Abstract
This study was conducted to describe demographics, clinical features, and outcomes of 3827 confirmed cases of Coronavirus Disease 2019 between March 12 and April 22, 2020 in the Emirates of Abu Dhabi, United Arab Emirates (UAE).
Data were extracted from the Infectious Diseases Notification Surveillance System of the Department of Health. The descriptive analysis was done using Statistical Package for Social Sciences v26 and reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
We analyzed 3827 cases; 82% were men, 18% women, 14% UAE citizens, and 86% were of other nationalities. Most cases (72%) had lower exposure to low-risk occupations of infectious disease as per the classification of the department of health while high exposure risk occupations, which included healthcare worker accounts only for 3%. While 43% of cases were asymptomatic, 57% displayed symptoms, which were mostly mild. Only 12% of patients had comorbidities, which were significantly higher in men (9%) than women (3%). Among those who have comorbid conditions; hypertension (27%) and diabetes (21%) were the most common comorbidities. Viral pneumonia (11%) was the most common sequela documented in records. Only 51 patients (4%) required admission to the intensive care units, and 4 patients died (0.1%).
The significant number of asymptomatic patients was identified by active case finding and contact tracing from the early period of the epidemic. A small percentage of severe, critical cases, and death reported in the Emirate of Abu Dhabi which may have been due to public health measures implemented for early detection, contact tracing, and treatment.
Keywords: Abu Dhabi, Coronavirus Disease 2019, epidemiology, pandemic, United Arab Emirates
1. Introduction
In December 2019, a novel coronavirus (2019-nCoV) was first identified in Wuhan, China.[1] This novel coronavirus was subsequently named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) due to its similarities with SARS-CoV previously identified in 2002 to 2003. The associated disease was later named Cornoavirus Disease 2019 (COVID-19).[2–4] This virus spread very rapidly in China and worldwide, creating an alarming international public health crisis. On March 11, 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a global pandemic.[5] By July 10, the WHO reported >12 million confirmed cases and over half a million deaths in 216 countries or territories.[6]
The United Arabe Emirates (UAE) was the first country in the Gulf Cooperation Council to report a COVID-19 case on January 29, 2020 linked to Wuhan.[7] Subsequently, cases were reported from the Middle East; in Iran on February 19, in Bahrain and Oman on February 24, in Kuwait on February 26, and in Saudi Arabia on March 2.[8]
The UAE is a federation of 7 Emirates: Abu Dhabi, Dubai, Ajman, Umm Al Quwain, Sharjah, Fujairah, and Ras Al Khaimah and located in the Middle East, on the Arabian Gulf between Oman, Saudi Arabia, and Qatar. It has a population of approximately 9.5 million people, of which around 81% are expatriates from nearly 200 countries.[9] The majority of expatriates are young men between the ages of 20 and 50, usually coming from India, Pakistan, Bangladesh, and the Philippines. Most men are employed in construction, sales, services, and small workshops, while women work as domestic staff and shop workers.[10] The Department of Health (DoH) in the Emirate of Abu Dhabi issued its first alert on January 21 to all healthcare facilities to immediately report any suspected or confirmed case of COVID-19 to the Infectious Diseases Notification Suveillance System (IDNSS). It advised activating the triage at the entry point of healthcare facilities, including the emergency departments. On April 19, DoH issued guidance on patient triage and transfer protocol, contact tracing, screening at the airports, and guidance to the laboratories for testing and reporting.[11]
Despite the early reporting of the first case, the number of COVID-19 cases remained low for at least 2 months (February and March), reaching its peak in May. The UAE Ministry of Health and Prevention reported a total of 52,068 cases and 324 deaths as of July 8.[12]
Since the start of this pandemic, the UAE health authorities have implemented several important public health actions, including the closure of borders, closure of educational institutions, shopping malls, restaurants, gyms, mass events (culture, sports, conferences, exhibitions, etc), the introduction of the remote working, the restriction of public movement (national sterilization program), the implementation of personal hygiene measures such as handwashing advice, the enforcement of wearing face coverings in public, encouraging social distancing, screening at the airports, contact tracing, and screening of asymptomatic cases.
The Public Health Authorities focused on active case finding of cases with a history of travel with mild or no symptoms. This unique ability to test a large number of suspected cases or those exposed to COVID-19 provided a wealth of data to analyze and learn about the disease epidemiology early on.
The objective of this study was to describe the current epidemiology of COVID-19 in the Emirate Abu Dhabi and to assess the associated contributing factors by age, sex, nationality, occupation, clinical characteristics, underlying comorbidities, and outcome. To our knowledge, this is the largest study that describes the epidemiological features of COVID-19-infected patients in the UAE and the region.
2. Methods
2.1. Study design
In this observational, retrospective descriptive study of confirmed positive COVID-19 patients in the Emirate of Abu Dhabi data from March 12 to April 22, 2020 were extracted from the IDNSS held at the Abu Dhabi Public Health Center (ADPHC). This electronic system is patient-based from all geographic areas of the Emirate of Abu Dhabi and contains information on demographics, clinical, and laboratory diagnosis of 56 notifiable infectious diseases including COVID-19.[13,14] Following the notification, Public Health Officers at the ADPHC collaborate with the reporting healthcare facility to gather further information regarding clinical details such as date of onset of symptoms, nature of symptoms, risk factors, comorbidities, etc, whether the case is in the community or the hospital. They also seek contact details of the case to get further information on the possible exposure, contacts, and advice on isolation/quarantine to the case and contacts. This study is reported following the STROBE Statement checklist.
