Abstract
Objective
To report the Mayo Clinic experience with coronavirus disease 2019 (COVID-19) related to patient outcomes.
Methods
We conducted a retrospective chart review of patients with COVID-19 diagnosed between March 1, 2020, and July 31, 2020, at any of the Mayo Clinic sites. We abstracted pertinent comorbid conditions such as age, sex, body mass index, Charlson Comorbidity Index variables, and treatments received. Factors associated with hospitalization and mortality were assessed in univariate and multivariate models.
Results
A total of 7891 patients with confirmed COVID-19 infection with research authorization on file received care across the Mayo Clinic sites during the study period. Of these, 7217 patients were adults 18 years or older who were analyzed further. A total of 897 (11.4%) patients required hospitalization, and 354 (4.9%) received care in the intensive care unit (ICU). All hospitalized patients were reviewed by a COVID-19 Treatment Review Panel, and 77.5% (695 of 897) of inpatients received a COVID-19–directed therapy. Overall mortality was 1.2% (94 of 7891), with 7.1% (64 of 897) mortality in hospitalized patients and 11.3% (40 of 354) in patients requiring ICU care.
Conclusion
Mayo Clinic outcomes of patients with COVID-19 infection in the ICU, hospital, and community compare favorably with those reported nationally. This likely reflects the impact of interprofessional multidisciplinary team evaluation, effective leveraging of clinical trials and available treatments, deployment of remote monitoring tools, and maintenance of adequate operating capacity to not require surge adjustments. These best practices can help guide other health care systems with the continuing response to the COVID-19 pandemic.
Abbreviations and Acronyms: APACHE IV, Acute Physiology and Chronic Health Evaluation IV; ARDS, acute respiratory distress syndrome; BMI, body mass index; CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; EAP, Expanded Access Program; ECMO, extracorporeal membrane oxygenation; EHR, electronic health record; ICD-10, International Classification of Diseases, Tenth Revision; ICU, intensive care unit; LOS, length of stay; NIH, National Institutes of Health; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment
By the time the World Health Organization declared coronavirus disease 2019 (COVID-19) to be a pandemic, the illness was already an unprecedented challenge for health care systems. Initial reports from Wuhan, China, suggested that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was highly transmissible and posed significant challenges related to prevention, containment, and treatment. Subsequent experience in Italy, Spain, Seattle, and New York City confirmed these challenges, and hospital systems throughout the world had to brace for the impact of COVID-19.
The Mayo Clinic is composed of sites in Phoenix/Scottsdale, Arizona, Jacksonville, Florida, Rochester, Minnesota, and additional hospitals in Minnesota and Wisconsin. The Mayo Clinic enterprise has 2680 licensed hospital beds and 392 regular intensive care unit (ICU) beds spanning 20 campuses in 4 states. The clinic served 1.2 million patients last year. Early during the pandemic and anticipating significant changes to the practice, Mayo Clinic leadership activated the Hospital Incident Command System and assembled several teams and task forces charged with developing processes and protocols to address the multifaceted pandemic response, including anticipated case surges.1
A multilayered integrated approach was taken to manage the COVID-19 pandemic at Mayo Clinic. Interprofessional work groups were developed across multiple impacted specialties to appraise the emerging literature on topics such as testing strategies2 and thromboprophylaxis.3 As necessary, providers with specific expertise in organ-specific complications such as nephrology, hematology, and neurology, among others, were invited to be part of the team and provide input as treatment algorithms were developed and matured. These best practices were compiled and shared with the Mayo Clinic staff in real time through the AskMayoExpert platform, a clinical knowledge resource that serves the Mayo Clinic practice. The Mayo Clinic COVID-19 Data Governance Task Force set up monitoring tools to inform the practice on resource use and forecast demand related to COVID-19. This allowed safe management of anticipated COVID-19 cases and ongoing medical practice needs.
A COVID-19 Research Task Force was created to select and execute treatment trials; coordinate biospecimen collection; create databases and advance augmented intelligence-facilitated analytics; prioritize preclinical biodiscovery science in immunology, virology, and vaccine development; and respond to the needs of minority populations disproportionately affected by COVID-19.4 Recognizing that treatments and treatment guidelines for COVID-19 infection would change over time, different processes were used to create a consistent system in which patients were screened for possible trial participation in the outpatient and inpatient settings.
Mayo Clinic saw its first COVID-19 case in March 2020, and all regions were actively engaged in caring for patients with COVID-19 infection by the end of that month. Patients with COVID-19 infection admitted to our hospitals were reviewed by treatment review panels at each site. This multidisciplinary group provided recommendations as to which clinical trials and emerging therapies would be most beneficial for patients with COVID-19 infection. The panels convened twice per day, and more frequently as needed. Every patient was assessed on hospital admission and consensus-driven recommendations were based on available treatment options through clinical trials, Emergency Use Authorization Expanded Access Programs (EAPs), or off-label use. Trials and programs had varying start times across Mayo Clinic, and the panels reviewed patients based on local availability and protocols for agents such as remdesivir and convalescent plasma. The panel directed antiviral and immunomodulatory treatments, as well as management of complications. Recommendations were provided to primary treatment teams and the patient and/or patient’s legally authorized representative in a shared decision-making process.4 The Treatment Review Panel also did weekly webcasts available to all providers to give up-to-date education as data emerged.
Mayo Clinic also developed a COVID-19 Frontline Care Team or COVID virtual clinic for outpatient care in conjunction with a nurse telephone line by which patients were telephonically triaged based on disease severity for testing at drive-through facilities or the emergency department.5 All SARS CoV-2–positive test results from outpatients at our institution were routed to this centralized team of physicians and nurses with the goal of contacting them in less than 10 hours. Patients deemed high risk are offered remote monitoring with Bluetooth-enabled pulse oximeters, blood pressure cuffs, and thermometers. All patients received initial nursing calls discussing the importance of isolation and follow-up calls (days 2, 7, and 14) to monitor for possible progression. Physicians assessed the need for care escalation when signs or symptoms worsened.6 Patients who declined remote monitoring or were lower risk received telephone follow-up with a dedicated nursing team.
In this article, we review the Mayo Clinic experience with COVID-19 from March 2020 through July 2020 to understand the outcomes of this integrated approach and identify best practices and lessons learned from our experience.
Methods
Study Design and Patients
We conducted a retrospective cohort study of all patients with COVID-19 infection from March 1, 2020, through July 31, 2020, at any of the Mayo Clinic sites. All Mayo Clinic sites use a single electronic health record (EHR) from Epic Systems Corporation. The Mayo Clinic Institutional Review Board approved this retrospective study that did not involve patient contact as a minimal risk protocol with waived informed consent. Patients who declined research authorization were excluded.
Case identification was performed using a registry set up in the “Healthy Planet” tool in the EHR. This registry flagged patients as having an “active” infection if a clinical diagnosis of COVID-19 was added to the problem list or a SARS-CoV-2 polymerase chain reaction test was positive in the EHR. Those without laboratory confirmation were reviewed to ensure that this was appropriate (eg, an outside laboratory result not visible in our chart). External results could satisfy registry metrics if they could be identified within the interoperable framework for data sharing across the EHR, Care Everywhere (Epic Systems Corporation). All outcomes and predictor variables were obtained from the EHR.
Outcomes
We defined the start of an episode of COVID-19 infection as the first time that either a positive test result was collected or the first time a clinical diagnosis was placed in the chart. The episode was censored either 30 days after the positive test or, if admitted to the hospital, 30 days after the last hospital admission encounter in which COVID-19 was addressed.
Complications for hospitalized patients were ascertained using International Classification of Diseases, Tenth Revision (ICD-10) codes to define acute respiratory distress syndrome (ARDS), septic shock, acute kidney injury, congestive heart failure, or thromboembolic disease, including acute cardiac and cerebrovascular thromboembolic events, during an episode of COVID-19 infection (Supplement 1, available online at http://www.mayoclinicproceedings.org). Complications of infection overall were defined as new diagnosis with ICD-10 codes being recorded between COVID-19 episode start (ie, date of first clinical diagnoses or collection of positive sample) and either 30 days after diagnosis or 30 days after last COVID-19–related hospital dismissal if a patient required admission or readmission.
