Abstract
Purpose
To compare 131I-therapy outcomes in high turnover and normal turnover Graves’ disease patients and predict optimal first 131I activity for high turnover patients.
Methods
Retrospective cohort design (1:2) validated by propensity score analysis. Cohort 1, high turnover (2-h RAIU/24-h RAIU ≥ 1), n = 104, and cohort 2, normal turnover (ratio < 1), n = 208, patients were compared for post 131I outcome. The cure was defined as a combined euthyroid and stable hypothyroid state following 131I treatment. Logistic regression analysis was used for identifying prognostic factors. The propensity score was applied; 77 matched pairs (1:1 ratio) of high and normal turnover patients were selected as a validation set.
Results
First 131I cure rates of 28% in high turnover and 66% in normal turnover groups (p = 0.001) were noted. The therapy cycles (median, 2 vs. 1) and cumulative 131I activity (median, 15 vs. 7 mCi) were required to cure hyperthyroidism in cohort 1 and cohort 2, respectively. Age (> 44 years), higher grade of goitre, and 2-h RAIU (> 37%) were associated with 131I therapy failure. The high turnover patients needed a factor of 1.5–2 times more 131I activity to achieve a similar cure rate compared to the normal turnover patients. The first-dose cure rate was 31% vs. 60% by propensity score analysis (n = 154), no way different (28% vs.66%) from the whole group of 312 patients.
Conclusion
High turnover Graves’ disease patients, if administered standard 131I activity, the outcomes shall be poor. To improve the success rate, 131I activity should be increased by 1.5 to 2 times in the high turnover patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13139-020-00674-3.
Keywords: Graves’ disease, Turnover, High turnover, Normal turnover, Radioiodine therapy
Introduction
Graves’ disease (GD) can be managed with antithyroid drugs (ATD), 131I radioiodine therapy (RIT), and surgery. All three treatment options are useful, though some consider choosing one option over the other [1–3]. Regarding RIT, the 131I activity to be administered can be planned by different methods, including administering fixed activity of 131Ior adjusting 131I activity based on the size of the gland and gland’s ability to trap radioactive iodine or a more complex method involving detailed pre-therapy dosimetry, requiring calculations of residence time (effective half-life) of 13II in the thyroid gland [4–11]. Regarding kinetics of 131I in the thyroid, around15% of GD patients can have a rapid turnover of 131I in the gland, and the effective half-life (Teff) for 131I clearance in this subgroup is significantly less [12–17]. The lower residence time (effective half-life) of 131I results in lesser radiation absorbed dose delivered to the thyroid gland (target organ), which probably leads to treatment failure in high turnover GD patients if routine activities of 131I are administered. For this subgroup, various regimens are tried, including the use of higher 131I activities, some supporting concurrent use of lithium, and some showing no benefit of adding lithium [18–21]. The optimal 131I activity used in the high turnover subgroup of GD patients is not well defined [22, 23]. With this background, this retrospective cohort study was planned. However, the retrospective cohort studies are fraught with a high degree of bias. To overcome the bias issue, the propensity score, a statistical technique, is applied to create unbiased data set by choosing matched pair for analysis.
Objectives
To compare the outcome of 131I RIT in high turnover and normal turnover GD patients and predict the optimal first 131I RIT activity to be used in high turnover GD patients. Results from the retrospective cohort study shall be verified by propensity score analysis.
Method
Study Design
Retrospective cohort design validated by propensity score analysis.
Setting
The institute ethics committee (Human studies), All India Institute of Medical Sciences, New Delhi, approved this retrospective cohort study (ref no: IECPG-598/24.10.2019). The patients were derived from a review of medical records of 5714 consecutive hyperthyroid patients who visited the Thyroid Clinic of Department of Nuclear Medicine, AIIMS, New Delhi, from February 1989 to August 2018 for 131I RIT.
Inclusion Criteria
GD patients with available baseline 2-h RAIU and 24-h RAIU values before RIT (n = 4800) and at least 1 year follow-up after RIT were included.
Exclusion Criteria
Patients with hyperthyroidism diagnosis other than Graves’ disease (toxic MNG and toxic adenomas) and the ones with incomplete follow-up at Thyroid Clinic (because of convenience, patients went back to the referring physicians/endocrinologists) after 131I RIT were excluded.
Definitions of Patient’s Groups (Cohorts) Based on the Turnover of the Thyroid Gland
Ratios of early RAIU to late RAIU values were taken as markers for the iodine turnover of the gland [13]. Although different centres use different time points (ranging from 2 to 6 h) for early RAIU [13, 24], as per our institutional practice, we used 2 h for early RAIU [25].
High turnover—defined as 2-h RAIU/24-h RAIU ratio ≥ 1
Normal turnover—defined as 2-h RAIU/24-h RAIU ratio < 1
Sample Size
Sample size estimation was done considering the primary objective, which is comparing outcome proportions. We used a formula for 1:2 group allocations for sample size [26, 27]. Considering significance criterion 0.05 (95%) (Zcrt α/2:1.96), statistical power 95% (Zpwr, β: 1.645), estimated P1 as 60%, and estimated P2 as 80%, the estimated sample size required was 102 patients in the test group [13, 20, 28].
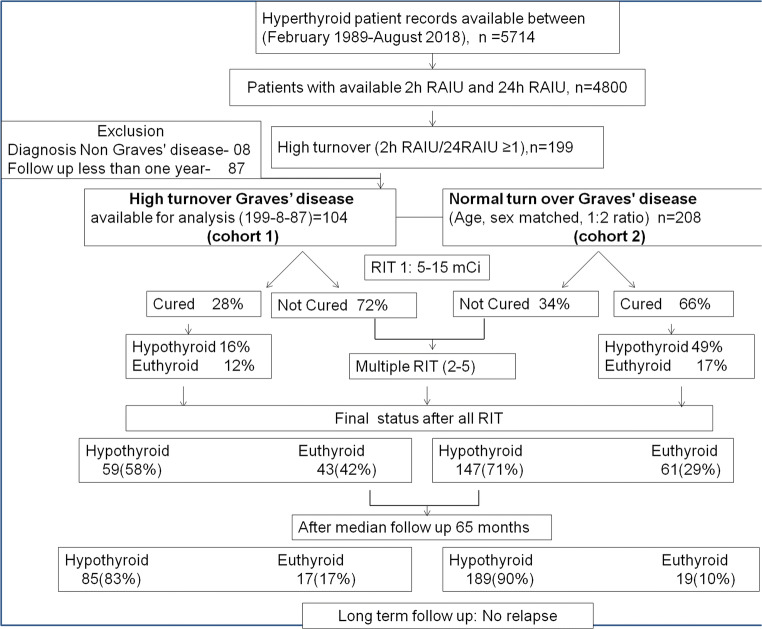
The patient selection chart is shown in Fig. 1. A total of 5714 hyperthyroid patients record were available for review. Out of these, 4800 patients had 2-h RAIU and 24-h RAIU values available. Among these, 199 patients had high turnover (2-h RAIU/24-h RAIU ratio ≥ 1). Out of these 199, eight patients on a thorough review of the case chart had a diagnosis of non-GD, and 87 were excluded as the follow-up was less than 1 year. Therefore, finally, 104 patients for high turnover cohort 1 were identified. For selecting the normal turnover cohort, which was age and sex-matched to a high turnover cohort, we first grouped all the case record into male and female groups and then further stratified all patients in the age group of + 1 year, for example, 19 + 1 (18–20) year, 22 + 1(21–23) year, and 25 + 1 (24–26) year. And then, for each high turnover patient in each slot, we selected 2 normal turnover patients randomly from the same strata resulting in 1:2 age and sex-matched groups. Finally, we included 104 patients in the high turnover group and 208 (1:2 ratio) in normal turnover as a control group.
