Abstract
Background
Worldwide, millions of women seek treatment for early pregnancy loss (EPL) annually. Medical management with misoprostol is widely used, but only effective 60% of the time. Pre-treatment with mifepristone prior to misoprostol might improve the success rate of medical management.
Methods
This was a multi-centre, double-blind, placebo-controlled randomised trial in 17 Dutch hospitals. Women with a non-viable pregnancy between 6 and 14 weeks of gestation were eligible for inclusion after at least one week of expectant management. Participants were randomised (1:1) between oral mifepristone 600 mg or an oral placebo tablet. Participants took 400 μg misoprostol orally, repeated after four hours on day two and, if necessary, day three. Primary outcome was expulsion of gestational sac and endometrial thickness <15 mm after 6–8 weeks. Analyses were done according to intention-to-treat principles. This trial is registered with ClinicalTrials.gov, NCT03212352.
Findings
Between June 28th 2018 and January 8th 2020, 175 women were randomised to mifepristone and 176 to placebo, including 344 in the intention-to-treat analysis. In the mifepristone group 136 (79•1%) of 172 participants reached complete evacuation compared to 101 (58•7%) of 172 participants in the placebo group (p<0•0001, RR 1•35, 95% CI 1•16–1•56). Incidence of serious adverse events was significantly lower in the mifepristone group with 24 (14%) patients affected versus 55 (32%) in the placebo group (p = 0•0005) (Table 3).
Interpretation
Pre-treatment with mifepristone prior to misoprostol was more effective than misoprostol alone in managing EPL.
Funding
Healthcare Insurers Innovation Foundation, Radboud University Medical Centre, Canisius Wilhelmina Hospital.
Keywords: Early pregnancy loss, Mifepristone, Misoprostol
Research in context.
Evidence before this study
Early pregnancy loss (EPL) occurs in 10 to 28% of all pregnancies. Medical treatment for EPL is found to be a safe and effective alternative for surgical treatment. Although a number of studies have been performed, there is limited evidence regarding the addition of mifepristone to treatment with misoprostol alone in case of EPL. This is reflected by different recommendations in international guidelines.
Added value of this study
This large, sufficiently powered, double-blinded placebo-controlled randomized trial demonstrates that the sequential combination of mifepristone with misoprostol is more effective than misoprostol alone in case of EPL.
Implications of all the available evidence
Our findings support the implementation of pre-treatment with mifepristone in case of EPL in (inter)national guidelines. The sequential combination of mifepristone and misoprostol is easy to use and more effective in reaching complete miscarriages, thereby preventing the need for uterine aspiration and its possible complications.
Alt-text: Unlabelled box
1. Introduction
Early pregnancy loss (EPL) is defined as a non-viable first trimester intra-uterine pregnancy, in which there may be an anembryonic gestation or embryonic death [1,2]. It is a common outcome of pregnancy, occurring in 10–28% of pregnancies with rates varying by age [3,4]. The estimated annual number of pregnancies worldwide is 227 million, meaning every year millions of women will seek treatment for EPL [5]. It is essential that EPL treatment is optimised, allowing women to receive the most effective and safe treatment while meeting their personal needs and rights.
Three treatment options exist for EPL: expectant, surgical, or medical. In many European countries, including the Netherlands, expectant management for at least one week is common practice, as it is well known that a spontaneous complete evacuation can occur in up to 50% of women [6,7]. However, after this period of expectant management, the remainder of women, in which no spontaneous evacuation occurred may require treatment. Although very successful in reaching complete evacuation, surgical management, i.e., uterine aspiration, is associated with risks of early and late complications, such as adhesion formation and increased risk of premature delivery in subsequent pregnancies, and higher costs [8], [9], [10]. Multiple international guidelines such as, for example from the American College of Obstetrics and gynecology (ACOG) and the guideline from the National Institute for Health and Care Excellence (NICE), currently recommend misoprostol as primary medical treatment of EPL, with some suggesting mifepristone pre-treatment and others advising against the use of mifepristone [2,11]. Misoprostol tablets, a prostaglandin E1 analogue, are widely used, easy to apply, proven safe, and do not require special storage or temperature conditions [2,11,12]. Medical management using misoprostol without previous expectant management may result in success rates of 66•0–88•5% [13,14]. After one week of expectant management, the success rate of misoprostol treatment decreases to 54% [15,16]. Thus, surgical treatment is associated with risks and higher costs, but medical treatment with misoprostol is limited in terms of its efficacy.
The sequential combination of mifepristone followed by misoprostol has been shown to be superior to misoprostol alone for the termination of viable first trimester pregnancies, and for labour induction following foetal death in utero in the second or third trimester [17,18]. Mifepristone acts by binding tightly to the progesterone receptor, thus blocking the action of progesterone. This results in the breakdown of the decidua, increasing prostaglandin levels and inducing a higher sensitivity of the uterus towards prostaglandins, leading to uterus contractions and the possible expulsion of the blastocyst [19]. It is therefore hypothesised that this combination will also be a more successful treatment of EPL than misoprostol alone.
Both a retrospective study and a pilot study (unpublished data) performed by our research group [16], and two recently published trials show an advantage of pre-treatment with mifepristone for the treatment of EPL [20,21]. However, the evidence supporting pre-treatment with mifepristone remains insufficient, leading to conflicting recommendations in the NICE and ACOG guidelines [2,11]. A recent systematic review and meta-analysis concluded that the current evidence for the addition of mifepristone is ‘limited’ [22], and the most recent Cochrane review classified the evidence level as ‘very low’ [23]. Taken together, evidence from a large, sufficiently powered, placebo-controlled, double-blinded randomised controlled trial (RCT) is needed.
The aim of the Triple M Trial (Mifepristone and Misoprostol for Miscarriage) was to compare the sequential combination of mifepristone with misoprostol with the use of a placebo followed by misoprostol in the treatment of EPL, in terms of complete evacuation, side effects and complications, in a placebo-controlled double-blind RCT.
