Abstract
Purpose:
To create and test a multi-purpose brachytherapy catheter prototype enabling intratumoral injection and brachytherapy following a single catheter insertion.
Methods and Materials:
The design of the prototype consists of an outer tube and an inner syringe tube that can be filled with injectable agent. The outer sheath and inner syringe tube were constructed using PTFE tubing and the other components were 3D printed using dental resin and PLA material. To demonstrate functionality we injected in vitro phantoms with dyed saline. For proof of concept we demonstrated the potential for the prototype to deliver cell therapy, enhance tumor delineation, deliver tattoo ink for pathology marking, avoid toxicity through local delivery of chemotherapy, and facilitate combination brachytherapy and immunotherapy.
Results:
The prototype enables accurate injection in vitro and in vivo without altering dosimetry. To illustrate the potential for delivery of cell therapies, we injected luciferase-expressing splenocytes and confirmed their delivery with IVIS imaging. To demonstrate feasibility of radiographically visualizing injected material we delivered iohexol contrast intratumorally and confirmed tumor retention using Faxitron x-ray imaging. Additionally, we show the potential of intratumoral administration to reduce toxicity associated with cyclophosphamide compared to systemic administration. To demonstrate feasibility, we treated tumor bearing mice with brachytherapy (192Ir source, 2 Gy to 5 mm) in combination with intratumoral injection of 375,000 U of interleukin 2 (IL-2) and observed no increased toxicity.
Conclusions:
These results demonstrate that a prototype multi-purpose brachytherapy catheter enables accurate intratumoral injection and support the feasibility of combining intratumoral injection with brachytherapy.
Keywords: Multi-purpose brachytherapy catheter, Brachytherapy, Intratumoral injection, Cell therapy, Interleukin 2, Contrast enhanced imaging
Introduction
Brachytherapy is a part of the standard of care treatment for many cancers including prostate and cervical, and plays a role as an adjuvant therapy for others such as breast and head and neck cancers1. High dose rate brachytherapy (HDR) typically requires placement of catheters within the tumor to enable safe delivery of radiation via an 192Ir source2. To our knowledge, brachytherapy has not been combined with local or intratumoral injection of additional diagnostic or therapeutic agents to date. However, the placement of a brachytherapy catheter could enable simultaneous intratumoral injection if a multi-purpose catheter were available for this application. Similar to the inherent normal tissue sparing afforded by brachytherapy, local delivery of injectable agents can reduce toxicity associated with systemic administration3. This potential for local dose escalation of both therapeutic radiation and injectable therapies with improved safety makes such combination treatment approaches an attractive strategy4.
There is growing interest in intratumoral approaches to delivering therapies for cancer5–10. In addition to the FDA-approved oncolytic virus T-VEC, which is administered via intratumoral injection11, there are a multitude of trials underway at various stages investigating intratumoral injection of immunotherapy classes that target both the innate and adaptive arms of the immune system12. Several of these immunotherapies are also being explored in combination with radiation13. Additionally, cell-based therapies administered via intratumoral injection are being explored, including dendritic cell and natural killer cell therapies14–17. Intratumoral administration may also enhance the efficacy of already established cell therapies including CAR-T cells which are approved for select hematologic malignancies18–22. To date, CAR-T cells have shown poor efficacy towards solid tumors, in part due to poor infiltration, and intratumoral administration may help to overcome this23.
In addition to directly delivering therapy, catheters placed for brachytherapy could enable new lines of experimentation to develop strategies to aid in imaging, pathology, and research. Intratumoral injection of contrast may allow for radiographic tracking of the spatio-temporal distribution of injected material. Contrast injection potentially could also assist in accurate tumor visualization and contouring during 2D treatment planning in resource poor settings where more advance imaging is not available or in patients with co-morbidities that prohibit systemic use of contrast. Additionally, it may be beneficial to mark the dwell position of a brachytherapy source and margins of the tumor with a biocompatible substance such as tattoo ink. This may be particularly useful for research purposes, quality improvement initiatives, and for assisting pathologic analysis of surgically resected tumors that have received brachytherapy or intratumoral injections. Here we describe the development and proof-of-concept testing of a multi-purpose brachytherapy catheter that enables brachytherapy and intratumoral injection from a single catheter insertion.
Materials and Methods
Design and use of multi-purpose brachytherapy catheter
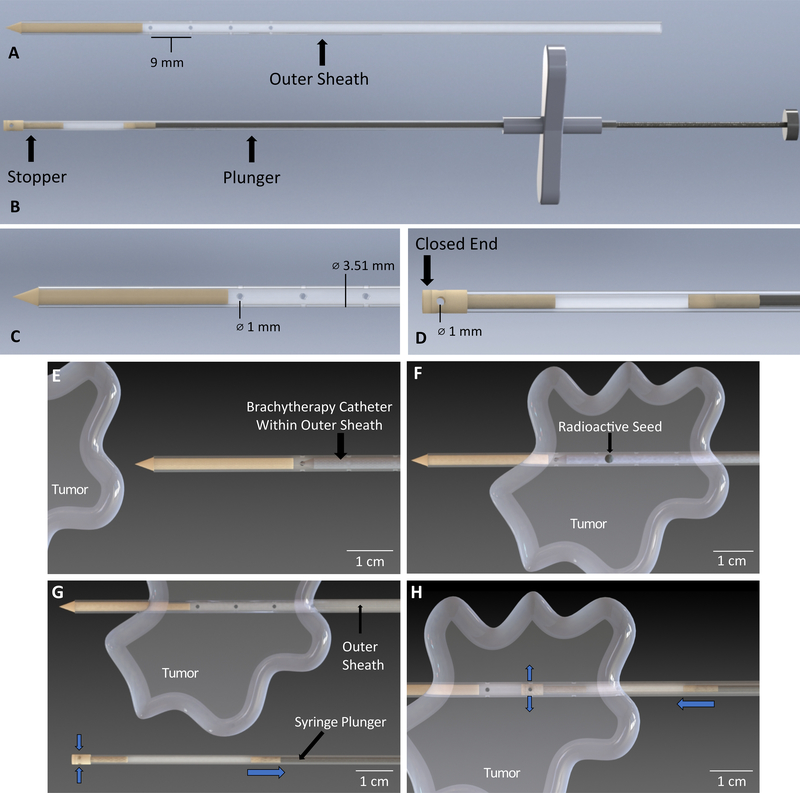
The multi-purpose brachytherapy catheter prototype consists of two main components, the outer sheath and inner tube syringe. All components in direct contact with the patient are biocompatible. The outer sheath consists of a larger polytetrafluoroethylene (PTFE) tube with a bonded dental resin tip used for puncturing through skin and tissue (Fig 1A). Sets of transverse holes are located along the tubing (Fig 1C). The syringe consists of a smaller PTFE tube with a hollow stopper bonded to the end of the tube (Fig 1B). This stopper also has transverse holes on its end and one closed face (Fig 1D). Within the inner tube is a stainless steel wire plunger with a dental resin plunger cap bonded to it. The plunger cap is flush with the inner tube, thus allowing liquid to be drawn into the tube via negative pressure when the plunger is pulled back. Figure 1, Supplemental Figure 1 additionally demonstrates use of the multi-purpose brachytherapy catheter during treatment. During the procedure, the outer sheath containing the brachytherapy catheter is placed in the tumor (Fig 1E,F). Brachytherapy is performed as done in current practice (Fig 1F). After treatment, the brachytherapy catheter is removed, with the outer sheath remaining in the tumor (Fig 1G). Before inserting the inner tube syringe into the outer sheath, the injectable agent is drawn up into the inner tube syringe to the appropriate volume using the μL markings of the syringe tube. The inner tube syringe is placed inside of the outer sheath (Fig 1H). The user aligns the holes on the stopper to holes on the outer sheath according to markings on the syringe tube and injects the agent.
