Abstract
The current review paper was written in collaboration with breast cancer surgeons from the European Breast Cancer Research Association of Surgical Trialists (EUBREAST), a breast pathologist from the Danish Breast Cancer Group (DBCG), and representatives from the European SocieTy for Radiotherapy & Oncology (ESTRO) breast cancer course. Herein we summarize the different mastectomies and reconstruction procedures and define high-risk anatomical areas for breast cancer recurrences, to further specify the challenges in the surgical procedure, histopathological evaluation, and target volumes in case of postmastectomy irradiation, as recommended by the ESTRO guidelines according to the surgical procedure. The paper has original figures and illustrations for all disciplines for in-depth understanding of the differences between the procedures.
Keywords: Breast cancer, Mastectomy, Reconstruction, Skin sparing, Nipple sparing, Radiation
Highlights
-
•
Mastectomy techniques and reconstruction evolved to improve cosmetic outcomes.
-
•
Different techniques maybe associated with different amount of residual breast tissue.
-
•
More data is needed to estimate who are the patients at risk for residual disease or recurrence.
-
•
Multidisciplinary work needed to individualise treatment for optimal oncological outcomes while maintaining the significant improvements in achieving better cosmesis for these patients.
1. Introduction
Radiation therapy (RT) is an important treatment modality for non-metastatic breast cancer. The selection of target volumes in case of non-metastatic disease, whether concerning the intact breast (in case of breast conservation surgery, BCS) or the chest-wall after mastectomy, with or without the regional lymph nodes, depends on individual patient- and disease-related features [1]. Most patients who undergo BCS are treated with RT, whereas the indication for postmastectomy RT (PMRT) is mainly based on tumour stage and the extent of lymph node involvement [2].
In the past years, the number of patients receiving PMRT has increased [3] following the publication of the results from an EBCTCG meta-analyses that reported improvements of disease free and breast cancer survival after PMRT for patients with involved axillary lymph nodes and in pN0 patients after inadequate axillary surgery [4]. Another reason for increased application of PMRT is that an axillary lymph node dissection is often replaced by sentinel lymph node biopsy and axillary RT [5,6]. Surgical techniques for mastectomy and reconstruction are constantly evolving to improve patient’s quality of life (QoL). Therefore, radiation oncologists should aspire to adapt the PMRT planning volumes. New radiation treatment planning and imaging systems allow dose distributions based on the anatomically defined estimated risk of residual cancer cells, and surgical techniques. Therein lays a need for better understanding of the surgical techniques to define the target volumes for PMRT [7].
The European SocieTy for Radiotherapy & Oncology (ESTRO) published guidelines for breast, chest wall, and locoregional irradiation volumes which are based on anatomical references (in contrast to bony landmarks), individual assessment of the patient and patterns of recurrence [1,[7], [8], [9]]. This work concerns a broad multidisciplinary effort consisting of ongoing step-wise research to improve our understanding of the areas that are at high-risk of local recurrence after mastectomy in order to optimize the target volumes for PMRT with or without immediate breast reconstruction (IBR) [7,[10], [11], [12]]. The current paper was written in collaboration with breast cancer surgeons from the European Breast Cancer Research Association of Surgical Trialists (EUBREAST), a breast pathologist from the Danish Breast Cancer Group (DBCG), and representatives from the ESTRO breast cancer course. Herein we summarize the common types of mastectomies and reconstruction procedures and define high-risk anatomical areas for breast cancer recurrences, to further specify clinical target volumes (CTV) in case of PMRT, as recommended by the ACROP-ESTRO guidelines [1,[7], [8], [9],12].
1.1. Anatomy of the female breasts
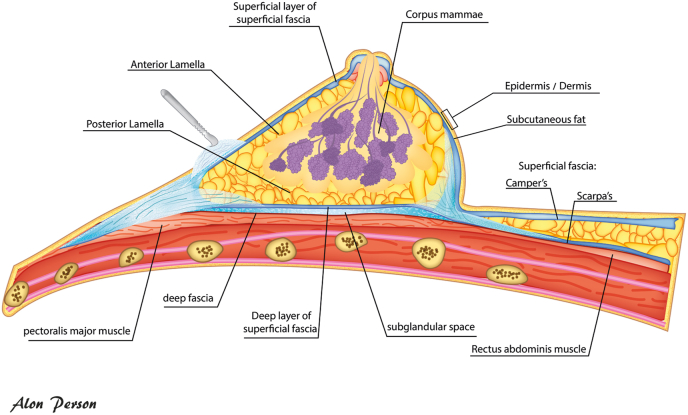
The breast glandular tissue (BGT, mammary gland) is not covered by an actual capsule or sheath [Fig. 1]. It lies in a delicate, closed 3-dimensional superficial fascia system, anterior to the ventral musculoskeletal part of the chest-wall (i.e., pectoral & intercostal muscles, ribs). The dorsal (posterior) border of the BGT is separated from the pectoralis muscles by the dorsal sheet/lamina posterior of the fascia (deep fascia), which is well defined and continuous. Between the BGT and deep fascia, there is an area of loose connective tissue that contains a thin layer of fatty tissue (retromammary space) [13]. The anterior border of the BGT is defined by the ventral sheet/lamina anterior of the fascia (superficial fascia) being a much more delicate and discontinuous structure. The deep and the superficial fascia fuse at the perimeter of the BGT and anchor the breast to the chest wall [13].
Fig. 1.
The 3-dimensional superficial fascia system of the mammary gland. Camper’s and Scarpa’s fascia are a thick superficial and deep layer, respectively, of the anterior abdominal wall.
The mammary gland is attached to the skin by vertical suspensory ligaments (Cooper’s ligaments), traversing the BGT and connecting the skin and the deep fascia. Between the BGT and the skin is a layer of fat of variable thickness; often 2–3 times thicker than the retromammary fat layer (fat pad in the pre-pectoral region/retromammary space).
Medially, the breast extends from nearby the sternum and projects laterally in a tail upwards into the axilla, as processus axillaris (or axillary tail of Spence), showing great anatomical variation.
The nipples (also called mammary papilla) are conical or cylindrical prominences. They are a projection of the skin, containing the outlets of the lactiferous ducts. The areola is containing numerous sebaceous glands. The BGT extends through the superficial fascia to the skin in the papilla, and underneath the area of the mammary gland (nipple, areola) where there is no fat. The distribution of BGT can differ within the breasts, with the superolateral quadrant of the breasts generally containing a larger amount of glandular tissue.
1.2. Mastectomy
Mastectomy is a complete surgical removal of the BGT with the aim to remove all in-breast neoplasia and/or glandular tissue. There are different types of mastectomy procedures which differ in the extent of resection of additional tissues. These include radical mastectomy, modified radical mastectomy (MRM), simple mastectomy, skin-sparing mastectomy (SSM), and nipple-areolar sparing mastectomy (NSM).
1.3. Radical and modified radical mastectomy
Radical mastectomy technique was published at 1894 [14]. It is also known as Halsted’s technique [15] and consists of “en-bloc” removal of the breast tissue, the overlying skin, the pectoral muscles (major, minor and fascia), and regional lymphatics (axillary level I, II, and III nodes). This extensive resection was originally proposed as an attempt to mechanically remove all potentially present tumour cells in the breast and the regional lymphatics, based on the hypothesis of centrifugal tumoural spread. It was believed that the lamina posterior below the parenchyma was partly anchored to the deep fascia by small sawtooth like junctions which could contain breast parenchyma and therefore its resection was part of removal of all potential breast parenchyma [15,16]. Later, even the resection of the internal mammary nodes (IMNs) was proposed (i.e., Extended Halsted mastectomy) [17], however extended Halsted was abandoned as it did not provide disease control advantage and was associated with increase morbidity [15,16]. Meanwhile, Halsted radical mastectomy remained the gold standard for the management of breast cancer for more than 70 years [15,16]. These two surgical approaches were developed at a time when neither adequate imaging, preoperative systemic therapy nor RT were used in breast cancer treatment, yet more extensive resection did not lead to improvements in overall survival [17]. Additional attempts to reduce postoperative morbidity combined with the understanding that no BGT is found beyond the pectoralis fascia, that the fascia is devoid of lymphatic vessels, and that true muscle recurrences are uncommon, led to less radical procedures such as modified radical mastectomy (MRM) initially described in 1934 [14,15,[18], [19], [20]]. These less aggressive procedures were reported to improved cosmesis and were associated with less pain, lymphedema, and upper limb mobility limitation without significantly compromising clinical outcome. MRM became widely acceptable surgical approach after the National Surgical Adjuvant Breast and Bowel (NASABP) B-04 clinical trial showed no survival advantage when compared with the radical mastectomy in the 1970s [14,15,[18], [19], [20]].
