Abstract
Objective
Pre-treatment with either fentanyl or midazolam has previously been used to prevent etomidate-induced myoclonus (EIM). The aim of the present study was to determine the effect of pre-treatment with a combination of midazolam and fentanyl in reducing the incidence and severity of EIM.
Methods
This prospective, randomised, double-blind study was conducted on 210 surgical patients allocated to three study groups. Group F patients received fentanyl 2 μg kg−1 and 5 mL saline. Group M patients received midazolam 0.03 mg kg−1 and 5 mL saline. Group FM patients received fentanyl 2 μg kg−1 plus midazolam 0.03 mg kg−1. The study drugs were administered intravenously over 30 s. Five minutes after study drug administration, etomidate 0.3 mg kg−1 was administered over 60 s. Patients were observed for 1 min for occurrence and severity of EIM.
Results
The incidence of EIM was 34/70 (48.6%), 55/70 (78.6%) and 11/70 (15.7%) in groups F, M and FM, respectively (p=0.001). Myoclonus of moderate or severe grade occurred in 23/70 (32.9%), 45/70 (64.3%) and 6/70 (8.6%) in groups F, M and FM, respectively (p=0.001). Patients who experienced myoclonus exhibited a significantly higher percentage change in post-induction heart rate (p=0.02), systolic blood pressure (p=0.001) and mean blood pressure (p=0.001) from pre-induction values than those who did not.
Conclusion
Pre-treatment with a combination of fentanyl and midazolam is more effective than that with fentanyl or midazolam alone in reducing the incidence and severity of EIM. Myoclonus is associated with a higher post-induction haemodynamic variation.
Keywords: Etomidate, fentanyl, midazolam, myoclonus
Introduction
Etomidate, a carboxylated imidazole derivative, is used as an IV anaesthetic induction agent. It has several advantages, such as a stable cardiovascular profile and minimal histamine release. However, its use is associated with bothersome myoclonic movements that can occur in 50%–80% of patients who do not receive pre-treatment prior to etomidate administration (1–4). This high incidence of etomidate-induced myoclonus (EIM) deters anaesthesiologists from using this drug. In addition, myoclonus may increase the risk of regurgitation and pulmonary aspiration in non-fasted patients (5, 6) and of vitreous prolapse in patients with open globe injury because of high intraocular pressure (7). Myoclonic movements may also interfere with patient monitoring by causing electrocardiogram (ECG) lead detachment (5).
Agents, such as opioids (3, 8, 9), benzodiazepines (1, 8), magnesium (10) and dexmedetomidine (11), have been used to decrease the incidence of EIM with variable results. Previous investigators have reported a decrease in the incidence of EIM to a level of 20%–25% with most of these pre-treatment agents (12). It has been suggested that the administration of both fentanyl and midazolam with etomidate induction may possibly eliminate EIM (13).
Pre-treatment with a combination of midazolam and fentanyl prior to the administration of etomidate is hypothesised to be better than that with either drug used alone in reducing the incidence and severity of EIM. The aim of this prospective, randomised, double-blind study was to compare the effect of pre-treatment with a combination of midazolam and fentanyl with either drug used alone on the incidence and severity of EIM in adult patients undergoing elective surgery under general anaesthesia.
Methods
This prospective, randomised, double-blind study was conducted on 210 American Society of Anaesthesiologists (ASA) physical status I and II adult patients, aged ≥18 years, undergoing elective surgery requiring general anaesthesia. The study was approved by the hospital ethics committee and DCGI. The study was registered with the Clinical Trials Registry-India (CTRI/2016/09/007324). Written informed consent was obtained from each patient.
Patients with allergy to benzodiazepines, opioids or etomidate with neuropsychological illness, seizure disorder, sepsis and a history of alcohol or drug abuse and those who had received analgesics or sedatives within the previous 24 h were excluded from the study. Patients on steroid medication or those who received steroids in the last 3 months and those with adrenocortical insufficiency were also excluded.