Figure 1.
Depiction of the multi-purpose brachytherapy catheter prototype design and use, showing A) The outer sheath, consisting of a tip used for puncturing skin and flexible tubing with four sets of transverse 1.00 mm holes separated by a distance of 9.00mm; B) The inner tube syringe with plunger shown to the right and the hollow stopper shown to the left; the syringe is used for drawing in and holding the immunotherapeutic fluid before injection; C) Close-up view of the outer sheath showing outer diameter of tube to be 3.51 mm and diameter of hole to be 1 mm; D) Close-up view of the inner tube syringe where the diameter of the hole on the stopper is 1 mm. E) The brachytherapy catheter is inserted into the tube of the outer sheath. The device was designed to accommodate an existing brachytherapy catheter that is commercially available and does not alter that brachytherapy catheter in any way; F) The outer sheath is inserted into the tumor and brachytherapy is performed; G) The brachytherapy catheter is removed from the outer sheath and fluid containing the immunotherapeutic agent is drawn into the syringe by pulling back the syringe plunger (blue arrows indicate direction of fluid flow); H) The syringe is inserted into the outer sheath and the holes are aligned using markings on the inner syringe tube. The plunger is pushed down to release fluid into the tumor (blue arrows indicate direction of fluid flow).
Fabrication
The syringe stopper, plunger cap, catheter tip, plunger, and syringe handles were designed in SolidWorks. An Ultimaker S5 (manufactured by Ultimaker) printer was used to print the plunger and syringe handles using PLA material per manufacturer recommendations. The other pieces were printed in a Form 2 printer (manufactured by FormLabs) using dental resin material per manufacturer recommendations. Materials were selected based on biocompatibility, expectation of minimal impact on brachytherapy dosimetry, and design strength. Supplemental Figure 1 depicts the workflow of the 3D printing process. Individual .STL files are included as a supplement.
The PTFE tubing used for the outer sheath has an outer diameter of 3.51 mm and the one used for the inner tube syringe has an outer diameter of 2.5 mm. Both tubes were cut to 15 cm length using scissors. The transverse holes on the outer sheath were perforated by hand, using a 1 mm diameter needle. The dental resin pieces, cone tip on outer sheath and stopper on syringe, were glued onto one end of the tubes using cyanoacrylate glue.
The plunger was fabricated out of a rigid stainless-steel wire with a diameter of 1.38mm. It was cut to 20 cm length and filed down on one end to a diameter of 0.96mm. The dental resin syringe cap was glued to the smaller diameter end of the wire using cyanoacrylate glue. The cap was also filed until it was flush with the inner tube syringe. The PLA parts were adhered to the inner tube syringe and stainless-steel plunger using cyanoacrylate glue.
Treatment planning and dosimetry analysis
We created a phantom consisting of a plastic reservoir filled with water. The multi-purpose catheter with and without the outer sheath was placed in the water reservoir and the chamber was closed with thermoplastic bolus molded to include a notch for reproducible thermoluminescent dosimeter (TLD) placement (n=3 TLDs per condition). A dose of 2 Gy was prescribed to the TLD using Oncentra software (Elekta). TLDs were analyzed by the University of Wisconsin-Madison Radiation Calibration Laboratory (Calibration Cert # 1664.01). For calculation of predicted attenuation of PTFE material compared to water we used the attenuation formula where N is beam energy through the material, N0 is the initial beam energy for 192Ir, μ/ρ is the mass attenuation coefficient, 𝜌 is density of material, and X is material thickness. An average beam energy of 354 keV as calculated from prior reports was used in the calculation24.
Fluid Distribution and Volume Delivery in Chicken Breast Tissue
Chicken breast was used as a phantom. Saline dyed with blue food coloring was used as the injection agent. The multi-purpose brachytherapy catheter was weighed before and after injection to determine liquid retention following injection. After injection, the fluid was allowed to distribute over about 15 seconds and the multi-purpose brachytherapy catheter was removed and weighed as mentioned above. The distribution of liquid was visualized by cutting through the insertion site of the multi-purpose brachytherapy catheter.
Tumor models
B78-D14 (B78) melanoma was obtained from Ralph Reisfeld (Scripps Research Institute) in 2002. B78 melanoma is a poorly immunogenic GD2+ cell line derived from B16 melanoma25. B78 cells are syngeneic for C57BL/6 mice and were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2mM L-glutamine, 100U/mL penicillin and 100μg/mL streptomycin. The MyC-CaP prostate cell line was obtained from ATCC. MyC-CaP cells are syngeneic for FVB mice and were grown in DMEM supplemented with 10% heat-inactivated FBS, 100U/mL penicillin and 100μg/mL streptomycin. Mycoplasma testing was done using PCR methodology previously described. All cells used were confirmed to be negative for Mycoplasma prior to injection into mice. Cell authentication was performed per ATCC guidelines using morphology, growth curves, and mycoplasma testing. Cells were typically cultured for no more than 5 passages prior to use (~ 2 weeks) and replicate freezer stocks were thawed and utilized for each experiment.
Animals
Mice were housed in accordance with the Guide for Care and Use of Laboratory Mice and experiments were performed under an animal protocol approved by the institutional animal care and use committee. C57BL/6 female mice and FVB male mice at age 5–7 weeks were purchased from Taconic. B78 and MyC-CaP tumors were engrafted by subcutaneous flank injection of 2×106 and 1×106 tumor cells respectively, diluted in 100 μL phosphate-buffered saline (PBS). Tumor size was determined by precision caliper measurement conducted by personnel blinded to treatment assignment. Tumor volume was approximated as (tumor volume in mm3) = [(tumor width in mm)2 x (tumor length in mm)]/2. Mice were randomized immediately prior to initiating treatment in all experiments. We defined the initial day of brachytherapy treatment as “day 1” and treatment of well-established tumors began ~ 5 weeks after primary tumor implantation.