Modified radical mastectomy includes complete removal of the breast, breast skin-envelop, and underlying fascia of the major pectoral muscle, along with the removal of the level I and II axillary lymph nodes. In the late 1990’s total/simple mastectomy came into practice with the advent of sentinel lymph node biopsy and omission of axillary dissection as standard procedure in all clinically node-negative breast cancer patients [21]. Total/simple mastectomy entails removal of the entire breast and skin but preservation of the pectoral muscles and the axillary lymphatics, making the main difference between MRM and simple/total mastectomy the omission of axillary dissection [Fig. 2] [15,18]. In some institutions the pectoralis fascia is removed in all cases of total/simple mastectomy, while in others it is preserved (unless the tumour is located near the muscle) [22].
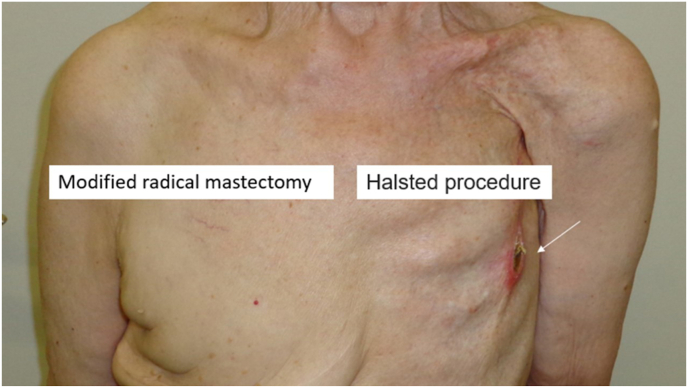
Fig. 2.
A patient who underwent Halsted’s mastectomy on the left side and modified radical mastectomy on the right. On the left, deformity from pectoral muscle removal is noted and the ribs are easily seen. The arrow on the left shows a local recurrence at the mastectomy scar, 25 years after the surgical procedure. On the right side, excess skin is noted.
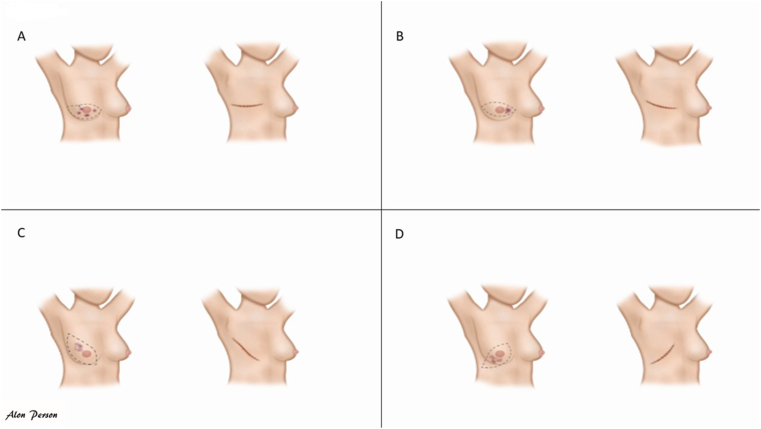
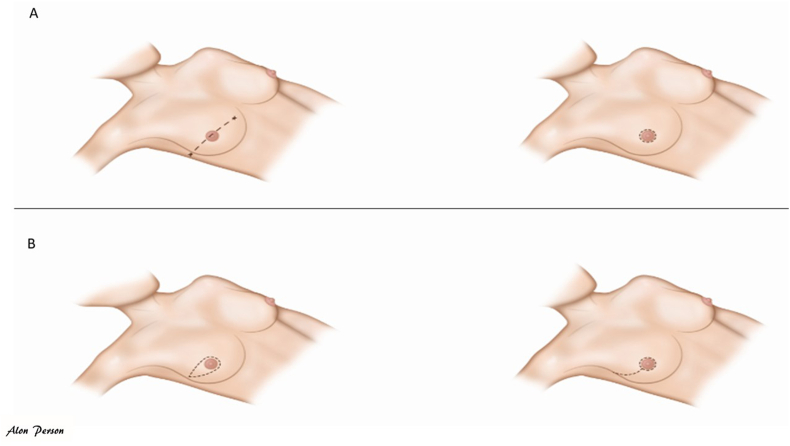
In general, in case of MRM or simple/total mastectomy without IBR, the most common incision is a horizontal elliptical incision with the aim to resecting the tumour and breast tissue (according to the relaxed skin tension lines) and allowing access to the axilla without extending the incision across the anterior axillary line (Fig. 3A and B). Fig. 3D shows an untypical incision, as the skin is not brought together according to the relaxed tension lines, which might be done in case of very exocentrically lower-outer located tumours.
Fig. 3.
A-D: Mastectomy incision according to tumour location.
As shown in Fig. 3 a large part of the breast skin is removed, reducing the size of the skin/subcutaneous part of the target volume of the postmastectomy chest wall compared to after BCS [1].
Regardless of the surgical procedure (even in BCS), per ACROP-ESTRO guidelines [1] the breast skin itself is not part of the CTV, except in patients with a T4b, T4c and T4d breast cancer [12]. However, the subcutaneous lymphatic plexus beneath the breast skin is part of the CTV regardless of the surgical procedure. Due to the nature of the MRM/total mastectomy procedure the remaining skin of the chest wall is pulled together and sutured, thus the subcutaneous plexus is approximated/relocated. Importantly, most of the local recurrences are subcutaneously in the chest wall, followed by the skin itself, and mostly at the area around the mastectomy scar (e.g., Fig. 2) [7]. The latter could be a result of tumour cell seeding at time of the mastectomy procedure, or a recurrence in the subcutaneous lymphatic plexus infiltrating the skin [[23], [24], [25], [26]]. The risk for local recurrences at the postmastectomy chest wall skin/scar was the leading concept of placing a bolus around the mastectomy scar for PMRT, to increase dose coverage of the skin and subcutaneous tissue [1], in some institutions complemented by boosting the mastectomy scar. But most important is that by far most local recurrences after mastectomy take place at the subcutaneous lymphatic plexus/skin at the level that was initially overlying the BGT and the draining lymphatics towards the axillary region [7].
Therefore, the CTV of the chest wall encompasses the volume of tissue beneath the skin that was preoperatively overlaying the breast (starting at least 3–5 mm beneath, depending on the thickness of the subcutis) with the posterior border being positioned ventral to the major pectoral muscle. In case the tumour is invading the muscle, inclusion of the pectoral muscle (if possible, partially, according to the location of the tumour) is recommended (Table 1, Table 2). In case of rib cage invasion (stage T4a and T4c), the ribs/intercostal muscles should also be focally included in the CTV [1], [8].
Table 1.
Key points to determine the clinical target volume in case of mastectomy.
| General points |
|---|
| The CTV after mastectomy should include any possible rBGT, and the superficial lymphatic plexus localized within the subcutaneous tissue, and all relevant histopathological information. Localisation of any possible rBGT is guided by a combination of the shape and site of the contralateral breast, palpable/visible (postoperative) signs, and imaging (preoperative, CT simulation). Marker wires can be put at time of CT-simulation to assist in CTV contouring. Special additional points of attention include the localisation of the primary tumour location according to preoperative imaging and that fatty tissue can be excluded, so BMI and potential natural folds of the body should be considered. |
CTV- clinical target volume; BMI- body mass index; rBGT-residual Breast Glandular Tissue.
Table 2.
Special considerations for clinical target volume according to procedure.
| Border per region | SSM/NSM with IBR and Retro-pectoral implant | SSM/NSM with IBR and Prepectoral implant | IBR-ABR |
|---|---|---|---|
| Cranial | maximally up to the caudal edge of the sterno-clavicular joint | ||
| Caudal | The reconstructed breast, guided by the contralateral breast | ||
| Ventral |
|
|
Depending on the mastectomy procedure and the type of ABR.
|
| Dorsal |
|
|
ventral side of the pectoral muscles or ribs and intercostal muscles where no muscle is present. |
| Medial | Lateral to the medial perforating mammary vessels. | ||
| Lateral | Typically, ventral to the mid-axillary line (important, location of most residual glandular tissue). Ventral to the lateral thoracic artery. | ||
SSM- Skin sparing mastectomy; NSM- Nipple Sparing Mastectomy; IBR- Immediate breast reconstruction; ABR- Autologous breast reconstruction.
