Abstract
Background:
ß-blockers have an uncertain effect in heart failure with a preserved ejection fraction of 50% or higher (HFpEF).
Methods:
We included patients with HFpEF from the Swedish Heart Failure Registry (SwedeHF) enrolled from 2011 through 2018. In a 2:1 propensity-score matched analysis (β-blocker use versus no-use), we assessed the primary outcome first HF hospitalization, the co-primary outcome cardiovascular (CV) death, and the secondary outcomes all-cause hospitalization and all-cause death. We performed an intention-to-treat and a per-protocol consistency analysis.
Results:
There were a total of 14,434 patients (median age [IQR], 79 [71-85] years; 51% women), 80% treated with a β-blocker at baseline. Treated patients were younger and had higher rates of atrial fibrillation and coronary artery disease, and higher NTproBNP levels. In the 4412:2206 patient matched cohort, at 5 years, 42% (95%-CI 40-44%) versus 44% (95%-CI 41-47%) had a HF admission and 38% [36-40%] versus 40% [36-42%] died from CV causes. In the intention-to-treat analysis β-blocker use was not associated with HF admissions [HR 0.95 (0.87-1.05, p=0.31] and CV death [HR 0.94 (0.85-1.03), p=0.19]. In the subgroup analyses, men appeared to have a more favorable association between β-blockers and outcomes than did women. There we no associations between β-blocker use and secondary outcomes.
Conclusions:
In patients with HFpEF β-blocker use is common but not associated with changes in HF hospitalization or cardiovascular mortality. In the absence of a strong rational and randomized control trials the case for β-blockers in HFpEF remains inconclusive.
Take Home Graphic

Graphical representation of Kaplan Meier curves of patients with or without beta-blocker and incidence of heart failure hospitalizations. CV = Cardiovascular, HFpEF = Heart failure with preserved ejection fraction, NTproBNP = N-terminal pro-B-type Natriuretic Peptide
Lay summary
The optimal treatment for heart failure with a preserved pump function remains unknown. Despite the lack of scientific studies, beta-blockers are very commonly used. When matching patients with a similar risk profile in a large heart failure registry, the use of beta-blockers for the treatment of heart failure with a preserved pump function was not associated with any changes in heart failure hospital admissions or cardiovascular death.
INTRODUCTION
Heart failure (HF) is a major cause of hospitalization and death and associated with a high socioeconomic burden (1, 2). HF with a preserved ejection fraction (HFpEF), is a syndrome with clinical signs and symptoms of HF, a left ventricular ejection fraction of 50% or higher, and frequently direct evidence of diastolic dysfunction (1). HFpEF accounts for about half of HF and the prevalence is increasing (2). Compared to HF with a reduced ejection fraction (HFrEF), rates of HF hospitalization, hospital length of stay and reduction in quality of life are comparable (1, 3, 4). There are no evidenced-based medical therapies for HFpEF and current treatment approaches are empirical.
ß-adrenergic receptor blockers (β-blockers) provide an unequivocal benefit in HFrEF, with a strong foundation of evidence that support their use. It has been suggested that this benefit extends to ejection fractions up to 50% but not above (5). A majority of patients with HFpEF are managed with β-blockers despite a lack of data, rationale or guideline recommendations (3, 6, 7). There is an emerging concern that β-blockers do not provide a benefit and may even be harmful in HFpEF through their effects on heart rate and diastolic function (8).
Therefore, we characterized β-blocker type and exposure and the association of β-blockers with multiple outcomes in HFpEF≥50%, using both censoring and competing risk modelling, in the contemporary Swedish Heart Failure Registry (SwedeHF).
METHODS
Study Design and Setting
SwedeHF is a large nationwide registry including heart failure (HF) patients based on clinicians’ diagnoses (9, 10). The protocol, registration form and annual reports are available at http://www.rikssvikt.se. EF is categorized as <30%, 30%-39%, 40%-49%, and ≥50%. The present analysis included patients with an EF of 50% or higher enrolled between January 1, 2011 and December 31, 2018 (Supplemental figure 1)
The index date was defined as the date of an outpatient visit or hospital discharge; patients who died during the index hospitalization were excluded. Patients were included starting January 1, 2011 in order to provide a contemporary cohort and to differentiate between loop and thiazide diuretics since this information was not entered into the case report form prior to April 2010. Follow-up was until December 31, 2018.
The Swedish Board of Health and Welfare (http://www.socialstyrelsen.se) maintains the Cause of Death Registry, the Patient Registry, and the Dispensed Drug Registry. As described elsewhere, the Cause of Death Registry provided date and causes of death, the Patient Registry provided additional baseline comorbidities as well as the outcome HF and all-cause hospitalization (11). In this registry, the diagnosis of HF was verified in between 86% and 91% of cases (12). Comorbidities at or prior to baseline were defined by corresponding ICD-9 and ICD-10 codes (see https://kiheartfailure.github.io/shfdb3/ for details). The outcomes of hospitalization and death were defined as between the day after the index date and end of follow-up, December 31, 2018, emigration or death, for which a HF diagnosis was required as the primary diagnosis to qualify as a HF hospitalization. Socioeconomic variables were obtained from Statistics Sweden.
All Swedish residents have unique personal identification numbers that enable linking of disease-specific health registries and governmental health and statistical registries. Establishment of the HF registry and this analysis with linking to the above registries were approved by a multisite ethics committee. Individual patient consent was not required, but patients were informed of entry into SwedeHF and allowed to opt out. As previously described, the dispensed drug registry contains details about every prescription filled (rather than merely prescribed) in Sweden and all pharmacies are required to participate by law, ensuring 100% complete data.
The prespecified primary outcome was first HF hospitalization and the co-primary outcome was cardiovascular (CV) death. Secondary outcomes were first all-cause hospitalization and all-cause mortality.
As a primary consistency analysis, patients were censored at crossover (on protocol analysis). In untreated patients, crossover was defined as new β-blocker (ATC C07) use in the dispensed drug registry. For treated patients, crossover was defined as no β-blocker use within 6 months from baseline or from the last prescription fill, where crossover was set 3 months from index or last refill.
Further consistency analyses were performed excluding N-terminal pro-B-type natriuretic peptide (NT-proBNP) in the imputation, matching and outcomes analyses, and EF partition values of ≥55% and ≥60% (Supplement for details).
Statistical analyses
Incidence per 1000 patient-years was calculated with 95% Poisson confidence intervals. Missing data was imputed with multiple imputation using MICE for 10 datasets and 10 iterations. Variables included in the model are reported in Table 1. The primary outcome, first heart failure hospitalization, was included as the Nelson-Aalen estimator, β-blocker use was not included in the imputation model.