Fig. 1.
Flowchart showing a selection of patient cohorts, follow-up, and outcome in high and normal turnover groups. Cohort 1 of 104 Graves’ disease patients with high turnover and cohort 2 as the control group (1:2 age/sex-matched normal turnover GD) were selected
Clinical Data
The patient’s data on baseline demographics, namely, thyroid function tests, number of 131I RIT given, and cumulative 131I activity administered, were extracted from the treatment chart.
For grading goitre severity, the World Health Organization (WHO) grading of goitre was used, which is described as grade 0, no goitre and not visible nor palpable; grade 1, palpable not visible gland; and grade 2, neck swelling visible when the neck is in normal position, corresponding to enlarged thyroid swelling on palpation [29]. For grading eye proptosis, grade 0, normal; grade 1, only stare present; and grade 2, proptosis present, were used.
131I Radioiodine Therapy and Follow-Up
Patients were per orally administered 131I activity ranging from 5 to 15 mCi. Radiation safety instructions were thoroughly explained, and written instructions were handed over to the patients to follow at home strictly. Normally antithyroid drugs are not prescribed routinely at our institution unless a severe comorbid condition was associated with GD. However, beta-blockers were prescribed to all if there were no contra-indication for the same for 6–8 weeks following RIT. Patients were followed at 3 monthly intervals with clinical and biochemical assessments at each follow-up. Further doses of 131I RIT were given in patients who had persistent hyperthyroidism.
Definitions of Euthyroid, Hypothyroid, and Hyperthyroid Status
The patient’s laboratory report and their respective laboratory ranges were used to define the biochemical status. For patients with overlapping or conflicting laboratory results and those who could not be categorized definitely in one particular category, the subsequent follow-up information was used to classifying them.
Outcome Definitions
Outcome After First 131I Radioiodine Therapy
Cured (First-Dose Success)
Patients who achieve either a euthyroid state (which is stable for at least 1 year without the requirement of repeat RAI or antithyroid medication) or a permanent hypothyroid state requiring thyroxine supplementation after 131I therapy were defined as cured. Thus, combined euthyroid and hypothyroid patients were considered cured.
Not Cured (First-Dose Failure)
Persistent hyperthyroid after 131I therapy necessitating further treatment with radioiodine/antithyroid drugs.
Cumulative Cure Rates After Successive131I RIT
Cumulative frequencies of 131I RIT cure rates after successive RIT sessions (2nd, 3rd, 4th, or more RIT) were noted.
Estimation of Optimal First 131I Activity for the Treatment of High Turnover GD Patients
Estimating a Correction Factor for Commonly Used 131I Activity Calculation Formulae
Commonly used Marinelli or simplified Marinelli formulae for 131I activity calculations were considered [10]. Activity to be administered is inversely proportional to radioiodine uptake and effective half-life [Activity∝ 1/RAIU (max)*effective half-life].
Activity to be administered in the high turnover group can be adjusted by multiplying a correction factor X, where X is the ratio of Teff for normal turnover GD and Teff for the rapid turnover gland. To obtain this ratio, we need to estimate the effective half-lives for the normal turnover and high turnover groups.
Effective Half-Life (Teff) Estimation
2-h RAIU and 24-h RAIU values were used to approximate the curve pattern. 2-h RAIU as the early time point seems more appropriate than 4–6 h, considering it is closer to the y-axis (as shown in supplementary figure 1, compared to 4 or 6 h) and shall provide a better approximation of uptake at time zero for curve fitting [30].
For effective half-life calculations, the exponential fitting method was used for curve fitting (an example is shown in supplemental figure 1). Since only two data points are used for curve fitting, only the subgroup of patients (55 patients) where the curve trend can be predicted (i.e., patients with 24-h RAIU less than 2-h RAIU showing a falling trend, were used). Patients with 24-h RAIU higher than or equal to or marginally less than 2 h were excluded (as Teff calculation was not possible). Non-linear curve fitting (exponential fitting) was applied between two time points that give the slope (λ) value. From this, Teff was obtained using formula Teff = 0.693/λ, as shown in supplementary figure 1.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows (SPSS Version 26.0) (IBM, Chicago, IL, USA). The normalcy of data was checked using the Shapiro-Wilk W test. Mann-Whitney test was used to compare the non-normally distributed continuous variables between two groups. A chi-square test was used to compare the categorical variables between the two groups. Considering the effect of other covariates (age, gender, duration of disease, goitre severity, prior antithyroid drug use, 131I RAI activity) on the outcome proportions in two groups, matching covariates analysis was done using the propensity score method. We calculated the propensity score using logistic regression (turnover group as the dependent variable) and other covariates mentioned above as an independent variable to get the predictive probability. We then matched the patients by propensity score, using the nearest-neighbour method, with an equal ratio of 1:1, and the match tolerance taken was 0.02 [31, 32]. For checking the association of risk factors and the outcome, univariate and multivariate analysis was used. For univariate analysis (to check the association between individual explanatory variable and outcome variable in the absence of other explanatory variables) (both representing proportions), chi-square test was used, and odds ratio was calculated. For multivariate analysis (to find independence of an explanatory variable in the presence of other confounding variables), binary logistic regression method was used to find out the best fitting model to describe the relationship between an outcome (dependent variable) and a set of explanatory variables (independent variables). Receiver operating characteristic (ROC) analysis was used to find the cutoff value for continuous variables, using the Youden index (sensitivity + specificity-1) [33]. p value < 0.05 was considered significant. Time to event analysis, for the spontaneous hypothyroid conversion of euthyroid patients in the long-term follow-up after all RIT, using Kaplan-Meier estimates with the comparison between curves was done using log-rank statistics.