All patients fasted overnight and received oral alprazolam 0.25 mg/0.5 mg (<50 kg or >50 kg body weight) the night prior to surgery. No premedication was given on the day of surgery. In the operating room, standard monitoring (ECG, non-invasive blood pressure, peripheral oxygen saturation (SpO2) and end-tidal carbon dioxide) was established. An intravenous (IV) line was secured.
Patients were randomised to one of the three groups using a computer-generated random number table. Group assignments were concealed within opaque envelopes. Group F patients received IV fentanyl 2 μg kg−1 and 5 ml saline. Group M patients received IV midazolam 0.03 mg kg−1 and 5 mL saline. Group FM patients received IV fentanyl 2 μg kg−1 plus midazolam 0.03 mg kg−1. The study drugs were prepared by an anaesthesiologist not involved in the study and diluted to 5 mL with normal saline to ensure blinding.
Baseline heart rate (HR), systolic (SBP), diastolic (DBP), mean arterial pressure (MAP) and SpO2 were noted. The study drug was administered intravenously over 30 s. Occurrence of hypotension and bradycardia or any other side effect, if any, after injection of the study drug was recorded. Five minutes after study drug administration, degree of sedation using Ramsay Sedation Scale (14) was noted, and etomidate 0.3 mg kg−1 was administered over 60 s. Any complaint of pain on injection of etomidate was recorded. Time to loss of verbal contact and time to loss of eyelash reflex were noted. Lungs were ventilated with nitrous oxide 50% in oxygen via face mask for a period of 1 min during which time an anaesthesiologist, blind to the treatment groups, observed for occurrence of myoclonus. Time to onset of myoclonus from the start of etomidate injection was noted. The severity of myoclonus was graded clinically by a scoring system used by Doenicke et al. (2), with 0=no myoclonus; 1=mild myoclonus, slight movement of a body segment (a finger or shoulder); 2=moderate myoclonus, slight movement of two different muscles or muscle groups (face and leg) 3=severe myoclonus, intense clonic movement in two or more muscle groups (fast abduction of a limb). The site of myoclonus (upper limb, lower limb, chest, jaw and shoulder) was noted.
Difficulty in ventilating the lungs because of myoclonus and need for administration of neuromuscular blocking agent to facilitate ventilation was also recorded. In case of difficulty in ventilating the lungs because of myoclonus, neuromuscular blocking agent was administered to facilitate ventilation, and this was noted. Occurrence of apnoea, hiccups or eye movements (nystagmus) following etomidate administration was also noted.
After the 1-minute observation period for EIM, neuromuscular blockade was provided with vecuronium 0.1 mg kg−1. Fentanyl 2 μg kg−1 was given in group M for analgesia. The lungs were ventilated with isoflurane 0.6% and nitrous oxide 66% in oxygen, and the airway was secured by tracheal intubation or ProSeal Laryngeal Mask Airway (ProSeal LMA) placement at 3 min. Time taken for placement of ProSeal LMA or tracheal tube was noted.
Haemodynamic parameters (HR, SBP, DBP and MAP) were monitored at 1-minute intervals and recorded at pre-induction (5 min after study medication), post-induction, 3 min post-induction and 1 min, 3 min and 5 min after intubation. The highest values observed in haemodynamic parameters within 3 min of induction were also noted. The study period ended after 5 min of tracheal intubation or ProSeal LMA placement. Anaesthesia was maintained as per standard of care.
Sample size determination
Using data from a previous study (3), pre-treatment with fentanyl decreased the incidence of myoclonus from 60% in the control group to 33% in the fentanyl group. Assuming that the administration of a combination of fentanyl and midazolam will reduce the incidence of myoclonus further to 15% (approximately 50% decrease), the number of patients per group required to detect this difference is 69, considering 80% power and 5% alpha level with one-sided test. A total of 70 subjects per group were studied.
Statistical analysis
IBM Statistical Package for the Social Sciences version 22.0 statistical package (IBM SPSS Corp.; Armonk, NY, USA) was used for analysis. One-way analysis of variance with post hoc (Bonferroni) tests were applied to determine the significant mean among the three groups. Chi-square tests were applied to determine the association among these groups for demographic and clinical characteristics. Descriptive statistics were expressed as mean and standard deviations for interval variables and as frequency with percentages for categorical variables. A p-value 0.05 (two tailed) was considered as statistically significant.