2. Methods
2.1. Study design
This randomised, placebo-controlled, double-blinded trial was performed in a total of 17 centres in the Netherlands, 1 district, 14 teaching and 2 tertiary referral hospitals. The participants were followed in an outpatient setting, unless admission was medically necessary. Ethical approval to conduct the study was obtained from the regional medical-ethical commission (Commissie Mensgebonden Onderzoek Arnhem-Nijmegen). The study protocol is available from https://doi.org/10.1186/s12884–019–2497-y.24
This trial was registered at the following registers:
Clinicaltrials.gov: NCT03212352.
Trialregister.nl: Trial NL 6366
EudraCT number: 2017-002694-19.
File number Commissie Mensgebonden Onderzoek: NL 62449.091.17.
2.2. Participants
Women 16 years of age or older, with an ultrasound examination showing a non-viable intra-uterine pregnancy between 6 and 14 weeks of gestation, who had been managed expectantly for at least one week, were eligible for inclusion. The diagnosis of EPL was made by ultrasonography describing: A crown-rump length (CRL) ≥ 6 mm and no cardiac activity, or a CRL <6 mm and no embryonic growth at least one week later, or a gestational sac with absent embryonic pole for at least 1 week.
In case of an obvious discrepancy of at least one week between the crown and rump length of the non-viable embryo and the calendar gestational age, women were also eligible for inclusion, as a week of expectant management had already been applied unknowingly.
Women who were haemodynamically unstable, or who showed signs of infection or incomplete miscarriage were excluded, as well as patients with contraindications to mifepristone or misoprostol. Women were also excluded if they had a known clotting disorder or used anticoagulants, had (risk factors for) cardiovascular disease, or if there was a language barrier hindering adequate counselling.
Eligible women were identified and approached to participate in the study by their treating physician. They were then counselled by trained staff informing them about the aims, methods, reasonable anticipated benefits, and potential hazards of the study medication, and were given the patient information letter. Patients were also informed about the off-label use of both mifepristone and misoprostol. Participation was voluntary, and patients could withdraw their consent to participate at any time during the study. The investigator could also decide to withdraw a participant from the study for urgent medical reasons. Baseline demographics, obstetric and medical history were recorded for all women at the time of randomisation using a case report form. Immediately after obtaining oral and written informed consent, randomisation was performed.
2.3. Randomisation and masking
Subjects were randomised in a 1:1 ratio to either mifepristone 600 mg orally or placebo pre-treatment using computerised randomisation tables. Randomisation was conducted by block randomisation, with a block size of eight, stratified by hospital. The randomisation tables were generated by two independent physicians, who had no further role in the execution of the trial.
After informed consent was obtained, the treating physician took the first available study number from the computerised randomisation table. Thus, a unique study number was assigned to each participant. This unique study number corresponded with identical looking medication jars available in the participating centres, containing the study medication.
For the purpose of this study, the Clinical Trials Unit in the Radboud university medical centre (Radboudumc) ordered batches of mifepristone and placebo tablets. The placebo tablets consisted mainly of maize starch and lactose, and were visually identical to mifepristone tablets, without containing active medication.
Three tablets were repackaged into identical medication jars, labelled with the unique study number, following the randomisation table. The content of each medication jar was unknown to all involved in the study, establishing double blinding. After repackaging and labelling, the blinded medication jars were distributed to all participating hospitals. A sealed list with the contents of each medication jar was available for deblinding in the Clinical Trials Unit of the Radboudumc in case of emergency. The data from this list was disclosed to the investigators not earlier than 16th April 2020, after the results of all outcome parameters had been collected for all participants.
Regarding misoprostol, the treating physician prescribed these tablets as usual, which were provided by the patients’ own/local pharmacies.
2.4. Procedures
The mifepristone tablets and visually identical placebo tablets were purchased at a regular price from Excelgyn (Groupe Nordic Pharma) for the purpose of this study. All medication was produced in Paris, France. Excelgyn had no further role in the design, conduct, or analysis of this trial.
After informed consent was obtained, and randomisation performed, each patient received three tablets containing either 200 mg mifepristone or the placebo. These three tablets were taken by the participant on day one.
With the exception of the blinded study medication intake, the further management of all participants was equal in both groups. At day three (36–48 h later), two doses of misoprostol 400 μg orally (four hours apart) were taken at home. Participants were instructed to observe their blood loss and loss of possible products of conception. If blood loss had not occurred, or if blood loss had occurred and the participant's subjective assessment was that no tissue had passed by day four, participants were instructed to take two more doses of misoprostol 400 μg orally (four hours apart).
To assess the treatment effect, a first follow-up visit including ultrasonography was performed between day 15 and 20, so after approximately two weeks. In case of an expulsed gestational sac and a total endometrial thickness (TED) < 15 mm determined using ultrasonography, no further evaluation was necessary, and the treatment was considered successful.
In the case of an expulsed sac but possible retained products of conception (RPOC) defined as TED > 15 mm, expectant management was advised, with consent from the patient, for another four weeks. If the gestational sac was still retained in utero after this period, or there was another reason for intervention, the treatment was considered unsuccessful and additional treatment was offered.
The primary outcome was assessed at six to eight weeks after the treatment start, when ultrasonography was performed to evaluate endometrial thickness. In case of an endometrial thickness > 15 mm, further treatment was performed according to local protocol and patient preference. Additional treatment was either expectant, medical (another course of misoprostol), or surgical (hysteroscopy or uterine aspiration).
During all follow-up visits, including unplanned and emergency visits, clinical signs and symptoms were assessed in addition to the ultrasonographic findings. When deemed necessary by the treating physician, additional treatment was offered and documented.
If successful uterine aspiration was performed after the primary medical treatment, no further examinations were scheduled for the purpose of this study. If the initial allocated treatment failed, patients were followed until complete evacuation of the uterus was established.
Anti-D prophylaxis was given as part of standard treatment to patients with an gestational age of at least 10 weeks, or if instrumentation took place, following the relevant Dutch guideline [25].
All participants were asked to fill out a patient diary, indicating how and when they took the medication. Additionally, they were asked to use the diary to report any adverse reaction after the administration of the study medication and/or misoprostol, up until the first follow-up visit. Participants were also asked to complete questionnaires regarding their quality of life and productivity loss at four different timepoints (T = 0, 1, 2 and 6 weeks after treatment start). The participants were able to contact their hospital 24 h a day in case of questions, complaints, or emergencies during the course of the study.