2.2. Setting: Emirate of Abu Dhabi
Abu Dhabi is the largest Emirate in the UAE, comprising 84% of the inland territory.[15] Abu Dhabi city is the capital of the UAE federation where all the government offices are based. It has 2nd largest population of the 7 Emirates, with an estimated population of 2,908,173 (1,857,618 men and 1,050,555 women), of which 81% (2,356,638) are expatriates.[10] There is also an unequal distribution among male and female expatriates where men account for roughly two-third of the expatriate population.[9]
2.3. Ethical approval
The study was approved by the “Abu Dhabi Health COVID-19 Research Ethics Committee” of the DoH with the reference no: DOH/CVDC/2020/701 on April 20, 2020.
2.4. Participants: case definition
Case definition was periodically updated included either clinical and/or laboratory criteria, as is described below:
2.4.1. “Clinical criteria”
For suspected COVID-19 cases: patient with upper or lower respiratory symptom with or without fever or at least 2 of the following symptoms: fever (measured or subjective), chills, rigors, myalgia, headache, sore throat, new olfactory, and taste disorder(s) OR at least one of the symptoms: cough, shortness of breath, or difficulty breathing OR severe respiratory illness with the presence of one of the clinical or radiographic features of pneumonia, or acute respiratory distress syndrome.
2.4.2. “Laboratory criteria"
Detection of SARS-CoV-2 virus by using real-time reverse polymerase chain reaction (RT-PCR) test in the nasopharyngeal and oropharyngeal samples.[11] Asymptomatic subjects were diagnosed by laboratory methods and criteria.
2.5. Demographics, clinical features, and outcomes
Variables selected to describe the demographic and clinical features of the cases were: age, sex, nationality, occupation, type of symptoms, the interval between onset of symptoms and notification, presence of comorbidities, sequelae, intensive care unit (ICU) admission, and death.
Nationality was categorized as citizens of UAE, other Arab countries, non-Arab countries, and unknown.
The occupation was categorized into 3 exposure risk levels (high risk, medium risk, or low risk) in keeping with the Occupational Safety and Health Administration.[16,17] Those without an occupation were classified as “other.” Members of this group included children aged 0 to 18 years, housewives, and the retired elderly.
COVID-19 disease severity was categorized into 5 groups, as per DoH and WHO guidance: asymptomatic, mild, moderate, severe, and critical.[11,18] In the asymptomatic disease laboratory test for COVID-19 was positive, and no symptoms were reported. Cases in mild disease were those with uncomplicated upper respiratory tract infection in addition to nonspecific symptoms such as sore throat, dry cough, fever, myalgia, arthralgia, and runny nose without shortness of breath. Moderate disease included cases with pneumonia who had symptoms of fever, cough, dyspnea, and fast breathing. In severe conditions, the patients had severe pneumonia to include moderate disease symptoms, with the addition of breathing rate >30 per minute or saturation of oxygen <90%. Critical disease included complications of acute respiratory distress syndrome (ARDS), sepsis, and septic shock.
The date of notification is usually based on a positive SARS-CoV-2 test result from the laboratory, which is reported to DoH or reported by a doctor from a health care facility. If the notification was from both laboratory and healthcare facilities, then the earlier date was accepted as a notification date. The interval between the date of onset of symptoms and date of notification was estimated.
2.6. Comorbidity status
Comorbidity status was verified by the review of the patient medical record by using International Classification of Disease version 10, 2015 (ICD-10).[19] Comorbidities variables were categorized, a person with no comorbidity, and the person with comorbidity, single and ≥2 comorbidities. The outcomes have reported no admission to hospital, admission to hospital, and deaths. The most common comorbidities were hypertension (27%) and diabetes (21%). Of those with comorbidities, (28%) cases had ≥2 comorbidities, with the most common being diabetes and hypertension.
2.7. Statistical analysis
The data were entered into Microsoft Excel, allowing for coding and cleaning variables that were considered essential for the best epidemiological description of the cases. Variables were then imported into Statistical Package for Social Sciences version 26 for statistical analysis. Qualitative variables were expressed as numbers and percentages, while quantitative variables were expressed as means, medians, and interquartile ranges (IQR). Student t test was used for continuous variables and the chi-square test for categorical variables. Logistic regression was used to predict factors that are associated with hospital admission. A P-value of ≤.05 was considered to indicate statistical significance.
3. Results
Data were collected between March 12 and April 22, 2020 on 3827 confirmed COVID-19 cases by RT-PCR test (nasopharyngeal and oropharyngeal swabs) notified to DoH from different healthcare facilities in the Emirate of Abu Dhabi.
The majority of cases were men (3157; 82%) compared with 670 women (18%). The age range was 0 to 90 years; with a mean of 35.8 years, standard deviation (SD) 12.6 and a median of 35 years. For male cases, the mean was 36.5 (SD 11.8), median 35 years, and IQR (29–44) years. For female subjects, the mean was 32.7 (SD 15.5), median 32, and IQR (25–41) years. Most cases (3105, 81%) were between the ages of 19 to 49 years, while 192 (5%) cases were aged 18 and less, and 530 (14%) subjects were aged 50 and above (Fig. 1).
Figure 1.
