Abstract
Purpose
We describe the large-scale self-initiated recruitment of patients to a self-monitoring initiative for macular pathologic features during the coronavirus disease 2019 (COVID-19) pandemic.
Design
Observational study with retrospective analysis.
Participants
A total of 2272 patients from the Singapore National Eye Centre (SNEC) whose visits were rescheduled over lockdown (April 13–June 1, 2020) were offered participation in a self-monitoring initiative administered by SNEC with the Alleye application (Switzerland) as the testing instrument.
Methods
This was an observational study with retrospective analysis. Demographics and characteristics were compared between those who signed up and those who did not. Similar comparisons were made between patients who complied with the initiative versus those who did not. Outcomes were tracked for 6 months starting from the commencement of lockdown.
Main Outcome Measures
Participation and compliance rates and characteristics of patients who were more likely to participate and comply with the initiative.
Results
Seven hundred thirty-two patients (32%) participated in this self-monitoring initiative. Those who participated were younger (62 years of age vs. 68 years of age; P < 0.001), men, and living with family. Patients not receiving treatment and those with poorer vision in the worse-seeing eye were more likely to participate. When grouped according to diagnosis, the proportion who participated was highest for diabetic macular edema (52%), nonneovascular age-related macular degeneration (AMD; 42%), diabetic retinopathy (35%), retinal vein occlusions (18%), and neovascular AMD (15%; P < 0.001). Testing compliance rate was 43% (315/732). Patients who complied with the initiative were older, were receiving treatment, and had poorer vision in the worse-seeing eye. Trigger events occurred in 33 patients, with 5 patients having clinically verified disease progression (1.6%).
Conclusions
We provide clinical data on characteristics of patients with stable retinal diseases who were offered, participated in, and complied with a self-monitoring program. The lower participation rate compared with standardized clinical studies reflects the difficulties in implementation for such initiatives in clinical settings. Despite this, self-monitoring continues to show promise in relieving clinic resources, suggesting the feasibility of scaling such programs beyond the COVID-19 pandemic.
Keywords: COVID-19, Digital, Mobile, Retina, Self-monitoring
Abbreviations and Acronyms: AMD, age-related macular degeneration; CI, confidence interval; COVID-19, coronavirus disease 2019; DME, diabetic macular edema; DR, diabetic retinopathy; OR, odds ratio; RVO, retinal vein occlusion; SNEC, Singapore National Eye Centre; VA, visual acuity
Early detection and treatment of disease activity are key principles in the management of the major retinal diseases, such as age-related macular degeneration (AMD), diabetic retinopathy (DR) including diabetic macular edema (DME), and retinal vein occlusion (RVO), to avoid irreversible vision loss.1, 2, 3 However, many patients with early or stable retinal diseases do not need active treatment (e.g., intravitreal injections) but rather need active monitoring for signs of disease progression or recurrence. These traditional face-to-face in-clinic monitoring visits contribute to a significant burden of care and resources.
Self-monitoring or home monitoring offers the potential for detecting disease progression before a patient becomes aware of functional deterioration.4, 5, 6 This strategy is appealing and can help to alleviate the significant burden on eye care services.7 However, self-monitoring programs have not been widely scaled or adopted. The coronavirus disease 2019 (COVID-19) global pandemic provides a setting in which an urgent need for self-monitoring arose because face-to-face visits to hospitals for monitoring were canceled or deferred in many countries as part of social distancing measures and lockdowns to minimize person-to-person interactions and the spread of the virus.8, 9, 10, 11, 12, 13 However, only a few studies have evaluated the clinical use of self-monitoring programs to understand challenges in implementation and adoption.14
In Singapore, a countrywide lockdown (the so-called circuit breaker period) was enforced from April 13, 2020, to June 1, 2020. During this period, hospital service was limited to provide only acute and essential care. In support of patients whose appointments had been affected, we initiated a self-monitoring program to provide patients with stable retinal diseases an alternative way to monitor their vision for new signs of disease activity. This report aims to describe the experience and characterize the profile of patients who agreed to participate in this program versus those who declined and, among the former, those who complied with this program. Our study provides one of the first clinical insights into the potential facilitators of and barriers to self-monitoring for stable retinal diseases, which may inform long-term, postpandemic implementation of such initiatives and strategies. Although this report highlights the findings from this extraordinary circumstance, it is applicable to a postpandemic new normal where such digital self-monitoring initiatives may represent new models of care that can help to reduce the burden of care.
Methods
We conducted an observational study of patients at a retinal specialist clinic in a large tertiary eye center in Singapore whose clinic visits were rescheduled during the Singapore lockdown period (April 13–June 1, 2020). These patients were offered a self-monitoring program with a digital application, or app (Alleye, Switzerland), as the testing instrument. The research described adhered to the tenets of the Declaration of Helsinki, and Singhealth institutional ethics approval was obtained. All patients gave informed consent by accepting the user agreement within the app during sign up, which allowed the use of their anonymized data for this analysis. Data for this analysis were obtained from a review of the electronic medical records from these patients.