Table 1 –
Baseline Characteristics
| Overall cohort | Matched cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Missing (%) | Bbl No | Bbl Yes | SMD | p-value | Missing (%) | Bbl No | Bbl Yes | SMD | p-value |
| n | 2444 | 11990 | 2206 | 4412 | ||||||
| Gender (Male 1 ) | 0.0 | 1216 (49.8) | 5800 (48.4) | 0.028 | 0.221 | 0.0 | 1101 (49.9) | 2184 (49.5) | 0.008 | 0.774 |
| Age 1 | 0.0 | 81.0 [73.0, 87.0] | 79.0 [71.0, 85.0] | 0.142 | <0.001 | 0.0 | 81.0 [72.2, 86.0] | 81.0 [73.0, 86.0] | 0.027 | 0.177 |
| Living conditions (living alone) 1 | 0.1 | 1407 (57.7) | 6363 (53.1) | 0.092 | <0.001 | 0.1 | 1245 (56.5) | 2535 (57.5) | 0.020 | 0.457 |
| Has children (Yes) 1 | 0.0 | 2039 (83.4) | 10234 (85.4) | 0.053 | 0.016 | 0.0 | 1848 (83.8) | 3675 (83.3) | 0.013 | 0.648 |
| Education 1 | 2.2 | 0.058 | 0.038 | 2.6 | 0.006 | 0.973 | ||||
| Compulsory school | 1152 (48.4) | 5390 (45.9) | 1023 (47.6) | 2052 (47.7) | ||||||
| Secondary school | 834 (35.0) | 4430 (37.7) | 769 (35.8) | 1524 (35.5) | ||||||
| University | 394 (16.6) | 1919 (16.3) | 359 (16.7) | 722 (16.8) | ||||||
| Disposable income 1 | 0.1 | 0.017 | 0.738 | 0.1 | 0.001 | 0.999 | ||||
| Low | 1001 (41.0) | 4815 (40.2) | 892 (40.5) | 1786 (40.5) | ||||||
| Medium | 904 (37.0) | 4486 (37.4) | 819 (37.2) | 1640 (37.2) | ||||||
| High | 535 (21.9) | 2679 (22.4) | 492 (22.3) | 982 (22.3) | ||||||
| BMI category ≥30kg/m 21 | 37.4 | 428 (28.7) | 2508 (33.2) | 0.098 | 0.001 | 38.1 | 395 (29.1) | 803 (29.3) | 0.004 | 0.941 |
| Outpatient Enrollment 1 | 0.0 | 1105 (45.2) | 6307 (52.6) | 0.148 | <0.001 | 0.0 | 1027 (46.6) | 2046 (46.4) | 0.004 | 0.910 |
| Index-year 2016-2018 1 | 0.0 | 982 (40.2) | 5086 (42.4) | 0.045 | 0.043 | 0.0 | 890 (40.3) | 1815 (41.1) | 0.016 | 0.554 |
| HF Nurse Follow-up 1 | 5.9 | 1057 (46.7) | 6014 (53.1) | 0.128 | <0.001 | 6.6 | 984 (48.1) | 1951 (47.1) | 0.020 | 0.468 |
| Follow-up location 1 | 3.6 | 0.179 | <0.001 | 4.0 | 0.021 | 0.723 | ||||
| Hospital | 1000 (43.1) | 6025 (51.9) | 943 (44.9) | 1867 (43.9) | ||||||
| Primary care | 1278 (55.1) | 5371 (46.3) | 1115 (53.1) | 2301 (54.1) | ||||||
| Other | 41 ( 1.8) | 206 ( 1.8) | 40 ( 1.9) | 85 ( 2.0) | ||||||
| Smoking 1 | 27.7 | 0.058 | 0.089 | 29.0 | 0.009 | 0.957 | ||||
| Current | 145 ( 8.5) | 712 ( 8.2) | 129 ( 8.3) | 261 ( 8.3) | ||||||
| 0Former | 671 (39.2) | 3671 (42.1) | 616 (39.5) | 1225 (39.0) | ||||||
| Never | 895 (52.3) | 4341 (49.8) | 815 (52.2) | 1652 (52.6) | ||||||
| HF duration >6 months 1 | 5.0 | 1348 (58.6) | 6828 (59.9) | 0.027 | 0.252 | 5.4 | 1239 (59.5) | 2508 (60.0) | 0.011 | 0.697 |
| NYHA class 1 | 43.0 | 0.099 | 0.008 | 46.9 | 0.041 | 0.727 | ||||
| I | 200 (16.4) | 1070 (15.3) | 177 (15.8) | 406 (17.0) | ||||||
| II | 563 (46.1) | 3314 (47.3) | 522 (46.7) | 1124 (46.9) | ||||||
| III | 406 (33.3) | 2445 (34.9) | 375 (33.5) | 783 (32.7) | ||||||
| IV | 52 ( 4.3) | 183 ( 2.6) | 44 ( 3.9) | 82 ( 3.4) | ||||||
| Continuous EF, % (SD) | 87.4 | 55.0 [50.0, 60.0] | 55.0 [50.0, 55.0] | 0.249 | <0.001 | 88.6 | 55.0 [50.0, 60.0] | 55.0 [50.0, 55.0] | 0.251 | 0.001 |
| Systolic BP, mmHg (SD) 1 | 2.5 | 130.0 [120.0, 146.0] | 130.0 [119.0, 145.0] | 0.057 | 0.003 | 2.6 | 130.0 [120.0, 146.0] | 130.0 [120.0, 147.0] | 0.021 | 0.515 |
| Diastolic BP, mmHg (SD) | 2.5 | 70.0 [62.0, 80.0] | 72.0 [65.0, 80.0] | 0.134 | <0.001 | 2.6 | 70.0 [63.0, 80.0] | 71.0 [65.0, 80.0] | 0.104 | <0.001 |
| MAP, mmHg (SD) | 2.5 | 91.7 [83.3, 100.0] | 93.0 [83.3, 100.7] | 0.048 | 0.033 | 2.6 | 91.7 [83.3, 100.0] | 93.3 [83.7, 101.7] | 0.074 | 0.004 |
| HR, bpm (SD) 1 | 3.4 | 72.0 [62.0, 82.0] | 72.0 [63.0, 82.0] | 0.064 | 0.250 | 3.6 | 72.0 [62.0, 82.0] | 71.0 [62.0, 82.0] | 0.023 | 0.242 |
| Hb g/l (SD) 1 | 6.6 | 126.0 [113.0, 137.0] | 128.0 [116.0, 140.0] | 0.129 | <0.001 | 6.4 | 126.0 [113.0, 138.0] | 126.0 [113.0, 137.0] | 0.026 | 0.338 |
| K mmol/l (SD) 1 | 10.8 | 4.1 [3.8, 4.4] | 4.2 [3.9, 4.5] | 0.079 | <0.001 | 11.3 | 4.1 [3.8, 4.4] | 4.1 [3.8, 4.4] | 0.002 | 0.886 |
| GFR CKD-EPI 1 | 3.3 | 58.6 [41.9, 77.5] | 57.2 [41.3, 75.2] | 0.066 | 0.014 | 3.4 | 58.0 [41.2, 77.2] | 57.6 [40.7, 75.9] | 0.031 | 0.251 |
| NT-proBNP pg/ml (SD) 1 | 36.5 | 1604.5 [582.5, 3719.0] | 1950.0 [838.0, 4040.0] | 0.064 | <0.001 | 37.7 | 1651.0 [615.8, 3786.5] | 1860.0 [695.0, 3900.0] | 0.070 | 0.030 |
