Abstract
As a non-canonical fibroblast growth factor, fibroblast growth factor 21 (FGF21) functions as an endocrine hormone that signals to distinct targets throughout the body. Interest in therapeutic applications for FGF21 was initially sparked by its ability to correct metabolic dysfunction and decrease body weight associated with diabetes and obesity. More recently, new functions for FGF21 signalling have emerged, thus indicating that FGF21 is a dynamic molecule capable of regulating macronutrient preference and energy balance. Here, we highlight the major physiological and pharmacological effects of FGF21 related to nutrient and energy homeostasis and summarize current knowledge regarding FGF21’s pharmacodynamic properties. In addition, we provide new perspectives and highlight critical unanswered questions surrounding this unique metabolic messenger.
Fibroblast growth factors (FGFs) have various structures, and their functions can broadly be described as paracrine, autocrine or endocrine1. The FGF19 subfamily, which consists of FGF15/19, FGF21 and FGF23, comprises endocrine factors that are able to leave their tissue of origin because of a diminished affinity for heparan sulfate glyocosaminoglycans1–3.
FGF21 was first characterized as a novel FGF expressed in the liver. It shares ~75% amino acid sequence identity between mice and humans4 (Fig. 1). Although Fgf21 mRNA can be detected in numerous tissues including the liver, pancreas, muscle and adipose tissues4,5, circulating levels of FGF21 are now known to be derived primarily, if not exclusively, from the liver under physiological conditions in both rodents6 and humans7. Although non-hepatic tissues, such as white and brown adipose tissues, may make negligible contributions to the circulating levels of FGF21, FGF21 derived from those tissues may still have important autocrine or paracrine roles8–10. Beyond its physiological production, FGF21 is also produced by non-hepatic tissues in non-physiological genetic models of altered mitochondrial function11–16, and circulating levels of FGF21 have been proposed to be a biomarker of mitochondrial disease in humans17–19. However, the relevance of elevated FGF21 to this disease state is unclear.
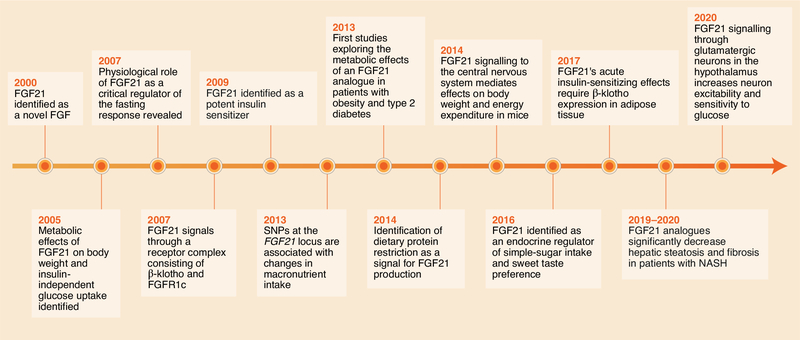
Fig. 1. Discovery of FGF21 as a metabolic messenger.
After the discovery of FGF21 in the year 2000, insight into FGF21’s function has advanced rapidly. FGF21 was first identified as a regulator of body weight and an insulin-independent modulator of glucose uptake (2005). Subsequent work revealed that FGF21 signals through a unique receptor complex (KLB–FGFR) (2007) and has potent insulin-sensitizing effects in obese rodents (2009). Physiological roles in which FGF21 regulates fasting (2007) and macronutrient intake (2013 and 2016) were later revealed, thus underscoring its function in energy and nutrient homeostasis. Important cellular targets, specifically the CNS (2014) and adipose tissues (2017), were then identified as direct targets mediating distinct aspects of FGF21’s metabolic effects. Clinical studies have revealed the therapeutic potential of FGF21 analogues in the treatment of diabetes and obesity (2013) and NASH (2019–2020). Most recently, direct central targets of FGF21’s actions have been shown to underlie its effects on neuronal function to suppress simple-sugar intake (2020).
In many tissues, including the liver, FGF21 levels are basally low and are markedly induced by a myriad of nutritional and cellular stress signals. For example, FGF21 was initially proposed to be a late-fasting/starvation factor, because it is transcriptionally induced during extended fasting, through a mechanism dependent on the transcription factor PPARα, and it regulates the adaptive fasting response20,21. However, FGF21 is also subject to transcriptional regulation mediated by nutritionally sensitive transcription factors. Hepatic mRNA and plasma protein levels of FGF21 are induced by high sugar intake through a mechanism dependent on the transcription factor ChREBP22–25 and protein restriction through pathways dependent on the transcription factors ATF4 and NRF26,27. FGF21 is maximally induced under a nutritional state of a combined high-sugar/low-protein condition28. Interestingly, the induction of FGF21 by both carbohydrate25 and protein26 requires PPARα in addition to the transcriptional mechanisms described above. However, although lipid consumption has been proposed to increase plasma FGF21 levels21,29, a thorough analysis of macronutrient intake has revealed that fat intake does not significantly alter FGF21 expression in rodents28. The importance of these transcriptional mechanisms in the induction of FGF21 is exemplified by their conservation in humans. Starvation30,31, high sugar intake32,33 and dietary protein restriction26 all induce circulating FGF21 levels in humans. In contrast, ketogenic diets do not induce plasma FGF21 levels in humans26,30,34, in agreement with the findings of more comprehensive macronutrient studies in mice28. Obesogenic diets, which are termed high-fat diets but are actually high-fat/high-carbohydrate diets, also significantly elevate plasma FGF21 levels35. The induction of FGF21 by obesogenic diets may be caused by numerous factors, including macronutrient levels, organelle stress including endoplasmic reticulum stress and mitochondrial stress36, and/or FGF21 resistance37,38. The diversity in the signals regulating FGF21 expression has made generating a unifying physiological model for FGF21 function complicated.