1.4. The postmastectomy chest wall
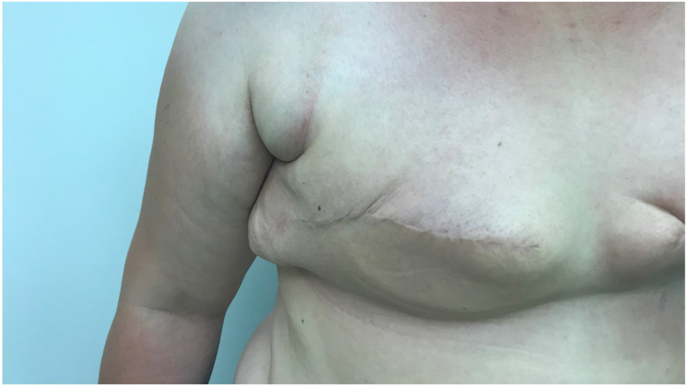
As a result of the mastectomy, patients, especially those with a thicker subcutaneous fat layer, like in case of a higher body mass index (BMI), might have a deformity at both the axillary and the medial parts of the incision scar (Fig. 2, Fig. 4), so-called “dog ears”. A “dog ear” is usually excessive tissue causing wrinkling at the edge of a scar because of residual subcutaneous fat from the chest wall that is usually not within the area of the original breast tissue. Different techniques were suggested to help reducing this postmastectomy deformity [Fig. 4]. For patients who are planned for a delayed reconstruction, these incisions in the primary procedure should be carefully planned for better outcomes after the final reconstruction procedure during which deformities such as “dog ears” can be corrected. Additionally, postmastectomy deformities (with potential rBGT) are more encountered in patients with higher BMI, where it is difficult even for experienced surgeons to obtain a flat chest wall. Even though there are no scientific data how to clinically evaluate these cases, if PMRT is indicated, we advise a careful physical evaluation of the patient. (e.g., CT-simulation or a MRI if highly suspicious for large amounts of rBGT). Moreover, consulting with the surgeon can help to understand the possibility of rBGT in areas, which is to be included in the CTV (Table 2).
Fig. 4.
Excessive tissue causing wrinkling at the edge of a scar as a result of residual subcutaneous fat from the chest wall (Dog ears).
1.5. Skin-and nipple sparing mastectomy
Earlier detection, improved understanding of disease biology and spread (which most often does not involve the skin), and improvement in surgical techniques, together with the desire for maintaining QoL of the patients with a breast reconstruction led to the use of subcutaneous mastectomy (i.e., SSM) with IBR for malignant disease.
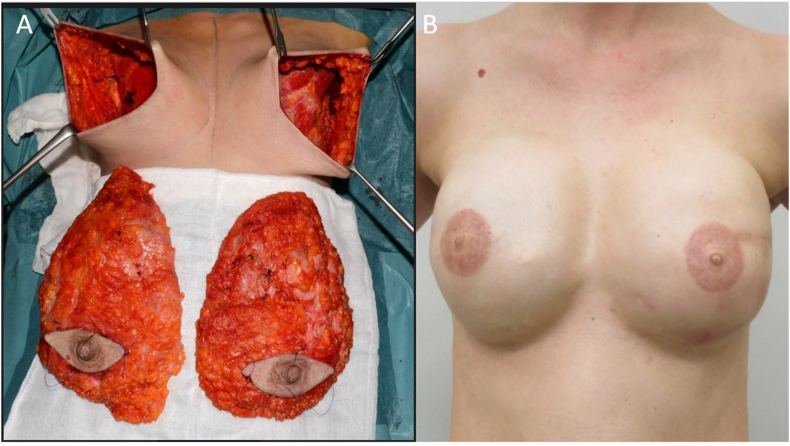
Skin-sparing mastectomy includes resection of the entire breast parenchyma with the nipple areola complex (NAC) while preserving most of the natural breast skin envelope. It is usually performed through a horizontal elliptic skin incision close around the NAC, resulting in a short horizontal scar, while the breast is losing its natural projection (Fig. 5A and B).
Fig. 5.
A, B: (A) Bilateral Skin sparing mastectomy (SSM) with (B) nipple reconstruction, all is native skin breast. The SSM was done via horizontal elliptical skin incision, which allows for a 360° freedom to resect the breast parenchyma, at all locations. It also allows full access to the axilla.
SSM aims to remove all BGT while preserving as much skin as possible to facilitate IBR (using the excess skin) with less deformity and scarring.
In case of SSM a subsequent procedure may consist of nipple and areolar reconstruction and/or tattooing [27]. Reconstruction of the NAC, requires supplementary efforts to achieve good cosmetic results including symmetry, size, shape, texture, and pigmentation and permanent projection (Fig. 5B) [27].
Nipple sparing mastectomy is a refinement of SSM procedures described in 1990 (around the time SSM were termed) [14], in which the NAC and the entire skin envelope are preserved to further maintain the contour and projection of the natural breast (Fig. 6). Only the major ducts (central ductal branch or the major ducts and sinuses by coring) from within the nipple lumen are resected (see Fig. 7).
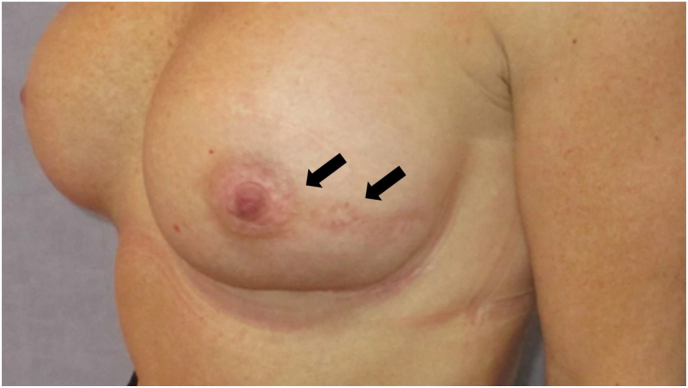
Fig. 6.
Nipple sparing mastectomy in a peri-areolar approach.
Fig. 7.
Nipple-sparing mastectomy (NSM) with a peri-areolar incision over 180°, with a radial elongation (a hockey-stick incision). It allows a direct access to the retro-areolar space and even to the medial quadrants.
At the time of surgery, some surgeons perform a histological assessment of the retro-areolar margin via an intraoperative biopsy while others await final pathology. This is often done by resection of a separate specimen to be sent for a definitive histology, with NAC excision in a second intervention if tumour cells are found at the level of the retro-areolar margin, as frozen sections have a high false negative rate. For example, DCIS (especially low-grade DCIS) may present as skip lesions, leaving the base of the nipple free of DCIS, but with a skip lesion in the ducts in the actual nipple core [28]. Additionally, patients with a centrally located breast cancer and advanced lymph node status (N2 and N3 stage) were found to have higher positive nipple margin rates in case of NSM, thus this should be taken into consideration when discussing the type of procedure [29].
NSM is often performed via inframammary fold incision (not shown in the figures). However, patients with pendulous/ptotic and/or large sized breast are not considered good candidates for NSM via the inframammary fold approach. In these cases, different incision patterns for the resection of excessive skin and a pedicled transition of the NAC are required [30]. The peri-areolar incision is reported to have a higher rate of nipple necrosis compared to inframammary approach [31,32]. Therefore, for NSM, the inframammary fold approach has become more popular access with an acceptable complications profile [31]. However, similar to SSM, NSM through an inframammary incision may limit the ability to resect the breast parenchyma at more distant locations of the breast, including the tail of Spence region. Moreover, this region is a conjunction of vessels (axillary, thoraco-epigastric), thus surgeons tend to perform a less aggressive resection of the parenchyma to avoid potential bleeding especially when visualization is limited. For pendulous/ptotic and/or large sized breasts, the optimal surgical technique for skin reducing nipple sparing mastectomy has not yet been defined. skin reducing nipple sparing mastectomy can be performed using different skin pattern including an inverted “T” mastopexy. Another approach is the Wise pattern for SSM/NSM [33]. This include a semi-circular region of skin at the base of the breast that is planned to be resected, de-epithelised and raised as a thin dermal flap. This relates to the area underlying the ‘T’-incision junction of the completed reconstruction. In case of SSM, the island of skin removed from the flap can be used to replace the resected nipple areola complex, similar to other techniques that use de-epithelialization of skin flaps for implant coverage (instead or combined with a mesh or acellular dermis). Skin reduction requires transposition of the NAC and accurate de-epithelialization of a skin pedicle, sparing the tissue-continuity of the subdermal vascular plexus. It is important to secure the blood supply of the nipple, by a medially pedicled dermal flap, to reduce the risk of necrosis. Henceforward, the removal of the entire BGT is performed according to the inverted “T” incision. This technique is highly delicate since it is important to remove the entire BGT while sparing the II-IV perforator vessels that assure the blood supply to the medial skin flap and the nipple. A possible option of skin reducing NSM in very large breasts with extreme ptosis is the use of invert “T” skin incision and the repositioning of the NAC as a full or partial thickness skin graft.
