Abstract
Objective
To investigate the effect of focused ultrasonography on clinical outcomes of septic shock.
Methods
Patients with septic shock were randomized into an integrated cardiopulmonary ultrasonography (ICUS) group and conventional (CON) group. Within 1 hour of admission, the ICUS group underwent ICUS examination for hemodynamic decision-making, while the CON group received standard treatment. The primary endpoint was 28-day mortality after admission. The secondary endpoints were cumulative fluid administration in the first 6, 24, and 72 hours; use of vasoactive drugs; lactate clearance; duration of ventilation; and ICU stay.
Results
Ninety-four qualified patients were enrolled (ICUS group, 49; CON group, 45). ICUS showed no significant effect on 28-day mortality. Within the initial 6 hours, the ICUS group tended to have a higher fluid balance and fluid intake than the CON group. The duration of vasopressor support was shorter in the ICUS group. There were no differences in the cumulative fluid infusion within 24 or 72 hours, lactate clearance, ICU stay, or duration of ventilation.
Conclusions
The initially focused ICUS did not affect the clinical outcomes of septic shock, but it tended to be associated with a higher fluid balance within the initial 6 hours and shorter duration of vasopressor support.
Keywords: Focused ultrasonography, early goal-directed therapy, severe sepsis, septic shock, hemodynamic monitoring, clinical outcome
Introduction
Sepsis is the third most lethal disease in China, contributing to more than 1 million deaths in 2015.1 Worldwide efforts have been made to standardize treatment and improve outcomes of sepsis. According to the latest Surviving Sepsis Campaign guidelines, the hour-1 bundle recommends the use of early fluid resuscitation and vasopressors for patients with hypotension.2 However, the resuscitation strategy remains controversial.3–7 In fact, patients with sepsis may share similar clinical manifestations but different mechanisms of circulatory compromise, including overlapping factors of hypovolemia, hypokinesis, and vasoplegia. Therefore, it is necessary to identify the major factor that contributes to successful treatment.
With its advantage of providing visual estimates of patients’ volume status and heart function, focused echocardiography has been regarded as the first-line modality for differentiating the causes of shock.8 When targeted at functional assessment rather than morphological abnormalities of the heart, focused echocardiography may influence clinical decisions based on the fluid requirement and inotropic or vasoactive choices. Multiple studies have focused on the prognostic value of specific echocardiographic measures in patients with sepsis or their influence on changes in the diagnostic and therapeutic plans; the incidences of such changes ranged from 17% to 67% in the intensive care unit (ICU) setting and even reached about 80% in anesthesia settings.9–11 However, randomized controlled trials are required to confirm whether these changes benefit patient outcomes. In a recent study, focused ultrasonography had little effect on the final outcomes among patients with undifferentiated shock.12 No studies to date have revealed the effect of focused ultrasonography on septic shock. Considering that the circulatory failure of sepsis is multifactorial and the presence of extravascular lung water impacts the hemodynamic treatment, we considered that the focused ultrasonography used for hemodynamic management in sepsis should be an integrated cardiopulmonary ultrasonography (ICUS) technique rather than echocardiography alone. This study was performed to investigate the effect of focused cardiopulmonary ultrasonography within the first hour of ICU admission on the clinical outcomes of septic shock.
Methods
Setting
This prospective randomized pilot study was conducted in a single center located in the division of critical care medicine at Xiangya Hospital, Central South University, Changsha City, Hunan Province, China.
The study protocol followed the requirements of the Declaration of Helsinki and was approved by the Ethics Committee of Xiangya Hospital (2017121158). This study was also registered in ClinicalTrials.gov (NCT number: 01920776). Written informed consent was obtained from the enrolled patients or their authorized family members.
Patient enrollment and definition of sepsis onset
From September 2014 to January 2016, patients diagnosed with sepsis were preliminary screened and were required to fulfill two or more conditions of systemic inflammatory response syndrome in the setting of suspected infection or organ dysfunction.13 The patients included in this study were required to meet at least one of the following inclusion criteria: (1) systolic blood pressure of ≤90 mmHg or a >40 mmHg decrease compared with baseline or mean arterial blood pressure of ≤65 mmHg after infusion of 20 mL/kg of crystalloid; (2) serum lactate concentration of ≥4 mmol/L; and (3) initiation of intravenous vasopressors. The exclusion criteria were (1) age of <18 years; (2) history of hospitalization due to chronic heart disease such as ischemic disease, valvular disease, dilated cardiomyopathy, or hypertrophic cardiomyopathy; (3) end-stage malignant tumor or irreversible terminal condition; and (4) pregnancy. Sepsis time 0 was defined as the time of admission to the ICU for sepsis treatment. For patients already in the ICU, sepsis time 0 was considered the earliest time at which the patients satisfied any of the inclusion criteria.
Study design
The patients were randomized in a 1:1 ratio into an ICUS group and conventional (CON) group using a random number table. Blood cultures and additional laboratory examinations were conducted in all patients. An attending physician was consulted to prescribe appropriate antibiotics based on each patient’s medication history, disease course, laboratory tests, imaging findings, and clinical severity. Analgesia and sedation were given to maintain a Critical-Care Pain Observation Tool score of 0 to 2 and a Richmond Agitation-Sedation Scale score of −2 to 0. Mechanical ventilation was set in standard mode.
