Abstract
Aging is associated with changes in regulation, particularly among diverse regulators in the brain. We assayed prominent regulatory elements in mouse brain to explore their relationship to one another, stress, and aging. Notably, unphosphorylated (activated) forkhead transcription factor 3a (uFOXO3a) expressed exponential decline congruent with increasing age-related mortality. Decline in uFOXO3a would impact homeostasis, aging rate, stress resistance, and mortality. We also examined other regulators associated with aging and FOXO3a: protein kinase B (PKB), the mechanistic target of rapamycin (mTOR), 70 kDa ribosomal S6 kinase (P70S6K), and 5’ AMP-activated protein kinase (AMPK). It would require powerful regulatory distortion, conflicting tradeoffs and/or significant damage to inflict exponential decline of a transcription factor as crucial as FOXO3a. No other regulator examined expressed an exponential pattern congruent with aging. PKB was strongly associated with decreases in uFOXO3a, but the aging pattern of PKB did not support a causal linkage. Although mTOR expressed a trend for age-related increase, this was not significant. We considered that the mTOR downstream element, P70S6K, might suppress FOXO3a, but remarkably, it expressed a strong positive association. The age-related pattern of AMPK was also incompatible. Literature suggested the immunological regulator NFĸB (nuclear factor kappa-light-chain-enhancer of activated B cells) increases with age and suppresses FOXO3a. This would inhibit apoptosis, autophagy, mitophagy, proteostasis, detoxification, antioxidants, chaperones, and DNA repair, thus exacerbating aging. We conclude that a key aspect of aging involves distortion of key regulators in the brain.
Keywords: PI3K-PKB, Immunity, Inflammation, NFκB, Stress resistance, Error catastrophes, FOXO3a, Aging
Introduction
Age-related mortality increases exponentially, suggesting that factors causally associated with aging are also likely to express exponential trajectories with age (de Magalhães et al. 2009, 2018). Increased signaling via the PI3K pathway to protein kinase B (PKB) is generally implicated in rising reactive oxygen species (ROS) in aging (e.g., Dröge and Schipper, 2007; Kim et al. 2008). Indeed, PI3K signaling to PKB impacts multiple critical regulators, including activation of the target of rapamycin (mTOR) and suppression of forkhead transcription factors (FOXOs) that critically regulate antioxidants, stress resistance, homeostasis, and aging (Kim et al. 2008; Manning and Cantley 2007; Manning and Toker 2017; Thapa et al. 2019). Dichotomous variation in these same pathways also underlie circadian organization and interspecific variation in body size as exemplified across dog breeds, giant versus small mice (Rollo 1994; Rollo et al. 1996; Rollo 2002; 2010; 2012), and humans (Samaras et al. 2002).
We examined crucial factors associated with aging in brains of C57BL/6J_SJL mice. Targets included unphosphorylated (active) FOXO3a, PKB, mTOR, 70 kDa ribosomal S6 kinase (P70S6K), and 5’ AMP-activated protein kinase (AMPK). FOXOs are known to decline with age, but here we document that FOXO3a decline, like aging, was exponential. Moreover, none of the important regulators we examined appeared causal, including PKB.
These results suggest that decline of FOXO3a and associated aging are driven by other key regulator(s). The immunological transcription factor NFĸB emerges as a likely culprit. NFĸB and FOXOs are mutually antagonistic. Kim et al. (2008) suggested that PKB suppression of FOXOs caused the age-related rise in NFĸB via disinhibition. Given that PKB could not explain the age-related decline of FOXO3a in our animals, rising NFĸB may be driven via other factors. Indeed, activation of NFĸB (and suppression of FOXO3a) is associated with increasing inflammatory stress and immunological distortion associated with changes in the microbiome with age (see Chiu et al. 2016).
