Key Points
Question
What is the optimal diagnostic strategy for mucous membrane pemphigoid?
Findings
This comparative diagnostic accuracy study of a cohort of 787 patients with suspected mucous membrane pemphigoid found high sensitivity of a mucosal direct immunofluorescence microscopic (DIF) biopsy—which is superior to serologic analysis; DIF on oral mucosa can be performed on both perilesional and normal buccal mucosa and additional DIF biopsy of another mucosal site or skin may increase the diagnostic yield.
Meaning
Performing DIF on a mucosal biopsy is recommended for diagnosing mucous membrane pemphigoid, and additional sequential biopsies from different sites, including the skin, is recommended in patients with a negative DIF result.
This cohort study examines the diagnostic accuracy of direct immunofluorescence microscopy on mucosal biopsy specimens and immunoserologic analysis in a large cohort of patients with suspected mucous membrane pemphigoid.
Abstract
Importance
An accurate diagnosis of mucous membrane pemphigoid (MMP) is essential to reduce diagnostic and therapeutic delay.
Objective
To assess the diagnostic accuracy of direct immunofluorescence microscopy on mucosal biopsy specimens and immunoserology in a large cohort of patients with suspected MMP.
Design, Setting, and Participants
This retrospective cohort study was carried out in a single tertiary care center for blistering diseases between January 2002 and March 2019. Eligible participants were patients with suspected MMP and paired data on at least a mucosal biopsy specimen for direct immunofluorescence microscopy (DIF) and indirect immunofluorescence microscopy (IIF) on a human salt-split skin substrate (SSS). In addition, an optional DIF test on a skin biopsy specimen and one or more performed routine immunoserologic tests were analyzed. Data analysis was conducted from April 2019, to June 2020.
Main Outcomes and Measures
Diagnostic accuracy of DIF, IIF SSS, and immunoblot for BP180 and BP230.
Results
Of the 787 participants, 121 (15.4%) received the diagnosis of MMP (50 men [41.3%], 71 women [58.7%]; mean [SD] age at diagnosis, 60.1 [17.7] years). Sixty-seven of the patients with MMP (55.4%) had monosite involvement, of which oral site was the most frequently affected (51 [42.1%]). No significant difference was found between the sensitivity of DIF on a perilesional buccal biopsy and a normal buccal biopsy (89.3% vs 76.7%). Three patients with solitary ocular involvement showed a positive DIF of only the oral mucosa. In 6 patients with a negative mucosal DIF, a skin biopsy confirmed diagnosis of MMP. Overall, IIF SSS was less sensitive (44.6%), but highly specific (98.9%). The sensitivity of immunoblot (66.1%) was higher compared to SSS, but with lower specificity (91.3%).
Conclusions and Relevance
This comparative diagnostic accuracy study of a cohort of 787 patients found a high sensitivity of a mucosal DIF biopsy for diagnosis of MMP, and lower sensitivity of serologic analysis. A biopsy can be taken from either perilesional or normal buccal mucosa. An additional DIF biopsy of another mucosal site or of affected or unaffected skin may increase the diagnostic yield and is recommended in patients with negative DIF results and high clinical suspicion.
Introduction
The clinical heterogeneity of mucous membrane pemphigoid (MMP) and challenging diagnosis often leads to a substantial diagnostic delay and suboptimal treatment.1 An optimal diagnostic strategy is therefore necessary for an accurate diagnosis. Mucous membrane pemphigoid is a group of autoimmune subepidermal blistering diseases predominantly affecting the mucosa with autoantibodies directed against different structural components of the epidermal basement membrane zone (EBMZ) including BP180, BP230, laminin 332, and type VII collagen.2 The oral mucosa is the most frequently affected site, followed by the ocular, nasal, nasopharyngeal, anogenital, laryngeal, and esophageal mucosa.3,4,5 Although mild involvement of the skin can be present, mucosal lesions are the leading clinical manifestation.2 Clinically, MMP is characterized by the presence of erythema, painful erosions, and blisters of mucosa with highly variable severity. Lesions tend to heal with scar formation, in particular in ocular and laryngeal MMP, resulting in blindness and, in rare cases, upper airway obstruction.6,7
Various terms were previously used to define MMP including benign mucous membrane pemphigoid and ocular cicatricial pemphigoid. Patients with predominant mucous membrane disease and targeted antigen of type VII collagen have been diagnosed as epidermolysis bullosa acquisita. The First International Consensus on MMP determined that mucous membrane pemphigoid was the appropriate covering terminology for this heterogenous group of diseases.2
No consensus reference standard for diagnosis of MMP has been established. According to the First International Consensus, clinical criteria and direct immunofluorescence microscopy (DIF) are essential for diagnosis.2 Direct immunofluorescence microscopy detects linear deposition of IgG, C3c, or IgA at the EBMZ in mucosa and skin biopsies.8,9 Moreover, circulating autoantibodies in serum can be detected by indirect immunofluorescence microscopy (IIF) and several immunoserologic tests.
In this study, the diagnostic accuracy of DIF on a mucosal biopsy and pairwise performed immunoserology were evaluated in a large cohort of patients with suspected MMP.