In case of NSM, the NAC is the area where rBGT is most frequently left behind, as the surgical procedure may be limited to preserve viability of the NAC. The axillary tail of the mammary gland is also considered as an area with a tendency of more rBGT, which may be due to mastectomy techniques noted above [34].
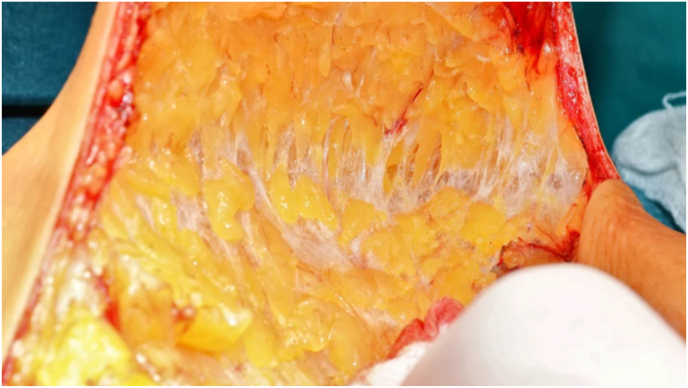
As indicated above, in both SSM and NSM the breast parenchyma needs to be accurately dissected from the covering skin at the level of the superficial fascia, as cleavage plane between the subcutis (not part of glandular tissue) and the BGT (Fig. 1). At time of surgery, identifying this border can be cumbersome and separating the subcutis from the glandular tissue can necessitate delicate incisions. A few reports indicated various amount of rBGT after SSM, and a tendency to leave more rBGT at the anterior border to preserve native-skin viability [[34], [35], [36]]. It was suggested that the thickness of the skin flap is highly dependent on surgeon’s expertise and techniques used [36]. The thickness of the skin-envelope depends on the thickness of the subcutaneous fat, which depends on factors including age and BMI. However, it often correlates with the amount of rBGT, except in the inframammary fold where there is relatively little BGT [37]. In patients with high BMI, the thickness of the subcutaneous fat layer can easily exceed 5 mm due to subcutaneous fat (Fig. 8) [13]. Often at least 5 mm of subcutaneous tissue is maintained to increase skin viability. Residual BGT can be palpable or better demonstrated on imaging in the early postoperative phase, because afterwards the compression of the implant might blur interpretation and even cause some atrophy.
Fig. 8.
Anterior plane of dissection in skin sparing and nipple sparing mastectomy, showing the subcutaneous fat. The thickness of the subcutaneous fat depends on the body mass index, but also varies according to different locations of the breast. The observation that there is a tendency to a thinner layer at the lower pole, assumed to be because of the weight of the mammary gland, explains, along with the gravitation force on the implant, why this area is more difficult to maintain the viability of the skin. Thus, the use of supportive material (such as a mesh) may be required to reducing the pressure.
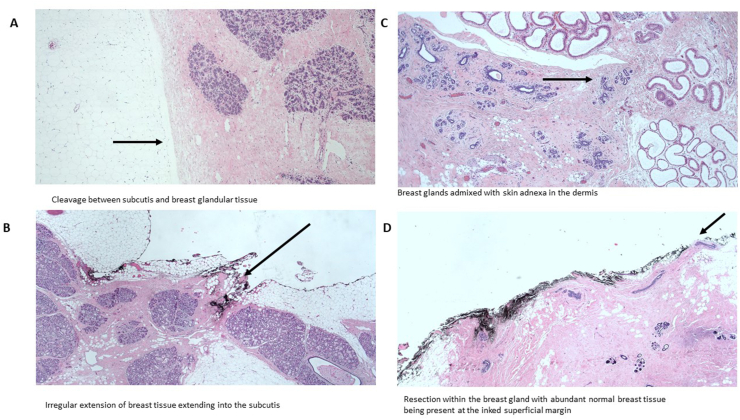
Evaluation of preoperative imaging of the breast to evaluate the amount and location of BGT can aid in evaluating the pre-existing thickness of the subcutaneous fat layer and thereby the distance between the skin and the BGT. Importantly, even if the resection is done perfectly at the cleavage between the subcutaneous fat and the fibroglandular tissue at the level of the superficial fascia (Fig. 1, Fig. 8, Fig. 9A), in some cases there might be irregular extensions of BGT into the subcutaneous tissue (Fig. 9B) and even into the dermis (Fig. 9C). Given that the surgical specimen sent to evaluation is limited to the resected tissue, these irregular extensions of the BGT at the anterior border often remain unnoticed. Presence of abundant normal breast glands may occasionally be observed at histopathological examination at the inked, superficial margin (Fig. 9D).
Fig. 9.
Microscopic view of the anterior resection margins of skin/nipple sparing mastectomy.
Thus, these are potential areas of rBGT or even residual disease in case of a superficial tumour location [38]. Additional potential challenges in these procedures are that, in contrast to non-SSM/NSM, the skin and subcutaneous tissue overlying the tumour or the skin at the area of the biopsy track are often not resected, and these areas may bear additional tumour cells as a result of a tumour foci [39,40] or seeding [41,42]. Evaluation of the superficial margins for rBGT in SSM, defined as the superficial area localized over the tumour showed that out of 168 SSMs, 64 (38%) had a positive superficial specimen margin for rBGT. A total of 14 patients out of 168 (8%) had even residual disease at the superficial extension, of whom in 13 cases (93%) rBGT was found in the superficial specimen taken above the tumour. This indicates that rBGT superficial to the tumour is associated with an increased risk for residual tumour foci (13 out of 64, 20%). Only one patient with a negative superficial specimen for rBGT had residual disease within the lymphatic spaces of the subcutaneous tissue. Importantly, 12 out of the 13 patients with residual disease at the superficial margins had DCIS, of which in 3 cases an invasive carcinoma component was present as well, and one case of invasive cancer without DCIS was found [43]. These results are aligned with the nature of DCIS, which growth pattern is associated with skip lesions making it difficult for the surgeon to predict its extension at the time of surgery [28]. Meticulous removal of all BGT above the tumour site is therefore imperative.
Even though SSM/NSM were reported to be oncologically safe, these data derive from retrospective studies [[44], [45], [46]] and it remains unclear to which extent the presence and amount of rBGT is associated with local recurrence risks and/or new primary tumours, especially in patients who are not scheduled for PMRT [7,10]. The relation between the skin flap thickness, which is related to the presence of rBGT and possibly residual disease, and the occurrence of recurrences is not yet well-defined. While some radiation oncologists recommend to consider PMRT in patients with a flap thickness of more than 5 mm [47], some surgeons, however, recommend routine MRI after NSM in order to assess rBGT [48]. Residual breast tissue as a sole indication for PMRT should be carefully weighed against impairment of QoL including cosmetic outcome of breast reconstruction. However, the benefit of PMRT for patients with rBGT, harbouring potential residual disease (e.g., rBGT in proximity of the tumour bed), or with other risk factors (multifocality, lymphovascular invasion, triple negative subtype, superficial localisation of the tumour) needs to be thoroughly considered. Therefore, we recommend discussing this on an individual base with the patient, using all possible risk factors combined. Due to the delicacy of NSM and IBR procedures, we do not recommend to use bolus to further increase the dose at the level of the NAC during PMRT, based on the risk of rBGT alone. This should, however, be considered in cases of suspected residual disease population in which NSM might better not have been advised.