2.5. Outcomes
The primary study outcome was the successful treatment or complete evacuation of the uterus (defined as an expelled gestational sac and an endometrial thickness < 15 mm determined using ultrasonography) using only the allocated therapy by randomisation, and was determined 6–8 weeks after the initial treatment [26].
The secondary outcomes included any additional interventions required to achieve complete evacuation, such as uterine aspiration, additional medical treatment, or hysteroscopy, as well as any adverse reactions. Safety outcomes consisted of (serious) adverse events (SAEs) and adverse reactions. An SAE is defined as an ‘undesirable medical event in a patient or test subject, not necessarily related to the treatment and results in: death, and/or is life-threatening, and/or requires inpatient hospitalization or causes prolongation of existing hospitalization, and/or results in persistent or significant disability/incapacity, and/or may have caused a congenital anomaly/birth defect, and/or requires intervention to prevent permanent impairment or damage [27,28]. An adverse reaction is defined as any adverse event for which there is a reasonable possibility that the drug caused the adverse event, but does not meet the criteria for an SAE, and in layman's terms is often referred to as a side-effect [27,28].
Participating centres were asked to promptly report any (suspected) SAE to the coordinating investigator and fill out a SAE form. Using this form, the coordinating investigator reported all SAEs to the Medical Ethical Committee within 15 days. An exception was made for the reporting of uterine aspirations, as this is a common procedure in the treatment process of EPL [29]. All cases of uterine aspiration were registered as SAEs and reported to the Medical Ethical Committee in the obligatory semi-annual safety reports.
Additional pre-specified outcomes such as the acceptability of treatment, quality of life, cost-effectiveness, and assessment of patient and clinical characteristics associated with complete evacuation were collected. These data are not presented here but are to be published in the near future.
2.6. Statistical analysis
The sample size calculation was based on data from a previous retrospective study, with a complete evacuation rate of 67% in the intervention group (mifepristone 600 mg orally 36–48 h before misoprostol) and 54% in the control group (misoprostol only) [16]. These findings are comparable with other data from the literature [15,30,31]. Based on these evacuation rates and an overall significance level of 5% (α = 0•05), in combination with a power of 80% (β = 0•20), the trial required 221 patients in each arm. Anticipating 4% lost to follow-up, based on the percentage of lost to follow-up in our pilot-study (2.5%), 230 patients had to be included per arm. Because of the intended execution of an interim analysis after inclusion and the outcome assessment of 50% of intended inclusions, the sample size was adjusted to 232 patients per arm (total 464), to maintain a sufficiently powered final analysis.
In case of missing data the specific hospital was contacted to retrieve the missing information as much as reasonably possible.
A Data Safety Monitoring Board oversaw the study and assessed the results of the interim analysis after 50% of inclusions regarding primarily effectivity and secondarily safety. A pre-defined stopping rule, according to O'Brien-Fleming, was used to assess the primary outcome, complete evacuation after 6–8 weeks [24]. Following this stopping rule, when analysis of the primary outcome would show a difference between both study groups with a p-value <0.0054, a premature halt of the trial would be justified.
Frequencies and proportions were calculated for categorical values. Means or medians were calculated for continuous variables, depending on normality.
The proportion of success in each arm was compared using a two-sample test for proportions (Chi-square or Fisher's exact test). The relative risks were calculated with a 95% confidence interval.
An intention-to-treat analysis was performed for both the primary outcome, and the secondary and safety outcomes. All analyses were performed using SPSS version 26 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.)
2.7. Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data of the study and had the final responsibility for the decision to submit for publication.
3. Results
Between 27th June 2018, and 8th January 2020, a total of 351 patients were enrolled and randomised into the treatment groups. Although the planned interim analysis was set at 50% (232 patients), an additional 119 patients were included before the trial was stopped due to the time lapse between the inclusion of the 232nd patient and the advice given by the Data Safety Monitoring Board to halt the trial. This time lag was caused by the follow-up period, acquiring the data, and performing the interim analysis. The Data Safety Monitoring Board advised the halting of the trial on 27th December 2019, based on the superiority of one of the treatments. There were no concerns reported with regards to patient safety. Data regarding all 351 included patients will be reported here.
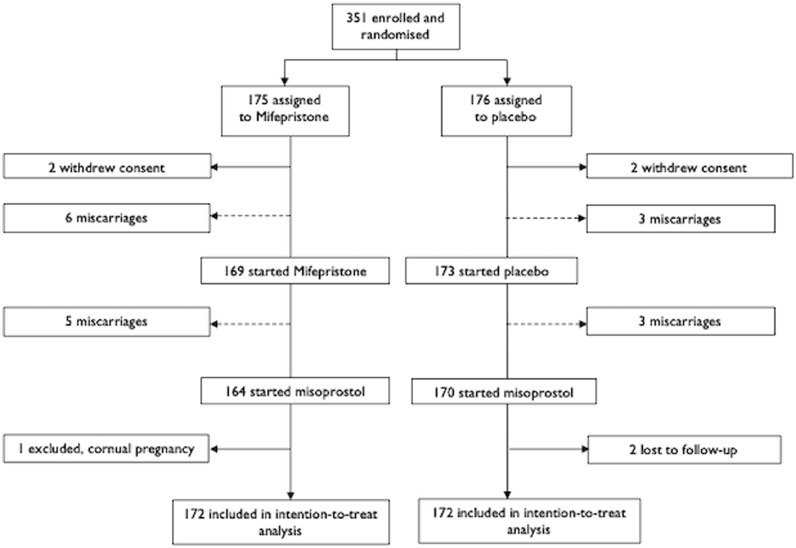
As shown in Fig. 1, a total of 175 patients were assigned to the treatment with mifepristone and 176 to the placebo, both followed by the standard misoprostol treatment. In each study arm, two patients withdrew consent a few hours after randomisation, before taking the blinded study medication; these patients were excluded from the analysis. In the mifepristone arm, one patient was excluded after randomisation, as she was found to have a cornual pregnancy not meeting the inclusion criteria. In the placebo arm, two patients were lost to the follow-up, as they did not attend their planned follow-up visits, nor responded to attempted contact by their doctor, and were excluded from the analysis. In both arms, 172 participants were included in the intention-to-treat analysis.