The two groups differed in the initial 6 hours of hemodynamic management, including the indications for intravenous fluid and vasoactive and inotropic agents. These decisions were made by one of the two assigned physicians who were available within 10 minutes, 24 hours per day, for bedside ultrasonographic examinations. Both of the on-call physicians had been trained and certified by the World Interactive Network Focused On Critical Ultrasound (WINFOCUS) after >150 examinations of practice. With their knowledge and skill qualifications, the two physicians were also trainers for the Chinese Critical Ultrasound Study Group. In the CON group, fluid infusion was guided by the central venous pressure (CVP). The patients immediately underwent CVP monitoring until stabilization of vital signs without a vasopressor. Crystalloids were used for resuscitation until the CVP reached ≥10 mmHg. After the required pressure had been reached, fluid infusion was restricted and inotropes were initiated if the central venous oxygen saturation was <70% or cardiac dysfunction was suspected. In the ICUS group, the CVP was measured 6 hours later. Hemodynamic management was based on combined echocardiography with a 2- to 5-MHz phase probe (Vivid i; General Electric Company, Boston, MA, USA) and lung ultrasonography with a 2- to 5-MHz convex probe, which were performed within 1 hour after sepsis time 0 with no interruption of the life-saving therapy. The prerequisite of the ultrasonographic protocol was identification of the subtype of septic shock based on the core components of Rapid Ultrasound for Shock and Hypotension protocol excluding the abdominal assessment.14 The hemodynamic decision-making procedures (Table 1) were based on the following four ultrasonographic views.
Table 1.
Decision-making procedure directed by focused cardiopulmonary ultrasonography.
| Ultrasonography Finding Question | Answer Choices |
|---|---|
| What is the IVC diameter? | <10 mm/≥20 mm*/other |
| What is the RV size? | Normal or dilated* |
| Is there evidence of diffuse interstitial syndrome? | Yes* or no |
| What is the LV systolic function? | Hyperdynamic/normal/moderately impaired*# /severely impaired*# |
*If none of these choices applied, fluid resuscitation was given at 30 mL/kg within 3 hours; if any of these choices applied, fluid resuscitation was cautiously performed; if two of these choices applied, fluid resuscitation was dispensed and vasopressor support was enhanced. #Inotropic support was initiated as needed.
IVC, inferior vena cava; RV, right ventricular; LV, left ventricular.
(1) Subcostal four-chamber and inferior vena cava (IVC) long-axis view: the IVC diameter was measured 2 cm from the right atrial junction at the end of expiration. Whether the respiratory variations were qualitatively defined was determined (no or yes). (2) Parasternal long- and short-axis views (at the level of the papillary muscles): the left ventricular (LV) contractility pattern was categorized as hyperdynamic [LV ejection fraction (LVEF) of ≥70%], normal (LVEF of 50%–70%), moderately depressed (LVEF of 30%–50%), or severely depressed (LVEF of ≤30%) by “eyeballing.” (3) Apical view: the right ventricular (RV)/LV telediastolic area was compared by eyeballing, and a ratio of ≥0.6 was considered to indicate RV dilation. (4) The bilateral lung ultrasound protocol was performed as described previously:15 Each of the 10 covered regions was assessed based on an A/B3/B7/C/P pattern with detailed pleural sliding and morphology.16
Generally, the diagnoses based on lung artifacts varied according to the definition established by Lichtenstein and Meziere.17 For example, multiple anterior diffuse B lines with lung sliding indicated pulmonary edema. If the ultrasonographic examination was not completed within 10 minutes, the examination was stopped. If indeterminate results were obtained, such as heterogeneous contractility or an obvious valve or chamber abnormality, an advanced echocardiographer was required for an official report. All videos and images were validated in a timely manner by an experienced senior physician who was a certified trainer of WINFOCUS.
From sepsis time 0, the first-choice vasopressor was norepinephrine, which was then continuously infused to maintain a mean arterial blood pressure of >65 mmHg in both groups. After the initial 6 hours, all patients received usual care and both groups were able to undergo sonographic examinations if needed. Pulse index continuous cardiac output (PiCCO) was available for patients with refractory shock. In this randomized controlled trial, only the data analysts were blinded. The demographic data, laboratory results, and related variables were collected at the time of admission. The primary endpoint was whether focused cardiopulmonary ultrasonography affected the 28-day mortality from sepsis 0. The secondary endpoints were the cumulative fluid intake and fluid balance in the first 6, 24, and 72 hours of mechanical ventilation and the ICU stay. The lactate clearance rate was calculated as the first measurement within 1 hour from sepsis time 0 minus the first measurement after the initial 6 hours of therapy, which was then was divided by the former value. Any changes in the diagnostic and therapeutic plans were recorded.
