Abstract
Background and Purpose
The promotion of hair regeneration and growth heavily depends on the activation of Wnt/β‐catenin signalling in the hair follicle, including dermal papilla (DP). KY19382, one of the newly synthesized analogues of indirubin‐3′‐monoxime (I3O), was identified as a Wnt/β‐catenin signalling activator via inhibition of the interaction between CXXC‐type zinc finger protein 5 (CXXC5) and dishevelled (Dvl). Given the close relationship between the Wnt/β‐catenin signalling and hair regeneration, we investigated the effect of KY19382 on hair regrowth and hair follicle neogenesis.
Experimental Approach
In vitro hair induction effects of KY19382 were performed in human DP cells. The hair elongation effects of KY19382 were confirmed through the human hair follicle and vibrissa culture system. In vivo hair regeneration abilities of KY19382 were identified in three models: hair regrowth, wound‐induced hair follicle neogenesis (WIHN) and hair patch assays using C57BL/6 mice. The hair regeneration abilities were analysed by immunoblotting, alkaline phosphatase (ALP) and immunohistochemical staining.
Key Results
KY19382 activated Wnt/β‐catenin signalling and elevated expression of ALP and the proliferation marker PCNA in DP cells. KY19382 also increased hair length in ex vivo‐cultured mouse vibrissa and human hair follicles and induced hair regrowth in mice. Moreover, KY19382 significantly promoted the generation of de novo hair follicles as shown by WIHN and hair patch assays.
Conclusion and Implications
These results indicate that KY19382 is a potential therapeutic drug that exhibits effective hair regeneration ability via activation of the Wnt/β‐catenin signalling for alopecia treatments.
Keywords: CXXC5, dermal papilla cells, GSK‐3β, neogenesis, Wnt/β‐catenin signalling
Abbreviations
- ALP
alkaline phosphatase
- CXXC5
CXXC‐type zinc finger protein 5
- DP
dermal papilla
- Dvl
dishevelled
- GSK‐3β
glycogen synthase kinase‐3β
- H&E
haematoxylin and eosin
- I3O
indirubin‐3′‐monoxime
- IHC
immunohistochemistry
- MNX
minoxidil
- NBT/BCIP
nitro blue tetrazolium/5‐bromo‐4‐chloro‐3′‐indolyl phosphate
- pNPP
p‐nitrophenyl phosphate
- WIHN
wound‐induced hair follicle neogenesis
What is already known
Wnt/β‐catenin signalling plays an important role in hair regrowth and regeneration.
The CXXC‐type zinc finger protein CXXC5 inhibits hair regeneration by suppressing the Wnt/β‐catenin signalling pathway.
What does this study add
Novel low‐MW compound promoted hair regeneration by inhibiting GSK‐3β activity and CXXC5–Dvl interaction, simultaneously.
This compound induced HFSC activation and hair induction marker, unlike other hair growth‐promoting agents.
What is the clinical significance
Drugs enhancing hair follicle neogenesis are not available but are needed for alopecia treatment.
Dual targeting of GSK‐3β and CXXC5–Dvl interaction could be a new treatment for baldness.
1. INTRODUCTION
Recent reports show a significant increase in the number of people with alopecia (Jang et al., 2013). In the skin of patients with alopecia, many hair follicles overstay in their resting stage (Eckert et al., 1968; Pratt et al., 2017) in which dermal papilla (DP) cells and keratinocytes, the two key players of hair cycle regulation, are inactive (Alonso & Fuchs, 2006; Botchkarev & Kishimoto, 2003; Sennett & Rendl, 2012). Various factors cause the inactivation of these cells, and this inactivation leads to miniaturized hair follicles, resulting in hair loss (Kligman, 1959; Price, 1999). Even though researchers have focused on developing drugs to cure alopecia, only two drugs, minoxidil (MNX) and finasteride, have been approved by the US Food and Drug Administration for clinical treatment of androgenic alopecia (Libecco & Bergfeld, 2004; Linas & Nies, 1981; Price, 1999). Although both drugs effectively promote hair growth, neither can initiate hair follicle neogenesis effectively due to the complicated process of hair regeneration (Paus, 2006).
The Wnt/β‐catenin signalling pathway plays an important role in hair morphogenesis, growth initiation and regeneration (Andl et al., 2002; Huelsken et al., 2001; Ito et al., 2007; Kishimoto et al., 2000). The activated Wnt/β‐catenin signalling pathway initiates embryonic hair formation in the epidermis and promotes the formation of dermal condensates (Andl et al., 2002; Huelsken et al., 2001). In addition, DP cells require activation of this pathway to initiate hair‐inducing activity that prolongs the anagen phase (Kishimoto et al., 2000). The hair‐inducing ability of DP cells can be confirmed by analysing the expression of alkaline phosphatase (ALP) (Iida et al., 2007; Lee et al., 2012). Recent studies showed that the activation of Wnt/β‐catenin signalling could restore ALP expression in long‐term cultured primary DP cells (Yamauchi & Kurosaka, 2009).
Hair growth‐promoting drugs targeting Wnt/β‐catenin signalling are not currently available. However, there is a genuine need for low MW compounds that, unlike MNX, can activate this pathway to enhance hair follicle neogenesis (Lee et al., 2017).
KY19382 is one of the newly synthesized analogues of indirubin‐3′‐monoxime (I3O), a glycogen synthase kinase‐3β (GSK‐3β) inhibitor, and has a significant ability to activate Wnt/β‐catenin signalling (Choi et al., 2019). Indirubin is an active ingredient of the indigo plant, Danggui Longhui Wan, used as traditional anti‐leukaemia medicine (Xiao et al., 2002). I3O is known to be a Wnt/β‐catenin activator that stabilizes β‐catenin via inhibition of GSK‐3β and has been shown to accelerate bone growth and inhibit adipogenesis (Cha & Choi, 2014; Choi et al., 2014; Zahoor et al., 2014). Furthermore, we recently found that KY19382 elongated tibial length by inactivating GSK‐3β and blocking the binding of CXXC‐type zinc finger protein 5 (CXXC5) and dishevelled (Dvl) (Choi et al., 2019). Moreover, PTD‐DBM, a peptide that interferes with CXXC5–Dvl interaction via binding to the PDZ domain of Dvl, stimulated wound‐induced hair follicle neogenesis (WIHN) and hair regrowth (Lee et al., 2017). However, this peptide is limited for routine application due to its cost and stability.
In this study, we selected KY19382 as an optimal compound that can stimulate hair growth. KY19382 activated the Wnt/β‐catenin signalling more effectively than I3O and showed low cytotoxicity in human DP cells. Exposure to KY19382 elongated rodent vibrissa and human hair shaft and mouse dorsal hair. Moreover, KY19382 significantly induced the hair follicle neogenesis as shown in hairless mice injected with dermal cells and keratinocytes and wounded mice treated with KY19382. Overall, KY19382 is an effective Wnt/β‐catenin signalling activator that can be used for the treatment of alopecia with high efficacy and safety.
2. METHODS
2.1. Cell culture and reagents
Primary human DP cells are described in our previous study (Shin et al., 2010). Primary human DP cells from passages 2 to 7 were used in this study. The cells were cultured in low‐glucose DMEM (Hyclone, Pittsburgh, USA) supplemented with 10% FBS (Gibco, Gaithersburg, USA), 1% antibiotic–anti‐mycotic (Gibco, Gaithersburg, USA), 1‐ng·ml−1 bFGF (Peprotech, Princeton, USA) and 5‐μg·ml−1 insulin (Gibco, Gaithersburg, USA) at 37°C in a humidified atmosphere of 5% CO2.
A rat vibrissa immortalized DP cell line was donated by the Skin Research Institute of the Amore Pacific Corporation R&D Center (Yongin, Korea). HEK293 Wnt/β‐catenin signalling reporter cell (HEK293 cells [ATCC Cat# CRL‐1573, RRID:CVCL_0045] containing a chromosomally integrated TOPflash reporter) is described in our previous study (Choi et al., 2019). Rat DP or HEK293 reporter cells were cultured in DMEM (Gibco, Gaithersburg, USA) containing 10% (v/v) FBS (Gibco, Gaithersburg, USA) and 100‐U·m−1 penicillin/streptomycin (Gibco, Gaithersburg, USA) at 37°C in a humidified atmosphere of 5% CO2. All compounds were dissolved in DMSO (Sigma‐Aldrich, St. Louis, USA), and the cells were incubated with 0.1% (v/v) DMSO or each compound in DMEM.
2.2. Cell viability assay
HEK293 reporter, human DP or rat DP cells were seeded in 24‐well plates and treated for 24 h for HEK293 reporter cells or for 48 h in DP cells with 0.1% (v/v) DMSO or 100‐μM minoxidil. The identically grown cells were also treated with KY19382 or I3O as the concentrations shown in Figures 1a, S1a and S2a. Cell viability was measured by the CellTiter‐Glo Luminescent Cell Viability Assay Kit (Promega, Madison, USA) as described in the manufacturer's instructions. The luminescence activities were measured using the FLUOstar OPTIMA luminometer (BMG Labtech, Offenburg, Germany).
FIGURE 1.
