Abstract
Trauma is a primary cause of death globally, with non‐compressible torso hemorrhage constituting an important part of “potentially survivable trauma death.” Resuscitative endovascular balloon occlusion of the aorta has become a popular alternative to aortic cross‐clamping under emergent thoracotomy for non‐compressible torso hemorrhage in recent years, however, it alone does not improve the survival rate of patients with severe shock or traumatic cardiac arrest from non‐compressible torso hemorrhage. Development of novel advanced maneuvers is essential to improve these patients’ survival, and research on promising methods such as selective aortic arch perfusion and emergency preservation and resuscitation is ongoing. This review aimed to provide physicians in charge of severe trauma cases with a broad understanding of these novel therapeutic approaches to manage patients with severe hemorrhagic trauma, which may allow them to develop lifesaving strategies for exsanguinating trauma patients. Although there are still hurdles to overcome before their clinical application, promising research on these novel strategies is in progress, and ongoing development of synthetic red blood cells and techniques that reduce ischemia‐reperfusion injury may further maximize their effects. Both continuous proof‐of‐concept studies and translational clinical evaluations are necessary to clinically apply these hemostasis approaches to trauma patients.
Keywords: Emergency medicine, hemorrhagic shock, ischemia‐reperfusion injury, oxygen carrier, torso hemorrhage, traumatic cardiac arrest
This review aimed to provide physicians in charge of severe trauma cases with a broad understanding of these novel therapeutic approaches to manage patients with severe hemorrhagic trauma, which may allow them to develop lifesaving strategies for exsanguinating trauma patients. Although there are still hurdles to overcome before their clinical application, promising research on these novel strategies is in progress.

Introduction
Trauma currently comprises 9% of causes of death worldwide. 1 Approximately 80% of the soldiers without head trauma died because of truncal injury during the Vietnam War, and many of those damages were technically repairable. 2 This trend remains the same today; non‐compressible torso hemorrhage (NCTH) is a significant cause of “potentially survivable trauma death.” 3 , 4 , 5 , 6 , 7 , 8 , 9 Hemorrhage‐induced traumatic cardiac arrest (HiTCA) with advanced hemorrhagic shock has an extremely low survival rate and is catastrophic, particularly in cases of blunt trauma. 10 , 11 , 12 Damage occurs immediately to important organs such as the brain and heart during warm ischemic time commencing with cardiac arrest; therefore, successful resuscitation is expected only within a short time period. 13 , 14 , 15 , 16 In addition, chest compressions are less effective in patients with hypovolemia, and blood loss is faster in cases where major vessels are injured. Patients with HiTCA have very poor outcomes; therefore, new management approaches are necessary to improve the tragic outcomes of those patients. To that end, some promising new approaches are currently being developed.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a technique used to gain endovascular hemorrhage control for decompensated hemorrhagic shock from subdiaphragmatic bleeding. It is designed to improve the survival rate of trauma patients and has become popular in recent decades. 17 There is currently no strong evidence that REBOA significantly improves survival in patients with severe traumatic hemorrhagic shock; on the contrary, one report suggests an association between REBOA and increased mortality. 18 Because REBOA is just a “bridge” until definitive hemostasis, it is important for saving patient to be brought to the suite where vascular embolization and/or surgery can be performed immediately after REBOA. 19 Further development and clinical application of advanced methods are required, and related research is currently progressing, mainly in the United States (US). Selective aortic arch perfusion (SAAP) is one such technique that simultaneously allows aortic occlusion and proximal aortic perfusion resuscitation to protect vital organs until definitive hemostatic surgery. 20 Emergency preservation and resuscitation (EPR) is another technique used to achieve similar organ protection by rapidly cooling the entire body via an aortic cannula to reduce metabolic energy demand. 16
It should be noted that the development of synthetic oxygen carriers and ischemia‐reperfusion injury reduction methods make these resuscitation procedures safer and more effective. To apply these hemostasis control and resuscitation concepts to severe trauma patients, it is necessary to move from concept studies to clinical trials. This article reviews the feasibility and viability of SAAP and EPR and presents the research findings related to integrated strategies and adjunct therapies that will definitely help emergency physicians save severe trauma patients.
SAAP
The survival rate of patients with traumatic cardiac arrest is poor. Attempting resuscitation on HiTCA patients was previously thought to be futile. 21 , 22 , 23
SAAP is a developing technique for patients with HiTCA for whom hemorrhage control is concurrently performed through thoracic aortic occlusion similar to Zone 1 REBOA and assisted perfusion of the heart and brain using oxygenated perfusate via the catheter to achieve restoration of spontaneous circulation (ROSC) (Fig. 1). 24 , 25 SAAP was first reported in the 1990s as a technique to improve ROSC in animal models of ventricular fibrillation that undergo non‐traumatic cardiac arrest. 24 , 26 , 27 It has also been shown to be effective in treating hemorrhagic traumatic cardiac arrest caused by NCTH 25 .
Fig. 1.