| Log NTproBNP | 36.5 | 7.4 [6.4, 8.2] | 7.6 [6.7, 8.3] | 0.185 | <0.001 | 37.7 | 7.4 [6.4, 8.2] | 7.5 [6.5, 8.3] | 0.086 | 0.030 |
| Loop diuretic (%) 1 | 0.3 | 1860 (76.2) | 9369 (78.4) | 0.051 | 0.022 | 0.3 | 1688 (76.7) | 3375 (76.8) | 0.002 | 0.953 |
| ARB/ACEI/ARNI (%) 1 | 0.5 | 1512 (62.1) | 8998 (75.5) | 0.291 | <0.001 | 0.6 | 1430 (65.1) | 2830 (64.6) | 0.011 | 0.688 |
| MRA (%) 1 | 0.4 | 702 (28.8) | 3798 (31.8) | 0.066 | 0.003 | 0.4 | 642 (29.2) | 1278 (29.1) | 0.001 | 0.994 |
| Digoxin (%) 1 | 0.2 | 183 ( 7.5) | 1611 (13.5) | 0.196 | <0.001 | 0.2 | 181 ( 8.2) | 352 ( 8.0) | 0.008 | 0.794 |
| ASA or antiplatelet (%) 1 | 0.3 | 744 (30.5) | 3764 (31.5) | 0.021 | 0.351 | 0.3 | 708 (32.2) | 1389 (31.6) | 0.013 | 0.638 |
| Anticoagulants (%) 1 | 0.3 | 944 (38.7) | 6603 (55.2) | 0.336 | <0.001 | 0.3 | 917 (41.6) | 1844 (41.9) | 0.006 | 0.840 |
| Statin (%) 1 | 0.2 | 811 (33.2) | 5573 (46.6) | 0.275 | <0.001 | 0.2 | 795 (36.1) | 1577 (35.8) | 0.005 | 0.860 |
| Nitrate (%) 1 | 0.2 | 288 (11.8) | 1693 (14.2) | 0.071 | 0.002 | 0.2 | 274 (12.4) | 568 (12.9) | 0.015 | 0.601 |
| Device: ICD/CRT (%) 1 | 3.3 | 25 ( 1.1) | 366 ( 3.2) | 0.146 | <0.001 | 3.4 | 25 ( 1.2) | 57 ( 1.3) | 0.014 | 0.695 |
| Hypertension (%) 1 | 0.0 | 1563 (64.0) | 8635 (72.0) | 0.174 | <0.001 | 0.0 | 1462 (66.3) | 2937 (66.6) | 0.006 | 0.832 |
| Diabetes (%) 1 | 0.0 | 595 (24.3) | 3389 (28.3) | 0.089 | <0.001 | 0.0 | 557 (25.2) | 1116 (25.3) | 0.001 | 0.992 |
| Prior MI (%) 1 | 0.0 | 537 (22.0) | 3586 (29.9) | 0.182 | <0.001 | 0.0 | 522 (23.7) | 1055 (23.9) | 0.006 | 0.846 |
| Prior PCI (%) 1 | 0.0 | 226 ( 9.2) | 1839 (15.3) | 0.186 | <0.001 | 0.0 | 221 (10.0) | 447 (10.1) | 0.004 | 0.920 |
| Prior CABG (%) 1 | 0.0 | 361 (14.8) | 2608 (21.8) | 0.181 | <0.001 | 0.0 | 351 (15.9) | 713 (16.2) | 0.007 | 0.822 |
| Peripheral artery disease (%) 1 | 0.0 | 230 ( 9.4) | 1155 ( 9.6) | 0.008 | 0.762 | 0.0 | 213 ( 9.7) | 414 ( 9.4) | 0.009 | 0.755 |
| Atrial fibrillation (%) 1 | 0.0 | 1160 (47.5) | 7692 (64.2) | 0.341 | <0.001 | 0.0 | 1129 (51.2) | 2266 (51.4) | 0.004 | 0.910 |
| History of stroke (%) 1 | 0.0 | 543 (22.2) | 2442 (20.4) | 0.045 | 0.042 | 0.0 | 490 (22.2) | 1012 (22.9) | 0.017 | 0.527 |
| History of severe bleeding (%) 1 | 0.0 | 616 (25.2) | 2876 (24.0) | 0.028 | 0.209 | 0.0 | 551 (25.0) | 1108 (25.1) | 0.003 | 0.928 |
| Valvular defect (%) 1 | 0.0 | 661 (27.0) | 3173 (26.5) | 0.013 | 0.570 | 0.0 | 605 (27.4) | 1191 (27.0) | 0.010 | 0.732 |
| COPD (%) 1 | 0.0 | 452 (18.5) | 1920 (16.0) | 0.066 | 0.003 | 0.0 | 395 (17.9) | 787 (17.8) | 0.002 | 0.973 |
| History of alcohol abuse (%) 1 | 0.0 | 61 ( 2.5) | 339 ( 2.8) | 0.021 | 0.400 | 0.0 | 58 ( 2.6) | 129 ( 2.9) | 0.018 | 0.546 |
| Musculoskeletal disease/connective tissue disease in the past 3 years (%) 1 | 0.0 | 979 (40.1) | 4626 (38.6) | 0.030 | 0.180 | 0.0 | 865 (39.2) | 1785 (40.5) | 0.025 | 0.343 |
| Cancer in the past 3 years (%) 1 | 0.0 | 457 (18.7) | 1980 (16.5) | 0.057 | 0.009 | 0.0 | 406 (18.4) | 792 (18.0) | 0.012 | 0.676 |
| Depression (%) 1 | 0.0 | 122 ( 5.0) | 488 ( 4.1) | 0.044 | 0.045 | 0.0 | 102 ( 4.6) | 210 ( 4.8) | 0.006 | 0.854 |
ACEI = Angiotensin Converting Enzyme Inhibitor, ARB= Angiotensin Receptor Blocker, ARNI = Angiotensin Receptor Neprilysin Inhibitor, ASA = Aspirin, BBl= beta-blocker, BMI = Body mass index, BP = Blood pressure, CABG = coronary artery bypass graft, CDK-EPI = Chronic Kidney Disease Epidemiology Collaboration, COPD = chronic obstructive pulmonary disease, EF = Ejection fraction, GFR = Glomerular filtration rate, Hb = hemoglobin, HF = Heart failure, HR = Heart rate, ICD/CRT= Intracardiac defibrillator/Cardiac resynchronization therapy, K = Potassium, log= logarithm, MAP = Mean arterial pressure, MI = Myocardial infarction, MRA = Mineralocorticoid receptor Antagonist, NYHA = New York Heart Association, NT-proBNP= N-terminal pro-B-type natriuretic peptide, PCI = percutaneous coronary intervention, SD = Standard deviation, SMD= Standardized mean difference. Categorical variables are presented with n(%) and tested with chi-square test and continuous variables with median[q1-q3]and tested with Mann-Whitney U test.
