Abstract
Objective:
To identify predictors of 30-day all-cause mortality for patients with cardiogenic shock secondary to acute coronary syndrome (ACS-CS) who require short-term mechanical circulatory support (ST-MCS).
Background:
ACS-CS mortality is high. ST-MCS is an attractive treatment option for hemodynamic support and stabilization of deteriorating patients. Mortality prediction modeling for ACS-CS patients requiring ST-MCS has not been well-defined.
Methods:
The Utah Cardiac Recovery (UCAR) Shock database was used to identify patients admitted with ACS-CS requiring ST-MCS devices between 05/2008 and 08/2018. Pre-ST-MCS clinical, laboratory, echocardiographic, and angiographic data were collected. The primary endpoint was 30-day all-cause mortality. A weighted score comprising of pre-ST-MCS variables independently associated with 30-day all-cause mortality was derived and internally validated.
Results:
A total of 159 patients (mean age, 61 years; 78% male) were included. Thirty-day all-cause mortality was 49%. Multivariable analysis resulted in four independent predictors of 30-day all-cause mortality: age, lactate, SCAI CS classification, and acute kidney injury. The model had good calibration and discrimination (area under the receiver operating characteristics curve 0.80). A predictive score (ranging 0–4) comprised of age ≥60 years, pre-ST-MCS lactate ≥2.5 mmol/L, AKI at time of ST-MCS implementation, and SCAI CS stage E effectively risk stratified our patient population.
Conclusion:
The ACS-MCS score is a simple and practical predictive score to risk-stratify CS secondary to ACS patients based on their mortality risk. Effective mortality risk assessment for ACS-CS patients could have implications on patient selection for available therapeutic strategy options.
Keywords: Cardiogenic Shock, Mechanical Circulatory Support, Acute Coronary Syndrome
Introduction
Cardiogenic shock (CS) is characterized by end-organ tissue hypoperfusion secondary to cardiac dysfunction and reduction in cardiac output (CO) (1,2). It is the most common cause of mortality in patients with acute coronary syndrome (ACS) and it complicates around 5–10% of all ACS cases (2–4). Despite improvement in ACS mortality rates with the routine use of revascularization therapy, overall ACS-CS 30-day mortality has remained high, ranging between 40–50% for the past two decades (3,5,6).
Besides the use of revascularization therapy and intra-aortic balloon pump (IABP), CS has been historically managed with vasoactive and inotropic agents which have been associated with increased myocardial oxygen consumption (7).The use of short-term mechanical circulatory support (ST-MCS) has been increasingly utilized in the treatment of CS for hemodynamic support, avoidance of escalation of vasoactive agents to toxically high doses, and stabilization of progressively deteriorating patients (8). Recent studies have shown that early initiation of MCS is associated with improved survival (9,10). However, so far there has been no significant evidence of acute mortality benefit with the use of ST-MCS in patients with CS (3,6,11–14). Moreover, ST-MCS devices carry a significant risk of complications such as bleeding events, cerebrovascular accidents (CVA), limb ischemia, and infections (7).
Despite the high mortality rates associated with ACS-CS and the increased utilization of ST-MCS devices in the treatment of ACS-CS, mortality prediction modelling for ACS-CS patients requiring ST-MCS has not been well-defined. Hence, we sought to identify predictors of 30-day all-cause mortality in patients with ACS-CS requiring ST-MCS and to develop a mortality prediction score by using pre-ST-MCS clinical variables.
Methods
This is a single-center, hybrid retrospective-prospective, observational study conducted at the University of Utah Health Sciences Center. The prospective part of the study was approved by the University of Utah’s Institutional Review Board (IRB_00080080) Utah Cardiac Recovery (UCAR) – Shock registry and the participants gave written informed consent. The retrospective part was approved with a waiver of informed consent by the IRB (IRB_00072747) Clinical Analyses in Cardiovascular Medicine.
Study population
The UCAR Shock database was used to identify consecutive patients admitted with ACS-CS requiring ST-MCS devices between 05/2008 and 08/2018.
The inclusion criteria were: i) Age ≥ 18 years, ii) ACS with CS, iii) Implementation of percutaneous ST-MCS device (selection of CS patients receiving ST-MCS was defined by the team of treating physicians, according to specific clinical indications and institutional practice, as described in Taleb, et al. (15)). Patients with CS secondary to non-ACS-related causes, including chronic heart failure exacerbation, acute myocarditis, acute allograft rejection, post-cardiotomy, or CS not otherwise specified were excluded. Patients who were in need of central extracorporeal membrane oxygenation support were also excluded.
Study data
Our institution’s database was queried for patient data collected at the pre-ST-MCS implantation time point. Baseline clinical data included patient’s age, gender, body mass index, prior medical history of hypertension, diabetes mellitus, tobacco use, hyperlipidemia, chronic obstructive lung disease, chronic kidney disease, atrial fibrillation, myocardial infarction, heart failure with reduced ejection fraction, and cerebrovascular accident (CVA). Other clinical data included need for cardiopulmonary resuscitation, need for mechanical ventilation, vasopressors use, ST-MCS device type, left anterior descending coronary artery involvement on coronary angiogram, and Society for Cardiovascular Angiography and Interventions (SCAI) classification of CS. Laboratory data included serum lactate, creatinine, and acid-base status as reflected by serum pH. Hemodynamic and echocardiographic data included systolic (SBP), diastolic, and mean arterial pressures (MAP), heart rate, cardiac index (CI), CO, pulmonary capillary wedge pressure (PCWP), systemic vascular resistance and left ventricular ejection fraction. Adverse events data collected included major bleeding, ischemic CVA, hemorrhagic CVA, transient ischemic attack, amputation, fasciotomy, vascular repair, and hemolysis.