Mice receiving brachytherapy were placed under isoflurane anesthesia and the multi-purpose catheter was inserted into the tumor. The catheter was cleansed between applications using 70% ethanol flush and wipe. The 192Ir high dose rate source was dispensed via Flexitron remote afterloader (Elekta) into a flexi-needle (Best Medical). Brachytherapy was prescribed as 2 Gy to a depth of 5 mm. Mice receiving IL-2 (acquired from NCI) were injected with 375,000 U immediately following brachytherapy using the multi-purpose brachytherapy catheter as described above (n=6 mice per group). We sacrificed mice when tumors exceeded 18 mm in any dimension, or whenever recommended by an independent animal health monitor for morbidity or moribund behavior. Mice receiving cyclophosphamide were injected either intraperitonally via standard 30 gauge needle and syringe or intratumorally with 500 mg/kg of cyclophosphamide using the multi-purpose brachytherapy catheter (n= 3 mice per group). Immediately prior to injection and 7 days post injection peripheral blood was collected via maxillary bleed and a complete blood count with differential was performed using a VetScan HM5 (Abaxis). In all experiments mice were monitored for toxicity at least twice weekly which consisted of evaluation of posture, overall behavior, activity level, skin appearance, and grooming.
Bioluminescence imaging
MyC-CaP tumor bearing mice were put under isoflurane anesthesia and placed in an In Vivo Imaging System (IVIS, Perkin Elmer). 107 luciferase-expressing murine splenocytes diluted in PBS were mixed with 4 mg luciferin (100 μL injection volume) immediately prior to intratumoral injection. Images were acquired 5, 10, and 15 minutes following injection. The experiment was conducted in triplicate with a representative image reported.
Faxitron imaging
B78 tumor bearing mice were put under isoflurane anesthesia and placed in a Faxitron imaging system (Faxitron UltrafocusDXA). Following intratumoral insertion of the multi-purpose brachytherapy catheter 100 μL of iohexol (Omnipaque, GE Healthcare) contrast was injected and x-ray images were acquired pre, intra, and immediately following injection. The experiment was conducted in triplicate with a representative image reported.
MicroCT imaging
B78 tumor bearing mice were anesthetized with isoflurane and placed on the scanner bed in a prone position. Following intratumoral insertion of the multi-purpose brachytherapy catheter 100 μL of iohexol (Omnipaque, GE Healthcare) contrast was injected and sequential CT (80 kVp; 1000 mAs; 220 angles) was acquired with an Inveon microPET/microCT scanner (Siemens Medical Solutions, Knoxville, TN) immediately following injection. The experiment was conducted in triplicate with a representative image reported.
Statistical methods
Tumor response plots are displayed as means +/− standard error. Survival curves were estimated with the Kaplan–Meier method and compared using log-rank tests. Baseline and day 7 lymphocyte counts were compared using two-tailed two-sample t-tests. Analyses were performed using GraphPad Prism 7 software.
Results
Multi-purpose brachytherapy catheter does not alter treatment planning dosimetry
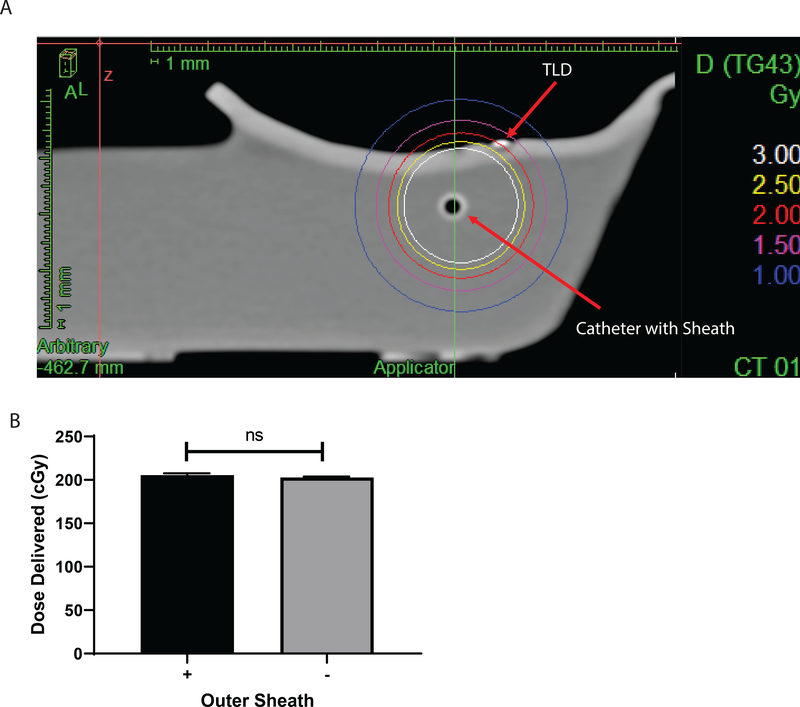
We developed a prototype for a multipurpose catheter that would enable delivery of brachytherapy and intratumoral injections following a single skin insertion (Fig 1). This prototype was developed and implemented here to test proof-of-concept applications. To determine whether the design of the multi-purpose catheter alters standard treatment brachytherapy dosimetry, we created a phantom consisting of a plastic reservoir filled with water. The multi-purpose catheter with and without the outer sheath was placed in the water reservoir and the chamber was closed with thermoplastic bolus molded to include a notch for reproducible TLD placement. A dose of 2 Gy was prescribed to the TLD (Fig 2A). Analysis of the dose delivered to the TLDs with and without the outer sheath presented demonstrated a 1.33% difference between groups which was within the margin of error for TLD measurement (k=2 coverage factor) and not statistically significant (Fig 2 B). Additionally, theoretical calculation for the attenuation of dose by PTFE, the material comprising the outer sheath, predicted approximately a 0.4% attenuation which is in agreement with the TLD findings and acceptable clinically.
Figure 2.
Dosimetry verification of the multi-purpose brachytherapy catheter. A) Treatment planning image of the in vitro phantom with the multi-purpose brachytherapy catheter inserted. A dose of 2 Gy was prescribed to the thermoluminescent dosimeter (TLD). B) Quantification of TLD exposure, n=3 TLDs per group.