Selection of Self-Monitoring Platform
Although a number of self-monitoring solutions are available, many required dedicated devices or comprehensive patient training, which would preclude rapid roll-out of the initiative during the COVID-19 lockdown.17, 18, 19 To meet our needs for a rapidly deployable, easy-to-use solution, we selected the Alleye mobile digital app as the detection tool. This smartphone-based app is a CE (European Conformity)-marked Class I device that received Food and Drug Administration 510(k) clearance for monitoring eyesight in AMD in 2018. It was intended for use to monitor patients with AMD and DR, for both detection of new disease and monitoring of treatment response.
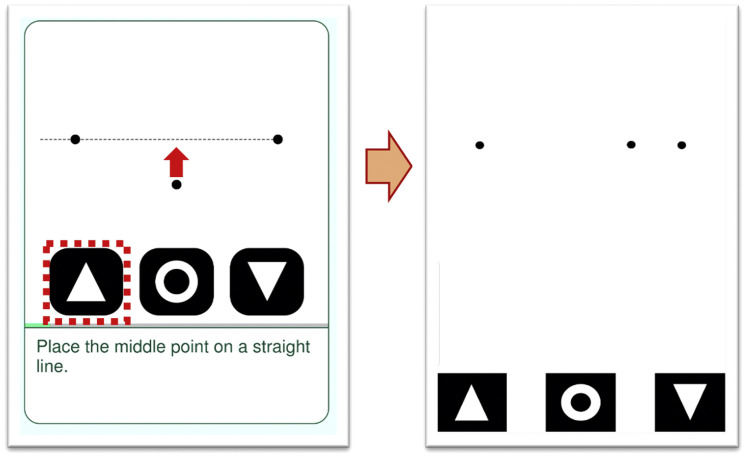
The app was designed as a mobile medical software application for the detection and characterization of metamorphopsia in the context of AMD. The principle of operations uses a dot alignment test of metamorphopsia and automatically triggers changes in score as a potential sign of worsening disease. Detailed use and validation of the app has been reported previously. In brief, the Alleye app was designed for home use by patients who can regularly perform the test on their personal mobile devices. The test consists of a dot alignment hyperacuity task. Patients perform the monocular task, which is to align a center dot to 2 fixed flanking dots to create an imaginary straight line (Fig 1 ). The task is repeated 12 times with flanking dots in different orientations and distances. In the pilot study, we advised the patients to perform the test twice weekly. The app displays a score reflecting the visual performance of the patient in relationship to a healthy individual. The maximum score is 100 and minimum is 0. A score of 100 indicates visual performance comparable with that of a healthy individual with no eye conditions. A drop in 25 points was determined to be a clinically relevant threshold to differentiate detection of worsening eye disease. The score of the last best test (score before a > 25-point drop) was used as a reference for subsequent test scores. Patients were advised to repeat a test if a drop of 25 points or more was detected. This was displayed to the patients on the app as a red circle. Three test scores consistently 25 points less than the reference constituted a trigger event.
Figure 1.
Diagram showing the hyperacuity dot test in the Alleye app. The user attempts to align the central dot to form a straight line in relationship to the other 2 dots. The degree of alignment will translate the subjective level of metamorphopsia to an objective score.
Patient Selection
During the lockdown (April 13–June 1, 2020), all nonessential visits to the eye center were deferred. The decision to reschedule was determined by the consultant in charge of each patient with appointments originally scheduled during the affected period and was based on the primary diagnosis, stability of disease, treatment regimen, and status of patients’ fellow eyes. Most patients receiving active treatment during the initial phase of neovascular AMD or those with persistently active disease were not deferred. Appointments for patients with other conditions like DME, RVO, or neovascular AMD observing longer, stable retreatment intervals were deferred if the benefit of deferral was deemed to outweigh the risk of visual loss. The rescheduling exercise occurred 2 weeks before lockdown, and for patients who were deemed suitable to be rescheduled, physicians could decide between a deferment period of 4 months or fewer (with a minimum of 2 months), 4 to 6 months, or 6 months or more. For the purpose of this analysis, we defined a long follow-up interval as more than 4 months.
Application Dissemination and Onboarding
A key difference in the methodology of this initiative compared with previously reported studies is that no face-to-face consultation or prescription of the app was performed. Because of the extenuating circumstances of the lockdown, patients from the retina clinic whose appointments were rescheduled were sent a text message (in their primary language as indicated during prior visits) to invite them to take part in the initiative and instruct them on the aims and use of the self-monitoring initiative. Patients were directed to a mobile device-friendly custom webpage for consent, instructions on app use, and information on the follow-up action in the case of disease deterioration. These instructions were delivered in a language of choice. On completing the mandatory instructional tutorial, a unique code to download the app was sent to patients to activate their personal accounts. The participation in the self-monitoring initiative, including the use of the app, was provided free of charge for all patients.
Manging Trigger Events: Monitoring and Integration into Hospital Appointments System
A dedicated team from the Singapore National Eye Centre (SNEC) Ocular Reading Centre was designated to monitor and manage the patients participating in this self-monitoring program. A standard operating procedure was established to detail the workflow and actions taken for a trigger event (Supplemental Appendix 1, available at www.ophthalmologyretina.org.). Briefly, a web-based dashboard, which was checked daily, was available for remote monitoring of all participating patients’ Alleye scores and triggers. This team was responsible for triaging patients who experienced a triggering event via a telephone consultation and to facilitate urgent appointments at the retina clinic at SNEC for further evaluation if deemed necessary.