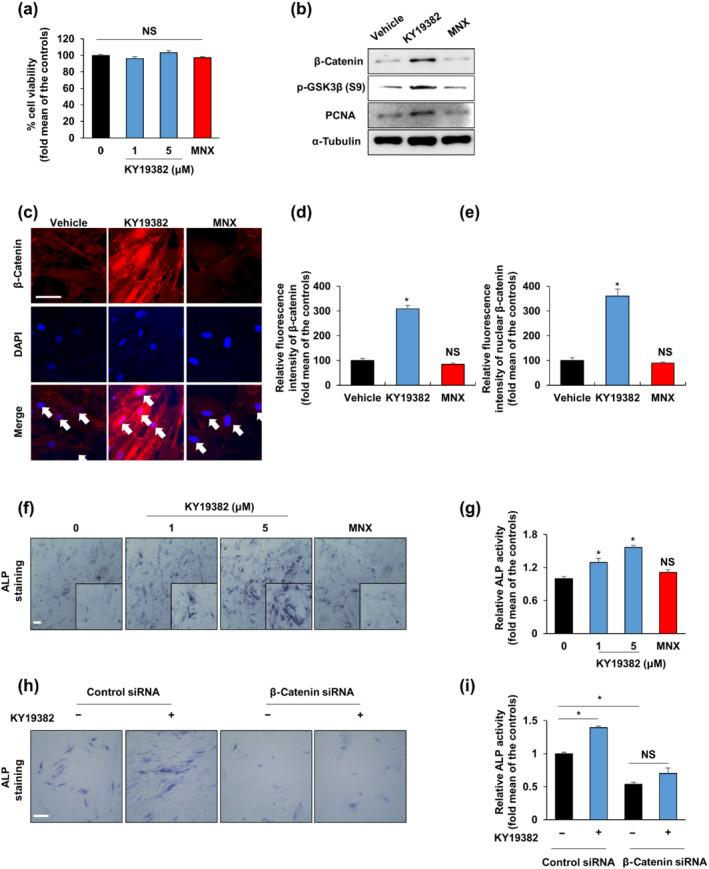
KY19382 increases hair induction activity via activation of the Wnt/β‐catenin pathway. (a) Cell viability of human dermal papilla (DP) cells treated with vehicle (0.1% [v/v] DMSO), 100‐μM minoxidil (MNX) or the shown concentrations of KY19382 for 48 h (n = 6). (b, c) Human DP cells were treated with vehicle (0.1% DMSO), 5‐μM KY19382 or 100‐μM minoxidil for 48 h. (b) Immunoblotting analyses for β‐catenin, α‐tubulin, p‐GSK3β and PCNA were analysed using human DP cells. (c) Immunocytochemical staining for β‐catenin. Nuclei were counterstained with DAPI. Arrows indicate nuclei. (d, e) Quantitative measurements of the total and nuclear β‐catenin intensities of human DP cells (n = 12 taken in three different images). (f, g) Human DP cells were treated with vehicle (0.1% DMSO), 100‐μM minoxidil or the marked concentrations of KY19382 for 48 h. (f) Alkaline phosphatase (ALP) staining and (g) ALP activity test (n = 5). (h, i) Human DP cells were transfected with β‐catenin siRNA or negative control for 12 h. After transfection, human DP cells were incubated with vehicle (0.1% DMSO) or 5‐μM KY19382 for 48 h. (h) Transfected cells were subjected to ALP staining. (i) ALP activity was quantified (n = 6). Scale bars = 100 μm. Values are expressed as means ± SEM. * P < .05, significantly different from vehicle, control or as indicated; NS, not significant
2.3. Luciferase reporter assay
HEK293 reporter cells were seeded in 24‐well plates, and the cells were treated for 24 h with the vehicle (0.1% (v/v) DMSO) or indicated concentrations of KY19382 or I3O (Figure 1a). The cells were washed with cold PBS and lysed in 55 μl of 1× lysis buffer (Promega, Madison, USA). The cell lysates were centrifuged at 15,920× g at 4°C for 15 min. Samples (30 μl) of each supernatant were transferred to 96‐well plates, and 15‐μl luciferin was added. The luciferase activity was measured at 485 nm using a FLUOstar OPTIMA luminometer (BMG Labtech, Offenburg, Germany).
2.4. Immunoblotting
The cells or tissues were washed twice with cold PBS and lysed in RIPA buffer (150‐mM NaCl; 10‐mM Tris, pH 7.2; 0.1% SDS; 1% Triton X‐100; 1% sodium deoxycholate; and 5‐mM EDTA). The lysates were centrifuged at 15,920× g at 4°C for 30 min. The equal amounts of protein (20 μg) were separated on 8–10% SDS‐PAGE gels and transferred onto Protran nitrocellulose membranes (Schleicher & Schuell Co., Keene, USA). Precision Plus Protein Standards (161–0373, Bio‐Rad, Hercules, USA) was used as an MW marker. After blocking with 5% skim milk for 1 h at room temperature, each membrane was blotted with the following primary antibodies: mouse anti‐β‐catenin (BD Biosciences Cat# 610154, RRID:AB_397555; 1:3000, Lexington, USA), mouse anti‐α‐tubulin (Cell Signaling Technology Cat# 3873, RRID:AB_1904178, 1:4000, Danvers, USA), rabbit anti‐ERK1/2 (Cell Signaling Technology Cat# 9102, RRID:AB_330744, 1:1000, Danvers, USA), rabbit anti‐p‐GSK‐3β (S9) (Cell Signaling Technology Cat# 9336, RRID:AB_331405, 1:1000, Danvers, USA), mouse anti‐PCNA (Santa Cruz Biotechnology Cat# sc‐56, RRID:AB_628110; 1:500, Dallas, USA), rabbit anti‐Fgf9 (Abcam Cat# ab71395, RRID:AB_2103075, 1:1000, Cambridge, USA) and rabbit anti‐cytokeratin 17 (Abcam Cat# ab53707, RRID:AB_869865, 1:1000, Cambridge, USA) at 4°C overnight. Each membrane was blotted with HRP‐conjugated anti‐mouse (Cell Signaling Technology Cat# 7076, RRID:AB_330924, 1:3000, Danvers, USA) or anti‐rabbit (Bio‐Rad Cat# 1706515, RRID:AB_11125142, 1:3000, Hercules, USA) IgG secondary antibody. The dilutions of antibody were maintained at −20°C and reused up to five times. The blots were visualized using enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK) and a luminescent image analyzer (LAS‐4000; Fujifilm, Tokyo, Japan). The Immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018).
2.5. Immunocytochemistry
Human or rat DP cells were seeded in a 12‐well plate on coverslips. The cells were incubated with 0.1% (v/v) DMSO as the vehicle, 1‐ or 5‐μM KY19382, or 100‐μM minoxidil for 48 h. Cultured cells were washed twice with cold PBS and were fixed in 4% paraformaldehyde (Wako, Osaka, Japan) or 10% formalin (Sigma‐Aldrich, St. Louis, USA) for 15 min at room temperature and then were washed with PBS and permeabilized with 0.2% Triton X‐100 for 15 min.
After blocking with 5% BSA in PBS for 30 min at room temperature, the cells were blotted with primary antibody: rabbit anti‐β‐catenin (Abcam Cat# 16051, RRID:AB_443301, 1:50, Cambridge, USA) overnight at 4°C. After washing with PBS, the cells were blotted with Alexa Fluor 488‐conjugated goat anti‐mouse antibody (Thermo Fisher Scientific Cat# A‐11001, RRID:AB_2534069, 1:300, Waltham, USA) or Alexa Fluor 555‐conjugated goat anti‐rabbit antibody (Thermo Fisher Scientific Cat# A‐21428, RRID:AB_2535849, 1:300, Waltham, USA) for 1 h at room temperature and counterstained with DAPI (Sigma‐Aldrich, St. Louis, USA) for 10 min at room temperature. Images were taken using an LSM510 confocal microscope (Carl Zeiss Inc., Germany). The fluorescence intensity was quantified using Zen software V3.1 software (ZEN Digital Imaging for Light Microscopy, RRID:SCR_013672, Germany).
2.6. ALP staining
For ALP staining in cells, human or rat DP cells were seeded in a 12‐well plate on coverslips. Cells were incubated with 0.1% (v/v) DMSO as the vehicle, 1‐ or 5‐μM KY19382, or 100‐μM minoxidil for 48 h and washed twice with cold PBS and were fixed in formalin for 15 min at room temperature. Then cells were incubated with nitro blue tetrazolium/5‐bromo‐4‐chloro‐3′‐indolyl phosphate solution (NBT/BCIP solution, Sigma‐Aldrich, St. Louis, USA). The reaction was stopped by washing with PBS. Dark blue staining was a positive signal for ALP.
For ALP staining of tissues, 20‐μm cryosections were dried for 4 h and then fixed in 4% paraformaldehyde for 5 min. After being washed with PBS, the sections were incubated in NBT/BCIP solution (NBT/BCIP tablets, Roche Diagnostics, Rotkreuz, Switzerland) for 30 min. The slides were counterstained with nuclear fast red solution (Sigma‐Aldrich, St. Louis, USA) for 30 s, washed in distilled water and dried for 2 h. The slides were incubated in 100% xylene for 30 s and then mounted using Permount (Thermo Fisher Scientific, Waltham, USA).