Phosphorylation (inactivation) of FOXO1 and activation of NFĸB were greater in old rats and NFĸB also induces pro-inflammatory cytokines likely to exacerbate redox stress (Kim et al. 2008). NFĸB impacts are likely to reinforce its own expression. Indeed, inhibition of FOXOs by NFĸB would reduce stress resistance, including antioxidants. Despite the general wisdom that elevated PI3K-PKB signaling is responsible for declining FOXO and increasing NFĸB, the age-related pattern of PKB in our mice was not compatible with exponential decline of FOXO3a. We argue that alterations in the microbiome and associated age-related elevation of NFĸB may suppress FOXOs and exacerbate aging (see Chiu et al. 2016).
Materials and methods
Random bred C57BL/6J_SJL mice were maintained in standard cages on a 12 h light:12 h dark photoperiod at 22o ± 2 °C. Teklad Rodent Chow and water were supplied ad libitum. All procedures and protocols were approved by the McMaster University Animal Research Ethics Board and adhered to Canadian Council on Animal Care Guidelines. Possible sources of variation in our data (besides age and gender) included circadian and ultradian rhythms, feeding/digestion, reproductive status, and perhaps some genetic variation associated with the hybrid background.
All assays were performed with whole-brain homogenate. Untreated mice of both genders (n = 38 males, n = 23 females) spanning the entire lifetime were acquired from our breeding colony during the afternoon (1:00 pm–5:00 pm). Animals were sacrificed and the brains were immediately removed, weighed, and snap frozen. Tissues were stored at −80 °C until processed. Tissue for processing was thawed for 10 min in cold homogenization buffer (PBS + 1% Triton + Protease Inhibitor Cocktail) at 1 ml/100 mg of tissue. The tissue was then homogenized and incubated for 4 h on ice. Samples were centrifuged at 4500 g (Allegra 6R, Beckman Coulter, Miami FL) for 20 min at 4 °C. The supernatant from each sample was transferred to micro-centrifuge tubes and centrifuged at 16,000 g for 15 min at 4 °C. A small aliquot of each sample was removed and total protein content was determined using a protein quantification kit (Bio-Rad Laboratories, Mississauga ON). The remaining supernatants were transferred into cryovials and stored at −80 °C until testing. Reagents were purchased from Sigma Aldrich (Oakville, ON) unless otherwise specified.
Total and phosphorylated signaling proteins were quantified in tissue homogenates using sandwich ELISA kits purchased from the following companies: total and phospho(S2448)-mTOR, Cell Signaling Technology (Whitby, Ontario); total and phospho(S253)-FOXO3a, Ray Biotech Kit (Norcross, GA), total and phospho(S485)-AMPKα1, total and phospho(S473)-Akt1, and total and phospho(T389)-P70S6 kinase kits were purchased from R&D Systems (Minneapolis, MN). Briefly, microplates pre-coated with capture antibody were provided in the kits. Samples or standards were added to designated wells and incubated overnight at 4 °C to bind analyte. Following incubation, plates were washed 3 times using provided wash buffer. A biotinylated detection antibody was diluted to appropriate concentration, added to each well and incubated for 2 h at room temperature.
Plates were washed three times with wash buffer to remove unbound detection antibody. Streptavidin-HRP was added to each well and incubated at room temperature for 1 h. Plates were again washed 3 times with wash buffer to remove unbound streptavidin-HRP. Tetramethylbenzidine substrate solution was added to each well and incubated at room temperature to allow development of blue color. Wells containing a standard dilution series were used to determine appropriate color intensity. Color development was stopped using 0.18 M H2SO4 and absorbance was measured at 450 nm using a SpectraMax plate reader® (Molecular Devices, Sunnyvale CA).
Statistical analyses and graphics were compiled with STATISTICA® software.
Results
It is important to emphasize that molecular biomarkers reflect changes across lifetimes and aging. Specific variation includes health, damage, regulatory distortions, gender, reproductive status, digestive status. The drastic decline in GH secretion with age is a notable example (Rudman et al. 1981). Collecting samples during a narrow circadian window ensured that age-related changes in expression are prominent.