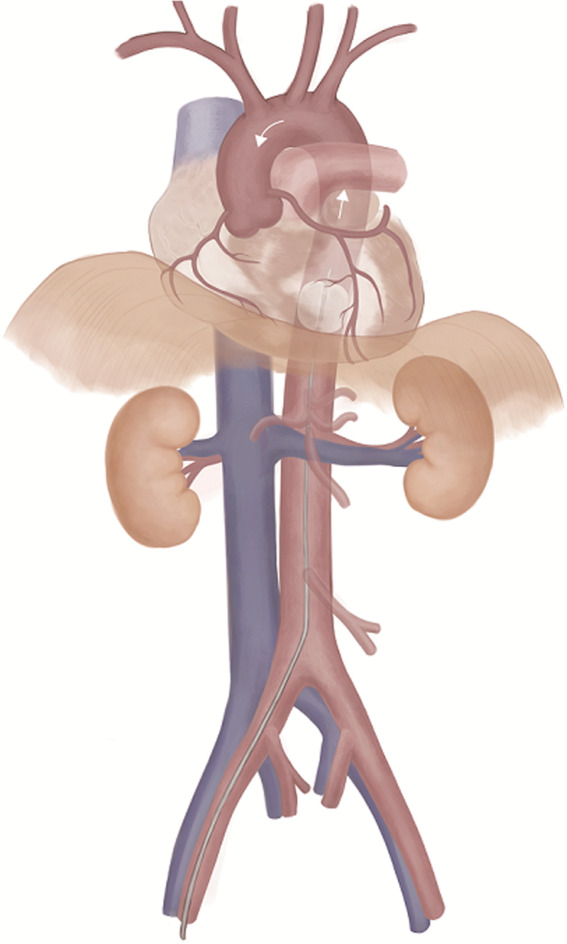
Selective aortic arch perfusion.
SAAP should be clearly indicated in HiTCA cases because this method simultaneously allows hemorrhage control and resuscitative perfusion of vital organs such as the heart and brain. When implementing SAAP, a large‐diameter occlusion balloon catheter is inserted from the femoral artery and advanced to the descending thoracic aorta. Similar to REBOA, in SAAP the balloon is placed between the left subclavian artery and the diaphragm to act as a thoracic aortic cross‐clamp, controlling peripheral hemorrhage when inflated. The SAAP catheter lumen permits extracorporeal circulation with oxygen carrier perfusates such as artificial hemoglobin, fluorocarbons, and natural blood. In previous studies, the SAAP catheter acted as an arterial blood cannula and could be assisted with a flow rate of 500 mL/min. 28 Therefore, extracorporeal circulation support of SAAP using an oxygen carrier such as whole blood may restore the normal electrical activity of the heart and rapidly replenish circulation volume. This catheter can also be used as an intra‐aortic route for administration of epinephrine or drugs capable of alleviating ischemia‐reperfusion injury. 29
After definitive hemorrhage control is established, the conversion to full‐flow extracorporeal life support (ECLS) has been demonstrated to be feasible. 28 Barnard and colleagues described a liver lobectomy‐induced HiTCA swine model. They classified transcatheter arterial hemorrhage into three groups to compare ROSC achievement rate and 60‐min survival rate: REBOA with fresh whole blood (FWB) intravenous administration (FWB‐REBOA group), SAAP using oxygenated Ringer’s solution (LR) (LR‐SAAP group), and SAAP using oxygenated FWB (FWB‐SAAP group). 20 ROSC achievement rates were 0% (95% confidence interval [CI], 0.00–30.9) in the FWB‐REBOA group, 60% in the LR‐SAAP group (95% CI, 26.2–87.8), and 100% in the FWB‐SAAP group (95% CI, 69.2–100.0), respectively (P < 0.001). The survival rate at 60 min, which was assumed as the transportation time until hospital arrival, was 0% (95% CI, 0.00–30.9) in the FWB‐REBOA group, 10% (95% CI, 0.25–44.5) in the LR‐SAAP group, and 90% (95% CI, 55.5–99.7) in the FWB‐SAAP group (P < 0.001). Of the nine FWB‐SAAP survivors, four (44%) achieved ROSC from asystole. SAAP using FWB as the perfusate demonstrated a significantly higher survival rate within 60 min than Zone 1 REBOA.
Remarkably, it has also been suggested that SAAP could be useful for achieving ROSC from hemorrhagic traumatic asystole, which has previous been considered a non‐viable state. Achievement of short‐term survival rate and ROSC with SAAP was significantly higher than with conventional cardiopulmonary resuscitation (CPR), 30 emergency thoracotomy, 31 , 32 and REBOA with balanced transfusion. 18 , 20 , 33 Although SAAP has been thoroughly evaluated in preclinical investigations, its effectiveness in clinical cases has not yet been reported. Device development is one of the hurdles for clinical application, as the SAAP circuit used in previous studies is bespoke and requires regulatory approval. 20
A further important hurdle is the complexity of administering many blood products into the aorta in a short time. Although the ROSC achievement rate is high when allogeneic whole blood or red blood cells (RBCs) are used as the SAAP perfusate, intra‐aortic administration of calcium is required to address hypocalcemia associated with citrate anticoagulants. Serum ionized calcium levels must be maintained within normal range to avoid the development of refractory ventricular fibrillation during resuscitation. 34 The concentration of ionized calcium in blood products anticoagulated with citric acid used for preservation is very low, so administration of large amounts causes marked hypo‐ionized calcemia. 35 , 36 In contrast to intravenous administration, with SAAP, perfusion is performed near the coronary arteries, so there is little time for the blood and perfusate to mix. For the calcium concentration to be homogenized, intra‐aortic calcium administration during resuscitation is necessary to prevent ventricular arrhythmia. Manning and colleagues demonstrated the need for calcium when using blood products as the perfusate for SAAP using a liver injury and massive hemorrhage HiTCA porcine model. 34 They observed severe hypo‐ionized calcium in the FWB‐SAAP group without co‐administration of calcium, and after SAAP, refractory ventricular fibrillation occurred and ROSC was not achieved. In contrast, in the FWB‐SAAP group that was co‐administered calcium, the chelating action of citric acid was effectively canceled, severe hypocalcemia was prevented, and spontaneous circulation was restored in all the animals. 34 They reported that the dose required to maintain normal serum ionized calcium levels was 7 mmol per 500 mL FWB, and 2–2.5 mmol for packed RBCs in which plasma components including citric acid were almost removed. However, this study’s authors only observed short‐term survival and not long‐term survival. It should also be noted that implementation of SAAP requires a considerable amount of perfusate with oxygen‐carrying capacity such as RBCs. This research demonstrated that SAAP using FWB was very effective, but the amount of oxygenated perfusate used was as much as 1600 mL per porcine with an average weight of 80 kg. 34 Because the ionized calcium in the perfusate is consumed by citric acid during preservation, additional administration of calcium is required; therefore, SAAP using FWB has challenges regarding logistics of preparation and storage. 37
Several animal studies of SAAP have used fluorocarbon and hemoglobin‐based oxygen carriers that are not currently approved for clinical use. 25 , 26 Artificial hemoglobin‐based oxygen carrier (HBOC) as SAAP perfusate achieved ROSC and short‐term survival rates similar to FWB with similar physiological adverse effects. 28 Hoops further suggested it may be easier to administer in clinical settings. The only significant difference in physiological derangement between HBOC and FWB was pulmonary hypertension, a known short‐term side effect of HBOC because of its vasoconstrictor and nitric oxide scavenging action, which was higher in the HBOC group, 28 and previous studies have shown that sodium nitrate might antagonize these actions. 38 As an artificial oxygen carrier product, HBOC‐201 has been used in many studies because it is stable at room temperature for 3 years, and its normal calcium concentration does not require simultaneous administration of perfusate or calcium supplementation. 38 These properties are beneficial when performing SAAP in environments where medical resources are not abundant, especially where blood products are not available.