For whole‐mount ALP staining, the wounded skin tissues were incubated 5‐mM EDTA in PBS at 37°C for 6 h. The dermis was separated under the stereomicroscope (SMZ 745T; Nikon, Tokyo, Japan). The dermis was fixed in 4% paraformaldehyde for 10 min, washed with PBS and then incubated in NBT/BCIP solution (NBT/BCIP tablets, Roche Diagnostics, Rotkreuz, Switzerland) for 10 min. The whole‐mount images of dermis were taken by using a stereomicroscope (Nikon, Tokyo, Japan). The number of ALP‐positive neogenic follicles was measured by counting dark blue dots.
2.7. ALP activity assay
Human or rat DP cells were seeded in 24‐well plates and incubated with 0.1% (v/v) DMSO as the vehicle control, 1‐ or 5‐μM KY19382, or 100‐μM minoxidil for 48 h. The cells were washed twice with cold PBS and lysed with 55 μl 1× reporter lysis buffer (Promega, Madison, USA) per well. Cell lysates were centrifuged at 10,000× g at 4°C for 30 min. Samples (30 μl) of each supernatant was incubated with 30 μl of p‐nitrophenyl phosphate (pNPP) liquid substrate (Sigma‐Aldrich, St. Louis, USA) for 1 h. ALP activity was measured at 405 nm using the FLUOstar OPTIMA luminometer and normalized by the protein concentration from the Bradford assay (Bio‐Rad, Hercules, USA).
2.8. siRNA preparation and transfection
The human DP cells were transfected with siRNA or the negative control (Bioneer, Daejeon, Korea) using Lipofectamine Plus (Invitrogen, Carlsbad, USA) in serum‐free Opti‐MEM (Gibco, Gaithersburg, USA) according to the manufacturer's instructions at a final concentration of 100 nM. The siRNA sequences targeting β‐catenin were 5′‐GAAACGGCTTTCAGTTGAG‐3′ and 5′‐AAACTACTGTGGACCACAAGC‐3′ (Bioneer, Daejeon, Korea). Twelve hours after transfection with β‐catenin siRNA, the human DP cells were treated with 0.1% DMSO as the vehicle or 5‐μM KY19382 for 48 h. ALP assay and immunoblotting were performed to examine ALP activity changes and confirm the transfection efficiency of β‐catenin siRNA.
2.9. Immunohistochemistry
Skin tissues were fixed in 4% paraformaldehyde or 10% formalin overnight at 4°C. Paraffin sections were deparaffinized and rehydrated. The slides were autoclaved or microwaved for 15 min in 10‐mM sodium citrate buffer for antigen retrieval. The samples were preincubated in PBS and then blocked with 5% BSA in PBS for 30 min at room temperature. The samples were incubated overnight at 4°C with the following primary antibodies: rabbit anti‐β‐catenin (Abcam Cat# 16051, RRID:AB_443301, 1:100, Cambridge, USA), mouse anti‐keratin 15 (Abcam Cat# ab80522, RRID:AB_1603675, 1:200, Cambridge, USA), anti‐PCNA (Santa Cruz Biotechnology Cat# sc‐56, RRID:AB_628110; 1:500, Dallas, USA), anti‐Ki67 (Abcam Cat# ab15580, RRID:AB_443209, 1:500, Cambridge, USA), anti‐Fgf9 (Abcam Cat# ab71395, RRID:AB_2103075, 1:200, Cambridge, USA) and anti‐cytokeratin 17 (Abcam Cat# ab53707, RRID:AB_869865, 1:400, Cambridge, USA). After washing with PBS, the slides were incubated with anti‐mouse Alexa Fluor 488 (Thermo Fisher Scientific Cat# A‐11001, RRID:AB_2534069, 1:500, Waltham, USA) or anti‐rabbit Alexa Fluor 555 (Thermo Fisher Scientific Cat# A‐21428, RRID:AB_2535849, 1:500, Waltham, USA) conjugated secondary antibody for 1 h at room temperature and counterstained with DAPI (Sigma‐Aldrich, St. Louis, USA) for 10 min. The images were taken using an LSM510 confocal microscope (Carl Zeiss Inc., Germany). The fluorescence intensity was measured using Zen software V3.1 software (ZEN Digital Imaging for Light Microscopy, RRID:SCR_013672, Germany). IHC analyses were conducted by following the BJP guidelines (Alexander et al., 2018).
2.10. Human hair follicle culture
Informed written consent was obtained from all patients. The Medical Ethical Committee of the Kyungpook National University Hospital approved all the procedures and the study was performed following the Declaration of Helsinki principles. The detailed method is described in detail in an earlier paper (Kang et al., 2019). Hair follicles were isolated from the occipital non‐balding scalps of androgenic alopecia male patients from two persons during hair transplantation. The hair follicles were incubated in Williams' medium E without phenol red (Sigma‐Aldrich, St. Louis, USA) at 37°C in a humidified atmosphere of 5% CO2. The human follicles were incubated with 0.1% DMSO as vehicle control, 5‐μM KY19382 or 100‐μM minoxidil.
2.11. X‐gal staining
Axin2LacZ/+ Wnt/β‐catenin signalling reporter mouse was purchased from Jackson Laboratory (Bar Harbor, USA). Vibrissa follicles were fixed in 0.4% paraformaldehyde for 3 h and 0.2% glutaraldehyde for 15 min and then washed with PBS. The tissues were incubated in X‐gal solution (Thermo Fisher Scientific, Waltham, USA) at 37°C overnight. Sky blue staining represents a positive signal for X‐gal.
2.12. Animal studies
All animal care and experimental procedures for anaesthetics, ex vivo vibrissa experiments, hair regrowth experiments, WIHN experiments and hair reconstitution assays were approved by the IACUC of Yonsei University (IACUC‐A‐201407‐260‐01, IACUC‐A‐201712‐672‐03, IACUC‐A‐201804‐721‐02, IACUC‐A‐201807‐761‐02, IACUC‐A‐201811‐823‐03, IACUC‐A‐201901‐857‐01, IACUC‐A‐201909‐954‐01 and IACUC‐A‐201907‐929‐01). Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020). Mice were bred in a microventilation cage system with a computerized environmental control system (Three‐Shine Inc., Seoul, Korea). Mice had free access to food and water. Temperature of the room was maintained at 24°C with a relative humidity of 40–70% and 12‐h light/dark cycle. The mice were housed with a standard diet and bedding from the Central Lab. Animal Inc. (Seoul, South Korea). Mice were housed with no more than five animals per cage.
Group sizes of animal experiments included more than five animals in at least three independent experiments. The group size for animal studies was based on previous studies (Lee et al., 2012, 2017). Considering the 3Rs (replacement, refinement or reduction) of animal use, we first conducted preliminary experiments to confirm the effect of KY19382 on hair growth through DP cell culture or ex vivo follicle culture and then conducted minimal animal experiments under optimal conditions.
2.13. In vivo hair growth test
Specific pathogen‐free 6‐week‐old C57BL/6N or C3H wild‐type male mice (approximately 22 g) were purchased from Koatech Co. (Gyeonggi‐do, Korea) or Orient Bio Co. (Gyeonggi‐do, Korea) and acclimated for 1 week. After anaesthesia with 2,2,2‐tribromoethanol (Sigma‐Aldrich, St. Louis, USA) through 400‐mg·kg−1 intraperitoneal injection, the hairs on the backs of 7‐week‐old mice, whose hair follicles were in the telogen phase, were shaved with a hair clipper. The mice were randomly separated into four groups following the BJP guidelines. For each of the mice, 300 μl of each drug was applied topically daily at an appropriate concentration (as described in figure legends) for up to 14 or 28 days. The indicated concentrations of KY19382 were dissolved in polyethylene glycol 400 (PEG400) (Sigma‐Aldrich, St. Louis, USA; Vehicle 1), and 100‐mM minoxidil was dissolved in 50% (v/v) ethanol (Duksan Pure Chemicals Co., Gyeonggi‐do, Korea), 30% water and 20% propylene glycol (Junsei Chemical Industry Co., Tokyo, Japan) (Vehicle 2). After the experiments were completed, the mice were killed using CO2 gas.
2.14. Haematoxylin and eosin staining
The tissues were dehydrated, paraffinized, embedded in paraffin and sliced into 4‐μm thickness. The paraffin sections were rehydrated through xylene and graded ethanol series. The slides were incubated in haematoxylin for 5 min and eosin for 1 min. The number of follicles was calculated by counting the hair follicles in haematoxylin and eosin (H&E) staining images. Dermal thickness was measured by using ImageJ (ImageJ, RRID:SCR_003070) software V1.48.
2.15. WIHN assay
Specific pathogen‐free 3‐week‐old C57BL/6N wild‐type male mice (approximately 11 g) were allowed to adapt to their new environment for 3 days. After anaesthesia with 2,2,2‐tribromoethanol (400 mg·kg−1, i.p.), 1‐cm2 full‐thickness wounds were generated on the backs of the mice under aseptic conditions. The mice were randomly distributed into four groups following the BJP guidelines; 20 μl of each drug was applied to the wounds daily for up to 14, 25 or 40 days. After the studies were completed, the mice were killed using CO2 gas.