Definitions
CS was defined using standard clinical and hemodynamic criteria, as previously described in other shock studies and major clinical trials:
SBP <90 mmHg or MAP <50 mmHg for >30 minutes or need for vasoactive agents infusion to maintain a SBP >90 mmHg or MAP >50 mmHg, and
one of the following: i) PCWP or left ventricular end diastolic pressure (LVEDP) >15 mmHg and CI <2.2 L/min/m2 despite optimal medical management including pressors and inotropes, ii) Clinical or radiological signs of pulmonary edema, iii) Impaired end-organ perfusion defined as: altered mental status; cold clammy skin and extremities; oliguria with urine output of <30 ml/hour.
ACS-CS was defined as CS with concomitant obstructive coronary artery disease with ≥70% stenosis in at least one major epicardial coronary artery, as evidenced during the cardiac catheterization, and positive myocardial biomarkers indicative of acute myocardial damage.
Acute kidney injury (AKI) was defined as an increase in serum creatinine by ≥0.3 mg/dL within 48 hours, or an increase to ≥1.5 times the baseline value, or a decrease in urine volume to <0.5 mL/Kg/hour over six hours since shock admission (16).
SCAI stages were defined as per description by Baran et al(5): stage A “At-risk”: patients who are at risk for CS, stage B “Beginning”: patients with evidence of hemodynamic instability (e.g. hypotension and tachycardia) but no evidence of hypoperfusion, stage C “Classic”: patients with evidence of hypoperfusion who require interventions beyond fluid resuscitation (e.g. inotropic and vasopressor medications, and mechanical circulatory support), stage D “Deteriorating”: patients failing to improve or stabilize despite appropriate initial therapy or escalation of therapy, and stage E “Extremis”: patient with circulatory collapse with on-going cardiopulmonary resuscitation or who are being supported with multiple simultaneous acute interventions.
Statistical methods:
The primary end point was 30-day all-cause mortality after enrollment. Patients’ baseline characteristics were summarized using standard summary statistics, including frequencies, percentages and means. Measures of variation were presented as the mean ± standard deviation. Comparison of categorical variables was performed using the chi-squared test or Fisher’s exact test as appropriate. Comparison between continuous variables was performed using two-group Student’s t-tests.
Univariable predictors of 30-days mortality were identified using logistic regression analysis that included pre-ST-MCS clinical, echocardiographic, hemodynamic, and laboratory variables, and the SCAI CS classification. For the development of the multivariable model, predictors significant at the p<0.20 level in unadjusted analyses were considered for inclusion, as were variables suggested to be significant based on previous studies. Missing data were imputed using the multivariate imputation by chained equations method of multiple multivariate imputation (17–19). Variables with >50% missing data were excluded from model consideration, and no relevant variables had >10% missing data. Collinearity among candidate variables was assessed using correlation, and variable inflation factor and tolerance analysis.
With the ultimate goal of creating a clinical predictive score, continuous and ordinal variables were dichotomized. Cutoff points were chosen such that they would best distinguish between 30-day mortality and survival, and if possible, agree with cutoff values reported in the literature. No dichotomized variables were entered into the model unless the original continuous/ordinal variables had already shown to be independent predictors of 30-day mortality.
Derivation and validation of the risk score:
Once variables associated with 30-day mortality were identified, a bootstrap inclusion fraction (BIF) was calculated for each potential variable, defined as the percentage of times that each variable would be retained in the model as a significant predictor in a large number of bootstrap resample, in which the variable selection is repeated (20,21). Variables with BIFs <50% were dropped from the model as unreliable, as these would not likely remain significant predictors in external data sets. The final prediction model was then internally validated with bootstrapping, allowing for the use of the entire study group to validate the model. We again performed internal validation of the predictive model using k-fold cross validation by dividing the original data set into five groups for receiver operating characteristic (ROC) curve comparison of the predictive model. To test calibration of the model, the goodness of fit of the model was confirmed using the Hosmer-Lemeshow test. We assigned weighted points to the predictive factors identified by multivariable analysis based on the β regression coefficient value (rounded to the nearest integer). A prediction score was calculated for each patient, ranging from 0–4. This score was then collapsed into a three-level risk stratification: low risk (ACS-MCS score 0–1), intermediate risk (ACS-MCS score 2), and high-risk (ACS-MCS score 3–4). The probabilities for the score 0–4 came from fitting the logistic regression model with 30-day all-cause mortality as the outcome and the prediction score as a continuous predictor. The probabilities of the three-level stratification came from fitting the logistic regression model with 30-day all-cause mortality as the outcome and the three-level stratification as a continuous predictor.
A Kaplan-Meier curve for the occurrence of all-cause mortality within the 30-day follow-up period stratified by risk score was plotted. A log-rank test was used to examine time to all-cause mortality differences by risk stratification.
A p value of <0.05 was considered statistically significant, and all reported p values were 2-tailed. All analyses were performed using STATA 16 (StataCorp, College Station, TX, USA).
Results
A total of 159 patients received ST-MCS for the treatment of ACS-CS at the University of Utah Medical Center, between May 2008 and August 2018. Table 1 shows the baseline characteristics of all patients included. The mean age was 61 ± 13 years and 78% of the patients were male. The etiology of ACS-CS was ST-elevation myocardial infarction in 120 patients (75.5%) and non-ST-elevation myocardial infarction in 39 patients (24.5%). The types of ST-MCS used were Intra-Aortic Balloon Pump (IABP) in 88 patients (55.4%), Impella device in 26 patients (16.4%, - Impella CP in 21 patients, Impella 2.5 in 3 patients, and Impella RP in 1 patient), and peripheral Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) in 7 patients (4.4%). Thirty-eight patients (23.9%) received a combination of ST-MCS devices. The mean duration of intensive care unit stay was 10 ± 16 days and the mean duration for total hospital stay was 13 ± 19 days. Thirty-day all-cause mortality, the primary outcome of our study, was 49% (n=78 patients).