Although it is difficult to judge if patients benefit clinically from SAAP, it allows for occlusion use such as REBOA before performing perfusion therapy. Therefore, in cases of severe hemorrhage shock progressing to HiTCA, SAAP has potential as a clinical methodology that begins with only occlusion and continues with selective perfusion as needed. 28 SAAP can be clinically applied merely with current technologies from a technical perspective. Before large clinical research trials may begin, a method to reduce ischemia‐reperfusion injury distal to the occlusion balloon must be developed. Additionally, there must be proper selection of safe and effective perfusate, and safety mechanisms to prevent air embolism in the circuit must be established.
EPR
Trauma surgeons who attempt to control hemorrhage of major injured vessels for a moribund patient often experience a situation where treatment is not applied in time for rapid exsanguination, resulting in the patient’s physiological collapse. If therapeutic hypothermia can be rapidly introduced during the initial treatment, such injuries could be treated before the patient cannot be resuscitated. EPR is an experimental resuscitation method in which rapid cooling of the whole body is used to reduce metabolic demand in patients with prolonged circulatory collapse. During the cooling period, surgical hemostatic repair is conducted, followed by delayed resuscitation using full cardiopulmonary bypass (CPB). 16 , 39 Hypothermia has long been known to prolong the intact survival time of organs 40 ; cardiac surgeons and neurosurgeons take advantage of this technique in their daily clinical practice. In clinical settings, especially in the emergency department, it is not possible to introduce full CPB within 5 min of cardiac arrest and fatal damage. However, even after ischemic conditions have already occurred, hypothermia minimizes oxygen demand, suppresses metabolism, and renders systemic resistance to ischemia. 41
The EPR resuscitation approach used in ongoing clinical research is described as follows (Fig. 2). 42 , 43 Patients undergo a left thoracotomy to occlude the descending thoracic aorta if REBOA has not been previously performed. To ensure a proper surgical field for EPR, it is necessary to transect the sternum from the left to the right, a procedure defined as a clamshell thoracotomy. The EPR arterial cannula (17–21 Fr) is then placed in the aorta via the Seldinger technique by a trauma surgeon, and the cannula is connected to the CPB system. The CPB roller pump rapidly injects ice cold 0.9% normal saline into the aorta at a flow rate of 2 L/min. The flow rate is then increased within a tolerable range to a maximum of 5 L/min. The total amount of normal saline typically required for sufficient cooling of a medium‐sized adult is 40–100 L. After the start of rapid infusion, the right atrial appendage is incised, and the perfusate is drained out of the body. When the tympanic temperature or nasopharyngeal temperature reaches 20°C, the aortic occlusion/clamp is slowly released, and cooling of the whole body is continued under CPB flow monitoring. After the injured sites are surgically controlled, whole‐body rewarming by reperfusion is initiated at a rate of 0.5°C/min. Ideally, the total time for deep hypothermic circulatory arrest should be <1 h.
Fig. 2.
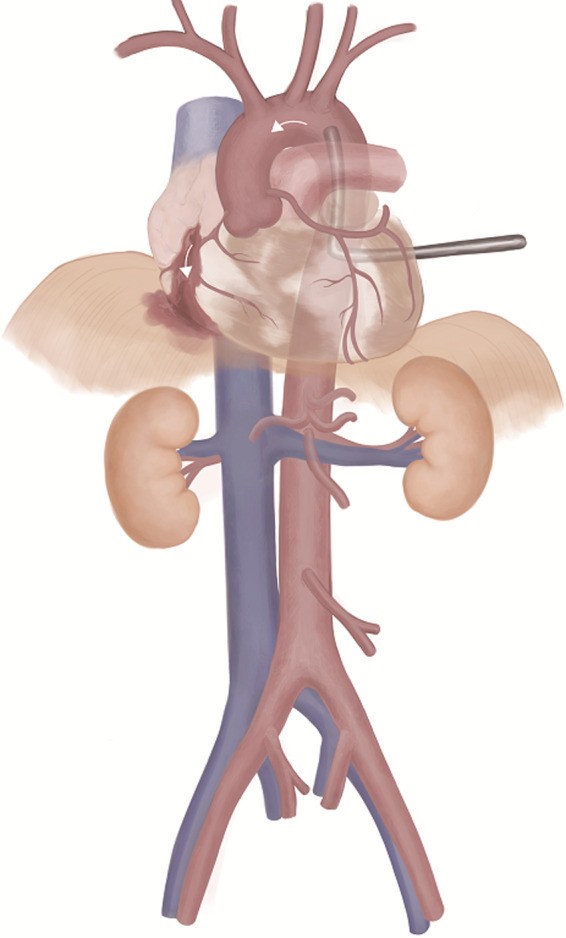
Emergency preservation and resuscitation.
