Abstract
Objective
To investigate both muscular manifestations and CK levels in a large cohort of patients with COVID-19 infection and to determine whether hyperckemia is associated with morbidity and mortality.
Methods
Data of 615 patients discharged from ASST Ovest Milanese (Milan, Lombardy, Italy) with final diagnosis of COVID-19 infection were retrospectively extracted from electronical medical records from 21 February to 1 May 2020. Patients were descriptively analyzed with respect to the following variables: sex, age, muscular manifestations (myalgia and/or arthralgia), fatigue, respiratory involvement (SARS pneumonia or respiratory failure) and history of falls. Association between patients’ characteristics and CK levels was investigated. In addition, the proportion of patients who died following access to the ER was calculated. Finally, the effect of CK levels and other patients’ features on mortality was estimated using a logistic regression model.
Results
176 (28.6%) patients had raised serum CK levels. CK levels were significantly associated with history of falls, male gender, SARS pneumonia, respiratory failure and in-hospital death. No correlation was found between hyperckemia and muscular manifestations.
Conclusions
Our study provides preliminary evidence that hyperckemia is associated with respiratory failure and fatal outcome in patients with COVID-19 infection.
In these patients, among other testing, CK dosage is recommended.
Key words: COVID-19, CK, myalgia, coronavirus
Introduction
As of April 15, 2020, Lombardy region accounted for 37% of Italian cases of COVID-19 infection and had 112.9 deaths per 100,000 population, almost six times higher than in the rest of Italy 1.
Although COVID-19 involves pulmonary system in the first place, in more than one-third of patients it causes neurological manifestations 2. Literature data report various symptoms affecting the central nervous system, the peripheral nervous system and the skeletal muscle 3,4. In particular, skeletal muscle injury has originally been defined by muscle pain associated with elevated serum creatine kinase (CK) level above 200 U/L 5.
During the ongoing pandemics of COVID-19 infection, descriptions of muscle symptoms have been recently reported 5-8. However, the correlation between serum CK levels and muscle injury has been poorly investigated 5,9,10.
Evidence of possible muscle involvement by coronaviruses dates back to 2003. During Severe Acute Respiratory Syndrome (SARS)-CoV outbreak in March 2003 muscle weakness and raised serum CK levels occurred in more than 30% of the SARS-infected patients 11.
In 2005 Leung et al. described a spectrum of myopathic changes associated with a SARS infection. Nevertheless, viral particle was not identified in the muscle 12. SARS-CoV-2 is structurally similar to SARS-CoV, and both bind to the angiotensin-converting enzyme 2 (ACE2) receptor to enter human cells. ACE2 is expressed in various human tissues in addition to the lungs, including muscle, although to a lesser extent 13, thus postulating a direct muscle involvement by SARS-CoV-2 in addition to immune-mediated muscle damage 3. These premises suggest a possible tropism of COVID-19 for muscle.
The primary aim of our study was to investigate both muscular manifestations and CK levels of a large cohort of patients with COVID infection in order to find a possible correlation among them. The secondary aim was to identify the association among CK levels, respiratory failure and in-hospital death.
Materials and methods
Data of 615 patients discharged from ASST Ovest Milanese (Milan, Lombardy, Italy) with final diagnosis of COVID-19 infection were collected from 21 February to 1 May 2020. Clinical data were retrospectively extracted from Emergency Room (ER) electronic records including laboratory findings and they were checked by two neurologists (ADR and EPV).
The institutional ethics board of ASST Grande Ospedale Metropolitano Niguarda, Milan, approved this study and due to the nature of retrospective chart review, waived the need for informed consent from individual patients (Approval number: 411-21072020).