Methods
Study Design and Participants
This single-center retrospective diagnostic accuracy study was performed at the Center for Blistering Diseases in Groningen, the national referral center for autoimmune bullous diseases in the Netherlands. The research design was similar to the study of Meijer et al10 in cutaneous pemphigoid. The study population consisted of consecutive patients from secondary and tertiary care hospitals throughout the Netherlands with a clinical suspicion of MMP, including the presentation of erythema, erosions, blisters, and/or scarring on mucosal sites, with or without skin lesions. Eligible participants were patients with paired data on at least (1) a mucosal biopsy for DIF, and (2) IIF on a human salt-split skin substrate (SSS) test performed between January 2002 and March 2019. In addition, data of an optional DIF on a skin biopsy specimen and 1 or more routinely performed immunoserologic tests (immunoblot, enzyme-linked immunosorbent assays, IIF on monkey esophagus) were analyzed. Keratinocyte footprint assay (KFA) was used to detect antilaminin-332 autoantibodies in patients’ sera.11 Samples were taken at time of first diagnosis, before introduction of immunosuppressive therapy, and within an inclusion window of a maximum of 4 weeks. Biopsy sites for DIF were defined as perilesional, lesional, and normal mucosa or skin.10 The medical ethical committee evaluated this study as outside the scope of the Medical Research Involving Human Subjects Act. Informed consent was waived because of the retrospective nature of the study and the use of anonymous data.
Diagnosis of MMP
Direct immunofluorescence microscopic results on mucosal and skin biopsies were considered positive when linear n-serrated/u-serrated depositions of IgG, IgA and/or C3c along the EBMZ were observed. The results of IIF on SSS were considered positive when IgG and/or IgA staining at the epidermal and/or dermal side were observed. Immunoblot was used to detect IgG and/or IgA against BP180 and/or BP230. Autoantibodies against the noncollagenous 16A domain of BP180 (NC16A) and BP230 were detected with commercially available enzyme-linked immunosorbent assays (ELISA) and were considered positive at a cutoff index of 9 U/mL or higher according to the manufacturer’s protocol (Medical and Biological Laboratories Co). Finally, IIF on ME substrate test results were considered positive when IgG was detected in a linear pattern along the EBMZ. Laboratory techniques DIF, IIF on SSS and ME substrate, and immunoblot were performed as previously described.10
The clinical diagnosis of MMP was made in the following cases: compatible clinical presentation and (1) a positive DIF biopsy result of the mucosa or (2) a positive DIF biopsy result of the skin or (3) a positive DIF biopsy result as well as a positive IIF SSS or (4) a positive IIF on SSS as well as positivity in at least 1 other immunoserologic test (immunoblot, ELISA, or IIF on ME). These criteria were largely based on The First International Consensus as well as on the study of Meijer et al.2,10 Clinical features and diagnostic test results of cases with indeterminate or a single positive test result were repeated and discussed among physicians (H.R., J.M., and B.H.), a pathologist (G.D.), and a biochemist (H.P.) to confirm or reject the diagnosis of MMP. Individuals were excluded in cases of suspected cutaneous pemphigoid or insufficient clinical information.
Statistical Analysis
Diagnostic accuracy was calculated with sensitivity, specificity, positive and negative predictive values (PPV and NPV), according to standardized formulas and with 95% CIs. The χ2 test was used to compare medians and proportions. Sensitivities of paired diagnostic tests were compared using the McNemar test. Statistical significance was defined by using 2-sided P ≤ .05 values. Statistical analyses were performed in SPSS Statistics statistical software (version 23, IBM).
Results
Baseline Characteristics
Data of 922 participants with suspected MMP with mucosal lesions and a performed DIF mucosal biopsy as well as IIF on SSS were analyzed. After exclusion of 135 patients, a total of 121 participants (15.4%) were diagnosed with MMP and compared with 666 controls (84.6%) (Figure 1). The mean (SD) age in the MMP group was 60.1 years (17.7; range, 5-88), and 55.0 years (16.3; range, 9-96) in the control group. Of the patients in the MMP group, 71 (58.7%) were women compared with 450 (67.6%) in the control group. Most patients with MMP presented with involvement of the oral mucosa (n = 105, 86.8%), followed by the ocular mucosa (n = 30, 24.8%), nasal mucosa (n = 16, 13.2%), anogenital mucosa (n = 24, 19.8%), pharyngeal mucosa (n = 8, 6.6%), laryngeal mucosa (n = 10, 8.3%), and esophageal mucosa (n = 1, 0.8%). Overall, 55.4% (n = 67) of the patients with MMP presented with monosite involvement, of which oral site was the most frequently affected (n = 51, 42.1%), and 44.6% (n = 54) presented with multisite involvement. In 18.2% (n = 22), skin involvement was observed. Table 1 depicts the clinical characteristics of both groups.
Figure 1. Study Flow Diagram.

Based on the index tests direct immunofluorescence (DIF) microscopy and indirect immunofluorescence on human salt-split skin (IIF SSS) substrate, eligible participants with other diagnoses were excluded. ELISA indicates enzyme-linked immunosorbent assays, ME indicates monkey esophagus.
Table 1. Clinical Characteristics of Patients With Mucous Membrane Pemphigoid (MMP) and Controls.
| Characteristic | No. (%) | |
|---|---|---|
| Patients with MMP (n = 121) | Controls (n = 666) | |
| Age at diagnosis, mean (SD) [range], y | 60.1 (17.7) [5-88] | 55.0 (16.3) [9-96] |
| Sex | ||
| Male | 50 (41.3) | 216 (32.4) |
| Female | 71 (58.7) | 450 (67.6) |
| Mucosal involvement | ||
| Oral | 105 (86.8) | 599 (89.9) |
| Ocular | 30 (24.8) | 53 (8.0) |
| Nasal | 16 (13.2) | 7 (1.1) |
| Anogenital | 24 (19.8) | 68 (10.2) |
| Pharyngeal | 8 (6.6) | 9 (1.4) |
| Laryngeal | 10 (8.3) | 0 (0) |
| Esophageal | 1 (0.8) | 4 (0.6) |
| Involvement | ||
| Multisite | 54 (44.6) | 88 (13.2) |
| Monosite | 67 (55.4) | 578 (86.8) |
| Oral only | 51 (42.1) | 514 (77.2) |
| Ocular only | 8 (6.6) | 31 (4.7) |
| Anogenital only | 8 (6.6) | 33 (5.0) |
| Skin involvement | 22 (18.2) | 33 (5.0) |
Direct Immunofluorescence Microscopy
A total of 823 mucosal DIF biopsies from 787 participants were analyzed. In 34 participants, a second sequential DIF mucosal biopsy from another site was performed, and in 1 patient, 3 sequential DIF mucosal biopsies were performed. Of the 823 mucosal DIF biopsies, 86.9% (n = 715) were taken from the oral mucosa, followed by ocular (6.4%, n = 53) and anogenital mucosa (6.4%, n = 53). In addition, 1 DIF biopsy was taken from pharyngeal mucosa (0.1%) and 1 from nasal mucosa (0.1%).