Results
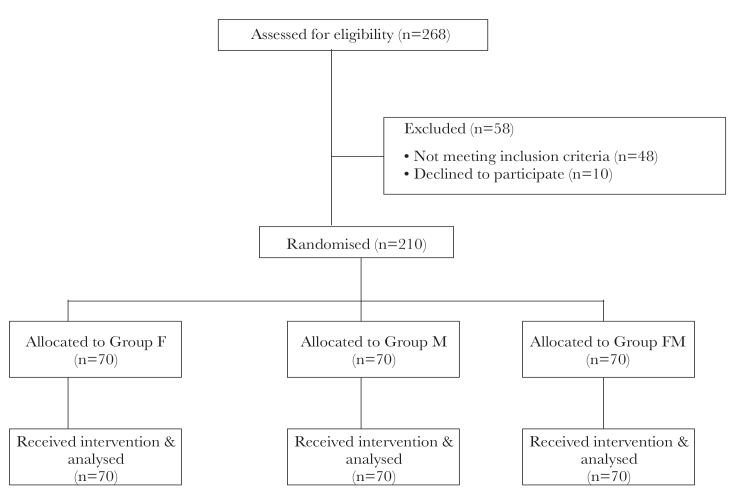
A total of 268 patients were assessed for eligibility. Of the 268 patients, 58 patients were excluded from the study (48 patients did not meet the inclusion criteria and 10 patients declined to participate). Finally, 210 patients were randomised into the three study groups, with 70 patients in each group (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram
Patient characteristics were comparable among the groups with regard to age, gender, ASA class, height, weight and BMI (Table 1). Following study drug administration, none of the patients developed bradycardia or hypotension in the three groups. Patients in group FM were significantly more sedated than patients in groups F and M (p=0.001). No patient experienced pain on injection of etomidate in our study. Difficulty in mask ventilation because of EIM (clenched jaw or chest wall rigidity) was observed in 6 (8.6%), 8 (11.4%) and 2 (2.9%) patients in groups F, M and FM, respectively (p=0.15). This necessitated neuromuscular blockade with vecuronium to facilitate lung ventilation.
Table 1.
Patient characteristics
| Parameter | Group F (n=70) | Group M (n=70) | Group FM (n=70) | p |
|---|---|---|---|---|
|
| ||||
| Age (year) | 37.04±12.40 | 38.44±10.86 | 36.81±13.04 | 0.69 |
| Gender (M/F) | 28/42 | 16/54 | 23/47 | 0.09 |
| ASA class I/II | 52/18 | 54/16 | 55/15 | 0.83 |
| Height (cm) | 1.60±0.10 | 1.58±0.09 | 1.58±0.09 | 0.18 |
| Weight (kg) | 61.44±13.22 | 57.64±8.91* | 58.11±11.25 | 0.10 |
| BMI (kg m−2) | 23.79±4.24 | 23.11±3.09 | 23.31±4.31 | 0.58 |
| LVC time (s) | 45.01±10.32 | 46.54±9.16 | 40.59±9.97*# | 0.001 |
| LOC time (s) | 54.43±10.0 | 55.83±9.33 | 46.93±10.27*# | 0.001 |
| TT/ProSeal LMA (n) | 31/39 | 24/46 | 30/40 | 0.43 |
| TT/ProSeal LMA time (s) | 10.79±1.93 | 10.79±2.78 | 10.44±1.69 | 0.56 |
Statistically significant (P≤0.05) when compared with group F.
Statistically significant (P≤0.05) when compared with group M. Values are expressed as mean±SD or numbers.