Superinfection, defined as infection by another organism occurring simultaneously with or after COVID-19 infection, was evaluated using positive cultures from blood and respiratory tract samples. Blood cultures with single positive results of common commensal organisms (likely contaminants) were discarded according to the National Healthcare Safety Network criteria used in laboratory-confirmed bloodstream infections.7 Likewise, respiratory cultures identifying “usual flora” and “yeast” were not categorized as positive for purposes of this analysis.
Mortality outcomes were assessed based on all-cause mortality during the COVID-19 infection episode and last follow-up performed on outpatients. In addition, patients were evaluated for outcomes based on their worst score in the National Institutes of Health (NIH) Ordinal Scale used in the Adaptive COVID-19 Treatment Trial for descriptive analysis.8 The NIH Ordinal Scale classifies outcomes as: (1) death; (2) hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); (3) hospitalized, receiving noninvasive ventilation or high-flow oxygen devices; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, not requiring supplemental oxygen, requiring ongoing medical care (COVID-19 related or otherwise); (6) hospitalized, not requiring supplemental oxygen, no longer requires ongoing medical care; (7) not hospitalized, limitation on activities and/or requiring home oxygen; and (8) not hospitalized, no limitations on activities.
Because we took the worst score the patient had during an episode of COVID-19 infection, no patients were scored at level 6 because all hospitalized patients required ongoing medical care at some point.
Predictor Variables
Demographic variables (age, sex, race, and Hispanic ethnicity), height, weight, and smoking history were obtained from the EHR. Charlson Comorbidity Index (CCI)9 data were digitally abstracted using a combination of the EHR data and data from the Mayo Clinic Unified Data Platform to obtain International Classification of Diseases, Ninth Revision 10 and ICD-10 codes identified before the initiation of each patient’s COVID-19 episode.11
Laboratory values were analyzed for the “worst” value occurring during the day of admission for inpatients. This definition regarded worst values as the highest alanine aminotransferase, aspartate aminotransferase, bilirubin, creatinine, C-reactive protein, ferritin, and neutrophil-lymphocyte ratio values and the lowest platelet count. For modeling, laboratory variables were classified as normal range vs abnormal based on sex-specific cut points based on our laboratory reference ranges.
Patients admitted to the ICU had their data set enriched by variables obtained from an ICU-specific data mart.12 The ICU data mart contains detailed treatment and ICU-specific intervention data, Sequential Organ Failure Assessment score,13 Acute Physiology and Chronic Health Evaluation (APACHE) IV score, and APACHE IV–predicted mortality and length-of-stay (LOS) information.14
Statistical Analyses
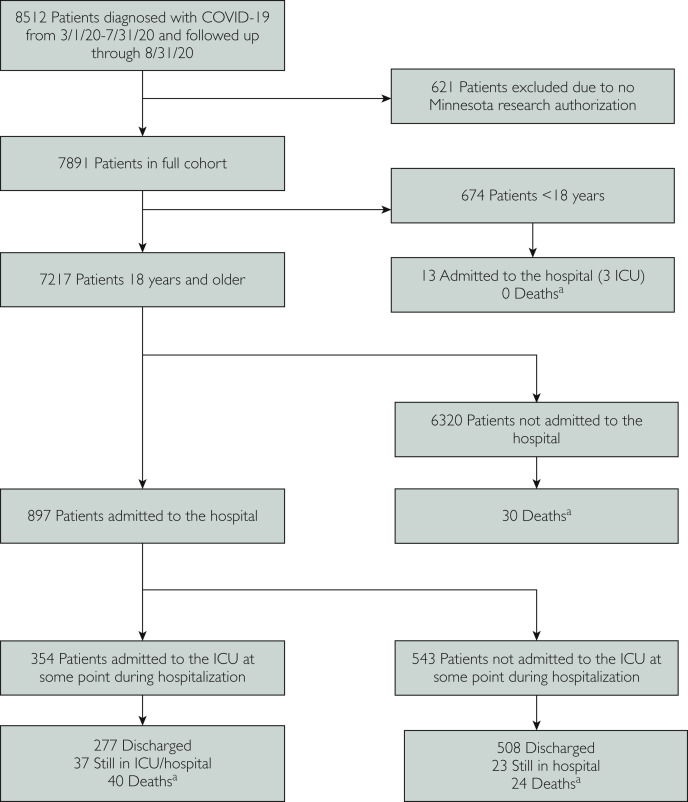
We used descriptive statistics (median and interquartile range for continuous variables and count and percent distributions for categorical variables) to characterize each cohort of patients: pediatric (aged <18 years) and adults (aged ≥18 years). For adult patients, we categorized outpatients vs hospitalized patients; among hospitalized patients, we assessed for ICU admission as part of their COVID-19 management (Figure 1 ). Missing data were not imputed and for categorical variables, a missing category was created.
Figure 1.
Study selection flowchart. aDeath during hospitalization or within 30 days of discharge (hospitalized patients) or within 30 days of coronavirus disease 2019 (COVID-10) diagnosis (outpatients). ICU, intensive care unit.
To identify factors associated with hospital admission, we used unconditional logistic regression to estimate odds ratios (ORs) and 95% CIs; all variables that were significant (P<.05) in univariate analysis were included in a multivariable model that also adjusted for Mayo region and prior registration in the Mayo system to account for incomplete comorbidity data for patients newly registered for the pandemic who may have had less rich comorbidity data in our system. For risk for hospitalization, these variables included demographic factors, body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared),15 smoking history,16 and CCI score.17
We also used logistic regression to identify factors among hospitalized patients that were associated with ICU admission. For risk for ICU admission among hospitalized patients, we evaluated laboratory values at first admission to the hospital (or ICU if directly admitted there). Factors associated with ICU admission that were statistically significant in univariate analysis were modeled in a multivariable model that also adjusted for Mayo Clinic center and prior registration in the Mayo Clinic system.
Finally, for adult hospitalized patients, we also conducted a survival analysis. We measured time to event in days from hospital admission to date of death due to any cause or date of last follow-up. We used Cox regression to estimate hazard ratios and 95% CIs in univariate analysis. Statistically significant variables were then included in a multivariable analysis, with the exception that we used a summary CCI score (0-1 vs 2+) instead of individual CCI comorbid conditions to reduce the number of covariates in the multivariable model, given the limited number of deaths in the analysis (N=64). Statistical analyses were performed using R, version 4.0.2 (R Core Team).
Results
From March 1, 2020, through July 31, 2020, Mayo Clinic cared for a total of 8512 patients with COVID-19 infection. Of these, 621 were excluded from the analysis because research authorization was not available. Of the remaining 7891 patients with COVID-19 infection, 674 were younger than 18 years and 7217 were 18 years or older (Figure 1).
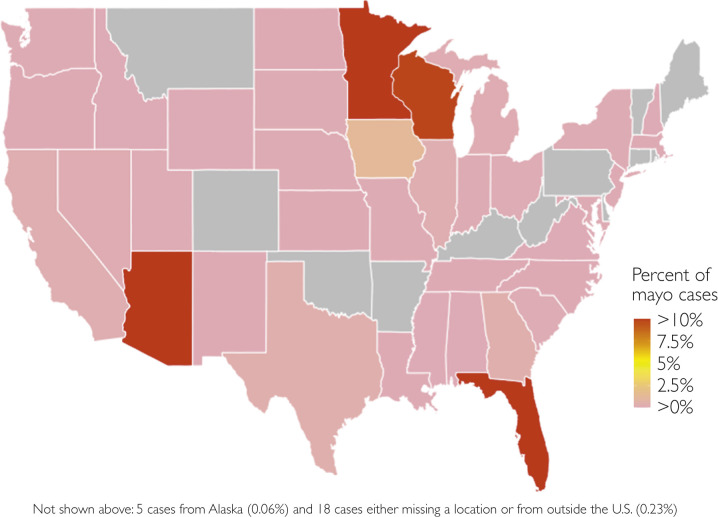
Most cases occurred between June 1, 2020, and July 31 2020, accounting for 87.5% (6905 of 7891) of total cases seen during the study period. Mayo Clinic in the Midwest saw 59% (4656 of 7891) of patients with COVID-19 infection, with Mayo Clinic in Arizona seeing 23% (1815 of 7891) and Mayo Clinic in Florida seeing 18% (1420 of 7891) during this period. Patients resided in Minnesota (N=3633), Arizona (N=1761), Florida (N=1404), Wisconsin (N=769), Iowa (N=94), and 32 other states (all N<34). States with cases seen are noted in Figure 2 . Among the 6971 adults with language data available, language other than English as a primary language was present in 14.5% (1010 of 6971) of patients overall and 16.3% (146 of 896) of hospital admissions.
