Abstract
BACKGROUND:
Rapid quantitative recovery of NK cells but slower recovery of T-cell subsets along with frequent viral infections are reported after umbilical cord blood (UCB) compared with matched sibling donor (MSD) hematopoietic cell transplantation (HCT). However, it remains unclear whether increased propensity for viral infections is also a result of slower recovery of virus-specific immunity after UCB as compared to MSD HCT.
OBJECTIVES:
We examined the differences in the function of virus-specific peripheral blood mononuclear cells (PBMC) after UCB (N=17) vs. MSD (N=9) using previously collected patient blood samples at various time points after HCT.
METHODS:
Interferon-gamma (IFN-γ) enzyme-linked immune absorbent spot (ELISpot) assay was used to quantify the PBMC frequencies that secrete IFN-γ in response to 11 immunopeptides from 5 common viruses. We included the patients who received the same reduced intensity conditioning regimen without ATG, no systemic glucocorticoids and had no relapse or acute/chronic graft-versus-host disease within 1 year after HCT.
RESULTS:
The CMV-reactive PBMC frequencies were higher in CMV seropositive vs. seronegative patients after HCT. Among CMV seropositive patients, the frequency of CMV-reactive PBMC was lower after UCB compared to MSD throughout one year of HCT. We observed no differences in virus-specific PBMC responses towards HHV6, EBV, BK, and adenovirus antigens between UCB and MSD.
CONCLUSION:
Our data demonstrate that the reconstitution of CMV-specific immunity is slower in CMV seropositive recipients of UCB vs. MSD HCT in contrast to other viruses which had similar recoveries. These study findings support implementation of more potent prophylactic strategies for preventing CMV reactivation in CMV seropositive patients receiving UCB HCT.
Keywords: umbilical cord blood (UCB), matched sibling donor (MSD), hematopoietic cell transplantation (HCT), IFN-γ ELISpot, immune reconstitution
INTRODUCTION
Delayed immune reconstitution is a serious complication after hematopoietic cell transplantation (HCT) that can result in increased mortality from serious infections.1-3 Cytomegalovirus (CMV) infection, in particular, remains one of the most fatal complications of HCT.4 We previously reported more rapid quantitative recovery of NK cells but slower recovery of T-cell (CD3+, CD8+, and CD4+) subsets along with higher frequency of viral infections after umbilical cord blood (UCB) compared with peripheral blood (PB) matched sibling donor (MSD) HCT.1 Increased viral infections after UCB HCT as compared to MSD HCT have been attributed to lack of transfer of memory immune cells from donor to recipient and delayed immune cell recovery after UCB HCT.2,5 However, prior studies focused on the quantitative recovery of immune cells after UCB compared to adult donor HCT.1,3 Thus, it remains unclear whether the increased propensity for viral infections in UCB as compared to T cell replete PB MSD recipients is primarily explained by the slower recovery of immune cell numbers or also by the slower recovery of virus-specific immune cell function among PB mononuclear cells (PBMC). We therefore examined virus-specific immune cell recovery on previously collected blood samples at scheduled time points after UCB and PB MSD HCT.