Fig. 1.
trial profile
Legend: -----> = included in intention-to-treat analysis
→ = excluded from intention-to-treat analysis.
The baseline characteristics of the study population were similar between both groups (Table 1). In 69% of patients, an embryo without cardiac activity was seen. EPL in at least one previous pregnancy had occurred in 30% of patients in both groups. In both groups, 20 patients (11•6%) had a gestational age above 12 weeks, with a mean crown-rump length of 22•24 mm (±25•98).
Table 1.
Baseline characteristics.
| Characteristic | Mifepristone and Misoprostol N = 172 | Placebo and Misoprostol N = 172 |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 32.95 (4.39) | 32.69 (4.30) |
| BMI (kg/m2) | ||
| Mean (SD) | 24.70 (4.44) | 24.08 (3.84) |
| Unknown | 28 | 34 |
| Race or ethnic group | ||
| Caucasian | 156 (90.7%) | 155 (90.1%) |
| Other | 12 (7.0%) | 13 (7.6%) |
| Unknown | 4 (2.3%) | 4 (2.3%) |
| Gravidity | ||
| 1 | 60 (34.9%) | 75 (43.6%) |
| 2 | 63 (36.6%) | 53 (30.8%) |
| ≥ 3 | 49 (28.5%) | 44 (25.6%) |
| Parity | ||
| 0 | 83 (48.3%) | 94 (54.7%) |
| 1 | 70 (40.7%) | 64 (37.2%) |
| 19 (11.0%) | 14 (8.1%) | |
| Gestational age based on amenorrhoea (days) | ||
| Mean (SD) | 71.22 (11.03) | 70.09 (11.57) |
| Unknown | 3 | 3 |
| Diagnosis | ||
| Embryo without cardiac activity | 123 (71.5%) | 115 (66.9%) |
| Anembryonic gestation | 49 (28.5%) | 57 (33.1%) |
| Prior miscarriage | 51 (29.7%) | 52 (30.2%) |
| Of these: misoprostol treatment for prior miscarriage | 13 (25.5%) | 19 (36.5%) |
| Of these: successful misoprostol treatment | 7 (53.8%) | 12 (63.2%) |
The primary outcome is presented in Table 2: at 6–8 weeks after the start of the treatment, using only the allocated therapy, 136/172 patients (79•1%) in the mifepristone group had reached complete evacuation versus 101/172 (58•7%) in the placebo arm (p<0•0001, RR 1•35, 95% CI 1•16–1•56). A sensitivity analysis that assumed the outcomes in the two women who were lost to follow-up were in favour of no treatment effect (p<0•0001, RR 1•95, 95% CI 1•38–2•74) did not alter the results. After the first follow-up visit at two weeks, 107/172 patients in the mifepristone group (62•2%) had already reached a complete evacuation versus 78/172 (45•3%) in the placebo group (p = 0•001, RR 1•37, 95% CI 1•12–1•68). A total of 59 patients (25 in the placebo group and 34 in the mifepristone group) were asked to return four weeks later because of suspected retained products of conception (RPOC), without applying a further intervention such as another course of medication or antibiotic prophylaxis. Spotting per vaginam was present in 36 (61•0%) of these patients. At the second follow-up an additional 41/59 (69•5%) patients had reached complete evacuation.
Table 2.
Clinical outcomes.
| Mifepristone and misoprostol (N = 172) | Placebo and misoprostol (N = 172) | p-value | |
|---|---|---|---|
| Primary outcome | |||
| Complete evacuation achieved with only allocated therapy | 136 (79.1%) | 101 (58.7%) | <0.0001 |
| Patients requiring additional intervention* | 36 | 71 | |
| Uterine aspiration | 19 (11.0%) | 51 (29.7%) | <0.0001 |
| Indication | |||
|
7 | 20 | |
|
5 | 12 | |
|
4 | 9 | |
|
3 | 7 | |
|
0 | 3 | |
| Emergency setting | 3 (1.7%) | 8 (4.7%) | |
| Extra course misoprostol after first follow-up | 14 (8.1%) | 27 (15.7%) | 0.022 |
| successful | 6 | 13 | |
| Hysteroscopy (operative) | 11 (6.4%) | 9 (5.2%) | 0.409 |
Some patients required more than one additional treatment to achieve complete evacuation. Therefore, the total number of additional treatments is higher than the number of patients undergoing these treatments.
Table 2 also lists the additional treatment given if the allocated therapy did not lead to a complete evacuation. The number of patients requiring at least one additional treatment was 36 in the mifepristone arm and in 71 the placebo arm. Uterine aspiration was performed less often in the mifepristone group (19/172 patients or 11•0%) than in the placebo group (51/172 patients or 29•7%) (p<0•0001, RR 0•37, 95% CI 0•23–0•60). Emergency uterine aspiration due to heavy vaginal bleeding was performed in three patients in the mifepristone group and eight patients in the placebo group.
Of the patients who did not reach a complete evacuation at the first follow-up visit at two weeks, 14 (8•1%) patients in the mifepristone group opted for another course of misoprostol, which was significantly fewer than the number in the placebo group (27, 15•7%) (p = 0•0305). This additional course of misoprostol led to complete evacuation in 6/14 (42•9%) in the mifepristone and 13/27 (48•1%) in the placebo group. The remaining patients required another additional treatment. The rate of hysteroscopies for the treatment of RPOC was similar between the mifepristone and the placebo group (6.4% and 5.2% respectively). All hysteroscopies were performed based on suspected intra-uterine remains visible on an ultrasound at the second follow-up appointment at 6–8 weeks after treatment start.
A total of 79 SAEs were recorded, as shown in Table 3. The SAEs consisted mainly of short stay hospital admissions of less than a day, for uterine aspiration, comprising 19/24 (79•2%) of the mifepristone group and 51/55 (92•7%) of the placebo group. Other reasons for admission were clinical hysteroscopy or the observation of excessive blood loss. Significantly fewer SAEs were observed in the mifepristone group (24 SAEs) than in the placebo group (55; p = 0•0005). The rate of SAEs other than hospital admission was 0.6% and 1.2% in the mifepristone and placebo group respectively. A pelvic infection was suspected in one patient in each study group (0•6%), and both were treated with oral antibiotics (Augmentin). One patient in the placebo group required a blood transfusion following heavy bleeding.