Included in the multiple imputation model (although not necessarily imputed if there is no missing data) and fully adjusted models
A propensity score for treatment with β-blockers was estimated for each patient with logistic regression for each of the 10 imputed datasets using the variables indicated in Table 1. Continuous variables were modeled using cubic splines with 3 degrees of freedom. 2:1 matching without replacement was thereafter performed on the average of the resulting 10 propensity scores (13). Matching was allowed if the propensity score differed by 0.01 or less. The ability of the propensity score matching to balance the baseline characteristics was assessed by standardized mean differences (Table 1).
The primary and secondary outcomes according to β-blocker status were assessed with Kaplan Meier analysis with log-rank test and Cox regression. Adjustments for confounders were made by assessing outcomes in the matched cohort (i.e. matched on baseline characteristics that were potential confounders), where the matched pairs were modeled using a frailty term. These were additionally partly adjusted for additional individual covariates (described below) in the overall cohort where the continuous variables were modeled using cubic splines with 3 degrees of freedom and location of enrollment and treatment with angiotensin receptor system (angiotensin receptor blocker or angiotensin converting enzyme blocker) or angiotensin-receptor-neprilysin-inhibitor (ARNI) were included as strata variables since they were deemed not to have proportional hazards.
The minimally adjusted models include age and sex. To account for confounding from comorbidities the partially adjusted models added prior acute myocardial infarction (AMI), prior percutaneous coronary intervention (PCI), prior coronary artery bypass graft (CABG), history of atrial fibrillation (AF), history of chronic obstructive pulmonary disease (COPD) and pre-existing hypertension to the minimally adjusted model. The fully adjusted models include the variables reported in Table 1.
The analyses were also performed using a sub-distributional hazards model where death was treated as a competing event (14). Subgroups for relevant clinical variables are presented in a Forest plot for the matched cohort using interaction effects. As some missing data was present for some variables, the matching (and thereby the adjustment) was not always complete.
All analyses were performed using R version 3.6.2 (2019-12-12). The R code for all data handling and statistical analyses is available at https://github.com/KIHeartFailure/bblEFover50. The level of significance was set to 5%, two-sided. No adjustment for multiple comparisons were made.
RESULTS
SwedeHF HFpEF Cohort
Between 2011 and 2018 there were 19,608 registrations, yielding 14,434 unique patients with HFpEF≥50%, 80% were treated with β-blockers. After 2:1 propensity score matching, 4412 treated patients were compared to 2206 untreated patients (Supplemental figure 1).
Baseline Characteristics
In the overall cohort, the median (IQR) age was 79 (71-85) years and 51% were women. Treated patients were younger and more obese, and more likely to have atrial fibrillation and prior myocardial infarction or revascularization. NTproBNP levels were significantly higher in those receiving β-blockers (Table 1). Heart rate was not significantly different between treated and untreated patients.
In the matched cohort, patients median age was 81 (73-86) years and 50% were women. The standardized differences between the groups were small, with none of the 44 variables used in the propensity score matching having a standardized difference of more than 10% and a majority in the range of 0% to 4% (Table 1).
Types and doses of ß-blockers
Specific ß-blockers and their doses are presented in Table 2. More than 90% percent of the patients received either metoprolol or bisoprolol, typically at submaximal doses.
Table 2 –
Beta-blocker treatment
| Variables | All | Matched |
|---|---|---|
| n | 11990 | 4412 |
| Type of beta-blocker used | ||
| Atenolol | 450 ( 3.8) | 219 (5.0) |
| Betaxolol | 0 ( 0.0) | 0 ( 0.0) |
| Bisoprolol | 5042 (42.1) | 1873 (42.5) |
| Carvedilol | 315 ( 2.6) | 114 (2.6) |
| Labetalol | 2 ( 0.0) | 0 ( 0.0) |
| Metoprolol | 6097 (50.9) | 2175 (49.3) |
| Pindolol | 3 ( 0.0) | 3 ( 0.1) |
| Propanolol | 35 ( 0.3) | 15 ( 0.3) |
| Sotalol | 39 ( 0.3) | 11 ( 0.2) |
| Timolol | 5 ( 0.0) | 1 ( 0.0) |
| % Target beta-blocker dose | 50 [20, 100] | 50 [20, 100] |
| Bisoprolol dose | 5.0 [2.5, 10.0] | 5.0 [2.5, 10.0] |
| Carvedilol dose | 37.5 [13.0, 50.0] | 25.0 [12.5, 50.0] |
| Metoprolol dose | 100.0 [50.0, 150.0] | 75.0 [50.0, 100.0] |
Outcomes in the Intention-to-Treat Analysis
In the overall cohort, the median follow-up time was approximately 2 years (range, 0-8 years), for a total of 36,583 patient-years of follow-up. At 5 years, 44% (95%-CI 41-47%) in the group without β-blocker versus 42% (95%-CI 40-44%) in the group with β-blocker had at least one HF admission. The unadjusted HR for risk of first HF hospitalization was 0.97 (0.90-1.05). In the matched cohort, the median follow-up time was about 2 years (range, 0-8 years), for a total of 16,223 patient-years of follow-up. There was no significant association between β-blockers and HF admissions (HR 0.95 (0.87-1.05) or CV death (HR 0.94 (0.85-1.03) (Table 3, Figure 1 and 2). There was a significant association between β-blockers and a lower risk for all cause hospital admissions and all-cause mortality in the matched model as shown in Table 2 and Supplemental Figure 2, but this was not confirmed in the per protocol consistency analysis (Table 4).