Results
A total of 312 patients recruited for this retrospective study: cohort 1 of 104 Graves’ disease patients with high turnover and cohort 2 as the control group (1:2 age/sex-matched normal turnover GD). The detailed flow chart is given in Fig. 1. The prevalence of high turnover GD in our population was 4%. The detailed characteristics of patients among the two groups are shown in Tables 1 and 2.
Table 1.
Baseline characteristics of patients in high turnover and normal turnover group included in the study
| High turnover | Normal turnover | p value | |
|---|---|---|---|
| Number of patients | n = 104 | n = 208 | |
| Age (years) (median, range) | 38.5 (16–66) | 38 (16–66) | 0.916 |
| Gender (male/female) | 31(30%)/73 (70%) | 66(32%)/142(68%) | 0.729 |
| Duration of disease (months) (median, range) | 24 (1–192) | 24 (1–150) | 0.236 |
| Prior antithyroid drug use | 95(91.3%) | 186(89%) | 0.592 |
| Duration of antithyroid drug use (months) (median, range) | 24 (0–192) | 18 (0–150) | 0.312 |
| Weight loss | 96(92.3%) | 183 (88%) | 0.241 |
| Palpitation | 97(93%) | 184(88.5%) | 0.181 |
| Increased sweating | 81(78%) | 168(81%) | 0.550 |
| Tremors | 88(85%) | 159(76%) | 0.094 |
| Diarrhoea | 47(45%) | 62(30%) | 0.007 |
| Proximal myopathy | 11(10.6%) | 13 (6.3%) | 0.176 |
| Thyroid eye disease | 40(38.5%) | 44(21%) | 0.001 |
| Proptosis grading | |||
| 0 | 64 (62%) | 164 (79%) | 0.004 |
| 1 | 22 (21%) | 28 (13%) | |
| 2 | 18 (17%) | 16 (8%) | |
| WHO goitre grading | |||
| 0 | 2 (2%) | 36(17%) | 0.001 |
| 1 | 22(21%) | 92 (44%) | |
| 2 | 80 (77%) | 80 (39%) | |
| First-dose RAI 1 activity (mCi) (median, range) | 7 (5–15) | 5 (5–15) | 0.001 |
| Radioiodine uptake | |||
| 2-h RAIU (median, range) | 62 (12–100) | 25 (3–83) | 0.001 |
| 24-h RAIU (median, range) | 56 (11–100) | 57.5(23–91) | 0.029 |
Bold numbers imply significant p values
Table 2.
Baseline characteristics of patients after matching (matched to potential confounders using propensity score method)
| High turnover | Normal turnover | p value | |
|---|---|---|---|
| Number of patients | n = 77 | n = 77 | |
| Age (years) (median, range) | 37 (16–66) | 38 (17–64) | 0.516 |
| Gender (male/female) | 21(27%)/56 (73%) | 21(27%)/56(73%) | 1.0 |
| Duration of disease (months) (median, range) | 24 (1–120) | 24 (1–150) | 0.928 |
| Prior antithyroid drug use | 69(90%) | 67(87%) | 0.616 |
| Duration of antithyroid drug use (months) (median, range) | 18 (0–120) | 24 (0–150) | 0.604 |
| WHO goitre grading | |||
| 0 | 2 (3%) | 3(4%) | 0.857 |
| 1 | 21(27%) | 19 (25%) | |
| 2 | 54 (70%) | 55 (71%) | |
| First-dose RAI 1 activity (mCi) (median, range) | 7 (5–15) | 5 (5–15) | 0.895 |
Two groups matched for potential confounders (age, sex, duration of disease, prior ATD use, goitre grading, RAI activity used)
Outcome After First RIT
The cure rate of 28% (16% hypothyroid and 12% euthyroid) in the high turnover cohort 1 compared to 66% (49% hypothyroid and 17% euthyroid) in the normal turnover cohort 2 was observed after the first RIT (p = 0.001). The detailed results are shown in Table 3. Matched pair analysis, based on the propensity score method, also showed similar results. The high turnover group’s cure rate was 31% compared to 61% in the normal turnover group (see Table 3).
Table 3.
Treatment outcomes, cure defined as hypothyroid or euthyroid at 12 months after first RIT
| Age and sex-matched groups | ||
| High turnover (n = 104) | Normal turnover (n = 208) | |
| Hypothyroid | 17 (16%) | 103 (49%) |
| Euthyroid | 12 (12%) | 35 (17%) |
| Cured* | 29 (28%) |
138 (66%) (p 0.001) |
| Complete multi-covariate# matched group (1:1) (after propensity score matching) | ||
| High turnover (n = 77) | Normal turnover (n = 77) | |
| Hypothyroid | 12 (15.5%) | 34 (44%) |
| Euthyroid | 12 (15.5%) | 13 (17%) |
| Cured* | 24 (31%) |
47(61%) (p 0.001) |
*Cure rate = hypothyroid rate + euthyroid rate
#Matched for age, sex, duration of disease, prior ATD use, goitre grading, RAI activity used
Cumulative Outcomes After Multiple RIT
In the high turnover cohort, patients required further RITs than the normal turnover group of patients. The median number of therapies, 2 RIT vs. 1 RIT, and median cumulated 131I activity (15 mCi vs. 7 mCi) were required in high turnover compared to normal turnover patients, as shown in Table 4.
Table 4.
Cumulative cure rates after multiple successive 131I radioiodine therapies
| Cumulative success after multiple RIT | High turnover (102)* | Normal turnover (208) | ||
|---|---|---|---|---|
| Cumulative frequency | Cumulative frequency | |||
| 1 RIT | 29 (28.4%) | 28.4% | 138(66.3%) | 66.3% |
| 2 RIT | 44 (43.1%) | 71.6% | 54(26%) | 92.3% |
| 3 RIT | 18 (17.6%) | 89.2% | 9 (4.3%) | 96.6% |
| ≥ 4 RIT | 11 (11.1%) | 100% | 7 (3.4%) | 100% |
| Median number of RIT required | 2 (1–5) | 1 (1–6) | (P 0.001) | |
| Median cumulative 131I activity required (mCi) | 15 (5–90) | 7 (5–100) | (P 0.001) | |
*2 patients, after they received their last RIT (which is after 12th month) did not follow up, hence could not be assessed for the final response (cured/not cured), but for treatment outcome at 12 months, they were taken as a failure for First RIT
Among cured patients in the high turnover group, the final clinical outcome at the time of writing was 58% in hypothyroid and 42% in euthyroid metabolic states. In the normal turnover group, 71% were finally hypothyroid, and 29% were euthyroid, as shown in Fig. 1. There was a significant difference in the distribution of hypothyroid and euthyroid status in the two groups (p = 0.025).