Unphosphorylated (activated) FOXO3a
Mortality increases exponentially with age, and consequently, factors associated with aging are also expected to show exponential trajectories (de Magalhães et al. 2009, 2018). FOXO3a is strongly linked to aging and lifespan, and is generally recognized to decline during aging. Whether such decline is exponential appears outstanding. Here we show that normal untreated mice express exponentially declining unphosphorylated uFOXO3a in the brain. Males and females showed that no significant difference (p = 0.513849) in regression lines and genders (23 females, 38 males) was consequently combined.
Simple linear regression found a significant age-related decline in uFOXO3a (p = 0.038281, r2 = 0.07074, d.f. = 1,59).
uFOXO = 0.3798–0.000246(age), where age is in units of days (uFOXO data were multiplied by 100 to remove leading zero values before analysis).
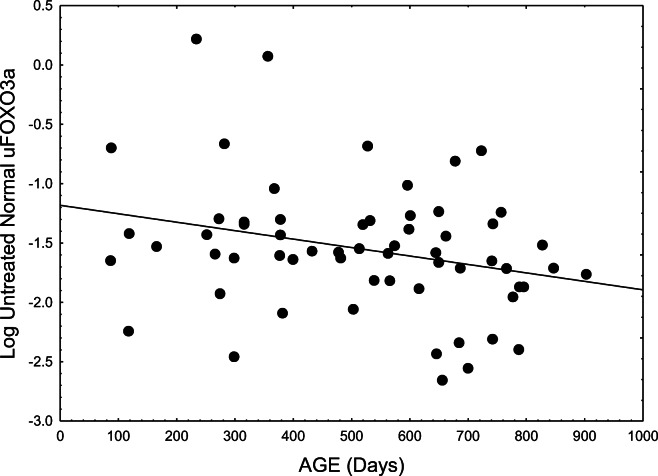
We also examined the pattern obtained with regression of logarithmically transformed uFOXO3a to determine whether it conformed to an exponential trajectory. This proved a better fit than the untransformed data in terms of both significance and correlation (p = 0.031465, r2 = 0.07605, d.f. = 1,59). Figure 1 presents the data and best-fitting line:
Fig. 1.
Levels of unphosphorylated uFOXO3a (the active form) declined exponentially with age in normal untreated mice (p = 0.03147, r2 = 0.07605, d.f. = 1,59)
Log(uFOXO) = −1.18222 − 0.00071(age).
A t-test performed on younger (<400 days old) versus older (>400 days old) Nr (normal) mice strongly reinforced the regression analysis (p < 0.025). Levels of uFOXO were 35.9% lower in older mice, indicating strongly compromised uFOXO3a in normal aging. Such decline would impact a multitude of critical stress resistance mechanisms regulated by this prominent transcription factor (see discussion). This result also emerged in the significant decline of the uFOXO/pFOXO ratio with age (p = 0.0019) (see below).
Phosphorylated (inactive) FOXO3a
Phosphorylated FOXO (pFOXO) showed an increasing trend with age (not shown), but linear regression was not significant (r2 = 0.0039, p = 0.6275). A logarithmic transformation improved the fit but also failed to reach significance (r2 = 0.0110, p = 0.4166). Failure to resolve pFOXO, and its relatively low levels compared to uFOXO, likely reflects that pFOXO translocates to the cytoplasm and is degraded by the proteasome.
Ratio of unphosphorylated uFOXO/phosphorylated pFOXO
Levels of uFOXO were consistently greater than those of pFOXO. Regression analysis obtained highly significant decline in the uFOXO/pFOXO ratio with age (r2 = 0.1517, p = 0.00192, d.f. = 1,59):
uFOXO/pFOXO = 6.41254 − 0.005068(age).