BMI: body mass index; ASA: American Society of Anaesthesiologists; LVC: time to loss of verbal command; LOC: time to loss of eyelash reflex; TT: tracheal tube; ProSeal LMA: ProSeal Laryngeal Mask Airway
Apnoea following etomidate administration was observed in 15 (21.4%), 1 (1.4%) and 20 (28.6%) patients in groups F, M and FM, respectively (p=0.001). Hiccups were observed in 2 (2.9%), 10 (14.3%) and 1 (1.4%) patients in groups F, M and FM, respectively (p=0.003). Nystagmus was observed in 25 (11.9%) out of 210 patients. Nystagmus was vertical in 17 (68%) and horizontal in 8 (32%) out of 25 patients. Position of the eyeball was central in 153 (72.8%), deviated upwards in 23 (10.9%) and deviated downwards in 34 (16.2%) out of 210 patients.
The incidence of EIM was significantly less in group FM (11/70 (15.7%)) than in group F (34/70 (48.6%)) (p=0.001) and group M (55/70 (78.6%)) (p=0.001); the difference between groups F and M was also significant (p=0.0004). Myoclonus of moderate or severe grade occurred in significantly fewer patients in group FM (8.6%) than those in group F (32.9%, p=0.007) and group M (64.3%, p=0.001); the difference between groups F and M was also significant (P=0.0003). There was no difference in time to onset of myoclonus between the three groups (p=0.62) (Table 2). The myoclonic movements were observed in the upper limb (88%), lower limb (65%), chest (17%), jaw (21%) and shoulder (4%). Haemodynamic changes (HR and mean blood pressure) at various timepoints in the three groups are shown in Figures 2 and 3. A higher percentage change in post-induction haemodynamic variables from pre-induction values was observed in patients who experienced myoclonus when compared to those who did not (Table 3). None of the patients showed any incidence of oxygen desaturation.
Table 2.
Myoclonus characteristics
| Parameter | Group F (n=70) | Group M (n=70) | Group FM (n=70) | p |
|---|---|---|---|---|
|
| ||||
| Incidence | 34 (48.6) | 55 (78.6) | 11 (15.7) | 0.001 |
| Onset time (s) | 101±40 | 96±36 | 107±31 | 0.62 |
| Severity grade | 0.001 | |||
| Grade 0 | 36 (51.4%) | 15 (21.4%) | 59 (84.3%) | |
| Grade 1 | 11 (15.7%) | 10 (14.3%) | 5 (7.1%) | |
| Grade 2 | 9 (12.9%) | 24 (34.3%) | 3 (4.3%) | |
| Grade 3 | 14 (20%) | 21 (30%) | 3 (4.3%) | |
Values are expressed as number (%) or mean±SD.
Figure 2.
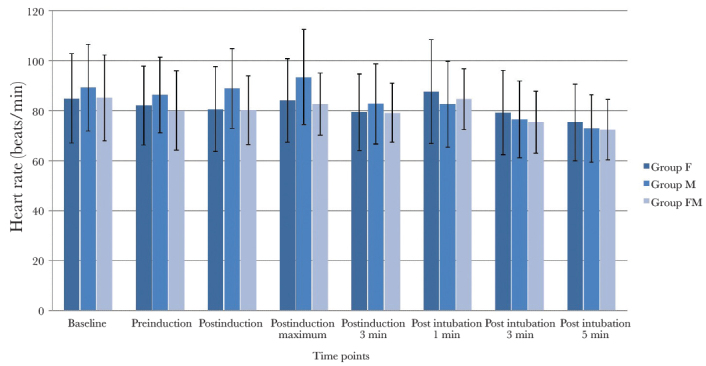
Heart rate changes at various time points in the three groups
Figure 3.
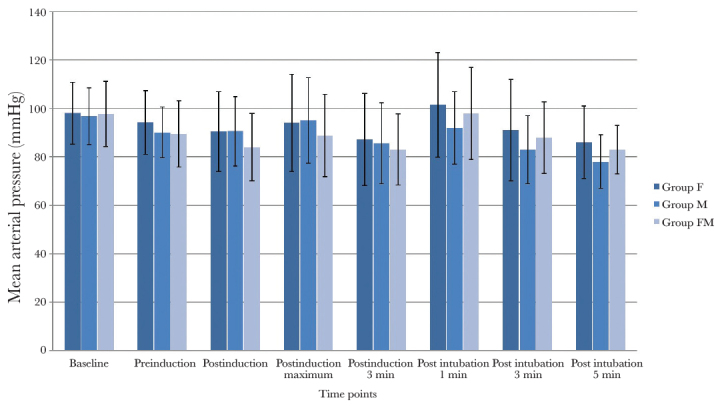
Mean arterial pressure changes at various time points in the three groups
Table 3.