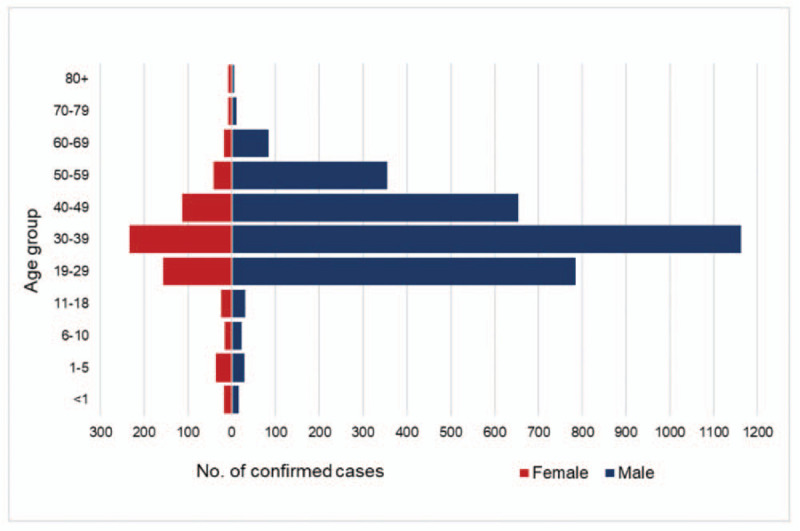
Age and sex distribution of COVID-19 confirmed cases in the Emirate of Abu Dhabi between March 12 and April 22, 2020 (n = 3827). COVID-19 = Coronavirus Disease 2019.
Among 3827 cases, 540 (14%) were UAE citizens, 435 (11%) were from other Arab countries, 2815 (74%) from non-Arab countries, and in 37 (1%) cases, their nationality was unknown (Table 1). All non-Arab cases were from 72 countries; 1517 (40%) cases were from India, 446 (12%) Pakistan, and 231 (6%) from the Philippines.
Table 1.
Demographic characteristics of COVID-19 confirmed cases (n = 3827) by age, sex, nationality, and workplace exposure levels, comorbidities, clinical presentation, and outcomes in the Emirate of Abu Dhabi between March 12 and April 22, 2020.
| Female (n = 670) | Male (n = 3157) | Total (N = 3827) | |||||
| n | (%) | n | (%) | N | (%) | P-value | |
| Age distributions | .000a | ||||||
| <1 | 17 | 0% | 16 | 0.4% | 33 | 1% | |
| 1–5 | 36 | 1% | 29 | 0.8% | 65 | 2% | |
| 6–10 | 16 | 0.4% | 22 | 0.6% | 38 | 1% | |
| 11–18 | 25 | 0.7% | 31 | 1% | 56 | 1% | |
| 19–29 | 156 | 4% | 785 | 21% | 941 | 25% | |
| 30–39 | 234 | 6% | 1163 | 30% | 1397 | 37% | |
| 40–49 | 113 | 3% | 654 | 17% | 767 | 20% | |
| 50–59 | 41 | 1% | 355 | 9% | 396 | 10% | |
| 60–69 | 18 | 0.5% | 85 | 2% | 103 | 3% | |
| 70–79 | 7 | 0.2% | 11 | 0.3% | 18 | 0.5% | |
| 80+ | 7 | 0.2% | 6 | 0.2% | 13 | 0.3% | |
| Nationality | .000a | ||||||
| UAE∗ | 160 | 4% | 380 | 10% | 540 | 14% | |
| Other Arab countries† | 124 | 3% | 311 | 8% | 435 | 11% | |
| Non-Arab countries | 381 | 10% | 2434 | 64% | 2815 | 74% | |
| Unknown | 5 | 0.1% | 32 | 1% | 37 | 1% | |
| COVID-19 workplace exposure risk levels | .000a | ||||||
| High exposure risk | |||||||
| Healthcare workers | 63 | 2% | 47 | 1% | 110 | 3% | |
| Medium exposure risk | |||||||
| Laborers and workers at high density workplaces | 61 | 2% | 567 | 15% | 628 | 16% | |
| Lower exposure risk | |||||||
| Office workers and workers with minimum contact with public | 409 | 11% | 2346 | 61% | 2755 | 72% | |
| Other‡ | 137 | 4% | 197 | 5% | 334 | 9% | |
| Comorbidities | .001a | ||||||
| No comorbidities | 566 | 15% | 2813 | 74% | 3379 | 88% | |
| With comorbidities | 104 | 3% | 344 | 9% | 448 | 12% | |
| Hypertension | 13 | 3% | 107 | 24% | 120 | 27%§ | |
| Diabetes | 4 | 1% | 92 | 21% | 96 | 21%§ | |
| Pregnancy | 36 | 8% | - | - | 36 | 8%§ | |
| Chronic pulmonary disease | 10 | 2% | 12 | 3% | 22 | 5%§ | |
| Cardiovascular disease | 4 | 1% | 14 | 3% | 18 | 4%§ | |
| Immunocompromised | 9 | 2% | 3 | 1% | 12 | 3%§ | |
| Chronic neurological/Neuromuscular disease | 3 | 1% | 6 | 1% | 9 | 2%§ | |
| Czhronic kidney disease | – | – | 5 | 1% | 5 | 1%§ | |
| Chronic liver disease | – | – | 5 | 1% | 5 | 1%§ | |
| Two or more comorbidities‖ | 25 | 6% | 100 | 22% | 125 | 28%a | |
| Time interval (date of onset of symptoms—date of notification) mean (SD∗) | 3.8 (3.4) | .000a | |||||
| Clinical presentation | .000a | ||||||
| Asymptomatic cases | 236 | 6% | 1407 | 37% | 1643 | 43% | |
| Symptomatic cases | 434 | 11% | 1750 | 46% | 2184 | 57% | |
| Mild | 244 | 11.2% | 1033 | 47.3% | 1277 | 58%¶ | |
| Cough | 31 | 1.4% | 129 | 5.9% | 160 | 7% | |
| Fever | 22 | 1.0% | 129 | 5.9% | 151 | 7% | |
| Headache | 1 | 0.05% | 29 | 1.3% | 30 | 1% | |
| Sore throat | 22 | 1.0% | 83 | 3.8% | 105 | 5% | |
| Myalgia | 5 | 0.2% | 17 | 0.8% | 22 | 1% | |