A logarithmic transformation did not improve the r2 or significance (r2 = 0.101096, p = 0.01252, d.f. = 1,59, Fig. 2):
Fig. 2.
Ratio of Log(uFOXO/pFOXO) with age (r2 = 0.101096, p = 0.01252, d.f. = 1,59)
Log(uFOXO/pFOXO) = 1.6004 − 0.000883(age).
The improved fit of the uFOXO/pFOXO ratio relative to uFOXO alone probably reflects that dividing the declining values of uFOXO by increasing values of pFOXO with age enhanced the negative slope of the line. Alternatively, declining proteasome function could slow pFOXO clearance. Figure 2 presents the log-transformed data as it better compares to Fig. 1.
Protein kinase B
Most studies suggest that PKB signaling increases with age, in which case phosphorylation of FOXOs by PKB could explain increasing nuclear exclusion and degradation of FOXOs with age. We found that PKB indeed showed a significant negative regulation of FOXO3a in a direct comparison (Fig. 3), but surprisingly, PKB failed to express a clear pattern of age-related increase in our study (Fig. 4). In fact, the age-related pattern appeared somewhat parabolic, with a peak at ~400 days and lower values in younger and older ages (i.e., increasing in youth, declining in older ages). Consequently, linear regression of pPKB against age was nearly flat-line (r2 = 0.002584, p = 0.6973). A polynomial fit was also not significant. Thus, the age-related pattern of phosphorylated PKB (and especially the lower values in old age) was not congruent with the age-related exponential decline of FOXO3a.
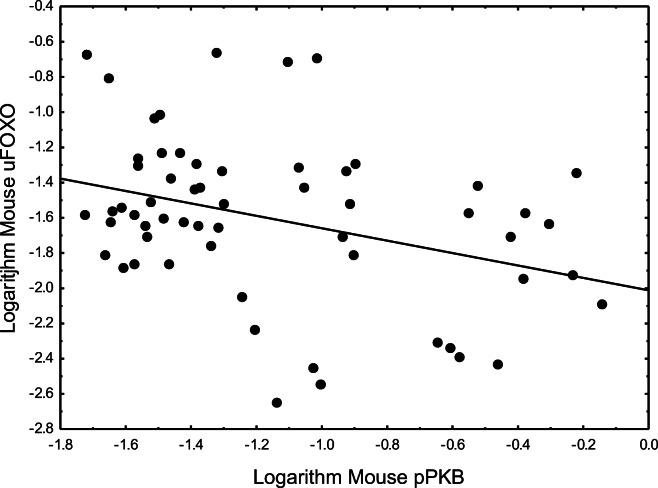
Fig. 3.
Phosphorylated pPKB impacts on unphosphorylated uFOXO (r2 = 0.1211, p = 0.006914)
Fig. 4.
pPKB had a somewhat parabolic pattern with age (p = 0.6973, d.f., 1,59)
There was a clear negative relationship between phosphorylated pPKB and unphosphorylated uFOXO which is well known. The best fitting analysis, however, was a highly significant exponential (log-log) relationship (r2 = 0.1211, p = 0.006914, Fig. 3):
Log (uFOXO) = −2.01173 − 0.3523(Log(pPKB)).
The fitted linear regression of pPKB against age was nearly flat-line and was not statistically resolved:
pPKB = 0.381393 − 0.000044 (age), p = 0.6973, d.f., 1,59, Fig. 4.
A polynomial fit was also not significant. Moreover, pPKB showed a weak trend for decline after 400 days that was inconsistent with deriving the exponential decline of uFOXO with age.
The fitted regression for PKB with age was flat-line. PKB showed a relatively weak decline after 400 days, but this was inconsistent with the exponential decline of uFOXO.