Table 3 –
Outcomes in the main analysis
| Outcome | Model | Overall cohort | Matched cohort | ||
|---|---|---|---|---|---|
| Bbl No | Bbl Yes | Bbl No | Bbl Yes | ||
| First HF hospitalization | % (95% CI) 1 yr | 20 (19-22) | 20 (19-21) | 20 (19-22) | 20 (19-21) |
| % (95% CI) 3 yr | 34 (32-36) | 34 (33-35) | 34 (32-37) | 33 (32-35) | |
| % (95% CI) 5 yr | 43 (40-46) | 42 (41-44) | 44 (41-47) | 42 (40-44) | |
| Incidence (no events, sum py, rate/1000py (95% CI)) | 714, 4856, 147 (136-158) | 3536, 25083, 141 (136-146) | 663, 4472, 148(137-160) | 1268, 8940, 142 (134-150) | |
| Crude HR (95% CI), p-value | ref | 0.97 (0.90-1.05), 0.486 | |||
| Adj mini HR (95% CI), p-value | ref | 1.03 (0.95-1.12), 0.414 | |||
| Adj partial HR (95% CI), p-value | ref | 0.96 (0.88-1.04), 0.271 | |||
| Adj full HR (95% CI), p-value | ref | 0.91 (0.84-1.00), 0.038 | ref | 0.95 (0.87-1.05), 0.311 | |
| CV death | % (95% CI) 1 yr | 13 (12-15) | 10 (10-11) | 12 (11-14) | 12 (11-13) |
| % (95% CI) 3 yr | 28 (26-31) | 24 (23-24) | 28 (26-30) | 26 (25-28) | |
| % (95% CI) 5 yr | 40 (37-43) | 35 (33-36) | 40 (36-42) | 38 (36-40) | |
| Incidence (no events, sum py, rate/1000py (95% CI)) | 653, 5806, 112 (104-121) | 2753, 30776, 89 (86-93) | 590, 5355, 110 (101-119) | 1119, 10868, 103 (97-109) | |
| Crude HR (95% CI), p-value | ref | 0.80 (0.73-0.87), <0.001 | |||
| Adj mini HR (95% CI), p-value | ref | 0.95 (0.87-1.03), 0.201 | |||
| Adj partial HR (95% CI), p-value | ref | 0.88 (0.81-0.97), 0.006 | |||
| Adj full HR (95% CI), p-value | ref | 0.86 (0.78-0.94), 0.001 | ref | 0.94 (0.85-1.03), 0.194 | |
| First all-cause hospitalization | % (95% CI) 1 yr | 60 (58-62) | 56 (55-57) | 59 (57-62) | 58 (56-59) |
| % (95% CI) 3 yr | 83 (81-85) | 79 (78-80) | 83 (81-85) | 79 (78-81) | |
| % (95% CI) 5 yr | 91 (89-93) | 88 (87-89) | 91 (89-93) | 88 (87-90) | |
| Incidence (no events, sum py, rate/1000py (95% CI)) | 1816, 2437, 745 (711-780) | 8574, 13220, 649 (635-662) | 1648, 2235, 738 (702-774) | 3166, 4790, 661 (638-684) | |
| Crude HR (95% CI), p-value | ref | 0.90 (0.85-0.95), <0.001 | |||
| Adj mini HR (95% CI), p-value | ref | 0.94 (0.89-0.99), 0.016 | |||
| Adj partial HR (95% CI), p-value | ref | 0.89 (0.85-0.94), <0.001 | |||
| Adj full HR (95% CI), p-vaiue | ref | 0.92 (0.87-0.97), 0.002 | ref | 0.92 (0.87-0.98), 0.008 | |
| All-cause death | % (95% CI) 1 yr | 22 (20-24) | 16 (16-17) | 20 (19-22) | 19 (18-20) |
| % (95% CI) 3 yr | 47 (45-49) | 37 (36-38) | 45 (43-48) | 41 (40-43) | |
| % (95% CI) 5 yr | 62 (60-65) | 53 (52-55) | 61 (58-64) | 59 (57-60) | |
| Incidence (no events, sum py, rate/1000py (95% CI)) | 1212, 5806, 209 (197-221) | 4845, 30776, 157 (153-162) | 1070, 5355, 200 (188-212) | 1976, 10868, 182 (174-190) | |
| Crude HR (95% CI), p-value | ref | 0.76 (0.71-0.81), <0.001 | |||
| Adj mini HR (95% CI), p-value | ref | 0.86 (0.81-0.92), <0.001 | |||
| Adj partial HR (95% CI), p-value | ref | 0.85 (0.79-0.90), <0.001 | |||
| Adj full HR (95% CI), p-value | ref | 0.83 (0.78-0.89), <0.001 | ref | 0.91 (0.84-0.98), 0.013 | |
Figure 1.