Table 2 and Figure 2 depict the diagnostic performance of the index test DIF on different mucosal sites. Direct immunofluorescence on oral mucosa (n = 715) showed a sensitivity and specificity of 87.9% (95% CI, 79.8%-93.1%) and 100% (95% CI, 99.2%-100%) respectively, and a PPV and NPV of 100% (95% CI, 95.1%-100%) and 97.9% (95% CI, 96.4%-98.8%), respectively. Comparison of DIF biopsy sites on oral mucosa showed no significant difference between the sensitivity of a perilesional buccal biopsy and a normal buccal biopsy (89.3%; 95% CI, 70.6%-97.2% vs 76.7%; 95% CI, 57.3%-89.4%; P = .30). In addition, no significant difference was seen in sensitivity of DIF between a perilesional buccal biopsy and a perilesional gingival biopsy (89.3%; 95% CI, 70.6%-97.2% vs 100%; 95% CI, 80.0%-100%; P = .22). Direct immunofluorescence on ocular mucosa (n = 53) showed a sensitivity of 66.7% and a PPV of 100%, but with relatively wide confidence intervals (95% CI, 38.7%-87.0% and 95% CI, 65.5%-100%, respectively). The specificity was 100% (95% CI, 88.6%-100%), and NPV 88.4% (95% CI, 74.1%-95.6%). The DIF on anogenital mucosa (n = 53) showed a sensitivity of 91.7% (95% CI, 59.8%-99.6%) and a PPV of 100% (95% CI, 67.9%-100%), also with wide confidence intervals. The specificity and NPV were 100% (95% CI, 89.3%-100%), and 97.6% (95% CI, 85.9%-99.9%), respectively.
Table 2. Diagnostic Performance of Direct Immunofluorescence Microscopic Test and Immunoserology in Study Participants With Suspected Mucous Membrane Pemphigoid.
| Test | No. (with MMP/controls) | % (95% CI) | |||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | ||
| DIF | |||||
| Oral mucosa | 715 (107/608) | 87.9 (79.8-93.1) | 100 (99.2-100) | 100 (95.1-100)a | 97.9 (96.4-98.8) |
| Perilesional buccal mucosa | 211 (28/183) | 89.3 (70.6-97.2) | 100 (97.4-100) | 100 (83.4-100)a | 98.4 (95.0-99.6) |
| Normal buccal mucosa | 95 (30/65) | 76.7 (57.3-89.4) | 100 (93.0-100) | 100 (82.2-100)a | 90.3 (80.4-95.7) |
| Perilesional gingival mucosa | 70 (20/50) | 100 (80.0-100) | 100 (91.1-100) | 100 (80.0-100)a | 100 (91.1-100) |
| Ocular mucosa | 53 (15/38) | 66.7 (38.7-87.0) | 100 (88.6-100) | 100 (65.5-100)a | 88.4 (74.1-95.6) |
| Anogenital mucosa | 53 (12/41) | 91.7 (59.8-99.6) | 100 (89.3-100) | 100 (67.9-100)a | 97.6 (85.9-99.9) |
| IIF SSS | 787 (121/666) | 44.6 (35.7-53.9) | 98.8 (97.5-99.4) | 87.1 (75.6-93.9) | 90.8 (88.4-92.7) |
| Immunoblot | 768 (121/647) | 66.1 (56.9-74.3) | 91.3 (88.8-93.3) | 58.8 (50.1-67.1) | 93.5 (91.2-95.3) |
| ELISA BP180 NC16A | 520 (121/399) | 32.2 (24.2-41.4) | 88.2 (84.5-91.1) | 45.3 (34.7-56.4) | 81.1 (77.0-84.6) |
| ELISA BP230 | 279 (67/212) | 10.4 (4.7-20.9) | 88.7 (83.4-92.5) | 22.6 (10.3-41.5) | 75.8 (69.9-80.9) |
| IIF ME | 729 (121/608) | 8.3 (4.3-15.0) | 98.8 (97.5-99.5) | 58.8 (33.5-80.6) | 84.4 (81.5-87.0) |
Abbreviations: DIF, direct immunofluorescence microscopy; ELISA, enzyme-linked immunosorbent assay; IIF ME, indirect immunofluorescence microscopy; MMP, mucous membrane pemphigoid; ME, monkey esophagus; NPV, negative predictive value; PPV, positive predictive value; SSS, salt-split skin.
0.5 is added to calculate the positive predictive value by using the Haldane-Anscombe correction.