EPR, which is currently undergoing clinical trials, requires a great deal of medical resources including surgeons, anesthesiologists, nurses, and technicians involved in trauma care who work closely with the cardiac surgery team. Unified team formation and careful training are essential for the implementation and success of EPR and to ensure the smooth performance of this novel method to save the lives of HiTCA patients. 42 EPR is a novel approach in the management of traumatic cardiac arrest patients, especially in cases of major intrathoracic hemorrhage or penetrating heart injury where conventional CPR, REBOA, or SAAP may be limited or even harmful. 44 Pioneering studies of EPR use the following case inclusion criteria 42 , 43 , 44 : age = 18–65 years, penetrating trauma with clinical suspicion of exsanguinating hemorrhage, at least one sign of life at the scene (respiratory efforts, pulse, or reactive pupil spontaneous movements), loss of pulse <5 min before emergency room arrival or in the emergency or operating room, and thoracotomy performed in the emergency room or without immediate return of a palpable pulse. Because EPR is an emergency experimental method performed on patients with sudden, unexpected, severe injuries, prior ethics committee reviews, consultation with the local community, and information disclosure are important and essential. 42
It is well known that clinical settings have many complex factors that differ from the controlled laboratory environment, including significant organ injury, which is always associated with massive hemorrhage in trauma patients. A canine study using the 1‐h EPR model demonstrated that animals with additional organ injury survived similarly to the group without organ injury, but had significantly more coagulopathy and multiple organ failures after resuscitation. 45 Furthermore, hypothermia is generally suggested to be independently associated with an increased adjusted odds of death following major trauma because core temperatures below 34°C cause generalized physiologic deceleration and homeostatic disturbances in all organ systems. 46 , 47 However, the profound hypothermia of 10°C–15°C introduced by EPR may be clinically and biochemically different from the hypothermia caused after trauma 48 .
Previous EPR‐related studies are preclinical studies, and there evidence of the efficacy of EPR in humans is insufficient. Based on data from these preclinical studies, the US Food and Drug Administration approved the emergency preservation and resuscitation cardiac arrest from trauma (EPRCAT) study (NCT01042015), a clinical trial on EPR safety and feasibility in HiTCA patients. 42 The primary endpoint is survival to hospital discharge without significant neurological sequelae. The secondary endpoints are 28‐day survival, neurologic function outcome at 6 and 12 months as measured by tools such as the Extension of Glasgow Outcome Scale and the 36‐item short‐form health survey, the presence of organ failure, and the complication of EPR procedures. The initial results of this study will be published in 2021.
Integrated Strategy and Vital Supplements
The resuscitative endovascular maneuvers described above are a “toolkit” for complementary life‐sustaining therapeutic interventions and to stabilize trauma patients with severe hemorrhagic shock or HiTCA patients with specific injury types such as penetrating trauma that do not respond to conventional resuscitation measures. 49 , 50 The application of these therapeutic interventions should be customized to the needs of each individual trauma patient. 39 According to the proposed integrated strategy, for trauma patients who show signs of decompensated circulatory insufficiency because of subdiaphragmatic NCTH, REBOA, and intravenous fluid resuscitation are first attempted to stabilize the hemodynamic state. The device that implements REBOA should be one that can be switched to SAAP later. In cases where trauma patients remain in impending refractory shock or progress to HiTCA despite REBOA and fluid resuscitation, SAAP is performed to achieve ROSC and quickly restore circulation to vital organs such as the brain and heart. The SAAP catheter balloon can also function as REBOA by continuing inflation after ROSC until surgical hemostasis is performed. If the patient is still unresponsive after SAAP or is not well‐adapted to it but may still survive, then EPR is indicated. The aim of this integrated strategy is to present a set of resuscitation choices that can be used based on the needs of individual trauma patients to increase their survival potential.