Multi-purpose brachytherapy catheter enables accurate delivery of injectable agents
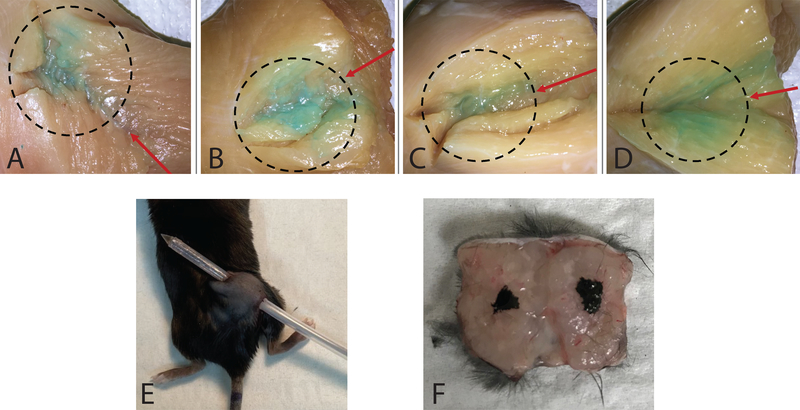
To demonstrate the capacity to enable intratumoral injection, the multi-purpose brachytherapy catheter was inserted into a chicken breast phantom and blue colored saline was injected. Visual inspection demonstrated that the fluid distribution followed the expected path considering the injection direction and method (Fig 3A-D). Using the difference in weight of the multi-purpose brachytherapy catheter before and after injection, we calculated the volume retention to be 4.03% ± 14.6%. To confirm these findings in vivo, we injected tattoo ink intratumorally in mice bearing B78 tumors and following euthanasia excised the tumor along the catheter insertion site (Fig 3E). The distribution of tattoo ink within the tumor confirms accurate injection (Fig 3F).
Figure 3.
Fluid distribution following injection of a tissue phantom. A-D) Blue colored saline was injected into chicken breast samples using the multi-purpose brachytherapy catheter. A cut was made along the entrance hole after injection to visualize fluid distribution. Red arrows indicate catheter entrance direction and dashed circles indicate concentration of fluid distribution. E) Mouse under anesthesia with multi-purpose brachytherapy catheter inserted. F) Tattoo ink for marking dwell positions of brachytherapy seed: tumor removed and cut along catheter insertion tract following injection of tattoo ink.
Multi-purpose brachytherapy catheter enables intratumoral injection of viable cells
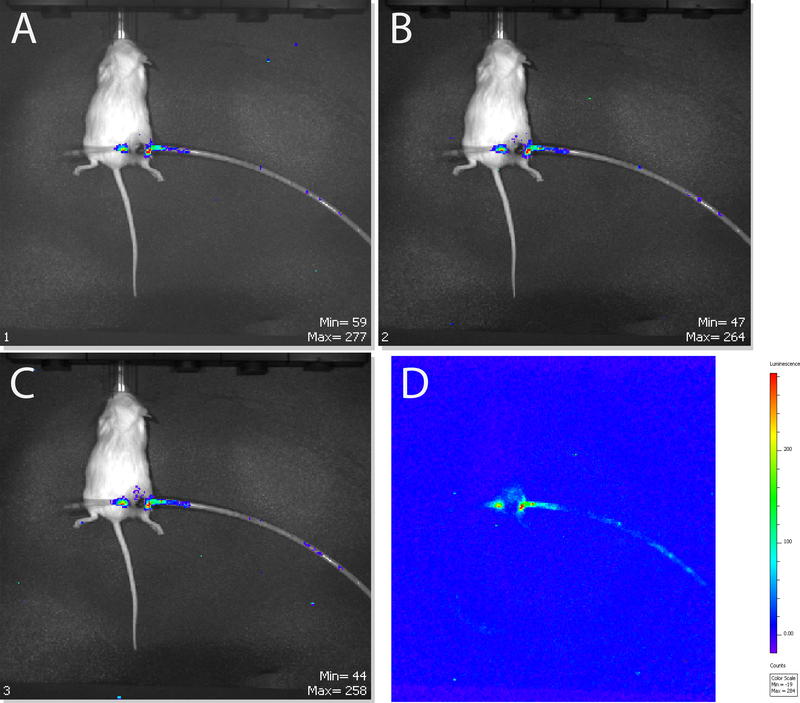
We next sought to determine whether the multi-purpose brachytherapy catheter could be used to deliver cell-based therapies directly to the tumor microenvironment. We used luciferase expressing murine splenocytes as a surrogate for cellular therapy. Mice bearing Myc-CaP tumors were anesthetized and the multi-purpose brachytherapy catheter was inserted into the tumor. IVIS imaging post intratumoral injection of splenocytes revealed detectable bioluminescence signal within the tumor 5 minutes following injection (Fig 4A) which continued to strengthen at 10 and 15 minutes following injection (Fig 4B-D).
Figure 4:
Representative time series IVIS bioluminescence scan of MyC-CaP tumor bearing mice following intratumoral injection of luciferase positive splenocytes at 5 (A), 10 (B), and 15 (C,D) minutes post injection.
Imaging injected material and implanted tumor using contrast
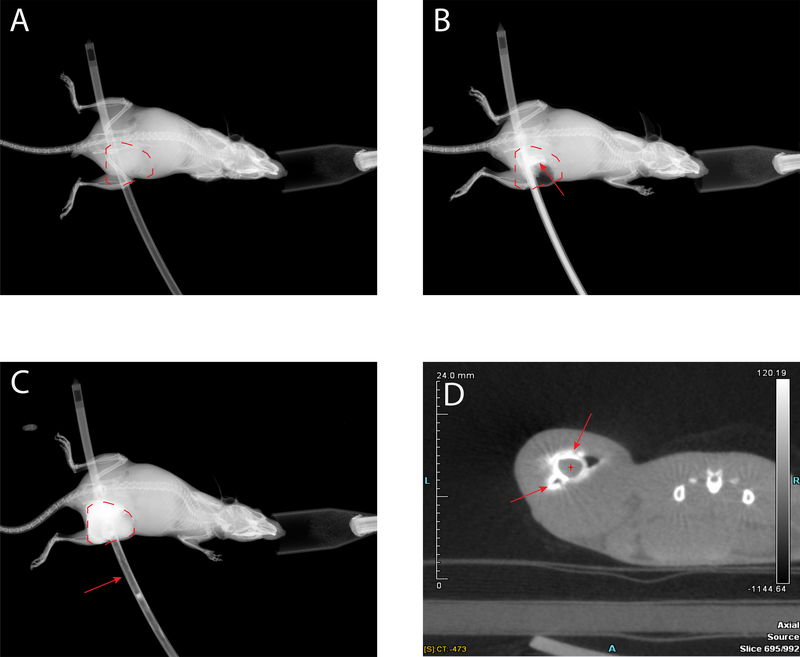
Radiographic visualization of injected material can allow time resolved evaluation of the special distribution of that material following intratumoral injection and this could have important research applications. In addition, adequate tumor visualization remains critical to ensure creation of an appropriate treatment plan that covers the full tumor volume while minimizing toxicity to surrounding normal tissue26, 27. To determine whether intratumoral injection of contrast can enhance tumor visualization with 2D methods that would be potentially applicable in resource-poor settings, we anesthetized mice bearing B78 tumors and using the multipurpose brachytherapy catheter injected iohexol contrast intratumorally. Faxitron x-ray imaging demonstrated enhanced visualization of the injected material and the implanted tumor volume following contrast injection (Fig 5C), compared to pre-contrast injection (Fig 5A, B).