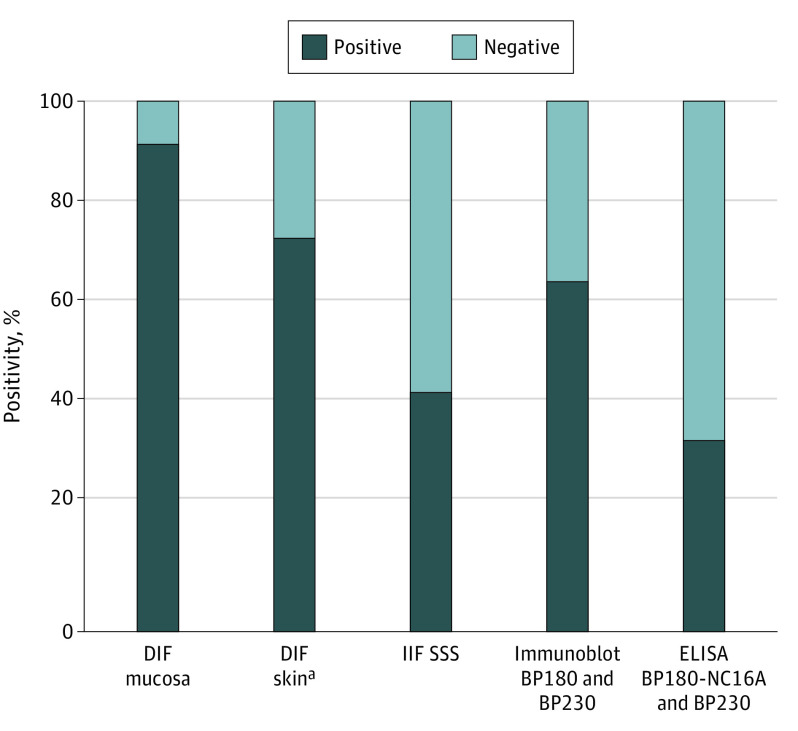
Figure 2. Percentage of Positive DIF, IIF SSS, and immunoserologic Results in Patients With MMP.
Skin biopsy for DIF was performed in 72 of 121 patients. DIF indicates direct immunofluorescence microscopy; ELISA, enzyme-linked immunosorbent assay; IIF SSS, indirect immunofluorescence microscopy on salt-split skin substrate; MMP, mucous membrane pemphigoid.
Direct Immunofluorescence Immunodepositions
The DIF in patients with MMP detected immunodepositions of IgG in 97 of 116 biopsies (83.6%), IgA in 71 biopsies (61.2%), C3c in 71 biopsies (61.2%), and IgM in 5 biopsies (4.3%). Table 3 depicts the immunodepositions of oral, ocular, anogenital, and skin biopsies. The linear n-serrated pattern was observed in 43 mucosal biopsies (37.6%), of which 41 were taken from oral mucosa (95.3%). In the remaining cases, the serration pattern was undetermined (n = 73, 62.9%). The linear n-serrated pattern was observed in 31 skin biopsies (58.5%) and linear u-serrated pattern in 3 biopsies (5.7%). The serration pattern was more often observed in skin biopsies compared with mucosal biopsies in patients with MMP with both biopsies performed (31 [66.0%] vs. 21 [44.7%]; P = .01).
Table 3. Immunodepositions of Direct Immunofluorescence Microscopic Testing on Mucosa and Skin in Patients Diagnosed With Mucous Membrane Pemphigoid.
| Variable | No. (%) | ||||
|---|---|---|---|---|---|
| All mucosal sites | Oral | Ocular | Anogenital | Skin | |
| Total | 135 (100) | 107 (100) | 15 (100) | 12 (100) | 72 (100) |
| Positive DIF | 116 (85.9) | 94 (87.9) | 10 (66.7) | 11 (91.7) | 53 (73.6) |
| IgG | 97 (83.6) | 78 (83.0) | 8 (80.0) | 10 (90.9) | 46 (86.8) |
| IgA | 71 (61.2) | 57 (60.6) | 8 (80.0) | 5 (45.5) | 30 (56.6) |
| C3c | 71 (61.2) | 56 (59.6) | 4 (40) | 10 (90.9) | 17 (32.1) |
| IgM | 5 (4.3) | 5 (5.3) | 0 | 0 | 3 (5.7) |
| N-serration | 43 (37.1) | 41 (43.6) | 0 | 2 (18.2) | 31 (58.5) |
| U-serration | 0 | 0 | 0 | 0 | 3 (5.7) |
| Serration undetermined | 73 (62.9) | 53 (56.4) | 10 (100) | 9 (81.8) | 19 (35.8) |
Abbreviations: DIF, direct immunofluorescence microscopy; Ig, immunoglobulin.
Sequential Biopsies and Skin Biopsies
In 13 patients with MMP (10.7%), multiple sequential DIF biopsies on different mucosal sites were performed (eTable in the Supplement). Notably, 3 patients with solitary ocular involvement showed a positive DIF of oral mucosa and a negative DIF of ocular mucosa. In 1 patient with MMP with oral and anogenital involvement, DIF results were only positive of oral mucosa. In 72 patients with MMP (59.5%), an additional DIF of a skin biopsy was performed and found positive results in 53 patients (73.6%). Of these, only 18 (34.0%) presented with skin lesions. Remarkably, in 6 patients with negative DIF on an oral mucosal biopsy, DIF on a skin biopsy was positive, of which 4 patients did not present with skin lesions (eFigure in the Supplement).
Indirect Immunofluorescence and Immunoserology
The index test IIF SSS (n = 787) showed a sensitivity and specificity of 44.6% (95% CI, 35.7%-53.9%), and 98.9% (95% CI, 97.7%-99.4%) respectively, and a PPV and NPV of 87.1% (95% CI, 75.6%-93.9%) and 90.8% (95% CI, 88.4%-92.7%), respectively. Compared with DIF, the sensitivity of IIF SSS was significantly lower (91.7% vs. 44.6%, respectively; P = <.001) (Figure 2). The sensitivity of immunoblot (was higher compared with IIF SSS (66.1%; 95% CI, 56.9%-74.3%; P = <.001), but with a substantial lower PPV 58.8% (95% CI, 50.1%-67.1%). The sensitivity and PPV of IIF ME, ELISA BP180 NC16A and BP230 were substantially lower compared with IIF SSS (Table 2). Patients with MMP with both oral and skin involvement showed more often NC16A reactivity by ELISA, compared with patients without MMP without any skin involvement (11 [50%] vs. 28 [28.3%]; P = .049).