Comparison of percentage change in post-induction haemodynamic variables from pre-induction values in patients who had myoclonus with those who did not have myoclonus in the entire study population
| Parameter | Myoclonus Present (n=100) | Myoclonus Absent (n=110) | p |
|---|---|---|---|
|
| |||
| Heart rate (beats min−1) | 8.8%±19.5 | 3.2%±14 | 0.02 |
| Systolic blood pressure (mmHg) | 5.0%±20.4 | −2.6%±11.4 | 0.001 |
| Diastolic blood pressure (mmHg) | 4.7%±19.2 | 0.30%±17.7 | 0.09 |
| Mean arterial pressure (mmHg) | 6.1%±19.5 | −2.0%±13.5 | 0.001 |
The percentage change is calculated as [(P ind mx–Pre-ind)/(Pre-ind)]*100, where P ind mx is the post-induction maximum value, and Pre-ind is the pre-induction value
Discussion
Etomidate administration is often associated with myoclonic movements that are not only bothersome but may also interfere with patient monitoring and have deleterious consequences as well. The exact mechanism of EIM is not clear. It has been suggested that large doses of etomidate depress cortical activity earlier than subcortical activity causing myoclonus (2). Pre-treatment with benzodiazepines or fentanyl, drugs that inhibit subcortical neuronal activity, may thus prevent EIM (2). Fentanyl and midazolam decrease the incidence of EIM by causing central nervous system inhibition, by their action on different receptors (15). Fentanyl decreases EIM by the stimulation of μ receptors on GABA-nergic neurons in the basal ganglia (2, 4, 6). Midazolam causes central nervous system inhibition, by its action on GABAA receptors (16).
The present data demonstrate that pre-treatment with a combination of fentanyl and midazolam is significantly more effective in reducing both the incidence and severity of EIM than that with either drug used alone. The incidence of myoclonus was 48.6%, 78.6% and 15.7% in patients receiving pre-treatment with fentanyl, midazolam and a combination of fentanyl and midazolam, respectively. Patients receiving fentanyl-midazolam combination experienced a significantly lower incidence of EIM severity grades 2 and 3 (8.6%) than patients receiving fentanyl (32.9%) or midazolam (64.3%).
Previous investigators also found a significantly lower incidence of EIM in patients receiving a combination of fentanyl and midazolam (25%) than those receiving fentanyl (40%) or midazolam (70%) as pre-treatment drugs (15). However, the incidence of EIM found in the fentanyl-midazolam combination group in their study was higher than that found in our study (25% vs. 15.7%). The lower incidence of EIM observed in our study is likely because of the larger doses of both fentanyl and midazolam used in the combination group in our study.
The role of midazolam pre-treatment has been investigated by researchers with conflicting reports on its efficacy in reducing EIM. A recent meta-analysis revealed that midazolam can effectively decrease the occurrence of EIM and lessen the severity of EIM (17). In some studies, the incidence of EIM following midazolam pre-treatment has been reported to range from 10% to 20% (1, 6, 8, 18). In contrast, the incidence of myoclonus in our study was 78.6% following pre-treatment with midazolam 0.03 mg kg−1 administered 5 min prior to etomidate. In addition, the ineffectiveness of midazolam in reducing EIM has been previously reported by other researchers. The incidence of EIM was 53%, 71.8% and 78% following pre-treatment with midazolam 0.02, 0.015 and 0.03 mg kg−1, respectively (13, 19, 20). The dose of midazolam administered in previous studies has varied from 0.015 to 0.05 mg kg−1 and has been administered 90–120 s prior to etomidate. In addition, etomidate has been administered over a period of 30–60 s. The rate of etomidate administration also affects the incidence of myoclonus (12). The differences in our results from those of previous authors might be related to differences in the dosage and timing of administration of pre-treatment agents, the rate of administration of etomidate and population characteristics.