| Runny nose | 9 | 0.4% | 14 | 0.6% | 23 | 1% | |
| Arthralgia | - | - | 1 | 0.05% | 1 | 0.05% | |
| More than one mild symptom | 154 | 7.1% | 631 | 28.9% | 785 | 36% | |
| Moderate | 110 | 5.0% | 420 | 19.2% | 530 | 24%¶ | |
| Abdominal pain | 1 | 0.05% | 5 | 0.2% | 6 | 0.3% | |
| Vomiting | 1 | 0.05% | 1 | 0.0% | 2 | 0.1% | |
| Diarrhea | 1 | 0.05% | 10 | 0.5% | 11 | 1% | |
| Viral pneumonia | 40 | 1.83% | 199 | 9.1% | 239 | 11% | |
| More than one moderate symptom | 67 | 3.1% | 205 | 9.4% | 272 | 12% | |
| Severe | 40 | 1.8% | 149 | 6.8% | 189 | 9% | |
| Shortness of breath | 3 | 0.1% | 10 | 0.5% | 13 | 0.6% | |
| Chest pain | 3 | 0.1% | 5 | 0.2% | 8 | 0.4% | |
| Severe viral pneumonia | 1 | 0.05% | 2 | 0.1% | 3 | 0.1% | |
| More than one severe symptom | 33 | 1.5% | 132 | 6.0% | 165 | 8% | |
| Critical | 10 | 0.5% | 37 | 1.7% | 47 | 2%¶ | |
| ARDS∗ | 7 | 0.3% | 26 | 1.2% | 33 | 2% | |
| Sepsis | 3 | 0.1% | 5 | 0.2% | 8 | 0.4% | |
| Renal failure | - | - | 6 | 0.3% | 6 | 0.3% | |
| Missing symptoms | 30 | 1.4% | 111 | 5.1% | 141 | 6%¶ | |
| Outcome | .004a | ||||||
| Not admitted to hospital | 298 | 8% | 1564 | 41% | 1862 | 49% | |
| Admitted to hospital | 269 | 7% | 1056 | 28% | 1325 | 35% | |
| Not admitted to ICU∗ | 264 | 20% | 1010 | 76% | 1274 | 96%# | |
| Admitted to ICU∗ | 5 | 0.4% | 46 | 3% | 51 | 4%# | |
| Missing | 103 | 3% | 537 | 14% | 640 | 17% | |
| Deaths | – | 4 | 0.1% | 4 | 0.1%∗∗ | ||
Percentages may not total 100 because of rounding.
Chi-square test for qualitative variables and t test for quantitative variables were performed to compare male cases to female cases.
UAE = United Arab Emirates, ARDS = acute respiratory distress syndrome, SD = Standard deviation, ICU = intensive care unit.
Including Comoros, Western Sahara Region, and South Sudan.
Housewives, retired elderly, and children aged 0–18 yrs.
Percentages were calculated out of 448.
Diabetes and hypertension were the most reported comorbidities (n = 47).
Percentages for symptomatic cases were calculated out of 2184.
Percentages were calculated out of 1325.
Percentage was calculated out of 3827.
Significance level at P-value < .05.
3.1. Occupation and workplace exposure levels
Of the 3827 cases, the majority (2755, 72%) were from the lower exposure risk occupations, comprised mostly of office workers and other workers with minimum contact with the public. 628 (16%) cases were from medium exposure risk, and only 110 (3%) cases had high exposure risk occupations, including healthcare workers. The remaining 334 (9%) cases included children aged 0 to 18, housewives, and retired elderly (Table 1).
3.2. Notifications and onset of symptoms
The interval between the onset of symptoms and date notification by the healthcare facilities ranged from 0 to 28 days (mean 3.84 days with SD of 3.4 days and median 3 days). In most cases, 3777 (98.7%) were notified within 14 days from the day of onset of symptoms. This interval was longer in 50 patients (1.3%) with mild clinical symptoms.
The daily number of notifications was low in the earlier study period but steadily increased with its peak seen in April, with 419 notifications on April 22 (Fig. 2).
Figure 2.
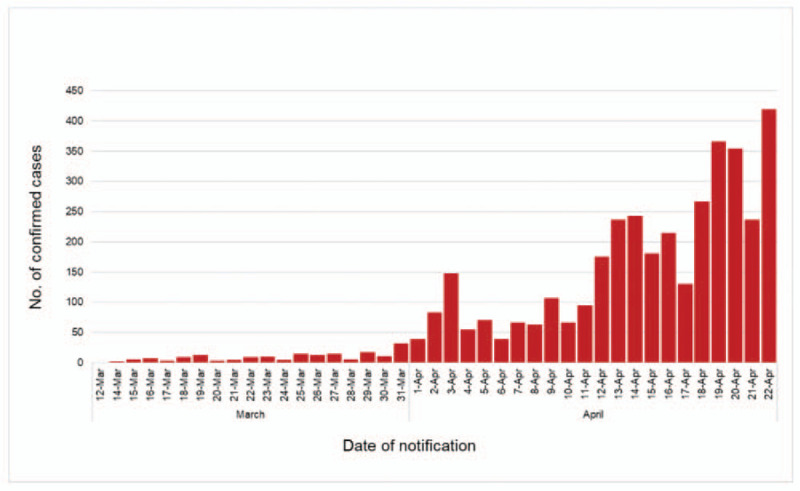
Date of notification and number of COVID-19 confirmed cases reported in the Emirate of Abu Dhabi between March 12 and April 22, 2020 (n = 3827). COVID-19 = Coronavirus Disease 2019.