The Fate of Euthyroid Patients After Completion of All RIT on Long-Term Follow-Up
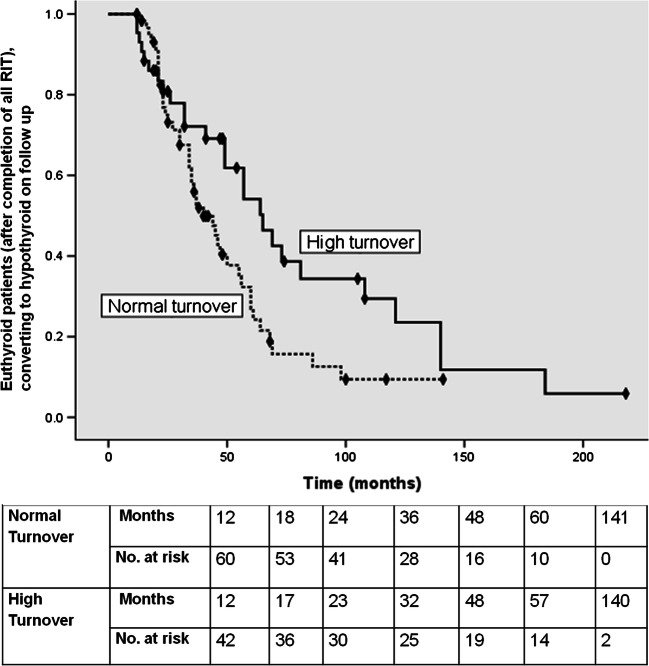
Interestingly, in patients who were euthyroid after completing all RIT, with a median time duration of 65 months, 60% showed spontaneous conversion to hypothyroid state in the high turnover group. With a median time duration of 40 months, 69% in the normal turnover group become hypothyroid (p = 0.03), as shown in Fig. 2.
Fig. 2.
Time to event analysis (Kaplan Meier curves) (event: spontaneous conversion to hypothyroid state in euthyroid patients after completion of all RIT) showing the fate of euthyroid patients after completion of all RIT on long-term follow-up
Association of Pre-therapy Factors and Treatment Failure Rate After First RIT
Univariate and Multivariate Analysis
Continuous variables were categorized into categorical variables for modelling into univariate and multivariate analysis. We used the receiver operating characteristic (ROC) method to find the cutoff for the continuous variables used in the univariate and multivariate analysis model. The cutoff values for the age, 44 years; duration of disease, 11 months; duration of ATD use, 11 months; 2-h RAIU, 37%; and 24-RAIU, 57%, were used.
In univariate analysis, eight factors were found significant, namely, age > 44 years, duration of disease > 11 months, goitre grading, prior ATD use, high turnover, 2-h RAIU > 37%, 24-h RAIU > 57%, and amount of RAI activities were significantly associated with the first-dose failure, while in multivariate analysis, only 4 factors were independently associated with first-dose failure, namely, the age > 44 years (odds ratio 2.6, p = 0.001), grade 2 goitre (odds ratio 2.8, p = 0.029), high turnover group (odds ratio 2.6, p = 0.007), and 2-h RAIU > 37% (odds ratio 2.7, p = 0.005); the details are shown in Table 5.
Table 5.
Univariate and multivariate analysis of the association between risk factors and treatment failure after first RIT
| Variables | First RAI outcome | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Not cured | Cured | Odds ratio for failure (CI) | (p value) | Adjusted odds ratio (CI) | (p value) | |
| Age | ||||||
|
≤ 44 years > 44 years |
80 (41%) 65 (56%) |
117 (59%) 50 (44%) |
1 1.9 (1.1–3.0) |
0.007 |
1 2.6 (1.4–4.5) |
0.001 |
| Gender | ||||||
|
Female Male |
100 (46%) 45 (46%) |
115 (54%) 52 (54%) |
1 0.9 (0.6–1.6) |
0.984 | – | |
| Duration disease | ||||||
|
≤ 11 months > 11 months |
26 (35%) 119 (50%) |
48 (65%) 119 (50%) |
1 1.8 (1.07–3.1) |
0.025 |
1 1.3 (0.6–2.5) |
0.483 |
| Goitre | ||||||
| 0 | 8 (21%) | 30 (79%) | 1 | |||
|
1 2 |
37(32%) 100 (62%) |
77 (68%) 60 (38%) |
n.a | 0.001 |
1.1 (0.4–2.9) 2.8 (1.1–7.4) |
0.782 0.029 |
| Prior ATD | ||||||
|
No Yes |
8 (26%) 137 (49%) |
23 (74%) 144 (51%) |
1 2.7 (1.1–6.3) |
0.015 |
1 2.6 (0.9–7.4) |
0.06 |
| Turnover | ||||||
|
Normal High |
70 (34%) 75 (72%) |
138 (66%) 29 (28%) |
1 5 (3–8.5) |
0.001 |
1 2.6 (1.2–5.2) |
0.007 |
| RAIU (2) | ||||||
|
≤ 37 > 37 |
43 (27%) 102 (68%) |
118 (73%) 49 (32%) |
1 5.7 (3.5–9.3) |
0.001 |
1 2.7 (1.3–5.5) |
0.005 |
| RAIU (24) | ||||||
|
≤ 57 > 57 |
62 (39%) 83 (55%) |
98 (61%) 69 (45%) |
1 1.9 (1.2–2.9) |
0.005 |
1 1.2(0.6–2.2) |
0.524 |
| RAI 1 (mCi) | ||||||
| 5–7 | 103 (43%) | 138 (57%) | 1 | |||
|
8–10 11–15 |
28 (55%) 14 (70%) |
23 (45%) 6 (30%) |
n.a | 0.027 |
0.7 (0.3–1.4) 0.7 (0.2–2.2) |
0.334 0.390 |
RAI radioactive iodine, OR odds ratio, CI confidence interval, ATD antithyroid drug, RAIU radioactive iodine uptake, mCi milli Curie. Calculation of odds ratio is possible in dichotomous variables; for goitre grading and RAI 1 activity variables, only significance value is mentioned; odds ratio is not mentioned
The median cumulated 131I activities required for the categorized groups based on these significant variables are shown in Table 7, which shows that the highest activity was needed for the high turnover group.
Table 7.