Figure 2.
Geographic distribution of coronavirus disease 2019 (COVID-19) cases (N=7891), Mayo Clinic, March through July 2020.
Pediatric Population
During the study period, 674 patients younger than 18 years were seen for COVID-19 infection, with characteristics summarized in Table 1 . The median age of pediatric patients cared for during the study period was 13 (range, <1-17) years. In terms of race and ethnicity, 36.9% (n=249) identified as non-Hispanic white; 23.4% (n=158), as Hispanic (all races); and 9.9% (n=67), as black non-Hispanic. Most of the study population was male (54.2%; n=365). Most (97.8%; n=659) did not have comorbid conditions on record from the CCI. A total of 70 (10.4%) had BMI of 25 kg/m2 or greater. During the study period, 13 children were admitted to the hospital (3 to the pediatric ICU). None required mechanical ventilation, vasopressors, or ECMO support. There were no pediatric deaths. Given the low rates of complications in the pediatric population, we excluded them from subsequent analyses.
Table 1.
Characteristics of Patients Younger Than 18 Years With COVID-19, Mayo Clinic, March Through July 2020a
| Characteristic | Total (N=674) |
|---|---|
| Age (y), median (range) | 13 (0-17) |
| Age category, no. (%) | |
| <1 y | 25 (3.7%) |
| 1-4 y | 69 (10.2%) |
| 5-9 y | 116 (17.2%) |
| 10-14 y | 182 (27.0%) |
| 15-17 y | 282 (41.8%) |
| Sex, no. (%) | |
| Female | 307 (45.5%) |
| Male | 365 (54.2%) |
| Race/ethnicity, no. (%) | |
| Hispanic (all races) | 158 (23.4%) |
| White, non-Hispanic | 249 (36.9%) |
| Black, non-Hispanic | 67 (9.9%) |
| Asian, non-Hispanic | 25 (3.7%) |
| All other, non-Hispanic | 8 (1.2%) |
| Missing | 167 (24.8%) |
| Month of diagnosis, no. (%) | |
| March | 4 (0.6%) |
| April | 10 (1.5%) |
| May | 40 (5.9%) |
| June | 249 (36.9%) |
| July | 371 (55.0%) |
| Body mass index (kg/m2), no. (%) | |
| <15 | 135 (20.0%) |
| 15.0-18.5 | 136 (20.2%) |
| 18.5-24.9 | 123 (18.2%) |
| ≥25 | 70 (10.4%) |
| Missing | 210 (31.2%) |
| Admitted to hospital, no. (%) | 13 (1.9%) |
| Admitted to ICU (as part of hospitalization), no. (%) | 3 (0.4%) |
| Death (30 d for outpatient, or during hospitalization or 30 d after discharge for hospitalized patients), no. (%) | 0 (0%) |
COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Risk for Hospitalization
A total of 7217 adult patients were included in our analyses. Among adults, 6320 (87.6%) patients did not require hospitalization. Of these outpatients, 30 (0.5%) died, including patients who died in hospice and skilled nursing facilities. Sixty-four (7.1%) hospitalized patients died during the follow-up period defined previously.
Adult demographic characteristics, comorbid conditions, and odds of hospitalization are summarized in Table 2 . Among adults, the median age of patients cared for was 59 (range, 18-99) years. Hospitalized patients were older, and the odds of hospital admission increased steeply with age. For example, patients 75 years and older had 24-fold higher odds of hospitalization compared with patients aged 18 to 34 years (unadjusted OR, 23.92; 95% CI, 18.2 to 31.60). There were slightly more male (50.8%; n=3667) than female (49.0%; n=3533) adult patients, and male sex was associated with greater odds of hospitalization (unadjusted OR, 1.19, 95% CI, 1.03 to 1.37). Most patients diagnosed during the study period identified as non-Hispanic white (56.8%; n=4097). Other groups included Hispanic all races (15.8%; n=1141) or black non-Hispanic (9.3%; n=668), Asian (4.2%;n=302), and all other/missing (14.0%; n=1009). Compared with non-Hispanic whites, both Hispanics (unadjusted OR, 1.31; 95% CI, 1.08 to 1.58) and Asians (unadjusted OR, 1.49; 95% CI, 1.07 to 2.03) had greater odds of hospitalization.
Table 2.
Risk for Hospital Admission for Patients 18 Years and Older With COVID-19, Mayo Clinic, March Through July 2020
| Characteristic | Total (N=7217) | Hospitalized (N=897) | Unadjusted Model |
Multivariable Model |
|||
|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | P | |||
| Age (y), median (range) | 39 (18-99) | 59 (18-99) | — | — | |||
| Age distribution (y), no. (%) | |||||||
| 18-34 | 3054 (42.3%) | 107 | 1 | Reference | 1 | Reference | |
| 35-49 | 1690 (23.4%) | 164 | 2.96 | 2.31-3.81 | 1.87 | 1.40-2.50 | <.001 |
| 50-64 | 1551 (21.5%) | 284 | 6.17 | 4.91-7.81 | 3.05 | 2.30-4.04 | <.001 |
| 65-74 | 524 (7.3%) | 157 | 11.78 | 9.02-15.44 | 5.26 | 3.74-7.39 | <.001 |
| ≥75 | 398 (5.5%) | 185 | 23.92 | 18.2-31.60 | 8.31 | 5.69-12.1 | <.001 |
| Sex, no. (%) | |||||||
| Female | 3533 (49.0%) | 406 | 1 | Reference | 1 | Reference | |
| Male | 3667 (50.8%) | 491 | 1.19 | 1.03-1.37 | 1.15 | 0.96-1.38 | .14 |
| Missing | 17 (0.2%) | 0 | |||||
| Month of COVID-19 diagnosis, no. (%) | |||||||
| March | 126 (1.7%) | 49 | 1 | Reference | 1 | Reference | |
| April | 189 (2.6%) | 79 | 1.13 | 0.71-1.79 | 0.99 | 0.54-1.81 | .98 |
| May | 614 (8.5%) | 118 | 0.37 | 0.25-0.57 | 0.32 | 0.19-0.56 | <.001 |
| June | 3059 (42.4%) | 301 | 0.17 | 0.12- 0.25 | 0.16 | 0.10-0.26 | <.001 |
| July | 3229 (44.7%) | 350 | 0.19 | 0.13- 0.28 | 0.15 | 0.09-0.24 | <.001 |
| Race/ethnicity, no. (%) | |||||||
| Hispanic (all races) | 1141 (15.8%) | 169 | 1.31 | 1.08-1.58 | 2.14 | 1.66-2.75 | <.001 |
| White, non-Hispanic | 4097 (56.8%) | 481 | 1 | Reference | 1 | Reference | |
| Black, non-Hispanic | 668 (9.3%) | 90 | 1.17 | 0.91-1.48 | 1.89 | 1.39-2.57 | <.001 |
| Asian, non-Hispanic | 302 (4.2%) | 50 | 1.49 | 1.07-2.03 | 2.56 | 1.70-3.86 | <.001 |
| All other/missing | 1009 (14.0%) | 107 | 0.89 | 0.71-1.11 | 2.85 | 2.07-3.93 | <.001 |
| CCI score, median (range) | 0 (0-19) | 3 (0-19) | — | — | — | — | — |
| CCI score, no. (%) | |||||||