Table 3.
Serious adverse events.
| Mifepristone and misoprostol (N = 172) | Placebo and misoprostol (N = 172) | p-value | |
|---|---|---|---|
| All Serious Adverse Events | 24 | 55 | 0.000487 |
| Hospital admissions | 23 (13.4%) | 53 (30.8%) | 0.000579 |
| Planned | 17 (9.9%) | 44 (25.6%) | |
| -uterine aspiration | 16 | 43 | |
| -clinical hysteroscopy | 1 | 1 | |
| Unplanned | 6 (3.5%) | 9 (5.2%) | |
| -uterine aspiration | 3 | 8 | |
| -observation blood loss | 3 | 1 | |
| No of other serious adverse events | 1(0.6%) | 2(1.2%) | 0.500 |
| Suspected pelvic infection requiring antibiotics | 1 (0.6%) | 1 (0.6%) | |
| Heavy blood loss requiring blood transfusion | 0 | 1 (0.6%) |
Table 4 shows the incidence of the six most frequent adverse reactions, as reported in the patient diaries kept for the first two weeks. This diary was completed and returned to their treating physician by 78% of patients, 133 in the mifepristone group and 137 in the placebo group. The diaries showed a medication compliance rate of 90.7% overall, comparable between both groups. In the mifepristone group, 80/133 (60•2%) patients reported at least one adverse reaction, which was significantly more than the 65/137 (47•7%) patients reporting an adverse reaction in the placebo group (p = 0•024, RR=1•27, 95% CI 1•01–1•59). The mean number of adverse reactions per patient did not differ significantly between both groups. The percentage of patients experiencing an adverse reaction from the study medication only was 15.9% overall, and not significantly different between groups (p = 0.0652). Nausea and dizziness were reported significantly more frequently in the mifepristone group (p = 0•007 and p = 0•034, respectively) during the misoprostol intake on days three and four. All other adverse reactions occurred in fewer than 5% of patients, and were similarly distributed between both treatment groups.
Table 4.
Most frequent adverse reactions as reported in patient diary.
| Mifepristone and misoprostol (N = 133) |
Placebo and misoprostol (N = 137) | p-value | |
|---|---|---|---|
| Patients reporting any adverse reaction | 80 (60.2%) | 65 (47.4%) | 0.024 |
| Total number of adverse reactions reported | 164 | 141 | |
| Mean per participant (SD) | 1.28 (1.39) | 1.03 (1.34) | 0.136 |
| Side effect - no of patients reporting this | |||
| Diarrhoea | 29 (21.8%) | 34 (24.8%) | 0.330 |
| Nausea | 34 (25.6%) | 18 (13.1%) | 0.007 |
| Severe cramping | 20 (15.0%) | 15 (10.9%) | 0.207 |
| Upset stomach | 10 (7.5%) | 12 (8.8%) | 0.441 |
| Headache | 10 (7.5%) | 8 (5.8%) | 0.379 |
| Dizziness | 16 (12.0%) | 7 (5.1%) | 0.034 |
*Patients were asked to report any adverse reaction they noticed. The total number of reported adverse reactions is therefore higher than the number of patients reporting adverse reactions.
4. Discussion
The results of this trial show that, after the expectant management of EPL for one week, the sequential combination of mifepristone and misoprostol is superior to a treatment of a placebo and misoprostol for reaching a complete evacuation of the uterus within six weeks, with a number needed to treat (NNT) of 4•9. Additionally, this combined treatment leads to significantly fewer patients requiring additional surgical treatment. The Triple M Trial was halted prematurely because the highly significant results of the pre-planned interim analysis met the pre-defined stopping rule (see study protocol). There were no concerns regarding safety of the participants, as SAEs occurred more often in the placebo group receiving current standard treatment, and adverse reactions (although occurring more often in the mifepristone group) were mild. Our findings extend those from previous trials, thus adding to the existing body of evidence in favour of mifepristone pre-treatment [21,32].
Pre-treatment with mifepristone was previously shown to improve uterine evacuation in cases of first trimester medical abortion and after second or third trimester foetal death [17,18]. The results of the present study are also in accordance with previous studies by both our own and other research groups on the efficacy of mifepristone in the treatment of EPL [16,20,21]. Our retrospective study and pilot study (unpublished data) both revealed an advantage of the addition of mifepristone to the treatment with misoprostol [16], with NNTs of 8•4 and 3•5, respectively. Schreiber et al. recently performed a RCT, without one week of expectant management prior to inclusion, of a comparable size (N = 300), finding an advantage of pre-treatment with mifepristone, with a NNT of 6; [21] however, no placebo was given to the control group, thus introducing potential bias as stated by the Cochrane group in their most recent systematic review regarding medical treatment of early foetal death [23]. In their study, successful treatment was defined as the loss of the gestational sac by day four, with no additional treatment within 30 days. Sinha et al. performed a smaller (N = 92) RCT, again without one week of expectant management, showing an advantage of pre-treatment with mifepristone, with a NNT of 3•5 [20]. Successful treatment was defined as the loss of the gestational sac and a TED < 15 mm on day 14.
Until now, irrefutable evidence regarding the role of mifepristone pre-treatment has been lacking, which may have led to the different recommendations in various guidelines. The ACOG recently revised its guideline regarding EPL, and now recommends that clinicians consider the use of mifepristone prior to treatment with misoprostol [11]. The ACOG does not recommend an expectant policy for the first week [11], which is more common in European guidelines. The NICE guideline recommends an expectant policy for at least one week; [2] however, this recently updated guideline still advices against the use of mifepristone.
This study is unique in terms of optimal design, which is a key strength enabling us to draw firm conclusions about the effect of mifepristone on the outcomes. The risk of bias was minimised by randomisation using a computerised number table, sequentially numbered drug containers of identical appearance, and the use of visually identical placebo tablets as study medication for the blinding of participants and medical personnel.