3.3. Comorbidity
Only 448 (12%) cases had reported comorbidities, which was higher in men (9%) compared with women (3%) (P = .001). The most common comorbidities were hypertension (27%) and diabetes (21%) (Table 1). Of those with comorbidities, 125 (28%) cases had ≥2 comorbidities, with the most common being diabetes and hypertension in 47 patients. Presence of chronic pulmonary disease, chronic liver disease, and having >1 comorbidity were significantly associated with the admission to the hospital (P = .038, .35, .019 respectively) (Table 2).
Table 2.
Multivariable factors of hospitalized versus non-hospitalized.
| Total (N = 3827) | 95% confidence interval | |||
| Gender | N (%) | P-value | Lower | Upper |
| Male | 3157 (82%) | .64 | −0.082 | 0.051 |
| Female | 670 (18%) | Reference | ||
| Age, y | ||||
| Median (IQR∗) | 35 (28–43) | .595 | −0.001 | 0.003 |
| Nationality | ||||
| UAE† | 540 (14%) | .735 | −0.295 | 0.208 |
| Other Arab countries | 435 (11%) | .394 | −0.363 | 0.143 |
| Non-Arab countries | 2815 (74%) | .411 | −0.347 | 0.142 |
| Unknown | 37 (1%) | Reference | ||
| COVID-19 workplace exposure risk levels | ||||
| High exposure risk | ||||
| Healthcare workers | 110 (3%) | .435 | −0.224 | 0.096 |
| Medium exposure risk | ||||
| Labourers and workers at high-density workplaces | 628 (16%) | .000a | −0.377 | −0.17 |
| Lower exposure risk | ||||
| Office workers and workers with minimum contact with public | 2755 (72%) | .348 | −0.132 | 0.046 |
| Other | 334 (9%) | Reference | ||
| Comorbidities | ||||
| No comorbidities | 3379 (88%) | Reference | ||
| With comorbidities | 448 (12%) | |||
| Hypertension | 120 (27%) | .099 | −0.022 | 0.252 |
| Diabetes | 96 (21%) | .088 | −0.020 | 0.284 |
| Pregnancy | 36 (8%) | .231 | −0.096 | 0.397 |
| Chronic pulmonary disease | 22 (5%) | .038a | 0.018 | 0.635 |
| Cardiovascular disease | 18 (4%) | .057 | −0.010 | 0.67 |
| Immunocompromised | 12 (3%) | .302 | −0.198 | 0.638 |
| Chronic neurological/neuromuscular disease | 9 (2%) | .097 | −0.073 | 0.885 |
| Chronic kidney disease | 5 (1%) | .619 | −0.481 | 0.808 |
| Chronic liver disease | 5 (1%) | .035a | 0.047 | 1.329 |
| Two or more comorbidities | 125 (28%) | .019a | 0.028 | 0.311 |
| Time interval (date of onset of symptoms–date of notification) (mean = 3.84) | ||||
| Median (IQR∗) | 3 (1–5) | .000a | 0.006 | 0.02 |
| Clinical presentation | ||||
| Asymptomatic cases | 1643 (43%) | .000a | 0.269 | 0.522 |
| Symptomatic cases | 2184 (57%) | |||
| Mild | 1277 (58%) | .000a | 0.187 | 0.443 |
| Moderate | 530 (24%) | .001a | 0.091 | 0.364 |
| Severe | 189 (9%) | .000a | 0.305 | 0.627 |
| Critical | 47 (2%) | .004a | 0.119 | 0.615 |
| Missing symptoms | 141 (6%) | Reference | ||
Percentages may not add to 100 because of rounding.
Multiple logistic regression was used to predict factors which may be associated with COVID-19 outcomes: admission to hospital.
Chronic pulmonary disease, and chronic liver disease individually contribute to the COVID-19 cases outcomes: admitted to hospital versus not admitted to hospital.
IQR = Interquartile range.
UAE = United Arab Emirates.
Significance level at P-value < .05.
3.4. Clinical characteristics and outcomes
There were 1643 (43%) cases with no symptoms. The proportion of asymptomatic and symptomatic cases was significantly higher in men than women (P = .0001); 37% versus 6% and 46% versus 11%, respectively. Out of 2184 (57%) symptomatic cases, 1277 (58%) patients had mild disease, 530 (24%) patients had moderate disease, 189 (9%) subjects had severe disease, 47 (2%) cases had the critical disease, and in 141 (6%) cases symptoms were not documented, (Table 1). Single symptoms reported were dry or productive cough in 160 cases (7%), fever in 151 (7%), and sore throat in 105 (5%) (Fig. 3). Multiple symptoms were reported in 1222 (56%) cases. There were 242 cases (11%) with viral pneumonia, and of these, only 3 patients had severe viral pneumonia. Complications in those with the critical disease were higher in men (P = .004) and included ARDS (2%), sepsis (0.4%), and renal failure (0.3%) of all symptomatic cases (Table 2).