| 0 | 4291 (59.5%) | 164 | 1 | Reference | — | — | — |
| 1 | 902 (12.5%) | 117 | 3.75 | 2.92-4.81 | — | — | — |
| ≥2 | 2024 (28.0%) | 616 | 11.01 | 9.20-13.25 | — | — | — |
| CCI diabetes mellitus, no. (%) | |||||||
| No | 6541 (90.6%) | 597 | 1 | Reference | 1 | Reference | |
| Yes | 676 (9.4%) | 300 | 7.94 | 6.68-9.45 | 2.50 | 1.99-3.13 | <.001 |
| CCI cardiovascular disease, no. (%) | |||||||
| No | 6916 (95.8%) | 760 | 1 | Reference | 1 | Reference | |
| Yes | 301 (4.2%) | 137 | 6.77 | 5.32-8.59 | 1.56 | 1.13-2.14 | .007 |
| CCI congestive heart failure, no. (%) | |||||||
| No | 6874 (95.2%) | 739 | 1 | Reference | 1 | Reference | |
| Yes | 343 (4.8%) | 158 | 7.09 | 5.65-8.88 | 1.14 | 0.83-1.57 | .42 |
| CCI peripheral vascular disease, no. (%) | |||||||
| No | 6771 (93.8%) | 668 | 1 | Reference | 1 | Reference | |
| Yes | 446 (6.2%) | 229 | 9.64 | 7.88-11.81 | 2.29 | 1.73-3.02 | <.001 |
| CCI chronic obstructive pulmonary disease, no. (%) | |||||||
| No | 6369 (88.2%) | 668 | 1 | Reference | 1 | Reference | |
| Yes | 848 (11.8%) | 229 | 3.16 | 2.66-3.74 | 1.46 | 1.16-1.84 | .001 |
| CCI malignancy, no. (%) | |||||||
| No | 6781 (94.0%) | 763 | 1 | Reference | 1 | Reference | |
| Yes | 436 (6.0%) | 134 | 3.50 | 2.81-4.34 | 1.30 | 0.98-1.71 | .07 |
| CCI liver, no. (%) | |||||||
| No | 6798 (94.2%) | 747 | 1 | Reference | 1 | Reference | |
| Yes | 419 (5.8%) | 150 | 4.52 | 3.64-5.59 | 1.68 | 1.28-2.20 | <.001 |
| CCI renal, no. (%) | |||||||
| No | 6748 (93.5%) | 679 | 1 | Reference | 1 | Reference | |
| Yes | 469 (6.5%) | 218 | 7.76 | 6.37-9.46 | 1.55 | 1.17-2.05 | .002 |
| Body mass index (kg/m2), no. (%) | |||||||
| <18.5 | 156 (2.2%) | 10 | 0.55 | 0.27-1.02 | 0.83 | 0.37-1.87 | .65 |
| 18.5-24.9 | 1444 (20.0%) | 159 | 1 | Reference | 1 | Reference | |
| 25.0-29.9 | 1441 (20.0%) | 238 | 1.60 | 1.29-1.99 | 1.21 | 0.92-1.58 | .17 |
| 30.0-39.9 | 1452 (20.1%) | 324 | 2.32 | 1.89-2.86 | 1.45 | 1.11-1.89 | .006 |
| ≥40 | 505 (7.0%) | 132 | 2.86 | 2.21-3.70 | 1.91 | 1.38-2.64 | <.001 |
| Missing | 2219 (30.7%) | 34 | 0.13 | 0.09-0.18 | 0.14 | 0.09-0.22 | <.001 |
| Smoking, no. (%) | |||||||
| Never | 3716 (51.5%) | 545 | 1 | Reference | 1 | Reference | |
| Former | 944 (13.1%) | 240 | 1.98 | 1.67-2.35 | 1.01 | 0.81-1.26 | .94 |
| Current | 645 (8.9%) | 84 | 0.87 | 0.68-1.11 | 1.14 | 0.84-1.55 | .38 |
| Missing | 1912 (26.5%) | 28 | 0.09 | 0.06-0.12 | 0.09 | 0.05-0.15 | <.001 |
CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019.
Of individual comorbid conditions, the most common were chronic obstructive pulmonary disease (11.8% [848 of 7217]), diabetes (9.4% [676 of 7217]; 98.7% [667 of 676] were type 2), renal disease (6.5% [469 of 7217]), and peripheral vascular disease (6.2% [446 of 7217]); the other comorbid conditions had a prevalence of 6.0% or lower. Each individual comorbid condition was significantly associated with odds of hospitalization, with the strong association observed for patients with peripheral vascular disease (unadjusted OR, 9.64; 95% CI, 7.88 to 11.81).
Of patients for whom BMI data were available, 27.1% were obese (BMI ≥30.0 kg/m2). Overweight individuals (25.1 < BMI <29.9 kg/m2) had increased odds of hospitalization of 1.60 (95% CI, 1.29 to 1.99) compared with normal BMI (18.5 < BMI <25.0 kg/m2). This increased in a stepwise manner for obese (OR, 2.32; 95% CI, 1.89 to 2.86) and morbidly obese individuals (BMI > 35 kg/m2; OR, 2.86; 95% CI, 2.21 to 3.70). Most patients were never (70.0%; n=3716 of 5305) or former (17.8%; n=944 of 5305) smokers, and smoking was not associated with odds of hospitalization, though this variable was frequently missing in outpatients.
In a multivariate model, increasing age, obesity, race/ethnicity, and most of the individual components of the CCI were significantly associated with increased odds of hospitalization. The month of diagnosis was also a significant covariate because odds of admission were lower in May, June, and July relative to March and April. The highest impact factors (largest ORs) were age older than 75 years (adjusted OR, 8.31; 95% CI, 5.69 to 12.1), age 65 to 74 years (adjusted OR, 5.26; 95% CI, 3.74 to 7.39), diabetes mellitus (OR, 2.50; 95% CI, 1.99 to 3.13), Hispanic ethnicity (OR, 2.14; 95% CI, 1.66 to 2.75), and Asian race (OR, 2.56; 95% CI, 1.70 to 3.86).
Risk for ICU Admission
Of the 897 requiring hospital admission, 354 (39.5%) required ICU admission. Risk for ICU admission was associated with age, with peak risk in ages 55 to 74 years (OR, 2.27; 95% CI, 1.33 to 3.57). Although males made up most ICU admissions (55.6%; n=197), this was not a statistically significant risk factor. Black race was associated with higher ICU admission (unadjusted OR, 1.93; 95% CI, 1.23 to 3.04), and Asian race was associated with the highest risk for ICU admission in the multivariate model (OR, 2.84; 95% CI, 1.33 to 6.08). A language other than English as a primary language was present in 14.5% (1010 of 6971) of patients overall and 16.3% (146 of 896) of hospital admissions. 62 of 301 adult patients admitted to the ICU with language data on file (20.6%) reported a language other than English as their primary language, indicating more than one third of those.
Elevated aspartate aminotransferase but not alanine aminotransferase level and an elevated creatinine level and neutrophil-lymphocyte ratio were associated with higher risk for ICU admission. Other characteristics of the complete blood cell count, such as absolute counts of lymphocytes, neutrophils, and eosinophils, were not associated with outcomes. These findings are summarized in Table 3 and Supplemental Tables 1 and 2 (available online at http://www.mayoclinicproceedings.org).
Table 3.