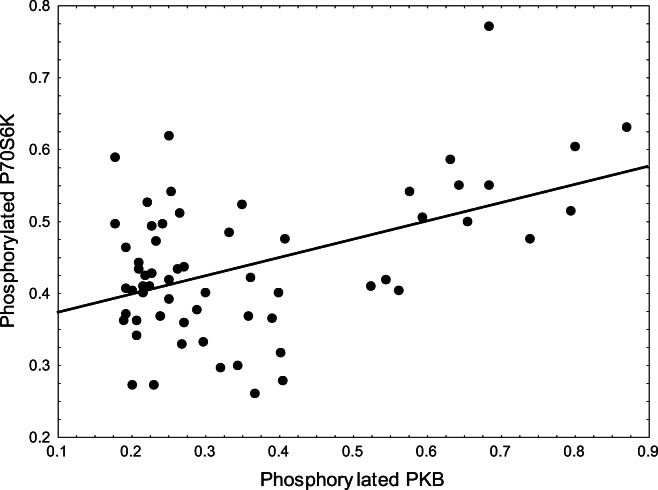
Interestingly, exploration revealed that PKB had a highly significant positive association with phosphorylated P70S6K (p = 0.000126, d.f. = 1,59, r2 = 0.22216, Fig. 5): Phosphorylated P70S6K = 0.3469753 + 0.253954(pPKB).
Fig. 5.
pPKB activity was closely associated with activation of pP70S6K (p = 0.000126, d.f. = 1,59, r2 = 0.22216)
mTOR (Mechanistic Target of Rapamycin)
We analyzed mTOR from a subset of the same group of mice. Data bracketing 100 to 800 days of age revealed a modest increase of mTOR with age but this was not significantly resolved (p < 0.5109, Fig. 6).
Fig. 6.
Phosphorylated mTOR showed a modest trend for age-related increase, but this was not statistically resolved (linear regression, p < 0.5109)
Moreover, the modest rise of mTOR with age was not congruent with the steep exponential decline of uFOXO. The r2 value for the mTOR regression was significant but weak (0.01617).
AMPK (5’ AMP-activated protein kinase)
As a biomarker of energy status, AMPK would be expected to contribute to regulation of biomarkers associated with production, stress resistance, and aging. AMPK is known to positively influence FOXO3. The pattern of phosphorylated AMPK with age was similar to that of PKB, weakly parabolic (Fig. 7).
Fig. 7.
The pattern of pAMPK with age was somewhat parabolic (p = 0.52798, r2 = 0.00678, d.f., 1,59)
The linear regression of pAMPK on age was virtually flat-line (p = 0.52798, r2 = 0.00678, d.f., 1,59). A weak polynomial fit was also not significant (Fig. 7). In ages over 600 days, there was a weak trend for decline. Although AMPK can activate uFOXOs that was not consistent with low levels of uFOXO at the highest levels of AMPK (p = 0.881552, r2 = 0.000392, d.f., 1,58, Fig. 8).
Fig. 8.
Activated uFOXO expressed a somewhat parabolic fit with activated pAMPK. Activity of uFOXO was higher at pAMPK levels of 0.1 to 0.2, but this was followed by lower responsiveness at higher levels of pAMPK (p = 0.881552, r2 = 0.000392, d.f., 1,58)
P70S6K (70 kDa ribosomal S6 kinase)
Given that P70S6K is a downstream target of mTOR, we expected that it would be negatively associated with uFOXO. The fact that P70S6K was strongly associated with higher levels of uFOXO seemed counter-intuitive. We previously argued that mTOR was likely associated with protein synthesis in early sleep, subsequently followed by uFOXO expression associated with stress resistance, and gluconeogenesis in preparation for waking (Rollo 2010, 2012). In this framework, P70S6K likely occupies a temporal window towards the end of mTOR activity, and preceding, and possibly overlapping increases in uFOXO activity. AMPK was not significantly associated with P70S6K (p = 0.1212, r2 = 0.202814, df = 1,58, Fig. 9).
Fig. 9.