These procedures and strategy may also be applicable to hemorrhagic diseases other than trauma that are followed by cardiac arrest. Given the widespread use of endovascular devices, ruptured abdominal aortic aneurysms that require urgent aortic replacement may also be a reasonable indication. Similarly, they may be applicable in other uncontrolled hemorrhage cases such as aortoenteric fistula, postpartum hemorrhage, and massive gastrointestinal hemorrhage. SAAP and EPR may save patients that would have otherwise died of hemorrhage by prolonging the survival time to surgical hemostasis of the vascular injury and other sources of bleeding. However, these new methods alone would not be able to achieve dramatic improvements in survival rates. Further development of the environment and ancillary products would be needed for this new therapeutic strategy to be used effectively and improve survival rates. A well‐trained and coordinated response team would be essential. In addition to the usual trauma staff, emergency physicians, trauma surgeons, and paramedics, the resuscitation team would likely require help from cardiovascular surgeons, anesthesiologists, and cardiopulmonary technicians, especially for EPR. Improving the operating environment is also important, and a “hybrid” design that allows for better integration of endovascular procedures and open surgery would support a much wider and more complex surgical procedure than is currently possible. 51
Moreover, current medical practice relies on donated blood to replenish blood lost from trauma, and there is a strong demand to find a suitable replacement. Storage and deployment of perfusate may be problematic, considering that a perfusate with oxygen‐carrying capability is required immediately for SAAP at 10 mL/kg/min and for EPR at the reperfusion phase. 29 , 42 Furthermore, there may be side effects associated with large‐scale use of the perfusate. Allogeneic donated blood products have multiple limitations, so it is important to develop effective oxygen carriers that can be used as an alternative to blood in an emergency. HBOCs both provide hemodynamic support and have oxygen‐carrying capacity. HBOCs could help mitigate the distribution limitations of whole blood products by acting as an “oxygen bridge” therapy in cases where transfusion is required because of severe acute anemia, but is not immediately available. Macko and colleagues used a severe hemorrhagic model to demonstrate that rats resuscitated with a PEGylated carboxyhemoglobin had a significantly better survival rate and increased mean arterial pressure than those with lactated Ringer’s solution, and there were no significant differences compared with the FWB group. 52 Additionally, Guo and colleagues reported that synthetic “rebuilt red blood cells” (RRBCs), based on the silica cell bioreplication approach, extensively and completely simulated the characteristics of natural RBCs. 53 RRBCs possess all the characteristics of natural RBCs, such as size, shape, oxygen transport ability, deformability to achieve long‐term circulation, and function of the membrane surface, which may be resistant to phagocytosis by immune cells. They also have the potential to acquire therapeutic drug delivery ability, magnetic manipulation capability, and ATP biosensors that could sense and detect toxins. In short, RRBCs are a class of artificial hybrid materials that have been shown to have a wide range of potential applications. Hagisawa and colleagues demonstrated that combination therapy with fibrinogen γ‐chain peptide‐coated, ADP‐encapsulated liposomes and hemoglobin vesicles can effectively and completely control hemorrhage from the liver for trauma‐induced massive hemorrhage in thrombocytopenic rabbits. 54 This product could be stored at normal temperature for more than a year and be used regardless of the patient’s blood type. Although there are promising candidates, all synthetic blood product treatments still have a long way to go before reaching clinical application. Extensive research is necessary to ensure that the substances carried by synthetic RBCs are released in target organs, and the cell manufacturing process needs to be commercially scalable. However, a large amount of research focuses on rebuilding synthetic blood cells, and there is no doubt that further progress will be made in this area in the future.
There must also be a focus on techniques to effectively control the adverse events associated with ischemia‐reperfusion injury in patients with HiTCA. Potential techniques include “post‐conditioning,” which aids in cytoprotection through the addition of lactate to the perfusate during post‐ischemia reperfusion to prevent rapid changes in lactate concentration, 55 targeted temperature management after resuscitation, 56 , 57 , 58 drugs with antioxidant effects such as edaravone 59 and melatonin, 60 TEMPOL (C9H18NO2), 61 and medical gases such as hydrogen and carbon monoxide. 62 , 63 , 64 These techniques may greatly contribute to the development of integrated therapeutic strategies for controlling ischemia‐reperfusion injury. 65
Drabek et al. and Alam et al. have been conducting research on the biological mechanisms underlying ischemia and reperfusion injury to maximize neuroprotective effects. 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 Studies of gene phenotypic changes caused by severe hypothermia may highlight new therapeutic targets for ischemic reperfusion injury, such as interleukins, the Akt survival pathway, and the mitogen‐activated protein kinase pathway. 69 , 70 The Pittsburgh research group is also investigating the biochemical mechanisms of EPR, focusing on inflammation and microglia. 71 , 72 More advanced methods that stimulate a specific hypothalamic neuronal circuit to induce a hibernation‐like state while introducing passive profound hypothermia such as EPR may have a clinical application in medicine in the future. 74
Treatment methods and strategies for patients with severe traumatic shock and HiTCA are changing, with clinical trials becoming more feasible as laboratory studies demonstrate promising results from these novel pharmacological approaches and special solutions. Novel devices that help accelerate the practice of SAAP and EPR also merit further research. In summary, “buying time” from the injury to definitive hemorrhage control and effectively suppressing adverse events after resuscitation will have significant effects on the prognosis and outcomes of severe trauma patients with exsanguination.
Conclusion
This article has comprehensively described the content, evidence, and limitations of cutting edge trauma resuscitation procedures, such as SAAP and EPR, with plentiful data from preclinical studies and for which clinical research is beginning. This review also discussed for the first time, the main hurdles for the clinical application of each novel approach and ways to address them. Because these novel exploratory approaches have revolved around preclinical studies, unknown adverse events may be discovered when applied clinically. However, current findings suggest that these strategies will effectively improve the survival of those with severe NCTH and HiTCA who have never had a way to be resuscitated. Appropriately designed multicenter clinical trials are necessary to establish the appropriate and optimal use of these complementary therapeutic approaches for patients with severe traumatic hemorrhage. This review aims to provide physicians with a basic knowledge of these novel therapeutic strategies, including SAAP and EPR, which may help save the lives of trauma patients with massive hemorrhage and HiTCA.
Disclosure
Approval of Research Protocol: Not applicable.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal on request.
Conflict of Interest: None declared.
Acknowledgements
We thank Christine Burr for English‐language editing.
Funding information
No funding information provided.