Characteristics of Patients 18 Years and Older Hospitalized With COVID-19, Overall and by ICU Status, Mayo Clinic, March Through July 2020
| Characteristic | Total (N=897) | ICU (N=354) | Unadjusted Model |
Multivariable Model |
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | P | |||
| Demographic variables | |||||||
| Age (y), median (range) | 59 (18-99) | 59.5 (20-99) | |||||
| Age distribution (y), no. (%) | |||||||
| 18-34 | 107 (11.9%) | 28 | 1 | Reference | 1 | Reference | |
| 35-49 | 164 (18.3%) | 69 | 2.05 | 1.21-3.52 | 2.32 | 1.04-5.20 | .04 |
| 50-64 | 284 (31.7%) | 123 | 2.16 | 1.33-3.57 | 2.33 | 0.95-5.73 | .07 |
| 65-74 | 157 (17.5%) | 70 | 2.27 | 1.34-3.91 | 2.06 | 0.76-5.58 | .16 |
| ≥75 | 185 (20.6%) | 64 | 1.49 | 0.89-2.55 | 1.49 | 0.55-4.06 | .43 |
| Sex, no. (%) | |||||||
| Female | 406 (45.3%) | 157 | 1 | Reference | 1 | Reference | |
| Male | 491 (54.7%) | 197 | 1.06 | 0.81-1.39 | 0.84 | 0.55-1.30 | .43 |
| Race/ethnicity, no. (%) | |||||||
| Hispanic (all races) | 169 (18.8%) | 65 | 1.05 | 0.73-1.51 | 1.64 | 0.97-2.77 | .07 |
| White, non-Hispanic | 481 (53.6%) | 179 | 1 | Reference | 1 | Reference | |
| Black, non-Hispanic | 90 (10.0%) | 48 | 1.93 | 1.23-3.04 | 1.34 | 0.65-2.76 | .43 |
| Asian, non-Hispanic | 50 (5.6%) | 23 | 1.44 | 0.79-2.58 | 2.84 | 1.33-6.08 | .007 |
| All other, non-Hispanic | 107 (11.9%) | 39 | 0.97 | 0.62-1.49 | 1.16 | 0.58-2.29 | .68 |
| Month of COVID-19 diagnosis, no. (%) | |||||||
| March | 49 (5.5%) | 23 | 1 | Reference | 1 | Reference | |
| April | 79 (8.8%) | 35 | 0.90 | 0.44-1.84 | 0.97 | 0.38-2.50 | .95 |
| May | 118 (13.2%) | 51 | 0.86 | 0.44-1.69 | 1.63 | 0.67-3.95 | .28 |
| June | 301 (33.6%) | 91 | 0.49 | 0.27-0.91 | 0.53 | 0.23-1.22 | .14 |
| July | 350 (39.0%) | 154 | 0.89 | 0.49-1.63 | 0.63 | 0.27-1.44 | .27 |
| Comorbidity and lifestyle | |||||||
| CCI score, median (range) | 3 (0-19) | 3 | — | ||||
| CCI score, no. (%) | |||||||
| 0 | 164 (18.3%) | 58 | 1 | Reference | 1 | Reference | |
| 1 | 117 (13.0%) | 50 | 1.36 | 0.84-2.22 | 1.02 | 0.44-2.40 | .96 |
| ≥2 | 616 (68.7%) | 246 | 1.22 | 0.85-1.75 | 0.97 | 0.44-2.18 | .95 |
| Body mass index (kg/m2), no. (%) | |||||||
| <18.5 | 10 (1.1%) | 4 | 1.23 | 0.30-4.47 | |||
| 18.5-24.9 | 159 (17.7%) | 56 | 1 | Reference | |||
| 25.0-29.9 | 238 (26.5%) | 94 | 1.20 | 0.79-1.83 | |||
| 30.0-39.9 | 324 (36.1%) | 130 | 1.23 | 0.83-1.83 | |||
| ≥40 | 132 (14.7%) | 58 | 1.44 | 0.90-2.32 | |||
| Missing | 34 (3.8%) | 12 | 1.00 | 0.45-2.15 | |||
| Smoking, no. (%) | |||||||
| Never | 545 (60.8%) | 207 | 1 | Reference | |||
| Former | 240 (26.8%) | 99 | 1.15 | 0.84-1.56 | |||
| Current | 84 (9.4%) | 35 | 1.17 | 0.73-1.86 | |||
| Missing | 28 (3.1%) | 13 | 1.42 | 0.65-3.04 | |||
| Laboratory results (admission), no. (%) | |||||||
| Platelets | |||||||
| Low | 238 (26.5%) | 89 | 0.89 | 0.65-1.21 | |||
| Normal range | 608 (67.8%) | 244 | 1 | Reference | |||
| High | 39 (4.3%) | 18 | 1.28 | 0.66-2.45 | |||
| Missing | 12 (1.3%) | 3 | |||||
| Aspartate aminotransferase, no. (%) | |||||||
| Normal range | 447 (53.9%) | 161 | 1 | Reference | 1 | Reference | |
| Elevated | 382 (46.1%) | 179 | 1.57 | 1.19-2.07 | 2.12 | 1.42-3.15 | <.001 |
| Missing | 68 | 14 | |||||
| Alanine aminotransferase, no. (%) | |||||||
| Normal range | 606 (73.2%) | 244 | 1 | Reference | |||
| Elevated | 222 (26.8%) | 96 | 1.13 | 0.83-1.54 | |||
| Missing | 69 | 14 | |||||
| Bilirubin, no. (%) | |||||||
| Normal range | 799 (96.3%) | 325 | 1 | Reference | |||
| Elevated | 31 (3.7%) | 16 | 1.56 | 0.76-3.22 | |||
| Missing | 67 | 13 | |||||
| Creatinine, no. (%) | |||||||
| Normal range | 563 (64.1%) | 211 | 1 | Reference | 1 | Reference | |
| Elevated | 315 (35.9%) | 143 | 1.39 | 1.05-1.84 | 1.66 | 1.04-2.64 | .03 |
| Missing | 19 | 0 | |||||
| Neutrophil-lymphocyte ratio, no. (%) | |||||||
| <6 | 427 (57.0%) | 126 | 1 | Reference | 1 | Reference | |
| ≥6 | 322 (43.0%) | 147 | 2.01 | 1.48-2.72 | 3.06 | 2.05-4.56 | <.001 |
| Missing | 148 | 81 | |||||
CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; ICU, intensive care unit; OR, odds ratio.
Outcomes in Critical Illness
A total of 354 patients were admitted to the ICU for care of COVID-19 infection across the enterprise. Median ICU LOS was 6.19 (range, 0.24-76.4) days. A total of 30.5% of ICU patients were receiving high-flow nasal cannula for a portion of their hospital stay, 23.2% required noninvasive mechanical ventilation, 29.9% required invasive mechanical ventilation, and 4.5% required ECMO. Median APACHE IV score was 57 (range, 9-180) and median admission Sequential Organ Failure Assessment score was 2 (range, 0-18). Overall mortality was 11.9%, and mean ICU LOS was 9.1 days. The predicted mortality by the APACHE IV model was 13.2% and LOS averaging 5.2 days, indicating a standardized mortality ratio of 0.90.
Therapeutics
Therapeutics were almost exclusively administered to hospitalized patients. A total of 695 of 897 (77.5%) patients received a COVID-19–directed treatment, with a higher proportion of ICU patients (89.3%; 316 of 354) receiving such treatments. A total of 370 (41.2%) patients received an antiviral, 433 (48.3%) received systemic steroids, 153 (17.1%) received an immunomodulatory monoclonal antibody, and 192 (21.4%) received convalescent plasma. Overall, 507 patients (56.5%) were enrolled in a clinical trial.
Several patients received combinations of therapies; 32 (3.6%) received all of these therapies; 119 (13.6%), a combination of 3; and 242 (26.9%), 2 therapies. The most common combinations paired steroids with one of the other treatments, most commonly antivirals, and all permutations of treatment classes were administered.
Complications
In 152 (16.9%) patients, ARDS was present, as defined by noting this in the patient’s problem list during their hospital stay. Acute kidney injury occurred in 185 (20.6%) inpatients during their COVID-19 episode. Diagnosed thrombotic and embolic complications were also common, occurring in 127 (14.2%) patients. Respiratory culture–confirmed bacterial superinfections were recorded in 141 (15.7%) patients. Bloodstream infections were present in 29 (3.2%) patients. All complications were more common in patients admitted to the ICU (Table 4 ).
Table 4.