The recorded data included the following: age, sex, muscular manifestations (myalgia and/or arthralgia), fatigue, history of falls (occurred at home before Hospital admission) and CK level. The occurrence of both respiratory involvement (SARS pneumonia or respiratory failure) and in-hospital deaths was collected from hospital discharge forms. CK levels were evaluated at the time of ER arrival. To simplify the results’ interpretation, with the aim of accounting for excessive heterogeneity of the CK level distribution, patients were stratified in four groups according to their CK values as in a previous paper 14: group 0 (CK < 200 U/L), group 1 (CK 200-554 U/L), group 2 (CK 555-1038 U/L) and group 3 (CK ≥ 1039 U/L).
Because CK levels can also raise during cardiac disorders, we selected patients without any reported history of myocarditis or peritonitis and with normal levels of troponin tested in ER.
Statistical analysis
Patients were descriptively analyzed with respect to the following variables: sex, age, muscular manifestations, fatigue, respiratory involvement, and history of falls according to the CK level. In addition, the proportion of patients who died following access to the ER was calculated. The association between patient characteristics, considered individually, and CK level was studied using proportional odds logistic regressions, one for each variable, and estimating the odds ratio and its 95% confidence interval (CI). The proportional odds model has the same structure as the binary logistic regression model but uses an ordinal outcome variable with more than two possible levels (such as the chosen categories of CK level) 14,15. It estimates a common odds ratio over all possible cut-offs of the outcome scale.
The association between clinical features and CK levels was also investigated through a multiple regression model. In this way, it was possible to identify the effect of each variable on the CK level accounting for the value of the other variables.
To verify the robustness of our findings and to overcome the arbitrary nature of CK level categorization, in a secondary analysis, the patients with elevated CK levels (≥ 200 U/L) were classified according to the tertiles of the CK level distributions: group 0 (CK < 200 U/L), group 1 (CK 200-288 U/L), group 2 (CK 289-501 U/L) and group 3 (CK ≥ 502 U/L) (see Supplementary material).
Finally, a logistic regression model was fitted to identify the predictors of in-hospital death. CK level was included in the model as a categorical variable. Adjustments were made for the above-mentioned variables.
The Statistical Analysis System Software (version 9.4; SAS Institute, Cary, North Carolina, USA) was used for the analyses. For all hypotheses tested, two-tailed P values less than 0.05 were considered as significant.
Results
Patients
A total of 615 hospitalized patients [mean age: 66.8 (± 16.2); males: 386 (62.8%)] with confirmed COVID-19 infection were included in the analysis. Of these patients, 17 (2.8%) had myalgia and/or arthralgia and 81 (13.2%) complained fatigue. Nobody reported cramps.
CK levels were dosed in all patients (11-12328, mean value: 251 ± 658 U/L): 176 (28.6%) patients had CK levels ≥ 200 U/L. Among these patients, 127 (20.7%) were included in group 1, 31 (5.0%) in group 2 and 18 (2.9%) in group 3. The demographic, clinical and laboratory characteristics are shown in Table I according to CK level.
Table I.
Characteristics of cohort members according to the creatine kinase (CK) levels.