Four cases of oral MMP diagnosis were made despite a negative DIF result, on the basis of a clinical presentation of erosions and blisters of the oral mucosa, and positive IIF SSS and additional confirmative immunoserology. There were false-positive results on IIF SSS in 1 control, diagnosed with erythema exudativum multiforme. In addition, single false-positive IIF SSS results were seen in 6 controls, clinically diagnosed with oral lichen planus (OLP) and with DIF revealing fibrin deposition in the EBMZ.
Epidermal binding of IgG was detected in 38 sera (70.4%) of 54 MMP sera with positive IIF SSS results, epidermal IgA in 17 sera results (31.5%), and combined epidermal binding of IgG and IgA in 6 sera results (11.1%). In 4 patients (7.4%), dermal binding of IgG was observed. In 3 of these, KFA results showed binding of IgG to laminin-332 footprints. In the remaining patient, DIF of the skin revealed a linear u-serrated immunodeposition along the EBMZ. Immunoblot testing detected reactivity of IgG against BP180 in 62 of 80 positive MMP sera results (77.5%), and IgA in 22 sera results (27.5%). In addition, IgG reactivity against BP230 was observed in 11 sera results (13.8%), and IgA in 1 serum result. In 6 sera results (7.5%), IgG reactivity against LAD-1 was observed and IgA in 4 sera results (5.0%).
Discussion
In this study, we analyzed the diagnostic value of DIF on mucosal and skin biopsies, and compared it with IIF and immunoserological tests in a large cohort of participants with clinically suspected MMP. Direct immunofluorescence on a mucosal biopsy showed the highest sensitivity and PPV for the diagnosis of MMP. A second biopsy from another mucosal site or from skin may increase the diagnostic yield. Consequently, these findings indicate that DIF of oral mucosa may diagnose MMP in patients with solitary ocular involvement, and a skin biopsy for DIF of affected or healthy skin may reveal MMP. Direct immunofluorescence on perilesional buccal biopsy was found to be equivalent to normal buccal biopsy for diagnosis of MMP. Moreover, IIF on SSS turned out not to be sufficient for diagnosis owing to low sensitivity.
The sensitivity of DIF calculated from all mucosal sites ranged between 66.7%-91.7%, which was higher than reported sensitivities in previous studies.12,13,14,15,16 Possible explanations may include sample error, technical difficulties in processing mucosal biopsies, and biopsy specimens taken from different mucosal sites. Most of the DIF tests in this study were performed on oral mucosa. We found no significant difference in sensitivity between perilesional buccal biopsy (89.3%) and normal buccal biopsy (76.7%), in line with the study of Carey et al.17 Use of DIF biopsy is preferably taken from perilesional buccal mucosa. However, if this is poorly accessible, a biopsy of normal buccal mucosa can be taken instead. Conflicting data exists about the accuracy of DIF on gingival mucosa, mainly owing to the frailty of the tissue and the technical difficulty of obtaining a gingival sample.13,17 In this study, we found no significant difference in sensitivity of DIF on gingival perilesional mucosa and buccal perilesional mucosa. Therefore, DIF is preferably performed on buccal mucosa, because of the difficulty of performing gingival biopsies. Previous studies have described the detection of immunoreactants by DIF in both affected and unaffected asymptomatic sites.6,18,19,20,21 In fact, we found 3 patients with monosite ocular MMP with a negative DIF result on ocular mucosa, but a positive DIF result on oral mucosa. According to Ong et al,19 patients with monosite ocular MMP were more likely to have negative DIF results on ocular mucosa. Little is known about the mechanisms that prevent individual sites from involvement of circulating and tissue-bound autoantibodies against components of the EBMZ. A possible explanation for these results may include poor biopsy site selection or unequal distribution of immunoreactants. Furthermore, factors involving the local cell-mediated inflammatory response and the differences in the balance between autoreactive T cells and regulatory T cells in different tissues in patients with MMP may contribute to different results in DIF.22
Shimanovich et al23 demonstrated that multiple and repeated biopsies increase the sensitivity of DIF for diagnosis of MMP. In this study, 6 patients (5%) were diagnosed with MMP based on a positive DIF result on a skin biopsy, demonstrating the importance of an additional DIF on a skin biopsy, in case of a negative mucosal DIF result. Unfortunately, repeated biopsies were not routinely performed in our MMP cohort.
Direct immunofluorescence on mucosa showed most often depositions of IgG, followed by IgA, and C3c.15,24,25,26 Serration pattern analysis is often not determinable in mucosal biopsies.27,28,29 In this study, serration pattern analysis was determined in 37.1% of all mucosal biopsies. In contrast, the serration pattern analysis was significantly more often determined in skin biopsies (66.0%), which is an additional reason to perform a skin biopsy for DIF.