2.16. Hair patch assay
Epidermal and dermal cells were isolated from neonatal C57BL/6N mice. The skin of neonatal mice was peeled and digested with 0.25% β‐trypsin to separate the epidermis and dermis. The separated dermal cells were seeded in a 100‐mm culture dish and incubated with 5‐μM KY19382 or 0.1% DMSO as vehicle control for 72 h; 1 × 106 epidermal and 1.5 × 106 dermal cells were injected subcutaneously into hairless mice. After the experiments were completed, the mice were killed using CO2 gas.
2.17. Ex vivo vibrissa follicle culture
Mouse vibrissa follicles were isolated from a C57BL/6N mouse. The mouse was killed (with CO2 ) and anagen follicles were isolated for organ culture under a stereomicroscope (Nikon, Tokyo, Japan). The isolated follicles were placed in 500‐μl DMEM supplemented with 100‐U·m−1 penicillin/streptomycin (Gibco, Gaithersburg, USA) and 12.5‐μg·m−1 gentamicin (Gibco, Gaithersburg, USA) in 24‐well plates. The vibrissa follicles were treated with 0.1% (v/v) DMSO as the vehicle control, 5‐μM KY19382 or 100‐μM MNX. The culture medium was changed every 2 days.
Rat vibrissa follicles were isolated from a 21‐day‐old male Wistar rat (Philpott et al., 1992). The rat was killed (with CO2 ) and anagen follicles were separated under a stereomicroscope (Nikon, Tokyo, Japan). The rat vibrissa follicles were treated with 1‐ or 5‐μM KY19382 or 0.1% DMSO as the vehicle control. The culture medium was changed every 4 days. Rat experiments have been approved by the Institutional Animal Care and Use Committee (IACUC) of Yonsei University (IACUC‐A‐201407‐260‐01).
2.18. Data and statistical analyses
In vivo and in vitro experiments were designed to establish equal size, blinding and randomization. Statistical analyses were performed only for experiments where group sizes (n) ≥ 5. All group sizes represent the numbers of experimental independent values, and these independent values were used to evaluate statistical analyses. All statistical data were presented as means ± SEM. Comparisons between two unpaired treatment groups were tested by Student's t test. One‐way ANOVA with Tukey's test was performed for comparisons between multigroup studies, and post hoc tests were conducted only if the F in ANOVA achieved the necessary statistical significance level (P < .05). There was no significant variance inhomogeneity. Prism software V5.01 (GraphPad Prism, RRID:SCR_002798, CA, USA) was used for statistical analyses. The threshold for statistical significance was set at P < .05.
To reduce unwanted sources of variation, data normalization was performed. For the experimental results including cell viability, quantification of images, ALP activity, TOPflash activity, elongation of vibrissa or hair follicles, and hair weight, each value was divided by the mean of the control values and indicated in the form of ‘% or relative’. For these results, the Y axis of the relevant figures was labelled ‘fold mean of the controls’. No data values were excluded in any statistical analysis. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.19. Materials
KY19382 (5, 6‐dichloroindirubin‐3′‐methoxime) was synthesized in our laboratory (Choi, Kim et al., 2019). Minoxidil was supplied by Tokyo Chemical Industry Co. (Tokyo, Japan) and 2,2,2‐tribromoethanol by Sigma Aldrich (St. Louis, MO, USA).
2.20. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. KY19382 induces ALP activity by activating Wnt/β‐catenin signalling in human DP cells
KY19382, a newly synthesized small molecule derived from I3O, was identified in a screen for interference with the CXXC5–Dvl protein–protein interaction, followed by activation of Wnt/β‐catenin signalling (Choi et al., 2019). KY19382 showed significantly higher Wnt activity than I3O in a concentration‐dependent manner in HEK293 reporter cells with low cytotoxicity (Figure S1a,b). To determine the highest concentration of KY19382 without toxic effects in DP cells, cell viability was preliminary measured over a range of concentrations in rat DP cells. Cell viability was not significantly affected at 1‐ and 5‐μM but was decreased at 10‐μM KY19382 for rat DP cells (Figure S2a). In addition, 1‐ and 5‐μM KY19382 did not show any toxicity in human DP cells (Figure 1a). Thus, 1‐ and 5‐μM KY19382 were used for subsequent in vitro studies.
To determine whether KY19382 activates Wnt/β‐catenin signalling via GSK‐3β inhibition, we examined the levels of β‐catenin and p‐GSK‐3β (S9), an inactive form of GSK‐3β, in DP cells. When treated with KY19382, the expression levels of β‐catenin, p‐GSK‐3β (S9) and proliferation marker, PCNA, were increased in DP cells (Figures 1b and S2b). The up‐regulation of Wnt/β‐catenin signalling was further confirmed by cytochemical analyses of DP cells treated with KY19382 (Figures 1c and S2c). Quantitative measurements of both total and nuclear β‐catenin intensities showed that the Wnt/β‐catenin signalling was activated by KY19382 treatment in DP cells (Figures 1d,e and S2d,e). To assess the effect of KY19382 on ALP activity induction, we performed ALP staining and ALP activity assays in DP cells. KY19382 treatment resulted in higher ALP staining intensity and activity, compared with those in the vehicle‐treated group (Figures 1f,g and S2f,g).
The effect of Wnt/β‐catenin signalling on KY19382‐induced ALP activity was confirmed in human DP cells following transfection with human β‐catenin siRNA. The knock‐down effect of β‐catenin siRNA transfection was confirmed with immunoblotting analyses (Figure S2h). KY19382‐induced ALP expression and activity were abolished by transfection with β‐catenin siRNA in human DP cells and such inhibitory effects were not shown in cells transfected with a control siRNA (Figure 1h,i).
Overall, these data showed that KY19382 significantly activated Wnt/β‐catenin signalling in both human and rat DP cells with low cytotoxicity. In addition, KY19382 treatment elevated ALP activity by activating Wnt/β‐catenin signalling.
3.2. KY19382 promotes hair growth and activates the Wnt/β‐catenin signalling in ex vivo‐cultured human scalp hair and rodent vibrissa follicles
To determine whether KY19382 promotes hair follicle growth, we treated human scalp hair and rodent vibrissa follicles with 0.1% DMSO as vehicle control, KY19382 or minoxidil, in ex vivo experiments. KY19382 treatment on human hair follicles for 3 days, mouse vibrissa follicles for 6 days and rat vibrissa follicles for 12 days significantly promoted follicle elongation (Figures 2a,e and S3b).
FIGURE 2.

KY19382 accelerates mouse vibrissa and human hair follicle elongation. (a–d) Mouse vibrissa follicles were cultured with vehicle (0.1% DMSO), 5‐μM KY19382 or 100‐μM minoxidil (MNX) for 6 days (n = 5). (a) The length of the vibrissa follicles was measured at 6 days after treatment. The elongation rate of mouse vibrissa follicles was evaluated as the difference in the length of vibrissa follicles, wherein the vibrissa follicle length in the control group at Day 6 was considered 100%. Scale bars = 200 μm. (b) Immunohistochemistry (IHC) analyses of hair bulb of mouse vibrissa follicle for Ki67 and β‐catenin. Dashed lines mean mouse vibrissa follicles. (c) Mouse vibrissa follicles were subjected to alkaline phosphatase (ALP) staining. Arrow indicates ALP‐positive region. (d) X‐gal staining of vibrissa follicles from Axin2 lacZ/+ mice. Arrow indicates X‐gal‐positive region. (b–d) Scale bars = 100 μm. (e) Human hair follicles were incubated with vehicle (0.1% DMSO), 5‐μM KY19382 or 100‐μM minoxidil for 3 days. The length of human hair follicles was evaluated at 3 days after treatment with 5‐μM KY19382 or 100‐μM minoxidil. The elongation rate of human follicles was calculated as the difference in the length of human follicles wherein the human follicle length in the control group at Day 3 was considered 100% (n = 15). Scale bars = 200 μm. Values are expressed as means ± SEM. * P < .05, significantly different from vehicle
To confirm the effect of KY19382 on the activation of Wnt/β‐catenin signalling in an ex vivo‐cultured system, we performed immunohistochemistry (IHC) analyses. An increase in β‐catenin was observed in human follicles and rat vibrissa follicles (Figure S3a,c,d). In addition, both the β‐catenin and proliferation marker, Ki67, increased in the KY19382‐treated mouse vibrissa follicles compared with those in vehicle‐treated follicles (Figure 2b). Moreover, increased ALP expression was detected in cultured mouse vibrissa follicles treated with KY19382, demonstrating the hair‐inducing effect of KY19382 (Figure 2c). Correspondingly, LacZ expression was highly increased at the base of mouse vibrissa follicles from Axin2 LacZ/+ mice after KY19382 treatment, showing its strong effect on Wnt/β‐catenin signalling activation (Figure 2d).
In summary, β‐catenin, Ki67, ALP and Axin2‐LacZ expression levels were concomitantly increased at the hair bulb when treated with KY19382, suggesting that KY19382 accelerated follicle growth and activated Wnt/β‐catenin signalling ex vivo for both rodent and human tissue samples.