Although transcriptional regulation of FGF21 expression is the primary mode of FGF21 production, plasma FGF21 levels may also be regulated at the level of secretion. YIPF6 is a membrane receptor that is associated with secretory vesicles and has been shown to interact with FGF21 in the endoplasmic reticulum, thus limiting its secretion39. YIPF6 also specifies packaging of FGF21 into coat-protein complex II vesicles39. Once secreted, FGF21 has a short half-life of approximately 0.5–1.5 hours (refs. 40,41). FGF21 is also subject to proteolytic cleavage by the serine proteases fibroblast activation protein (FAP) and dipeptidyl peptidase IV (DPP-IV)42,43. DPP-IV is responsible for the cleavage of human FGF21 on the amino terminus at residues 2 and 4, and FAP is responsible for the cleavage of FGF21 at residue 171 (refs. 42,43). While cleavage at residues 2 and 4 does not significantly impair FGF21 function, cleavage at residue 171 inactivates FGF21, because the last ten residues of FGF21 are critical for its interaction with the obligate FGF21 co-receptor β-klotho (KLB)44,45. Notably, human but not mouse FGF21 is cleaved and processed by FAP, because mouse FGF21 possesses a single–amino acid difference in the stringent FAP-recognition motif42,43.
FGF21 signals through a FGFR1-KLB receptor complex
Once secreted, FGF21 elicits its biological effects by binding and activating a receptor complex composed of the co-receptor KLB and a traditional FGF receptor, fibroblast growth factor receptor 1c (FGFR1c)46,47. Whereas FGFR1c exhibits a ubiquitous tissue expression pattern5, KLB expression is primarily restricted to specific metabolic tissues (for example, the liver, pancreas and adipose tissue)5, and lower expression is observed in the brain22,48–50. Despite some reports to the contrary51,52, KLB is not expressed in muscle5,41. Thus, KLB is believed to confer specificity for FGF21 signalling. Indeed, loss of KLB abolishes all effects of FGF21 in vitro and in vivo46,53–56. KLB is thought to function as a targeting receptor for FGF21, thus promoting association with FGFR1c, which functions as the effector receptor57. FGF21 binding promotes the formation of a dimeric FGFR1c–KLB signalling complex, which activates the intracellular tyrosine kinase domains of FGFR1c and propagates signalling through phosphorylation by the kinase ERK46,53,58,59 (Fig. 1). Although FGF21 signalling can promote post-translational protein modifications60,61 and alter gene expression62,63, little is known regarding the intracellular signalling pathways and mechanism of FGF21 action within cells.
Effects of FGF21 on metabolism
Through the years, FGF21 has been proposed to have multiple functions and has been given many designations, including a starvation hormone64 or stress hormone36. However, we propose that FGF21, rather than functioning as a master regulator of metabolism, instead functions as a ‘master sensitizer’ of specific hormonal signals regulating metabolism. Depending on the metabolic state, induction of FGF21 instructs the system to reestablish homeostasis through actions on multiple tissues. Although not meant to be exhaustive, this review highlights the main functions of FGF21 in regulating nutrient and energy homeostasis and describes the key target tissues mediating these effects. Specifically, we explore the effects of FGF21 on (1) enhancing insulin sensitivity, (2) increasing energy expenditure and weight loss, (3) decreasing hepatic triglycerides and (4) regulating macronutrient preference.
FGF21 enhances insulin sensitivity.
An interesting aspect in the discovery of FGF21’s metabolic actions is that FGF21 was identified in a screen for factors that increased white adipocyte glucose uptake independently of insulin58. Remarkably, however, FGF21 was subsequently discovered to possess potent insulin-sensitizing actions in vivo41. In fact, FGF21 is one of the most potent acute insulin sensitizers ever identified. A single injection of FGF21 can decrease plasma glucose levels by more than 50% in animal models with genetically induced and diet-induced obesity41,65. This decrease in plasma glucose levels occurs primarily through increased peripheral glucose disposal41,56,65, although decreases in hepatic glucose production have also been observed66. FGF21 acutely enhances insulin sensitivity through direct actions on adipose tissues65,67. While induction of adiponectin, an insulin-sensitizing hepatoprotective adipokine68, has been proposed to mediate these insulin-sensitizing effects of FGF21 (refs. 69,70), other studies have found that adiponectin is dispensable for the metabolic effects of FGF21 (refs. 65,71). Indeed, more recent studies have confirmed that adiponectin is not required for FGF21 signalling but instead have suggested that adiponectin may induce physiological FGF21 production from the liver10.
Interestingly, in lean, ad libitum–fed wild-type mice, a single intraperitoneal injection of FGF21 has no effect on plasma glucose levels65. However, coadministration of FGF21 with insulin synergistically increases plasma glucose disposal many fold beyond that induced by insulin administration alone65. This insulin-sensitizing effect of FGF21 may function physiologically during the fasted-to-refed state, thus maximizing nutrient uptake after the depletion of energy stores during a prolonged fast6. Therefore, FGF21 may function by regulating glucose and lipid homeostasis during late fasting62, then function acutely during refeeding through enhancing insulin-stimulated glucose uptake6 and suppressing lipolysis72. FGF21 may consequently serve as a metabolic switch that maintains nutrient homeostasis by acting as a master sensitizer of endocrine signals during fasting (for example, glucagon) and different endocrine signals during refeeding (for example, insulin), thereby facilitating the transition from the fasted to the refed state3.
Given the extent of the decrease in plasma glucose, a tissue such as the liver might logically be assumed to be involved in FGF21’s metabolic actions. However, loss of KLB from hepatocytes is dispensable for the insulin-sensitizing effects of FGF21 (ref. 67). Instead, loss of FGF21 signalling to adipose tissues, as accomplished by crossing Klbfl/fl mice with adiponectin-Cre mice65,67, abolishes the insulin-sensitizing effects of FGF21 in both lean and diet-induced obese (DIO) mice, thus demonstrating the importance of direct FGF21 signalling to adipose tissues in mediating this insulin-sensitizing effect. Thermogenic (UCP1+) adipocytes are particularly important, because the insulin-sensitizing effects of FGF21 are also impaired in mice lacking KLB in UCP1+ adipocytes (Klbfl/fl;Ucp1-Cre)65. Importantly, positron emission tomography/computed tomography studies using radiolabelled [18F]fluorodeoxyglucose in mice have found that coadministration of FGF21 and insulin markedly increases glucose uptake in brown adipose tissue, but not muscle or white adipose tissue65. The capacity of brown adipose tissue, when activated, to dispose of glucose is substantial, accounting for a large fraction of ingested glucose in rodents73,74. In agreement with the in vivo fluorodeoxyglucose data65 and previous in vitro results in white adipocytes58, FGF21 treatment of isolated primary brown adipocytes, but not white adipocytes, enhances insulin-stimulated glucose uptake6. Finally, mice with acute ablation of UCP1+ adipocytes75 or those lacking UCP1 exhibit impaired FGF21-mediated regulation of glucose homeostasis76. Together, these data reveal that FGF21 enhances insulin sensitivity through direct actions on adipose tissues (Fig. 2).