References
- 1. World Health Organization . Injuries and Violence. The Facts. 2014. [Accessed 04 Nov 2020]. Available from: https://www.who.int/violence_injury_prevention/media/news/2015/Injury_violence_facts_2014/en/
- 2. Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil. Med. 1984; 149: 55–62. [PubMed] [Google Scholar]
- 3. Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J. Trauma Inj. Infect. Crit. Care. 2006; 60: S3–11. [DOI] [PubMed] [Google Scholar]
- 4. Holcomb JB, McMullin NR, Pearse L et al. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001–2004. Ann. Surg. 2007; 245: 986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly JF, Ritenour AE, McLaughlin DF et al. Injury severity and causes of death from operation Iraqi freedom and operation enduring freedom: 2003–2004 versus 2006. J. Trauma Inj. Infect. Crit. Care. 2008; 64: S21–S27. [DOI] [PubMed] [Google Scholar]
- 6. Eastridge BJ, Mabry RL, Seguin P et al. Death on the battlefield (2001–2011). J. Trauma Acute Care Surg. 2012; 73: S431–S437. [DOI] [PubMed] [Google Scholar]
- 7. Morrison JJ, Noncompressible RTE, Hemorrhage T. A review with contemporary definitions and management strategies. Surg. Clin. N. Am. 2012; 92: 843–58. [DOI] [PubMed] [Google Scholar]
- 8. Kisat M, Morrison JJ, Hashmi ZG, Efron DT, Rasmussen TE, Haider AH. Epidemiology and outcomes of non‐compressible torso hemorrhage. J. Surg. Res. 2013; 184: 414–21. [DOI] [PubMed] [Google Scholar]
- 9. Morrison JJ, Stannard A, Rasmussen TE, Jansen JO, Tai NRM, Midwinter MJ. Injury pattern and mortality of noncompressible torso hemorrhage in UK combat casualties. J. Trauma Acute Care Surg. 2013; 75: 263–8. [DOI] [PubMed] [Google Scholar]
- 10. Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J. Am. Coll. Surg. 2000; 190: 288–98. [DOI] [PubMed] [Google Scholar]
- 11. Morrison JJ, Poon H, Rasmussen TE et al. Resuscitative thoracotomy following wartime injury. J. Trauma Acute Care Surg. 2013; 74: 825–9. [DOI] [PubMed] [Google Scholar]
- 12. Khorsandi M, Skouras C, Shah R. Is there any role for resuscitative emergency department thoracotomy in blunt trauma? Interact. Cardiovasc. Thorac. Surg. 2013; 16: 509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole SL, Corday E. Four‐minute limit for cardiac resuscitation. JAMA 1956; 161: 1454–8. [DOI] [PubMed] [Google Scholar]
- 14. Jennings RB, Reimer KA, Steenbergen C. Complete global myocardial ischemia in dogs. Crit. Care Med. 1988; 16: 988–96. [DOI] [PubMed] [Google Scholar]
- 15. Radovsky A, Safar P, Sterz F et al. Regional prevalence and distribution of ischemic neurons in dog brains 96 hours after cardiac arrest of 0 to 20 minutes. Stroke 1995; 26: 2127–33. [DOI] [PubMed] [Google Scholar]
- 16. Tisherman SA. Salvage techniques in traumatic cardiac arrest: thoracotomy, extracorporeal life support, and therapeutic hypothermia. Curr. Opin. Crit. Care. 2013; 19: 594–8. [DOI] [PubMed] [Google Scholar]
- 17. Pieper A, Thony F, Brun J et al. Resuscitative endovascular balloon occlusion of the aorta for pelvic blunt trauma and life threatening hemorrhage: a 20‐year experience in a level I trauma center. J. Trauma Acute Care Surg. 2018; 84: 449–53. [DOI] [PubMed] [Google Scholar]
- 18. Tatsuya N, Cameron C, Yusuke T. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score‐adjusted untreated patients. J. Trauma Acute Care Surg. 2015; 78: 721–8. [DOI] [PubMed] [Google Scholar]
- 19. Kimura A, Iwase H, Tanaka H, et al. Japan Trauma Evaluation and Care (Kimura A, ed), 2nd edn. Tokyo: Herusu syuppan, 2018; 73. (in Japanese) [Google Scholar]
- 20. Barnard EBG, Manning JE, Smith JE, Rall JM, Cox JM, Ross JD. A comparison of selective aortic arch perfusion and resuscitative endovascular balloon occlusion of the aorta for the management of hemorrhage‐induced traumatic cardiac arrest: a translational model in large swine. PLoS Medicine 2017; 14: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reul GJ Jr, Mattox KL, Beall AC Jr, Jordan GL Jr. Recent advances in the operative management of massive chest trauma. Ann. Thorac. Surg. 1973; 16: 52–66. [DOI] [PubMed] [Google Scholar]
- 22. Lockey D, Crewdson K, Davies G. Traumatic cardiac arrest: who are the survivors? Ann. Emerg. Med. 2006; 48: 240–4. [DOI] [PubMed] [Google Scholar]
- 23. Rosemurgy AS, Norris PA, Olson SM, Hurst JM, Albrink MH. Prehospital traumatic cardiac arrest: the cost of futility. J. Trauma Acute Care Surg. 1993; 35: 468–73. [PubMed] [Google Scholar]
- 24. Manning JE, Murphy CA, Hertz CM, Perretta SG, Mueller RA, Norfleet EA. Selective aortic arch perfusion during cardiac arrest: a new resuscitation technique. Ann. Emerg. Med. 1992; 21: 1058–65. [DOI] [PubMed] [Google Scholar]
- 25. Manning JE, Katz LM, Pearce LB et al. Selective aortic arch perfusion with hemoglobin‐based oxygen carrier‐201 for resuscitation from exsanguinating cardiac arrest in swine. Crit. Care Med. 2001; 29: 2067–74. [DOI] [PubMed] [Google Scholar]
- 26. Manning JE, Batson DN, Gansman TW, Murphy CA, Perretta SG, Norfleet EA. Selective aortic arch perfusion using serial infusions of perflubron emulsion. Acad. Emerg. Med. 1997; 4: 883–90. [DOI] [PubMed] [Google Scholar]