1.6. The common dissection planes
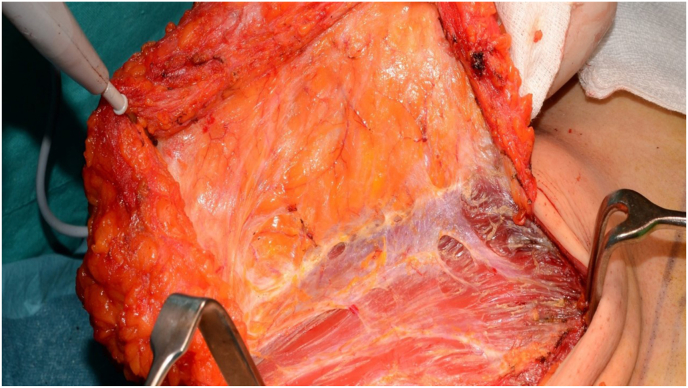
In all types of mastectomies there are several planes in which the surgeon dissects the breast tissue. Regardless of the procedure, the dissection from the chest wall is needed in all. The breast tissue is dissected off the muscle up to the fascia (Fig. 10). Nowadays, the dorsal fascia is not routinely removed by all surgeons. Removal of the dorsal fascia with the breast tissue is done depending on the tumour location and degree of invasion close to or in the muscle, as it is a rare event that breast ducts or glandular tissue will be found beyond the dorsal fascia of the breast [49,50]. However, surgical protocols vary among centres or National guidelines, for example in Denmark, the dorsal fascia (but not the muscle) is removed in most cases. Data is scarce about the clinical yield of removing the fascia, and it can potentially increase surgical complications, thus there is no consensus about the need to excises the pectoralis muscle fascia, unless needed to achieve a clear margin [22]. In any case, the final surgical and histopathological report should indicate if the pectoral dorsal fascia was removed. As indicated above, per ACROP-ESTRO guidelines, in case of absence of tumour invasion into the pectoralis muscle, the pectoral muscle is not part of the CTV (Table 1) [1,7,51].
Fig. 10.
Posterior resection border, the breast glandular tissue resected including the pectoralis major fascia. The figure shows the delicate fascia encapsulating the glandular tissue at the posterior plane and the perforating vessels.
1.7. Reconstruction after mastectomy
The option for either immediate or delayed breast reconstruction should be considered in all patients who are scheduled for mastectomy, and well prior to any surgical procedure. In this, factors such as the type and timing of reconstruction must be considered, taking into account also a possible indication for PMRT. The patient needs to be informed that PMRT can be associated with increased severity and rates of complications, including impaired aesthetic outcomes, all of which are highly related to the type of reconstruction [52,53]. The best timing of PMRT in the setting of any method of reconstruction is controversial [35,53,54]. Moreover, the need for PMRT often cannot be determined until the final pathologic evaluation is complete, thereby after surgery, unless a sentinel node biopsy or axillary dissection is performed prior to the breast surgery.
Breast reconstruction following mastectomy can be performed using various techniques, including positioning a tissue expander that is replaced by a permanent implant in a second procedure before or after PMRT [55] (2-stage procedure with expander and implant, TE/I). Breast reconstruction can be performed by one stage implant-based reconstruction (single-stage direct-to-implant, DTI), autologous tissue breast reconstruction (ABR), or a combination of expander/implant and autologous tissue [53,55]. The type of reconstruction depends on patient-related factors, such as BMI, smoking, body habitus (i.e., breasts size and shape, excess skin quality, need for reduction/mastopexy of the contralateral breast, donor sites (like amount of fat in the abdominal area and/or thighs), comorbidity and surgeon’s expertise with any given technique. Autologous-based reconstruction is reported to have lower rates of complications and better cosmetic outcomes in the setting of PMRT, compared to implant-based reconstruction [52]. Autologous breast reconstruction, however, demands specific expertise and is associated with additional donor site morbidity and may delayed oncologic treatment in case of severe complications.
Implant-based reconstructions (i.e, prosthetic) include different techniques that vary with regards to the number of procedures (single-stage, two-stage procedure), type of implant and supportive material, and location of implant in relation to the pectoralis major muscle.
In case of DTI, a permanent implant is inserted at the time of the mastectomy (pre-pectoral or sub (post/retro)-pectoral) whereas in a two-stage procedure (TE/I) a tissue expander is placed (usually sub-pectoral) at the time of surgery and replaced by a permanent implant in a second procedure, generally several months later after completion of radiation [55].
The prosthetic devices (tissue expander and/or implant) can be placed behind the pectoralis major muscle (sub-pectoral) or anterior to the pectoralis major muscle (pre-pectoral).
Single or two-stage procedures of implant-based IBRs are increasing compared to autologous breast reconstruction (ABR), following the increased popularity of SSM and NSM and the availability of supportive materials such as biological/synthetic mesh or acellular dermal matrix (ADM) [56].
Supportive materials facilitated both the single-stage and two-stage procedure by serving as additional “tissue” (both in pre-pectoral and sub-pectoral implants) to create a pocket to stabilize the implant and to minimize its dislocation (Fig. 11) [57]. The use of ADM was also suggested to reduce the risks of PMRT complications in the setting of IBR, a hypothesis that remains to be validated [58]. An alternative technique to synthetic supportive material is the use of autologous de-epithelialized dermal grafts (Fig. 12) [59,60], mostly harvested from the ipsilateral side from the lower pole of the breast skin [60]. These dermal grafts are used to create a pocket for the implant at the time of IBR, to stabilize the implant and to minimize dislocation.
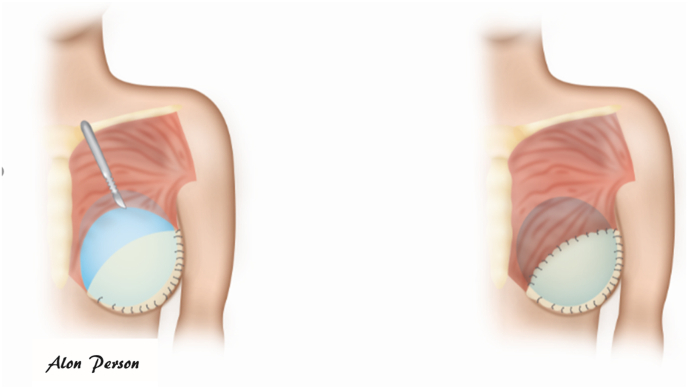
Fig. 11.
A,B: The use of supportive mesh to complete the pectoralis muscle deficit at the lower pole and create a pocket to hold the subpectoral implant.
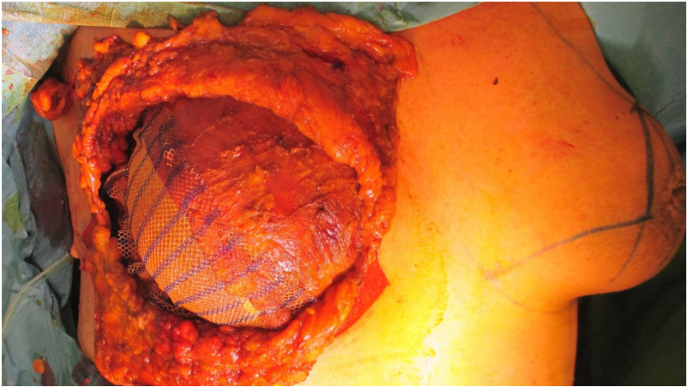
Fig. 12.
Skin sparing mastectomy with skin reducing procedure for mastopexy, using an inverted T incision, with preserving of autologous de-epithelialized dermal graft on the lower pole, to serve as a dermal sling. Immediate reconstruction was done using anatomical shaped silicone implant, 610 cc, placed posterior to the pectoral major muscle (sub-pectoral) which is covering the cranial pole of the implant. The infero-lateral pole is covered by partly absorbable synthetic mesh, and on top of the mesh the dermal sling. All is then covered by the native-breast skin flap adjusted from the medial and lateral sides, with a visible scar in the form of inverted “T”.
These techniques are often used to facilitate the DTI technique as in female the pectoral major muscle is generally not well-developed or, in case of TE/I with an expander located sub-pectoral, to complete full coverage of the implant especially at the lower pole. Therefore, ADM or dermal graft can be used if the muscle and skin are not sufficiently expanded (or in a one stage procedure) to close the “defect” and cover the implant or, in case of a pre-pectoral position, to provide coverage instead of the pectoral muscle. However, in case of pre-pectoral implants, there is a tendency to leave more subcutaneous tissue for support of the implant to create a more natural looking breast mound, i.e., more potential rBGT, and potential residual tumour foci.