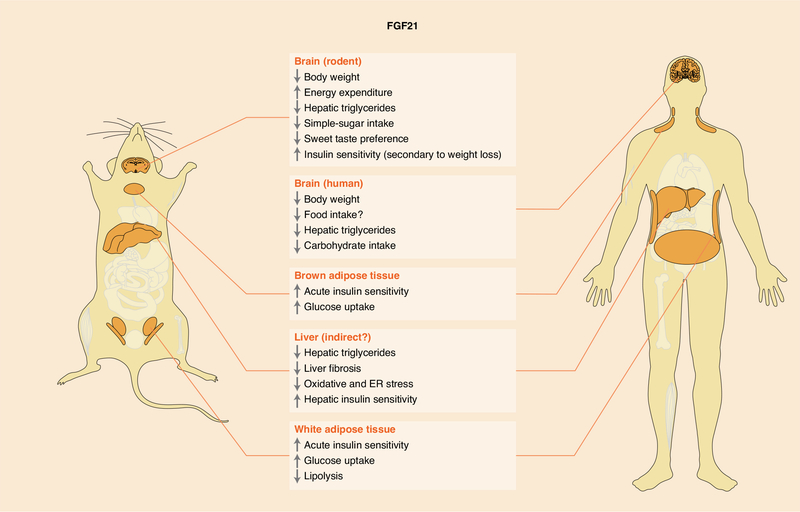
Fig. 2. Target tissues and metabolic activities of FGF21.
The metabolic effects of FGF21 in regulating body weight, hepatic triglycerides and macronutrient preference are conserved. However, species-specific differences in FGF21’s glucose-lowering effects have been observed. Most of FGF21’s metabolic effects are mediated through direct signalling to the brain and adipose tissues. Although FGF21 signalling to other tissues indirectly affects hepatic metabolism, whether FGF21 analogues signal directly to the liver is unclear. Evidence in rodents suggests FGF21’s abilities to regulate macronutrient preference, decrease body weight and hepatic triglycerides, and secondarily increase insulin sensitivity (associated with weight loss) are mediated through direct FGF21 signalling to the central nervous system. FGF21’s acute insulin-sensitizing effects are mediated through direct signalling to adipose tissues. ER, endoplasmic reticulum.
FGF21 regulates energy homeostasis.
In contrast to its acute actions, prolonged administration of FGF21 or FGF21 analogues has important metabolic effects, including a marked decrease in body weight in rodents and non-human primates, and more modest effects on body weight in humans3,40,58,77,78. Pharmacological administration of FGF21 to obese rodents reverses diabetes and obesity through increasing energy expenditure77,78. Prolonged FGF21 administration to DIO mice significantly decreases body weight, adiposity, and hepatic triglycerides and cholesterol; reverses plasma hyperglycaemia and hypertriglyceridaemia; and secondarily increases insulin sensitivity78. In addition, prolonged overexpression of FGF21 in mice increases insulin sensitivity and extends lifespan64,79. These effects of extended FGF21 signalling in promoting weight loss through increased energy expenditure are mediated through direct actions on the central nervous system67,80–84, not adipose tissues65,67,85 (Fig. 2). In DIO non-human primates, similarly to rodents, FGF21 or FGF21-analog administration also promotes weight loss40,81,86. Notably, however, whereas some studies have reported FGF21-mediated weight loss without effects on food intake40,86, another study using an FGF21 analogue (PF-05231023) in non-human primates has reported that the weight loss is attributable to significant decreases in food intake81. In agreement with the differences observed in non-human primates between administration of FGF21 and an FGF21 analogue, administration of various FGF21 analogues to humans also has been reported to result in differences in body weight and insulin sensitivity. Independent administration of two different FGF21 analogues, PF-05231023 (ref. 81) and LY2405319 (ref. 87), to people with obesity and type 2 diabetes significantly decreases body weight and improves lipid profiles. In contrast, two other FGF21 analogues, AKR-001 (also known as efruxifermin)88 and pegbelfermin89,90, have not been found to significantly decrease body weight in people with obesity or type 2 diabetes. Beyond the differing body-weight regulation among FGF21 analogues, the effects of different compounds on glucose homeostasis and insulin sensitivity also vary: some compounds do not significantly affect insulin sensitivity81, while others significantly increase insulin sensitivity in people with type 2 diabetes87,88. Nevertheless, the magnitude of the glucose-lowering effects of FGF21 analogues is clearly lower in humans than rodents81,87–91, perhaps because of differences in brown adipose tissue metabolism between species65. However, all analogues consistently have a pronounced ability to decrease plasma triglyceride levels, in many cases by as much as ~70% (refs. 81,87–91). The differences in reported outcomes with the various FGF21 analogues may reflect differences in analogue development and/or study design. Thus, until studies are conducted with recombinant FGF21 protein in humans, the analogue-specific versus FGF21-specific effects on metabolism may not be known with certainty.
The same holds true for differences observed in the potential adverse effects observed with different FGF21 analogues versus FGF21 administration: while most FGF21 analogues are generally well tolerated, one discontinued FGF21 analogue increases heart rate and blood pressure92. In contrast, these effects have not been observed with other analogues88,89, and administration of FGF21 is actually cardioprotective in numerous rodent models93–95. Another potential but controversial adverse effect of FGF21 is bone loss. In mice, FGF21 has been reported to promote bone loss96,97, but this effect has not been independently validated in rodents98 or obese non-human primates99. In humans, administration of FGF21 analogues has conflicting effects on bone turnover: PF-05231023 has been found to increase markers of bone turnover in humans81, whereas AKR-001 (ref. 88) and pegbelfermin89 have not. Because PF-05231023 affects body weight, and AKR-001 and pegbelfermin do not, changes in markers of bone turnover might possibly be an indirect consequence of weight loss. Finally, most adverse effects associated with FGF21-analogue administration are gastrointestinal81,87,88. Although the exact cause of these gastrointestinal effects is unclear, it may be a consequence of FGF21-analogue interference with FGF19-mediated regulation of bile acid metabolism100.