- 27. Manning JE, Batson DN, Payne FB et al. Selective aortic arch perfusion during cardiac arrest: enhanced resuscitation using oxygenated perflubron emulsion, with and without aortic arch epinephrine. Ann. Emerg. Med. 1997; 29: 580–7. [DOI] [PubMed] [Google Scholar]
- 28. Hoops HE, Manning JE, Graham TL et al. Selective aortic arch perfusion with fresh whole blood or HBOC‐201 reverses hemorrhage‐induced traumatic cardiac arrest in a lethal model of noncompressible torso hemorrhage. J. Trauma Acute Care Surg. 2019; 87: 263–73. [DOI] [PubMed] [Google Scholar]
- 29. Manning JE, Murphy CA, Neil Batson D, Perretta SG, Mueller RA, Norfleet EA. Aortic arch versus central venous epinephrine during CPR. Ann. Emerg. Med. 1993; 22: 703–8. [DOI] [PubMed] [Google Scholar]
- 30. Leis CC, Hernández CC, Blanco MJ, Paterna PC, Hernández RE, Torres EC. Traumatic cardiac arrest: should advanced life support be initiated? J. Trauma Acute Care Surg. 2013; 74: 634–8. [DOI] [PubMed] [Google Scholar]
- 31. Barnard E, Yates D, Edwards A, Fragoso‐Iñiguez M, Jenks T, Smith JE. Epidemiology and aetiology of traumatic cardiac arrest in England and Wales ‐ A retrospective database analysis. Resuscitation. 2017; 110: 90–4. [DOI] [PubMed] [Google Scholar]
- 32. Cureton EL, Yeung LY, Kwan RO et al. The heart of the matter: utility of ultrasound of cardiac activity during traumatic arrest. J. Trauma Acute Care Surg. 2012; 73: 102–10. [DOI] [PubMed] [Google Scholar]
- 33. Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Funabiki T. Partial occlusion, conversion from thoracotomy, undelayed but shorter occlusion: resuscitative endovascular balloon occlusion of the aorta strategy in Japan. Eur. J. Emerg. Med. 2018; 25: 348–54. [DOI] [PubMed] [Google Scholar]
- 34. Manning JE, Ross JD, McCurdy SL, True NA. Aortic hemostasis and resuscitation: preliminary experiments using selective aortic arch perfusion with oxygenated blood and intra‐aortic calcium coadministration in a model of hemorrhage‐induced traumatic cardiac arrest. Acad. Emerg. Med. 2016; 23: 208–12. [DOI] [PubMed] [Google Scholar]
- 35. Zaloga GP. Hypocalcemia in critically ill patients. Crit. Care Med. 1992; 20: 251–62. [DOI] [PubMed] [Google Scholar]
- 36. Denlinger JK, Nahrwold ML, Gibbs PS, Lecky JH. Hypocalcaemia during rapid blood transfusion in anaesthetized man. Br. J. Anaesth. 1976; 48: 995–1000. [DOI] [PubMed] [Google Scholar]
- 37. Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell‐free hemoglobin‐based blood substitutes and risk of myocardial infarction and death: a meta‐analysis. JAMA 2008; 299: 2304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paula MM, Anke S, Françoise A et al. The effect HBOC‐201 and sodium nitrite resuscitation after uncontrolled haemorrhagic shock in swine. Injury 2012; 43: 638–47. [DOI] [PubMed] [Google Scholar]
- 39. Kutcher ME, Forsythe RM, Tisherman SA. Emergency preservation and resuscitation for cardiac arrest from trauma. Int. J. Surg. 2016; 33: 209–12. [DOI] [PubMed] [Google Scholar]
- 40. Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia; its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperatures. Ann. Surg. 1950; 132: 849–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor M, Elrifai A, Bailes J. Hypothermia in relation to the acceptable limits of ischemia for bloodless surgery. In: Advances in Low‐Temperature Biology, Volume 3. Amsterdam: Elsevier, 1996. p. 1–64. [Google Scholar]
- 42. Tisherman SA, Alam HB, Rhee PM et al. Development of the emergency preservation and resuscitation for cardiac arrest from trauma clinical trial. J. Trauma Acute Care Surg. 2017; 83: 803–9. [DOI] [PubMed] [Google Scholar]
- 43. Ravi C, Samuel AT, Bianca C, Fouche‐Weber LY. Anesthesia for emergency preservation and resuscitation (EPR) for traumatic cardiac arrest. A Brief Review. Curr. Anesthesiol. Rep. 2018; 8: 59–62. [Google Scholar]
- 44. James EM, Todd ER, Samuel AT, Jeremy WC. Emerging hemorrhage control and resuscitation strategies in trauma: endovascular to extracorporeal. J. Trauma Acute Care Surg. 2020; 89: S50–S58. [DOI] [PubMed] [Google Scholar]
- 45. Nozari A, Safar P, Wu X et al. Suspended animation can allow survival without brain damage after traumatic exsanguination cardiac arrest of 60 minutes in dogs. J. Trauma. 2004; 57: 1266–75. [DOI] [PubMed] [Google Scholar]
- 46. Patt A, McCroskey BL, Moore EE. Hypothermia‐induced coagulopathies in trauma. Surg. Clin. North Am. 1988; 68: 775–85. [DOI] [PubMed] [Google Scholar]
- 47. Henry EW, Clifton WC, Andrew BP, Samuel AT. Admission hypothermia and outcome after major trauma. Crit. Care Med. 2005; 33: 1296–301. [DOI] [PubMed] [Google Scholar]
- 48. Tisherman SA. Hypothermia and injury. Curr. Opin. Crit. Care. 2004; 10: 512–9. [DOI] [PubMed] [Google Scholar]
- 49. True NA, Siler S, Manning JE. Endovascular resuscitation techniques for severe hemorrhagic shock and traumatic arrest in the presurgical setting. J. Spec. Oper. Med. 2013; 13: 33–7. [DOI] [PubMed] [Google Scholar]
- 50. Belenkiy SM, Batchinsky AI, Rasmussen TE, Cancio LC. Resuscitative endovascular balloon occlusion of the aorta for hemorrhage control: past, present, and future. J. Trauma Acute Care Surg. 2015; 79: S236–S242. [DOI] [PubMed] [Google Scholar]