Statistical analysis
All analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). With the assumption of a 15% absolute reduction of 28-day mortality, a sample size of 94 patients was projected to have a power of 80% and an alpha error of 0.05 (two-tailed). Considering that 15% of patients might decline further treatment or be lost to follow-up, the projected sample size was 100. The 28-day mortality was compared with a log-rank test, and the survival curve was analyzed with the Kaplan–Meier method. All continuous variables were explored with the Kolmogorov–Smirnov test. Parametric variables are reported as mean ± standard deviation, and group comparisons were performed with the independent-samples t test. Nonparametric variables are expressed as median (interquartile range), and group comparisons were performed using the Wilcoxon rank sum test. Categorical variables are reported as frequency (percentage), and group differences were compared using the chi-square test or Fisher’s exact test (if any n < 5). All comparisons were two-tailed, and p < 0.05 was considered to exclude the null hypothesis.
Results
Baseline characteristics of study population
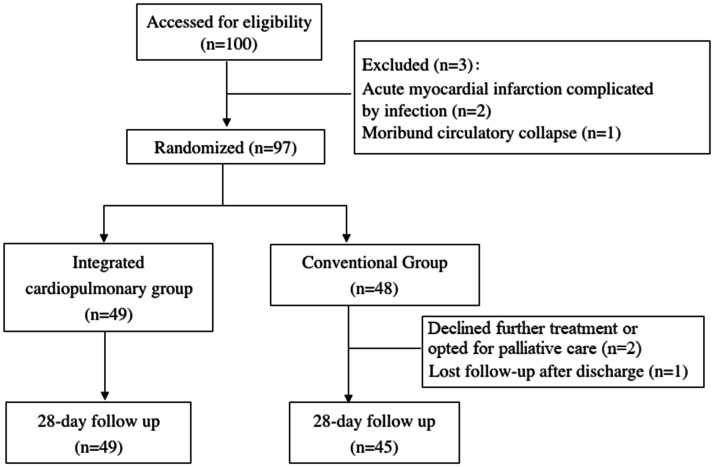
During the study period, 100 patients were screened and 97 were considered eligible. The participant flow chart is shown in Figure 1. Data of 94 patients were collected; of these, 2 patients voluntarily dropped out of the study and 1 patient was lost to follow-up. Finally, the ICUS group comprised 49 patients and the CON group comprised 45 patients. The demographic characteristics of the patients are presented in Table 2. Thirty-six (73.5%) patients in the ICUS group and 34 (75.6%) patients in the CON group received assisted mechanical ventilation. No patient received controlled ventilation or muscle relaxants. Twenty-four (49.0%) patients in the ICUS group and 30 (66.7%) patients in the CON group underwent surgery. Twelve patients in each group (ICUS vs. CON group: 24.5% vs. 26.7%) developed hospital-acquired infections. Negative culture was more frequent in the ICUS group than CON group [28 (57.1%) vs. 16 (35.6%), respectively; p = 0.04].
Figure 1.
Flow diagram of study.
Table 2.
Demographic and clinical characteristics of patients at time of admission to intensive care unit.
| Items | ICUS group (n = 49) | CON group (n = 45) | p value |
|---|---|---|---|
| Sex, male/female | 24/25 | 28/17 | |
| Age, years | 54.5 ± 15.2 | 56.7 ± 11.0 | 0.51 |
| BMI, kg/m2 | 23.1 ± 3.3 | 23.6 ± 4.6 | 0.51 |
| Comorbidities | |||
| Diabetes | 4 (8.2) | 5 (11.1) | 0.73 |
| Hypertension | 8 (16.3) | 9 (20.0) | 0.79 |
| Chronic pulmonary disease | 4 (8.2) | 1 (2.2) | 0.36 |
| History of operation | 5 (10.2) | 11 (24.4) | 0.10 |
| Coronary artery disease | 5 (10.2) | 1 (2.2) | 0.21 |
| Hepatology | 4 (8.2) | 6 (13.3) | 0.51 |
| Renal disease | 3 (6.1) | 9 (20.0) | 0.06 |