Table 1:
Patient characteristics
| Variable | 30-day Survivors (n=81) | 30-day Non-Survivors (n=78) | p-value |
|---|---|---|---|
| Age, years | 58 ± 13 | 63 ± 13 | 0.006 |
| Male sex, n (%) | 62 (76.5) | 63 (80.7) | 0.52 |
| BMI, kg/m2 | 30.8 ± 6.6 | 30.4 ± 6.0 | 0.68 |
| Previous Medical History | |||
| Hypertension, n (%) | 40 (49.4) | 41 (52.5) | 0.69 |
| Diabetes Mellitus, n (%) | 27 (33.3) | 28 (35.9) | 0.74 |
| Hyperlipidemia, n (%) | 32 (39.5) | 29 (37.2) | 0.76 |
| Tobacco use, n (%) | 38 (46.9) | 32 (41.0) | 0.46 |
| Chronic obstructive lung disease, n (%) | 7 (8.6) | 6 (7.7) | 0.83 |
| Chronic kidney disease, n (%) | 6 (7.4) | 9 (11.5) | 0.37 |
| Heart failure with reduced ejection fraction, n (%) | 9 (11.1) | 10 (12.8) | 0.74 |
| Atrial fibrillation, n (%) | 7 (8.6) | 10 (12.8) | 0.39 |
| Prior myocardial infarction, n (%) | 12 (14.8) | 18 (23.1) | 0.18 |
| History of stroke, n (%) | 5 (6.2) | 3 (3.9) | 0.50 |
| SCAI CS Classification | |||
| SCAI Stage C | 27 (33.3) | 11 (14.1) | |
| SCAI Stage D | 31 (38.3) | 20 (25.6) | |
| SCAI Stage E | 23 (28.4) | 47 (60.3) | < 0.001 |
| Additional Therapies on Admission to Hospital | |||
| CPR prior to presentation, n (%) | 30 (37.0) | 44 (56.4) | 0.01 |
| Mechanical ventilation, n (%) | 52 (64.2) | 59 (75.6) | 0.12 |
| Total number of vasoactive agents | 1.0 ± 1.0 | 1.5 ± 1.3 | 0.01 |
| Pre-MCS hemodynamic data | |||
| MAP, (mmHg) | 78.9 ± 19.8 | 72.0 ± 15.36 | 0.01 |
| Heart rate, (bpm) | 99.4 ± 24.6 | 101.9 ± 26.20 | 0.58 |
| Right atrial pressure, (mmHg) | 16.6 ± 10.1 (n=62) | 16.0 ± 5.11 (n=55) | 0.78 |
| Pulmonary capillary wedge pressure, (mmHg) | 24.8 ± 9.9 (n=62) | 23.7 ± 8.42 (n=55) | 0.56 |
| Systemic vascular resistance, | 1444.9 ± 670.0 (n=62) | 1379.8 ± 577.8 (n=55) | 0.71 |
| CO (L/min) | 4.7 ± 3.6 (n=62) | 4.1 ± 2.3 (n=55) | 0.37 |
| CI, (L/min/m2) | 2.3 ± 1.6 (n=62) | 2.0 ± 1.2 (n=55) | 0.31 |
| Left ventricular EF, % | 28.0 ± 15.5 | 29.6 ± 13.3 | 0.60 |
| Pre-MCS biochemical data | |||
| Lactic acid level, (mg/dl) | 3.8 ± 3.2 | 7.6 ± 5.0 | < 0.001 |
| pH | 7.2 ± 0.2 | 7.2 ± 0.2 | 0.12 |
| Pre-MCS end-organ damage | |||
| AKI, n (%) | 25 (32.9) | 46 (65.7) | < 0.001 |
| Abnormal liver function, n (%) | 45 (69.2) | 43 (72.9) | 0.66 |
| Anoxic brain injury, n (%) | 3 (3.7) | 20 (25.6) | < 0.001 |
| MCS type | |||
| IABP, n (%) | 42 (51.9) | 46 (59.0) | |
| Impella, n (%) | 14 (17.3) | 12 (15.4) | |
| VA-ECMO, n (%) | 2 (2.5) | 5 (6.4) | |
| Combination of MCS devices, n (%) | 23 (28.4) | 15 (19.2) | 0.37 |
| Outcomes | |||
| Total MCS duration (hours) | 125.5 ± 173.5 | 75.7 ± 102.5 | 0.03 |
| Intensive care unit stay, (days) | 14.8 ± 20.8 | 5.26 ± 5.3 | < 0.001 |
| Total hospital stay, (days) | 20.67 ± 23.7 | 5.65 ± 5.6 | < 0.001 |
| Adverse Events | |||
| Major bleeding | 27 (33.3) | 22 (28.2) | 0.48 |
| Ischemic CVA | 9 (11.1) | 4 (5.1) | 0.25 |
| Hemorrhagic CVA | 1 (1.2) | 2 (2.6) | 0.62 |
| TIA | 0 | 0 | |
| Amputation | 2 (2.5) | 1 (1.3) | 0.99 |
| Fasciotomy | 3 (3.7) | 0 | 0.25 |
| Vascular repair | 4 (5.0) | 3 (3.9) | 0.99 |
| Hemolysis | 6 (7.4) | 2 (2.6) | 0.28 |
BMI: Body mass index; CI: Cardiac index; CO: Cardiac output; CPR: cardiopulmonary resuscitation; CS: Cardiogenic shock; CVA: Cerebrovascular accident; IABP: Intra-aortic balloon pump; MAP: Mean arterial pressure; MCS: Mechanical circulatory support; SCAI: Society for Cardiovascular Angiography and Interventions; TIA: transient ischemic attack; VA-ECMO: Veno-arterial extracorporeal membrane oxygenation.