- 51. Andrew WK, Christine V, Mirette D et al. The evolution of a purpose designed hybrid trauma operating room from the trauma service perspective: the RAPTOR (Resuscitation with angiography percutaneous treatments and operative Resuscitations). Injury 2014; 45: 1413–21. [DOI] [PubMed] [Google Scholar]
- 52. Antoni M, Forest RS, William HN, Abe A, Bjorn KS. Improved hemodynamic recovery and 72‐hour survival following low‐volume resuscitation with a PEGylated carboxyhemoglobin in a rat model of severe hemorrhagic shock. Mil. Med. 2020; 17: usz472. [DOI] [PubMed] [Google Scholar]
- 53. Jimin G, Jacob OA, Rita S et al. Biomimetic rebuilding of multifunctional red blood cells: modular design using functional components. ACS Nano 2020; 14: 7847–59. [DOI] [PubMed] [Google Scholar]
- 54. Kohsuke H, Manabu K, Masato T et al. Combination therapy using fibrinogen γ‐chain peptide‐coated, ADP‐encapsulated liposomes and hemoglobin vesicles for trauma‐induced massive hemorrhage in thrombocytopenic rabbits. Transfusion 2019; 59: 3186–96. [DOI] [PubMed] [Google Scholar]
- 55. Koyama T, Niikura H, Shibata M et al. Impact of ischemic postconditioning with lactate‐enriched blood on early inflammation after myocardial infarction. IJC Metab. Endocr. 2014; 2: 30–4. [Google Scholar]
- 56. Group HaCAS . Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002; 346: 549–56. [DOI] [PubMed] [Google Scholar]
- 57. Stephen AB, Timothy WG, Michael DB et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002; 346: 557–63. [DOI] [PubMed] [Google Scholar]
- 58. Jean‐Baptiste L, Hamid M, Amélie LG et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N. Engl. J. Med. 2019; 381: 2327–37. [DOI] [PubMed] [Google Scholar]
- 59. Masanobu T, Masaru U, Koji D et al. Edaravone reduces ischemia‐reperfusion injury mediators in rat liver. J. Surg. Res. 2007; 137: 69–74. [DOI] [PubMed] [Google Scholar]
- 60. Cuzzocrea S, Reiter RJ. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur. J. Pharmacol. 2001; 426: 1–10. [DOI] [PubMed] [Google Scholar]
- 61. Wilhelm B, Peter S, Rainer K et al. Antioxidant tempol enhances hypothermic cerebral preservation during prolonged cardiac arrest in dogs. J. Cereb. Blood Flow Metab. 2002; 22: 105–17. [DOI] [PubMed] [Google Scholar]
- 62. Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J. Cell Mol. Med. 2006; 10: 650–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nakao A, Kaczorowski DJ, Sugimoto R, Billiar TR, McCurry KR. Application of heme oxygenase‐1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2008; 42: 78–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tomohiro K, Chien‐Sheng H, Naobumi T et al. Inhaled hydrogen gas therapy for prevention of lung transplant‐induced ischemia/reperfusion injury in rats. Transplantation 2010; 90: 1344–51. [DOI] [PubMed] [Google Scholar]
- 65. Hiromichi N, Tsuyoshi N, Noritomo F et al. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 2020; 7: e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Drabek T, Han F, Garman RH et al. Assessment of the delta opioid agonist DADLE in a rat model of lethal hemorrhage treated by emergency preservation and resuscitation. Resuscitation. 2008; 77: 220–8. [DOI] [PubMed] [Google Scholar]
- 67. Han F, Drabek T, Stezoski J et al. Protein nitration and polyADP‐ribosylation in brain after rapid exsanguination cardiac arrest in a rat model of emergency preservation and resuscitation. Resuscitation. 2008; 79: 301–10. [DOI] [PubMed] [Google Scholar]
- 68. Lahoud‐Rahme MS, Stezoski J, Kochanek PM, Melick J, Tisherman SA, Drabek T. Blood‐brain barrier integrity in a rat model of emergency preservation and resuscitation. Resuscitation. 2009; 80: 484–8. [DOI] [PubMed] [Google Scholar]
- 69. Shuja F, Tabbara M, Li Y et al. Profound hypothermia decreases cardiac apoptosis through Akt survival pathway. J. Am. Coll. Surg. 2009; 209: 89–99. [DOI] [PubMed] [Google Scholar]
- 70. Alam HB, Hashmi S, Finkelstein RA et al. Alterations in gene expression after induction of profound hypothermia for the treatment of lethal hemorrhage. J. Trauma. 2010; 68: 1084–98. [DOI] [PubMed] [Google Scholar]
- 71. Drabek T, Janata A, Jackson EK et al. Microglial depletion using intrahippocampal injection of liposome‐encapsulated clodronate in prolonged hypothermic cardiac arrest in rats. Resuscitation. 2012; 83: 517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Drabek T, Janata A, Wilson CD et al. Minocycline attenuates brain tissue levels of TNF‐α produced by neurons after prolonged hypothermic cardiac arrest in rats. Resuscitation. 2014; 85: 284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Drabek T, Wilson CD, Janata A et al. Unique brain region–dependent cytokine signatures after prolonged hypothermic cardiac arrest in rats. Ther. Hypothermia Temp. Manag. 2015; 5: 26–39. [DOI] [PubMed] [Google Scholar]
- 74. Tohru MT, Genshiro AS, Shingo S et al. A discrete neuronal circuit induces a hibernation‐like state in rodents. Nature 2020; 583: 109–14. [DOI] [PubMed] [Google Scholar]