METHODS
We analyzed 107 PB specimens from 26 adults that were prospectively collected for immune monitoring at days 60, 100, 180, and 360 after HCT. All study patients provided written informed consent prior to HCT and this study was approved by the University of Minnesota Institutional Review Board. In order to have a homogeneous patient population and to avoid factors affecting immune reconstitution we included only those patients who received the same reduced intensity conditioning (RIC) regimen without ATG, no systemic glucocorticoids and had >95% CD3 and CD33 PB donor chimerisms by day 60, no relapse or acute/chronic graft-versus-host disease (GVHD) within 1 year after HCT. Conditioning regimen consisted of fludarabine 150 mg/m2, cyclophosphamide 50 mg/kg and 200cGy total body irradiation, and GVHD prophylaxis of cyclosporine and mycophenolate mofetil.6 Recipients of UCB and MSD HCT received the same supportive care, including acyclovir dose of 800 mg 5 times a day for CMV seropositive (CMVpos) and 400 mg twice daily for CMV seronegative (CMVneg) patients through day 100 of HCT, then all received acyclovir 400 mg twice daily for at least one year after HCT.1,6,7
Interferon-gamma (IFN-γ) enzyme-linked immune absorbent spot assay (ELISpot) was performed using IFN-γ ELISpot kits (Bio-Techne Inc) to quantitate the frequency of PBMC that secrete IFN-γ in response to 11-mer overlapping immunopeptides: IE1 and pp65 for CMV, U90 for human herpesvirus 6 (HHV6), EBNA1, LMP2 and BZLF1 for Epstein-Barr virus (EBV), VP1 and large T for BK virus (BKV), and hexon and penton for adenovirus (HAdV3) (JPT Peptide Technologies, Gmbh). PBMC were thawed and kept overnight in L-Glutamine containing RPMI-1640 medium supplemented with 10% fetal bovine serum (Invitrogen) and 10 ng/mL human recombinant interleukin 2 (Bio-techne). The following day the PBMC were seeded at the 108 cells/L concentration into duplicate wells of 96-well plate (200 microL volume), pulsed with peptides (concentration adjusted per JPT manufacturer recommendations), and incubated for 24 hours. Stimulation with the recombinant CD3/CD28 antibodies (100 ng/mL, Bio-Techne), lipopolysaccharides (10 ng/mL, Sigma-Aldrich) served as positive controls, while the dimethyl sulfoxide (0.01%) (Sigma-Aldrich) served as a negative control. Plates were developed according to the manufacturer’s instruction (Bio-Techne), scanned on Cellular Technology Limited (CTL) instrument, and the images were analyzed using SmartCount™ software (ImmunoSpot).
Immune reconstitution parameters after UCB and MSD HCT were summarized by median (95% CI, range) at each time point after HCT. Wilcoxon Rank Sum test and Kruskal-Wallis test for nonparametric data were used for the comparison between the groups. The cut-off significance level for all p-values was 0.05. All statistical analyses were implemented using Statistical Analysis System software version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
We examined the differences in quantitative and virus-specific immunity after UCB (N=17) versus PB MSD (N=9) after HCT for hematological malignancies. The recovery of blood NK cells was more rapid and particularly pronounced after UCB compared to MSD at day 100 and day 180 after HCT (Table). In contrast, when comparing T-cells after UCB versus MSD at day 60 after HCT, we observed lower median absolute counts (x106/L) of blood CD3+ (208.8 vs. 408.3, p=0.03), CD8+ (39.1 vs. 112.1, p=0.02) and CD4+ (136.1 vs. 264.1; p=0.11). In subgroup analyses, the recovery of absolute T-cell counts was not significantly different in CMVpos versus CMVneg recipients when UCB and MSD cohorts were examined separately (data not shown).
Table.
Absolute immune cell count recovery after UCB vs. MSD HCT
| Immune cell type | UCB N=17 |
MSD N=9 |
p-value |
|---|---|---|---|
| Median number x106/L (range) | |||
| Day 60 after HCT | |||
| NK cells | 238.7 (99.6-1002.0) | 196.5 (74.4-306.9) | 0.08 |
| CD3+ T cells | 208.8 (0.6-930.0) | 408.3 (263.2-3279.6) | 0.03 |
| CD8+ T cells | 39.1 (0.0-144.3) | 112.1 (37.9-350.9) | 0.02 |
| CD4+ T cells | 136.1 (0.1-791.4) | 264.1 (182.4-2794.2) | 0.11 |
| Day 100 after HCT | |||
| NK cells | 306.0 (167.2-1221.7) | 114.9 (18.1-277.2) | <0.01 |
| CD3+ T cells | 266.8 (17.3-729.0) | 452.9 (50.7-2324.4) | 0.14 |
| CD8+ T cells | 57.6 (3.2-437.4) | 223.7 (20.5-358.3) | 0.10 |
| CD4+ T cells | 230.4 (3.6-370.0) | 278.4 (18.0-1994.3) | 0.22 |
| Day 180 after HCT | |||
| NK cells | 300.6 (135.3-1400.0) | 111.6 (19.8-218.0) | <0.01 |
| CD3+ T cells | 447.2 (181.9-3792.9) | 546.3 (118.4-1375.6) | 0.86 |
| CD8+ T cells | 166.3 (14.9-2693.0) | 140.1 (46.3-521.4) | 0.79 |
| CD4+ T cells | 285.0 (131.4-834.4) | 293.8 (67.8-740.1) | 0.86 |
| Day 365 after HCT | |||
| NK cells | 252.0 (133.3-1014.0) | 188.3 (114.4-548.1) | 0.44 |
| CD3+ T cells | 624.0 (321.9-1951.7) | 760.1 (191.8-804.3) | 0.44 |
| CD8+ T cells | 242.0 (34.8-1362.3) | 342.8 (63.3-423.1) | 0.95 |
| CD4+ T cells | 326.9 (166.3-926.3) | 328.2 (115.3-335.2) | 0.51 |
UCB, umbilical cord blood. MSD, matched sibling donor. HCT, hematopoietic cell transplantation.