The ESTRO-ACROP guidelines in the setting of implant–based IBR discuss the location of CTV in accordance with the different locations of the implant (pre-or subpectoral-pectoral) [11]. In case of sub-pectoral implant, if there is no tumour involvement of the dorsal fascia, the CTV of the chest wall does not include the deep lymphatic plexus and therefore only includes the rim of tissue ventral to the major pectoral muscle and the implant. Except at the medial, lateral and caudal borders, where it may extend to the ventral side of the chest wall where it is not covered by the major pectoral muscle [1,[7], [8], [9],12]. In case of a pre-pectoral positioned implant, the CTV of the chest wall is divided into two volumes of interest by the implant: the ventral part between the skin and the implant, containing the subcutaneous lymphatic plexus and eventual residual glandular tissue and the dorsal part between the implant and the pectoral muscle/chest wall, containing the deep lymphatic plexus and eventual rBGT. While the implant can be largely excluded from the CTV of the chest wall, due to the position of the implant, irradiation of the implant to a dose similar to the prescribed dose might be inevitable (Table 1) [12].
Autologous flap-based reconstruction usually follows simple/total mastectomy and can be performed immediately at the time of primary surgery or as a delayed procedure (even years after mastectomy, with/without PMRT) [55]. Delayed autologous reconstruction can help to replace fibrotic skin after surgery and PMRT and is considered as the preferred method for reconstruction in case of severe late complications. Autologous reconstruction can be performed with the use of pedicled or free flaps with microsurgical anastomosis of vessels. Different donor sites are available to harvest skin and fat tissue (most often abdominal wall and upper thigh, though many other procedures have been described) [61]. The most common flaps for breast reconstruction are created from the abdominal wall and include the (free) deep inferior epigastric artery perforator (DIEP) flap and the (pedicled) transverse rectus abdominis musculocutaneous (TRAM) flap. Flaps that origin in the back include the (pedicled) latissimus dorsi (LAD) flap using skin, fat, and muscle from the back, whereas the thoracodorsal artery perforator flap (TDAP) uses skin and fat only, both due to the small volume of donor tissue usually combined with an implant and/or with oncoplastic procedures (mostly LAD). Flaps from buttocks or thigh muscles are usually free flaps including gluteal musculocutaneous and/or perforator flaps (SGAP and IGAP) as well as the thigh-based flaps such as the transverse gracilis (TUG) and Profunda artery perforator (PAP) flaps [62].
Autologous flap reconstruction is a more extensive and delicate procedure than implant reconstruction. Flap-failure may leave the patient without other options for salvage reconstructive procedures. When immediate ABR is performed and RT is indicated, the autologous flap (skin and soft tissue) is not part of the CTV since there is no rBGT tissue nor tumour cells in the volume of the neo-breast. Residual glandular tissue, if found, is usually located at the area of the native breast/chest wall-skin that is connected to the flap. This area is mostly the area of local recurrences in these types of reconstruction [7]. In rare cases, if the BGT was not completely dissected from the pectoral muscle, rBGT and a subsequent recurrence can occur behind the flap [7]. Whereas often the autologous flap can be recognised thanks to a different tissue density, especially in the first period after reconstruction, for easier recognition the scars and flap should be marked prior to planning CT to facilitate correct delineation.
1.8. Bolus
Bolus is used as a tissue equivalent material placed on the skin during PMRT to increase the dose to the chest wall skin and subcutaneous tissue to reduce the risk of local recurrences [63]. However, bolus use, and protocols (thickness and schedule) vary significantly between institutions [[64], [65], [66], [67]]. Bolus was found to be the most important independent risk factor for severe skin toxicity in case of PMRT, due to the increase in skin surface volume receiving higher radiation doses [63]. Acute skin toxicity may result in treatment interruption or early cessation of radiation, which eventually may impact chest wall recurrence [68,69]. In case of simple/total mastectomy a quite large part of the native-breast skin is removed including subcutaneous tissue and within its lymphatic plexus, which is not the case of SSM/NSM. There are no recommendations on if/when to use bolus after SSM/NSM. In some institutions, bolus is routinely used in any IBR (because the skin and subcutaneous tissue are preserved and considered a high-risk volume, and due to the IBR stretched in a thinned layer over the implant) to allow full coverage of these volumes (within the 95% isodose line). In many other institutions, however, the use of bolus is restricted to high-risk cases for local recurrences including T4b,c,d primary tumours. Based on dosimetric evaluation by the DBCG, which is planned to be clinically validated in a randomised DBCG RT Recon trial [NCT03730922], due to the shape of the reconstructed breast which resembles the shape of the native breast, using tangential field-in-field planning, only the lateral side of the reconstructed breast tends to have the skin-sparing effect compared to the apex of the breast mound (NAC areas). While PMRT is a strong risk factor for breast reconstruction failure especially in case of an implant-based reconstruction, and acute toxicity may result in late skin sequela (fibrosis, severe telangiectasia), it is unknown if omitting the bolus may reduce these complications. Therefore, until further data become available, the routinely use of a bolus in case of IBR is not recommended and should be considered on an individual basis if there is a concern for a high-risk area that is not getting full dose coverage [11]. Therefore, we recommend that when planning such cases for PMRT, the radiation oncologist should consider 95% dose in such areas, and that may in some cases lead to either low energy (6 MV) or a bolus if the area is known. It is recommended to ask for advice of the surgeon where are the areas of close superficial margins in case of a bolus, and if the location is not clearly identified, use low energy (6 MV) in the quadrant, to increase the dose to the surface.
1.9. PMRT boost
The use of a boost in case of mastectomy via an additional radiation dose to the chest wall scar has been applied in many institutions [70]. A retrospective study by the Massachusetts General Hospital [70] evaluated whether delivery of a chest wall boost to the mastectomy scar or chest wall is independently associated with reconstruction complications in the setting of breast reconstruction (autologous, DTI, TE/I). It confirmed that a radiation boost was significantly associated with infection, skin necrosis, and implant exposure. For implant-based reconstruction patients, the boost was independently associated with increased risks of implant failure. Importantly, the addition of the boost was not associated with improving local tumour control, even in high-risk subgroups [55]. Therefore, we do not recommend routine use of chest wall or mastectomy scar boost in case of PMRT, with or without IBR.
1.10. Future of mastectomy and RT
Currently there are several trials aiming to improve the outcomes of patients who are planned for mastectomy. These include changing the sequence of treatments and performing locoregional irradiation prior to surgery, for example the PRADA trial (Primary Radiotherapy And DIEP flAp Reconstruction Trial) [NCT02771938], which aims to evaluate preoperative radiation in patients who are planned for mastectomy and autologous-based reconstruction.
2. Summary
Our paper shows how mastectomy techniques and reconstruction evolved to improve cosmetic outcomes and its potential implications on rBGT, residual disease and recurrences. Breast surgeons, radiation oncologists, pathologists, radiologists, and medical/clinical oncologists should work together to individualise the treatment for optimal oncological outcomes while maintaining the significant improvements in achieving better cosmesis for these patients. Surgeons should be aware of the challenges related to each procedure and strive to remove all BGT and reduce the risk of residual disease. The surgical and histopathology reports should indicate features such as the extent of the procedure, resection of the fascia, rBGT in the anterior border, excision of the biopsy site. Radiation oncologists should be aware of potential rBGT, and tumour cell seeding, helping to define the areas to be included in the target volume. The type of mastectomy and reconstruction maybe associated with different high-risk areas linked to variable incidences for the presence of rBGT. Importantly, it should be noted that the indication of PMRT and, more challenging, which regions are considered at higher risk for recurrence are related to the individual tumour and reconstruction-linked factors. Per similar disease stage, some patients will strongly benefit from PMRT whilst other have no gain at all, therefore much more research is needed to individualise PMRT strategy to improve overall outcomes. Research to improve our understanding of the role of tumour biology, molecular phenotype, resistance to therapy, local recurrence patterns and response to PMRT, including the use of bolus and/or scar boost is still ongoing. Nevertheless, familiarity with the patient’s surgical procedures is essential when moving forward along the path towards fully volume based PMRT.