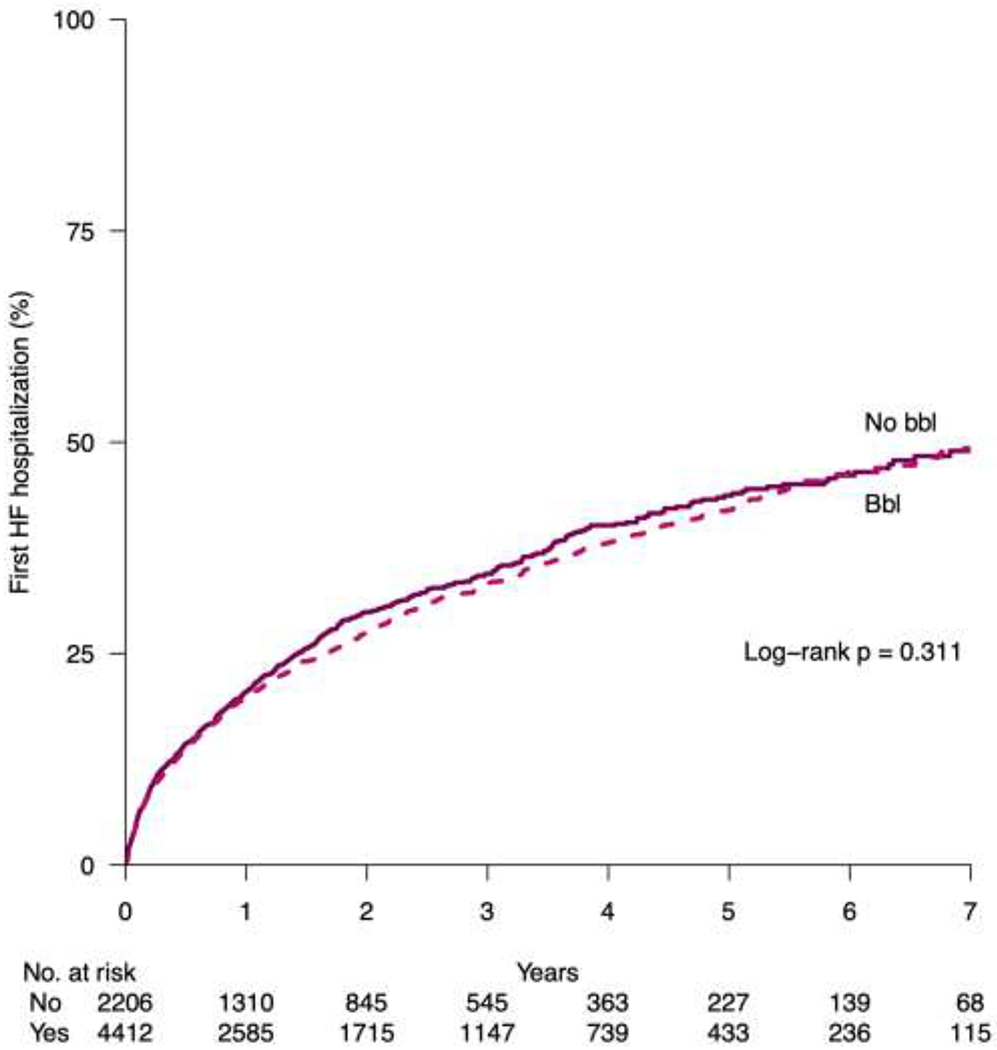
Kaplan Meier plot for first heart failure admission in the matched cohort. Bbl, beta-blocker; HF, heart failure
Figure 2.

Kaplan Meier plot for cardiovascular death in the matched cohort. Bbl, beta-blocker; CVD, cardiovascular death
Table 4 –
Consistency analysis : Censoring patients at crossover
| Outcome | Model | Bbl No | Bbl Yes | Bbl No | Bbl Yes |
|---|---|---|---|---|---|
| First HF hospitalization, β-blocker crossover censored | Adj full HR (95% CI), p-value | ref | 1.09 (0.98-1.22), 0.116 | ||
| CV death, β-blocker crossover censored | Adj full HR (95% CI), p-value | ref | 1.06 (0.94-1.20), 0.343 | ||
| First hospitalization, β-blocker crossover censored | Adj full HR (95% CI), p-value | ref | 0.94 (0.88-1.00), 0.064 | ||
| Death, β-blocker crossover censored | Adj full HR (95% CI), p-value | ref | 0.99 (0.90-1.08), 0.758 | ||
Outcomes in the Per Protocol Consistency Analysis
Censoring occurred when the β-blocker status changed from non-use to use of ß-blockers in 396 of 2206 patients (18%) at a median of 100 days (interquartile range [IQR], 31-368) and from use to non-use in 450 of 4412 (10%) at 339 days (IQR, 164-677). As shown in Table 4 there was no association between β-blocker use and the primary or secondary outcomes.
All-cause Death as a Competing Risk
When all-cause death was considered a competing risk, there were no relevant differences as compared with the main intention-to-treat analysis (Table 5).
Table 5 –
Consistency analysis accounting for all-cause death as a competing risk in the matched cohort
| Matched cohort | ||
|---|---|---|
| Outcome | Bbl No | Bbl Yes |
| First HF hospitalization, all-cause death as competing risk | ref | 0.96 (0.88-1.06) 0.450 |
| CV death death, all-cause as competing risk | ref | 0.96 (0.87-1.06) 0.400 |
| First hospitalization, all-cause death as competing risk | ref | 0.94 (0.88-0.99) 0.029 |
Subgroup Interactions
For HF hospitalizations and CV death, there was a significant sex interaction (Figure 3A and B). In men β-blocker use was associated with lower hazard ratios for heart failure admissions and CV deaths whereas women demonstrated a trend towards higher hazard ratios.
Figure 3.

Subgroup analysis for A) first HF Hospitalization and B) CV death. BP, blood pressure; CV, cardiovascular; COPD, chronic obstructive pulmonary disease; HF, heart failure; PAD, peripheral artery disease.
Additional Consistency Analyses
Consistency analyses excluding NT-proBNP values in matched model and outcomes analyses at higher EF thresholds (55% and 60%) are presented in Supplemental tables 1 and 2. These analyses did not change the observed outcomes or were inconclusive due to small sample sizes.
DISCUSSION
In this large propensity score—matched registry analysis of unselected patients with HFpEF, β-blockers were not associated with an increased or decreased risk for HF hospitalization or cardiovascular death (Take Home Graphic), ß-blocker use was associated with higher NTproBNP levels. An association with a lower risk of all-cause hospitalization and all-cause death in the intention-to-treat analysis was not confirmed in the per protocol consistency analysis. While the between β-blockers and outcomes were not different in most subgroups there was a significant sex interaction, with men but not women having a favorable association between ß-blocker use and heart failure admissions and CV death.
HFpEF in SwedeHF
Compared to clinical trials, SwedeHF provides a detailed real-life generalizable insight into the HFpEF population. For instance, in TOPCAT and PARAGON-HF, the median age was 68 and 72 years, respectively, with mostly NYHA class II symptoms and a median NTproBNP level of about 900 and 940ng/L (7, 15). In comparison, patients with HFpEF in SwedeHF had a median age of 79 years, a third have NYHA class III/IV symptoms and median NTproBNP levels were above 1500ng/L. This difference confirms that many elderly patients with more advanced HFpEF are currently excluded from clinical trials. This is also reflected in a 5-year all-cause mortality that exceeds 50%.