Figure 5:
Contrast enhanced tumor imaging. Dotted red line marks tumor volume. Faxitron x-ray image of mouse with empty catheter inserted (A), catheter loaded with Omnipaque iohexol contrast beginning injection, red arrow points to intratumoral contrast (B), and immediately post injection of contrast, red arrow points to empty catheter (C). D) MicroCT axial image following intratumoral injection of iohexol contrast. Red arrows point to injected contrast, red cross denotes lumen of multi-purpose catheter.
To confirm our 2D imaging findings and demonstrate retention of injected contrast within the tumor, we again anesthetized mice bearing B78 tumors and placed them on the bed of a microCT scanner in the prone position. We used the multi-purpose brachytherapy catheter to inject iohexol contrast intratumorally. MicroCT imaging confirmed intratumoral retention of injected contrast (Fig 5D).
Intratumoral injection enables delivery of therapy with reduced toxicity
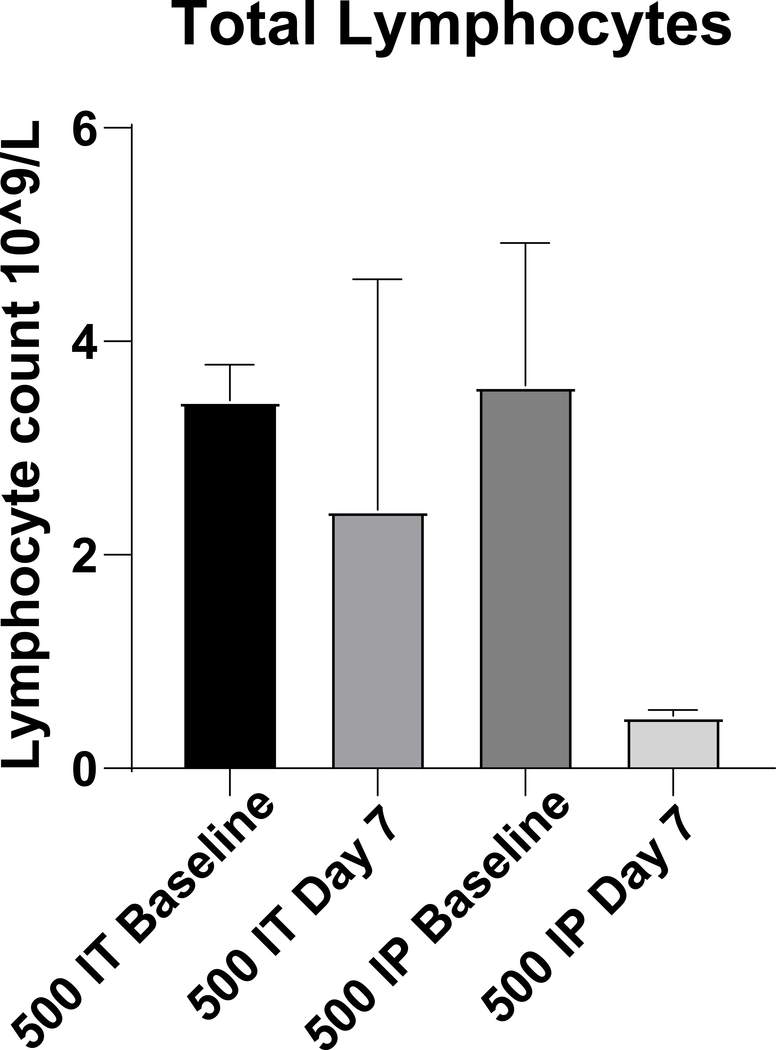
Toxicity associated with systemic administration can limit the use of agents, including certain chemotherapeutics, that might otherwise potentiate the effects of brachytherapy28, 29. To illustrate this point, we tested whether local delivery of cyclophosphamide could avoid systemic toxicity, namely lymphodepletion. Importantly, intratumoral cyclophosphamide has potential therapeutic applications as an immunomodulatory approach to depleting tumor infiltrating regulatory T cells30, 31. Mice bearing B78 tumors were randomized to receive cyclophosphamide 500 mg/kg intratumorally or intraperitoneally. Following intratumoral injection, there was no significant difference in lymphocyte count prior to and 7 days following injection (p= 0.686). In contrast, in mice receiving intraperitoneal cyclophosphamide there was a trend towards a significant reduction in lymphocyte count 7 days following injection compared to baseline (p=0.081, Fig 6).
Figure 6:
Quantification of lymphocytes following systemic or local delivery of cyclophosphamide. Mice were treated with 500 mg/kg cyclophosphamide. n=3/group
To demonstrate a proof-of-concept that brachytherapy and intratumoral immunotherapy can be safely co-administered and feasibly performed for clinical benefit, we randomized mice bearing a B78 melanoma tumor to brachytherapy (2 Gy to depth of 5 mm) or sham catheter insertion. Mice receiving brachytherapy were also injected with 375,000 U of IL-2 using the multi-purpose brachytherapy catheter. IL-2 is FDA approved for treatment of metastatic melanoma32, however severe toxicity observed with systemic administration limits its use33. Prior studies have suggested a therapeutic synergy between external beam radiation therapy and intratumoral IL210 and clinical studies are investigating such approaches (e.g. NCT03958383, NCT01416831)34. Here, in mice treated with a combination of brachytherapy and intratumoral IL2, no mice receiving combination therapy experienced any toxicity as measured by an independent blinded observer. Combination therapy reduced tumor growth compared to sham catheter insertion (Supplementary Fig 2A) and prolonged survival (Supplementary Fig 2B). Log-rank analysis of survival data revealed a significant difference in survival between combination of brachytherapy and intratumoral IL2 versus sham catheter insertion (p=0.0031).
To further evaluate the safety of our multi-purpose catheter, we compared bleeding and wound healing rates between standard brachytherapy catheters and our working prototype following catheter insertion. Mice were placed under anesthesia and either a standard brachytherapy catheter or our working prototype was percutaneously placed. Following placement, catheters were removed and wound surface area and time to hemostasis were recorded. We observed a significant difference in wound surface area between the standard brachytherapy catheter and our multi-purpose catheter (mean SA Standard catheter 3.8 mm2 vs SA multi-purpose catheter 6.3 mm2; p = 0.0154, Supplementary Fig 2C). We observed no significant difference in time to hemostasis between standard catheter placement and our multi-purpose catheter (Supplementary Fig 2D, Supplementary Video 2,3). Additionally, we tracked wound healing over time and observed no significant differences in wound healing between standard catheter placement and our multi-purpose catheter (Supplementary Fig 2E).
Discussion
To our knowledge this is the first report describing a device prototype that enables intratumoral injection and brachytherapy through a single catheter insertion. Intratumoral approaches to delivering a variety of therapies have become increasingly popular due to potential for local dose escalating while mitigating systemic toxicities. Advances in real time image guidance have made these approaches feasible. The nature of interstitial brachytherapy lends itself well to combination with intratumoral therapy as catheters placed for brachytherapy could be used for intratumoral injection. Because interstitial brachytherapy treatment plans are commonly optimized by using a sufficient number of needle insertions to minimize excessive dose heterogeneity in achieving target coverage35 and because excessive needle placement is positively correlated with iatrogenic trauma and toxicity36, we reasoned that an approach using a single catheter insertion would be critical to making such a combination of brachytherapy and intratumoral injection safe and potentially translatable to clinical settings. Here, we report the design, prototype development, and proof-of-concept testing of a such catheter to facilitate this dual use application.