The mechanism underlying FGF21-mediated weight loss through actions in the central nervous system (CNS) is incompletely understood. While increases in sympathetic-nerve activity and adipose tissue thermogenesis have commonly been proposed to mediate FGF21’s effect in promoting weight loss80,101, several lines of evidence suggest that FGF21-mediated control of energy homeostasis is not one dimensional. An effective example has recently been demonstrated in an exploration of the metabolic effects of FGF21 in mice in which thermogenic (UCP1+) adipocytes were acutely ablated75. Notably, in these studies, FGF21 significantly decreased body weight to the same extent in wild-type mice and mice acutely lacking thermogenic adipose tissues. A particularly interesting finding was the mechanism underlying the weight loss. Whereas FGF21 increased energy expenditure and caused significant weight loss in wild-type mice, as expected, mice with acute ablation of thermogenic adipose tissues exhibited no increase in energy expenditure but still exhibited increased weight loss through increased physical activity and a trend towards decreased food intake75. Relatedly, two independent studies have revealed that FGF21 significantly decreases body weight in the absence of UCP1 (refs. 102,103). Notably, while one study found that deletion of UCP1 strongly blunts the effects of FGF21 on energy expenditure and appears to mediate its effects on body weight through decreased food intake103, the other study observed a strong trend in increased physical activity and the maintenance of increased energy expenditure after FGF21-analogue administration102. Indeed, administration of high doses of FGF21 has been shown to increase both energy expenditure and physical activity78. Thus, we speculate that FGF21 may act on higher-order neural circuits in the brain, thereby coordinating a resetting of body-weight homeostasis by effectively modulating energetic balance in a species-specific and context-specific manner. For example, although a common neural circuit may exist between species, rodents possess more brown adipose tissue than humans relative to total body weight, and increasing energy expenditure may provide a more effective way to achieve a negative energy balance and return to metabolic homeostasis. If this primary mode is disrupted, alternative mechanisms (such as food intake or physical activity) may be used by these circuits (Fig. 3a). Conversely, because non-human primates and humans have relatively less thermogenic adipose tissue than rodents, body-weight regulation may be mediated by a coordinated effect on caloric intake and/or physical activity, thus promoting a negative energy balance.
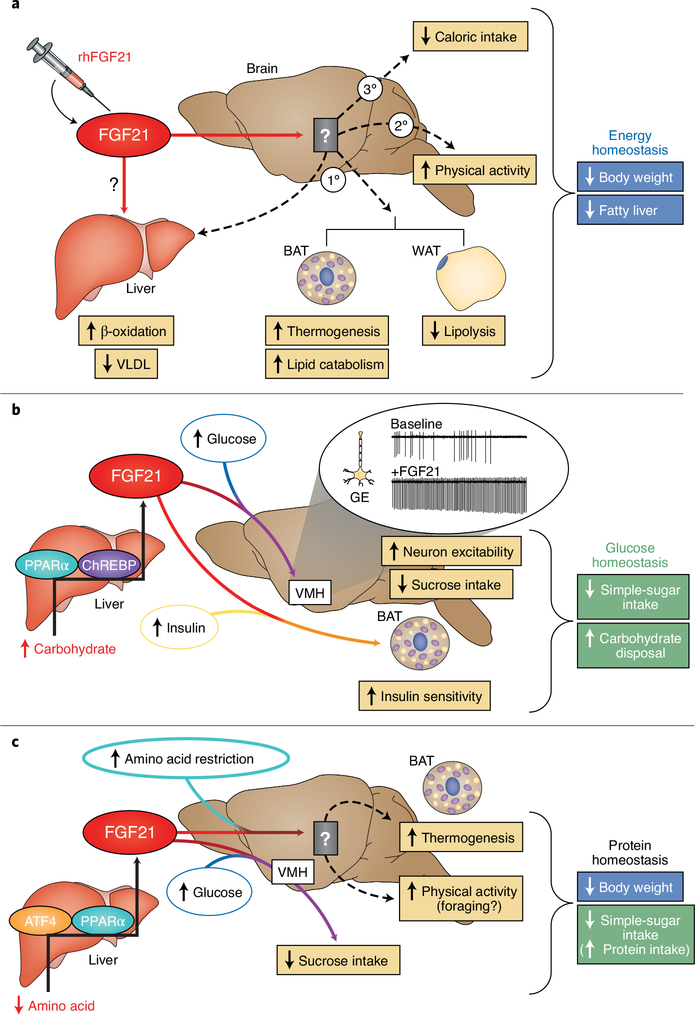
Fig. 3. FGF21 modulates signals, thus regulating energy and nutrient homeostasis.
a, In rodents, pharmacological administration of recombinant human FGF21 (rhFGF21) signals to unknown regions in the brain and invokes the primary (1°) energy-expending effects of FGF21 in decreasing body weight and liver triglycerides. However, in rodents lacking thermogenic adipose tissues, or in primates and humans, which have lower thermogenic adipose capacity than rodents, secondary (2°) and tertiary (3°) mechanisms increase physical activity and decrease caloric intake, thereby decreasing body weight. Direct actions of FGF21 on the liver in humans are unclear. b, FGF21’s ability to regulate glucose homeostasis is bimodal. FGF21 production from the liver is increased in a ChREBP- and PPARα-dependent transcriptional response to excess hepatic carbohydrate levels. Once secreted, FGF21 signals to the CNS and consequently enhances the excitability of glucose-excited (GE) neurons in the VMH in response to glucose. This increase in glucose sensitivity signals the suppression of simple-sugar intake. To complement FGF21’s effects on simple-sugar consumption, FGF21 also promotes carbohydrate disposal by enhancing insulin sensitivity in adipose tissues. c, FGF21 is produced by the liver in response to amino acid restriction through transcriptional regulation mediated by ATF4 and PPARα. Liver-derived FGF21 then enters the circulation and signals the brain to modulate macronutrient preference and energy expenditure. FGF21 signalling to the brain suppresses sugar intake, which secondarily promotes the intake of other macronutrients (such as protein). The brain regions mediating FGF21’s effects on thermogenesis and physical activity during amino acid dilution remain to be identified. BAT, brown adipose tissue; WAT, white adipose tissue; VLDL, very low-density lipoprotein.