The titer of circulating autoantibodies in patients with MMP sera is often not detectable.2 This may be owing to lower concentration of autoantibodies in sera, and the heterogeneity of the target antigens. In this study, 4 (3.3%) patients with erosions of the oral mucosa were diagnosed with oral MMP based on a positive IIF SSS and positive additional serological test results, despite negative DIF results. According to the First International Consensus, immunoserology is not considered an absolute criterion for diagnosis of MMP.2 However, IIF on SSS results has shown high specificity in variants of bullous pemphigoid for detection of pemphigoid-specific autoantibodies and is confirmative for diagnosis.10 Therefore we included immunoserologic analysis as a criterion in the methodology for the diagnosis of MMP to properly assess the diagnostic accuracy. Previous studies reported sensitivity rates of IIF SSS on normal human skin of approximately 50%-85%.8,9,30,31,32,33,34 Indeed, we found lower sensitivity of IIF SSS sensitivity (44.6%) in comparison with DIF on oral mucosa (87.9%), but with a high specificity (98.8%). In addition, the sensitivity of immunoblot (66.1%) was higher than IF SSS. Sixty-two immunoblot-positive MMP sera results (77.5%) showed IgG autoantibodies to BP180, compared with 11 (13.8%) with IgG autoantibodies to BP230, and 22 (27.5%) with IgA autoantibodies to BP180, in line with previous studies.8,35,36,37,38,39 This study showed that IIF ME had no significant additional value for diagnosis of MMP.9,34
The sensitivity of ELISA for IgG against the NC16A domain of BP180 was 32.2%. Previous studies reported a sensitivity ranging between 30%-53%.8,9,31 BP180 is the main autoantigen targeted by the sera of patients with MMP. However, several studies reported circulating IgG autoantibodies in patients with MMP against epitopes other than NC16A, in particular the C-terminal portion.40,41,42 No commercially available ELISAs for the C-terminal portion are available for MMP testing. The presence of both oral and skin involvement was associated with NC16A reactivity, in line with findings by Cozzani et al.9 It is worth noting that 7 patients diagnosed with OLP showed circulating basement membrane zone antibodies. However, DIF results showed fibrin deposition in the EBMZ. Oral MMP and OLP may show similar clinical features and are therefore difficult to distinguish.43 Previously, Buijsrogge et al44 demonstrated that low circulating anti–BP180 IgG titers by immunoblot were present in 17% of patients with OLP. Cooper et al45 demonstrated positive circulating basement membrane zone antibodies in 56% of patients with OLP. Disruption of the EBMZ is often seen in lichen planus, with the potential of a secondary autoantibody response to epitopes.46,47 The presence of circulating basement membrane zone autoantibodies in lichen planus may be the result of epitope spreading and are not necessarily pathogenic.45
Limitations
The absence of diagnostic criteria as a reference standard for the diagnosis of MMP and consequently inclusion of index tests in diagnostic criteria are a limitation that may influence high specificity. Another limitation is the retrospective design and limited sample size in subgroups and therefore low statistical power.
Conclusions
The results of this study found that DIF on a mucosal biopsy was the most sensitive method for the diagnosis of MMP and was superior to serologic analysis. Direct immunofluorescence on oral mucosa can be performed on both perilesional and normal buccal mucosa. Performing additional sequential biopsies from different sites, including the perilesional affected or unaffected skin, is recommended in patients with a single negative DIF result.
eTable. Study patients with MMP with multiple sequential DIF biopsies from different mucosal sites
eFigure. Ratios of immunoreactivity in various diagnostic tests in patients with MMP
References
- 1.Laskaris G, Sklavounou A, Stratigos J. Bullous pemphigoid, cicatricial pemphigoid, and pemphigus vulgaris. a comparative clinical survey of 278 cases. Oral Surg Oral Med Oral Pathol. 1982;54(6):656-662. doi: 10.1016/0030-4220(82)90080-9 [DOI] [PubMed] [Google Scholar]
- 2.Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138(3):370-379. doi: 10.1001/archderm.138.3.370 [DOI] [PubMed] [Google Scholar]
- 3.Smith RJ, Sessions RB, Bean SF, Pingree TJK. Benign mucous membrane pemphigoid. Ann Otol Rhinol Laryngol. 1982;91(2 Pt 1):142-144. doi: 10.1177/000348948209100203 [DOI] [PubMed] [Google Scholar]
- 4.Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. 2004;111(1):45-52. doi: 10.1016/j.ophtha.2003.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed AR, Kurgis BS, Rogers RSI III. Cicatricial pemphigoid. J Am Acad Dermatol. 1991;24(6 Pt 1):987-1001. doi: 10.1016/0190-9622(91)70159-Y [DOI] [PubMed] [Google Scholar]
- 6.Fleming TE, Korman NJ. Cicatricial pemphigoid. J Am Acad Dermatol. 2000;43(4):571-591. doi: 10.1067/mjd.2000.107248 [DOI] [PubMed] [Google Scholar]
- 7.Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc. 1986;84:527-663. [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt E, Skrobek C, Kromminga A, et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol. 2001;145(5):778-783. doi: 10.1046/j.1365-2133.2001.04471.x [DOI] [PubMed] [Google Scholar]