In vitro fluid distribution testing demonstrated that the multi-purpose brachytherapy catheter prototype design is viable. Several key observations were made while testing the device prototype using raw chicken breast as a substitute for the in vivo tumor environment. As intended, fluid could be drawn into the inner tube syringe and released through holes on the device and into the surrounding tissue as the user pushed down on the plunger (Fig 3). Ideally, fluid retention should remain at 5% or lower for all devices37, 38. This is critical for ensuring accurate dosage and for minimizing waste of the injectable agent. On average, our multipurpose brachytherapy catheter prototype retained 4.03% of fluid post-injection, which complies with the necessary design specification. The volume of fluid delivered during this test ranged from approximately 250–400 microliters. There were no large variations in percent retention based on starting volume for these trials.
The advent of cell-based therapies such as CAR-T cells have revolutionized the approach to treating hematological malignancies. However, success of such therapy in the treatment of solid tumors remains limited, in part due to poor intratumoral infiltration23. Using lucerifase-expressing splenocytes, we demonstrated the potential for successful intratumoral injection of immune cells using the multi-purpose brachytherapy catheter (Fig 4). Additionally, growing evidence demonstrates that radiation modulates the tumor immune microenvironment with several radiation mediated effects occurring minutes to hours following treatment, including inducing pro-inflammatory cytokine production and eradicating suppressive immune cell populations39–45. Therefore, by utilizing a multi-purpose brachytherapy catheter it is not only possible to ensure intratumoral delivery of viable immune cells, but potentially to also convert the tumor microenvironment to a pro-inflammatory state with brachytherapy and enable enhanced immune-mediated killing of tumor cells.
Cytokine-based treatments such as interleukin 2 (IL-2) have shown success in patients with metastatic melanoma and renal cell carcinoma32, 46. These therapies are toxic when delivered systemically, which limits their use despite demonstrated efficacy. Local delivery allows for therapeutic dosing while avoiding dose-limiting toxicity3, 12 and here we observe in murine models that this can be combined safely with brachytherapy. In melanoma tumor bearing mice treated with combination IL-2 and brachytherapy we observed a reduction in tumor growth and significantly prolonged survival, without signs of toxicity, supporting the feasibility of combined intratumoral injection with interstitial brachytherapy (Supplementary Fig 2). Further studies beyond the scope of this manuscript will be needed to evaluate the efficacy and mechanisms of potential cooperative therapeutic interaction between brachytherapy and intratumoral IL2 or other immunotherapies. Others have investigated combining radiotherapy with IL-2 including preclinical work demonstrating intratumoral injection of an IL-2 immunocytokine construct enhanced the in situ vaccination effect generated by radiotherapy10. Additionally, a phase 1 trial demonstrated that combination stereotactic body radiotherapy and IL-2 was safe and 8 of 12 patients treated with combination therapy experienced a complete or partial response34. These promising results have led to an active phase 2 trial further investigating combination stereotactic body radiotherapy and IL-2 (NCT01416831). Taken together, this opens the possibility of further preclinical and translational studies investigating brachytherapy in combination with IL-2 using our multifunction catheter.
Chemotherapy induced toxicity, including lymphodepletion, limits the potential for combination therapy with other chemotherapeutics and radiation47. Chemotherapeutics such as cyclophosphamide can function as radiosensitizers and therapeutic dose levels can be achieved locally with intratumoral administration while reducing systemic lymphodepletion48. Using our multi-purpose brachytherapy catheter, we demonstrated the potential to avoid lymphodepletion with intratumoral injection of cyclophosphamide. Mice treated with an identical dose systemically experienced a trend towards significant reduction in their lymphocyte count, which is a poor prognostic factor for treatment success (Fig 6).
The utility of intratumoral injection can extend beyond directly administering therapeutics. MRI is currently the standard imaging modality used for contouring tumor volumes. However, in resource limited areas and developing nations access to MRI is not routinely available. In these settings 2D imaging becomes relevant and is suboptimal by comparison but could potentially be enhanced with intratumoral injection of contrast after catheter placement. As shown in our x- ray images, intratumoral injection of Omnipaque contrast enhanced visualization of the tumor compared to surrounding tissue, and tumor retention of contrast was confirmed with microCT imaging (Fig 5).
Precise intratumor injection may also have important potential research applications. Injection of contrasted material or tattoo ink may be useful in certain settings. This could enable radiographic or histologic marking of brachytherapy catheter position, brachytherapy source dwell positions, or intratumoral injection site locations after catheter removal. This capability could be critical in enabling in vivo studies of brachytherapy dose response and in facilitating spatial and temporal monitoring of the diffusion and movement of materials injected intratumor. These approaches could be critical in future applications of this multifunctional catheter, enabling precise radiographic and histologic correlate studies in preclinical and clinical applications.
Our working prototype, while sufficient for proof-of-concept testing in preclinical settings, has several limitations and requires further refinement before translation to early phase testing in humans. Our fabrication techniques were influenced by the physical limitations of our academic manufacturing facility. We were only able to 3D print several individual components of this prototype, rather than a complete prototype. This resulted in a need for component assembly and this aspect of design is not desirable for large scale production or clinical application. Additionally, we lacked sufficient laser cutting capabilities and were required to create perforations for injection in the outer sheath manually using a needle which can alter the reproducibility of injection between replicates. We further acknowledge that the outer diameter of our working prototype could be difficult for clinical translation and might carry risk for traumatic needle injury. However, the size of the outer diameter of our working prototype is within the range of several FDA approved catheters. Small bore percutaneous nephrostomy tubes are 8–14 F (2.67–4.67 mm), gastrostomy tubes have diameters of 16–20F (5.33–6.67 mm), and Cordis central line catheters have diameters between 8–11F (2.67–3.67 mm). The results of our animal testing showed that despite an increase in wound surface area induced by our working prototype, we observed no increase in time to hemostasis or wound healing complications between standard brachytherapy catheter placement and our working prototype. Additionally, we are confident that with industrial manufacturing support and expertise the outer diameter of this prototype can be reduced sufficiently to ensure patient tolerability and to limit toxicity risks.
Conclusion
We report the design, prototype development, and proof-of-concept testing of a multi-purpose catheter for brachytherapy and intratumor injection. The growing interest in intratumoral approaches to administering cancer therapies presents a unique opportunity for combination with brachytherapy. Further preclinical studies will be required to determine optimal approaches to such combinations and to test whether these may improve the clinical efficacy and/or reduce the toxicity of cancer treatment.