| Others | 17 (34.7) | 8 (17.8) | 0.10 |
| Mechanical ventilation | 36 (73.5) | 34 (75.6) | 0.60 |
| PaCO2 | 34.0 (28.0–42.5) | 39.0 (31.3–47.5) | 0.16 |
| PaO2/FiO2 | 190.0 (126.7–241.3) | 170.0 (115.00–225.8) | 0.24 |
| WBCs, ×109/L | 12.9 (6.9–21.2) | 13.3 (5.8–18.2) | 0.44 |
| Platelets, ×109/L | 92.0 (58.0–149.5) | 122.00 (67.5–166.5) | 0.22 |
| Total bilirubin, mmol/L | 24.7 (12.9–56.1) | 24.0 (13.1–37.3) | 0.67 |
| Serum creatinine, mmol/L | 147.6 (88.0–255.4) | 179.3 (140.6–311.6) | 0.05 |
| APACHE II score | 20.8 ± 8.2 | 21.8 ± 6.7 | 0.75 |
| SOFA score | 13.2 ± 4.8 | 13.2 ± 4.1 | 0.86 |
| Etiology | |||
| Urosepsis | 4 (8.2) | 10 (22.2) | 0.08 |
| Pneumonia | 10 (20.4) | 6 (13.3) | 0.42 |
| Bloodstream | 23 (46.9) | 20 (44.4) | 0.84 |
| Peritonitis | 20 (40.8) | 20 (44.4) | 0.84 |
| Meningitis | 5 (10.2) | 2 (4.4) | 0.44 |
| Cholangitis | 2 (4.1) | 2 (4.4) | 1.0 |
| Microorganisms | |||
| Hospital-acquired infection | 12 (24.5) | 12 (26.7) | 0.82 |
| Gram-positive bacilli | 11 (22.4) | 14 (31.1) | 0.36 |
| Gram-negative bacilli | 22 (44.9) | 28 (62.2) | 0.10 |
| Fungi | 5 (10.2) | 2 (4.4) | 0.44 |
| No microorganisms found | 28 (57.1) | 16 (35.6) | 0.04 |
| Heart rate, beats/minute | 129.0 (119.0-139.0) | 126.5 (104.5-140.0) | 0.30 |
| Respiratory rate, breaths/minute | 26.9 ± 7.0 | 24.7 ± 6.8 | 0.13 |
| MAP, mmHg | 69.8 ± 13.4 | 66.9 ± 15.1 | 0.36 |
| Temperature, °C | 40.0 ± 1.2 | 37.7 ± 1.3 | 0.30 |
| Number of SIRS presentations | |||
| 2 | 11 (22.4) | 12 (26.7) | 0.81 |
| 3 | 24 (49.0) | 18 (40.0) | 0.41 |
| 4 | 14 (28.6) | 15 (33.3) | 0.66 |
| Lactate, mmol/L | 4.2 (2.3–6.9) | 4.0 (2.6–5.6) | 0.66 |
| Procalcitonin, ng/mL | 14.9 (2.9–80.9) | 35.9 (6.7–103.3) | 0.15 |
| NT-proBNP, pg/mL | 4746.5 (1207.3–7941.0) | 5728.0 (1066.0–25000.0) | 0.42 |
Values are reported as n (%), median (interquartile range), or mean ± standard deviation.
ICUS, integrated cardiopulmonary ultrasonography; CON, conventional; BMI, body mass index; APACHE II, Acute Physiology and Chronic Health Evaluation II; PaCO2, partial pressure of carbon dioxide in arterial blood; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen in arterial blood; SOFA, Sequential Organ Failure Assessment; WBC, white blood cell; SIRS, systemic inflammatory response syndrome; MAP, mean arterial pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Primary and secondary endpoints
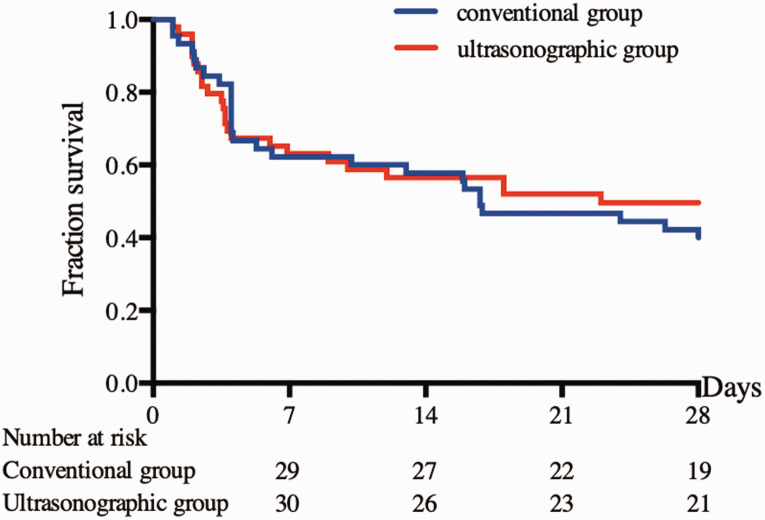
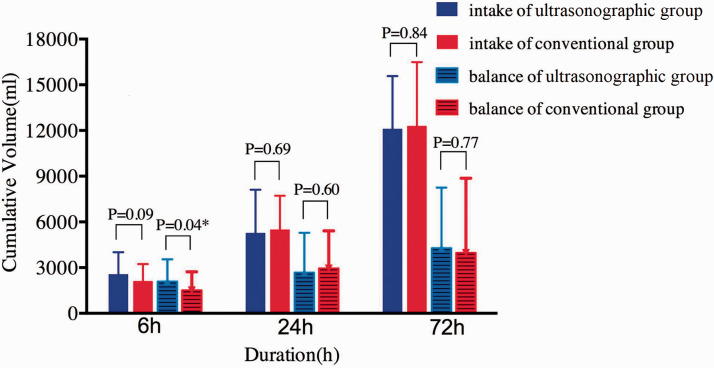
The 28-day mortality rate was not significantly different between the two groups (50.6% vs. 60.0%) (Figure 2). The fluid balance volume within the initial 6 hours in the ICUS group was almost 600 mL more than that in the CON group (2092.0 ± 1452.9 vs. 1508.9 ± 1211.8 mL, respectively; p = 0.04) (Figure 3), while the fluid intake volume was 450 mL more (2568.2 ± 1442.6 vs. 2111.1 ± 1115.5 mL, respectively) without a significant difference.