- 9.Cozzani E, Di Zenzo G, Calabresi V, et al. Autoantibody profile of a cohort of 78 Italian patients with mucous membrane pemphigoid: correlation between reactivity profile and clinical involvement. Acta Derm Venereol. 2016;96(6):768-773. [DOI] [PubMed] [Google Scholar]
- 10.Meijer JM, Diercks GFH, de Lang EWG, Pas HH, Jonkman MF. Assessment of diagnostic strategy for early recognition of bullous and nonbullous variants of pemphigoid. JAMA Dermatol. 2019;155(2):158-165. doi: 10.1001/jamadermatol.2018.4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giurdanella F, Nijenhuis AM, Diercks GFH, Jonkman MF, Pas HH. Keratinocyte footprint assay discriminates antilaminin-332 pemphigoid from all other forms of pemphigoid diseases. Br J Dermatol. 2020;182(2):373-381. Published online January 9, 2019. doi: 10.1111/bjd.18129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers RS III, Van Hale HM. Immunopathologic diagnosis of oral mucosal inflammatory diseases. Australas J Dermatol. 1986;27(2):51-57. doi: 10.1111/j.1440-0960.1986.tb00288.x [DOI] [PubMed] [Google Scholar]
- 13.Helander SD, Rogers RS III. The sensitivity and specificity of direct immunofluorescence testing in disorders of mucous membranes. J Am Acad Dermatol. 1994;30(1):65-75. doi: 10.1016/S0190-9622(94)70010-9 [DOI] [PubMed] [Google Scholar]
- 14.Bean SF. Cicatricial pemphigoid. Immunofluorescent studies. Arch Dermatol. 1974;110(4):552-555. doi: 10.1001/archderm.1974.01630100012002 [DOI] [PubMed] [Google Scholar]
- 15.Griffith MR, Fukuyama K, Tuffanelli D, Silverman S Jr. Immunofluorescent studies in mucous membrane pemphigoid. Arch Dermatol. 1974;109(2):195-199. doi: 10.1001/archderm.1974.01630020011001 [DOI] [PubMed] [Google Scholar]
- 16.Sano SM, Quarracino MC, Aguas SC, et al. Sensitivity of direct immunofluorescence in oral diseases. study of 125 cases. Med Oral Patol Oral Cir Bucal. 2008;13(5):E287-E291. doi: 10.1016/s1077-9108(08)79026-1 [DOI] [PubMed] [Google Scholar]
- 17.Carey B, Joshi S, Abdelghani A, Mee J, Andiappan M, Setterfield J. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182(3):747-753. Published online January 7, 2019. doi: 10.1111/bjd.18032 [DOI] [PubMed] [Google Scholar]
- 18.Mehra T, Guenova E, Dechent F, et al. Diagnostic relevance of direct immunofluorescence in ocular mucous membrane pemphigoid. J Dtsch Dermatol Ges. 2015;13(12):1268-1274. doi: 10.1111/ddg.12716 [DOI] [PubMed] [Google Scholar]
- 19.Ong HS, Setterfield JF, Minassian DC, Dart JK; Mucous Membrane Pemphigoid Study Group 2009–2014 . Mucous membrane pemphigoid with ocular involvement: the clinical phenotype and its relationship to direct immunofluorescence findings. Ophthalmology. 2018;125(4):496-504. doi: 10.1016/j.ophtha.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 20.Fine JD, Neises GR, Katz SI. Immunofluorescence and immunoelectron microscopic studies in cicatricial pemphigoid. J Invest Dermatol. 1984;82(1):39-43. doi: 10.1111/1523-1747.ep12259075 [DOI] [PubMed] [Google Scholar]
- 21.Chan LS, Yancey KB, Hammerberg C, et al. Immune-mediated subepithelial blistering diseases of mucous membranes: pure ocular cicatricial pemphigoid is a unique clinical and immunopathological entity distinct from bullous pemphigoid and other subsets identified by antigenic specificity of autoantibodies. Arch Dermatol. 1993;129(4):448-455. doi: 10.1001/archderm.1993.01680250060007 [DOI] [PubMed] [Google Scholar]
- 22.Dart JK. The 2016 Bowman Lecture: conjunctival curses: scarring conjunctivitis 30 years on. Eye (Lond). 2017;31(2):301-332. doi: 10.1038/eye.2016.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimanovich I, Nitz JM, Zillikens D. Multiple and repeated sampling increases the sensitivity of direct immunofluorescence testing for the diagnosis of mucous membrane pemphigoid. J Am Acad Dermatol. 2017;77(4):700-705.e3. doi: 10.1016/j.jaad.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 24.Rogers RS III, Perry HO, Bean SF, Jordon RE. Immunopathology of cicatricial pemphigoid: studies of complement deposition. J Invest Dermatol. 1977;68(1):39-43. doi: 10.1111/1523-1747.ep12485162 [DOI] [PubMed] [Google Scholar]
- 25.Fine JD, Neises GR, Katz SI. Immunofluorescence and immunoelectron microscopic studies in cicatricial pemphigoid. J Invest Dermatol. 1984;82(1):39-43. doi: 10.1111/1523-1747.ep12259075 [DOI] [PubMed] [Google Scholar]
- 26.Venning VA, Frith PA, Bron AJ, Millard PR, Wojnarowska F. Mucosal involvement in bullous and cicatricial pemphigoid: a clinical and immunopathological study. Br J Dermatol. 1988;118(1):7-15. doi: 10.1111/j.1365-2133.1988.tb01744.x [DOI] [PubMed] [Google Scholar]
- 27.Terra JB, Pas HH, Hertl M, Dikkers FG, Kamminga N, Jonkman MF. Immunofluorescence serration pattern analysis as a diagnostic criterion in antilaminin-332 mucous membrane pemphigoid: immunopathological findings and clinical experience in 10 Dutch patients. Br J Dermatol. 2011;165(4):815-822. doi: 10.1111/j.1365-2133.2011.10474.x [DOI] [PubMed] [Google Scholar]
- 28.Vodegel RM, Jonkman MF, Pas HH, de Jong MCJM. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151(1):112-118. doi: 10.1111/j.1365-2133.2004.06006.x [DOI] [PubMed] [Google Scholar]