P70S6K surprisingly showed a strong positive association with uFOXO. This was most significant with a log-log transform (r2 = 0.1218967, p = 0.00625, d.f. 1,58)
Cao et al. (2006) described various feedback loops in which FOXO1 increased phosphorylation of P70S6K and serine 307 of IRS-1. This reduced phosphorylation of PKB (inhibiting) as well as uFOXO1 (thus activating itself). Others recognized that negative feedback from P70S6K can suppress PI3K signaling (via impacts on the insulin response substrate, IRS) that otherwise drives mTOR-P70S6K activation (e.g., Tremblay and Marette 2001; Harrington et al. 2004; Manning 2004; Shah et al. 2004; Um et al. 2004; Klionsky et al. 2005).
Liu et al. (2010) found that TOR activity was associated with PHLPP phosphatases that mediated negative feedback to PI3K-PKB. P70S6K was associated with expression of these phosphatases (Liu et al. 2010) but these phosphatases may also suppress P70S6K (Mathur et al. 2017). Critical aspects associated with inhibition of PI3K-PKB are insulin resistance and associated generation of reactive oxygen species (ROS) (Mathur et al. 2017).
There was no significant relationship of P70S6K with age, the line showing a slight negative slope (p = 0.2403491, d.f. = 1,59):
P70S6K = 0.475615 − 0.000073(age).
The positive relationship with uFOXO and lack of a congruent age-related pattern reject P70S6K as a candidate explaining exponential decline of uFOXO with age.
Discussion
Although there are many types of regulators, we chose to examine a primary regulator of stress-resistance and aging, uFOXO3a. Given that levels of FOXO3a mRNA do not decrease with age (Zemva et al. 2012), age-related decline of uFOXO function must reflect post-transcriptional events and interactions with higher-order organization. Decline of FOXO3a-mediated stress resistance allows accumulation of damage and associated aging (Gurkar et al. 2018). Since FOXO provides stress resistance and ameliorates damage, decline may engage a negative feedback loop that exacerbates progressive FOXO loss.
FOXO3a
FOXO3a is strongly linked to slower aging and longer life, but FOXOs generally decline with age. Increasing free radical levels with age, perhaps reflecting deterioration of mitochondrial DNA, have been suggested to elevate PI3K-PKB activity that could then suppress FOXO3a (Dröge and Schipper 2007). Suppression of FOXOs would reduce a myriad of critical functions including apoptosis, autophagy, mitophagy, proteostasis, detoxification, antioxidants, chaperones, and DNA repair (see Kim et al. 2014; Gurkar et al. 2018; Wang et al. 2018). This has the potential to fuel a vicious cycle. Results with various regulators all expressed considerable variation likely tracing to gender, body mass, digestive status, and other physiological and behavioral aspects. Of all the regulators examined, the uFOXO/pFOXO ratio explained the greatest amount of variation with age (r2 = 0.1517, p = 0.00192). Gender was not a significant source of variation for the uFOXO/pFOXO ratio.
The exponential nature of FOXO3a decline appears previously undocumented. Exponential decline is of considerable interest (Fig. 1) given that numerous important aspects express exponential changes with age. These include cellular senescence (Herbig et al. 2006), cancer (Wu et al. 2016), and mortality rates (Harman 1983; de Magalhães et al. 2009, 2018). Given critical regulation of stress resistance homeostasis and longevity by FOXO3a, understanding what regulators and/or aging aspects are associated with FOXO3A is paramount. Interestingly, decline of FOXO3a with age does not involve reduction of RNA (Zemva et al. 2012). We expected that examining a broad range of regulatory factors likely to impact FOXO3a would identify a way forward. Instead, even well-known candidates suggested to impact FOXOs (such as PI3K-PKB) were not congruent with the exponential decline of uFOXO3a in our mice (see Adler et al. 2007; Dröge and Schipper 2007; Kim et al. 2008).