| CK level (U/L) | OR (95% CI) | P-value | ||||
|---|---|---|---|---|---|---|
| 0-199 (n = 439) |
200-554 (n = 127) |
555-1038 (n = 31) |
≥ 1039 (n = 18) |
|||
| Sex | ||||||
| Female | 184 (41.9) | 34 (26.8) | 8 (25.8) | 3 (16.7) | Ref. | |
| Male | 255 (58.1) | 93 (73.2) | 23 (74.2) | 15 (83.3) | 2.11 (1.44-3.10) | < 0.001 |
| Age (years) | ||||||
| 0-49 | 69 (15.7) | 18 (14.2) | 2 (6.5) | 3 (16.7) | Ref. | |
| 50-64 | 115 (26.2) | 29 (22.8) | 9 (29.0) | 3 (16.7) | 1.09 (0.60-1.96) | 0.780 |
| 65-74 | 92 (21.0) | 34 (26.8) | 3 (9.7) | 5 (27.8) | 1.34 (0.74-2.42) | 0.336 |
| ≥75 | 163 (37.1) | 46 (36.2) | 17 (54.8) | 7 (38.9) | 1.33 (0.77-2.30) | 0.300 |
| Muscular manifestations | ||||||
| No | 424 (96.6) | 126 (99.2) | 30 (96.8) | 18 (100.0) | Ref. | |
| Yes | 15 (3.4) | 1 (0.8) | 1 (3.2) | 0 (0.0) | 0.34 (0.08-1.45) | 0.144 |
| Fatigue | ||||||
| No | 378 (86.1) | 112 (88.2) | 29 (93.6) | 15 (83.3) | Ref. | |
| Yes | 61 (13.9) | 15 (11.8) | 2 (6.5) | 3 (16.7) | 0.79 (0.47-1.35) | 0.397 |
| Respiratory involvement | ||||||
| No | 78 (17.8) | 9 (7.1) | 3 (9.7) | 1 (5.6) | Ref. | |
| SARS pneumonia | 250 (56.9) | 79 (62.2) | 8 (25.8) | 7 (38.9) | 2.15 (1.15-4.04) | 0.017 |
| Respiratory failure | 111 (25.3) | 39 (30.7) | 20 (64.5) | 10 (55.6) | 4.00 (2.08-7.67) | < 0.001 |
| History of falls | ||||||
| No | 429 (97.7) | 120 (94.5) | 30 (96.8) | 14 (77.8) | Ref. | |
| Yes | 10 (2.3) | 7 (5.5) | 1 (3.2) | 4 (22.2) | 3.54 (1.60-7.85) | 0.002 |
Abbreviations: CK: creatine kinase; OR: odds ratios; CI: confidence interval; Ref.: reference interval
Variables associated with CK levels
Bivariate association analysis results are reported in Table I. Muscular manifestations, fatigue and age were not significantly associated with CK level.
Conversely, sex, respiratory involvement and history of falls were related with hyperckemia. Men prevalence was 58.1% among patients with normal CK level and 83.3% among those with CK level ≥ 1039 U/L.
Among patients with normal CK levels, 25.3% had respiratory failure whereas this figure was greater than 50% among those with CK levels higher than 554 U/L, independently from SARS pneumonia. On the opposite side, the prevalence of SARS pneumonia was higher among patients who had CK levels lower than 555 U/L. A graphical representation of the relationship between respiratory involvement and CK level is provided in Figure 1.
Figure 1.
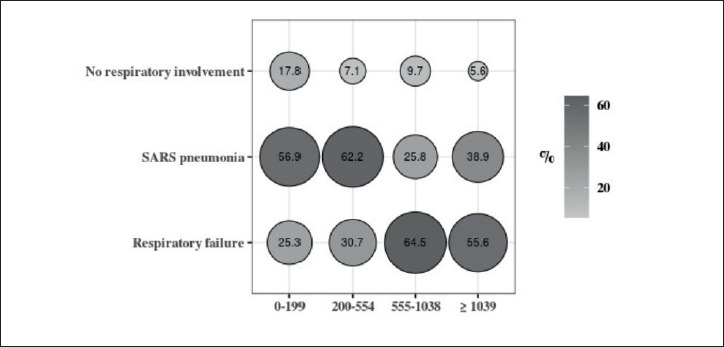
Percentage distribution of respiratory involvement according to CK levels.
Patients who fell at home before hospitalization had CK levels significantly higher than others. In particular, the percentages of those who fell were 2.3 and 22.2% among patients with CK < 200 and ≥ 1039, respectively.
Multivariate analysis confirmed these results. Table II shows the adjusted estimates of association between CK levels and the other variables. Respiratory failure was associated with hyperckemia (OR: 3.67, 95% CI 1.86 to 7.24, p = 0.036), together with history of falls (OR: 3.66, 95% CI 1.61 to 8.35, p < 0.001), SARS pneumonia (OR: 2.00, 95% CI 1.05 to 3.82, p = 0.002) and male gender (OR: 1.88, 95% CI 1.26 to 2.79, p = 0.002).