Outcome Measures
Clinical and demographic data were collected retrospectively from the electronic medical record of the baseline visit, defined as the last visit recorded before lockdown. Patients were then tracked from the commencement of the initiative, April 3, 2020, through September 1, 2020. Demographic characteristics compared for this per-patient analysis consisted of age, gender, race, living arrangements, and the primary diagnosis of retinal pathologic feature. Clinical data such as whether the patient was receiving active treatment (defined as having a treatment administered 3 months before the beginning of lockdown), intended rescheduled interval (divided into ≤ 4 months or > 4 months), and visual acuity (VA) in the better eye and worse eye (expressed as the number of letters read on a logarithm of the minimum angle of resolution VA chart and recorded by whichever reading was best: uncorrected, corrected, or pinhole) were also recorded. If the patients had more than 1 retinal diagnosis (regardless of eye), the primary diagnosis was assigned based on a hierarchy. The diagnosis of a condition for which the patient was receiving treatment took precedence. Next, if the patient was not receiving active treatment, the diagnosis of highest severity that was threatening vision was selected. Diagnosis was grouped into: neovascular AMD, nonneovascular AMD (comprising both early and late AMD, including eyes with macular atrophy and scars), DME, DR, RVO, and all other retinal pathologic features (others).
Trigger events were divided into true-positive triggers, defined as trigger events that resulted in an urgent consultation and disease progression on clinical examination, or false-positive triggers, defined as (1) trigger events that resulted in an urgent consultation, but no disease progression on clinical examination, or (2) trigger events resulting from misuse of the app as determined during telephone triage (examples include, but were not restricted to, wrong eye tested, wrong patient, or improper use of the app).
In this analysis, 3 main comparisons were performed. First, we compared characteristics of patients who signed up for this self-monitoring program versus those who did not. Next, among those who signed up, we further compared characteristics between compliant and noncompliant users. Compliant users were defined as patients who performed the recommended number of tests (at least 2) per week until the time of analysis (September 1, 2020), as stipulated in the instructions. Finally, among the patients who experienced a trigger event (defined as 3 test scores consistently 25 points less than the patient’s reference), we compared the characteristics between patients who experienced true-positive trigger events and those who did not.
Statistical Analysis
Descriptive data included the mean ± standard deviation (SD) and percentages where appropriate. The Student t test and chi-square test were used where appropriate to compare characteristics between groups. A logistic regression model correcting for age and gender was used to compare the diagnosis between groups, and a linear or logistic regression model correcting for age, gender, and diagnosis was used (as appropriate) to compare the intended follow-up and VA of the better and worse eye between groups. A P value of less than 0.05 was considered statistically significant. All analyses were conducted using R software version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
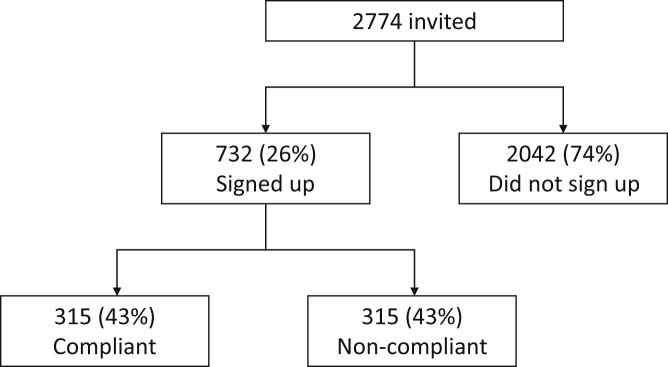
Invitations via text message to participate in the self-monitoring initiative over the period of lockdown (April 13–June 1, 2020) were sent to 2774 patients. Among these 2774 patients, the mean ± SD age was 68 ± 13 years and 45% were women. The most common retinal diagnoses were neovascular AMD (881 patients [32%]), DR (571 patients [21%]), RVO (400 patients [14%]), nonneovascular AMD (330 patients [12%]), and DME (263 patients [9%]). Seven hundred thirty-two patients (26%) signed up for the self-monitoring initiative (Fig 2 ). The most common retinal diagnoses of this subgroup were DR (201 patients [27%]), nonneovascular AMD (138 patients [19%]), neovascular AMD (136 patients [19%]), and DME (136 patients [19%]).
Figure 2.
Flow chart showing disposition of patients who were invited to participate, those who signed up, and those who complied with the initiative.
Comparing the Characteristics of Patients Who Signed Up versus Those Who Declined
The comparison between characteristics of patients who signed up versus those who did not are summarized in Table 1 . Characteristics of patients who signed up include younger age (mean ± SD, 62 ± 14 years vs. 69 ± 13 years; P < 0.021), fewer women (38.1% vs. 46.9%; P < 0.001), and more living with family or friends (98.2% vs. 93.4%; P = 0.021).
Table 1.