3.3. KY19382 promotes hair regrowth in mice
To find the optimum concentration of KY19382 in vivo, a preliminary experiment was conducted in which various concentrations of KY19382 were topically applied daily to the shaved dorsal skin of 7‐week‐old C3H mice for 28 days. The most significant hair regrowth effect was observed after treatment with 0.5‐mM KY19382 (Figure S4a,b). To compare the effects of KY19382 and minoxidil on mouse hair regrowth, Vehicle 1, Vehicle 2, KY19382 or minoxidil was topically applied to the dorsal skins of 7‐week‐old C57BL/6N mice daily for 14 or 28 days. Minoxidil was used as the positive control (Davies et al., 2005; Lee et al., 2012; Park et al., 2015; Sun et al., 2013; Takahashi et al., 2003). After 28 days, KY19382 promoted hair regrowth more efficiently than minoxidil (Figure 3a). The more effective hair growth promotion effect by KY19382 than by minoxidil was also shown by measurement of the weight of newly grown hair (Figure 3b). H&E staining showed that hair follicles in the control group were still in the telogen phase, but hair follicles of skin treated with KY19382 or minoxidil for 28 days entered the anagen phase (Figure 3c). Increase of dermal thickness and hair follicle numbers indicates the telogen–anagen transition (Lee et al., 2017). The more effective transition to anagen by KY19382 than by minoxidil was shown by measurement of the relative hair follicle numbers and dermal thickness in H&E staining images (Figure 3d,e). To confirm KY19382‐induced changes in proliferation or Wnt/β‐catenin signalling in the bulge, IHC analyses were performed on skin tissues treated for 14 days. Proliferation markers, Ki67 and PCNA, were specifically increased in keratin 15‐positive bulge stem cells of the KY19382‐treated group (Figures 3f and S4c). β‐Catenin was also increased only in the KY19382‐treated group in bulge stem cells (Figure 3f). Quantitative analyses suggested that the Wnt/β‐catenin signalling in bulge stem cells was activated by KY19382 treatment (Figure 3g,h). Moreover, ALP, a critical marker for hair induction, was highly expressed in the DP cells of skins treated with KY19382 for 14 days (Figure 3i). Similarly, Western blot analyses showed that the levels of β‐catenin and PCNA were significantly increased in the KY19382‐treated group (Figure 3j). These data collectively suggested that KY19382 promoted hair regrowth and increased markers for hair growth promotion, such as β‐catenin, PCNA and ALP more efficiently than minoxidil.
FIGURE 3.

KY19382 stimulates hair regrowth in vivo. C57BL/6N mice were treated with Vehicle 1 (PEG400), Vehicle 2 (50% [v/v] ethanol, 30% water and 20% propylene glycol), 0.5‐mM KY19382 or 100‐mM minoxidil (MNX) for 14 or 28 days (n = 5). (a) The gross image showed hair regrowth in mice treated with each drug for 28 days. (b) Quantitative measurements of the weight of regrown hairs. (c) Haematoxylin and eosin (H&E) staining to evaluate the hair follicles of skins with different treatments. (d, e) Quantitative analyses of the relative number of hair follicles and dermal thickness of H&E staining images (n = 5). (f) Immunohistochemistry (IHC) staining for keratin 15, β‐catenin and Ki67 using the dorsal skin of mice treated with each drug for 14 days. Lines show keratin 15‐positive bulge stem cells region. (g, h) Quantitative estimations of the total and nuclear β‐catenin intensities in bulge stem cells (n = 15 taken in five different images). (i) Alkaline phosphatase (ALP) staining was performed to evaluate the ALP activity of hair follicles with different treatments. Arrow points to the ALP‐positive region. (j) Immunoblotting analyses for β‐catenin, PCNA and ERK. Scale bars = 100 μm. Values are expressed as means ± SEM. * P < .05, significantly different as indicated ; NS, not significant
3.4. KY19382 enhances WIHN
Considering that KY19382 stimulated hair growth in vitro, ex vivo and in vivo systems via activation of Wnt/β‐catenin signalling, the effect of KY19382 on WIHN was tested in mice.
To confirm this, we cut 1‐cm2 full‐thickness wounds in 3‐ to 4‐week‐old C57BL/6N mice and applied the drugs daily for 14, 25 and 40 days after wounding. The KY19382‐treated group increased the number of newly formed follicles compared with those in the vehicle‐ or in the minoxidil‐treated group, as confirmed by whole‐mount ALP staining (Figure 4a). The ALP‐positive signals, indicated by dark blue dots, showed that the neogenic hair growth was induced by treatment of KY19382 (Figure 4b). In addition, H&E staining showed that KY19382 induced the formation of neogenic follicles, 14 days after wounding (Figure 4c). IHC analyses showed that keratin 17, a marker for intermediate filament keratin protein (Ito et al., 2007), was specifically increased in neogenic follicles of the KY19382‐treated group (Figure 4d). β‐Catenin and proliferation markers, Ki67 and PCNA, were increased in the KY19382‐treated group, especially in the neogenic follicle regions (Figures 4d and S4d). Quantitative measurements showed that the Wnt/β‐catenin signalling activation and proliferation in neogenic follicles were elevated by KY19382 treatment (Figure 4e,f,h). Fgf9 involved in WIHN in wound fibroblasts (Lee et al., 2017) was also increased in the dermis of wounds treated with KY19382 (Figure 4d,g). The newly formed white hairs at the wound sites were found only in the KY19382‐treated group (Figure 4i). Furthermore, Western blot analyses confirmed that the markers associated with hair follicle neogenesis were increased in wounded skin treated with KY19382, but not minoxidil (Figure 4j). Taken together, KY19382 markedly induced WIHN and WIHN‐related markers by significantly activating the Wnt/β‐catenin pathway.
FIGURE 4.

KY19382 stimulates WIHN in vivo. C57BL/6N mouse wounds were treated with 0.5‐mM KY19382, 100‐mM minoxidil (MNX), Vehicle 1 (PEG400) or Vehicle 2 (50% [v/v] ethanol, 30% water and 20% propylene glycol) for 14, 25 or 40 days (n = 6). (a) Alkaline phosphatase (ALP) staining to evaluate the neogenic follicles of mice treated with each drug for 25 days. (b) Quantitative measurements of ALP‐positive neogenic follicles. Dashed lines show ALP‐positive neogenic hair follicles. (c) H&E staining to evaluate newly formed hair follicles in mice treated with each drug for 14 days. Dashed lines mean the boundary between the epidermis and dermis. Arrows show newly formed follicles. (d) Immunohistochemistry (IHC) staining for Fgf9, keratin 17, β‐catenin and Ki67. (e, f) Quantitative measurement of the nuclear and total β‐catenin intensities of neogenic hair follicle cells (n = 15 taken in five different images). (g) Quantitative analyses of Fgf9‐positive dermal cells and (h) Ki67‐positive newly formed hair follicle cells (each n = 5). (i) Gross images showed newly formed hair in mice after treatment for 40 days. (j) Immunoblotting analyses for Fgf9, keratin 17, β‐catenin, PCNA and ERK for 14 days. Scale bars = 100 μm. Values are expressed as means ± SEM. * P < .05, significantly different as indicated; NS, not significant)
3.5. KY19382 promotes hair follicle neogenesis in patch assays
To investigate the therapeutic effect of KY19382, we utilized a hair patch assay system. Mouse dermal cells were cultured with 0.1% DMSO as vehicle control or 5‐μM KY19382 for 72 h before transplantation and then mixed with epithelial cells for injection into hairless mice.
Fourteen days after injection, reconstituted hair follicles were observed on the skin of hairless mice where cells were injected (Figure 5a). The magnified images showed that the number and density of neogenic hair follicles generated in the tissue injected with the KY19382‐treated cells were significantly higher than those injected with the vehicle‐treated control cells (Figure 5b). As quantitatively analysed, the KY19382 treatment increased the number of newly formed hair follicles (Figure 5c). Moreover, the expression levels of β‐catenin and Ki67 were greatly increased in neogenerated hair follicles induced by KY19382, as demonstrated by IHC analyses (Figure 5d). The intensity of nuclear β‐catenin and the number of Ki67‐positive cells in neogenic hair follicles were elevated by KY19382 (Figure 5e,f).
FIGURE 5.

KY19382 induces hair follicle neogenesis in hairless mice. Mouse dermal cells were cultured with vehicle (0.1% DMSO) or 5‐μM KY19382 for 72 h and then subcutaneously injected with epidermal cells into hairless mice. The hair follicle neogenesis was analysed at 14 days after transplantation (n = 5). (a) Hair follicle neogenesis at the injected area of hairless mice. Arrow indicates the hair regeneration area on the skin. (b) Magnified image of area exhibiting hair follicle neogenesis. (c) Quantitative analyses of regenerated hair follicles. (d) Immunohistochemistry (IHC) analyses for β‐catenin and Ki67. (e, f) Quantitative analyses of the nuclear β‐catenin intensity and Ki67‐positive cells of regenerated hair follicles. Dashed lines mean hair follicles. Scale bars = 50 μm. Values are expressed as means ± SEM. * P < .05, significantly different from control (0 μM KY19382)
4. DISCUSSION
Currently, available drugs for treating alopecia are limited by their inability to regenerate hair follicles. Minoxidil and finasteride can promote hair growth when the hair follicle is present, but they are not effective in patients with severe alopecia (Libecco & Bergfeld, 2004; Messenger & Rundegren, 2004; Price, 1999; Rossi et al., 2016). Existing drugs that control the proliferation of hair cells can be difficult for treating patients with miniaturized or absent hair follicles (Han et al., 2004). Therefore, we aimed to develop a drug that is effective in promoting hair regrowth and hair follicle neogenesis by inducing markers for hair induction such as ALP and activating hair follicle stem cells via the Wnt/β‐catenin pathway.