Factors Associated with Increased Mortality:
An exploratory univariable logistic regression analysis was performed in thirty potential clinical, laboratory, hemodynamic, and echocardiographic covariates shown to impact the incidence of 30-day all-cause mortality in ACS-CS patients (Table 2). Twelve covariates statistically significant at the p<=0.20 level (Table 2), as well as variables that are known to have a clinical association with 30-day all-cause mortality were evaluated as potential confounders in the multivariable model.
Table 2:
Exploratory univariable analysis of baseline demographic and clinical characteristics
| Covariate | Univariable analysis OR (95% CI) | p-value |
|---|---|---|
| Age (per 1-y increase) | 1.035 (1.009–1.062) | 0.008 |
| Age >60 years | 2.103 (1.116–3.962) | 0.021 |
| Male sex | 1.287 (0.601–2.759) | 0.52 |
| BMI (per 1-Kg/m2 unit increase) | 0.989 (0.939–1.042) | 0.68 |
| Medical History | ||
| Hypertension | 1.136 (0.609–2.116) | 0.69 |
| Diabetes Mellitus | 1.120 (0.584–2.154) | 0.73 |
| Hyperlipidemia | 0.906 (0.478–1.718) | 0.76 |
| Tobacco use | 0.787 (0.420–1.475) | 0.46 |
| Chronic obstructive lung disease | 0.881 (0.282–2.748) | 0.83 |
| Chronic kidney disease | 1.630 (0.552–4.818) | 0.38 |
| Heart failure with reduced ejection fraction | 1.176 (0.451–3.071) | 0.74 |
| Atrial fibrillation | 1.555 (0.560–4.313) | 0.40 |
| Prior myocardial infarction | 1.725 (0.769–3.871) | 0.19 |
| History of stroke | 0.608 (0.140–2.635) | 0.51 |
| SCAI Classification E vs. other classes | 3.823 (1.971–7.416) | <0.001 |
| Additional Therapies on Admission | ||
| CPR pre-MCS (yes vs no) | 2.2 (1.165–4.153) | 0.015 |
| Pre-MCS intubation (yes vs no) | 1.732 (0.870–3.446) | 0.118 |
| Number of pressors pre-MCS (per 1 medication increase) | 1.422 (1.072–1.886) | 0.015 |
| Hemodynamics | ||
| MAP | 0.977 (0.956–0.999) | 0.040 |
| Heart rate | 1.004 (0.990–1.017) | 0.58 |
| Right atrial pressure | 0.991 (0.933–1.053) | 0.77 |
| Pulmonary capillary wedge pressure | 0.987 (0.946–1.030) | 0.56 |
| Systemic vascular resistance | 0.999 (0.999–1.000) | 0.70 |
| CO | 0.929 (0.790–1.093) | 0.37 |
| CI | 0.838 (0.592–1.186) | 0.32 |
| Left ventricular ejection fraction | 1.007 (0.978–1.036) | 0.59 |
| Pre-MCS biochemical data | ||
| Lactic acid | 1.251 (1.121–1.397) | <0.001 |
| pH | 0.147 (0.012–1.745) | 0.13 |
| Pre-MCS End Organ Damage | ||
| Abnormal liver function | 1.194 (0.548–2.603) | 0.66 |
| AKI | 3.777 (1.958–7.287) | <0.001 |
| Anoxic brain injury | 8.966 (2.543–31.613) | 0.001 |
BMI: Body mass index; CI: Cardiac index; CO: Cardiac output; CPR: cardiopulmonary resuscitation; CS: Cardiogenic shock; CVA: Cerebrovascular accident; IABP: Intra-aortic balloon pump; MAP: Mean arterial pressure; MCS: Mechanical circulatory support; SCAI: Society for Cardiovascular Angiography and Interventions; TIA: transient ischemic attack; VA-ECMO: Veno-arterial extracorporeal membrane oxygenation.
The ACS-MCS mortality predictive score:
After application of the described methods of variable selection, multivariable analysis showed the variables: age, lactate, acute kidney injury, and SCAI classification, to be significant independent predictors of 30-day mortality. After dichotomizing the variables, a model including age ≥60, lactate ≥2.5 mmol/L, SCAI CS stage E, and presence of AKI was derived (Table 3). Model performance was very good, with a discrimination area under the ROC curve of 0.80. The cross-validated mean AUC for the predictive model on 5-fold cross validation was 0.77 (95% CI: 0.68–0.81), and is the best estimate of future performance of the clinical prediction model.
Table 3:
Multivariable predictors included in the ACS-MCS mortality risk score.
| Variable | Odds Ratio (95% CI) | p-value | β-regression coefficient | Points |
|---|---|---|---|---|
| Age ≥ 60 | 2.66 (1.24–5.69) | 0.01 | 0.98 | 1 |
| Lactate ≥ 2.5 | 4.05 (1.72–9.53) | <0.01 | 1.40 | 1 |
| AKI | 3.15 (1.51–6.57) | <0.01 | 1.15 | 1 |
| SCAI stage E | 2.42 (1.10–5.32) | 0.03 | 0.88 | 1 |
AKI: Acute kidney injury; SCAI: Society for Cardiovascular Angiography and Interventions
The model also had good calibration, confirmed by the Hosmer-Lemeshow test (p=0.93), with the non-significant p-value indicating lack of evidence against goodness of fit.