Use of ß-blockers in HFpEF
The current analysis confirms that β-blockers are extensively used in HFpEF patients (15, 16). In this SwedeHF 2011-2018 cohort, 4 out of 5 HFpEF patients were treated with a β-blocker, identical to the 2000-2014 use in a previous analysis of SwedeHF patients with EF≥40% (11). Such a high propensity towards the prescription of β-blockers may either reflect a perceived benefit, derived from HFrEF studies, or an assumption that β-blockers are advantageous in treating common co-morbidities. Since most patients in SwedeHF received β-blockers that are recommended in HFrEF, sometimes together with digoxin, it is conceivable that many clinicians, without evidence, extrapolate their benefits from HFrEF to HFpEF.
Evidence-basis for β-blockers in HFpEF
There is no conclusive evidence of a benefit or harm of β-blockers in patients with HFpEF. The SENIORS randomized controlled trial of nebivolol on the effects on mortality and cardiovascular admissions revealed an overall benefit in patients with an EF>35% (17). However, only patients with an EF<50% derived a benefit from β-blockers, whereas the patients with an EF≥50% did not (5). The Japanese Diastolic Heart Failure Study (J-DHF) investigated carvedilol in HFpEF but did not find an advantage of low doses of carvedilol (18). The ß-PRESERVE HFpEF trial of β-blockers that was announced in 2010 did not report any results (19, 20). In summary, in contrast to the unequivocal benefits of β-blockers in HFrEF that included more than 18,000 patients in randomized controlled trials, β-blocker use in HFpEF≥50% does not appear to provide a benefit in the randomized studies that included fewer than 500 patients (5, 18).
As large clinical trials of β-blockers in HFpEF are missing, there have been attempts to evaluate β-blockers in observational data. These include secondary analyses of prospective studies, often summarized into meta-analyses, and cohort studies such as SwedeHF (21–23). The first β-blocker analysis of the SwedeHF cohort in 2014 included patients with an EF of 40% or higher (11). Consistent with the present results, this prior analysis did not reveal a benefit of β-blocker regarding heart failure hospitalizations but did suggest a small associated reduction in all-cause mortality (one death per year avoided in 100 treated patients). A recent SwedeHF analysis of ß-blocker use in advanced CKD suggested that a beneficial association with outcomes in HFrEF but not in HFpEF (24). While randomized studies were neutral and observational analyses did not show any consistent association, secondary analyses of the TOPCAT trial suggested an association of β-blockers with excess HF hospitalizations and higher NTproBNP levels (23, 25). In SwedeHF, β-blocker use was also associated with increased NTproBNP levels. Although this finding does not establish a causal relationship it confirms studies that demonstrated that β-blockers elevate NTproBNP, a marker of HF severity and enrollment criterion in clinical trials (26–28). While the current analysis does not suggest any adverse clinical associations of β-blockers in HFpEF, the absence of an unequivocal benefit and established side effects are still casting doubts on the current prescription practice.
HFpEF Comorbidities and β-blockers
Hypertension, atrial fibrillation and coronary artery disease are common comorbidities that may be perceived to benefit from β-blockers in HFpEF. However, the evidence-basis for these indications is either declining or was never established. The LIFE hypertension trial demonstrated an excess of cardiovascular events with atenolol when compared to losartan, which led to a downgrade of β-blockers from the group of preferred antihypertensive agents (29). In addition, recent trials of β-blockers in atrial fibrillation suggest an inferiority to calcium channel blockers and/or digoxin and myocardial infarction studies in the era of reperfusion and myocardial salvage revealed that β-blockers increase the risk for heart failure without providing a survival benefit (30–34).
β-blockers in Women
While most subgroups did not show any interaction with β-blocker use, women may be unfavorably affected by β-blockers when compared to men. While women are underrepresented in most HFrEF trials, several studies have highlighted their tendency to have more adverse drug effects than men and the need to reduce their medication doses (35, 36). Considering an even representation of women in HFpEF studies our result calls for close attention towards the sex interaction of medications.
Limitations
The analysis of the SwedeHF registry is limited by bias, confounding and a regional patient base and prescription patterns. While the data contains many baseline characteristics and comorbidities, allowing for propensity score matching based on 44 variables, it is impossible to avoid residual imbalances in the measured and unmeasured confounders. The rational for β-blocker prescriptions and crossovers were not available. Moreover, we could not account for the duration and dosing of β-blockers. While the unexpected finding that heart rates were identical in patients with and without β-blocker maybe explained by the differences in baseline characteristics, suboptimal compliance cannot be excluded.
Conclusion
Despite a lack of evidence or indication, β-blockers are still extensively prescribed in HFpEF. We did not observe any consistent associations between β-blockers and outcomes. In the absence of a strong rationale and sufficiently powered clinical trials the case for β-blockers in HFpEF≥50% is not compelling and the adverse β-blocker effects should be considered in a patient group that is prone to polypharmacy (37).
Supplementary Material
Bullet points.
The effect of beta-blockers in HFpEF≥50% is uncertain.
In a propensity score matched HFpEF analysis in the SwedeHF registry, beta-blockers were not associated with a change in risk for HF admissions or CV deaths.
Funding/Support:
This study received support from the EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking BigData@Heart grant [n° 116074]; the Swedish Research Council [grant 523-2014-2336]; and the Swedish Heart Lung Foundation [grants 20150557 and 20170841] in the form of grants to LHL. Dr. Meyer was supported by grant R01 HL-122744 from the National Institutes of Health.