Among CMVpos patients, there were 4 cases of CMV reactivation out of 10 UCB and none out of 4 MSD. The frequencies of CMV-reactive PBMC were higher in CMVpos as compared to CMVneg patients (Figure). However, among CMVpos patients, particularly in those with CMV reactivation, the frequency of CMV-reactive PBMC was lower after UCB compared with MSD throughout one year of HCT.
Figure. Virus-specific immune reconstitution after UCB vs. MSD HCT by CMV serostatus.
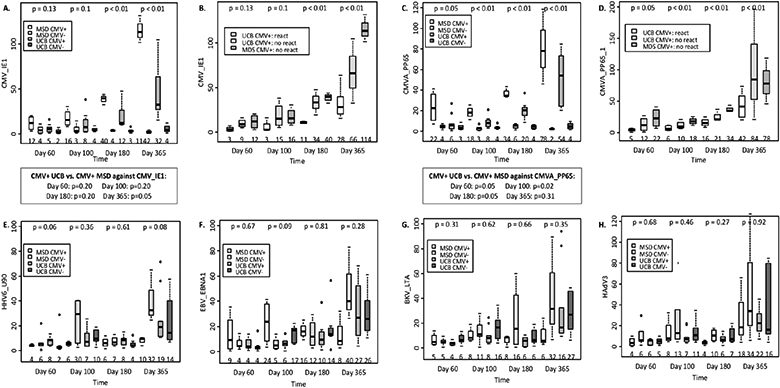
The boxes show the interquartile range of spot-forming cell count (SFC) per 100,000 PBMC specific to each viral antigen by MSD vs. UCB donor types and by CMV serostatus. The bold horizontal lines inside the boxes and the numbers at the bottom of each panel indicate the median SFC per 100,000 PBMC; p-values on top compare the donor type/CMV serostatus groups. The whiskers represent 1.53 the height of the box (or minimum/maximum values if there is no value in that range); and the circles indicate the outliers. The individual figures show the SFC specific to (A) CMV_IE1 peptide by donor type and CMV serostatus; (B) CMV_IE1 peptide in CMV seropositive patients by CMV reactivation status; (C) CMVA_PP65 peptide by donor type and CMV serostatus; (D) CMVA_PP65 peptide in CMV seropositive patients by CMV reactivation status; (E) HHV6_U90 by donor type and CMV serostatus; (F) EBV_EBNA1 peptide by donor type and CMV serostatus, the results against EBV_LMP2 and EBV_BZLF1 peptides were similar to EBV_EBNA1 peptide (not shown); (G) BKV_LTA peptide by donor type and CMV serostatus, the results against BKV_VP1 peptide was similar to BKV_LTA (data not shown); and (H) HAdV3 peptide by donor type and CMV serostatus, the results against HAdV5 peptide was similar to HAdV3 (data not shown).
Other viral events included 6 HHV6 and 1 adenovirus reactivation in UCB recipients, and 1 EBV reactivation in MSD recipients. We detected no differences in virus-specific PBMC responses towards HHV6, EBV, BK and adenovirus antigens between UCB and MSD recipients throughout one year of HCT.
