Introduction
Surveillance guidelines following polypectomy promote cost-effective reductions in future colorectal cancer (CRC) risk, but high non-adherence rates1 can have negative consequences on costs and effectiveness. Professional societies recommend a 3-year interval for patients with advanced colorectal polyps (ACPs), though relatively few studies report provider adherence to surveillance intervals.2 This study evaluated rates and predictors of guideline-discordant recommendations for patients with ACPS.
Methods
This retrospective cohort study included patients with removal of ≥1 ACP at University of Colorado Hospital from 6/2012–6/2017. Patients were excluded for: personal history of CRC, hereditary CRC syndrome, inflammatory bowel disease, life-limiting medical problem that impacted surveillance, poor prep, and age>85 years. An ACP was defined as 1)tubular adenoma (TA) or sessile serrated polyp (SSP) ≥10mm; 2)TA with villous histology or high-grade dysplasia, or SSP with dysplasia; 3) traditional serrated adenoma of any size.3
The surveillance interval captured was provided by the endoscopist after the pathology resulted. The primary outcome was the proportion of patients who received a surveillance interval recommendation discordant from the 2012 USMSTF guidelines and whether it was shorter or longer than recommended.2 Of note, the 2020 USMSTF guidelines provide the same recommendations (3 year interval) for advanced polyps.4 Multivariable logistic regression analyses that included available polyp and patient factors was performed. A sensitivity analysis on size of the largest polyp and total number of polyps was performed to determine the cutoffs in the multivariable models. Alpha level was 0.05 and all tests were two-tailed.
Results
This study included 1120 patients: 38.8% had 1–2 total polyps, 27.6% had ≥1 SSP, 24.2% had a histologically advanced polyp, 16.3% ≥1 polyp resected piecemeal, and 59.4% had all polyps ≤ 12mm. The median size of the largest polyp was 12mm, and this cutoff was used in analyses. The indications for colonoscopy were screening (52%), surveillance (26.8%), and diagnostic (21.2%).
Guideline-discordant surveillance intervals
There were 207 patients (18.5%) who received a guideline discordant surveillance interval. These patients were more likely to have had HGD (OR 2.50, 95%CI: 1.33–4.70), flat polyps (OR 1.54, 95%CI: 1.04–2.28), or all polyps ≤12mm (OR 1.64, 95%CI: 1.14–2.36). Patients with polyps removed piecemeal (OR 0.22, 95%CI: 0.11–0.44), with villous histology (OR 0.49, 95%CI: 0.28–0.84), and where a fellow was involved (OR 0.41, 95%CI 0.24–0.68) were less likely to receive a guideline-discordant surveillance interval. Patients had lower odds of receiving an inaccurate interval with increasing number of polyps. Sex, age, BMI, and bowel preparation quality were not associated with recommendation concordance.
Predictors of surveillance intervals longer than recommended
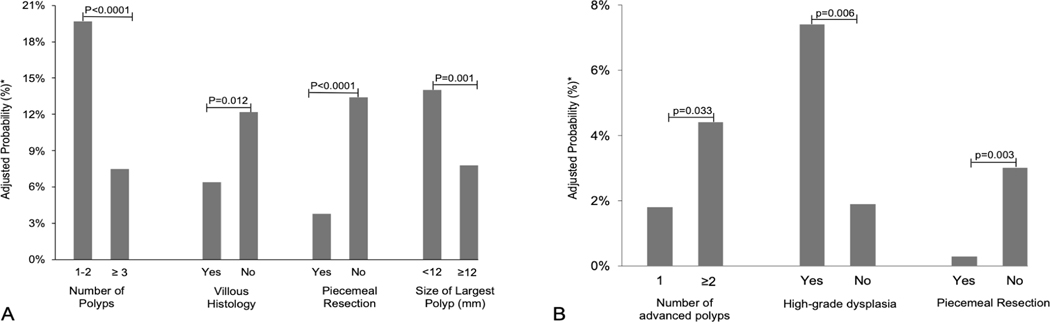
There were 175 patients (15.6%) who received an interval longer than recommended. Having ≤2 total polyps (OR 3.00; 95%CI: 2.11–4.25), all polyps ≤12mm (OR 1.91, 95%CI: 1.28–2.84), or a flat polyp (OR 1.65, 95%CI: 1.09–2.49) were predictive of an inappropriately long interval (Figure 1). Factors associated with lower odds of receiving an interval longer than recommended were villous histology (OR 0.49, 95%CI: 0.27–0.89), piecemeal resection (OR 0.25, 95%CI: 0.12–0.52), and fellow involvement in the procedure (OR 0.44, 95%CI: 0.25–0.77).