Conflict of Interest Disclosures
Dr. Meyer reported having licensed patents on the use of pacemakers to prevent and treat heart failure with preserved ejection fraction, outside the submitted work. Dr. Lund reports research grants from AstraZeneca, Novartis, Boehringer Ingelheim, Vifor-Fresenius, and Boston Scientific, and consulting or speaker’s honoraria from AstraZeneca, Novartis, Boehringer Ingelheim, Vifor-Fresenius, Bayer, Sanofi, Merck, Myokardia, Orion Pharma, MedScape, Radcliffe Cardiology, Lexicon, and Respicardia, and stock ownership in AnaCardio, outside the submitted work. Dr Dahlström reports research grants from Boehringer Ingelheim, Pfizer, AstraZeneca, Boston Scientific, Vifor Pharma and Roche Diagnostics and consulting or speaker’s honoraria from AstraZeneca, Novartis and Amgen, outside the submitted work. Dr. Savarese reports grants and personal fees from Vifor, grants and non-financial support from Boehringer Ingelheim, personal fees from Societa’ Prodotti Antibiotici, grants and personal fees from AstraZeneca, personal fees from Roche, personal fees from Servier, grants from Novartis, personal fees from GENESIS, personal fees from Cytokinetics, personal fees from Medtronic, grants from Boston Scientific, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9. [DOI] [PubMed] [Google Scholar]
- 3.Loop MS, Van Dyke MK, Chen L, Brown TM, Durant RW, Safford MM, et al. Comparison of Length of Stay, 30-Day Mortality, and 30-Day Readmission Rates in Medicare Patients With Heart Failure and With Reduced Versus Preserved Ejection Fraction. Am J Cardiol. 2016;118(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis EF, Lamas GA, O’Meara E, Granger CB, Dunlap ME, McKelvie RS, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9(1):83–91. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Investigators ASACa. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84. [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, McMurray JJV, Investigators P-HSCa. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. Reply. N Engl J Med. 2020;382(12):1182–3. [DOI] [PubMed] [Google Scholar]
- 8.Meyer M, LeWinter MM. Heart Rate and Heart Failure With Preserved Ejection Fraction: Time to Slow β-Blocker Use? Circ Heart Fail. 2019;12(8):e006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savarese G, Vasko P, Jonsson Å, Edner M, Dahlström U, Lund LH. The Swedish Heart Failure Registry: a living, ongoing quality assurance and research in heart failure. Ups J Med Sci. 2019;124(1):65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson A, Edner M, Alehagen U, Dahlström U. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail. 2010;12(1):25–31. [DOI] [PubMed] [Google Scholar]
- 11.Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312(19):2008–18. [DOI] [PubMed] [Google Scholar]
- 12.Ingelsson E, Arnlöv J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7(5):787–91. [DOI] [PubMed] [Google Scholar]
- 13.Mitra R, Reiter JP. A comparison of two methods of estimating propensity scores after multiple imputation. Stat Methods Med Res. 2016;25(1):188–204. [DOI] [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 15.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92. [DOI] [PubMed] [Google Scholar]
- 16.Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, et al. Echocardiographic Features of Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2019;74(23):2858–73. [DOI] [PubMed] [Google Scholar]
- 17.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215–25. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Origasa H, Hori M, Investigators J-D. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail. 2013;15(1):110–8. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J, et al. Rationale and design of the beta-blocker in heart failure with normal left ventricular ejection fraction (beta-PRESERVE) study. Eur J Heart Fail. 2010;12(2):181–5. [DOI] [PubMed] [Google Scholar]
- 20.Martin N, Manoharan K, Thomas J, Davies C, Lumbers RT. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2018;6:CD012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53(2):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bavishi C, Chatterjee S, Ather S, Patel D, Messerli FH. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev. 2015;20(2):193–201. [DOI] [PubMed] [Google Scholar]
- 23.Silverman DN, Plante TB, Infeld M, Callas PW, Juraschek SP, Dougherty GB, et al. Association of β-Blocker Use With Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients With Heart Failure With a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw Open. 2019;2(12):e1916598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu EL, Uijl A, Dekker FW, Lund LH, Savarese G, Carrero JJ. Association Between β-Blocker Use and Mortality/Morbidity in Patients With Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction and Advanced Chronic Kidney Disease. Circ Heart Fail. 2020;13(11):e007180. [DOI] [PubMed] [Google Scholar]
- 25.Tsujimoto T, Kajio H. Beta-blocker use and cardiovascular event risk in patients with heart failure with preserved ejection fraction. Sci Rep. 2018;8(1):9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergström A, Andersson B, Edner M, Nylander E, Persson H, Dahlström U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC). Eur J Heart Fail. 2004;6(4):453–61. [DOI] [PubMed] [Google Scholar]
- 27.Edelmann F, Musial-Bright L, Gelbrich G, Trippel T, Radenovic S, Wachter R, et al. Tolerability and Feasibility of Beta-Blocker Titration in HFpEF Versus HFrEF: Insights From the CIBIS-ELD Trial. JACC Heart Fail. 2016;4(2):140–9. [DOI] [PubMed] [Google Scholar]
- 28.Nambiar L, Silverman D, Vanburen P, LeWinter M, Meyer M. Beta-Blocker Cessation in Stable Outpatients With Heart Failure With a Preserved Ejection Fraction. J Card Fail. 2020;26(3):281–2. [DOI] [PubMed] [Google Scholar]
- 29.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. [DOI] [PubMed] [Google Scholar]
- 30.Kotecha D, Bunting KV, Gill SK, Mehta S, Stanbury M, Jones JC, et al. Effect of Digoxin vs Bisoprolol for Heart Rate Control in Atrial Fibrillation on Patient-Reported Quality of Life: The RATE-AF Randomized Clinical Trial. JAMA. 2020;324(24):2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1622–32. [DOI] [PubMed] [Google Scholar]
- 32.Bangalore S, Makani H, Radford M, Thakur K, Toklu B, Katz SD, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. 2014;127(10):939–53. [DOI] [PubMed] [Google Scholar]
- 33.Motivala AA, Parikh V, Roe M, Dai D, Abbott JD, Prasad A, et al. Predictors, Trends, and Outcomes (Among Older Patients ≥65 Years of Age) Associated With Beta-Blocker Use in Patients With Stable Angina Undergoing Elective Percutaneous Coronary Intervention: Insights From the NCDR Registry. JACC Cardiovasc Interv. 2016;9(16):1639–48. [DOI] [PubMed] [Google Scholar]
- 34.Ulimoen SR, Enger S, Pripp AH, Abdelnoor M, Arnesen H, Gjesdal K, et al. Calcium channel blockers improve exercise capacity and reduce N-terminal Pro-B-type natriuretic peptide levels compared with beta-blockers in patients with permanent atrial fibrillation. Eur Heart J. 2014;35(8):517–24. [DOI] [PubMed] [Google Scholar]
- 35.Bots SH, Groepenhoff F, Eikendal ALM, Tannenbaum C, Rochon PA, Regitz-Zagrosek V, et al. Adverse Drug Reactions to Guideline-Recommended Heart Failure Drugs in Women: A Systematic Review of the Literature. JACC Heart Fail. 2019;7(3):258–66. [DOI] [PubMed] [Google Scholar]
- 36.Bots SH, den Ruijter HM. Recommended Heart Failure Medications and Adverse Drug Reactions in Women. Circulation. 2019;139(12):1469–71. [DOI] [PubMed] [Google Scholar]
- 37.Unlu O, Levitan EB, Reshetnyak E, Kneifati-Hayek J, Diaz I, Archambault A, et al. Polypharmacy in Older Adults Hospitalized for Heart Failure. Circ Heart Fail. 2020;13(11):e006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