Comparison of Patients Who Participated Compared with Those Who Did Not Participate
| Total (n = 2274) | Signed Up (n = 732) | Declined (n = 2042) | P Value | Adjusted P Value | |
|---|---|---|---|---|---|
| Age (yrs), mean ± SD | 68 ± 13 | 62 ± 14 | 69 ± 13 | 0.021 | |
| Female gender, no. (%) | 1237 (45) | 279 (38) | 958 (47) | < 0.001 | |
| Race, no. (%) | |||||
| Chinese | 1992 (72) | 398 (54) | 1594 (78) | 0.021 | 0.12∗ |
| Indian | 245 (9) | 65 (9) | 180 (9) | ||
| Malay | 235 (8) | 57 (8) | 178 (9) | ||
| Others | 302 (11) | 212 (29) | 90 (4) | ||
| Living with family, no. (%) | 2626 (95) | 719 (98) | 1907 (93) | 0.014 | 0.021∗ |
| Diagnosis, no. (%) | |||||
| DME | 263 (9) | 136 (19) | 127 (6) | < 0.001 | < 0.001† |
| DR | 571 (21) | 201 (27) | 370 (18) | ||
| Neovascular AMD | 881 (32) | 136 (19) | 745 (36) | ||
| Nonneovascular AMD | 330 (12) | 138 (19) | 192 (9) | ||
| RVO | 400 (14) | 73 (10) | 327 (16) | ||
| Other | 329 (12) | 48 (7) | 281 (14) | ||
| Bilateral eyes affected, no. (%) | 1017 (37) | 296 (40) | 721 (35) | 0.072 | 0.32‡ |
| VA (letters) mean ± SD | |||||
| Better eye | 70 ± 13 | 69 ± 14 | 74 ± 12 | < 0.001 | 0.93‡ |
| Worse eye | 50 ± 25 | 49 ± 25 | 61 ± 22 | < 0.001 | < 0.001‡ |
| Patients with intended follow-up (> 4 mos), no. (%) | 803 (29) | 395 (54) | 408 (20) | < 0.001 | < 0.001‡ |
| Patients receiving active treatment, no. (%) | 984 (35) | 152 (21) | 832 (41) | < 0.001 | 0.023‡ |
AMD = age-related macular degeneration; DME = diabetic macular edema; DR = diabetic retinopathy; RVO = retinal vein occlusion; SD = standard deviation; VA = visual acuity.
Adjusted for age and gender.
Adjusted for age, gender, and ethnicity.
Adjusted for age, gender, ethnicity, and diagnosis.
After adjusting for age, gender, and race, the retinal diagnosis was significantly different among patients who signed up versus those who did not. When grouped according to retinal diagnosis, the proportion that signed up was 52% among DME patients, 35% among DR patients, 15% among neovascular AMD patients, 42% among nonneovascular AMD patients, and 18% among RVO patients (P < 0.001).
After adjusting for age, gender, and diagnosis, those who signed up had worse vision in the worse-seeing eye (mean ± SD, 49 ± 25 letters vs. 61 ± 22 letters; P < 0.001). Every 1 line of worse presenting vision was associated with a 1.3-fold increased likelihood of signing up (odds ratio [OR], 1.3; 95% CI, 1.1–1.4; P = 0.013). Patients with an intended longer follow-up (defined as > 4 months) were more likely to sign up (OR, 3.2; 95% CI, 1.2–5.1; P ≤ 0.001), whereas those undergoing active treatment were less likely to sign up (OR, 3.3; 95% CI, 0.4–6.3; P < 0.001).
Characteristics of Patients Who Were Compliant versus Noncompliant
Among the 732 participants who signed up, 315 patients (43%) complied with the initiative. The clinical characteristics comparing the 2 groups are summarized in Table 2 . Characteristics of those who complied include older age (mean ± SD, 64 ± 13 years vs. 60 ± 14 years; P = 0.032).
Table 2.
Comparison of Patients Who Complied versus Those Who Did Not Comply
| Total (n = 732) | Complied (n = 315) | Did Not Comply (n = 417) | P Value | Adjusted P Value | |
|---|---|---|---|---|---|
| Age (yrs), mean ± SD | 62 ± 13 | 64 ± 13 | 60 ± 14 | 0.032 | |
| Gender (female), no. (%) | 286 (39) | 138 (44) | 148 (35) | 0.23 | |
| Race, no. (%) | |||||
| Chinese | 398 (54) | 132 (42) | 266 (64) | 0.023 | 0.36∗ |
| Indian | 65 (9) | 31 (10) | 34 (8) | ||
| Malay | 57 (8) | 34 (11) | 23 (6) | ||
| Other | 212 (29) | 118 (37) | 94 (22) | ||
| Living with family, no. (%) | 719 (98) | 311 (99) | 408 (98) | 0.21 | 0.84∗ |
| Diagnosis, no. (%) | |||||
| DME | 136 (19) | 71 (23) | 65 (16) | < 0.001 | 0.023† |
| DR | 201 (27) | 41 (13) | 160 (38) | ||
| Neovascular AMD | 136 (19) | 81 (26) | 55 (13) | ||
| Nonneovascular AMD | 138 (19) | 80 (25) | 58 (14) | ||
| RVO | 73 (10) | 25 (8) | 48 (12) | ||
| Other | 48 (7) | 17 (5) | 31 (7) | ||
| Bilateral eyes affected, no. (%) | 428 (58) | 212 (67) | 216 (52) | 0.23 | 0.45‡ |
| VA (letters), mean ± SD | |||||
| Better eye | 74 ± 12 | 74 ± 12 | 74 ± 13 | 0.79 | 0.21‡ |
| Worse eye | 57 ± 23 | 53 ± 25 | 63 ± 20 | 0.023 | 0.042‡ |
| Intended follow-up (> 4 mos) , no. (%) | 395 (54) | 181 (57) | 214 (51) | 0.24 | < 0.001‡ |
| Receiving active treatment, no. (%) | 152 (21) | 92 (29) | 60 (14) | 0.032 | < 0.001‡ |
AMD = age-related macular degeneration; DME = diabetic macular edema; DR = diabetic retinopathy; RVO = retinal vein occlusion; SD = standard deviation; VA = visual acuity.