Opioids have been demonstrated to decrease the incidence of myoclonus. Pre-treatment with fentanyl 2 μg kg−1 resulted in an incidence of EIM of 5.6% (18) and 6.7% (21). Fentanyl 100 μg administered 90 s prior to etomidate 0.3 mg kg−1 reduced the incidence of myoclonus to 24% compared to 82% in patients receiving saline (22). On the other hand, fentanyl 100 μg was found to be ineffective in decreasing EIM (23). Stockham et al. (3), using three doses of fentanyl, demonstrated that patients receiving fentanyl 500 μg experience apnoea but no myoclonus compared to patients receiving 100 μg fentanyl with 33% incidence of myoclonus.
Several agents have been investigated to reduce the incidence and severity of EIM with varied results. Dexmedetomidine (0.5 μg kg−1) and thiopental (1.0 mg kg−1) reduced the incidence of myoclonus from 64% to 34% and 36%, respectively (24). Magnesium sulphate (2.48 mmol) reduced the incidence of myoclonus from 72% in a placebo group to 24% (10). Lidocaine 20 mg reduced the incidence from 83.3% to 56.6% (25). The combined use of fentanyl and midazolam resulted in an EIM incidence of 15.7% in our study.
Although EIM is a temporary phenomenon, it can have serious consequences in patients with hypertension, aneurysms and coronary artery disease. Myoclonic muscle contractions increase myocardial oxygen consumption that further compromises patients with limited cardiovascular reserve (19). Patients with EIM were observed to have a higher HR and blood pressure than those with no myoclonus. Analysis of our data revealed that there was a significantly greater percentage change in post-induction haemodynamic variables (HR, SBP and MAP) from pre-induction values in patients who experienced myoclonus when compared to those who did not.
Nystagmus is one of the adverse effects of etomidate administration. In a study on the ocular effects of etomidate, the occurrence of low-frequency pendular nystagmus was observed in 7.14% of patients in the first 30 s after etomidate injection (26). In our study, nystagmus was observed in 25 (11.9%) out of 210 patients; nystagmus was vertical in 17 (68%) and horizontal in 8 (32%) out of 25 patients. The eyeball was central in 153 (72.9%), deviated upwards in 23 (11.0%) and deviated downwards in 34 (16.2%) out of 210 patients. In addition, upward deviation of the eyeball (Bell’s phenomenon) and downward deviation (“inverse” Bell’s phenomenon) were noted following etomidate administration in 8.57% and 10% of patients, respectively (26). Premedication with fentanyl has been reported to decrease EIM in a dose-dependent manner with an increased risk of apnoea (3). Apnoea was observed to occur in 21.4%, 1.4% and 28.6% in patients receiving fentanyl, midazolam and fentanyl-midazolam combination, respectively. In our study, no patient experienced pain on injection of etomidate. The replacement of the vehicle propylene glycol with a fat emulsion has almost eliminated the pain due to etomidate injection (2).
Our study has few limitations. First, we compared the effectiveness and safety of fentanyl and midazolam as pre-treatment drugs and did not include a “control group” as it was considered unethical to deviate from standard of care. Second, grading the severity of myoclonus was subjective as literature search did not reveal any other accurate and convenient monitoring indicator for EIM.
Conclusion
Pre-treatment with a combination of fentanyl and midazolam prior to etomidate administration is more effective in decreasing the incidence and severity of EIM during induction of general anaesthesia in adults than pre-treatment with fentanyl alone. Midazolam is ineffective in reducing myoclonus. Myoclonus is associated with a higher post-induction haemodynamic variation.
Acknowledgements
We gratefully acknowledge Prakhar Prakash (Systems Engineer, GE Healthcare, Waukesha, WI 53188, USA) for assistance in preparing figures for the manuscript.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of V.M.M.C & Safdarjung Hospital (No. IEC/SJH/VMMC/Project/January-2014/517 dated 14/7/2015).