Figure 3.
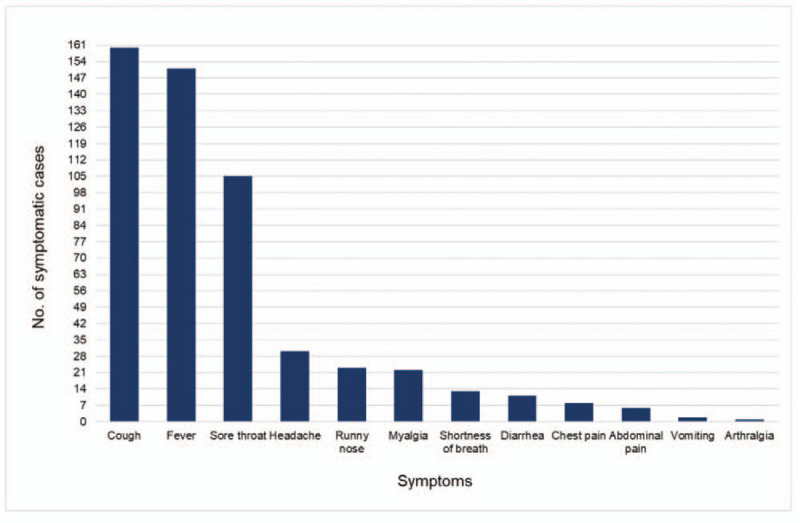
Types of single symptom reported of COVID-19 confirmed cases in the Emirate of Abu Dhabi between March 12 and April 22, 2020 (n = 532). COVID-19 = Coronavirus Disease 2019.
There were 1862 (49%) cases that did not require hospital admission whereas, 1325 (35%) patients required hospital admission, and in the remaining 640 cases (17%), this information was missing (Table 1). Among those admitted to the hospital, only 51 (4%) required ICU admission, of whom 3 patients died (6%). Of those admitted to ICU, 16 cases had comorbidities while 35 had none. Of the 16, diabetes was the most common comorbidity (10/16 patients, 63%), with cardiovascular disease, the second most common (5/16 cases, 31%). There were only 4 deaths in our dataset: all men; 3 patients died in the ICU, and 1 died in the community.
4. Discussion
To the best of our knowledge, this study is the first descriptive analysis of COVID-19 in the UAE, using a dataset extracted from the IDNSS. There was a low number of notifications at the beginning of our study period in March 2020. However, numbers increased gradually, with its peak seen in April 2020. Most of the cases were men (82%) with a median age of 35 years.
The highest number of cases were aged between 19 and 49 years (81%), which is reflected mainly by the UAE population's demographic structure, where most of the expatriate population are between 20 and 50 years. Furthermore, we observed highest infections rate among men in comparison with women. The large young male expatriate population is usually employed in the private sector in sales and services, construction, hospitality, domestic, and retail.[10] Majority of expatriate men tend to live in dormitories and camps with a high chance of transmitting the infection rapidly. In comparison, female expatriates are predominantly demostic staff and shop workers,[9] thus having low chance of infection than their male counterparts.
Transmission among healthcare workers seems to be very minimal (110 cases, 3%), which is very low compared with other countries such as in China 6.7% and 16% among healthcare professionals in the United States of America (USA).[20,21] This may be due to the easy access and availability of personal protective equipment to all frontline healthcare workers and strict infection control measures.
At least 43% of our cases were asymptomatic with the potential to transmit their infection to others.[22] A similar finding was reported from Iceland.[23] However, we do not know if these patients were actually at a presymptomatic stage who developed symptoms later. Other countries like China have reported only 1.2% to be asymptomatic.[24] Hence, it is crucial that public health measures are followed strictly by asymptomatic cases to prevent its spread to other people in the community. A high percentage of asymptomatic patients compared with other countries is attributed to the availability of testing, active case finding among high-risk occupations, or contact tracing process.
According to an earlier study from China, 81% of people with COVID-19 had mild or moderate disease (including people without pneumonia and people with mild pneumonia), 14% had severe disease, and 5% had a critical illness.[25] In our study, 83% of symptomatic cases had either mild or moderate disease, which is in line with the available literature. Patients with mild symptoms usually do not need additional evaluation and advised self-isolation.[26] In our study, 49% were not admitted to the hospital, and only 35% of the cases required hospital admission for further monitoring and management. In 645 cases (17%), this information was missing or referred for the home isolation program.
The interval between onset of symptoms and its notification to the DoH is crucial to identifying the source of infections and taking public health actions. Although the range of this period was from 0 to 28 days, the median was only 3 days. This interval has been reported much higher for measles, mumps, and pertussis in a study in the Netherlands: median of 11.5 days, 19 days, and 9 days respectively.[27]
The most common comorbidities in our COVID-19 cases were hypertension and diabetes (27% and 21%, respectively), which were higher than in a study from Saudi Arabia.[28] Diabetes was the most common comorbidity in patients admitted to ICU (10/16 cases, 63%). A meta-analysis of 8 studies, including 46,248 patients with laboratory-confirmed COVID-19, indicated that those with the most severe disease were more likely to have hypertension, diabetes, respiratory disease, and cardiovascular disease.[29] Obesity and smoking were also associated with an increased risk of COVID-19,[30,31] but this information was lacking in our data.