Adjusted for age and gender.
Adjusted for age, gender, and ethnicity.
Adjusted for age, gender, ethnicity, and diagnosis.
After adjusting for age, gender, and race, the retinal diagnosis was significantly different among patients who complied and those who did not. When grouped according to retinal diagnosis, the proportion who complied was 52% (71/136) among DME patients, 20% (41/201) among DR patients, 60% (81/136) among neovascular AMD patients, 59% (80/138) among nonneovascular AMD patients, and 34% (25/73) among RVO patients (P < 0.023).
After adjusting for age, gender, and diagnosis, the VA in patients who complied was worse in the worse-seeing eye (mean ± SD, 53[25] letters vs. 63 ± 20 letters; P = 0.042) but similar in the better-seeing eye. Every line of worse presenting vision was associated with a 1.4-fold increased likelihood compliance (OR, 1.4; 95% CI, 1.7–1.1; P < 0.001). Patients who had longer intended follow-up (OR, 2.2; 95% CI, 1.2–3.4; P < 0.001) and were receiving active treatment (OR, 2.5; 95% CI, 1.7–3.4; P < 0.001) were more likely to comply.
Trigger Events
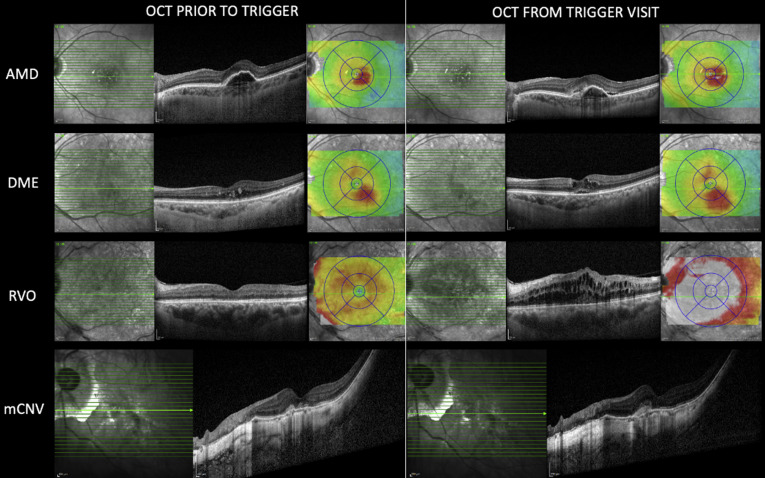
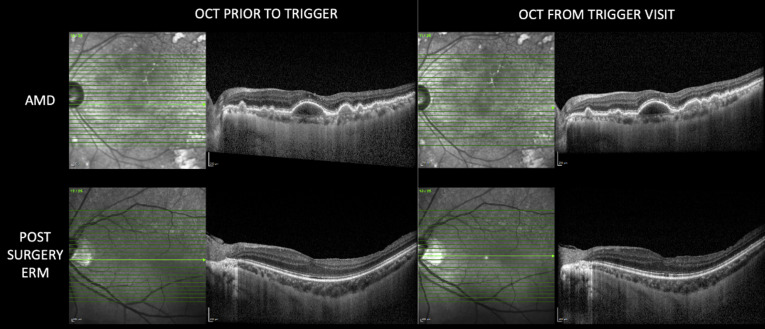
A total of 33 trigger events from 33 patients (10.5%) were detected over the study period. Each patient was called by the clinical team from SNEC Ocular Reading Centre. Based on assessment according to the standard operating procedure, 7 patients were given urgent appointments and attended at SNEC within a mean of 6 days (range, 2–10 days). Of these 7 of 33 patients (21%), 5 patients (2 with DME, 1 with RVO, 1 with neovascular AMD, and 1 with myopic choroidal neovascularization) were confirmed to have disease progression on clinical examination that resulted in active intervention (Table 3 ; Fig 3 ). The remaining 2 patients were found to have no progression of disease, and on further questioning, it was discovered that they had difficulty using the app (Fig 4 ). None of the patients who used the app correctly reported a false trigger. Of the remaining 26 of 33 patients (79%), 15 of 26 patients (58%) reported that they had not performed the test at the prescribed interval, 6 of 26 patients (23%) reported that they had tested the wrong eye, and 5 of 26 patients (19%) reported difficulties using the test. By the end of the analysis period, 20 of the 26 patients had attended their rescheduled appointments and were found to have no disease progression. Comparison of characteristics of patients with verified disease progression versus those without is summarized in Table 4 . These clinical characteristics were compared between the most recent visit before lockdown and the trigger-related visit.