DISCUSSION
Our data suggest that, despite more rapid quantitative recovery of NK cells after UCB HCT, reconstitution of CMV-specific adaptive immunity is slower within first year of HCT in CMVpos patients transplanted with UCB versus PB MSD. Thus, higher frequency of viral reactivations/infections after UCB HCT are explained not only by the slower recovery of T-cell numbers but also by the slower recovery of CMV-specific T-cell immunity. Relative protection from excessive viral infections in UCB recipients is likely explained by robust recovery of NK cells after UCB HCT.1-3 NK cells are among the earliest cell types to respond to latent viral reactivations via secretion of IFN-γ, among other cytokines.8 However, without additional signals from T cells (e.g. IL-2 and IL-12) IFN-γ production by NK cells remains suppressed.9 We observed longitudinal increase in IFN-γ spot-forming units toward CMV antigens over time, which is likely related to an improvement in communication between innate and adaptive immune cells as previously reported.10
Promising results against CMV infection have been reported with use of novel antiviral prophylactic agents11-13 and immunotherapy strategies.14-16 Emerging prevention strategies also include CMV peptide vaccines and virus-specific T-cell adoptive transfer.14-18 Poxvirus vectored triplex CMV vaccine administrated to CMV seropositive patients after HCT was recently reported to be effective in reducing the CMV viremia events.16 Similarly, adoptively transferred donor-derived multi-antigen- or CMV-specific cytotoxic T lymphocytes after HCT resulted in lower rates of CMV replication and tissue infection.15,19
Our study findings support the implementation of more effective antiviral prophylactic strategies in order to reduce the risk of viral infections and thereby improve survival in CMVpos patients receiving UCB HCT.
Highlights.
The CMV-reactive PBMC frequencies are higher in CMV seropositive vs. CMV seronegative patients after HCT.
Among CMV seropositive patients, the frequency of CMV-reactive PBMC is lower after UCB compared to MSD throughout one year of HCT.
We observed no differences in virus-specific PBMC responses towards HHV6, EBV, BK, and adenovirus antigens between UCB and MSD.
Acknowledgements:
This work was supported in part by the University of Minnesota BMT Program Marrow On The Move grant; National Institutes of Health, National Cancer Institute grant P01 CA65493 (C.G.B., J.S.M and J.E.W.); and P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota (Q.C.).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Bejanyan N, Brunstein CG, Cao Q, et al. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv 2018;2:909–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saliba RM, Rezvani K, Leen A, et al. General and Virus-Specific Immune Cell Reconstitution after Double Cord Blood Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015;21:1284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2012;18:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood 2013;122:3359–64. [DOI] [PubMed] [Google Scholar]
- 5.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy 2007;9:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejanyan N, Rogosheske J, DeFor T, et al. Higher Dose of Mycophenolate Mofetil Reduces Acute Graft-versus-Host Disease in Reduced-Intensity Conditioning Double Umbilical Cord Blood Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015;21:926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejanyan N, Rogosheske J, DeFor TE, et al. Sirolimus and Mycophenolate Mofetil as Calcineurin Inhibitor-Free Graft-versus-Host Disease Prophylaxis for Reduced-Intensity Conditioning Umbilical Cord Blood Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2016;22:2025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohei H, Kellampalli U, Vlasova-St. Louis I. Immune Reconstitution Disorders: Spotlight on Interferons. Int J Biomed Investig 2019;2:2581–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He XS, Draghi M, Mahmood K, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest 2004;114:1812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapp M, Wiedemann GM, Sun JC. Memory responses of innate lymphocytes and parallels with T cells. Semin Immunopathol 2018;40:343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, Gakhar N, MacDonald J, et al. Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone marrow transplantation 2020;55:780–6. [DOI] [PubMed] [Google Scholar]
- 12.Johnsrud JJ, Nguyen IT, Domingo W, Narasimhan B, Efron B, Brown JW. Letermovir Prophylaxis Decreases Burden of Cytomegalovirus (CMV) in Patients at High Risk for CMV Disease Following Hematopoietic Cell Transplant. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2020;26:1963–70. [DOI] [PubMed] [Google Scholar]
- 13.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. The New England journal of medicine 2017;377:2433–44. [DOI] [PubMed] [Google Scholar]
- 14.Hanley PJ, Cruz CR, Shpall EJ, Bollard CM. Improving clinical outcomes using adoptively transferred immune cells from umbilical cord blood. Cytotherapy 2010;12:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013;121:3745–58. [DOI] [PubMed] [Google Scholar]
- 16.Aldoss I, La Rosa C, Baden LR, et al. Poxvirus Vectored Cytomegalovirus Vaccine to Prevent Cytomegalovirus Viremia in Transplant Recipients: A Phase 2, Randomized Clinical Trial. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood 2009;114:1958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013;121:5113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roex MCJ, van Balen P, Germeroth L, et al. Generation and infusion of multi-antigen-specific T cells to prevent complications early after T-cell depleted allogeneic stem cell transplantation-a phase I/II study. Leukemia 2020;34:831–44. [DOI] [PubMed] [Google Scholar]