Table II.
Association between patients’ characteristics and CK levels.
| OR (95% CI) | P-value | |
|---|---|---|
| Sex | ||
| Female | Ref. | |
| Male | 1.88 (1.26-2.79) | 0.002 |
| Age (years) | ||
| 0-49 | Ref. | |
| 50-64 | 0.87 (0.47-1.60) | 0.648 |
| 65-74 | 0.96 (0.51-1.78) | 0.890 |
| ≥75 | 0.96 (0.54-1.72) | 0.900 |
| Muscular manifestations | ||
| No | Ref. | |
| Yes | 0.44 (0.10-1.96) | 0.282 |
| Fatigue | ||
| No | Ref. | |
| Yes | 0.84 (0.48-1.45) | 0.531 |
| Respiratory involvement | ||
| No | Ref. | |
| SARS pneumonia | 2.00 (1.05-3.82) | 0.002 |
| Respiratory failure | 3.67 (1.86-7.24) | 0.036 |
| History of falls | ||
| No | Ref. | |
| Yes | 3.66 (1.61-8.35) | < 0.001 |
Abbreviations: CK: creatine kinase; OR: odds ratios; CI: confidence interval; Ref.: reference interval
CK level and death
One hundred-two patients (16.6%) died. The distribution of deaths occurred within CK level categories is reported in Figure 2. Table III shows adjusted estimates of the risk of death according to the characteristics shown in Table I. CK levels were significantly associated with death. Compared with the normal level group, an increased risk of 51, 172 and 230% was observed among patients with CK 200-554 U/L, 555-1038 U/L and ≥ 1039 U/L, respectively (p = 0.011). When patients with elevated CK levels (≥ 200 U/L) were combined into one group, the risk of death was increased by 88% (95% CI, 6-232%).
Figure 2.
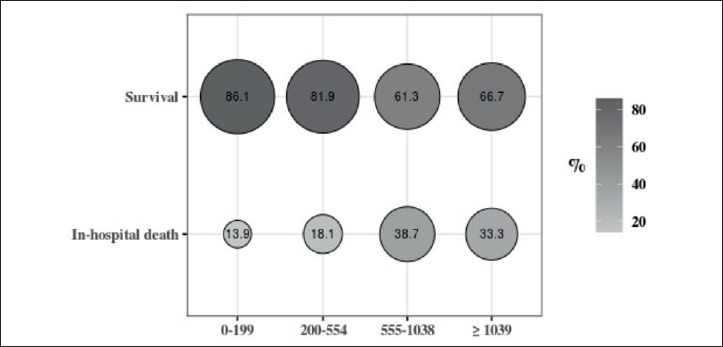
Percentage distribution of in-hospital death according to CK levels.
Table III.
Association between patients’ characteristics, including CK levels, and in-hospital death.
| OR (95% CI) | P-value | |
|---|---|---|
| Sex | ||
| Female | Ref. | |
| Male | 1.94 (1.07-3.50) | 0.029 |
| Age (years) | 1.17 (1.13-1.22) | < 0.001 |
| Muscular manifestations | ||
| No | Ref. | |
| Yes | 4.08 (0.38-44.34) | 0.249 |
| Fatigue | ||
| No | Ref. | |
| Yes | 0.75 (0.32-1.76) | 0.509 |
| Respiratory involvement | ||
| No | Ref. | |
| SARS pneumonia | 0.42 (0.17-1.02) | 0.055 |
| Respiratory failure | 2.71 (1.15-6.41) | 0.023 |
| History of falls | ||
| No | Ref. | |
| Yes | 0.78 (0.24-2.52) | 0.683 |
| CK level (U/L) | ||
| 0-199 | Ref. | |
| 200-554 | 1.51 (0.78-2.92) | 0.225 |
| 555-1,038 | 2.72 (1.00-7.42) | 0.051 |
| ≥ 1,039 | 3.30 (0.85-12.87) | 0.086 |
| P-value trend | 0.011 | |
Abbreviations: CK: creatine kinase; OR: odds ratios; CI: confidence interval; Ref.: reference interval
Sex, age, and respiratory failure were also associated with mortality risk.