Supplementary Material
Supplemental Video 1: Animation depicting use of the multi-purpose catheter prototype to deliver brachytherapy and intratumoral immunotherapy with a single catheter insertion technique.
Supplemental Video 2: Visual measurement of hemostasis following removal of standard brachytherapy catheter
Supplemental Video 3: Visual measurement of hemostasis following removal of multi-purpose brachytherapy catheter
Supplemental Figure 2: Brachytherapy was performed with a standard brachytherapy catheter inserted into the outer sheath of the multi-purpose brachytherapy catheter. The outer sheath remained in place, the standard brachytherapy catheter was replaced with the inner tube syringe and 375,000 U IL-2 was injected into the tumor. Combined brachytherapy and intratumoral IL-2 slows tumor growth compared to sham catheter insertion in B78 melanoma (A), and results in improved survival (B). n=6/group ** denotes p-value < 0.01. C) Quantification of wound surface area comparing standard brachytherapy catheter (left) to the multi-purpose brachytherapy catheter (right). N=5/group, surface area was estimated using the surface area of ellipse equation: a x b x π. Mean surface area was compared using Student’s T test. D) Time to hemostasis quantification comparing standard brachytherapy catheter placement (left) to the multi-purpose brachytherapy catheter (right). N=5/group, time to hemostasis was compared using Student’s T test. E) Representative image comparing wound healing between standard brachytherapy catheter placement (left) and the multi-purpose brachytherapy catheter (right). Images were taken 3 days following catheter placement.
Supplemental Figure 1: 3D printing workflow. 3D printing converts computer aided designs (CAD), 3D scans, or tomography data into physical objects. The 3D model obtained from a CAD design, 3D scans, or tomography data is saved as an STL file which is digitally sliced. The object is printed layer by layer without the need of molds or machining. Icons made by Kirahshastry and Freepik from www.flaticon.com
Acknowledgements
The authors would like to thank Anna Keller, Rebecca Gillis, Jiacomo Beckman, and Jason Wang for their contributions to early designs of the prototype. The author’s work is supported in part by grants from NIH P30 CA014520, NIH P50 DE026787, NIH 1DP5OD024576, NIH T32GM008692, NIH TL1TR002375, and the Shaw Scientist Award. The authors would like to acknowledge the University of Wisconsin Small Animal Imaging & Radiotherapy Facility and NIH S10OD028670-01 for supporting this work.
Funding
The author’s work is supported in part by grants from NIH P30 CA014520, NIH P50 DE026787, NIH 1DP5OD024576, NIH T32GM008692, NIH TL1TR002375, and the Shaw Scientist Award
Footnotes
Disclosures
Mr. Jagodinsky, Ms. Medeiros, Ms. Raj, Ms. Dempsy, Ms. Locsin, and Ms. Razuan have a patent P190102US01 pending to WARF.
Dr. Morris reports advisory roles and equity interests in Archeus Technologies and Seneca Therapeutics, outside the submitted work; In addition, Dr. Morris has a patent App No. 15/652,400 with royalties paid to WARF, a patent App No. 15/658,535 with royalties paid to WARF, a patent P180116US01 pending to WARF, a patent App No. 62/728645 pending to WARF, and a patent P190102US01 pending to WARF.
References
- 1.Chargari C, Deutsch E, Blanchard P, Gouy S, Martelli H, Guérin F, et al. Brachytherapy: An overview for clinicians. CA Cancer J Clin. 2019;69(5):386–401. Epub 2019/07/30. doi: 10.3322/caac.21578. [DOI] [PubMed] [Google Scholar]
- 2.Skowronek J Current status of brachytherapy in cancer treatment - short overview. J Contemp Brachytherapy. 2017;9(6):581–9. Epub 2017/12/30. doi: 10.5114/jcb.2017.72607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aznar MA, Tinari N, Rullan AJ, Sanchez-Paulete AR, Rodriguez-Ruiz ME, Melero I. Intratumoral Delivery of Immunotherapy-Act Locally, Think Globally. J Immunol. 2017;198(1):31–9. Epub 2016/12/21. doi: 10.4049/jimmunol.1601145. [DOI] [PubMed] [Google Scholar]
- 4.Patel RB, Baniel CC, Sriramaneni RN, Bradley K, Markovina S, Morris ZS. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy. 2018;17(6):995–1003. Epub 2018/08/02. doi: 10.1016/j.brachy.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–903. Epub 2008/11/24. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meric-Bernstam F, Sandhu SK, Hamid O, Spreafico A, Kasper S, Dummer R, et al. Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. Journal of Clinical Oncology. 2019;37(15_suppl):2507-. doi: 10.1200/JCO.2019.37.15_suppl.2507. [DOI] [Google Scholar]
- 7.Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK, et al. Vaccination with a TLR9 Agonist and Local Low-Dose Radiation Induces Systemic Responses in Untreated Indolent Lymphoma. Cancer Discov. 2018;8(10):1258–69. Epub 2018/08/28. doi: 10.1158/2159-8290.CD-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018,’10(426). doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ursu R, Carpentier A, Metellus P, Lubrano V, Laigle-Donadey F, Capelle L, et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric, randomised study. Eur J Cancer. 2017;73:30–7. Epub 2017/01/28. doi: 10.1016/j.ejca.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Morris ZS, Guy EI, Francis DM, Gressett MM, Werner LR, Carmichael LL, et al. In Situ Tumor Vaccination by Combining Local Radiation and Tumor-Specific Antibody or Immunocytokine Treatments. Cancer Res. 2016;76(13):3929–41. Epub 2016/05/06. doi: 10.1158/0008-5472.CAN-15-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33(25):2780–8. Epub 2015/05/26. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 12.Hong WX, Haebe S, Lee AS, Westphalen B, Norton JA, Jiang W, et al. Intratumoral immunotherapy for early stage solid tumors. Clin Cancer Res. 2020. Epub 2020/02/18. doi: 10.1158/1078-0432.CCR-19-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagodinsky JC, Harari PM, Morris ZS. The Promise of Combining Radiation Therapy with Immunotherapy. Int J Radiat Oncol Biol Phys. 2020. Epub 2020/04/23. doi: 10.1016/j.ijrobp.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melero I, Duarte M, Ruiz J, Sangro B, Galofré J, Mazzolini G, et al. Intratumoral injection of bone-marrow derived dendritic cells engineered to produce interleukin-12 induces complete regression of established murine transplantable colon adenocarcinomas. Gene Ther. 1999;6(10):1779–84. doi: 10.1038/sj.gt.3301010. [DOI] [PubMed] [Google Scholar]
- 15.Nishioka Y, Hirao M, Robbins PD, Lotze MT, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999;59(16):4035–41. [PubMed] [Google Scholar]
- 16.Mazzolini G, Alfaro C, Sangro B, Feijoó E, Ruiz J, Benito A, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23(5):999–1010. Epub 2004/12/14. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 17.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4(5):522–6. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–102. Epub 2010/07/28. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. Epub 2011/08/10. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–700. Epub 2016/07/13. doi: 10.1182/blood-2016-04-711903. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. Epub 2017/11/20. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yong CSM, Dardalhon V, Devaud C, Taylor N, Darcy PK, Kershaw MH. CAR T-cell therapy of solid tumors. Immunol Cell Biol. 2017;95(4):356–63. Epub 2016/12/22. doi: 10.1038/icb.2016.128. [DOI] [PubMed] [Google Scholar]