Table 3.
Characteristics of Patients Who Experienced Triggers That Detected Disease Progression
| Gender | Age (yrs) | Diagnosis | Side | Characteristics at Last Visit before Lockdown |
Characteristics at Visit as a Result of Trigger |
Days from Last Visit to Trigger | Days from Trigger to Presentation | ||
|---|---|---|---|---|---|---|---|---|---|
| Visual Acuity | Central Retinal Thickness | Visual Acuity | Central Retinal Thickness | ||||||
| Male | 64 | DME | Left | 69 | 288 | 68 | 330 | 111 | 10 |
| Female | 65 | DME | Right | 70 | 331 | 75 | 368 | 102 | 6 |
| Female | 66 | mCNV | Left | 62 | 269 | 58 | 312 | 151 | 7 |
| Female | 73 | AMD | Left | 75 | 347 | 72 | 391 | 85 | 2 |
| Male | 58 | RVO | Left | 64 | 265 | 55 | 653 | 121 | 6 |
AMD = age related macular degeneration; DME = diabetic macular edema; mCNV = myopic choroidal neovascularisation; RVO = retinal vein occlusion.
Figure 3.
OCT scans from patients obtained before the trigger visit to the trigger visit. The top row shows a patient with neovascular age-related macular degeneration (AMD) whose disease was inactive and who was in the midst of a stable 10-week treatment interval. The interval was extended by 4 weeks to avoid the coronavirus 2019 lockdown; however, a trigger event occurred at day 85 from the last visit (approximately 12 weeks from last treatment). New subretinal hyperreflective material can be seen around the pigment epithelial detachment on the OCT scan obtained at the trigger visit. The second row shows a patient receiving treatment for diabetic macular edema (DME). The patient’s prior treatment interval was 8 to 10 weeks and a trigger event was noted approximately 14 weeks from the last treatment. The presence of new intraretinal and subretinal fluid with overall thickening of the retina can be seen in comparison with the scan obtained before the trigger. The third row shows a patient with an old quiescent central retinal vein occlusion (RVO). This patient had no active intervention at the prior visit, but the trigger event detected new activation of disease after approximately 17 weeks from the last visit. The last row shows a patient with myopic maculopathy with a previously treated myopic choroidal neovascularization (mCNV). No active intervention was administered at the last visit, and the patient was in the midst of a stable monitoring interval of 4 to 6 months previously. The trigger detected new mCNV activity associated with a drop of 4 letters.
Figure 4.
OCT scans from 2 patients who experienced trigger events but showed no disease progression. The top row shows an 85-year-old patient with intermediate age-related macular degeneration (AMD), large drusenoid pigment epithelial detachment that was largely unchanged from the 2 visits approximately 4 months apart. The patient expressed difficulties in using the app. The bottom row shows a 45-year-old patient who had undergone macular surgery for an epiretinal membrane (ERM) 1 year previously. No disease progression was noted on OCT scans at the 2 visits. The patient acknowledged excessive use of the app, performing 3 to 4 tests per day.
Table 4.
Comparison of Characteristics between Patients with True and False Triggers
| True-Positive Triggers (n = 5) | False-Positive Trigger (n = 28) | P Value | |
|---|---|---|---|
| Age (yrs), mean ± SD | 62 ± 4 | 67 ± 12 | 0.44 |
| Gender (female), no. (%) | 2 (40) | 16 (57) | 0.92 |
| Diagnosis, no. (%) | 0.81 | ||
| AMD | 1 (20) | 15 (55.6) | |
| DME | 2 (40) | 8 (29.6) | |
| RVO | 1 (20) | 2 (7.4) | |
| others | 1 (20) | 2 (7.4) | |
| VA (letters), mean ± SD | |||
| Better eye | 77 ± 5 | 74 ± 9 | 0.36 |
| Worse eye | 47 ± 21 | 64 ± 14 | 0.031 |
| Tests performed/wk | 4 ± 2 | 3 ± 1 | 0.42 |
AMD = age-related macular degeneration; CRT = central retinal thickness; DME = diabetic macular edema; RVO = retinal vein occlusion; SD = standard deviation; VA = visual acuity.
Discussion
This report describes the rapid deployment of and clinical data from the uptake of a digital self-monitoring initiative for patients with retinal conditions during the COVID-19 lockdown in Singapore. The impetus for this initiative arose from the sudden need to defer all nonessential hospital visits in an effort to curb the spread of the virus while providing an alternative solution for disease monitoring. Our experience highlights the importance of active initial engagement from physicians and health care workers in the introductory process for a self-monitoring initiative. Although this report highlights the findings from this extraordinary circumstance, it is applicable to a new normal after the COVID-19 pandemic when such digital self-monitoring initiatives may represent new models of care that can help to reduce the burden of care.