Median cumulated 131I activity required for cure in categorical groups of independently significant variables
| Independently significant variables in multivariate analysis | Significance (p value) | Median cumulated 131I activity ultimately required for cure in categorized groups |
|---|---|---|
| Age | ||
|
≤ 44 years > 44 years |
0.001 |
7 mCi 10 mCi |
| Goitre | ||
|
0 1 2 |
0.029 |
5 mCi 7 mCi 12.5 mCi |
| Turnover | ||
|
Normal High |
0.007 |
7 mCi 15 mCi |
| RAIU (2) | ||
|
≤ 37 > 37 |
0.005 |
5 mCi 14 mCi |
For the high turnover group, the adjusted odds ratio in multivariate analysis (multivariate-adjusted) was 2.6 (95% CI: 1.2–5.2), p = 0.007, as shown in Table 5. This finding was also confirmed using another method using propensity score-matched data (PSM adjusted odds ratio was 3.5 (95% CI: 1.7–8.2, p = 0.001) (details shown in Table 6).
Table 6.
Analysis to find association between risk factors and treatment failure after first RIT in propensity score matched data
| Variables | First RAI outcome | Univariate analysis on propensity score matched | ||
|---|---|---|---|---|
| Not cured | Cured | (PSM) data PSM adjusted odds ratio for failure (CI) (p value) |
||
| Age | ||||
|
≤ 44 years > 44 years |
48 (48%) 35 (65%) |
52 (52%) 19 (35%) |
1 1.9 (1.008–3.9) |
0.046 |
| Gender | ||||
|
Female Male |
62 (55%) 21 (50%) |
50 (45%) 21 (50%) |
1 0.8 (0.4–1.6) |
0.553 |
| Duration disease# | ||||
|
≤ 11 months > 11 months |
17 (45%) 66 (57%) |
21 (55%) 50 (43%) |
1 1.6 (0.8–3.4) |
0.192 |
| Goitre | ||||
| 0 | 8 (21%) | 30 (79%) | ||
|
1 2 |
37(32%) 100 (62%) |
77 (68%) 60 (38%) |
n.a | 0.01 |
| Prior ATD | ||||
|
No yes |
6 (33%) 77 (57%) |
12 (67%) 59 (43%) |
1 2.6 (0.9–7.3) |
0.06 |
| Turnover | ||||
|
Normal High |
30 (39%) 53 (69%) |
47 (61%) 24 (31%) |
1 3.5 (1.7–8.2) |
0.001 |
| RAIU(2) | ||||
|
≤ 37 > 37 |
15 (27%) 68 (70%) |
41 (73%) 30 (30%) |
1 6 (3–12) |
0.001 |
| RAIU (24) | ||||
|
≤ 57 > 57 |
33 (50%) 50 (57%) |
33 (50%) 38 (43%) |
1 1.3 (0.7–2.5) |
0.41 |
| RAI 1 (mCi) | ||||
| 5–7 | 63 (54%) | 54 (46%) | ||
|
8–10 11–15 |
14 (50%) 6 (67%) |
14 (50%) 3 (33%) |
n.a | 0.683 |
PSM propensity score matching, CI confidence interval
Prediction of Optimal First Dose of RIT to Be Used in High Turnover Group
The mean effective half-life in the high turnover group obtained by the exponential fitting method was 3.5 days (standard deviation: +1.79 days). This Teff was consistent with the values mentioned in the literature, which is the effective half-life of 2.8 days in the high turnover gland and around 5.5 days in the normal turnover gland [14–16].
Estimation of the Correction Factor for Dose Calculation in High Turnover GD
The correction factor was defined as the ratio of Teff for normal turnover GD to Teff for the rapid turnover gland. Using, Teff for normal turnover GD as 5.5 days, as taken from the literature [14–16], we get a multiplication factor of 1.5 (when Teff for high turnover taken as 3.5 days, which we got in our study) and 1.96 (when Teff for high turnover taken as 2.8 days which is mentioned in published literature) [14–16]. Hence, the correction factor is around 1.5–2.
Discussion
A high turnover of iodine is clinically significant because the decreased retention time of 131I not only reduces the radiation dose delivered to the thyroid gland (target organ) that results in therapeutic failure but also results in increased radiation dose to non-target (blood and bone marrow) tissues [12, 34, 35].
It has been noted that up to 12–20% of GD patients may have a rapid 131I turnover in the thyroid gland [13, 36, 37]. In our study, we got a rapid turnover prevalence rate of around 4% of cases of GD, which is significantly less as compared to the values mentioned above in the literature. The probable reason could be due to the different definition of rapid turnover based on early and late RAIU ratio used in various studies. In our research, we have used 2 h as early RAIU, while in the study by Aktay et al., authors have taken 5 h as early RAIU [13]. RAIU measurement strongly depends on iodine levels that vary with the environmental iodine content of a geographic region’s food and drinks which can produce disparity in RAIU values [25, 38, 39].
Studies evaluating the effects of prior ATD have shown conflicting results, with one group of investigators showing negative impact [14] and others leading no effect [40, 41]. In our study, prior ATD use was not significant in multivariate analysis; however, it significantly impacted RIT results in normal turnover patients when analysed separately in each cohort, but not in high turnover patients.
Few prior studies have suggested that in patients with young onset of GD, there is an increased likelihood of relapse after ATD treatment [42, 43]. Interestingly, in our study, we observed that older age (> 44 years) patients had 2.6 times more odds of failure to first RIT than younger age (18–44 years) patients.
Zhang et al., in their study, showed a goitre size of more than 56 g which is associated with a high turnover of the gland [30]. In their research, DeGroot et al. also concluded that larger thyroid volumes need to be treated with higher dosages of 131I per unit volume [44], and this has been confirmed by other studies too [45]. In our previous study, Damle et al. [46], studied the effect of RAIU on 131I therapy outcomes where the authors administered 5 mCi 131I (mean activity) for the treatment of GD and reported the first-dose success rate of 81.7% in patients with 24-h RAIU < 50% and 68.6% in the group with 24-h RAIU > 50% (p < 0.001).
RAIU is an important predictive factor for determining treatment outcome. Only a single RAIU value does not give information about the thyroid gland’s iodine kinetics, only give an idea about iodine avidity. Rather, the ratio of early to late RAIU can give an idea about the gland’s turnover. Initially, it was believed that maximum uptake of RAI occurs at 24 h, and few studies evaluated the impact of a single RAIU (24 h) on RIT outcome. In our previous study, as reported by Damle et al., the effect of RAIU on 131I therapy outcomes [46]. The authors administered 5 mCi 131I (mean activity) for the treatment of GD and reported the first-dose success rate of 81.7% in patients with 24-h RAIU < 50% and 68.6% in the group with 24-h RAIU > 50% (p < 0.001). Similar results were shown in another study by Kristoffersen et al. [47]. In our research, though the significant association between 24-h RAIU and RIT failure was found in univariate analysis (p 0.005), on multivariate analysis, it was not significant (p 0.52), rather only 2-h RAIU was significantly associated with treatment failure in multivariate analysis {OR-2.7 (95% CI: 1.3–5.5), p = 0.005}. Both these studies mentioned above showed the importance of RAIU in estimating RIT administered activity, but the importance of early RAIU was not evaluated previously; this is the first study highlighting the value of early 2-h RAIU in Graves’ disease patients. However, a high 131I turnover has been demonstrated as a strong predictor of RIT failure in several studies [13, 48, 49].