The ACS-MCS predictive score, created as reported in the statistical methods section, ranged from 0 to 4. Performance of the predictive score was very good with a discrimination area under the ROC curve of 0.78 (Figure 1). The cross-validated mean AUC for the predictive model on 5-fold cross validation was 0.79 (95% CI: 0.66–0.83). The score also had good calibration confirmed by the Hosmer-Lemeshow test (p=0.98). The probabilities for the full score, and the average probabilities for the three-level risk stratification in shown in Table 4: low risk (ACS-MCS score 0–1), intermediate risk (ACS-MCS score 2), and high-risk (ACS-MCS score 3–4). The observed and predicted 30-day all-cause mortality rates were similar for each score grade and significantly increased with higher risk scores (Figure 2) (Table 4). A Kaplan-Meier curve for the occurrence of all-cause mortality within the 30-day follow-up period stratified by risk score (low, intermediate and high-risk) is shown in Figure 3.
Figure 1:

Performance of the ACS-MCS risk score on discriminating 30-day all-cause mortality. ROC: receiver operating characteristic.
Table 4:
Probabilities for the full ACS-MCS score, and the average probabilities for the three-level stratification: Low, Medium and High risk scores
| ACS-MCS Score | Observed % Mortality | Predicted Mortality Rate* | Risk Stratification | Observed % Mortality | Predicted Mortality Rate |
|---|---|---|---|---|---|
| 0 | 7.14% | 8.59% | Low (0–1) | 19.24% | 18.52% |
| 1 | 23.68% | 21.99% | |||
| 2 | 44.19% | 45.82% | Medium (2) | 44.19% | 45.89% |
| 3 | 72.34% | 71.73% | High (3–4) | 76.56% | 75.99% |
| 4 | 88.24% | 88.39% |
Rate expressed as a percentage for ease of comparison
Figure 2:

Predicted and observed 30-day all-cause mortality according to the ACS-MCS risk score.
Figure 3:

Kaplan-Meier curve for the occurrence of all-cause mortality within the 30-day follow-up period stratified by risk score: low risk (ACS-MCS score 0–1), intermediate risk (ACS-MCS score 2), and high-risk (ACS-MCS score 3–4).
Discussion
The main finding of our study is that despite the overall increased mortality associated with ACS-CS, individualized 30-day all-cause mortality in patients receiving ST-MCS can be predicted using a relatively simple predictive risk score. Our score had very good discrimination, robust to internal cross validation, and good calibration. Moreover, it is parsimonious, with only four variables, and easily calculated at the bedside in patients with ACS-CS.
Few studies have proposed risk scores for patients with CS (22–28). Some of these predictive algorithms were developed in the pre-percutaneous coronary interventions (PCI) era, such as the algorithm introduced by Hasdai et al. (24). Other scores such as the one proposed by Harjola et al.(27) included patients with CS regardless of specific shock etiology. Poss et al. derived a 30-day mortality risk-stratification score from data from the IABP-SHOCK II (Intra-aortic Balloon Pump in CS) trial and identified six variables associated with increased 30-day mortality: age >73, history of stroke; creatinine >1.5 mg/dl at admission, serum glucose >191 mg/dl at admission, arterial lactate >5 mmol/l at admission, and post-PCI “thrombolysis in myocardial infarction” (TIMI) flow grade <3 (28). We have identified several variables to predict 30-day all-cause mortality among ACS-CS patients requiring ST-MCS. Our final multivariate Cox regression model included age ≥60 years, pre-ST-MCS lactate ≥2.5 mmol/L, AKI at time of ST-MCS implementation, and SCAI CS stage E.
Age:
Despite an overall trend of improved mortality in elderly patients with CS with the introduction of revascularization therapies (29,30), older age has been recognized to be associated with worse outcomes (26,31). In addition, in everyday practice, advanced MCS therapies might be precluded in older patients. Our risk score shows that age is one of the variables contributing to increased mortality and thus should be weighted as such when considering individualized prognosis and further advanced therapies.
Serum lactate:
Elevated serum lactate levels have been found to predict short- (27,28) and long-term mortality (32) in patients with CS. It is thought to reflect systemic hypoperfusion, tissue hypoxia, and end-organ dysfunction. In CS patients with Impella devices, elevated serum lactate was found to be a predictor of 30-day mortality (33,34). In our study, the average serum lactate level was 7.58 mmol/L among non-survivors and 3.77 mmol/L among survivors. A serum lactate level of >2.5 mmol/L was a significant predictor of 30-day all-cause mortality (OR 4.05, 95% CI 1.72–9.53, p <0.01)
Acute kidney injury:
AKI is a common complication of CS (35,36). CS related-AKI is thought to be a result of reduced renal perfusion, secondary systemic hypoperfusion, and severe renal congestion (36,37). In the case of VA-ECMO or other MCS, other factors have been postulated to contribute to AKI development such as systemic inflammation, hypercoagulable state, hemolysis and hemoglobinuria, and ischemia/reperfusion-associated AKI secondary to rapid hemodynamic fluctuations induced by alterations in vasopressors/inotropes (38). AKI, as evidenced by creatinine levels according to the Kidney Disease Improving Global Outcomes guidelines, has been shown to be a strong and independent predictor of 90-day mortality in patients with CS (35).