Our study's case fatality rate was 0.1%, which includes 3 deaths in the ICU and 1 death in the community, but as reported elsewhere, it can reach 1%.[32,33] It is possible that data on the ICU and hospital admissions, and deaths may not be captured in our IDNSS as information is collected at the time of reporting. In the UAE, 40% of deaths due to COVID-19 has been linked to diabetes.[34] Cumulative confirmed COVID-19 deaths per million population up to July 11, 2020 for the UAE is low (33) compared with United Kingdom (657), Italy (578), France (460), and USA of (405).[35] This may be explained by the UAE's smaller number of the elderly and high-risk populations and the strict implementation of public health measures at the early stage of pandemic and its monitoring.
In the UAE, the COVID-19 testing program includes testing for all symptomatic, asymptomatic cases, and their contacts; a total of 4,508,928 tests were done from January 29 to July 19, 2020 (45,588.9 per 100,000 population) with the positivity rate of 0.4%.[36]
Public Health measures are strictly monitored and include surveillance, contact tracing, borders closure, strict lockdown, personal hygiene measures (handwashing, wearing face masks in public, social distancing), screening at the airports, and provision of facilities like screening, testing, isolation, quarantine, and hospital care.
Our study has many strengths. It is based on routinely collected data, which tends to be complete, comprehensive, regularly updated, and therefore reflective of current trends, and contains information on demographics, clinical characteristics, and outcomes.
This study has certain limitations as it was retrospective and contains information collected at the time of COVID-19 case notification for public health purposes. We could not fully assess the final outcomes at the end of the illness. Symptoms were self-reported and may be subject to recall bias, especially regarding their onset and type. Although these notifications are compulsory, data may still be incomplete or absent.
In conclusion, this study describes the demographic data, clinical features, and outcome of the 3827 confirmed cases of COVID-19. The Emirate of Abu Dhabi has implemented aggressive measures at the early stages of the pandemic to contain local transmission of COVID-19 such as wearing of face masks in public, social distancing, drive-through COVID-19 testing, strict lockdown measures, movement restrictions, and border closures, which reduced the spread of COVID-19 and led to fewer deaths. Given the constantly evolving nature of COVID-19 pandemic, and public health value of surveillance data, we recommend the application of rigorous measures to maintain high quality surveillance data. Furthermore, non-pharmaceutical interventions such as mandate to wear facial covering, emphasize on social distancing, personal hygiene, awareness, and education campaigns, and in certain cases stay-at-home orders will eventually contribute to control the pandemic in the Emirates of Abu Dhabi and across the entire UAE.
Acknowledgments
The authors are thankful to Prof Nico Nagelkerke for the manuscript review. Dr Walid A. Zaher, Dr Rami Beiram, and Prof Abdul Souid for their constructive guidance and suggestions during this research planning and development.
They also thank the staff of the Department of Health and The Abu Dhabi Public Health Center frontline public health workers who collected these essential data.
Author contributions
Conceptualization: Farida Al Hosani, Bashir Aden, Shammah Al Memari, Shereena Al Mazrouei, Ahmed R. Alsuwaidi, Michal Grivna, Mohamud Sheek-Hussein.
Data curation: Suad Ajab.
Formal analysis: Suad Ajab.
Methodology: Michal Grivna, Marília Silva Paulo, Muhammad Abid, Mohamud Sheek-Hussein.
Project administration: Mohaud Sheek-Hussein.
Validation: Suad Ajab, Bashir Aden, Michal Grivna, Marília Silva Paulo, Muhammad Abid, Mohamud Sheek-Hussein.
Writing – original draft: Suad Ajab, Mohamud Sheek-Hussein.
Writing – review & editing: Bashir Aden, Ahmed R. Alsuwaidi, Michal Grivna, Marília Silva Paulo, Muhammad Abid, Mohamud Sheek-Hussein.
Footnotes
Abbreviations: 2019-nCoV = 2019 Novel Coronavirus, ADPHC = Abu Dhabi Public Health Center, ARDS = acute respiratory distress syndrome, COVID-19 = Coronavirus Disease 2019, DoH = Department of Health, ICU = intensive care unit, IDNSS = Infectious Diseases Notification Surveillance System, IQR = interquartile ranges, RT-PCR = real-time reverse transcription polymerase chain reaction, SARS-CoV-2 = Severe Acute Respiratory Syndrome Coronavirus 2, SD = standard deviation, STROBE = Strengthening Reporting of Observational Sutdies in Epidemiology, UAE = United Arab Emirates, USA = United States of America, WHO = World Health Organization.
How to cite this article: Hosani FA, Aden B, Memari SA, Mazrouei SA, Ajab S, Abid M, Alsuwaidi AR, Grivna M, Paulo MS, Sheek-Hussein M. Epidemiology of asymptomatic and symptomatic Coronavirus Disease 2019 confirmed cases in the Emirate of Abu Dhabi, United Arab Emirates: Observational study. Medicine. 2021;100:12(e25219).
The authors declare that they have no competing interests.