The digital instrument selected in this study, the Alleye app, assesses hyperacuity15 as a qualitative determination of metamorphopsia that is superior to Amsler grid testing and is a proxy for retinal disease.16 In a previous trial, the application demonstrated good performance in differentiating eyes with wet AMD from those of age-matched normal control participants (area under the receiver operating characteristic curve, 0.84). Although the use of digital instruments to detect new disease activity is not novel, the mode and speed of mass deployment in clinical practice has not been reported previously.
Overall, we reported a low rate (approximately 25%) of sign up and low compliance rate (approximately 40%). This was likely because of the circumstances and methods of recruitment, which lacked detailed counseling and close trial engagement, which were found to be key drivers for compliance in prior studies.17 , 18 The closest comparisons to this study, 2 clinical implementations of the ForeseeHome device (Notal Vision, Ltd), reported that 25% to 50% of participants were noncompliant after the first year of use.14 , 19
It was not surprising that younger patients and those with family support were more likely to sign up and were more compliant with testing than their older counterparts. This could be related to familiarity with digital technology among the younger patients. We also found no racial predilection to signing up or compliance, suggesting the importance of the primary functional language of the initiative.
Patients with neovascular AMD and nonneovascular AMD were found to be more compliant in this cohort. First, it is likely that patients who had experienced vision loss with conditions that required active treatment, such as neovascular AMD and DME, would be more willing to monitor their vision actively to detect early changes. Second, it is also likely that patients with nonneovascular AMD in one eye could likely have sustained vision loss in the fellow eye and hence were more likely to monitor the better-seeing eye. This hypothesis is supported further by our results showing that patients with poorer vision in the worse-seeing eye were more likely to sign up and comply, presumably to monitor the better-seeing eye.
Over the course of 6 months, a total of 33 triggers were identified, and 5 of 33 (15%) accurately detected worsening of disease. This translated to a 9% (28/315) false-trigger rate compared with a true detection rate of 1.6% (5/315). Although the accurate trigger rate was low, the absolute number of patients who potentially returned to hospital was low, and we consider this false-trigger rate acceptable in exchange for safely deferring hospital appointments for the large proportion (89%) of the patients in exchange. Only 1 of 5 patients experienced a dramatic drop in vision on subsequent examination, suggesting that in most of these patients, the disease activity was detected early. No comparable studies exist that have reported the true trigger rate of these new initiatives in a heterogenous cohort like ours. The trigger rate reported here was 3.2%/year in a population with varied clinical characteristics, treatment statuses, and diagnoses, whereas prior studies reported rates only on specific study populations, like patients whose AMD converted from early to wet forms of the disease.19
From our experience, we suggest that a self-monitoring strategy to augment the management of retina disease is feasible and requires 3 key components for success: First, patients must be identified and educated on these initiatives. Family and carer involvement is important in improving participation and compliance. Second, an easy-to-use ubiquitous test platform that is translated easily across key languages for each community should be considered. The Alleye app is currently available in 10 languages: English, Chinese, Spanish, French, Italian, Dutch, Polish, Arab, Czech, and German. This test platform should be robust enough to detect prefunctional change in visual disability but not too onerous or costly. Finally, the interpretation of test results and triggers should be easily accessible to health care providers to allow for prompt follow-up. The Alleye app was chosen for its simple-to-use interface, good detection rate, and ease of integration into our existing digital infrastructure. In addition, a comprehensive logistics and infrastructure was set up and provided by the SNEC Ocular Reading Centre to ensure the monitoring of test scores and ad hoc appointments appropriately scheduled for patients with triggers of potential disease deterioration.
The strengths of this study include its clinical nature, allowing for a diverse and large cohort to be examined. This highlights the differences in groups who would or would not participate in a self-monitoring initiative for retina disease and guides strategies to address these cohorts. The limitations of this study include a lack of randomized control participants, for example, a group monitored with Amsler grid testing, which we acknowledge will be necessary to validate this intervention. An additional benefit of this home monitoring system over conventional monitoring is the ability for clinicians to monitor adherence and identify triggers remotely, thus ensuring that potentially sight-threatening events are identified early and access to care is provided in a timely fashion. We were also unable to accurately determine the false-negative rate of the intervention because formal visits for nontrigger patients were not tracked consistently. Only 13 patients returned earlier than their intended deferral, of whom 2 had documented worsening of disease, but neither exhibited a drop in VA. In the remaining patients, the reasons for early attendance were attributed to patient preference and the earlier-than-expected lifting of restrictions and availability of early clinic appointments.
In conclusion, this article describes the first experience of our cohort of deploying a large-scale home monitoring initiative. Significant challenges exist to deploy the initiative on a large scale; although mass communications tools are useful in reaching a great number of people, a need exists for targeted counseling and education to ensure that patients use these tools correctly and appropriately. More importantly, these findings provide further insights into how best to deploy such digital self-monitoring initiatives, which are likely to continue as novel models of care even after the pandemic to reduce the overall health care burden.