Despite improvements in systemic perfusion with ST-MCS, evidence suggests that AKI affects as much as 60% of CS patients receiving ST-MCS (36). Importantly, severe AKI has been shown to be a significant predictor of in-hospital mortality and long-term mortality in CS patients requiring ST-MCS (36,37). In our study, 65.7% of patients who died at 30-days had AKI at the time of ST-MCS implementation. Early and intensive continuous veno-venous hemofiltration has been shown to be associated with better in-hospital and long-term survival in patients with AKI secondary to cardiac surgery post-operative shock (39). Thus, one can infer that early recognition of AKI in ACS-CS is crucial and could indicate the need for an earlier implementation of therapeutic strategies.
The SCAI classification of CS:
The SCAI developed a classification system for CS describing five stages of shock (A to E) based on clinical, biochemical, and hemodynamic parameters (5). Other studies have validated SCAI classification of CS in clinical practice (40,41). In a large retrospective study, comprised of 1,007 patients with CS (815 patients), of which 58% were secondary to ACS, or large myocardial infarction (192 patients), Schrage et al. showed that higher SCAI classification was significantly associated with increased 30-day mortality (40). In our study, 69.3% of patients who died at 30-days were at a SCAI CS stage E compared to only 28.4% of patients who survived. SCAI CS stage E describes patients with circulatory collapse requiring multiple interventions. Typically, patients with SCAI CS stage E have elevated lactate >5 and declining kidney function. Our findings support the application of the SCAI classification of CS, among other variables, in risk-stratifying patients based individualized mortality risk. Large prospective studies are needed to further validate the SCAI classification system of CS and to study the relationship between SCAI classification stages and ST-MCS use.
Management of ACS-CS patients is a continually evolving field of care, and the deployment of specialized “Shock Teams” is one approach being utilized to improve patient outcomes (15). However, not all centers managing AMI-CS patients have Shock Team (ST) strategies available. We applied our score in a subset of our patients treated by a ST approach, and a subset of patients treated without a ST approach and performed well in both groups, AUC=0.7539, and AUC=0.8103, respectively.
The ACS-MCS mortality predictive score is simple and is based on only four variables, which makes it convenient for the clinician in the acute setting. All four variables can be obtained from a proper clinical assessment and an arterial blood gas, including lactate and creatinine levels. The score could aid clinicians in personalizing CS treatment strategies based on individualized mortality risk (Figure 4). In our study, more patients in the survival group (28.4%) received a combination of ST-MCS devices compared to patients in the non-survival group (19.2%). Although no significant association between ST-MCS devices type and mortality was found in our study, we look forward to the results from ongoing randomized controlled trials assessing survival after ST-MCS compared to medical management alone. In addition, our mortality risk score can be used to risk-stratify patients with ACS-CS before the implementation of ST-MCS and this could help set realistic expectations for patients and their families and importantly allow the treating physicians to weigh all available therapeutic options.
Figure 4:
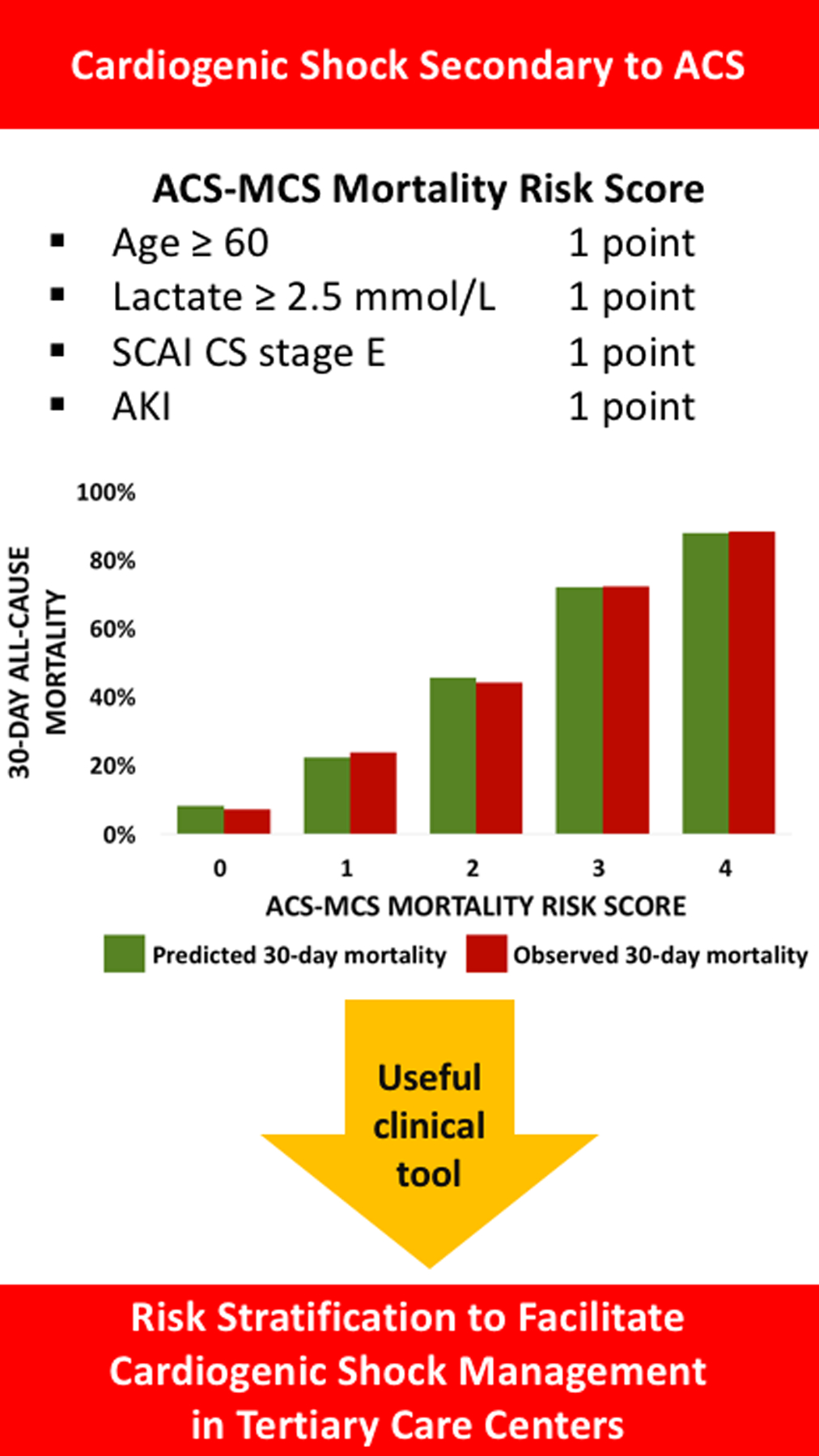
The ACS-MCS score as a useful tool in the contemporary management of cardiogenic shock (CS) secondary to acute coronary syndromes (ACS) in tertiary care centers. SCAI: Society for Cardiovascular Angiography and Interventions, AKI: Acute kidney injury.