Complications, Length of Stay, and Mortality for Patients 18 Years and Older Hospitalized With COVID-19, Overall and by ICU Status, Mayo Clinic, March Through July 2020
| Outcomes | Total (N=897) | No ICU (N=543) | ICU (N=354) | P |
|---|---|---|---|---|
| Complications, no. (%) | ||||
| Acute kidney injury | 185 (20.6%) | 76 (14.0%) | 109 (30.8%) | <.001 |
| Thromboembolic | 127 (14.2%) | 53 (9.8%) | 74 (20.9%) | <.001 |
| Cardiovascular | 11 (1.2%) | 6 (1.1%) | 5 (1.4%) | .68 |
| Urinary tract infections | 140 (15.6%) | 83 (15.3%) | 57 (16.1%) | .74 |
| Respiratory tract infections | 51 (5.7%) | 8 (1.5%) | 43 (12.1%) | <.001 |
| Bloodstream infections | 29 (3.2%) | 8 (1.5%) | 21 (5.9%) | <.001 |
| Length of stay (cumulative, d), median (range) | 5 (0-84) | 5 (0-84) | 7.5 (0-68) | <.001 |
| Mortality, in hospital or 30 d after discharge, % | 7.1% (64) | 4.1% (22) | 11.9% (42) | <.001 |
COVID-19, coronavirus disease 2019; ICU, intensive care unit.
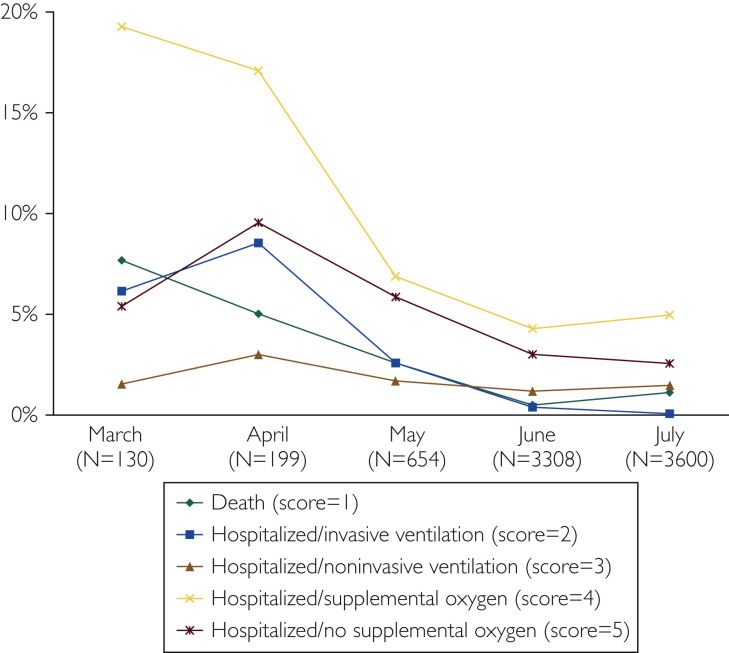
NIH Ordinal Scale
For the entire cohort, outcomes by month using the NIH Ordinal Scale indicated a decrease in the worst severe outcomes (levels 1-5, hospitalizations required) through July (Figure 3 ). In this overall cohort, 94 patients died.
Figure 3.
Crude rates by month for National Institutes of Health Ordinal Scale outcomes (1-5) for 7891 patients, Mayo Clinic, March through July 2020.
Survival Analyses
We conducted a survival analysis among hospitalized patients, calculating 30-day mortality rates (based on Kaplan-Meier estimates) for select patient characteristics, summarized in Table 5 overall and in Supplemental Table 1 for individual CCI elements. In the multivariable model, older age, earlier month of diagnosis, high CCI score (≥2), ever smoking, elevated creatinine level, and higher neutrophil-lymphocyte ratio were all associated with lower rates of survival.
Table 5.
Risk for Death for Hospitalized Patients 18 Years and Older With COVID-19, Mayo Clinic, March Through July 2020a
| Characteristic | N (N=897) | Deaths |
Unadjusted Model |
Adjusted Model |
||||
|---|---|---|---|---|---|---|---|---|
| Deaths (N=64) | 30-d Mortality Rate (95% CI) | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | P | ||
| Age (y) | ||||||||
| 18-34 | 107 | 0 | 0.4% (0%-1.1%) | 1 | Reference | 1 | Reference | |
| 35-49 | 164 | 4 | ||||||
| 50-64 | 284 | 7 | 1.4% (0%-2.8%) | 1.67 | 0.49-5.71 | 0.58 | 0.15-2.23 | .43 |
| 65-74 | 157 | 13 | 6.4% (2.5%-10.2%) | 5.75 | 1.88-17.65 | 0.71 | 0.17-3.00 | .65 |
| ≥75 | 185 | 40 | 19.8% (13.8%-25.4%) | 16.86 | 6.03-47.13 | 3.67 | 1.06-12.71 | .04 |
| Sex | ||||||||
| Female | 406 | 19 | 3.7% (1.9%-5.6%) | 1 | Reference | |||
| Male | 491 | 45 | 7.4% (5.1%-9.7%) | 1.98 | 1.16-3.39 | |||
| Race/ethnicity | ||||||||
| Hispanic (all races) | 169 | 7 | 2.4% (0%-4.7%) | 0.41 | 0.18-0.90 | |||
| White, non-Hispanic | 481 | 47 | 8.4% (5.9%-10.9%) | 1 | (reference) | |||
| Black, non-Hispanic | 90 | 3 | 3.4% (0%-7.1%) | 0.33 | 0.10-1.05 | |||
| Asian, non-Hispanic | 50 | 4 | 6.2% (0%-12.7%) | 0.81 | 0.29-2.24 | |||
| All other, non-Hispanic | 107 | 3 | 0.9% (0%-2.8%) | 0.27 | 0.08-0.87 | |||
| Month of COVID-19 diagnosis | ||||||||
| March | 49 | 8 | 14.3% (3.9%-23.6%) | 1 | Reference | 1 | Reference | |
| April | 79 | 4 | 5.1% (0.1%-9.8%) | 0.28 | 0.08-0.93 | 0.31 | 0.09-1.09 | .07 |
| May | 118 | 9 | 6.8% (2.2%-11.3%) | 0.43 | 0.17-1.11 | 0.33 | 0.12-0.92 | .03 |
| June | 301 | 15 | 3.3% (1.3%-5.4%) | 0.28 | 0.12-0.66 | 0.24 | 0.09-0.62 | .004 |
| July | 350 | 28 | 6.5% (3.8%-9.1%) | 0.50 | 0.23-1.10 | 0.32 | 0.13-0.79 | .01 |
| Charlson Comorbidity Index score | ||||||||
| 0-1 | 281 | 2 | <1% | 1 | Reference | 1 | Reference | |
| ≥2 | 616 | 62 | 8.4% (6.1%-10.6%) | 14.76 | 3.61-60.34 | 4.25 | 0.80-22.59 | .09 |
| Body mass index (kg/m2) | ||||||||
| <18.5 | 10 | 1 | 12.1% (6.9%-17.1%) | 0.81 | 0.11-6.06 | |||
| 18.5-24.9 | 159 | 19 | 10.0% (0%-26.8%) | 1 | Reference | |||
| 25.0-29.9 | 238 | 15 | 4.3% (1.7%-6.9%) | 0.50 | 0.25-0.98 | |||
| 30.0-39.9 | 324 | 25 | 5.3% (2.8%-7.7%) | 0.61 | 0.34-1.11 | |||
| ≥40 | 132 | 3 | 2.3% (0.0%-4.9%) | 0.18 | 0.05-0.60 | |||
| Missing | 34 | 1 | 2.9% (0.0%-8.5%) | 0.23 | 0.03-1.68 | |||
| Smoking | ||||||||
| Never | 545 | 20 | 2.2% (1.0%-3.5%) | 1 | Reference | 1 | Reference | |
| Ever | 324 | 39 | 10.9% (7.4%-14.2%) | 3.48 | 2.03-5.96 | 2.16 | 1.18-3.93 | .01 |
| Missing | 28 | 5 | 14.7% (0.3%-27.0%) | 5.61 | 2.11-14.96 | 1.18 | 0.32-4.28 | .80 |
| Platelets | ||||||||
| Low | 238 | 28 | 9.8% (5.9%-13.6%) | 2.37 | 1.42-3.96 | |||
| Normal range | 608 | 31 | 4.0% (2.4%-5.5%) | 1 | Reference | |||
| High | 39 | 4 | 7.7% (0%-15.7%) | 2.11 | 0.75-5.99 | |||
| Missing | 12 | 1 | ||||||
| Aspartate aminotransferase | ||||||||
| Normal range | 447 | 29 | 5.4% (3.3%-7.5%) | 1 | Reference | |||
| Elevated | 382 | 33 | 7.2% (4.6%-9.8%) | 1.39 | 0.85-2.30 | |||
| Missing | 68 | 2 | ||||||
| Alanine aminotransferase | ||||||||
| Normal range | 606 | 47 | 6.7% (4.6%-8.6%) | 1 | Reference | |||
| Elevated | 222 | 15 | 5.1% (2.1%-8.0%) | 0.88 | 0.49-1.58 | |||
| Missing | 69 | 2 | ||||||
| Bilirubin | ||||||||
| Normal range | 799 | 58 | 6.0% (4.3%-7.6%) | 1 | Reference | |||
| Elevated | 31 | 4 | 12.9% (0.3%-23.9%) | 1.83 | 0.66-5.04 | |||
| Missing | 67 | 2 | ||||||
| Creatinine | ||||||||
| Normal range | 563 | 23 | 3.6% (2.0%-5.1%) | 1 | Reference | 1 | Reference | |
| Elevated | 315 | 41 | 9.9% (6.5%-13.1%) | 3.23 | 1.94-5.37 | 1.98 | 1.10-3.57 | .02 |
| Missing | 19 | 0 | ||||||
| Neutrophil-lymphocyte ratio | ||||||||
| <6 | 322 | 19 | 3.6% (1.8%-5.3%) | 1 | Reference | 1 | Reference | |
| ≥6 | 427 | 34 | 8.1% (5.1%-11.1%) | 2.42 | 1.38-4.24 | 1.78 | 1.00-3.19 | .05 |
| Missing | 148 | 11 | ||||||
| Ferritin | ||||||||
| Normal | 137 | 11 | 6.6% (2.3%-10.7%) | 1 | Reference | |||
| High | 278 | 20 | 5.5% (2.7%-8.1%) | 0.92 | 0.44-1.91 | |||
| Missing | 482 | 33 | ||||||
COVID-19, coronavirus disease 2019.