Therefore, determining the central targets of FGF21 action is critical. Attempts to address this question have been hampered by the very low level of KLB expression in different cells in the CNS. Recently, however, studies that have crossed mice expressing Cre recombinase under the control of the endogenous Klb locus (Klb-Cre) with mice expressing the tdTomato reporter (Klb-Cre;tdTomato) have revealed critical sites of KLB expression in the hypothalamus48. Moreover, through single-cell RNA sequencing of cells from the hypothalamus in Klb-Cre;tdTomato mice, FGF21’s hypothalamic target cells have been identified. In contrast to the reported restriction of KLB expression to the suprachiasmatic nucleus49, KLB is expressed in numerous cell types and regions throughout the hypothalamus48 (and CNS). Future work is needed to identify which neural circuits coordinate FGF21-mediated regulation of energy homeostasis and how FGF21 sensitizes these neurons to respond to metabolic cues.
FGF21 reverses fatty liver.
Physiological circulating levels of FGF21 are produced primarily by the liver in response to homeostatic imbalance, perhaps partly to protect the liver itself from metabolic or cellular stress3,104. As mentioned in the previous section, FGF21 has potent lowering effects on hepatic and plasma triglycerides in obese rodents and humans, and FGF21 mimetics are actively being pursued as therapeutic agents for the treatment of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NASH)95,105. An important contributor to the progression from non-alcoholic fatty liver disease to NASH is altered lipid metabolism106,107. Administration of the FGF21 analogues AKR-001 (ref. 88) and pegbelfermin89 in humans with NASH results in marked absolute decreases in liver fat (up to 70%) and significant amelioration of liver fibrosis88,89. FGF21’s potent triglyceride-lowering effects are mediated by altered adipose and liver metabolism. Pharmacological administration of FGF21 or FGF21 analogues exerts lipid-lowering effects through accelerated lipoprotein catabolism108, suppression of postprandial lipolysis in adipose tissues88, decreased hepatic endoplasmic reticulum and oxidative stress109,110, and increased beta-oxidation of lipids in the liver62 (Figs. 2 and 3a). The mechanisms underlying these effects have been controversial, and both the liver and CNS have been proposed as the responsible target tissue in which direct signalling occurs. Whereas some studies have shown that FGF21 signals directly to the hepatocytes or liver111,112, others have found that FGF21 does not signal directly to hepatocytes50,62,113. Importantly, FGFR1c, FGF21’s primary target, is not expressed in primary hepatocytes46,114. However, FGFR1 is expressed in immortalized hepatocyte cell lines (HepG2 cells)115, thus raising doubts regarding the relevance of these models in the assessment of FGF21 function112. Although FGF21 may be capable of binding and signalling through other FGF receptors (FGFR2 and FGFR3)46 in hepatocytes, KLB is absolutely required for signaling46,53–56. Notably, loss of KLB in hepatocytes does not alter the FGF21-mediated decreases in body weight, and the improvements in both insulin sensitivity and glucose and lipid homeostasis in DIO mice67. Instead, FGF21 signals directly to the CNS (Fig. 3a), at least in rodents, and consequently regulates energy expenditure and improves glucose and lipid homeostasis80,82.
Importantly, although FGF21’s effects on hepatic lipid metabolism appear to require FGF21 signalling in the brain, no studies have directly assessed whether FGF21 signalling to the brain is required for FGF21’s pharmacological ability to reverse NASH. FGF21 or FGF21 analogues could possibly act on the liver in mediating the beneficial effects on NASH. Although hepatocytes do not express FGFR1 under normal conditions46,114, FGFR1 expression in hepatocytes could potentially be induced during NASH, similarly to its induction during hepatocarcinogenesis115, thus making it a target for FGF21 signalling. Alternatively, FGF21 analogues, whose receptor binding profiles may differ from those of recombinant FGF21 protein95, might possibly mediate their effects through signalling to the liver. Finally, clinical studies in patients with obesity and type 2 diabetes have found that FGF21-analogue administration markedly induces plasma adiponectin levels81,87–89, and this induction has be proposed to contribute to FGF21-mediated effects in ameliorating hepatic steatosis and inflammation during NASH116. The potential contribution of adiponectin to mediating the beneficial effects of FGF21 against NASH in vivo warrants further investigation. These studies highlight the clinically meaningful therapeutic potential of targeting FGF21 signalling in the treatment of fatty liver disease and reveal important unanswered questions surrounding the mechanisms of these pharmacological agents.
Macronutrient imbalance and FGF21.
A major physiological role of FGF21 is the regulation of nutrient homeostasis. Genome-wide association studies correlating changes in macronutrient intake with genetic variants have identified single-nucleotide polymorphisms at the FGF21 locus that significantly correlate with changes in carbohydrate intake117,118. These single-nucleotide polymorphisms are also associated with increases in sweet-taste preference119 and greater waist-to-hip ratios120. In agreement with these human data, mice lacking FGF21 also exhibit a greater preference for sucrose22. Subsequent work has revealed that FGF21 is induced by simple-sugar intake in both mice and humans22,32,33, and that sugar-induced production of FGF21 from the liver occurs in a ChREBP-dependent manner22,121. Once in circulation, FGF21 signals to the CNS and consequently suppresses simple-sugar intake and sweet-taste preference22,122, but not protein or lipid intake22,84. In agreement with the findings in rodents, administration of an FGF21 analogue to people with obesity decreases the preference for sweet-tasting food and carbohydrate intake123. Thus, increased FGF21 production from the liver in response to carbohydrate intake is a negative feedback loop regulating carbohydrate intake22.