Figure 2.
Comparison of 28-day mortality with Kaplan–Meier estimate (p = 0.58).
Figure 3.
Comparison of cumulative volume intake and balance over time. *p < 0.05. At 24 and 72 hours, the data for patients who were dead or had been discharged from the hospital before that were analyzed as missing.
The lactate reduction rate, cumulative fluid intake, and fluid balance within 24 and 72 hours were not significantly different between the two groups. Although the ICUS group tended to have a shorter duration of vasopressor support than the CON group [48 hours (interquartile range, 24.0–83.5 hours) vs. 69 hours (interquartile range, 34.0–97.9 hours)], the duration of mechanical ventilation and ICU stay showed no significant differences between the groups. The details regarding the secondary endpoints and hemodynamic interventions are shown in Table 3. All patients received norepinephrine to maintain perfusion. The administration of other vasoactive or inotropic drugs was similar between the two groups. Nine patients in each group (ICUS vs. CON group: 18.4% vs. 20.0%) showed cardiac dysfunction by echocardiography or PiCCO. Eleven patients in the ICUS group also had PiCCO measurements; however, the cardiac output as measured by ultrasonography was not totally consistent with that measured by PiCCO, which may have been due to the smaller sample size.
Table 3.
Comparison of administrations, interventions, and secondary endpoints
| Items | ICUS group (n = 50) | CON group (n = 44) | p value |
|---|---|---|---|
| Vasoactive and inotropic administrations | |||
| Maximum dose of norepinephrine,* µg/kg/minute | 0.75 (0.20–2.0) | 1.00 (0.35–2.0) | 0.15 |
| Dobutamine | 4 (8.2) | 2 (4.4) | 0.68 |
| Vasopressin | 1 (2.0) | 4 (8.89) | 0.19 |
| Others# | 8 (16.3) | 5 (11.1) | 0.49 |
| Cardiac dysfunction | 9 (18.4) | 9 (20.0) | 0.80 |
| CRRT | 24 (49.0) | 26 (57.8) | 0.58 |
| PiCCO | 11 (22.5) | 10 (22.2) | 0.97 |
| Secondary outcomes | |||
| Lactate clearance rate$ | 0.13 (−0.34–0.41) | 0.17 (−0.23–0.44) | 0.78 |
| Time to lactate normalization, hours | 18.5 (7.3–69.0) | 19.0 (11.5–213.0) | 0.61 |
| Duration of vasopressor use, hours | 48.0 (24.0–83.5) | 69.0 (34.0–97.9) | 0.08 |
| ICU stay, hours | 108.0 (63.5–286.8) | 130.1 (96.0–296.7) | 0.38 |
| Duration of mechanical ventilation, hours | 60.00 (18.7–152.6) | 62.0 (24.0–120.0) | 0.72 |
Data are presented as n (%) or median (interquartile range).
*The maximum dose did not include the use of vasopressors at the time of death.
#Others included digitalin, natriuretic peptide, adrenaline, dopamine, and esmolol.
$Lactate clearance rate = (first measurement within 1 hour after ICU admission − first measurement 6 hours after initial therapy) / first measurement within 1 hour after ICU admission.
ICUS, integrated cardiopulmonary ultrasonography; CON, conventional; CRRT, continuous renal replacement therapy; PiCCO, pulse index continuous cardiac output; ICU, intensive care unit.
Ultrasonography findings
For four patients without adequate subcostal views, transhepatic views of the IVC were used as alternate views. For all patients, standard images were obtained in both the parasternal and apical views. No obvious errors were observed through subsequent validation. The major findings are shown in Table 4. Five patients had moderate LV depression, two patients had an RV/LV ratio of >0.6, and six patients had diffuse pulmonary edema. Two of these patients had LV depression combined with diffuse pulmonary edema. No patients had an LVEF of ≤30%, IVC of >20 mm, or absence of respiratory variation. As a result, 38 (77.6%) patients in the ICUS group and 28 (62.2%) patients in the CON group received >30 mL/kg crystalloid fluid for resuscitation within the initial 6 hours. Two patients with an obviously enlarged left atrium underwent consultation with an advanced echocardiographer, and both were diagnosed with mitral disease. In addition, five (10.2%) patients underwent bronchoscopy or thoracentesis because of ultrasonographic atelectasis or mass pleural effusion. Such pathologies were located in a unilateral lung; therefore, these patients were not considered to have diffuse pulmonary edema.
Table 4.
Findings of focused cardiopulmonary ultrasonography.
| Ultrasonography findings | Measurements (n) |
|---|---|
| IVC diameter | <10 mm/≥20 mm/other: 10/0/39 |
| RV size | Normal/dilated: 47/2 |
| Diffuse interstitial syndrome | Yes/no: 6*/43 |
| LV systolic function | Hyperdynamic/normal/moderately impaired/severely impaired: 2/42/5/0 |
*Two patients had combined moderate LV depression.