There was no funding for this research study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for Global Health Governance. JAMA 2020;323:709–10. [DOI] [PubMed] [Google Scholar]
- [2].Uddin M, Mustafa F, Rizvi TA, et al. SARS-CoV-2/COVID-19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses 2020;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou Y, Hou Y, Shen J, et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 2020;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khan G, Sheek-Hussein M, Al Suwaidi A, et al. Novel coronavirus pandemic: a global health threat. Turkish J Emerg Med 2020;20:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization. WHO/Europe Coronavirus disease (COVID-19) outbreak - about the virus; 2020. Available at: http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/novel-coronavirus-2019-ncov. Accessed April 27, 2020. [Google Scholar]
- [6].World Health Organization (WHO). Coronavirus Disease: Situation Report 172. 2020. [Google Scholar]
- [7].Turak N. First Middle East cases of coronavirus confirmed in the UAE. CNBC; 2020. Available at: https://www.cnbc.com/2020/01/29/first-middle-east-cases-of-coronavirus-confirmed-in-the-uae.html. Accessed June 1, 2020. [Google Scholar]
- [8].Duddu P. Covid-19 in the Middle East: Coronavirus-affected countries. Pharmaceutical Techonology; 2020. Available at: https://www.pharmaceutical-technology.com/features/coronavirus-affected-countries-middle-east-covid-19/. Accessed December 27, 2020. [Google Scholar]
- [9].Paulo MS, Loney T, Lapão LV. The primary health care in the emirate of Abu Dhabi: are they aligned with the chronic care model elements? BMC Health Serv Res 2017;17:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Statistics Center of Abu Dhabi. 3. Population and Demography Population Vital Statistics. Abu Dhabi; 2019. Available at: https://www.scad.gov.ae/Release Documents/Statistical Yearbook Population_2019_Annual_Yearly_en.pdf. Accessed April 27, 2020. [Google Scholar]
- [11].Department of Health - Abu Dhabi. Circular n. 33. 19 April 2020. [Google Scholar]
- [12].Abu Dhabi Public Health Center. Scientific Research Monitoring on COVID-19. Dep Heal. 2020. Available at: https://doh.gov.ae/en/covid-19/Healthcare-Professionals/Scientific-Publication. Accessed December 27, 2020. [Google Scholar]
- [13].Department of Health - Abu, Dhabi. Communicable diseases bulletin. Qtly Summ Rep 2018;8:1–26. [Google Scholar]
- [14].World Health Organization. WHO Guidance for the Use of Annex 2 of the International Health Regulations (2005). WHO Libr Cat Data. 2010:1–60. Available at: http://www.who.int/ihr/revised_annex2_guidance.pdf?ua=1. Accessed December 27, 2020. [Google Scholar]
- [15].UAE Government. Abu Dhabi - The Official Portal of the UAE Government; 2020. Available at: https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi. Accessed June 1, 2020. [Google Scholar]
- [16].U.S. Department of Labor. Guidance on preparing workplaces for COVID-19. OSHA 2020;35:1–35. [Google Scholar]
- [17].World Health Organization. Considerations for public health and social measures in the workplace in the context of COVID-19. World Heal Organ. 2020:1–7. Available at: https://www.who.int/publications/i/item/considerations-for-public-health-and-social-measures-in-the-workplace-in-the-context-of-covid-19. Accessed December 27, 2020. [Google Scholar]
- [18].Varghese G, John R, Manesh A, et al. Clinical management of COVID-19. Indian J Med Res 2020;151:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].International Classification of Diseases. ICD-10 Version: 2015. IDC-10; 2015. Available at: https://icd.who.int/browse10/2015/en#/I. Accessed December 27, 2020. [Google Scholar]
- [20].Centers for Disease Control and Prevention. CDC COVID Data Tracker. Centers Dis Control Prev. 2020;2020:6–7. Available at: https://covid.cdc.gov/covid-data-tracker/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-updates%2Fcases-in-us.html#cases_casesper100klast7days. Accessed December 27, 2020. [Google Scholar]
- [21].CDC Weekly C. The epidemiological characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — China, 2020. China CDC Wkly 2020;2:113–22. [PMC free article] [PubMed] [Google Scholar]
- [22].WHO. Coronavirus disease: situation report 73. World Heal Organ 2020;2019:1–4. [Google Scholar]
- [23].Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med 2020;382:2302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zazhi Z liu xing bing xue za zhi = Z liuxingbingxue. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. CDC China. 2020;41:145–151. [DOI] [PubMed] [Google Scholar]
- [25].Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- [26].Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid-19. N Engl J Med 2020;383:1757–66. [DOI] [PubMed] [Google Scholar]
- [27].Marinović AB, Swaan C, van Steenbergen J, et al. Quantifying reporting timeliness to improve outbreak control. Emerg Infect Dis 2015;21:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alsofayan YM, Althunayyan SM, Khan AA, et al. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health 2020;13:920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huang R, Zhu L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi-center study. Santiago H da C, ed. PLoS Negl Trop Dis 2020;14:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ferguson N, Laydon D, Nedjati Gilani G, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand; 2020:1–20. Available at: https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf. Accessed December 27, 2020. [Google Scholar]
- [33].Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med 2020;26:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gautam S. Coronavirus: about 40% of UAE deaths linked to diabetes - The National. The National; 2020. Available at: https://www.thenationalnews.com/uae/health/coronavirus-about-40-of-uae-deaths-linked-to-diabetes-1.1034114. Accessed December 27, 2020. [Google Scholar]
- [35].Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. Coronavirus Pandemic (COVID-19) - Statistics and Research - Our World in Data. Our World Data; 2020. Available at: https://ourworldindata.org/coronavirus. Accessed December 27, 2020. [Google Scholar]
- [36].UAE Ministry of Cabinet Affairs. UAE Covid 19 Updates; 2020. Available at: https://fcsa.gov.ae/en-us/Pages/Covid19/UAE-Covid-19-Updates.aspx. Accessed December 27, 2020. [Google Scholar]