Hair follicle neogenesis could be an important strategy for alopecia treatment (Ito et al., 2007). Previous studies have shown that hair follicle neogenesis depends crucially on the activation of Wnt/β‐catenin signalling in DP cells and keratinocytes, along with activation of hair follicle stem cells (Enshell‐Seijffers et al., 2010; Huelsken et al., 2001; Ito et al., 2007; Waters et al., 2007). Wnt/β‐catenin signalling plays essential roles in maintaining the hair‐inducing ability of DP cells and promoting hair follicles to the anagen phase (Andl et al., 2002; Kishimoto et al., 2000; Sick et al., 2006). In addition, published data have suggested that Wnt/β‐catenin signalling activators, such as valproic acid (Lee et al., 2012), Aconiti ciliare tuber extract (Park et al., 2012) and Malva verticillata seed extract (Lee et al., 2016), are potential candidates for alternative hair growth treatments, as they induce the expression of hair‐inducing markers in DP cells. Therefore, it is important to use Wnt/β‐catenin signalling activators that activate both hair induction markers and stem cells to promote hair growth and regeneration effectively.
Although direct Wnt/β‐catenin signalling activators, such as valproic acid, promote hair growth, they fail to sustain hair growth and often show marginal effects in clinical tests (Jo et al., 2013, 2014). This marginal and limited effect may be attributed to functions of negative feedback regulators such as CXXC5 or DKK1 (Kwack et al., 2012; Lee et al., 2017). We found that CXXC5, a negative feedback regulator of Wnt/β‐catenin signalling, is specifically increased in the miniaturized follicles of bald scalps, and CXXC5 knockout mice exhibited enhanced hair growth (Lee et al., 2017). PTD‐DBM, a peptide that interfered with CXXC5–Dvl protein interaction, enhanced hair growth, and the combinatory treatment of PTD‐DBM and valproic acid synergistically increased hair growth and the WIHN (Lee et al., 2017). Similarly, KY19382, which strongly activated the Wnt/β‐catenin signalling via interference with the CXXC5–Dvl interaction and inhibition of GSK‐3β (Choi et al., 2019), critically enhanced hair regrowth, as well as WIHN in vivo. The increased CXXC5 in bald scalps and the effectiveness of PTD‐DBM or KY19382 on hair growth in mice suggested the potential use of KY19382 as a clinical treatment for hair loss.
In normal physiology, the Wnt/β‐catenin pathway plays roles in tissue regeneration including neogenic hair growth, but its aberrant activation by mutations such as loss of function mutation of APC and gain‐of‐function mutation of β‐catenin (non‐degradable form as a protein) often results in cancer (Fodde, 2002; Li et al., 2004). Our approach for activation of the Wnt/β‐catenin pathway using KY19382 is not via direct activation, but via the release of the negative feedback mechanism through interference of the Dvl binding function of CXXC5. Therefore, we suggested that this approach activating the Wnt/β‐catenin pathway by blocking the negative feedback mechanism would be safe, and this is supported by successful Food and Drug Administration (FDA) approval of the antibody against sclerostin, a LRP5/6 receptor‐binding protein inhibiting the Wnt/β‐catenin pathway (Shakeri & Adanty, 2020).
In this study, we confirmed that the levels of β‐catenin, p‐GSK3β (S9) and the proliferation marker, PCNA, were increased in human DP cells treated with KY19382. Increased ALP activity after KY19382 treatment suggested that KY19382 increased the hair‐inducing ability in human DP cells. Compared with the vehicle‐treated group, mouse vibrissa follicles treated with KY19382 for 6 days significantly promoted elongation of the hair shaft. Furthermore, KY19382 increased the length and expression levels of β‐catenin in human hair follicles. The levels of β‐catenin, Ki67 and PCNA were increased in the keratin 15‐positive bulge of mouse skin treated with KY19382, suggesting a positive effect of KY19382 on the activity of hair follicle stem cells. Moreover, KY19382 regenerated a number of neogenic follicles in the WIHN assay, and histological images showed higher expression levels of β‐catenin, proliferation markers and markers for hair follicle neogenesis, Fgf9 and keratin 17. These results indicate that KY19382 treatment may be a possible therapy for baldness. In the hair patch assay, KY19382 regenerated a greater number of neogenic hair follicles, and histological evaluation revealed higher expression levels of β‐catenin and Ki67 than control, demonstrating that pretreatment with KY19382 enhanced the hair‐inducing ability of dermal cells. Therefore, our results suggest that KY19382 may be useful for the treatment of hair loss and baldness via its effective dual‐targeting ability to inhibit both GSK‐3β and CXXC5–Dvl interactions.
AUTHOR CONTRIBUTIONS
Y.C.R., D‐H.L., J.S. and J.P. performed and analysed the experiments. Y‐R.K., S.C. and S.S.B. helped in the in vitro and in vivo experiments. Y.C.R., J.S., S‐H.L. and K‐Y.C. wrote the manuscript. Y.K.S., S‐H.L. and K‐Y.C. supervised the study.
CONFLICT OF INTEREST
K‐Y.C. is the CEO of CK Biotech. Inc. (Seoul, Korea), which has a licence to develop and use the compounds disclosed in the publication. The authors have no further conflicts of interest to declare.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1. KY19382 elevates the Wnt/β‐catenin signaling more significantly than I3O. (a) Cell viability was analyzed in HEK293 reporter cells treated with vehicle (0.1% (v/v) DMSO) or the indicated concentrations of I3O or KY19382 for 24 hours (n = 5). (b) TOPflash activity of HEK293 reporter cells treated with vehicle (0.1% DMSO) or the indicated concentrations of KY19382 or I3O for 24 hours (n = 6). Values are expressed as means ± SEM (* P < .05; NS, not significant).
Figure S2. KY19382 elevates hair induction markers through activating Wnt/β‐catenin signaling. (a‐g) Rat DP cells were treated with vehicle (0.1% DMSO) or the indicated concentrations of KY19382 for 48 hours. (a) Cell viability was measured. (b) Immunoblotting was performed to detect β‐catenin and PCNA. (c) Immunocytochemical analyses for β‐catenin of rat DP cells. (d‐e) Quantitative analyses of the total and nuclear β‐catenin of rat DP cells (n = 5). (f) Rat DP cells were subjected to ALP staining via incubation with BCIP/NBT. Arrow indicated ALP‐positive rat DP cells. (g) The ALP activity was quantified and normalized with respect to total protein concentration (n = 6). (h) Human DP cells were treated with siRNA of β‐catenin or negative control for 12 hours. After transfection, 5 μM KY19382 or vehicle (0.1% DMSO) was treated to cells for 48 hours. Transfection of β‐catenin siRNA was confirmed through immunoblotting. Scale bars = 50 μm. Values are expressed as means ± SEM (* P < .05; NS, not significant).
Figure S3. KY19382 accelerates human hair and rat vibrissa follicle elongation. (a) IHC staining of human hair bulb for β‐catenin. Dashed lines indicate human hair follicles. Scale bars = 100 μm. (b‐d) Rat vibrissa follicles from Wistar were cultured with 1 or 5 μM KY19382 in DMEM for 12 days. (b) Elongation rate of rat vibrissa follicles was calculated as the difference in the length of vibrissa follicles wherein vibrissa follicle length in the control group at day 12 was considered 100% (n = 5). (c) IHC analyses of hair bulb of rat vibrissa follicle for β‐catenin. Dashed lines were rat vibrissa follicles. (d) IHC analyses of rat vibrissa follicle bulge for β‐catenin. Arrows indicate β‐catenin positive nuclei. (c‐d) Scale bars = 50 μm. Values are expressed as means ± SEM (* P < .05).
Figure S4. Treatment with KY19382 increases the proliferation marker in the dorsal skins and wounds of mice. (a) The gross image represented hair regrowth treated with the indicated dose of KY19382 for 28 days in C3H mice. (b) Quantitative calculation of the weight of regrown hairs. (c) C57BL/6N mouse dorsal skins were treated for 14 days. IHC staining for PCNA. (d) C57BL/6N mouse wounds were treated for 14 days. IHC analyses for PCNA. Scale bars = 100 μm.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2019R1A2C3002751 and 2020M3E5E2040018). Y.C.R. was supported by a Brain Korea 21 (BK21) FOUR studentship from the NRF.
Ryu, Y. C. , Lee, D.‐H. , Shim, J. , Park, J. , Kim, Y.‐R. , Choi, S. , Bak, S. S. , Sung, Y. K. , Lee, S.‐H. , Choi, K.‐Y. . KY19382, a novel activator of Wnt/β‐catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br J Pharmacol. 2021;178:2533–2546. 10.1111/bph.15438
Funding information Brain Korea 21 (BK21) FOUR; National Research Foundation of Korea, Grant/Award Numbers: 2019R1A2C3002751, 2020M3E5E2040018
Contributor Information
Soung‐Hoon Lee, Email: sexyondal@gmail.com.