Discussion
A total of 7891 patients with confirmed COVID-19 infection received care at Mayo Clinic between March 1, 2020, and July 31, 2020. Among these patients, 897 (11.4%) required hospitalization and 354 (4.9%) required ICU care. We observed an overall mortality of 1.2% (94 of 7891), with 7.1% (64 of 897) mortality in hospitalized patients and 11.3% (40 of 354) in patients requiring ICU care.
Our observed mortality rates are lower than the national average and lower than what has been reported from various observational cohorts across the country, large clinical trials, and EAPs. The acuity of our patients, as indicated by APACHE IV scores, was moderately severe, but the standardized mortality ratio indicates modestly reduced mortality compared with what would be expected. As of September 4, 2020, a total of 6,132,074 cases of COVID-19 have been reported in the United States, with 186,173 deaths (3%).18
Rates of mortality reported in hospitalized patients vary widely but have consistently been higher than what was observed here. In a cohort study that included 11,210 adult patients with confirmed COVID-19 infection who presented to 92 hospitals of a health care organization located in 12 states, 7139 (63%) required hospitalization; among these hospitalized patients, 2866 (40%) required ICU care. An overall in-hospital mortality of 20.3% and mortality rate of 34.7% among those with an ICU stay were reported.19 A prospective cohort study from a health care organization in New York City reported that 51.9% of patients required hospitalization, 36.1% required ICU, and 24.3% overall mortality.20 Other New York–based studies indicated 21% mortality21 and 22% mortality22 among hospitalized patients. Connecticut reported 13.5% mortality in a hospital-based study.23
Nationally, the multicenter open-label EAP of convalescent plasma in hospitalized adults with COVID-19 infection involved 35,322 patients receiving care at 1809 sites across the United States. In this study, 30-day mortality of hospitalized patients was 24.5% during the study period. In the double-blind, randomized, placebo-controlled trial of intravenous remdesivir treatment in adults hospitalized with COVID-19 infection, the following mortality rates at day 14 were reported: overall, 86 of 1059 (8%); placebo arm, 54 of 521 (10%); and remdesivir arm, 32 of 538 (6%).8 A study of mortality across countries found that age demographic characteristics accounted for most of the observed variation in fatality rates. In this study, fatalities ranged from 0.002% in those younger than 34 years and 28.3% in those 85 years and older.24 Our observed mortality was lower in each age bracket.
The observed decline in mortality over time has been previously observed. One possible explanation for this is that it reflects selection bias. Early in the outbreak, testing was prioritized to those with specific symptoms, thereby selecting for those with more severe illness. Later in the epidemic, testing included asymptomatic individuals, who would be expected to have less severe illness. Second, because the Mayo Clinic sites were not involved in the initial wave of infections within North America, we had the advantage of learning from the clinical experience and insights of our colleagues worldwide. Most of the comparators cited were in the East Coast and New York in particular, which bore the brunt of the first wave with disruptions to every aspect of local health care.
Another factor may have been our outpatient COVID-19 management teams and remote monitoring capabilities, allowing complications and deterioration to be detected earlier and interventions put in place in a more timely manner. The high proportion of our patients able to participate in these programs was likely a contributor to our outcomes.
A possible contributor to decreased mortality over time was the attenuating effects of masks and social distancing, reducing inoculum of exposure and potentially reducing the severity of illness.25 Also, differential mortality among strains of SARS-CoV-2 has been postulated, and the impact of different substrains on population outcomes and shifts in strains over time and space are not yet understood.26
Finally, best practices in critical care and excellent multidisciplinary ICU care were maintained according to Mayo Clinic’s model of care. In the early epidemic, early intubation was a more common practice based on the assumption that patients would always decline rapidly27 and to limit exposure of health care workers to aerosol-generated procedures.28 However, we learned very quickly that although some patients deteriorated rapidly and required intubation, most of them would be placed early on high-flow nasal cannula, less often on noninvasive ventilation, encouraged to self-prone, and ultimately would not require intubation, though the effect of individual aspects of this bundled approach cannot be analyzed here and some elements of it have recently been subject to controversy.29 Among those intubated, adherence to best supportive care was encouraged early.30 Special emphasis was made on lung protective strategy, early prone positioning, conservative fluid strategy, judicious use of sedative and neuromuscular blocking agents, and management of the hypercoagulable state,3 as well as adherence to the modified ABCDEF bundle (assess and manage pain, both spontaneous awakening and breathing trial, choice of sedation, delirium assessment, early mobility and exercise, and family communication and involvement).31 Although ECMO use was infrequent, going to ECMO early when it was determined to be indicated may have contributed as well.
Critical to our outcomes was the ability to confirm the diagnosis of COVID-19 quickly following sample collection within 12 to 24 hours. Therefore, patients were evaluated early during their COVID-19 infection. Patients not requiring hospitalization were closely monitored from home by a structured telemedicine system. Each and every hospitalized patient was reviewed by the multidisciplinary Treatment Review Panel that, in collaboration with the primary team, made recommendations on the best supportive care based on evidence as they became available and progressed with time8 , 32 and offered COVID-19–directed therapies, clinical trial options, and general infectious disease consultation. These therapies were communicated to the patient by an infectious disease provider. This method, although requiring significant resources, ensured high-quality communication with the primary teams and the application of latest evidence available as reviewed by the multidisciplinary team.
Trials of antiviral therapy were available early, which accounts for a large proportion of patients who received them (41% [368 of 897] of hospitalized patients). Despite our individualized approach, with emphasis on limiting their use to those with rapid deterioration on imaging, oxygenation, or clinical appearance or those with a propensity to do so or those who had an established indication (eg, chronic obstructive pulmonary disease, asthma, long-term use, pregnancy, and septic shock), a fair proportion (50.1%; 449 of 897) received glucocorticoids. The use of convalescent plasma was more restrained and more common in those with contraindications to remdesivir (eg, abnormal liver function or renal failure). In some instances, the use of antivirals was limited due to temporary lack of availability. The use of specific immunomodulatory agents (eg, interleukin 6 inhibitors) was limited (16.9%; 152 of 897). It was initially used under emergency Investigational New Drug authorization and later mostly in randomized controlled trial only and rarely under an EAP.33 The Treatment Review Panel was available after hours, providing real-time guidance and expertise. The multidisciplinary nature of this group helped ensure that differential diagnoses overlapping with COVID-19 infection would be considered as part of a holistic treatment approach, such as E-cigarette or vaping product use–associated lung injury34 or ARDS.