- 24.Medich DC, Munro JJ. Monte Carlo characterization of the M-19 high dose rate Iridium-192 brachytherapy source. Med Phys. 2007;34(6):1999–2006. doi: 10.1118/1.2733809. [DOI] [PubMed] [Google Scholar]
- 25.Haraguchi M, Yamashiro S, Yamamoto A, Furukawa K, Takamiya K, Lloyd KO, et al. Isolation of GD3 synthase gene by expression cloning of GM3 alpha-2,8-sialyltransferase cDNA using anti-GD2 monoclonal antibody. Proc Natl Acad Sci U S A. 1994;91(22):10455–9. doi: 10.1073/pnas.91.22.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyal S, Kataria T. Image guidance in radiation therapy: techniques and applications. Radiol Res Pract. 2014;2014:705604. Epub 2014/12/17. doi: 10.1155/2014/705604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray J, Griffin C, Gulliford S, Syndikus I, Staffurth J, Panades M, et al. A randomised assessment of image guided radiotherapy within a phase 3 trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2020;142:62–71. Epub 2019/11/22. doi: 10.1016/j.radonc.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol. 2015;38(3):259–65. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revannasiddaiah S, Susheela SP. Chemically enhanced radiotherapy: visions for the future. Ann Transl Med. 2016;4(3):52. doi: 10.3978/j.issn.2305-5839.2015.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsung K, Meko JB, Tsung YL, Peplinski GR, Norton JA. Immune response against large tumors eradicated by treatment with cyclophosphamide and IL-12. J Immunol. 1998;160(3):1369–77. [PubMed] [Google Scholar]
- 31.Pardoll DM. New strategies for enhancing the immunogenicity of tumors. Curr Opin Immunol. 1993;5(5):719–25. doi: 10.1016/0952-7915(93)90127-e. [DOI] [PubMed] [Google Scholar]
- 32.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. Epub 1999/11/24. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz RN, Stover L, Dutcher JP. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park). 2002;16(11 Suppl 13):11–20. [PubMed] [Google Scholar]
- 34.Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med. 2012;4(137):137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 35.Chicheł A, Kanikowski M, Skowronek J. Vital role of volume and number of needles in HDR brachytherapy (HDR-BT) of prostate cancer. J Contemp Brachytherapy. 2009;1(3):145–50. Epub 2009/10/08. [PMC free article] [PubMed] [Google Scholar]
- 36.Eapen L, Kayser C, Deshaies Y, Perry G, E C, Morash C, et al. Correlating the degree of needle trauma during prostate brachytherapy and the development of acute urinary toxicity. Int J Radiat Oncol Biol Phys. 2004;59(5):1392–4. doi: 10.1016/j.ijrobp.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 37.Küme T, Sişman AR, Solak A, Tuğlu B, Cinkooğlu B, Coker C. The effects of different syringe volume, needle size and sample volume on blood gas analysis in syringes washed with heparin. Biochem Med (Zagreb). 2012;22(2):189–201. [PMC free article] [PubMed] [Google Scholar]
- 38.Jarrahian C, Rein-Weston A, Saxon G, Creelman B, Kachmarik G, Anand A, et al. Vial usage, device dead space, vaccine wastage, and dose accuracy of intradermal delivery devices for inactivated poliovirus vaccine (IPV). Vaccine. 2017;35(14):1789–96. Epub 2017/02/08. doi: 10.1016/j.vaccine.2016.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. Epub 2012/11/12. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 41.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. Epub 2014/04/25. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Ruiz ME, Garasa S, Rodriguez I, Solorzano JL, Barbes B, Yanguas A, et al. Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule Are Induced by Ionizing Radiation on Lymphatic Endothelium. Int J Radiat Oncol Biol Phys. 2017;97(2):389–400. Epub 2016/11/07. doi: 10.1016/j.ijrobp.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 43.Waldmann TA. Cytokines in Cancer Immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12). Epub 2017/11/05. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81(4):1128–35. Epub 2010/11/26. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012;2:191. Epub 2012/12/17. doi: 10.3389/fonc.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fyfe GA, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1996;14(8):2410–1. Epub 1996/08/01. doi: 10.1200/JCO.1996.14.8.2410. [DOI] [PubMed] [Google Scholar]
- 47.Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8(69):114268–80. Epub 2017/12/14. doi: 10.18632/oncotarget.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CS, Jounaidi Y, Su T, Waxman DJ. Enhancement of intratumoral cyclophosphamide pharmacokinetics and antitumor activity in a P450 2B11-based cancer gene therapy model. Cancer Gene Ther. 2007;14(12):935–44. Epub 2007/09/14. doi: 10.1038/sj.cgt.7701092. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1: Animation depicting use of the multi-purpose catheter prototype to deliver brachytherapy and intratumoral immunotherapy with a single catheter insertion technique.
Supplemental Video 2: Visual measurement of hemostasis following removal of standard brachytherapy catheter
Supplemental Video 3: Visual measurement of hemostasis following removal of multi-purpose brachytherapy catheter
Supplemental Figure 2: Brachytherapy was performed with a standard brachytherapy catheter inserted into the outer sheath of the multi-purpose brachytherapy catheter. The outer sheath remained in place, the standard brachytherapy catheter was replaced with the inner tube syringe and 375,000 U IL-2 was injected into the tumor. Combined brachytherapy and intratumoral IL-2 slows tumor growth compared to sham catheter insertion in B78 melanoma (A), and results in improved survival (B). n=6/group ** denotes p-value < 0.01. C) Quantification of wound surface area comparing standard brachytherapy catheter (left) to the multi-purpose brachytherapy catheter (right). N=5/group, surface area was estimated using the surface area of ellipse equation: a x b x π. Mean surface area was compared using Student’s T test. D) Time to hemostasis quantification comparing standard brachytherapy catheter placement (left) to the multi-purpose brachytherapy catheter (right). N=5/group, time to hemostasis was compared using Student’s T test. E) Representative image comparing wound healing between standard brachytherapy catheter placement (left) and the multi-purpose brachytherapy catheter (right). Images were taken 3 days following catheter placement.
Supplemental Figure 1: 3D printing workflow. 3D printing converts computer aided designs (CAD), 3D scans, or tomography data into physical objects. The 3D model obtained from a CAD design, 3D scans, or tomography data is saved as an STL file which is digitally sliced. The object is printed layer by layer without the need of molds or machining. Icons made by Kirahshastry and Freepik from www.flaticon.com