaterials (Basel) 2020;10:2224. doi: 10.3390/nano10112224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., Ziya Gencer M., Ammari A., Sadeghi A., Roshangar L., Aslani S., Esmaeilzadeh A., Ghaebi M., Valizadeh S., Ahmadi M. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020;89:107088. doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Saber-Moghaddam N., Salari S., Hejazi S., Amini M., Taherzadeh Z., Eslami S., Rezayat S.M., Jaafari M.R., Elyasi S. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial. Phytother. Res. 2021 doi: 10.1002/ptr.7004. [DOI] [PubMed] [Google Scholar]
- 462.Tahmasebi S., El-Esawi M.A., Mahmoud Z.H., Timoshin A., Valizadeh H., Roshangar L., Varshoch M., Vaez A., Aslani S., Navashenaq J.G., Aghebati-Maleki L., Ahmadi M. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J. Cell. Physiol. 2020 doi: 10.1002/jcp.30233. [DOI] [PubMed] [Google Scholar]
- 463.Liu Z., Ying Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. Front. Cell Dev. Biol. 2020;8:479. doi: 10.3389/fcell.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Soni V.K., Mehta A., Ratre Y.K., Tiwari A.K., Amit A., Singh R.P., Sonkar S.C., Chaturvedi N., Shukla D., Vishvakarma N.K. Curcumin, a traditional spice component, can hold the promise against COVID-19? Eur. J. Pharmacol. 2020;886:173551. doi: 10.1016/j.ejphar.2020.173551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., Lee C.K., Chang S.T., Kuo C.J., Lee S.S., Hou C.C., Hsiao P.W., Chien S.C., Shyur L.F., Yang N.S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 466.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Li Y., Wang J., Liu Y., Luo X., Lei W., Xie L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020;101:1079–1084. doi: 10.1099/jgv.0.001466. [DOI] [PubMed] [Google Scholar]
- 468.Kandeel M., Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251:117627. doi: 10.1016/j.lfs.2020.117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Rajagopal K., Varakumar P., Baliwada A., Byran G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach. Futur. J. Pharm. Sci. 2020;6:104. doi: 10.1186/s43094-020-00126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Adhikari B., Marasini B.P., Rayamajhee B., Bhattarai B.R., Lamichhane G., Khadayat K., Adhikari A., Khanal S., Parajuli N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: a review. Phytother. Res. 2020 doi: 10.1002/ptr.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Ibrahim M.A.A., Abdelrahman A.H.M., Hussien T.A., Badr E.A.A., Mohamed T.A., El-Seedi H.R., Pare P.W., Efferth T., Hegazy M.F. In silico drug discovery of major metabolites from spices as SARS-CoV-2 main protease inhibitors. Comput. Biol. Med. 2020;126:104046. doi: 10.1016/j.compbiomed.2020.104046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Basu S., Veeraraghavan B., Ramaiah S., Anbarasu A. Novel cyclohexanone compound as a potential ligand against SARS-CoV-2 main-protease. Microb. Pathog. 2020;149:104546. doi: 10.1016/j.micpath.2020.104546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Umadevi P., Manivannan S., Fayad A.M., Shelvy S. In silico analysis of phytochemicals as potential inhibitors of proteases involved in SARS-CoV-2 infection. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1866669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Kumar G., Kumar D., Singh N.P. Therapeutic approach against 2019-nCoV by inhibition of the ACE-2 receptor. Drug. Res. (Stuttg.) 2020 doi: 10.1055/a-1337-4462. [DOI] [PubMed] [Google Scholar]
- 475.Barh D., Tiwari S., Weener M.E., Azevedo V., Góes-Neto A., Gromiha M.M., Ghosh P. Multi-omics-based identification of SARS-CoV-2 infection biology and candidate drugs against COVID-19. Comput. Biol. Med. 2020;126:104051. doi: 10.1016/j.compbiomed.2020.104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Mrityunjaya M., Pavithra V., Neelam R., Janhavi P., Halami P.M., Ravindra P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 2020;11:570122. doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Rocha F.A.C., de Assis M.R. Curcumin as a potential treatment for COVID-19. Phytother. Res. 2020;34:2085–2087. doi: 10.1002/ptr.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-RasadiK Banach M., Sahebkar A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020;34:2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Rahban M., Habibi-Rezaei M., Mazaheri M., Saso L., Moosavi-Movahedi A.A. Anti-viral potential and modulation of Nrf2 by curcumin: pharmacological implications. Antioxidants (Basel) 2020;9:1228. doi: 10.3390/antiox9121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Soni V.K., Mehta A., Shukla D., Kumar S., Vishvakarma N.K. Fight COVID-19 depression with immunity booster: curcumin for psychoneuroimmunomodulation. Asian J. Psychiatr. 2020;53:102378. doi: 10.1016/j.ajp.2020.102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Keflie T.S., Biesalski H.K. Micronutrients and bioactive substances: their potential roles in combating COVID-19. Nutrition. 2020;84:111103. doi: 10.1016/j.nut.2020.111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Plos, Variants, 3 New Covid Vaccines, and Contested Efficacy Claims: A Month of Seismic Shifts and Confusion, https://absolutelymaybe.plos.org/2021/01/31/variants-3-new-covid-vaccines-and-contested-efficacy-claims-a-month-of-seismic-shifts-and-confusion/ (2021, accessed 27 January 2021).
- 484.GoodRx, The Latest Research on COVID-19 Treatments and Medications in the Pipeline, https://www.goodrx.com/blog/coronavirus-treatments-on-the-way/ (2021, accessed 25 January 2021).