Recent work has demonstrated that FGF21 signals directly to glutamatergic, but not GABAergic or dopaminergic, neurons in regulating simple-sugar intake and sweet-taste preference48. While physiological FGF21 signalling to glutamatergic neurons in the paraventricular nucleus regulates basal sucrose intake22,48, FGF21 signalling to glutamatergic neurons in the ventromedial hypothalamus (VMH) is required for FGF21-mediated suppression of simple-sugar intake48. In agreement with our proposed role of FGF21 as a master sensitizer, FGF21 increases the sensitivity of both glucose-excited and glucose-inhibited neurons in the VMH in response to increased, but not decreased, glucose concentrations48. FGF21 accomplishes this action by increasing the excitability of KLB+ neurons in the VMH48. By enhancing the excitability of KLB+ glucose-responsive neurons in response to high, but not low, glucose concentrations, FGF21 achieves specificity in suppressing simple-sugar intake under conditions of elevated plasma glucose and not during fasting. Importantly, however, these same neurons are dispensable in FGF21-mediated increases in energy expenditure. These findings indicate that FGF21 signals through distinct populations of neurons, thereby mediating effects on macronutrient intake and energy expenditure48. The production of FGF21 in the liver in response to excess carbohydrate intake functions in a feedback loop by signalling to the hypothalamus to suppress further carbohydrate intake22. In addition, FGF21 signalling to adipose tissues promotes the disposal of excess carbohydrates41,65. Thus, FGF21 production from the liver, in combination with elevated glucose levels, protects the liver from metabolic stress through a coordinated regulation of carbohydrate intake and disposal (Fig. 3b).
Beyond the response to high carbohydrate, FGF21 is induced in response to protein imbalance, specifically dietary-protein dilution26,27,124. During amino acid restriction, mammals increase their consumption of diets low in protein to acquire sufficient protein to satisfy organismal requirements, despite increasing their overall caloric intake125–129. Physiological induction of FGF21 during dietary-protein dilution, which by definition involves higher carbohydrate or lipid consumption, is likely to coordinate an increase in en energy expenditure to offset the increased caloric intake and maintain body-weight homeostasis. FGF21 is required for the increases in energy expenditure and the prevention of weight gain associated with low-protein diets26,27. In addition, as expected according to the mechanism of FGF21’s action in increasing energy expenditure, FGF21 signalling to the CNS is required for the weight loss associated with a low-protein diet83,84. Interestingly, similarly to its effects in suppressing simple-sugar intake and sweet-taste preference, FGF21 signalling to glutamatergic, but not GABAergic, neurons is required for the weight loss associated with dietary-protein dilution84. Therefore, induction of FGF21 from the liver, in combination with low amino acid levels, signals the CNS to increase energy expenditure and protect against weight gain from excess caloric intake. In addition, FGF21 may promote foraging for other macronutrients (that is, protein and lipids) by suppressing carbohydrate intake22,84 (Fig. 3c).
Critical questions for future research
Great strides have been made in deciphering the mechanisms underlying FGF21’s metabolic effects. Although specific sites of action that coordinate FGF21’s effects have broadly been identified, the mechanisms of action within these targets remain unclear. For example, how FGF21 signals within target cells and what pathways are required for FGF21 signalling are unknown. In addition, how FGF21 alters the sensitivity, or impinges on the signalling, of a diverse set of metabolic cues is an important unanswered question. Interestingly, both FGF21 and another endocrine FGF, FGF15/19, share a requirement for KLB for signaling46,54, but what cross-talk, if any, exists between these signalling pathways in both physiological and pharmacological settings is unclear. Beyond the identification of FGF21 targets and signalling cross-talk, whether FGF21 signalling pathways are impaired during obesity and diabetes remains unresolved38,61,130. Because circulating levels of FGF21 are significantly elevated during obesity and diabetes3, and given the known metabolic effects of FGF21, FGF21’s action might logically be reasoned to somehow be impaired, or ‘FGF21 resistant’, during obesity37. Importantly, in contrast to their leptin resistance131, obese rodents, primates and humans are responsive to pharmacological levels of exogenous FGF21 or FGF21 analogues in this potentially FGF21-resistant state.
Similarly to its regulation of nutrient homeostasis, FGF21 production by the liver is also increased in response to alcohol intake132,133, and administration of FGF21 suppresses alcohol intake122,134. In agreement with findings in other models described above, FGF21 signalling in response to alcohol consumption may function as a feedback loop that protects the liver from damage. Whether these effects on alcohol intake are mediated through the same central targets that exert FGF21’s effect on suppressing sugar intake remains to be determined. Although many of FGF21’s functions require KLB expression in the brain, the cellular targets of FGF21 signalling in the CNS have only recently been discovered. Because distinct cellular targets and brain regions coordinate different effects of FGF21, identification of the neural substrates and circuits regulating these processes is likely to provide novel insights into FGF21’s function and reveal previously unrecognized circuits controlling higher-order regulation of energy homeostasis and reward-seeking pathways. The ability to define how central and peripheral FGF21 target tissues coordinate FGF21-mediated responses in a context specific manner, perhaps through communication via both the afferent and efferent nervous system, will be a critical research area in the future.
Acknowledgements
The authors were supported by US National Institutes of Health (NIH) grants R01DK106104 (M.J.P.), R01AA027654 (M.J.P.), T32 DK112751 (K.H.F.) and T32 HL007121 (K.H.F.), and the Veterans Affairs Merit Review Program I01BX004634 (M.J.P.).
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information Nature Metabolism thanks Aimin Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt.