Manuscript no. ORET-D-20-00972.
Footnotes
Supplemental material available at www.ophthalmologyretina.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): K.Y.C.T.: Financial support (to institution) - visionsave, L.M.B.: Board member, Employee, and Equity owner - Oculocare Medical, Inc.; Founder - Alleye app, G.S.W.T.: Financial support (to institution) - visionsave
Supported by Visionsave, Singhealth SNEC/2020/IFC/C2/01, Singapore National Eye Centre, Singapore, Republic of Singapore. The sponsor had no role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Singhealth approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Teo, Lee, Cheung, G.S.W.Tan
Analysis and interpretation: Teo, Bachmann, Sim, Cheung, G.S.W.Tan
Data collection: Teo, Bachmann, A.Tan, Wong, G.S.W.Tan
Obtained funding: Teo, G.S.W.Tan
Overall responsibility: Teo, Bachmann, Sim, Lee, A.Tan, Wong, Cheung, G.S.W.Tan
Supplementary Data
References
- 1.Cornish E.E., Teo K.Y., Nguyen V., et al. Five-year incidence and visual acuity outcomes for intravitreal therapy in bilateral neovascular age-related macular degeneration: Fight Retinal Blindness! Project. Retina. 2021;41:118–124. doi: 10.1097/IAE.0000000000002798. [DOI] [PubMed] [Google Scholar]
- 2.Stem M.S., Moinuddin O., Kline N., et al. Outcomes of anti-vascular endothelial growth factor treatment for choroidal neovascularization in fellow eyes of previously treated patients with neovascular age-related macular degeneration. JAMA Ophthalmol. 2018;136:820–823. doi: 10.1001/jamaophthalmol.2018.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veluswamy B., Lee A., Mirza R.G., Gill M.K. Correlation of baseline visual acuity with outcomes of treatment with anti-VEGF in neovascular age-related macular degeneration. Clin Ophthalmol. 2020;14:1565–1572. doi: 10.2147/OPTH.S256009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faes L., Bachmann L.M., Sim D.A. Home monitoring as a useful extension of modern tele-ophthalmology. Eye. 2020;34:1950–1953. doi: 10.1038/s41433-020-0964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faes L., Bodmer N.S., Bachmann L.M., et al. Diagnostic accuracy of the Amsler grid and the preferential hyperacuity perimetry in the screening of patients with age-related macular degeneration: systematic review and meta-analysis. Eye (Lond) 2014;28:788–796. doi: 10.1038/eye.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sim D.A., Mitry D., Alexander P., et al. The evolution of teleophthalmology programs in the United Kingdom: beyond diabetic retinopathy screening. J Diabetes Sci Technol. 2016;10:308–317. doi: 10.1177/1932296816629983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Writing Committee for the UKA-RMDEMRUG The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections. Report 1: visual acuity. Ophthalmology. 2014;121:1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Cowling B.J., Ali S.T., Ng T.W.Y., et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romano M.R., Montericcio A., Montalbano C., et al. Facing COVID-19 in ophthalmology department. Curr Eye Res. 2020;45:653–658. doi: 10.1080/02713683.2020.1752737. [DOI] [PubMed] [Google Scholar]
- 10.Teo K.Y.C., Chan R.V.P., Cheung C.M.G. Keeping our eyecare providers and patients safe during the COVID-19 pandemic. Eye. 2020;34:1161–1162. doi: 10.1038/s41433-020-0960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teo K.Y.C., Nguyen V., Barthelmes D., et al. Extended intervals for wet AMD patients with high retreatment needs: informing the risk during COVID-19, data from real-world evidence. Eye (Lond) 2020;34:1161–1162. doi: 10.1038/s41433-020-01315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickham L., Hay G., Hamilton R., et al. The impact of COVID policies on acute ophthalmology services-experiences from Moorfields Eye Hospital NHS Foundation Trust. Eye (Lond) 2020;34:1189–1192. doi: 10.1038/s41433-020-0957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27:1–4. doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H.J., Kiernan D.F., Eichenbaum D., et al. Home Monitoring of Age-Related Macular Degeneration: Utility of the ForeseeHome Device for Detection of Neovascularization. Ophthalmol Retina. 2021;5(4):348–356. doi: 10.1016/j.oret.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westheimer G. Editorial: visual acuity and hyperacuity. Invest Ophthalmol. 1975;14:570–572. [PubMed] [Google Scholar]
- 16.Simunovic M.P. Metamorphopsia and its quantification. Retina. 2015;35:1285–1291. doi: 10.1097/IAE.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 17.Chew E.Y., Clemons T.E., Bressler S.B., et al. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design—HOME Study report number 1. Contemp Clin Trials. 2014;37:294–300. doi: 10.1016/j.cct.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser P.K., Wang Y.Z., He Y.G., et al. Feasibility of a novel remote daily monitoring system for age-related macular degeneration using mobile handheld devices: results of a pilot study. Retina. 2013;33:1863–1870. doi: 10.1097/IAE.0b013e3182899258. [DOI] [PubMed] [Google Scholar]
- 19.Group AHSR. Chew E.Y., Clemons T.E., et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535–544. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.