A limitation of this trial might be the fact that our choices concerning inclusion criteria, route and dosages of medication, and the timing of follow-up are not applicable in all settings, as healthcare may be arranged differently, and circumstances may vary, in different countries. We will now elucidate the choices we made in designing our trial. The fact that women were only eligible for participation after at least one week of expectant management may be seen by some as a limitation of this study, as it is not recommended in all current guidelines. However, this can also be considered a strength, as it shows the effect of mifepristone in a group of women who are unlikely to spontaneously miscarry. As women who miscarry spontaneously are excluded by this week of expectant management, unnecessary treatment is prevented.
The dosage of mifepristone 600 mg is higher than the WHO-recommended 200 mg for medical abortions [33]. We followed the manufacturers recommendation regarding the dosage of mifepristone. Additionally, one could argue that non-viable pregnancies may require higher dosages of mifepristone after one week of expectant management, as they remain intra-uterine regardless of foetal demise. This might especially be the case for our population, with whom a week of expectative management had already been applied. Furthermore, a dosage of 600 mg mifepristone may not necessarily lead to more side effects compared to 200 mg. In fact, and surprisingly, the higher dosage of 600 mg has even been reported to cause significantly fewer side effects and less pain during the expulsion of the products of conception [34]. Both Sinha et al. and Schreiber et al. used 200 mg mifepristone, and report far higher frequencies of the most common patient-reported adverse reactions (up to 79•2% and 42•2%, respectively) [20,21], compared with the 25•6% frequency reported in this study. Future research in this particular population may provide clarity about the preferable dosage in relation to efficacy and adverse reactions.
Regarding misoprostol, many different treatment regimens are being used, as the superior regimen regarding dosage and route of administration for misoprostol remains unclear to date [23]. We chose the oral admission of 800 μg misoprostol, divided over two doses per day. A split dosage of misoprostol (two or three doses of 400 μg) has been reported to be similarly successful to a protocol using 800 μg at once [35]. As gastrointestinal side effects are dose- and interval-dependent, we chose a split dosage with the aim of reducing side effects as far as possible [36]. This also gives patients the option to omit the second dose of 400 μg if a strong reaction has occurred to the first dose. In addition, the oral route is more practical and might be preferred by patients, compared to the vaginal route, although evidence is conflicting [37,38]. The additional second-day administration of misoprostol is common practice in the Netherlands. This procedure is based on the self-assessment of loss of tissue by the prior-instructed patient. The administration of misoprostol on day four was thus not seen as a failure.
The primary outcome was determined as an ultrasonographically confirmed expulsion of the gestational sac, with an endometrial thickness of < 15 mm. Previous studies do not provide any clear evidence about which ultrasonographic endometrial thickness best corresponds to the presence of retained products of conception (RPOCs) [39,40]. Based on the results of their study, Lavecchia et al. concluded that a maximum anterior–posterior diameter of 15 mm or less meant that RPOCs were less likely to be confirmed histologically [26]. Several studies published in the last decade report the use of an endometrial thickness of <15 mm to assess the presence of RPOC [41], [42], [43]. This was also found in a recent systematic scoping review, investigating the assessment of complete uterine evacuation or the presence of RPOC [44]. The primary outcome was assessed at 6–8 weeks after the start of the treatment, which is a substantially longer follow-up period than the aforementioned studies. All participants were followed until compete evacuation was achieved. This longer follow-up period was chosen as it allows for a more reserved policy, preventing possible unnecessary additional interventions, as recently shown by the MisoREST study [45]. In this trial, Lemmers et al. studied the treatment of women with an endometrium of 10 mm or more on ultrasound one to two weeks after the treatment of EPL with misoprostol. These women were then randomised to undergo either expectant or surgical management. Their results show that an expectant policy is fully justifiable for at least six weeks, and was found to be just as safe as prompt uterine aspiration planned after two weeks, thus preventing surgery in 85% of these women. Additionally, this policy was preferred by most women, showing a significantly lower quality of life in the group receiving prompt uterine aspiration compared to women receiving expectant management [46]. It has been proven that patients who get to choose their preferred treatment have a better health-related quality of life [47]. As early pregnancy loss is an intense and distressing life event, it is of utmost importance to take patient preferences into account.
In addition to these considerations, it is important to realize that, in contrast to other countries, in the Netherlands a surgical uterine evacuation involves, although usually short stay, hospitalization of the patient. This will have led to a higher amount of SAE's in our trial compared to other trials, especially in the placebo group. If this fact is not taken into account it might lead to a distorted picture of the amount and ratio of SAE's in both study groups. The low rate of SAE's other than hospitalization for surgical uterine evacuation is comparable to that of other studies [20,21,32].
In spite of differences regarding treatment regimens, setting and arrangement of healthcare, our robust design ensures our results are generalizable to the general population. We included a broad range of patients suffering an EPL, in different settings regarding the kind of hospital patients are referred to, and applied current practice as the control group.
The growing evidence supporting mifepristone pre-treatment in EPL has important implications worldwide. Mifepristone was first introduced in France in 1987, and since the patent expired prices have already dropped drastically. Both mifepristone and misoprostol do not require special storage conditions and can be kept at room temperature, enabling a more widespread use in low-income countries.
More effective medical treatment of EPL after a period of expectant management, followed by a more conservative follow-up such as in this trial, may lead to fewer early and late complications, as (surgical) interventions can often be prevented.
In conclusion, this double-blind placebo-controlled RCT shows that pre-treatment with mifepristone prior to misoprostol significantly increases the number of patients with a complete evacuation of the uterus in the case of a medical treatment of EPL after one week of expectant management. The implementation of this proven safe regimen into (inter)national guidelines may lead to an extensive improvement in the medical treatment of EPL worldwide.
Declaration of Competing Interest
Dr. Hamel reports grants from Healthcare Insurers Innovation Foundation, during the conduct of the study; meant to cover costs of performing the trial, no involvement in any other aspect of the trial such as study design, data gathering/analysis, manuscript preparation. All other authors have nothing to declare.
Acknowledgments
Contributors
CH was the coordinating investigator. FV was the principal investigator. The idea for the study originally came from MS. FV, MS, SC, JB, and CH adapted the study protocol to its final form. JB contributed significantly to the execution of this study by coordinating the pilot study and acquiring funding. EH, RO, PK, AM, RL, PG, MR, EN, CS, IG, and BT were clinical principal investigators responsible for recruitment. CH and FV wrote the first draft of the manuscript. All authors contributed to approval of the final manuscript, and were all responsible for the decision to submit the manuscript.