Study limitations
The findings of this study have to be considered in light of some limitations. First, this is a partially retrospective study and thus is subject to limitations related to the study design. Second, we only included patients with ACS-CS and thus our results cannot be generalized to patients with CS secondary to non-ACS etiology. Third, the choice of ST-MCS device followed local protocols and left in the discretion of our Shock Team’s consensus (15), which might have introduced selection bias. Fourth, we did not perform an external validation using a dataset from another study site. Since our k-fold validation used the same dataset the model and prediction score were derived on, it was only an internal validation. Internal validations measure the reproducibility of the prediction model and prediction score’s accuracy, without directly validating their generalizability to new patients. However, since internal validity is required for external validity, it supports the generalizability of the model (42). Fifth, our score assigns a small mortality risk to patients with a score of zero, meaning that other risk factors not included in our model might impact mortality risk as well. Besides, the sample size does not allow subgrouping based on different device types and assessment of the outcomes and predictors for each subgroup. Despite the practicality and convenience of predicting mortality based on a scoring system composed of clinical and laboratory variables, clinical decision making is far more complicated and would take multiple medical and non-medical (e.g. social) aspects into consideration. Finally, our primary outcome was 30-day all-cause mortality and thus this score does not reflect long-term outcomes.
Conclusion
In conclusion, mortality rates vary among patients with ACS-CS who require ST-MCS. We propose a simple and practical predictive score to risk stratify patients based on their 30-day mortality risk. The proposed score could have significant clinical implications when managing patients with ACS-CS in terms of individualizing treatment strategies and predicting prognosis.
Sources of Funding:
Dr Drakos was supported by AHA Heart Failure Strategically Focused Research Network, 16SFRN29020000, NHLBI R01 HL135121-01, NHLBI R01 HL132067-01A1 and Nora Eccles Treadwell Foundation. Dr. Tonna is supported by a career development award (K23HL141596) from the National Heart, Lung, And Blood Institute (NHLBI) of the NIH. Dr. Taleb was supported by NHLBI T32HL007576.
Disclosure:
Dr. Welt has served on the advisory board of Medtronic. Dr Tonna has received modest support from LivaNova and Philips Healthcare as a speaking honorarium in relation to this work. Dr Drakos is a consultant to Abbott. All other authors report no conflicts.
Abbreviations:
- ACS
Acute coronary syndrome
- AKI
Acute kidney injury
- AUC
Area under the curve
- BIF
Bootstrap inclusion fraction
- CI
Cardiac index
- CO
Cardiac output
- CS
Cardiogenic shock
- CVA
Cerebrovascular accident
- IABP
Intra-aortic balloon pump
- IRB
Institutional review board
- MAP
Mean arterial pressure
- MCS
Mechanical circulatory support
- PCI
Percutaneous coronary intervention
- PCWP
Pulmonary capillary wedge pressure
- ROC
Receiver operating characteristic
- SBP
Systolic blood pressure
- SCAI
Society for Cardiovascular Angiography and Interventions
- ST-MCS
Short-Term Mechanical Circulatory Support
- VA-ECMO
Veno-arterial extracorporeal membrane oxygenation
References:
- 1.Hollenberg SM, Kavinsky CJ, Parrillo JE. Cardiogenic shock. Ann Intern Med 1999;131:47–59. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008;117:686–97. [DOI] [PubMed] [Google Scholar]
- 3.Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999;341:625–34. [DOI] [PubMed] [Google Scholar]
- 4.Vahdatpour C, Collins D, Goldberg S. Cardiogenic Shock. J Am Heart Assoc 2019;8:e011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baran DA, Grines CL, Bailey S et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 6.Schrage B, Ibrahim K, Loehn T et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019;139:1249–1258. [DOI] [PubMed] [Google Scholar]
- 7.Subramaniam AV, Barsness GW, Vallabhajosyula S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiol Ther 2019;8:211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal S, Sud K, Martin JM, Menon V. Trends in the Use of Mechanical Circulatory Support Devices in Patients Presenting With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv 2015;8:1772–4. [DOI] [PubMed] [Google Scholar]
- 9.Basir MB, Schreiber TL, Grines CL et al. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am J Cardiol 2017;119:845–851. [DOI] [PubMed] [Google Scholar]
- 10.Basir MB, Schreiber T, Dixon S et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv 2018;91:454–461. [DOI] [PubMed] [Google Scholar]
- 11.Ouweneel DM, Eriksen E, Sjauw KD et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol 2017;69:278–287. [DOI] [PubMed] [Google Scholar]
- 12.Thiele H, Zeymer U, Neumann FJ et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 13.Thiele H, Akin I, Sandri M et al. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N Engl J Med 2018;379:1699–1710. [DOI] [PubMed] [Google Scholar]