These findings did not substantially change by modifying the criteria for categorization of CK levels (Supplementary material, Table S1 and S2).
Table S1.
Association between patients’ characteristics and CK levels.
| OR (95% CI) | P-value | |
|---|---|---|
| Sex | ||
| Female | Ref. | |
| Male | 1.89 (1.27-2.80) | 0.002 |
| Age (years) | ||
| 0-49 | Ref. | |
| 50-64 | 0.82 (0.45-1.51) | 0.525 |
| 65-74 | 0.92 (0.50-1.70) | 0.788 |
| ≥ 75 | 0.96 (0.54-1.69) | 0.874 |
| Muscular manifestations | ||
| No | Ref. | |
| Yes | 0.44 (0.10-1.96) | 0.280 |
| Fatigue | ||
| No | Ref. | |
| Yes | 0.78 (0.45-1.36) | 0.389 |
| Respiratory involvement | ||
| No | Ref. | |
| SARS pneumonia | 2.10 (1.10-4.01) | 0.025 |
| Respiratory failure | 3.66 (1.86-7.23) | < 0.001 |
| History of falls | ||
| No | Ref. | |
| Yes | 4.11 (1.82-9.30) | 0.001 |
Abbreviations: CK: creatine kinase; OR: odds ratios; CI: confidence interval; Ref.: reference interval
Patients were classified into the following groups: group 0 (CK < 200 U/L), group 1 (CK 200-288 U/L), group 2 (CK 289-501 U/L) and group 3 (CK ≥ 502 U/L)
Table S2.
Association between patients’ characteristics, including CK levels, and in-hospital death.
| OR (95% CI) | P-value | |
|---|---|---|
| Sex | ||
| Female | Ref. | |
| Male | 1.97 (1.09-3.56) | 0.024 |
| Age (years) | 1.17 (1.13-1.22) | < 0.001 |
| Muscular manifestations | ||
| No | Ref. | |
| Yes | 4.05 (0.38-43.68) | 0.249 |
| Fatigue | ||
| No | Ref. | |
| Yes | 0.76 (0.32-1.77) | 0.519 |
| Respiratory involvement | ||
| No | Ref. | |
| SARS pneumonia | 0.42 (0.17-1.04) | 0.060 |
| Respiratory failure | 2.71 (1.14-6.42) | 0.024 |
| History of falls | ||
| No | Ref. | |
| Yes | 0.77 (0.24-2.52) | 0.670 |
| CK level (U/L) | ||
| 0-199 | Ref. | |
| 200-288 | 1.62 (0.69-3.83) | 0.267 |
| 289-501 | 1.37 (0.52-3.59) | 0.524 |
| ≥ 502 | 2.62 (1.18-5.80 | 0.018 |
| P-value trend | 0.019 | |
Abbreviations: CK: creatine kinase; OR: odds ratios; CI: confidence interval; Ref.: reference interval
Discussion
In this study we evaluated the CK levels of a large cohort of COVID-19 patients focusing on muscle symptoms and clinical outcome.
To our knowledge, few studies analysed the correlation between CK levels and muscle injury 5,9,10.