Authors contribution
Conceptualisation, OKP, BVO, LB, PHP, DdR.; Methodology, All authors.; Data Curation, OKP.; Writing – OKP; Writing – Review & Editing, All.; Supervision BVO, LB, PHP, DdR.
Declaration of competing interest
None relevant for the contents of this paper.
References
- 1.Offersen B.V., Boersma L.J., Kirkove C. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 2016;118:205–208. doi: 10.1016/j.radonc.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Marks L.B., Kaidar-Person O., Poortmans P. Regarding current recommendations for postmastectomy radiation therapy in patients with one to three positive axillary lymph nodes. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.71.0764. [DOI] [PubMed] [Google Scholar]
- 3.Frasier L.L., Holden S., Holden T. Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines. JAMA Oncol. 2016;2:95–101. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebctcg McGale P., Taylor C. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donker M., van Tienhoven G., Straver M.E. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sávolt Á., Péley G., Polgár C. Eight-year follow up result of the OTOASOR trial: the Optimal Treatment of the Axilla - surgery or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol. 2017;43:672–679. doi: 10.1016/j.ejso.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Kaidar-Person O., Poortmans P., Offersen B.V. Breast Cancer Res Treat; 2020. Spatial location of local recurrences after mastectomy: a systematic review. [DOI] [PubMed] [Google Scholar]
- 8.Offersen B.V., Boersma L.J., Kirkove C. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen H.M., Offersen B.V. Regional recurrence after adjuvant breast cancer radiotherapy is not due to insufficient target coverage. Radiother Oncol. 2015;114:1–2. doi: 10.1016/j.radonc.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Kaidar-Person O., Poortmans P., Offersen B.V. What are the guidelines for immediate breast reconstruction? Eur J Surg Oncol. 2020 doi: 10.1016/j.ejso.2020.03.226. [DOI] [PubMed] [Google Scholar]
- 11.Kaidar-Person O., Vrou Offersen B., Hol S. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol. 2019;137:159–166. doi: 10.1016/j.radonc.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Kaidar-Person O., Nissen H.D., Yates E.S. Clinical Oncology; 2020. Postmastectomy radiation therapy planning after immediate implant-based reconstruction using the European society for Radiotherapy and oncology-advisory committee in radiation Oncology practice consensus guidelines for target volume delineation. [DOI] [PubMed] [Google Scholar]
- 13.Rehnke R.D., Groening R.M., Van Buskirk E.R., Clarke J.M. Anatomy of the superficial fascia system of the breast: a comprehensive theory of breast fascial anatomy. Plast Reconstr Surg. 2018;142:1135–1144. doi: 10.1097/PRS.0000000000004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones C., Lancaster R. Evolution of operative technique for mastectomy. Surg Clin. 2018;98:835–844. doi: 10.1016/j.suc.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Freeman M.D., Gopman J.M., Salzberg C.A. The evolution of mastectomy surgical technique: from mutilation to medicine. Gland Surg. 2018;7:308–315. doi: 10.21037/gs.2017.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halsted W.S.I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46:1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veronesi U., Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer. 1981;47:170–175. doi: 10.1002/1097-0142(19810101)47:1<170::aid-cncr2820470128>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Zurrida S., Bassi F., Arnone P. The changing face of mastectomy (from mutilation to aid to breast reconstruction) Int J Surg Oncol. 2011;2011:980158. doi: 10.1155/2011/980158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veronesi U., Cascinelli N., Mariani L. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B., Anderson S., Bryant J. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 21.Takei H., Kurosumi M., Yoshida T. Current trends of sentinel lymph node biopsy for breast cancer —a surgeon’s perspective. Breast Cancer. 2007;14:362–370. doi: 10.2325/jbcs.14.362. [DOI] [PubMed] [Google Scholar]
- 22.Suijker J., Blok Y.L., de Vries R. Pectoral fascia preservation in oncological mastectomy to reduce complications and improve reconstructions: a systematic review. Plastic and reconstructive surgery. Global open. 2020;8 doi: 10.1097/GOX.0000000000002700. e2700-e2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson J., Esserman L., Ewing C. Sentinel lymph node mapping in post-mastectomy chest wall recurrences: influence on radiation treatment fields and outcome. Ann Surg Oncol. 2016;23:715–721. doi: 10.1245/s10434-015-4971-8. [DOI] [PubMed] [Google Scholar]
- 24.Noone R.B., Frazier T.G., Noone G.C. Recurrence of breast carcinoma following immediate reconstruction: a 13-year review. Plast Reconstr Surg. 1994;93:96–106. ; discussion 107-108. [PubMed] [Google Scholar]
- 25.Stanec Z., Zic R., Budi S. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg. 2014;73:485–491. doi: 10.1097/SAP.0b013e31827a30e6. [DOI] [PubMed] [Google Scholar]
- 26.Liebens F., Carly B., Cusumano P. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas. 2009;62:113–123. doi: 10.1016/j.maturitas.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Nimboriboonporn A., Chuthapisith S. Nipple-areola complex reconstruction. Gland Surg. 2014;3:35–42. doi: 10.3978/j.issn.2227-684X.2014.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tramm T., Zuckerman K., Tavassoli F.A. Skip lesion of DIN (DCIS) in the nipple in a case of breast cancer. Int J Surg Pathol. 2011;19:817–821. doi: 10.1177/1066896909339737. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberg R.E., Chan J.S., Swistel A.J., Hoda S.A. Pathological evaluation of nipple-sparing mastectomies with emphasis on occult nipple involvement: the Weill-Cornell experience with 325 cases. Breast J. 2014;20:15–21. doi: 10.1111/tbj.12199. [DOI] [PubMed] [Google Scholar]
- 30.Ujhelyi M., Savolt A., Fulop R. Evaluation of the medially pedicled skin-reducing nipple-sparing mastectomy as a standard mastectomy technique for large and ptotic breasts. Breast J. 2020;26(11):2276–2279. doi: 10.1111/tbj.13985. [DOI] [PubMed] [Google Scholar]
- 31.Daar D.A., Abdou S.A., Rosario L. Is there a preferred incision location for nipple-sparing mastectomy? A systematic review and meta-analysis. Plast Reconstr Surg. 2019;143:906e–919e. doi: 10.1097/PRS.0000000000005502. [DOI] [PubMed] [Google Scholar]
- 32.Park S., Yoon C., Bae S.J. Comparison of complications according to incision types in nipple-sparing mastectomy and immediate reconstruction. Breast. 2020;53:85–91. doi: 10.1016/j.breast.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thuman J., Freitas A.M., Schaeffer C., Campbell C.A. Prepectoral wise-pattern staged implant-based breast reconstruction for obese or ptotic patients. Ann Plast Surg. 2019;82 doi: 10.1097/SAP.0000000000001791. S404–s409. [DOI] [PubMed] [Google Scholar]
- 34.Kaidar-Person O., Boersma L.J., Poortmans P. Residual glandular breast tissue after mastectomy: a systematic review. Ann Surg Oncol. 2020;27:2288–2296. doi: 10.1245/s10434-020-08516-4. [DOI] [PubMed] [Google Scholar]
- 35.Papassotiropoulos B., Guth U., Chiesa F. Prospective evaluation of residual breast tissue after skin- or nipple-sparing mastectomy: results of the SKINI-trial. Ann Surg Oncol. 2019;26:1254–1262. doi: 10.1245/s10434-019-07259-1. [DOI] [PubMed] [Google Scholar]
- 36.Papassotiropoulos B., Guth U., Dubsky P., Tausch C. ASO author reflections: a call for surgeon experience and surgical radicality to prevent residual breast tissue after skin- and nipple-sparing mastectomy. Ann Surg Oncol. 2019;26(5):1254–1262. doi: 10.1245/s10434-019-07259-1. [DOI] [PubMed] [Google Scholar]
- 37.Giannotti D.G., Hanna S.A., Cerri G.G., Bevilacqua J.L.B. Analysis of skin flap thickness and residual breast tissue after mastectomy. Int J Radiat Oncol Biol Phys. 2018;102:82–91. doi: 10.1016/j.ijrobp.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Kaidar-Person O., Kuhn T., Poortmans P. Should we worry about residual disease after mastectomy? Lancet Oncol. 2020;21:1011–1013. doi: 10.1016/S1470-2045(20)30331-4. [DOI] [PubMed] [Google Scholar]