References
- 1.Beenken A & Mohammadi M The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beenken A & Mohammadi M The structural biology of the FGF19 subfamily. Adv. Exp. Med. Biol. 728, 1–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BonDurant LD & Potthoff MJ Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu. Rev. Nutr. 38, 173–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura T, Nakatake Y, Konishi M & Itoh N Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 1492, 203–206 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Fon Tacer K et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 24, 2050–2064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markan KR et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63, 4057–4063 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen JS et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab. 4, 551–560 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justesen S, Haugegaard KV, Hansen JB, Hansen HS & Andersen B The autocrine role of FGF21 in cultured adipocytes. Biochem. J. 477, 2477–2487 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Huang Z et al. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunit y. Cell Metab 26, 493–508.e4 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Han MS et al. A feed-forward regulatory loop in adipose tissue promotes signalling by the hepatokine FGF21. Genes Dev. 35, 133–146 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumiya Y et al. FGF21 is an Akt-regulated myokine. FEBS Lett 582, 3805–3810 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tezze C et al. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab 25, 1374–1389.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keipert S et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 306, E469–E482 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Pereira RO et al. OPA1 deficiency promotes secretion of FGF21 from muscle that prevents obesity and insulin resistance. EMBO J. 36, 2126–2145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keipert S et al. Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol. Metab. 4, 537–542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restelli LM et al. Neuronal mitochondrial dysfunction activates the integrated stress response to induce fibroblast growth factor 21. Cell Rep. 24, 1407–1414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morovat A et al. Use of FGF-21 as a biomarker of mitochondrial disease in clinical practice. J. Clin. Med. 6, E80 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehtonen JM et al. FGF21 is a biomarker for mitochondrial translation and mtDNA maintenance disorders. Neurology 87, 2290–2299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovadi E et al. Elevated FGF 21 in myotonic dystrophy type 1 and mitochondrial diseases. Muscle Nerve 55, 564–569 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Inagaki T et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Badman MK et al. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437 (2007). [DOI] [PubMed] [Google Scholar]
- 22.von Holstein-Rathlou S et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 23, 335–343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iizuka K, Takeda J & Horikawa Y Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 583, 2882–2886 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Sánchez J, Palou A & Picó C Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology 150, 5341–5350 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Iroz A et al. A specific ChREBP and PPARα cross-talk is required for the glucose-mediated FGF21 response. Cell Rep. 21, 403–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laeger T et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 124, 3913–3922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maida A et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Invest. 126, 3263–3278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solon-Biet SM et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 24, 555–565 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Badman MK, Koester A, Flier JS, Kharitonenkov A & Maratos-Flier E Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150, 4931–4940 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gälman C et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell Metab. 8, 169–174 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Fazeli PK et al. FGF21 and the late adaptive response to starvation in humans. J. Clin. Invest. 125, 4601–4611 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dushay JR et al. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab. 4, 51–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lundsgaard AM et al. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol. Metab. 6, 22–29 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christodoulides C, Dyson P, Sprecher D, Tsintzas K & Karpe F Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J. Clin. Endocrinol. Metab. 94, 3594–3601 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Vienberg SG et al. Impact of short-term high-fat feeding and insulin-stimulated FGF21 levels in subjects with low birth weight and controls. Eur. J. Endocrinol. 167, 49–57 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Kim KH & Lee MS FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metab. J. 38, 245–251 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satapati S et al. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes 57, 2012–2021 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher FM et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59, 2781–2789 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L et al. YIPF6 controls sorting of FGF21 into COPII vesicles and promotes obesity. Proc. Natl Acad. Sci. USA 116, 15184–15193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kharitonenkov A et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148, 774–781 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Xu J et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models: association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab 297, E1105–E1114 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Dunshee DR et al. Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J. Biol. Chem. 291, 5986–5996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhen EY, Jin Z, Ackermann BL, Thomas MK & Gutierrez JA Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem. J. 473, 605–614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Micanovic R et al. Different roles of N- and C- termini in the functional activity of FGF21. J. Cell. Physiol. 219, 227–234 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Yie J et al. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 583, 19–24 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Kurosu H et al. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki M et al. βKlotho is required for fibroblast growth factor (FGF) 21 signalling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 22, 1006–1014 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen-Cody SO et al. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metab. 32, 273–286.e276 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bookout AL et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, 1147–1152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Q et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Patel V et al. Novel insights into the cardio-protective effects of FGF21 in lean and obese rat hearts. PLoS ONE 9, e87102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benoit B et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat. Med. 23, 990–996 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Ogawa Y et al. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl Acad. Sci. USA 104, 7432–7437 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X et al. Co-receptor requirements for fibroblast growth factor-19 signalling. J. Biol. Chem. 282, 29069–29072 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Adams AC, Cheng CC, Coskun T & Kharitonenkov A FGF21 requires βklotho to act in vivo. PLoS ONE 7, e49977 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding X et al. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 16, 387–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S et al. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553, 501–505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kharitonenkov A et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yie J et al. Understanding the physical interactions in the FGF21/FGFR/β-Klotho complex: structural requirements and implications in FGF21 signalling. Chem. Biol. Drug Des. 79, 398–410 (2012). [DOI] [PubMed] [Google Scholar]