Data sharing statement
Individual participant data that underlie the results reported in this article will be shared, after de-identification (text, tables, figures, and appendices). Additionally, the study protocol and statistical analysis plan will be made available. Data will be available beginning three months and ending five years following article publication. Data will be provided to researchers who provide a methodologically sound proposal, to achieve the aims stated in an approved proposal. Proposals should be directed to lotte.hamel@radboudumc.nl to gain access, data requestors will need to sign a data access agreement.
Funding
This study was funded by the Healthcare Insurers Innovation Foundation (project number: 3080 B15–191). In addition, departmental funds from the Department of Obstetrics and Gynaecology from both Radboud university medical centre and Canisius Wilhelmina Hospital, both Nijmegen, the Netherlands, were used.
References
- 1.Neilson J.P., Gyte G.M.L., Hickey M., Vazquez J.C., Dou L. John Wiley & Sons, Ltd; Chichester, UK: 2009. Medical treatments for incomplete miscarriage (less than 24 weeks) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NICE guideline Ectopic pregnancy and miscarriage: diagnosis and initial management. Natl Inst Heal Care Excell. 2019 https://www.nice.org.uk/guidance/ng126/chapter/Recommendations#symptoms-and-signs-of-ectopic-pregnancy-and-initial-assessment [Google Scholar]
- 3.Ammon Avalos L., Galindo C., Li D.K. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res Part A - Clin Mol Teratol. 2012;94:417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 4.Buck Louis G.M., Sapra K.J., Schisterman E.F. Lifestyle and pregnancy loss in a contemporary cohort of women recruited before conception: the LIFE Study. Fertil Steril. 2016;106:180–188. doi: 10.1016/j.fertnstert.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://data.guttmacher.org/. 2020.
- 6.Luise C., Jermy K., May C., Costello G., Collins W.P., Bourne T.H. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873–875. doi: 10.1136/bmj.324.7342.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieringa-de Waard M., Vos J., Bonsel G.J., Bindels P.J.E., Ankum W.M. Management of miscarriage: a randomized controlled trial of expectant management versus surgical evacuation. Hum Reprod. 2002;17:2445–2450. doi: 10.1093/humrep/17.9.2445. [DOI] [PubMed] [Google Scholar]
- 8.You J.H.S., Chung T.K.H. Expectant, medical or surgical treatment for spontaneous abortion in first trimester of pregnancy: a cost analysis. Hum Reprod. 2005;20:2873–2878. doi: 10.1093/humrep/dei163. [DOI] [PubMed] [Google Scholar]
- 9.Niinimäki M., Jouppila P., Martikainen H., Talvensaari-Mattila A. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367–372. doi: 10.1016/j.fertnstert.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 10.Lemmers M., Verschoor M.A.C., Hooker A.B. Dilatation and curettage increases the risk of subsequent preterm birth: a systematic review and meta-analysis. Hum Reprod. 2016;31:34–45. doi: 10.1093/humrep/dev274. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Clinical management guidelines for obstetrician – gynecologists. Obstet Gynecol. 2019;133:168–186. [Google Scholar]
- 12.Pfizer Limited. Cytotec UK SPC (Summary of Product Characteristics). https://www.medicines.org.uk/emc/product/1642/smpc.
- 13.Fernlund A., Jokubkiene L., Sladkevicius P., Valentin L. Misoprostol treatment vs expectant management in women with early non-viable pregnancy and vaginal bleeding: a pragmatic randomized controlled trial. Ultrasound Obstet Gynecol. 2018;51:24–32. doi: 10.1002/uog.18940. [DOI] [PubMed] [Google Scholar]
- 14.Bagratee J.S., Khullar V., Regan L., Moodley J., Kagoro H. A randomized controlled trial comparing medical and expectant management of first trimester miscarriage. Hum Reprod. 2004;19:266–271. doi: 10.1093/humrep/deh049. [DOI] [PubMed] [Google Scholar]
- 15.Graziosi G.C.M., Mol B.W.J., Reuwer P.J.H., Drogtrop A., Bruinse H.W. Misoprostol versus curettage in women with early pregnancy failure after initial expectant management: a randomized trial. Hum Reprod. 2004;19:1894–1899. doi: 10.1093/humrep/deh344. [DOI] [PubMed] [Google Scholar]
- 16.Van Den Berg J., Van Den Bent J.M., Snijders M.P., De Heus R., Coppus S.F., Vandenbussche F.P. Sequential use of mifepristone and misoprostol in treatment of early pregnancy failure appears more effective than misoprostol alone: a retrospective study. Eur J Obstet Gynecol Reprod Biol. 2014;183:16–19. doi: 10.1016/j.ejogrb.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Who; 2014. Clinical practice handbook for safe abortion; p. 64. [PubMed] [Google Scholar]
- 18.Exelgyn. Mifegyne 200mg tablets: summary of product characteristics (SmPC). https://www.medicines.org.uk/emc/product/3783/smpc.
- 19.Corey E.J., Czakó B., László K. Molecules and medicine. 2007.
- 20.Sinha P., Suneja A., Guleria K., Aggarwal R., Vaid N.B. Comparison of mifepristone followed by misoprostol with misoprostol alone for treatment of early pregnancy failure: a randomized double-blind placebo-controlled trial. J Obstet Gynecol India. 2018;68:39–44. doi: 10.1007/s13224-017-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber C.A., Creinin M.D., Atrio J., Sonalkar S., Ratcliffe S.J., Barnhart K.T. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161–2170. doi: 10.1056/NEJMoa1715726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Wattar B.H., Murugesu N., Tobias A., Zamora J., Khan K.S. Management of first-trimester miscarriage: a systematic review and network meta-analysis. Hum Reprod Update. 2019;25:362–374. doi: 10.1093/humupd/dmz002. [DOI] [PubMed] [Google Scholar]
- 23.Lemmers M., Verschoor M.A.C., Kim B.V. Medical treatment for early fetal death (Less than 24 weeks) Cochrane Database Syst Rev. 2019;2019 doi: 10.1002/14651858.CD002253.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Den Berg J., Hamel C.C., Snijders M.P., Coppus S.F., Vandenbussche F.P. Mifepristone and misoprostol versus misoprostol alone for uterine evacuation after early pregnancy failure: study protocol for a randomized double blinded placebo-controlled comparison (Triple M Trial) BMC Pregnancy Childbirth. 2019;19:1–8. doi: 10.1186/s12884-019-2497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbussche F.P.H.A., Klumper F.J.C.M. NVOG richtlijn Erytrocytenimmunisatie en zwangerschap. 2009.