Kang‐Yell Choi, Email: kychoi@yonsei.ac.kr.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Sharman, J. L. , Southan, C. , Davies, J. A. , & CGTP Collaborators . (2019). The concise guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Mangum, J. , Wonnacott, S. , & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, L. , & Fuchs, E. (2006). The hair cycle. Journal of Cell Science, 119(Pt 3), 391–393. 10.1242/jcs02793 [DOI] [PubMed] [Google Scholar]
- Andl, T. , Reddy, S. T. , Gaddapara, T. , & Millar, S. E. (2002). WNT signals are required for the initiation of hair follicle development. Developmental Cell, 2(5), 643–653. 10.1016/s1534-5807(02)00167-3 [DOI] [PubMed] [Google Scholar]
- Botchkarev, V. A. , & Kishimoto, J. (2003). Molecular control of epithelial–mesenchymal interactions during hair follicle cycling. The Journal of Investigative Dermatology. Symposium Proceedings, 8(1), 46–55. 10.1046/j.1523-1747.2003.12171.x [DOI] [PubMed] [Google Scholar]
- Choi, O. M. , Cho, Y. H. , Choi, S. , Lee, S. H. , Seo, S. H. , Kim, H. Y. , Han, G. , Min, D. S. , Park, T. , & Choi, K. Y. (2014). The small molecule indirubin‐3′‐oxime activates Wnt/β‐catenin signaling and inhibits adipocyte differentiation and obesity. International Journal of Obesity, 38(8), 1044–1052. 10.1038/ijo.2013.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S. , Kim, H. Y. , Cha, P. H. , Seo, S. H. , Lee, C. , Choi, Y. , Shin, W. , Heo, Y. , Han, G. , Lee, W. , & Choi, K. Y. (2019). CXXC5 mediates growth plate senescence and is a target for enhancement of longitudinal bone growth. Life Science Alliance, 2(2), e201800254. 10.26508/lsa.201800254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Sobey, C. G. , Stanford, S. C. , Teixeira, M. M. , Wonnacott, S. , & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, G. C. , Thornton, M. J. , Jenner, T. J. , Chen, Y. J. , Hansen, J. B. , Carr, R. D. , & Randall, V. A. (2005). Novel and established potassium channel openers stimulate hair growth in vitro: Implications for their modes of action in hair follicles. The Journal of Investigative Dermatology, 124(4), 686–694. 10.1111/j.0022-202X.2005.23643.x [DOI] [PubMed] [Google Scholar]
- Eckert, J. , Church, R. E. , & Ebling, F. J. (1968). The pathogenesis of alopecia areata. The British Journal of Dermatology, 80(4), 203–210. 10.1111/j.1365-2133.1968.tb11960.x [DOI] [PubMed] [Google Scholar]
- Enshell‐Seijffers, D. , Lindon, C. , Kashiwagi, M. , & Morgan, B. A. (2010). β‐Catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Developmental Cell, 18(4), 633–642. 10.1016/j.devcel.2010.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde, R. (2002). The APC gene in colorectal cancer. European Journal of Cancer, 38(7), 867–871. 10.1016/s0959-8049(02)00040-0 [DOI] [PubMed] [Google Scholar]
- Han, J. H. , Kwon, O. S. , Chung, J. H. , Cho, K. H. , Eun, H. C. , & Kim, K. H. (2004). Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. Journal of Dermatological Science, 34(2), 91–98. 10.1016/j.jdermsci.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Huelsken, J. , Vogel, R. , Erdmann, B. , Cotsarelis, G. , & Birchmeier, W. (2001). β‐Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell, 105(4), 533–545. 10.1016/s0092-8674(01)00336-1 [DOI] [PubMed] [Google Scholar]
- Iida, M. , Ihara, S. , & Matsuzaki, T. (2007). Hair cycle‐dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Development, Growth & Differentiation, 49(3), 185–195. 10.1111/j.1440-169X.2007.00907.x [DOI] [PubMed] [Google Scholar]
- Ito, M. , Yang, Z. , Andl, T. , Cui, C. , Kim, N. , Millar, S. E. , & Cotsarelis, G. (2007). Wnt‐dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature, 447(7142), 316–320. 10.1038/nature05766 [DOI] [PubMed] [Google Scholar]
- Jang, W. S. , Son, I. P. , Yeo, I. K. , Park, K. Y. , Li, K. , Kim, B. J. , Seo, S. J. , Kim, M. N. , & Hong, C. K. (2013). The annual changes of clinical manifestation of androgenetic alopecia clinic in Korean males and females: A outpatient‐based study. Annals of Dermatology, 25(2), 181–188. 10.5021/ad.2013.25.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, S. J. , Choi, S. J. , Yoon, S. Y. , Lee, J. Y. , Park, W. S. , Park, P. J. , Kim, K. H. , Eun, H. C. , & Kwon, O. (2013). Valproic acid promotes human hair growth in in vitro culture model. Journal of Dermatological Science, 72(1), 16–24. 10.1016/j.jdermsci.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Jo, S. J. , Shin, H. , Park, Y. W. , Paik, S. H. , Park, W. S. , Jeong, Y. S. , Shin, H. J. , & Kwon, O. (2014). Topical valproic acid increases the hair count in male patients with androgenetic alopecia: A randomized, comparative, clinical feasibility study using phototrichogram analysis. The Journal of Dermatology, 41(4), 285–291. 10.1111/1346-8138.12422 [DOI] [PubMed] [Google Scholar]
- Kang, Y. R. , Bak, S. S. , Kim, M. K. , Joo, H. W. , Mali, N. M. , Shin, M. J. , Kim, M. K. , Kim, J. C. , Oh, J. W. , & Sung, Y. K. (2019). Expression level of prostaglandin D2 receptor 2 regulates hair regression. The Journal of Investigative Dermatology, 139(8), 1824–1828e1822. 10.1016/j.jid.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Kishimoto, J. , Burgeson, R. E. , & Morgan, B. A. (2000). Wnt signaling maintains the hair‐inducing activity of the dermal papilla. Genes & Development, 14(10), 1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kligman, A. M. (1959). The human hair cycle. The Journal of Investigative Dermatology, 33, 307–316. 10.1038/jid.1959.156 [DOI] [PubMed] [Google Scholar]
- Kwack, M. H. , Kim, M. K. , Kim, J. C. , & Sung, Y. K. (2012). Dickkopf 1 promotes regression of hair follicles. The Journal of Investigative Dermatology, 132(6), 1554–1560. 10.1038/jid.2012.24 [DOI] [PubMed] [Google Scholar]
- Lee, E. Y. , Choi, E. J. , Kim, J. A. , Hwang, Y. L. , Kim, C. D. , Lee, M. H. , Roh, S. S. , Kim, Y. H. , Han, I. , & Kang, S. (2016). Malva verticillata seed extracts upregulate the Wnt pathway in human dermal papilla cells. International Journal of Cosmetic Science, 38(2), 148–154. 10.1111/ics.12268 [DOI] [PubMed] [Google Scholar]
- Lee, S. H. , Seo, S. H. , Lee, D. H. , Pi, L. Q. , Lee, W. S. , & Choi, K. Y. (2017). Targeting of CXXC5 by a competing peptide stimulates hair regrowth and wound‐induced hair neogenesis. The Journal of Investigative Dermatology, 137(11), 2260–2269. 10.1016/j.jid.2017.04.038 [DOI] [PubMed] [Google Scholar]
- Lee, S. H. , Yoon, J. , Shin, S. H. , Zahoor, M. , Kim, H. J. , Park, P. J. , Park, W. S. , Min, D. S. , Kim, H. Y. , & Choi, K. Y. (2012). Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One, 7(4), e34152. 10.1371/journal.pone.0034152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. M. , Kim, C. E. , Margolin, A. A. , Guo, M. , Zhu, J. , Mason, J. M. , Hensle, T. W. , Murty, V. V. V. S. , Grundy, P. E. , Fearon, E. R. , D'Agati, V. , Licht, J. D. , & Tycko, B. (2004). CTNNB1 mutations and overexpression of Wnt/β‐catenin target genes in WT1‐mutant Wilms' tumors. The American Journal of Pathology, 165(6), 1943–1953. 10.1016/s0002-9440(10)63246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libecco, J. F. , & Bergfeld, W. F. (2004). Finasteride in the treatment of alopecia. Expert Opinion on Pharmacotherapy, 5(4), 933–940. 10.1517/14656566.5.4.933 [DOI] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. , Cirino, G. , Docherty, J. R. , George, C. H. , Insel, P. A. , Izzo, A. A. , Ji, Y. , Panettieri, R. A. , Sobey, C. G. , Stefanska, B. , Stephens, G. , Teixeira, M. , & Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology. 177(16), 3611–3616. 10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linas, S. L. , & Nies, A. S. (1981). Minoxidil. Annals of Internal Medicine, 94(1), 61–65. 10.7326/0003-4819-94-1-61 [DOI] [PubMed] [Google Scholar]
- Messenger, A. G. , & Rundegren, J. (2004). Minoxidil: Mechanisms of action on hair growth. The British Journal of Dermatology, 150(2), 186–194. 10.1111/j.1365-2133.2004.05785.x [DOI] [PubMed] [Google Scholar]