Informed Consent: Written informed consent was obtained from all patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.P.; Design - S.P., P.M., P.V., V.T., R.S.; Supervision - S.P., P.M., V.T.; Resources - VMMC & Safdarjung Hospital Materials - VMMC & Safdarjung Hospital; Data Collection and/or Processing - S.P., P.M., P.V., V.T., R.S.; Analysis and/or Interpretation - S.P., P.M., P.V., V.T., R.S.; Literature Search - S.P., P.M., P.V., V.T., R.S.; Writing Manuscript - S.P., P.M.; Critical Review - S.P., P.M., P.V., V.T., R.S.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: All expenses were borne by Vardhman Mahavir Medical College and Safdarjung Hospital.
References
- 1.Schwarzkopf KR, Hueter L, Simon M, Fritz HG. Midazolam pretreatment reduces etomidate-induced myoclonic movements. Anaesth Intensive Care. 2003;31:18–20. doi: 10.1177/0310057X0303100103. [DOI] [PubMed] [Google Scholar]
- 2.Doenicke AW, Roizen MF, Kugler J, Kroll H, Foss J, Ostwald P. Reducing myoclonus after etomidate. Anesthesiology. 1999;90:113–9. doi: 10.1097/00000542-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Stockham RJ, Stanley TH, Pace NL, Gillmor S, Groen F, Hilkens P. Fentanyl pretreatment modifies anesthetic induction with etomidate. Anaesth Intensive Care. 1998;16:171–6. doi: 10.1177/0310057X8801600207. [DOI] [PubMed] [Google Scholar]
- 4.Giese JL, Stockham RJ, Stanley TH, Pace NL, Nelissen RH. Etomidate versus thiopental for induction of anesthesia. AnesthAnalg. 1985;64:871–6. doi: 10.1213/00000539-198509000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Van Keulen SG, Burton JH. Myoclonus associated with etomidate for ED procedural sedation and analgesia. Am J EmergMed. 2003;21:556–8. doi: 10.1016/j.ajem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Huter L, Schreiber T, Gugel M, Schwarzkopf K. Low-dose intravenous midazolam reduces etomidate-induced myoclonus: aprospective, randomized study in patients undergoing electivecardioversion. Anesth Analg. 2007;105:1298–302. doi: 10.1213/01.ane.0000287248.25610.c0. [DOI] [PubMed] [Google Scholar]
- 7.Berry JM, Merin RG. Etomidate myoclonus and the openglobe. Anesth Analg. 1989;69:256–9. doi: 10.1213/00000539-198908000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Hwang JY, Kim JH, Oh AY, Do SH, Jeon YT, Han SH. A comparison of midazolam with remifentanil for the prevention ofmyoclonic movements following etomidate injection. J Int MedRes. 2008;36:17–22. doi: 10.1177/147323000803600103. [DOI] [PubMed] [Google Scholar]
- 9.Khalil SN, Lawson KS, Hanis CL, Lemak NA, Ruiz RS. Alfentanil decreases myoclonus caused by etomidate. Middle East JAnesthesiol. 1999;15:185–92. [PubMed] [Google Scholar]
- 10.Guler A, Satilmis T, Akinci SB, Celebioglu B, Kanbak M. Magnesium sulphate pretreatment reduces myoclonus after etomidate. Anesth Analg. 2005;101:705–9. doi: 10.1213/01.ANE.0000160529.95019.E6. [DOI] [PubMed] [Google Scholar]
- 11.Dey S, Kumar M. Comparison of pretreatment with dexmedetomidine with midazolam for prevention of etomidate-inducedmyoclonus and attenuation of stress response at intubation: Arandomized controlled study. J Anaesthesiol Clin Pharmacol. 2018;34:94–8. doi: 10.4103/joacp.JOACP_297_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do SH, Han SH, Park SH, Kim JH, Hwang JY, Son IS, Kim MS. The effect of injection rate on etomidate-induced myoclonus. Korean J Anethesiol. 2008;55:305–7. doi: 10.4097/kjae.2008.55.3.305. [DOI] [Google Scholar]