Figure 1. Adjusted probabilities for predictors of surveillance intervals longer (A*) and shorter (B**) than USMSTF recommendations.
*Adjusted for number of polyps, size of largest polyp, villous histology, high grade histology, morphology (flat, pedunculated), piecemeal resection, fellow involvement, & bowel preparation quality
**Adjusted for number of polyps, high-grade dysplasia, and piecemeal resection
Predictors of surveillance intervals shorter than recommended
There were 32 patients (2.8%) who received an interval shorter than recommended. The presence of multiple ACPs (OR 2.28, 95%CI: 1.01–5.18) or HGD (OR 4.33, 95%CI: 1.75–10.72) were associated with an inappropriately short surveillance interval (Figure 1).
Discussion
In the largest reported cohort of only patients with ACPs, gastroenterologists provided guideline discordant surveillance recommendations in 18% of patients. We identified novel predictors of this non-adherence, uniquely identifying that endoscopists appeared to be assessing factors related to overall polyp burden when making their recommendation. Specifically, patients with ≤2 total polyps and those whose largest polyp was ≤12mm were more likely to receive an interval longer than recommended (almost always 5 years), while those with ≥2 ACPs were more likely to receive an interval shorter than recommended. Additionally, HGD was associated with intervals that were too short, whereas the absence of advanced histology was associated with intervals that were too long. Compared to a recent meta-analysis,1 our rate of intervals that were too long was similar while the rate of intervals that were too short was lower.
The present study highlights the importance of guideline-discordant surveillance recommendations given the high future CRC risk in patients with an ACP5, 6 and surveillance colonoscopy as a risk reduction strategy.7–9 Additional strengths of our study include accounting for more lenient recommendations for polyps resected piecemeal (up to 12 months per guidelines, which may explain why our rate of non-adherence to interval guidelines was lower than other publications) and those with suboptimal (but not poor) bowel preparations. A limitation is that we cannot account for individual provider clinical judgment. This study identified novel predictors that can inform quality improvement initiatives to promote stronger guideline adherence. This is critical to optimize resource utilization, limit cost to society, and prevent CRC.
Sources of support:
JMK was supported in part by the NIH Gastrointestinal Diseases Training Grant (T32-DK007038)
Abbreviations:
- CRC
colorectal cancer
- ACP
advanced colorectal polyp
- AA
advanced adenoma
- ASP
advanced serrated polyp
- SSP
sessile serrated polyp
- TSA
traditional serrated adenoma
Footnotes
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Djinbachian R, Dube AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy 2019;51:673–683. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 3.Kolb JM, Molmenti CL, Patel SG, et al. Increased Risk of Colorectal Cancer Tied to Advanced Colorectal Polyps; An Untapped Opportunity to Screen First-Degree Relatives and Decrease Cancer Burden. American Journal of Gastroenterology 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Click B, Pinsky PF, Hickey T, et al. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. JAMA 2018;319:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song M, Emilsson L, Bozorg SR, et al. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study. The Lancet Gastroenterology & Hepatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. The Lancet Oncology 2017;18:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins EC, Wooldrage K, Stenson I, et al. Heterogeneity in colorectal cancer incidence among people recommended 3-yearly surveillance post-polypectomy: a validation study. Endoscopy 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross AJ, Robbins EC, Pack K, et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut 2020;69:1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]