PKB/Akt
Besides potentially mediating direct suppression of FOXOs, PKB also mediates suppression of the FOXO activator, AMPK (Salminen et al. 2016). Indeed, the direct suppression of FOXO3a by PKB reflected a highly significant and exponential relationship (p = 0.006914, Fig. 3). Regardless, the somewhat parabolic relationship of PKB with age was not congruent with the exponential decline of uFOXO3a (Fig. 4) since, rather than strongly increasing in older ages, PKB was declining (Fig. 4). Moreover, although pPKB is well established to phosphorylate FOXO, pPKB was not significantly related to phosphorylated pFOXO (p = 0.3741). This likely reflects that pFOXO is rapidly degraded.
mTOR
Generally, the balance between TOR and FOXOs is highlighted as associated with aging processes and longevity. Thus, inhibition of TOR increases the lifespan of mice (Harrison et al. 2009; Miller et al. 2011, 2014; Zhang et al. 2014). Although mTOR has been viewed as a strong regulator of FOXOs (and it showed a modest trend for age-related increase here: Fig. 6), this trend was not significant in our study and it appears too weak to derive the strong exponential decline of FOXO3a.
AMPK
The AMPK-FOXO3 pathway critically regulates energy metabolism, activates FOXO3 (and its diverse functions), and suppresses NFkB (Greer et al. 2007; Jeon 2016). Consequently, AMPK is associated with stress resistance, healthy aging, reduced inflammation, and extended longevity (see Salminen and Kaarniranta 2012; Salminen et al. 2016; Jeon 2016). Moreover, pAMPK expressed a parabolic activity pattern with age (Fig. 7) with declines beginning in middle age. A specific range of lower AMPK levels was most strongly associated with uFOXO3a (Fig. 8), but high levels were associated with lower FOXO3a. Given the positive impact of AMPK on FOXOs, the age-related decline of pAMPK could partly contribute to lower FOXO3a in older ages.
P70S6K
Decreases in PI3K-PKB and mTOR activity could disinhibit expression of uFOXO, but given the strength of the positive P70S6K-FOXO3a association (Fig. 6), it seems likely that P70S6K may contribute to direct activation of uFOXO. This would go a long way towards explaining the controversy regarding regulation of autophagy by P70S6K. P70S6K is a downstream target of TOR signaling but mTOR itself generally suppresses autophagy (Heras-Sandoval et al. 2014). It is generally considered that it is uFOXO that mediates autophagy (see Klionsky et al. 2005; Martins et al. 2016). Thus the association of P70S6K with activation of uFOXO could also explain the association of P70S6K with autophagy.
Given the critical regulation and ubiquity of FOXOs in stress resistance and aging, this transcription factor may well reflect what Pearl (1928) perceived as a vital or inherent essence.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adler AS, Sinha S, Kawahara TLA, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Kamioka Y, Yokoi N, Kobayashi T, Hino O, Onodera M, Mochizuki N, Nakae J. Interaction of FoxO1 and TSC2 induces insulin resistance through activation of mTOR/p70S6K pathway. J Biol Chem. 2006;281:40242–40251. doi: 10.1074/jbc.M608116200. [DOI] [PubMed] [Google Scholar]
- Chiu CF, Chang YW, Kuo KT, Shen YS, Liu CY, Yu YH, Cheng CC, Lee KY, Chen FC, Hsu MK, Kuo TC, Ma JT, Su JL. NF-κB–driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. PNAS. 2016;113:E2526–E2535. doi: 10.1073/pnas.1522612113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, Curado J, Church GM. Meta-analysis of age related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, Thompson L, de Lima I, Gaskill D, Li X, Thornton D, Yang C, Palmer D. A reassessment of genes modulating aging in mice using demographic measurements of the rate of aging. Genetics. 2018;208:1617–1630. doi: 10.1534/genetics.118.300821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282(41):30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Gurkar AU, Robinson AR, Cui Y, Li X, Allani SK, Webster A, Muravia M, Fallahi M, Weissbach H, Robbins PD, Wang Y. Dysregulation of DAF-16/FOXO3A-mediated stress responses accelerates oxidative DNA damage induced aging. Redox Biol. 2018;18:191–199. doi: 10.1016/j.redox.