Mao et al. studied the neurological manifestations of 214 patients with Coronavirus disease showing that patients with muscle injury (i.e. those with skeletal muscle pain and serum CK greater than 200 U/L) had significantly higher levels of CK (median: 400 U/L [range 203.0-12216.0] vs median: 58.5 U/L [range 8.8-212.0]; p < .001) compared with other subjects. Therefore, they speculated that muscle symptoms were owing to skeletal muscle injury and significantly elevated proinflammatory cytokines in serum may cause skeletal muscle damage 5. Conversely, Vacchiano et al. analyzed 108 COVID-19 patients finding that muscle pain was not associated with CK high levels, supporting the notion that this symptom was not directly accounted for by muscle injury and making a direct viral mechanism unlikely 9. As the Italian colleagues, we did not find significant correlation among muscle symptomatology and CK levels. Instead, we found that patients with history of falls (i.e. patients admitted to hospital after falling at home) had CK levels higher than others (p < 0.002) even if there was no clinical evidence of muscle trauma on ER reports.
Regarding the clinical aspects, in literature the rate of cases presenting muscular symptoms varies from 63% 7 to 26% 8. In our population only 2.8% patients presented myalgia and/or arthralgia. This percentage rises to 15.9% if we include fatigue in the muscular manifestations.
Fatigue is a highly non-specific symptom, and it is hard to assess retrospectively if this figure was related to the infection itself or to a hidden muscle injury.
The low percentage of muscular symptoms observed could be due to the extreme hospital circumstances at the peak of this pandemic for which ER clinicians reported only the prevalent symptoms of the patients. Moreover, a proportion of subjects were unconscious at the time of arrival in the hospital or suffered of previous severe cognitive impairment so it was hard to access if neuromuscular symptoms were present or not. Furthermore, electrophysiological studies that could have been done to evaluate myopathy were infeasible during the COVID-19 epidemic.
In our cohort the mortality rate was 16.6% in line with previous studies 16. Interestingly, we found that elevated CK levels were related with respiratory failure rather than with SARS pneumonia, in accordance with literature data 5. Although it is impossible to establish a time-dependent relationship between respiratory failure and hyperckemia because of the retrospective nature of the study, we found that CK levels were significantly correlated to fatal outcome. Previous studies conducted during SARS-CoV outbreak found that CK is an important predictor of oxygenation failure and death in patients with Severe Acute Respiratory Syndrome 17,18. In 2007 Chan et al. made a retrospective analysis of 1312 patients affected with SARS showing that CK is one of the predictive elements of oxygenation failure and poor outcome in these patients 19. The most recent literature regarding SARS-CoV-2 infection reported raised serum CK levels in 33% of patients, reaching 46% for Intensive care unit patients 6. A Chinese retrospective study confirmed that median CK levels (normal values < 190) were higher in deceased patients (189 U/L) than in the other patients (84 U/L) 20. Recently, Bonetti et al. found that CK represents one of the laboratory predictors of death from Covid-19 21 while Pitscheider et al. showed a strong correlation among CK levels, disease severity and markers of inflammation 10. There are currently also a few published case reports of rhabdomyolysis with myalgia and fatigue associated with severe SARS-CoV-2 infection 22 with potential implications on renal function 23. Considered all together the above considerations, we thus can conclude that CK levels are correlated with the severity of COVID infection, more likely as an expression of myopathic involvement of skeletal and respiratory muscles during COVID-19 infection, rather than of a distress condition due to the strenuous respiratory muscle effort secondary to SARS interstitial pneumonia.
Conclusions
Our study provides evidence that hyperckemia is associated with respiratory failure and fatal outcome. Muscle manifestations are not associated with the raise of CK levels; however, because of the extreme hospital circumstances, they can be easily misdiagnosed. In patients with COVID-19 infection, among other testing, CK dosage is recommended. Further prospective studies to document the frequency of COVID-associated muscle damage are warranted.