- 13.Hodgson RE, Burrows RC. Midazolam and etomidate for induction of anaesthesia in ophthalmic surgery. South Afr J Anaesth Analg. 2002;8:4–7. doi: 10.1080/22201173.2002.10872966. [DOI] [Google Scholar]
- 14.Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alphaxolone-alphadalone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isitemiz I, Uzman S, Toptaş M, Vahapoglu A, Gül YG, Inal FY, et al. Prevention of etomidate-induced myoclonus: Whichis superior: Fentanyl, midazolam, or a combination? A Retrospective comparative study. Med Sci Monit. 2014;20:262–7. doi: 10.12659/MSM.889833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White PF, Eng MR. Intravenous anesthetics. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, editors. Clinical Anesthesia. Philadelphia: Lippincott Williams and Wilkins; 2009. pp. 444–65. [Google Scholar]
- 17.Zhou C, Zhu Y, Liu Z, Ruan L. Effect of pretreatment with midazolam on etomidate-induced myoclonus: A meta-analysis. J Int Med Res. 2017;45:399–406. doi: 10.1177/0300060516682882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khteishat B, Salaytah W, Qatawneh T, Alsagheer M. The effect of preinduction with midazolam, fentanyl, and ketamine on etomidate induced myoclonic muscle movements. J Royal Med Services. 2011;18:38–41. [Google Scholar]
- 19.Sedighinejad A, Naderi Nabi B, Haghighi M, Biazar G, Imanta-lab V, Rimaz S, et al. Comparison of the effects of low-dose mid-azolam, magnesium sulfate, remifentanil and low-dose etomidate on prevention of etomidate-induced myoclonus in orthopedic surgeries. Anesth Pain Med. 2016;6:e35333. doi: 10.5812/aapm.35333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasinwong W, Uakritdathikarn T, Kovitwanawong N, Pakam P. Prevention of etomidate-induced myoclonic movement af-ter midazolam co-induction with low-dose etomidate. Songkla Med J. 2011;29:1–9. [Google Scholar]
- 21.Yang YY, Choi SJ, Kim HJ, Son SC. The effect of midazolam, fentanyl and a small dose of etomidate for prevention of myoc-lonus during induction of anesthesia with etomidate. Korean J Anesthesiol. 2000;39:166–71. doi: 10.4097/kjae.2000.39.2.166. [DOI] [Google Scholar]
- 22.Zhao X, Bao R, Zhu J, Liu Z, Meng Y, Fan X, Li J. Pretreat-ment with butorphanol reduces myoclonus after etomidate. J Anesthesiol Clin Sci. 2013 doi: 10.7243/2049-9752-2-2. doi: 10.7243/2049-9752-2-2. Available from http://www.hoajon-line.com/journals/pdf/2049-9752-2-2.pdf. [DOI] [Google Scholar]
- 23.Fassoulaki A, Pateras C, Kaniaris P. Fentanyl in the prevention of etomidate-induced myoclonus. Cah Anesthesiol. 1987;35:201–2. [PubMed] [Google Scholar]
- 24.Mizrak A, Koruk S, Bilgi M, Kocamer B, Erkutlu I, Ganidagli S, et al. Pretreatment with dexmedetomidine or thiopental de-creases myoclonus after etomidate: A randomized, double blind controlled trial. J Surg Res. 2010;159:e11–6. doi: 10.1016/j.jss.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Gultop F, Akkaya T, Bedirli N, Gumus H. Lidocaine pretreat-ment reduces the frequency and severity of myoclonus induced by etomidate. J Anesth. 2010;24:300–2. doi: 10.1007/s00540-010-0869-6. [DOI] [PubMed] [Google Scholar]
- 26.Oji EO, Holdcroft A. The ocular effects of etomidate. Anaesthesia. 1979;34:245–9. doi: 10.1111/j.1365-2044.1979.tb06302.x. [DOI] [PubMed] [Google Scholar]