IVC, inferior vena cava; RV, right ventricular; LV, left ventricular.
Discussion
In the present study, early focused sonography demonstrated little effect on 28-day mortality among patients with septic shock; however, it still tended to enhance fluid resuscitation within the initial 6 hours, and it shortened the duration of vasopressor support. To the best of our knowledge, this is the first randomized controlled study to investigate the effect of bedside focused cardiopulmonary sonography on patients with sepsis.
Our study results revealed that focused cardiopulmonary ultrasonography did not result in beneficial clinical outcomes in patients with septic shock. Several factors led to death, and the hemodynamic monitoring tool was not sufficient to influence the outcomes.18 Only 11 (22.4%) patients had abnormal ultrasound findings, and this percentage was less than expected, thus weakening the clinical effects. Another explanation is that our ultrasonographic protocol was too simple to guide delicate hemodynamic treatment. Notably, the 28-day mortality rate in our study was much higher than that in several other multicenter sepsis trials.3–5 This might be attributable to the relatively lower medical level in our department, which is located in an underdeveloped area in China. In previous studies, the mortality rate of hospitalized patients with septic shock varied from 31.1% to 75.9% in different countries.19,20 A recent international study demonstrated that when using the same focused ultrasonography technique among patients with undifferentiated shock, patients recruited from South Africa had a 20% higher mortality rate than those from North America.12
This study examined the effect of focused cardiopulmonary sonography for initial evaluation of septic shock to answer specific questions regarding fluid requirement and vasoactive choices and to categorize the shock. Although the increased fluid administration based on sonography findings showed very good agreement with large-database research from real-world evidence,21 this increase did not continue beyond 3 days. In total, 11 (22.4%) patients in the ICUS group and 17 (37.8%) patients in the CON group received restrictive resuscitation. Perhaps focused sonography enhanced the confidence of the physician in diagnosing septic shock while helping to exclude shock of cardiac origin in most cases. However, this needs further clarification because when early transesophageal echocardiography and Surviving Sepsis Campaign guidelines were compared, the results showed obvious disagreement with the prescription of fluid loading in patients with septic shock.22 Notably, whether the increased fluid balance was associated with the patients’ good clinical outcomes remains unclear; this might have been because there was no significant improvement in lactate clearance or organ function in the ultrasonographic group. Whether early resuscitation resulted in a shorter vasopressor duration also requires further investigation.
Prompt and adequate fluid resuscitation is a fundamental but challenging problem in patients with septic shock. Both deficient and excessive fluid resuscitation are associated with a poor prognosis.23 According to the latest international guidelines, infusion of 30 mL/kg of a crystalloid within the hour-1 bundle is recommended2; however, this might not be appropriate for all patients with hypotensive sepsis. In fact, the determinant of fluid tolerance majorly relies on individual heart function. Sepsis-related myocardial dysfunction reportedly occurs in as many as 20% to 65% of patients.24 Even at the Mayo Clinic, a study showed that the intensivists misjudged the ventricular function in about half of patients with septic shock before echocardiographic examination.11 More importantly, the echocardiography findings changed the therapeutic plan in nearly one-third of the patients.11 Because of the small sample size of this study, the differences in the initial fluid intake and fluid balance should be carefully estimated. Because no harm occurred, it would be worthwhile to perform a similar assessment in a larger clinical trial.
Dynamic values such as the IVC collapsibility/distensibility index are reportedly more instructive than the static IVC diameter, but their application is rigorous.26 In the current study, about 75% of patients received assisted mechanical ventilation, which prevented monitoring of the IVC collapsibility/distensibility index. The diameter and variation of the IVC depend on many factors, including the preload status, right heart function, difference between the thoracic and abdominal pressure, and others; therefore, the IVC alone is not a reliable index to predict preload.27 Instead, the fluid resuscitation was guided by a combination of the LVEF and IVC parameters in this study. In another cohort study, the physicians changed the treatment plans in more than half of patients with suspected sepsis guided by a combination of the LVEF and IVC parameters.25
One advantage of the present study is that lung ultrasound was integrated into the hemodynamic decision-making procedure. The amount of lung sonographic artifacts was well correlated with the extravascular lung water.17,28 Lung ultrasonography significantly influenced the therapeutic decisions in about 85.6% of mechanically ventilated critically ill patients.16 Fluid administration may be limited by lung sonography because of acute circulatory failure.29–31 One study showed that instantaneous ICUS was associated with a shorter time to diagnosis and smaller fluid infusion volume in critically ill patients with acute pulmonary edema.32 Considering the above points, we intentionally chose ICUS to guide our hemodynamic management. However, few patients were affected, and several patients required urgent treatment because of atelectasis or mass pleural effusion.