- 39.Holland R., Veling S.H., Mravunac M., Hendriks J.H. Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979–990. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 40.Bosma S.C.J., Leij F., Vreeswijk S. Five-Year results of the preoperative accelerated partial breast irradiation (PAPBI) trial. Int J Radiat Oncol Biol Phys. 2020;106:958–967. doi: 10.1016/j.ijrobp.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 41.Uriburu J.L., Vuoto H.D., Cogorno L. Local recurrence of breast cancer after skin-sparing mastectomy following core needle biopsy: case reports and review of the literature. Breast J. 2006;12:194–198. doi: 10.1111/j.1075-122X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 42.Franchelli S., Canavese G., Murolo C., Barabino P. Local recurrence of breast cancer around a prosthesis dome. Br J Plast Surg. 2002;55:685–686. doi: 10.1054/bjps.2002.3942. [DOI] [PubMed] [Google Scholar]
- 43.Cao D., Tsangaris T.N., Kouprina N. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol. 2008;15:1330–1340. doi: 10.1245/s10434-007-9795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlson G.W., Styblo T.M., Lyles R.H. Local recurrence after skin-sparing mastectomy: tumor biology or surgical conservatism? Ann Surg Oncol. 2003;10:108–112. doi: 10.1245/aso.2003.03.053. [DOI] [PubMed] [Google Scholar]
- 45.Meretoja T.J., von Smitten K.A., Leidenius M.H. Local recurrence of stage 1 and 2 breast cancer after skin-sparing mastectomy and immediate breast reconstruction in a 15-year series. Eur J Surg Oncol. 2007;33:1142–1145. doi: 10.1016/j.ejso.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Woitek R., Pfeiler G., Farr A. MRI-based quantification of residual fibroglandular tissue of the breast after conservative mastectomies. Eur J Radiol. 2018;104:1–7. doi: 10.1016/j.ejrad.2018.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hehr T., Baumann R., Budach W. Radiotherapy after skin-sparing mastectomy with immediate breast reconstruction in intermediate-risk breast cancer : indication and technical considerations. Strahlenther Onkol. 2019;195:949–963. doi: 10.1007/s00066-019-01507-9. [DOI] [PubMed] [Google Scholar]
- 48.Zippel D., Tsehmaister-Abitbol V., Rundstein A. Magnetic resonance imaging (MRI) evaluation of residual breast tissue following mastectomy and reconstruction with silicone implants. Clin Imag. 2015;39:408–411. doi: 10.1016/j.clinimag.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Dalberg K., Krawiec K., Sandelin K. Eleven-year follow-up of a randomized study of pectoral fascia preservation after mastectomy for early breast cancer. World J Surg. 2010;34:2539–2544. doi: 10.1007/s00268-010-0737-4. [DOI] [PubMed] [Google Scholar]
- 50.Temple W.J., Lindsay R.L., Magi E., Urbanski S.J. Technical considerations for prophylactic mastectomy in patients at high risk for breast cancer. Am J Surg. 1991;161:413–415. doi: 10.1016/0002-9610(91)91100-w. [DOI] [PubMed] [Google Scholar]
- 51.Pifer P.M., Bice R.P., Jacobson G.M. The lack of consensus of international contouring guidelines for the dorsal border of the chest wall clinical target volume: what is the impact on organs at risk and relationships to patterns of recurrence in the modern era? Adv Radiat Oncol. 2019;4:35–42. doi: 10.1016/j.adro.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooke A.L., Diaz-Abele J., Hayakawa T. Radiation therapy versus No radiation therapy to the neo-breast following skin-sparing mastectomy and immediate autologous free flap reconstruction for breast cancer: patient-reported and surgical outcomes at 1 Year-A mastectomy reconstruction outcomes consortium (MROC) substudy. Int J Radiat Oncol Biol Phys. 2017;99:165–172. doi: 10.1016/j.ijrobp.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Naoum G.E., Salama L., Niemierko A. Single stage direct-to-implant breast reconstruction has lower complication rates than tissue expander and implant and comparable rates to autologous reconstruction in patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2020;106:514–524. doi: 10.1016/j.ijrobp.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Santosa K.B., Chen X., Qi J. Postmastectomy radiation therapy and two-stage implant-based breast reconstruction: is there a better time to irradiate? Plast Reconstr Surg. 2016;138:761–769. doi: 10.1097/PRS.0000000000002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho A.Y., Hu Z.I., Mehrara B.J., Wilkins E.G. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol. 2017;18:e742–e753. doi: 10.1016/S1470-2045(17)30617-4. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal S., Kidwell K.M., Farberg A. Immediate reconstruction of the radiated breast: recent trends contrary to traditional standards. Ann Surg Oncol. 2015;22:2551–2559. doi: 10.1245/s10434-014-4326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee C.H., Cheng M.H., Wu C.W. Nipple-sparing mastectomy and immediate breast reconstruction after recurrence from previous breast conservation therapy. Ann Plast Surg. 2019;82:S95–S102. doi: 10.1097/SAP.0000000000001696. [DOI] [PubMed] [Google Scholar]
- 58.Craig E.S., Clemens M.W., Koshy J.C. Outcomes of acellular dermal matrix for immediate tissue expander reconstruction with radiotherapy: a retrospective cohort study. Aesthetic Surg J. 2019;39:279–288. doi: 10.1093/asj/sjy127. [DOI] [PubMed] [Google Scholar]
- 59.Davis C., Boyd C., Mateo de Acosta Andino D.A. Dermal autografts in breast reconstruction: a review of past and current trends. Ann Plast Surg. 2020;84:618–622. doi: 10.1097/SAP.0000000000002128. [DOI] [PubMed] [Google Scholar]
- 60.Lynch M.P., Chung M.T., Rinker B.D. Dermal autografts as a substitute for acellular dermal matrices (ADM) in tissue expander breast reconstruction: a prospective comparative study. J Plast Reconstr Aesthetic Surg. 2013;66:1534–1542. doi: 10.1016/j.bjps.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Lee Y.J., Kim S., Moon S.H. Unilateral autologous breast reconstruction with unipedicled and bipedicled deep inferior epigastric artery perforator flap: a review of 168 cases over 3 years. Microsurgery. 2020;40(6):663–669. doi: 10.1002/micr.30601. [DOI] [PubMed] [Google Scholar]
- 62.Meattini I., De Santis M.C., De Rose F. Local treatment of the axilla in early breast cancer: so many questions, still few answers. Clin Oncol. 2020;32:e37–e38. doi: 10.1016/j.clon.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Shiba S., Okamoto M., Kiyohara H. Clinical advantage of chest-wall post-mastectomy radiation therapy without bolus. Vivo. 2018;32:961–965. doi: 10.21873/invivo.112335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner J.Y., Zeniou A., Williams A., Jyothirmayi R. Technique and outcome of post-mastectomy adjuvant chest wall radiotherapy-the role of tissue-equivalent bolus in reducing risk of local recurrence. The British Journal of Radiology. 2016:20160060. doi: 10.1259/bjr.20160060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yap M.L., Tieu M., Sappiatzer J. Outcomes in patients treated with post-mastectomy chest wall radiotherapy without the routine use of bolus. Clin Oncol. 2018;30:427–432. doi: 10.1016/j.clon.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura N., Arahira S., Zenda S. Post-mastectomy radiation therapy without usage of a bolus may be a reasonable option. J Radiat Res. 2017;58:66–70. doi: 10.1093/jrr/rrw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aristei C., Kaidar-Person O., Tagliaferri L. The assisi think tank meeting and survey of post MAstectomy radiation therapy after breast reconstruction: the ATTM-SMART report. Eur J Surg Oncol. 2018;44:436–443. doi: 10.1016/j.ejso.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Tieu M.T., Graham P., Browne L., Chin Y.S. The effect of adjuvant postmastectomy radiotherapy bolus technique on local recurrence. Int J Radiat Oncol Biol Phys. 2011;81 doi: 10.1016/j.ijrobp.2011.01.002. e165–171. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura N., Arahira S., Wada N. Post-mastectomy radiation therapy without a bolus may not increase the risk of local recurrence. Int J Radiat Oncol Biol Phys. 2015;93:E9. [Google Scholar]
- 70.Naoum G.E., Salama L., Ho A. The impact of chest wall boost on reconstruction complications and local control in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2019;105:155–164. doi: 10.1016/j.ijrobp.2019.04.027. [DOI] [PubMed] [Google Scholar]