We found significantly decreased success rates after the first RIT in the high turnover group (28%) compared to the normal turnover group (66%) (p = 0.001). Aktay et al., in their study, found that up to 50% of the GD patients with high131I turnover failed to respond to the initial 131I therapy, hence required multiple RAI therapies [13].
The amount of administered 131I activity plays a significant role in GD treatment success in the high turnover group, as shown in Table 7. In this context, Aktay reported that a mean dose of 28 mCi was required to treat patients with high turnover [13]. A recent multi-arm RCT that compared low dose RIT (3.7 MBq/g) with lithium, moderate dose 131I (5.55 MBq/g) with lithium, against the high dose RIT (7.4 MBq/g) without lithium. Among the groups, patients belonging to the third group had the best outcome where the median dose of 131I was 25 mCi [20].. The authors achieved cure rates of 80% with the median use of 25 mCi (12–49 mCi). In our study, the median cumulative activity of 15 mCi was required in the high turnover group compared to the median 7 mCi in the normal turnover group to achieve a cure.
Higher 131I activity results in higher cure rates but shall result in a more hypothyroid state [18, 50]. Achieving euthyroidism following RIT is the best outcome; however, it is challenging to predict whether patients shall achieve euthyroid or hypothyroid metabolic state [51, 52]. We too observed that among all cured patients in our series, more patients were hypothyroid in the normal turnover group (71%) than in the high turnover group (58%) after completion of all RITs; another interesting fact is that the patients who were euthyroid after completion of all RIT, when followed for longer duration, had higher hypothyroid conversion rate and also took shorter duration to achieve that feat (median time 40 months versus 65 months, respectively). These observations could be explained by the fact that rapid turnover kinetics does not allow radioiodine to stay in the gland for a longer duration to deliver higher radiation absorbed dose to the thyroid gland; thus, actually lesser absorbed dose is delivered. However, in the normal turnover gland, a higher absorbed dose is delivered (mCi-to-mCi basis) compared to the rapid turnover gland, resulting in quick cure and more hypothyroidism for similar administered 131I activity keeping all other variables identical.
No precise dose calculation can secure RIT’s dual purpose in Graves’ disease, i.e. achieving a high first-dose 131I success rate and long-term euthyroidism; hence, it is found acceptable to offer a fixed quantity of RAI. The administered RAI should be corrected for high turnover because these patients frequently show a failure to activities routinely administered for normal turnover patients. The administered activity can be done by multiplying a correction factor if high turnover is present. The correction factor of 1.5–2 should be multiplied to fixed-dose activity calculations for high turnover GD. This proposed correction factor derived from the effective half-life ratio and activity calculation formulas correlated well with the results of actual treatment and outcomes results seen in our study. In our research to achieve a cure in 90% of patients, the median cumulative activity required was 15 mCi in the high turnover group, approximately two times of 7 mCi required in the normal turnover group. The contribution of each variable is depicted in Table 7.
The highest cumulated activity was required for high turnover group patients, among other independently significant variables. Using a correction factor to adjust for high turnover will take care of other variables while planning to administer 131I activity.
Strength of the Study
The strength of our study is that a large number of patients to compare two groups (high turnover cases and normal turnover controls) were included, assuming the power of study at 95% and type 1 error at 5%, making it a robust study from a statistical point of view. The effects of covariates were studied and confirmed using two robust statistical methods: (a) using a logistic regression model in heterogeneous groups/cohorts’ original data and (b) baseline matched cohorts using propensity score matching to get uniform groups.
Limitations
The study is not without limitations; first, the study design is a retrospective cohort study. There was a lack of data on anti-thyrotrophin receptor antibody (TRAb) status. For effective half-life calculations, only two time points, 2 h and 24 h, were used. The patients’ exact iodine status, whether the patients were iodine sufficient or iodine-deficient at the time of treatment, was not assessed.
Conclusion
High turnover Graves’ disease differs significantly from a normal turnover counterpart in post 131I outcomes. Factors associated with high failure rate are higher age (> 44 years), higher grade of goitre, higher 2-h RAIU (> 37%), and high turnover of gland. Based on the gland’s turnover, first administered 131I activity can be increased by 1.5 to 2 times in the high turnover group to improve the 131I outcome, which adjusts for other variables.
Supplementary Information
(PDF 145 kb)
Acknowledgements
We want to thank Dr. M.A Khan and Mr. Hem Chandra Sati, from Department of Biostatistics, AIIMS, New Delhi, for their help related to statistical analysis.
Authors’ Contributions
Both authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Saurabh Arora and Chandrasekhar Bal. The first draft of the manuscript was written by Saurabh Arora, and Chandrasekhar Bal commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Data Availability
Original raw data is available on request to corresponding author.