- 29.Meijer JM, Atefi I, Diercks GFH, et al. Serration pattern analysis for differentiating epidermolysis bullosa acquisita from other pemphigoid diseases. J Am Acad Dermatol. 2018;78(4):754-759.e6. doi: 10.1016/j.jaad.2017.11.029 [DOI] [PubMed] [Google Scholar]
- 30.Jindal A, Rao R, Bhogal BS. Advanced diagnostic techniques in autoimmune bullous diseases. Indian J Dermatol. 2017;62(3):268-278. doi: 10.4103/ijd.IJD_196_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayakawa T, Furumura M, Fukano H, et al. Diagnosis of oral mucous membrane pemphigoid by means of combined serologic testing. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(4):483-496. doi: 10.1016/j.oooo.2013.12.402 [DOI] [PubMed] [Google Scholar]
- 32.Setterfield J, Shirlaw PJ, Kerr-Muir M, et al. Mucous membrane pemphigoid: a dual circulating antibody response with IgG and IgA signifies a more severe and persistent disease. Br J Dermatol. 1998;138(4):602-610. doi: 10.1046/j.1365-2133.1998.02168.x [DOI] [PubMed] [Google Scholar]
- 33.Carrozzo M, Cozzani E, Broccoletti R, et al. Analysis of antigens targeted by circulating IgG and IgA antibodies in patients with mucous membrane pemphigoid predominantly affecting the oral cavity. J Periodontol. 2004;75(10):1302-1308. doi: 10.1902/jop.2004.75.10.1302 [DOI] [PubMed] [Google Scholar]
- 34.Maglie R, Borgi A, Caproni M, Antiga E. Indirect immunofluorescence in mucous membrane pemphigoid: which substrate should be used? Br J Dermatol. 2019;180(5):1266-1267. doi: 10.1111/bjd.17694 [DOI] [PubMed] [Google Scholar]
- 35.Bernard P, Prost C, Durepaire N, Basset-Seguin N, Didierjean L, Saurat JH. The major cicatricial pemphigoid antigen is a 180-kD protein that shows immunologic cross-reactivities with the bullous pemphigoid antigen. J Invest Dermatol. 1992;99(2):174-179. doi: 10.1111/1523-1747.ep12616797 [DOI] [PubMed] [Google Scholar]
- 36.Balding SD, Prost C, Diaz LA, et al. Cicatricial pemphigoid autoantibodies react with multiple sites on the BP180 extracellular domain. J Invest Dermatol. 1996;106(1):141-146. doi: 10.1111/1523-1747.ep12329728 [DOI] [PubMed] [Google Scholar]
- 37.Murakami H, Nishioka S, Setterfield J, et al. Analysis of antigens targeted by circulating IgG and IgA autoantibodies in 50 patients with cicatricial pemphigoid. J Dermatol Sci. 1998;17(1):39-44. doi: 10.1016/S0923-1811(97)00067-4 [DOI] [PubMed] [Google Scholar]
- 38.Christophoridis S, Büdinger L, Borradori L, Hunziker T, Merk HF, Hertl M. IgG, IgA and IgE autoantibodies against the ectodomain of BP180 in patients with bullous and cicatricial pemphigoid and linear IgA bullous dermatosis. Br J Dermatol. 2000;143(2):349-355. doi: 10.1046/j.1365-2133.2000.03661.x [DOI] [PubMed] [Google Scholar]
- 39.Oyama N, Setterfield JF, Powell AM, et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: the pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol. 2006;154(1):90-98. doi: 10.1111/j.1365-2133.2005.06998.x [DOI] [PubMed] [Google Scholar]
- 40.Lee JB, Liu Y, Hashimoto T. Cicatricial pemphigoid sera specifically react with the most C-terminal portion of BP180. J Dermatol Sci. 2003;32(1):59-64. doi: 10.1016/S0923-1811(03)00035-5 [DOI] [PubMed] [Google Scholar]
- 41.Calabresi V, Carrozzo M, Cozzani E, et al. Oral pemphigoid autoantibodies preferentially target BP180 ectodomain. Clin Immunol. 2007;122(2):207-213. doi: 10.1016/j.clim.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 42.Yasukochi A, Teye K, Ishii N, Hashimoto T. Clinical and immunological studies of 332 japanese patients tentatively diagnosed as anti-BP180-type mucous membrane pemphigoid: a novel BP180 C-terminal domain enzyme-linked immunosorbent assay. Acta Derm Venereol. 2016;96(6):762-767. doi: 10.2340/00015555-2407 [DOI] [PubMed] [Google Scholar]
- 43.Fukuda A, Himejima A, Tsuruta D, et al. Four cases of mucous membrane pemphigoid with clinical features of oral lichen planus. Int J Dermatol. 2016;55(6):657-665. doi: 10.1111/ijd.12884 [DOI] [PubMed] [Google Scholar]
- 44.Buijsrogge JJA, Hagel C, Duske U, et al. IgG antibodies to BP180 in a subset of oral lichen planus patients. J Dermatol Sci. 2007;47(3):256-258. doi: 10.1016/j.jdermsci.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 45.Cooper SM, Dean D, Allen J, Kirtschig G, Wojnarowska F. Erosive lichen planus of the vulva: weak circulating basement membrane zone antibodies are present. Clin Exp Dermatol. 2005;30(5):551-556. doi: 10.1111/j.1365-2230.2005.01866.x [DOI] [PubMed] [Google Scholar]
- 46.Thornhill MH. Immune mechanisms in oral lichen planus. Acta Odontol Scand. 2001;59(3):174-177. doi: 10.1080/000163501750266774 [DOI] [PubMed] [Google Scholar]
- 47.Cooper SM, Prenter A, Allen J, Dean D, Wojnarowska F. The basement membrane zone and dermal extracellular matrix in erosive lichen planus of the vulva: an immunohistochemical study demonstrating altered expression of hemidesmosome components and anchoring fibrils. Clin Exp Dermatol. 2005;30(3):277-281. doi: 10.1111/j.1365-2230.2005.01751.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Study patients with MMP with multiple sequential DIF biopsies from different mucosal sites
eFigure. Ratios of immunoreactivity in various diagnostic tests in patients with MMP