- 26.Lavecchia M., Klam S., Abenhaim H.A. Effect of uterine cavity sonographic measurements on medical management failure in women with early pregnancy loss. J Ultrasound Med. 2016;35:1705–1710. doi: 10.7863/ultra.15.09063. [DOI] [PubMed] [Google Scholar]
- 27.Central Committee on Research Involving Human Subjects. Central committee on research involving human subjects. 2020. https://english.ccmo.nl/investigators/during-and-after-the-research/saes-susars-and-sades (accessed Dec 18, 2020).
- 28.Food and Drug Administration (FDA). CFR Code of Federal Regulations. 2019 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=170.3&SearchTerm=170.3.
- 29.Trinder J., Brocklehurst P., Porter R., Read M., Vyas S., Smith L. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (miscarriage treatment (MIST) trial) BMJ. 2006;332:1235–1240. doi: 10.1136/bmj.38828.593125.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen S., Hahlin M., Platz-Christensen J. Randomised trial comparing expectant with medical management for first trimester miscarriages. BJOG An Int J Obstet Gynaecol. 1999;106:804–807. doi: 10.1111/j.1471-0528.1999.tb08401.x. [DOI] [PubMed] [Google Scholar]
- 31.Stockheim D., Machtinger R., Wiser A. A randomized prospective study of misoprostol or mifepristone followed by misoprostol when needed for the treatment of women with early pregnancy failure. Fertil Steril. 2006;86:956–960. doi: 10.1016/j.fertnstert.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Chu J.J., Devall A.J., Beeson L.E. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)31788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Medical management of abortion. Geneva, 2018 https://apps.who.int/iris/bitstream/handle/10665/278968/9789241550406-eng.pdf. [PubMed]
- 34.Saurel-Cubizolles M.J., Opatowski M., David P., Bardy F., Dunbavand A. Pain during medical abortion: a multicenter study in France. Eur J Obstet Gynecol Reprod Biol. 2015;194:212–217. doi: 10.1016/j.ejogrb.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Kulier R., Kapp N., Gülmezoglu A.M., Hofmeyr G.J., Cheng L., Campana A. Medical methods for first trimester abortion. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD002855.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen R., O'Brien B.M. Uses of misoprostol in obstetrics and gynecology. Rev Obstet Gynecol. 2009;2:159–168. [PMC free article] [PubMed] [Google Scholar]
- 37.Arvidsson C., Hellborg M., Gemzell-Danielsson K. Preference and acceptability of oral versus vaginal administration of misoprostol in medical abortion with mifepristone. Eur J Obstet Gynecol Reprod Biol. 2005;123:87–91. doi: 10.1016/j.ejogrb.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Perriera L.K., Reeves M.F., Chen B.A., Hohmann H.L., Hayes J., Creinin M.D. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143–149. doi: 10.1016/j.contraception.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Rulin M.C., Bornstein S.G., Campbell J.D. The reliability of ultrasonography in the management of spontaneous abortion, clinically thought to be complete: a prospective study. Am J Obstet Gynecol. 1993;168:12–15. doi: 10.1016/s0002-9378(12)90877-7. [DOI] [PubMed] [Google Scholar]
- 40.Creinin M.D., Harwood B., Guido R.S., Fox M.C., Zhang J. Endometrial thickness after misoprostol use for early pregnancy failure. Int J Gynecol Obstet. 2004;86:22–26. doi: 10.1016/j.ijgo.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Ma'ani W., Solomayer E.F., Hammadeh M. Expectant versus surgical management of first-trimester miscarriage: a randomised controlled study. Arch Gynecol Obstet. 2014;289:1011–1015. doi: 10.1007/s00404-013-3088-1. [DOI] [PubMed] [Google Scholar]
- 42.Nadarajah R., Quek Y.S., Kuppannan K., Woon S.Y., Jeganathan R. A randomised controlled trial of expectant management versus surgical evacuation of early pregnancy loss. Eur J Obstet Gynecol Reprod Biol. 2014;178:35–41. doi: 10.1016/j.ejogrb.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Wijesinghe P.S., Padumadasa G.S., Palihawadana T.S., Marleen F.S. A trial of expectant management in incomplete miscarriage. Ceylon Med J. 2011;56:10–13. doi: 10.4038/cmj.v56i1.2888. [DOI] [PubMed] [Google Scholar]
- 44.Hamel C.C., van Wessel S., Carnegy A. Towards well-defined diagnostic criteria for retained products of conception (RPOC): a systematic scoping review. Manuscr Submitt Publ. 2020 [Google Scholar]
- 45.Lemmers M., Verschoor M.A.C., Oude Rengerink K. MisoREST: surgical versus expectant management in women with an incomplete evacuation of the uterus after misoprostol treatment for miscarriage: a cohort study. Eur J Obstet Gynecol Reprod Biol. 2017;211:83–89. doi: 10.1016/j.ejogrb.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Lemmers M., Verschoor M.A.C., Broekhuijsen K. Quality of life for curettage versus expectant management in women with incomplete evacuation of the uterus after treatment with misoprostol for miscarriage: the misorest trial. Hum Reprod. 2015;30 doi: 10.1093/humrep/30.Supplement-1.1. [DOI] [Google Scholar]
- 47.Wieringa-De Waard M., Hartman E.E., Ankum W.M., Reitsma J.B., Bindels P.J.E., Bonsel G.J. Expectant management versus surgical evacuation in first trimester miscarriage: health-related quality of life in randomized and non-randomized patients. Hum Reprod. 2002;17:1638–1642. doi: 10.1093/humrep/17.6.1638. [DOI] [PubMed] [Google Scholar]