Compliance with Ethical Standards
Conflict of Interest
Saurabh Arora and Chandrasekhar Bal declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration as revised in 2013 and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed Consent
The institute ethics committee (Human studies), All India institute of Medical Sciences, New Delhi, approved this retrospective study (ref no: IECPG-598/24.10.2019) and the requirement to obtain informed consent was waived.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Törring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine-a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab. 1996;81:2986–2993. doi: 10.1210/jcem.81.8.8768863. [DOI] [PubMed] [Google Scholar]
- 2.Abraham P, Avenell A, McGeoch SC, Clark LF, Bevan JS. Antithyroid drug regimen for treating Graves’ hyperthyroidism. Cochrane Database Syst Rev. 2010;2010:CD003420. doi: 10.1002/14651858.CD003420.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wartofsky L, Glinoer D, Solomon B, Nagataki S, Lagasse R, Nagayama Y, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129–135. doi: 10.1089/thy.1991.1.129. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij A, Vandenbroucke JP, Smit JW, Stokkel MP, Dekkers OM. Clinical outcomes after estimated versus calculated activity of radioiodine for the treatment of hyperthyroidism: systematic review and meta-analysis. Eur J Endocrinol. 2009;161:771–777. doi: 10.1530/EJE-09-0286. [DOI] [PubMed] [Google Scholar]
- 5.Jaiswal AK, Bal C, Damle NA, Ballal S, Goswami R, Hari S, et al. Comparison of clinical outcome after a fixed-dose versus dosimetry-based radioiodine treatment of Graves’ disease: results of a randomized controlled trial in Indian population. Indian J Endocrinol Metab. 2014;18:648–654. doi: 10.4103/2230-8210.139222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarlov AE, Hegedus L, Kristensen LO, Nygaard B, Hansen JM. Is the calculation of the dose in radioiodine therapy of hyperthyroidism worthwhile? Clin Endocrinol. 1995;43:325–329. doi: 10.1111/j.1365-2265.1995.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters H, Fischer C, Bogner U, Reiners C, Schleusener H. Radioiodine therapy of Graves’ hyperthyroidism: standard vs calculated 131iodine activity. Results from a prospective, randomized, multicentre study. Eur J Clin Investig. 1995;25:186–193. doi: 10.1111/j.1365-2362.1995.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters H, Fischer C, Bogner U, Reiners C, Schleusener H. Reduction in thyroid volume after radioiodine therapy of Graves’ hyperthyroidism: results of a prospective, randomized, multicentre study. Eur J Clin Investig. 1996;26:59–63. doi: 10.1046/j.1365-2362.1996.98243.x. [DOI] [PubMed] [Google Scholar]
- 9.Stokkel MP, HandkiewiczJunak D, Lassmann M, Dietlein M, Lusteret M. EANM procedure guidelines for therapy of benign thyroid disease. Eur J Nucl Med Mol Imaging. 2010;37:2218–2228. doi: 10.1007/s00259-010-1536-8. [DOI] [PubMed] [Google Scholar]
- 10.Marinelli LD, Quimby EH, Hine GJ. Dosage determination with radioactive isotopes; practical considerations in therapy and protection. Am J Roentgenol Radium Ther. 1948;59:260–281. [PubMed] [Google Scholar]
- 11.Leslie WD, Ward L, Salamon EA, Ludwig S, Rowe RC, Cowden EA. A randomized comparison of radioiodine doses in Graves' hyperthyroidism. J Clin Endocrinol Metab. 2003;88:978–983. doi: 10.1210/jc.2002-020805. [DOI] [PubMed] [Google Scholar]
- 12.Berg GEB, Michanek AMK, Holmberg ECV, Fink M. Iodine-131 treatment of hyperthyroidism: the significance of effective half-life measurements. J Nucl Med. 1996;37:228–232. [PubMed] [Google Scholar]
- 13.Aktay R, Rezai K, Seabold JE, Bar RS, Kirchner PT. Four- to twenty-four-hour uptake ratio: an index of rapid iodine-131 turnover in hyperthyroidism. J Nucl Med. 1996;37:1815–1819. [PubMed] [Google Scholar]
- 14.Clerc J, Izembart M, Dagousset F, Jaïs JP, Heshmati HM, Chevalier A, et al. Influence of dose selection on absorbed dose profiles in radioiodine treatment of diffuse toxic goitres in patients receiving or not receiving carbimazole. J Nucl Med. 1993;34:387–393. [PubMed] [Google Scholar]
- 15.Kobe C, Eschner W, Wild M, Rahlff I, Sudbrock F, Schmidt M, et al. Radioiodine therapy of benign thyroid disorders: what are the effective thyroidal half-life and uptake of 131I? Nucl Med Commun. 2010;31:201–205. doi: 10.1097/MNM.0b013e328333d303. [DOI] [PubMed] [Google Scholar]
- 16.Becker DV, Hurley JR. The impact of technology on clinical practice in Graves' disease. Mayo Clin Proc. 1972;47:835–847. [PubMed] [Google Scholar]
- 17.Harbert JC. Radioiodine therapy of hyperthyroidism. In: Harbert JC, Eckelman WC, Neumann RD. eds. Nuclear medicine diagnosis and therapy. New York: Thieme Medical Publishing Inc.: 1996:951–973.
- 18.de Jong JA, Verkooijen HM, Valk GD, Zelissen PM, de Keizer B. High failure rates after (131)I therapy in Graves hyperthyroidism patients with large thyroid volumes, high iodine uptake, and high iodine turnover. Clin Nucl Med. 2013;38:401–406. doi: 10.1097/RLU.0b013e3182817c78. [DOI] [PubMed] [Google Scholar]
- 19.Sekulic V, Rajic M, Vlajkovic M, Ilić S, Stević M, Kojić M. The effect of short-term treatment with lithium carbonate on the outcome of radioiodine therapy in patients with long-lasting Graves’ hyperthyroidism. Ann Nucl Med. 2017;31:744–751. doi: 10.1007/s12149-017-1206-z. [DOI] [PubMed] [Google Scholar]
- 20.Thamcharoenvipas S, Kerr SJ, Tepmongkol S. Finding the best effective way of treatment for rapid I-131 turnover Graves’ disease patients: a randomized clinical trial. Medicine (Baltimore) 2019;98:15573. doi: 10.1097/MD.0000000000015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bal CS, Kumar A, Pandey RM. A randomized controlled trial to evaluate the adjuvant effect of lithium on radioiodine treatment of hyperthyroidism. Thyroid. 2002;12:399–405. doi: 10.1089/105072502760043486. [DOI] [PubMed] [Google Scholar]
- 22.Kalinyak JE, McDougall IR. How should the dose of iodine-131 be determined in the treatment of Graves’ hyperthyroidism? J Clin Endocrinol Metab. 2003;88:975–977. doi: 10.1210/jc.2002-021801. [DOI] [PubMed] [Google Scholar]
- 23.Peters H, Fischer C, Bogner U, Reiners C, Schleusener H. Treatment of Graves’ hyperthyroidism with radioiodine: results of a prospective randomized study. Thyroid. 1997;7:247–251. doi: 10.1089/thy.1997.7.247. [DOI] [PubMed] [Google Scholar]
- 24.Van Isselt JW, de Klerk JMH, Koppeschaar HPF, Koppeschaar HP, Van Rijk PP. Iodine-131 uptake and turnover rate vary over short intervals in Graves’ disease. Nucl Med Commun. 2000;21:609–616. doi: 10.1097/00006231-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Ballal S, Soundararajan R, Bal C. Re-establishment of normal radioactive iodine uptake reference range in the era of universal salt iodization in the Indian population. Indian J Med Res. 2017;145:358–364. doi: 10.4103/ijmr.IJMR_1158_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–126. doi: 10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleiss JL. Statistical methods for rates and proportions. 2. New York: Wiley; 1981. p. 45. [Google Scholar]
- 28.Braga M, Walpert N, Burch HB, Solomon BL, Cooper DS. The effect of methimazole on cure rates after radioiodine treatment for Graves’ hyperthyroidism: a randomized clinical trial. Thyroid. 2002;12:135–139. doi: 10.1089/105072502753522365. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization, International Council for Control of Iodine Deficiency Disorders & United Nations Children's Fund (UNICEF). Indicators for assessing iodine deficiency disorders and their control through salt iodization. World Health Organization; 1994. https://apps.who.int/iris/handle/10665/70715.