A previous study indicated a strong positive correlation (p < 0.001, r = 0.985) between the cardiac output values measured by critical care echocardiography and PiCCO in pediatric patients.33 In the present study, however, one main purpose for the use of ultrasonography for treatment guidance in the ICU was to reduce the need for invasive procedures. Therefore, PiCCO was not considered a routine monitoring item. In addition, only 11 (22%) of the 49 patients in the ultrasonographic group underwent PiCCO monitoring, but the cardiac output values measured by ultrasonography were not totally consistent with those measured by PiCCO. One reason for the discrepancy between our study and the previous study of pediatric patients might be the higher quality and measurement accuracy of pediatric patients’ cardiac ultrasound images. The present study may have contained some errors in the cardiac output measurement by echocardiography; this was also reported in a previous study.34 Further studies involving larger sample sizes are needed.
This study has several limitations that should be noted. First, the Sepsis-3 criteria were not used to screen patients for eligibility because the participants were recruited before documentation of the new criteria. Second, the intervention was not blinded to the patients or attending intensivist; however, the latter might have acted more proactively to the therapeutic response. Third, the outcomes of a more detailed examination of echocardiographic effects was not performed. In particular, measurement of LV diastolic function should be involved in the decision-making process because LV diastolic function occurs more often than systolic depression and has prognostic value in sepsis-related myocardial dysfunction.24,35,36 The lack of diastolic function evaluation partly explains the low incidence of sepsis-related myocardial dysfunction in the present study. Moreover, repetitive echocardiographic examinations might assist in rapid evaluation of the responses to initial resuscitation and provide more reference for subsequent treatment, which may in turn help to improve the prognosis. Fourth, dynamic parameters of fluid responsiveness were not used to guide fluid management. For the convenience of comparison and decision-making, the CVP was not initially monitored in the ultrasonographic group; therefore, we cannot make conclusions regarding the relationship between ultrasonographic signs and CVP. Finally, this study would have been more powered if the sample size had been larger.
In conclusion, initial focused ICUS evaluation had no effect on the clinical outcomes of patients with severe sepsis and septic shock, although it probably enhanced fluid administration and shortened vasopressor support. A multicenter or more productive randomized controlled trial is required to verify these results.
Acknowledgments
We acknowledge Songyun Deng (Department of Critical Care Medicine, Xiangya Hospital, Central South University, Changsha, China) for providing assistance with formatting the references. The members of the Chinese Critical Ultrasound Study Group are as follows: Dawei Liu, Xiaoting Wang, Hongmin Zhang, Qing Zhang, Xin Ding, and Huan Chen (Department of Critical Care Medicine, Peking Union Medical College Hospital, Beijing, China); Yangong Chao and Qinbing Zeng (Department of Critical Care Medicine, the First Affiliated Hospital of Tsinghua University, Beijing, China); Ran Zhu, Mingming Chen, and Xiaohan Yin (Department of Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China); Lixia Liu and Yan Huo (Department of Critical Care Medicine, Hebei Medical University Fourth Hospital, Shijiazhuang, China); Wei He and Na Liu (Department of Critical Care Medicine, Beijing Tongren Hospital, Capital Medical University, Beijing, China); Lina Zhang, Li Huang, and Li Li (Department of Critical Care Medicine, Xiangya Hospital, Central South University, Changsha, China); Wanhong Yin, Yi Li, and Xueying Zeng (Department of Critical Care Medicine, West China Hospital, Sichuan University, Sichuan, China); Jun Wu and Jingyi Wu (Department of Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University, Shanghai, China); Xiang Si (Department of Critical Care Medicine, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China); and Haitao Liu (Department of Critical Care Medicine, The Third Affiliated Hospital of Harbin Medical University, Harbin, China).
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Li Li https://orcid.org/0000-0003-2972-1883
References
- 1.Weng L, Zeng XY, Yin P, et al. Sepsis-related mortality in China: a descriptive analysis. Intensive Care Med 2018; 44: 1071–1080. DOI: 10.1007/s00134-018-5203-z. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017; 43: 304–377. DOI: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015; 41: 1549–1560. DOI: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 4.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372: 1301–1311. DOI: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 5.Rowan KM, Angus DC, Bailey M, et al. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med 2017; 376: 2223–2234. DOI: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 6.Simpson N, Lamontagne F, Shankar-Hari M. Septic shock resuscitation in the first hour. Curr Opin Crit Care 2017; 23: 561–566. DOI: 10.1097/mcc.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 7.Mallat J, Reddi BJ. Do not abandon monitoring the central venous pressure during fluid resuscitation of septic shock patients. Intensive Care Med 2018; 44: 2012–2013. DOI: 10.1007/s00134-018-5387-2. [DOI] [PubMed] [Google Scholar]