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging: consequences of mitochondrial aging. Age. 1983;6(3):86–94. doi: 10.1007/BF02432509. [DOI] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I. The TSC1-2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/PKB/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311(5765):1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(7):e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim JY, Yu BP, Chung HY. The activation of NF-κB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology. 2008;9:33–47. doi: 10.1007/s10522-007-9114-6. [DOI] [PubMed] [Google Scholar]
- Kim J, Ishihara N, Lee TR. A DAF-16/FoxO3a-dependent longevity signal is initiated by antioxidants. Biofactors. 2014;40(2):247–257. doi: 10.1002/biof.1146. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Meijer AJ, Codogno P, Neufeld TP, Scott RC. Autophagy and p70S6 kinase. Autophagy. 2005;1:59–61. doi: 10.4161/auto.1.1.1536. [DOI] [PubMed] [Google Scholar]
- Liu J, Stevens PD, Gao T. mTOR-dependent regulation of PHLPP expression controls the rapamycin sensitivity in cancer cells. J Biol Chem. 2010;286:6510–6520. doi: 10.1074/jbc.M110.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins R, Lithgow GJ, Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15:196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Pandey VK, Kakkar P. PHLPP: a putative cellular target during insulin resistance and type 2 diabetes. J Endocrinol. 2017;233:R185–R198. doi: 10.1530/JOE-17-0081. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl R. The rate of living. New York: Alfred A. Knopf, Publisher; 1928. [Google Scholar]
- Rollo, C.D. 1994. Phenotypes: their epigenetics, ecology and evolution. Chapman and hall 463.
- Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Rollo CD. Aging and the mammalian regulatory triumvirate. Aging Dis. 2010;1:105–138. [PMC free article] [PubMed] [Google Scholar]
- Rollo CD. Circadian redox regulation. In: Schipper HM, editor. Pantopoulos, K. Principles of free radical biomedicine: Nova Science Publ; 2012. pp. 575–627. [Google Scholar]
- Rollo CD, Carlson J, Sawada M. Accelerated aging of giant transgenic mice is associated with elevated free radical processes. Can J Zool. 1996;74:606–620. doi: 10.1139/z96-070. [DOI] [Google Scholar]
- Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67:1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A. Age-related changes in AMPK activation: role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res Rev. 2016;28:15–26. doi: 10.1016/j.arr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Samaras TT, Storms LH, Elrick H. Longevity, mortality and body weight. Ageing Res Rev. 2002;1(4):673–691. doi: 10.1016/S1568-1637(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Thapa N, Horn HT, Anderson RA. Phosphoinositide spatially free AKT/PKB activation to all membrane compartments. Adv Biol Regul. 2019;72:1–6. doi: 10.1016/j.jbior.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age-and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Wang N, Zhang J, Qin M, Yi W, Yu S, Chen Y, Guan J, Zhang R. Amelioration of streptozotocin-induced pancreatic β cell damage by morin: involvement of the AMPK-FOXO3-catalase signaling pathway. Int J Mol Med. 2018;41(3):1409–1418. doi: 10.3892/ijmm.2017.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Cao N, Fenech M, Wang X. Role of sirtuins in maintenance of genomic stability: relevance to cancer and healthy aging. DNA Cell Biol. 2016;35(10):542–575. doi: 10.1089/dna.2016.3280. [DOI] [PubMed] [Google Scholar]
- Zemva J, Schilbach K, Stöhr O, Moll L, Franko A, Krone W, et al. Central FoxO3a and FoxO6 expression is downregulated in obesity induced diabetes but not in aging. Exp Clin Endocrinol Diabetes. 2012;120(06):340–350. doi: 10.1055/s-0031-1297970. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]