Figures and tables
References
- 1.Odone A, Delmonte D, Scognamiglio T, et al. COVID-19 deaths in Lombardy, Italy: data in context. Lancet Public Health 2020;5:e310. https://doi.org/10.1016/S2468-2667(20)30099-2 10.1016/S2468-2667(20)30099-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin X, Lian J-S, Hu J-H, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020;69:1002-1009. https://doi.org/10.1136/gutjnl-2020-320926 10.1136/gutjnl-2020-320926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paliwal VK, Garg RK, Gupta A, et al. Neuromuscular presentations in patients with COVID-19. Neurol Sci 2020;41:3039-3056. https://doi.org/10.1007/s10072-020-04708-8 10.1007/s10072-020-04708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Giorgio MR, Di Noia S, Morciano C, et al. The impact of SARS-CoV-2 on skeletal muscles. Acta Myol 2020;39:307-312. https://doi.org/10.36185/2532-1900-034 10.36185/2532-1900-034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol 2020. https://doi.org/10.1001/jamaneurol.2020.1127 10.1001/jamaneurol.2020.1127 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020;395:497-506. https://doi.org/10.1016/S0140-6736(20)30183-5 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med 2020;288:335-344. https://doi.org/10.1111/joim.13089 10.1111/joim.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet 2020;395:1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vacchiano V, Riguzzi P, Volpi L, et al. Early neurological manifestations of hospitalized COVID-19 patients. Neurol Sci 2020. https://doi.org/10.1007/s10072-020-04525-z 10.1007/s10072-020-04525-z [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitscheider L, Karolyi M, Burkert FR, et al. Muscle involvement in SARS-CoV-2 infection. Eur J Neurol 2020. https://doi.org/10.1111/ene.14564 10.1111/ene.14564 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 2003;348:1986-1994. https://doi.org/10.1056/NEJMoa030685 10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- 12.Leung TW. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol 2005;62:1113-1117. https://doi.org/10.1001/archneur.62.7.1113 10.1001/archneur.62.7.1113 [DOI] [PubMed] [Google Scholar]
- 13.Li M-Y, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. https://doi.org/10.1186/s40249-020-00662-x 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002;249:305-311. https://doi.org/10.1007/s004150200010 10.1007/s004150200010 [DOI] [PubMed] [Google Scholar]
- 15.Musumeci O, la Marca G, Spada M, et al. LOPED study: looking for an early diagnosis in a late-onset Pompe disease high-risk population. J Neurol Neurosurg Psychiatry 2016;87:5-11. https://doi.org/10.1136/jnnp-2014-310164 10.1136/jnnp-2014-310164 [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. https://doi.org/10.1001/jama.2020.1585 10.1001/jama.2020.1585 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsui PT, Kwok ML, Yuen H, et al. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis 2003;9:1064-1069. https://doi.org/10.3201/eid0909.030362 10.3201/eid0909.030362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler RA, Lapinsky SE, Hallett D, et al. Critically ill patients with severe acute respiratory syndrome. JAMA 2003;290:367-373. https://doi.org/10.1001/jama.290.3.367 10.1001/jama.290.3.367 [DOI] [PubMed] [Google Scholar]
- 19.Chan JCK, Tsui ELH, Wong VCW, Hospital Authority SARS Collaborative Group . Prognostication in severe acute respiratory syndrome: a retrospective time-course analysis of 1312 laboratory-confirmed patients in Hong Kong. Respirol Carlton Vic 2007;12;531-542. https://doi.org/10.1111/j.1440-1843.2007.01102.x 10.1111/j.1440-1843.2007.01102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368. https://doi.org/10.1136/bmj.m1091 10.1136/bmj.m1091 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonetti G, Manelli F, Patroni A, et al. Laboratory predictors of death from coronavirus disease 2019 (COVID-19) in the area of Valcamonica, Italy. Clin Chem Lab Med 2020;58:1100-1105. https://doi.org/10.1515/cclm-2020-0459 10.1515/cclm-2020-0459 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Shan KS, Minalyan A, et al. A rare presentation of coronavirus disease 2019 (COVID-19) induced viral myositis with subsequent rhabdomyolysis. Cureus 2020;12:e8074. https://doi.org/10.7759/cureus.8074 10.7759/cureus.8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 2020;26. https://doi.org/10.3201/eid2607.200445 10.3201/eid2607.200445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