- 8.Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014; 40: 1795–1815. DOI: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanji HD, McCallum J, Sirounis D, et al. Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care 2014; 29: 700–705. DOI: 10.1016/j.jcrc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Heiberg J, El-Ansary D, Canty DJ, et al. Focused echocardiography: a systematic review of diagnostic and clinical decision-making in anaesthesia and critical care. Anaesthesia 2016; 71: 1091–1100. DOI: 10.1111/anae.13525. [DOI] [PubMed] [Google Scholar]
- 11.Sekiguchi H, Harada Y, Villarraga HR, et al. Focused cardiac ultrasound in the early resuscitation of severe sepsis and septic shock: a prospective pilot study. J Anesth 2017; 31: 487–493. DOI: 10.1007/s00540-017-2312-8. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson PR, Milne J, Diegelmann L, et al. Does point-of-care ultrasonography improve clinical outcomes in emergency department patients with undifferentiated hypotension? An international randomized controlled trial from the SHoC-ED Investigators. Ann Emerg Med 2018; 72: 478–489. DOI: 10.1016/j.annemergmed.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–1256. DOI: 10.1097/01.ccm.0000050454.01978.3b. [DOI] [PubMed] [Google Scholar]
- 14.Perera P, Mailhot T, Riley D, et al. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically ill. Emerg Med Clin North Am 2010; 28: 29–56, vii. DOI: 10.1016/j.emc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Liu D, He H, et al. Using critical care chest ultrasonic examination in emergency consultation: a pilot study. Ultrasound Med Biol 2015; 41: 401–406. DOI: 10.1016/j.ultrasmedbio.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Xirouchaki N, Kondili E, Prinianakis G, et al. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med 2014; 40: 57–65. DOI: 10.1007/s00134-013-3133-3. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134: 117–125. DOI: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinsky MR. Hemodynamic evaluation and monitoring in the ICU. Chest 2007; 132: 2020–2029. DOI: 10.1378/chest.07-0073. [DOI] [PubMed] [Google Scholar]
- 19.Shankar-Hari M, Harrison DA, Rubenfeld GD, et al. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth 2017; 119: 626–636. DOI: 10.1093/bja/aex234. [DOI] [PubMed] [Google Scholar]
- 20.Baykara N, Akalin H, Arslantas MK, et al. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit Care 2018; 22: 93. DOI: 10.1186/s13054-018-2013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng M, McSparron JI, Kien DT, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 2018; 44: 884–892. DOI: 10.1007/s00134-018-5208-7. [DOI] [PubMed] [Google Scholar]
- 22.Bouferrache K, Amiel JB, Chimot L, et al. Initial resuscitation guided by the Surviving Sepsis Campaign recommendations and early echocardiographic assessment of hemodynamics in intensive care unit septic patients: a pilot study. Crit Care Med 2012; 40: 2821–2827. DOI: 10.1097/CCM.0b013e31825bc565. [DOI] [PubMed] [Google Scholar]
- 23.Benes J, Kirov M, Kuzkov V, et al. Fluid therapy: double-edged sword during critical care? Biomed Res Int 2015; 2015: 729075. DOI: 10.1155/2015/729075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallabhajosyula S, Pruthi S, Shah S, et al. Basic and advanced echocardiographic evaluation of myocardial dysfunction in sepsis and septic shock. Anaesth Intensive Care 2018; 46: 13–24. DOI: 10.1177/0310057x1804600104. [DOI] [PubMed] [Google Scholar]
- 25.Haydar SA, Moore ET, Higgins GL, 3rd, et al. Effect of bedside ultrasonography on the certainty of physician clinical decisionmaking for septic patients in the emergency department. Ann Emerg Med 2012; 60: 346–358.e344. DOI: 10.1016/j.annemergmed.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Yildizdas D, Aslan N. Ultrasonographic inferior vena cava collapsibility and distensibility indices for detecting the volume status of critically ill pediatric patients. J Ultrason 2020; 20: e205–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orso D, Paoli I, Piani T, et al. Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: a systematic review and meta-analysis. J Intensive Care Med 2020; 35: 354–363. [DOI] [PubMed] [Google Scholar]
- 28.Volpicelli G, Skurzak S, Boero E, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 2014; 121: 320–327. DOI: 10.1097/aln.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 29.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest 2009; 136: 102–109. DOI: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015; 147: 1659–1670. DOI: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 31.Lee CW, Kory PD, Arntfield RT. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care 2016; 31: 96–100. DOI: 10.1016/j.jcrc.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Wang XT, Liu DW, Zhang HM, et al. Integrated cardiopulmonary sonography: a useful tool for assessment of acute pulmonary edema in the intensive care unit. J Ultrasound Med 2014; 33: 1231–1239. DOI: 10.7863/ultra.33.7.1231. [DOI] [PubMed] [Google Scholar]
- 33.Aslan N, Yildizdas D, Horoz OO, et al. Comparison of cardiac output and cardiac index values measured by critical care echocardiography with the values measured by pulse index continuous cardiac output (PiCCO) in the pediatric intensive care unit: a preliminary study. Ital J Pediatr 2020; 46: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jozwiak M, Mercado P, Teboul JL, et al. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care 2019; 23: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanfilippo F, Corredor C, Fletcher N, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med 2015; 41: 1004–1013. DOI: 10.1007/s00134-015-3748-7. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez C, Begot E, Dalmay F, et al. Prognostic impact of left ventricular diastolic function in patients with septic shock. Ann Intensive Care 2016; 6: 36. DOI: 10.1186/s13613-016-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]