- 14.Rios SA, Bravo CA, Weinreich M et al. Meta-Analysis and Trial Sequential Analysis Comparing Percutaneous Ventricular Assist Devices Versus Intra-Aortic Balloon Pump During High-Risk Percutaneous Coronary Intervention or Cardiogenic Shock. Am J Cardiol 2018;122:1330–1338. [DOI] [PubMed] [Google Scholar]
- 15.Taleb I, Koliopoulou AG, Tandar A et al. Shock Team Approach in Refractory Cardiogenic Shock Requiring Short-Term Mechanical Circulatory Support: A Proof of Concept. Circulation 2019;140:98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681–94. [DOI] [PubMed] [Google Scholar]
- 18.Royston P Multiple imputation of missing values. Stata J 2004;4:227–241. [Google Scholar]
- 19.Royston P Multiple imputation of missing values: further update of ice, with an emphasis on categorical variables. Stata J 2009;9:466–477. [Google Scholar]
- 20.Royston P, Sauerbrei W. Bootstrap assessment of the stability of multivariable models. Stata J 2009;9:547–570. [Google Scholar]
- 21.Vittinghoff EG, Shiboski David V., McCulloch Stephen C., Charles E. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. New York: Springer, 2005:134. [Google Scholar]
- 22.Garcia-Alvarez A, Arzamendi D, Loma-Osorio P et al. Early risk stratification of patients with cardiogenic shock complicating acute myocardial infarction who undergo percutaneous coronary intervention. Am J Cardiol 2009;103:1073–7. [DOI] [PubMed] [Google Scholar]
- 23.Klein LW, Shaw RE, Krone RJ et al. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol 2005;96:35–41. [DOI] [PubMed] [Google Scholar]
- 24.Hasdai D, Holmes DR, Califf RM et al. Cardiogenic shock complicating acute myocardial infarction: predictors of death. GUSTO Investigators. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. Am Heart J 1999;138:21–31. [DOI] [PubMed] [Google Scholar]
- 25.Sutton AG, Finn P, Hall JA, Harcombe AA, Wright RA, de Belder MA. Predictors of outcome after percutaneous treatment for cardiogenic shock. Heart 2005;91:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sleeper LA, Reynolds HR, White HD, Webb JG, Dzavík V, Hochman JS. A severity scoring system for risk assessment of patients with cardiogenic shock: a report from the SHOCK Trial and Registry. Am Heart J 2010;160:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harjola VP, Lassus J, Sionis A et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015;17:501–9. [DOI] [PubMed] [Google Scholar]
- 28.Pöss J, Köster J, Fuernau G et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol 2017;69:1913–1920. [DOI] [PubMed] [Google Scholar]
- 29.Dauerman HL, Goldberg RJ, Malinski M, Yarzebski J, Lessard D, Gore JM. Outcomes and early revascularization for patients > or = 65 years of age with cardiogenic shock. Am J Cardiol 2001;87:844–8. [DOI] [PubMed] [Google Scholar]
- 30.Dauerman HL, Ryan TJ, Piper WD et al. Outcomes of percutaneous coronary intervention among elderly patients in cardiogenic shock: a multicenter, decade-long experience. J Invasive Cardiol 2003;15:380–4. [PubMed] [Google Scholar]
- 31.Dauerman HL, Goldberg RJ, White K et al. Revascularization, stenting, and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol 2002;90:838–42. [DOI] [PubMed] [Google Scholar]
- 32.Thiele H, Zeymer U, Neumann FJ et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638–45. [DOI] [PubMed] [Google Scholar]
- 33.Lauten A, Engström AE, Jung C et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail 2013;6:23–30. [DOI] [PubMed] [Google Scholar]
- 34.Rohm CL, Gadidov B, Leitson M, Ray HE, Prasad R. Predictors of Mortality and Outcomes of Acute Severe Cardiogenic Shock Treated with the Impella Device. Am J Cardiol 2019;124:499–504. [DOI] [PubMed] [Google Scholar]
- 35.Tarvasmäki T, Haapio M, Mebazaa A et al. Acute kidney injury in cardiogenic shock: definitions, incidence, haemodynamic alterations, and mortality. Eur J Heart Fail 2018;20:572–581. [DOI] [PubMed] [Google Scholar]
- 36.Abadeer AI, Kurlansky P, Chiuzan C et al. Importance of stratifying acute kidney injury in cardiogenic shock resuscitated with mechanical circulatory support therapy. J Thorac Cardiovasc Surg 2017;154:856–864.e4. [DOI] [PubMed] [Google Scholar]
- 37.Marenzi G, Assanelli E, Campodonico J et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med 2010;38:438–44. [DOI] [PubMed] [Google Scholar]
- 38.Askenazi DJ, Selewski DT, Paden ML et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 2012;7:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li SY, Yang WC, Chuang CL. Effect of early and intensive continuous venovenous hemofiltration on patients with cardiogenic shock and acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 2014;148:1628–33. [DOI] [PubMed] [Google Scholar]
- 40.Schrage B, Dabboura S, Yan I et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv 2020. [DOI] [PubMed] [Google Scholar]
- 41.Jentzer JC, van Diepen S, Barsness GW et al. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J Am Coll Cardiol 2019;74:2117–2128. [DOI] [PubMed] [Google Scholar]
- 42.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med 1999;130:515–24. [DOI] [PubMed] [Google Scholar]