This study is limited by its retrospective nature. It is possible that certain comorbid conditions thus may have been omitted if not appropriately documented, especially for patients who were new to our system because of the COVID-19 epidemic who did not have pre-existing documentation in the EHR. The geography of our patient referral base also means that our description and findings may not translate to other populations throughout the United States with different comorbid conditions and resources at their disposal. Critically, no location in our system was challenged with an overwhelming surge on the level of New York City during this time frame.
Another limitation to the generalizability of this study is that our enterprise is a consortium comprising multiple tertiary referral centers. Other health care organizations may not have the bandwidth for extending tertiary care expertise and sharing logistical and planning support across states.
Conclusion
Multidisciplinary team–based consensus-driven treatment approaches to COVID-19 infection, complemented with aggressive diagnostic capabilities and outpatient monitoring, are associated with low rates of hospitalization and mortality. These best practices learned over time could serve as a template for an effective continuing response to the pandemic.
Acknowledgments
We thank Nathan W. Cummins, MD; Gianrico Farrugia, MD; Gregory J. Gores, MD; Bobbie S. Gostout, MD; Richard J. Gray, MD; and Kent R. Thielen, MD, for their contributions in revising this manuscript and all the health care providers, researchers, patients, and families who have made these outcomes possible.
Footnotes
Potential Competing Interests: Dr O’Horo has been paid consulting fees by Elsevier Inc and Bates College not directly pertaining to the presented work; Dr Cerhan has grant support from NanoString, Celgene, and Genentech not related to presented work; Dr Ebbert serves as a consultant for Nesmah; Dr Levy has received research grants (funds to the institution) for clinical trials related to critical illness and immunobiology of pediatric coronavirus disease 2019; Dr Tande has received honoraria from UpToDate.com, not related to this work; Dr Razonable has received research grants (funds to the institution) on clinical trials on interleukin 6 inhibitors and anti-spike monoclonal antibody against coronavirus disease 2019 (Roche and Regeneron); Dr Rizza has received research grants from Gilead Sciences for the clinical trial of Remdesivir; Dr Badley is supported by grants from National Institute of Allergy and Infectious Diseases (grants AI110173 and AI120698), Amfar (#109593), and Mayo Clinic (HH Shieck Khalifa Bib Zayed Al-Nahyan Named Professorship of Infectious Diseases) and is a paid consultant for Abbvie, is a paid member of the data and safety monitoring board for Corvus Pharmaceuticals, owns equity for scientific advisory work in Zentalis and Nference, and is founder and President of Splissen therapeutic; and Dr Libertin has received research grants (funds to the institution) on clinical trials on an antiviral compound and an engineered anti-human granulocyte macrophage-colony stimulating factor monoclonal antibody (ViralClear and Humanigen).
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Berbari E.F., Williams A.W., Williamson M.J., Caine N.A., Nath K.A., Farrugia G. Mayo Clinic strategies for COVID-19: introduction. Mayo Clin Proc. 2020;95(9):S1–S2. doi: 10.1016/j.mayocp.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah A.S., Tande A.J., Challener D.W., O’Horo J.C., Binnicker M.J., Berbari E.F. Diagnostic stewardship: an essential element in a rapidly evolving COVID-19 pandemic. Mayo Clin Proc. 2020;95(9):S17–S19. doi: 10.1016/j.mayocp.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBane R.D., Torres Roldan V.D., Niven A.S. Anticoagulation in COVID-19: a systematic review, meta-analysis and rapid guidance from the Mayo Clinic. Mayo Clin Proc. 2020;95(11):2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger C.D., Mikhail A.E., Orenstein R., Ebbert J.O., Vergidis P., Badley A.D. Research response to SARS-CoV-2/COVID-19. Mayo Clin Proc. 2020;95(9):S52–S55. doi: 10.1016/j.mayocp.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane S.J., Ganesh R., Post J.A., Jacobson N.A. Telemedicine consultations and follow-up of patients with COVID-19. Mayo Clin Proc. 2020;95(9):S33–S34. doi: 10.1016/j.mayocp.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah A., Challener D., Tande A.J. Drive-through testing: a unique, efficient method of collecting large volume of specimens during the SARS-CoV-2 (COVID-19) pandemic. Mayo Clin Proc. 2020;95(7):1420–1425. doi: 10.1016/j.mayocp.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN) Patient Safety Component Manual. 2020. https://www.cdc.gov/nhsn/pdfs/validation/2020/pcsmanual_2020-508.pdf
- 8.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 — preliminary report [letter] N Engl J Med. 2020;383:992–994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 9.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 11.Quan H., Sundararajan V., Halfon P. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 12.Herasevich V., Kor D.J., Li M., Pickering B.W. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28(11) 42, 44-45. [PubMed] [Google Scholar]
- 13.Jones A.E., Trzeciak S., Kline J.A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37(5):1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 15.Du Y., Lv Y., Zha W., Zhou N., Hong X. Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. https://doi.org/10.1016/j.metabol.2020.154373 Metabolism. Published online September 16, 2020. [DOI] [PMC free article] [PubMed]
- 16.Reddy R.K., Charles W.N., Sklavounos A., Dutt A., Seed P.T., Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2020;93(2):1045–1056. doi: 10.1002/jmv.26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji W., Huh K., Kang M. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention CDC COVID-19 Data Tracker. https://covid.cdc.gov/covid-data-tracker/#cases Published 2020. Accessed September 4, 2020.
- 19.Yehia B.R., Winegar A., Fogel R. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8):e2018039. doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPadden J., Warner F., Young H.P. Clinical characteristics and outcomes for 7,995 patients with SARS-CoV-2 infection. https://doi.org/10.1101/2020.07.19.20157305 medRxiv. Preprint posted July 21, 2020. [DOI] [PMC free article] [PubMed]
- 24.Levin A.T., Hanage W.P., Owusu-Boaitey N., Cochran K.B., Walsh S.P., Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. medRxiv. Preprint posted July 23, 2020. [DOI] [PMC free article] [PubMed]
- 25.Lu Y.-T., Chen P.-J., Sheu C.-Y., Liu C.-L. Viral load and outcome in SARS infection: the role of personal protective equipment in the emergency department. J Emerg Med. 2006;30(1):7–15. doi: 10.1016/j.jemermed.2005.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyoshima Y., Nemoto K., Matsumoto S., Nakamura Y., Kiyotani K. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet. 2020;65:1075–1082. doi: 10.1038/s10038-020-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10(1):78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung J.C.-H., Ho L.T., Cheng J.V., Cham E.Y.K., Lam K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020;8(4):e19. doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrando C., Mellado-Artigas R., Gea A., COVID-19 Spanish ICU Network Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care. 2020;24(1):597. doi: 10.1186/s13054-020-03314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alhazzani W., Møller M.H., Arabi Y.M. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48(6):e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marra A., Ely E.W., Pandharipande P.P., Patel M.B. The ABCDEF bundle in critical care. Crit Care Clin. 2017;33(2):225–243. doi: 10.1016/j.ccc.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. https://doi.org/10.1056/NEJMoa2021436 N Engl J Med. Published online July 17, 2020.
- 33.Temesgen Z., Assi M., Vergidis P. First clinical use of lenzilumab to neutralize GM-CSF in patients with severe COVID-19 pneumonia. https://doi.org/10.1101/2020.06.08.20125369 medRxiv. Preprint posted July 14, 2020.
- 34.The Lancet Respiratory Medicine The EVALI outbreak and vaping in the COVID-19 era. Lancet Respir Med. 2020;8(9):831. doi: 10.1016/S2213-2600(20)30360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.