- 30.Zhang R, Tan J, Wang R, Zhang G, Jia Q, Meng Z, et al. Analysis of risk factors of rapid thyroidal radioiodine-131 turnover in Graves’ disease patients. Sci Rep. 2017;7:8301. doi: 10.1038/s41598-017-08475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Z, Gardiner JC, Bradley CJ. Applying propensity score methods in medical research: pitfalls and prospects. Med Care Res Rev. 2010;67:528–554. doi: 10.1177/1077558710361486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: a propensity analysis. JAMA. 2001;286:1187–1194. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]
- 33.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 34.Green M, Fisher M, Miller H, Wilson GM. Blood radiation dose after I therapy of thyrotoxicosis. Calculations with reference to leukemia. Br Med J. 1961;2:210–215. doi: 10.1136/bmj.2.5246.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rail JE, Sonenberg MS, Robbins J, Lazerson R, Rawson RW. The blood level as a guide to therapy with radioiodine. J Clin Endocrinol Metab. 1953;13:1369–1377. doi: 10.1210/jcem-13-11-1369. [DOI] [PubMed] [Google Scholar]
- 36.Pinyowatanasilp P, Uaratanawong S. Therapy dose calculation in hyperthyroidism using the 3-hour early i-131 uptake measurements. Vajira Med J. 2005;49:147–152. [Google Scholar]
- 37.Morris LF, Waxman AD, Braunstein GD. Accuracy considerations when using early (four- or six-hour) radioactive iodine uptake to predict twenty-four-hour values for radioactive iodine dosage in the treatment of Graves’ disease. Thyroid. 2000;10:779–787. doi: 10.1089/thy.2000.10.779. [DOI] [PubMed] [Google Scholar]
- 38.Baczyk M, Junik R, Ziemnicka K, Sowiński J. Iodine prophylaxis intensification. Influence on radioiodine uptake and activity of 131I used in the treatment of hyperthyroid patients with Graves' disease. Nuklearmedizin. 2005;44:197–199. [PubMed] [Google Scholar]
- 39.Zimmermann MB. Iodine deficiency and endemic cretinism. In: Braverman LE, Cooper DS, editors. Werner & Ingbar’s the thyroid a fundamental and clinical text. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 217–241. [Google Scholar]
- 40.Cunnien AJ, Hay ID, Gorman CA, Offord KP, Scanlon PW. Radioiodine-induced hypothyroidism in Graves’ disease: factors associated with the increasing incidence. J Nucl Med. 1982;23:978–983. [PubMed] [Google Scholar]
- 41.Andrade VA, Gross JL, Maia AL. The effect of methimazole pretreatment on the efficacy of radioactive iodine therapy in Graves’ hyperthyroidism: one-year follow-up of a prospective, randomized study. J Clin Endocrinol Metab. 2001;86:3488–3493. doi: 10.1210/jcem.86.8.7707. [DOI] [PubMed] [Google Scholar]
- 42.Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SC, Franklyn JA. Age and gender predict the outcome of treatment for Graves' hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1038–1042. doi: 10.1210/jcem.85.3.6430. [DOI] [PubMed] [Google Scholar]
- 43.Bonnema SJ, Bennedbaek FN, Veje A, Marving J, Hegedüs L. Propylthiouracil before 131I therapy of hyperthyroid diseases: effect on cure rate evaluated by a randomized clinical trial. J Clin Endocrinol Metab. 2004;89:4439–4444. doi: 10.1210/jc.2004-0247. [DOI] [PubMed] [Google Scholar]
- 44.DeGroot LJ, Stanbury JB. Graves’ disease: diagnosis and treatment. In: DeGroot LJ, Stanbury JB, editors. The thyroid and its diseases. 4. New York: John Wiley & Sons; 1975. pp. 314–367. [Google Scholar]
- 45.De Bruin TWA, Croon CDL, de Klerk JMH, van Isselt JW. Standardized radioiodine therapy in Graves’ disease: the persistent effect of thyroid weight and radioiodine uptake on the outcome. J Intern Med. 1994;236:507–513. doi: 10.1111/j.1365-2796.1994.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 46.Damle N, Bal C, Kumar P, Reddy R, Virkar D. The predictive role of 24h RAIU with respect to the outcome of low fixed-dose radioiodine therapy in patients with diffuse toxic goiter. Hormones (Athens) 2012;11:451–457. doi: 10.14310/horm.2002.1377. [DOI] [PubMed] [Google Scholar]
- 47.Kristoffersen US, Hesse B, Rasmussen AK, Kjaer A. Radioiodine therapy in hyperthyroid disease: poorer outcome in patients with high 24 hours radioiodine uptake. Clin Physiol Funct Imaging. 2006;26:167–170. doi: 10.1111/j.1475-097X.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 48.van Isselt JW, Broekhuizen-de Gast HS. The radioiodine turnover rate as a determinant of radioiodine treatment outcome in Graves’ disease. Hell J Nucl Med. 2010;13:2–5. [PubMed] [Google Scholar]
- 49.Marcocci C, Gianchecchi D, Masini I, Golia F, Ceccarelli C, Bracci E, et al. A reappraisal of the role of methimazole and other factors on the efficacy and outcome of radioiodine therapy of Graves’ hyperthyroidism. J Endocrinol Investig. 1990;13:513–520. doi: 10.1007/BF03348615. [DOI] [PubMed] [Google Scholar]
- 50.Allahabadia A, Daykin J, Shappard MC, Gough SC, Franklyn JA. Radioiodine treatment of hyperthyroidism- prognostic factors and outcome. J Clin Endocrinol Metab. 2001;86:3611–3617. doi: 10.1210/jcem.86.8.7781. [DOI] [PubMed] [Google Scholar]
- 51.Metso S, Jaatinen P, Huhtala H, Luukkaala T, Oksala H, Salmi J. A long-term follow-up study of radioiodine treatment of hyperthyroidism. Clin Endocrinol. 2004;61:641–648. doi: 10.1111/j.1365-2265.2004.02152.x. [DOI] [PubMed] [Google Scholar]
- 52.Nygaard B, Hegedü SL, Gervil M, Hjalgrim H, Hansen BM, Søe-Jensen P, et al. Influence of compensated radioiodine therapy on thyroid volume and incidence of hypothyroidism in Graves’ disease. J Intern Med. 1995;238:491–497. doi: 10.1111/j.1365-2796.1995.tb01230.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 145 kb)
Data Availability Statement
Original raw data is available on request to corresponding author.