- 60.Chau MD, Gao J, Yang Q, Wu Z & Gromada J Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc. Natl Acad. Sci. USA 107, 12553–12558 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markan KR et al. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Mol. Metab. 6, 602–610 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potthoff MJ et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl Acad. Sci. USA 106, 10853–10858 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Byun S et al. Fasting-induced FGF21 signalling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat. Commun. 11, 807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.BonDurant LD et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 25, 935–944.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berglund ED et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150, 4084–4093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lan T et al. FGF19, FGF21, and an FGFR1/beta-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26, 709–718.e703 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turer AT & Scherer PE Adiponectin: mechanistic insights and clinical implications. Diabetologia 55, 2319–2326 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Holland WL et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17, 790–797 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Z et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Kolumam G et al. Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βKlotho complex. EBioMedicine 2, 730–743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arner P et al. FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett. 582, 1725–1730 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Bartelt A et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun. 8, 15010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nedergaard J, Bengtsson T & Cannon B New powers of brown fat: fighting the metabolic syndrome. Cell Metab 13, 238–240 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Challa TD et al. A genetic model to study the contribution of brown and brite adipocytes to metabolism. Cell Rep. 30, 3424–3433.e3424 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Kwon MM, O’Dwyer SM, Baker RK, Covey SD & Kieffer TJ FGF21-mediated improvements in glucose clearance require uncoupling protein 1. Cell Rep. 13, 1521–1527 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Coskun T et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Xu J et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Youm YH, Horvath TL, Mangelsdorf DJ, Kliewer SA & Dixit VD Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc. Natl Acad. Sci. USA 113, 1026–1031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Owen BM et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20, 670–677 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talukdar S et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23, 427–440 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Ameka M et al. Liver derived FGF21 maintains core body temperature during acute cold exposure. Sci. Rep. 9, 630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hill CM et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 27, 2934–2947.e2933 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flippo KH, Jensen-Cody SO, Claflin KE & Potthoff MJ FGF21 signalling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Sci. Rep. 10, 19521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen MZ et al. FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/βKlotho complex in non-adipocytes. Mol. Metab. 6, 1454–1467 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murray SA et al. Whole transcriptome analysis and validation of metabolic pathways in subcutaneous adipose tissues during FGF21-induced weight loss in non-human primates. Sci. Rep. 10, 7287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaich G et al. The effects of LY2405319, an FGF21 analogue, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Kaufman A, Abuqayyas L, Denney WS, Tillman EJ & Rolph T AKR-001, an Fc-FGF21 analog, showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type 2 diabetes patients. Cell Rep. Med. 1, 100057 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanyal A et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 392, 2705–2717 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Charles ED et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity (Silver Spring) 27, 41–49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harrison SA et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 391, 1174–1185 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Kim AM et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes. Metab. 19, 1762–1772 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Planavila A, Redondo-Angulo I & Villarroya F FGF21 and cardiac physiopathology. Front. Endocrinol. (Lausanne) 6, 133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanajak P, Chattipakorn SC & Chattipakorn N Effects of fibroblast growth factor 21 on the heart. J. Endocrinol. 227, R13–R30 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Geng L, Lam KSL & Xu A The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat. Rev. Endocrinol. 16, 654–667 (2020). [DOI] [PubMed] [Google Scholar]
- 96.Wei W et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc. Natl Acad. Sci. USA 109, 3143–3148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X, Wei W, Krzeszinski JY, Wang Y & Wan Y A liver-bone endocrine relay by IGFBP1 promotes osteoclastogenesis and mediates FGF21-induced bone resorption. Cell Metab. 22, 811–824 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X et al. FGF21 is not a major mediator for bone homeostasis or metabolic actions of PPARα and PPARγ agonists. J. Bone Miner. Res. 32, 834–845 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Andersen B et al. FGF21 decreases body weight without reducing food intake or bone mineral density in high-fat fed obese rhesus macaque monkeys. Int. J. Obes. (Lond.) 42, 1151–1160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J et al. Chronic over-expression of fibroblast growth factor 21 increases bile acid biosynthesis by opposing FGF15/19 action. EBioMedicine 15, 173–183 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Douris N et al. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology 156, 2470–2481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Véniant MM et al. Pharmacologic effects of FGF21 are independent of the “browning” of white adipose tissue. Cell Metab. 21, 731–738 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Samms RJ et al. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 11, 991–999 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Jensen-Cody SO & Potthoff MJ Hepatokines and metabolism: deciphering communication from the liver. Mol. Metab. 44, 101138 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henriksson E & Andersen B FGF19 and FGF21 for the treatment of NASH-two sides of the same coin? Differential and overlapping effects of FGF19 and FGF21 from mice to human. Front. Endocrinol. (Lausanne) 11, 601349 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marra F & Lotersztajn S Pathophysiology of NASH: perspectives for a targeted treatment. Curr. Pharm. Des. 19, 5250–5269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen JC, Horton JD & Hobbs HH Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schlein C et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab. 23, 441–453 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Ye D et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology 60, 977–989 (2014). [DOI] [PubMed] [Google Scholar]
- 110.Kim SH et al. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia 58, 809–818 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Fisher FM et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 152, 2996–3004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gong Q et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 64, 425–438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Foltz IN et al. Treating diabetes and obesity with an FGF21-mimetic antibody activating the βKlotho/FGFR1c receptor complex. Sci. Transl. Med. 4, 162ra153 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Kan M, Wu X, Wang F & McKeehan WL Specificity for fibroblast growth factors determined by heparan sulfate in a binary complex with the receptor kinase. J. Biol. Chem. 274, 15947–15952 (1999). [DOI] [PubMed] [Google Scholar]
- 115.Huang X et al. Resident hepatocyte fibroblast growth factor receptor 4 limits hepatocarcinogenesis. Mol. Carcinog. 48, 553–562 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bao L et al. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br. J. Pharmacol. 175, 3379–3393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chu AY et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum. Mol. Genet. 22, 1895–1902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tanaka T et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 97, 1395–1402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Søberg S et al. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 25, 1045–1053.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 120.Frayling TM et al. A common allele in FGF21 associated with sugar intake is associated with body shape, lower total body-fat percentage, and higher blood pressure. Cell Rep. 23, 327–336 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fisher FM et al. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol. Metab. 6, 14–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Talukdar S et al. FGF21 regulates sweet and alcohol preference. Cell Metab. 23, 344–349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baruch A et al. Antibody-mediated activation of the FGFR1/Klothoβ complex corrects metabolic dysfunction and alters food preference in obese humans. Proc. Natl Acad. Sci. USA 117, 28992–29000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Laeger T et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep 16, 707–716 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simpson SJ & Raubenheimer D Obesity: the protein leverage hypothesis. Obes. Rev. 6, 133–142 (2005). [DOI] [PubMed] [Google Scholar]
- 126.Sørensen A, Mayntz D, Raubenheimer D & Simpson SJ Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 16, 566–571 (2008). [DOI] [PubMed] [Google Scholar]
- 127.Raubenheimer D & Simpson SJ Protein leverage: theoretical foundations and ten points of clarification. Obesity (Silver Spring) 27, 1225–1238 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Martinez-Cordero C et al. Testing the Protein Leverage Hypothesis in a free-living human population. Appetite 59, 312–315 (2012). [DOI] [PubMed] [Google Scholar]
- 129.Gosby AK, Conigrave AD, Raubenheimer D & Simpson SJ Protein leverage and energy intake. Obes. Rev. 15, 183–191 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Hale C et al. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology 153, 69–80 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Friedman J The long road to leptin. J. Clin. Invest. 126, 4727–4734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Desai BN et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol. Metab. 6, 1395–1406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Søberg S et al. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol. Metab. 11, 96–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schumann G et al. KLB is associated with alcohol drinking, and its gene product β-Klotho is necessary for FGF21 regulation of alcohol preference. Proc. Natl Acad. Sci. USA 113, 14372–14377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]