- Park, G. H. , Park, K. Y. , Cho, H. I. , Lee, S. M. , Han, J. S. , Won, C. H. , Chang, S. E. , Lee, M. W. , Choi, J. H. , Moon, K. C. , Shin, H. , Kang, Y. J. , & Lee, D. H. (2015). Red ginseng extract promotes the hair growth in cultured human hair follicles. Journal of Medicinal Food, 18(3), 354–362. 10.1089/jmf.2013.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, P. J. , Moon, B. S. , Lee, S. H. , Kim, S. N. , Kim, A. R. , Kim, H. J. , Park, W. S. , Choi, K. Y. , Cho, E. G. , & Lee, T. R. (2012). Hair growth‐promoting effect of Aconiti Ciliare Tuber extract mediated by the activation of Wnt/β‐catenin signaling. Life Sciences, 91(19–20), 935–943. 10.1016/j.lfs.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Paus, R. (2006). Therapeutic strategies for treating hair loss. Drug Discovery Today: Therapeutic Strategies, 3(1), 101–110. 10.1016/j.ddstr.2006.03.004 [DOI] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , Browne, W. J. , Clark, A. , Cuthill, I. C. , Dirnagl, U. , Emerson, M. , Garner, P. , Holgate, S. T. , Howells, D. W. , Karp, N. A. , Lazic, S. E. , Lidster, K. , MacCallum, C. J. , Macleod, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott, M. P. , Green, M. R. , & Kealey, T. (1992). Rat hair follicle growth in vitro. The British Journal of Dermatology, 127(6), 600–607. 10.1111/j.1365-2133.1992.tb14873.x [DOI] [PubMed] [Google Scholar]
- Pratt, C. H. , King, L. E. Jr. , Messenger, A. G. , Christiano, A. M. , & Sundberg, J. P. (2017). Alopecia areata. Nature Reviews. Disease Primers, 3, 17011–17012. 10.1038/nrdp.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, V. H. (1999). Treatment of hair loss. The New England Journal of Medicine, 341(13), 964–973. 10.1056/nejm199909233411307 [DOI] [PubMed] [Google Scholar]
- Rossi, A. , Anzalone, A. , Fortuna, M. C. , Caro, G. , Garelli, V. , Pranteda, G. , & Carlesimo, M. (2016). Multi‐therapies in androgenetic alopecia: Review and clinical experiences. Dermatologic Therapy, 29, 424–432. 10.1111/dth.12390 [DOI] [PubMed] [Google Scholar]
- Sennett, R. , & Rendl, M. (2012). Mesenchymal–epithelial interactions during hair follicle morphogenesis and cycling. Seminars in Cell & Developmental Biology, 23(8), 917–927. 10.1016/j.semcdb.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri, A. , & Adanty, C. (2020). Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: A review. Journal of Population Therapeutics and Clinical Pharmacology, 27(1), e25–e31. 10.15586/jptcp.v27i1.655 [DOI] [PubMed] [Google Scholar]
- Shin, H. , Kwack, M. H. , Shin, S. H. , Oh, J. W. , Kang, B. M. , Kim, A. A. , Kim, J. , Kim, M. K. , Kim, J. C. , & Sung, Y. K. (2010). Identification of transcriptional targets of Wnt/β‐catenin signaling in dermal papilla cells of human scalp hair follicles: EP2 is a novel transcriptional target of Wnt3a. Journal of Dermatological Science, 58(2), 91–96. 10.1016/j.jdermsci.2010.02.011 [DOI] [PubMed] [Google Scholar]
- Sick, S. , Reinker, S. , Timmer, J. , & Schlake, T. (2006). WNT and DKK determine hair follicle spacing through a reaction‐diffusion mechanism. Science, 314(5804), 1447–1450. 10.1126/science.1130088 [DOI] [PubMed] [Google Scholar]
- Sun, Y. N. , Cui, L. , Li, W. , Yan, X. T. , Yang, S. Y. , Kang, J. I. , Kang, H. K. , & Kim, Y. H. (2013). Promotion effect of constituents from the root of Polygonum multiflorum on hair growth. Bioorganic & Medicinal Chemistry Letters, 23(17), 4801–4805. 10.1016/j.bmcl.2013.06.098 [DOI] [PubMed] [Google Scholar]
- Takahashi, T. , Kamimura, A. , Hamazono‐Matsuoka, T. , & Honda, S. (2003). Phosphatidic acid has a potential to promote hair growth in vitro and in vivo, and activates mitogen‐activated protein kinase/extracellular signal‐regulated kinase kinase in hair epithelial cells. The Journal of Investigative Dermatology, 121(3), 448–456. 10.1046/j.1523-1747.2003.12426.x [DOI] [PubMed] [Google Scholar]
- Waters, J. M. , Richardson, G. D. , & Jahoda, C. A. (2007). Hair follicle stem cells. Seminars in Cell & Developmental Biology, 18(2), 245–254. 10.1016/j.semcdb.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Xiao, Z. , Hao, Y. , Liu, B. , & Qian, L. (2002). Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leukemia & Lymphoma, 43(9), 1763–1768. 10.1080/1042819021000006295 [DOI] [PubMed] [Google Scholar]
- Yamauchi, K. , & Kurosaka, A. (2009). Inhibition of glycogen synthase kinase‐3 enhances the expression of alkaline phosphatase and insulin‐like growth factor‐1 in human primary dermal papilla cell culture and maintains mouse hair bulbs in organ culture. Archives of Dermatological Research, 301(5), 357–365. 10.1007/s00403-009-0929-7 [DOI] [PubMed] [Google Scholar]
- Zahoor, M. , Cha, P. H. , & Choi, K. Y. (2014). Indirubin‐3′‐oxime, an activator of Wnt/β‐catenin signaling, enhances osteogenic commitment of ST2 cells and restores bone loss in high‐fat diet‐induced obese male mice. Bone, 65, 60–68. 10.1016/j.bone.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Zahoor, M. , Cha, P. H. , Min, D. S. , & Choi, K. Y. (2014). Indirubin‐3′‐oxime reverses bone loss in ovariectomized and hindlimb‐unloaded mice via activation of the Wnt/β‐catenin signaling. Journal of Bone and Mineral Research, 29(5), 1196–1205. 10.1002/jbmr.2147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. KY19382 elevates the Wnt/β‐catenin signaling more significantly than I3O. (a) Cell viability was analyzed in HEK293 reporter cells treated with vehicle (0.1% (v/v) DMSO) or the indicated concentrations of I3O or KY19382 for 24 hours (n = 5). (b) TOPflash activity of HEK293 reporter cells treated with vehicle (0.1% DMSO) or the indicated concentrations of KY19382 or I3O for 24 hours (n = 6). Values are expressed as means ± SEM (* P < .05; NS, not significant).
Figure S2. KY19382 elevates hair induction markers through activating Wnt/β‐catenin signaling. (a‐g) Rat DP cells were treated with vehicle (0.1% DMSO) or the indicated concentrations of KY19382 for 48 hours. (a) Cell viability was measured. (b) Immunoblotting was performed to detect β‐catenin and PCNA. (c) Immunocytochemical analyses for β‐catenin of rat DP cells. (d‐e) Quantitative analyses of the total and nuclear β‐catenin of rat DP cells (n = 5). (f) Rat DP cells were subjected to ALP staining via incubation with BCIP/NBT. Arrow indicated ALP‐positive rat DP cells. (g) The ALP activity was quantified and normalized with respect to total protein concentration (n = 6). (h) Human DP cells were treated with siRNA of β‐catenin or negative control for 12 hours. After transfection, 5 μM KY19382 or vehicle (0.1% DMSO) was treated to cells for 48 hours. Transfection of β‐catenin siRNA was confirmed through immunoblotting. Scale bars = 50 μm. Values are expressed as means ± SEM (* P < .05; NS, not significant).
Figure S3. KY19382 accelerates human hair and rat vibrissa follicle elongation. (a) IHC staining of human hair bulb for β‐catenin. Dashed lines indicate human hair follicles. Scale bars = 100 μm. (b‐d) Rat vibrissa follicles from Wistar were cultured with 1 or 5 μM KY19382 in DMEM for 12 days. (b) Elongation rate of rat vibrissa follicles was calculated as the difference in the length of vibrissa follicles wherein vibrissa follicle length in the control group at day 12 was considered 100% (n = 5). (c) IHC analyses of hair bulb of rat vibrissa follicle for β‐catenin. Dashed lines were rat vibrissa follicles. (d) IHC analyses of rat vibrissa follicle bulge for β‐catenin. Arrows indicate β‐catenin positive nuclei. (c‐d) Scale bars = 50 μm. Values are expressed as means ± SEM (* P < .05).
Figure S4. Treatment with KY19382 increases the proliferation marker in the dorsal skins and wounds of mice. (a) The gross image represented hair regrowth treated with the indicated dose of KY19382 for 28 days in C3H mice. (b) Quantitative calculation of the weight of regrown hairs. (c) C57BL/6N mouse dorsal skins were treated for 14 days. IHC staining for PCNA. (d) C57BL/6N mouse wounds were treated for 14 days. IHC analyses for PCNA. Scale bars = 100 μm.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


