Abstract
Purpose:
Retinal vascular occlusion is a leading cause of profound irreversible visual loss, but the understanding of the disease is insufficient. We systematically investigated the age, gender, and laterality at the onset of retinal artery occlusion (RAO) and retinal vein occlusion (RVO) in the IRIS® Registry (Intelligent Research in Sight).
Design:
A retrospective registry cohort.
Participants:
Retinal vascular occlusion cases participating in the IRIS Registry.
Methods:
All cases diagnosed as retinal vascular occlusion in the IRIS Registry between 2013 and 2017 were included. Cases with unspecified gender or laterality were excluded when conducting the relevant analyses. Cases were categorized based on diagnosis codes into RAO, with subtypes transient retinal artery occlusion (TRAO), partial retinal artery occlusion (PRAO), branch retinal artery occlusion (BRAO), and central retinal artery occlusion (CRAO), and into RVO, with subtypes venous engorgement (VE), branch retinal vein occlusion (BRVO), and central retinal vein occlusion (CRVO). Age was evaluated as a categorical variable (5-year increments). We investigated the association of age, gender, and laterality with the onset frequency of retinal vascular occlusion subtypes.
Main outcome measures:
The frequency of onset of RAO and RVO subtypes by age, gender and laterality.
Results:
A total of 1,251,476 retinal vascular occlusion cases were included, 23.8% of which were RAO, while 76.2% were RVO. 1,248,656 and 798,089 cases were selected for analysis relevant to gender and laterality, respectively. The onset frequency of all subtypes increased with age. PRAO, BRAO, CRAO, and CRVO presented more frequently in men (53.5%, 51.3%, 52.6%, 50.4%), while TRAO, VE, and BRVO presented more frequently in women (54.9%, 56.0%, 54.5%). BRVO and all RAO subtypes showed a right-eye onset preference (BRVO 51.0%, TRAO 51.7%, PRAO 54.4%, BRAO 53.5%, CRAO 53.4%), while VE and CRVO exhibited a left-eye onset preference (VE 53.3%, CRVO 50.9%).
Conclusions:
While retinal vascular occlusion incidence increases with age regardless of subtypes, we found various subtype-specific disease onset differences related to gender and, in particular, ocular laterality. These findings may improve understanding of the specific etiology of retinal vascular occlusions of different subtypes and their relationship with structural and anatomic asymmetries of the vascular system.
Keywords: retinal vascular occlusion, retinal artery occlusion, retinal vein occlusion, laterality, age, gender, big data, epidemiology, demographics
Introduction
Retinal vascular occlusion, one of the leading causes of profound irreversible visual loss, is comprised of a group of retinopathies characterized by blood flow blocked in arteries or veins. According to the vessels affected, retinal vascular occlusions can be categorized into retinal artery occlusion (RAO) and retinal vein occlusion (RVO). Collectively, retinal vascular occlusion (both RAO and RVO) is one of the most common causes of visual disability in the world’s middle-aged and elderly population.1,2
The American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) is an extensive, comprehensive clinical eye disease data registry.3,4 It consists of data from more than 349 million patient visits contributed by more than 18,000 contracted healthcare providers in the United States (as of September 1, 2020). The database was launched on March 24, 2014, and continues to grow each day.5 The IRIS Registry platform provides a large-scale glimpse into eye disease trends, features, and real-world information such as patients’ diagnoses, treatment choices, and outcomes.3–9 By compiling visit and surgical history from a diverse set of patients across the United States, the IRIS Registry dataset allows further study of existing clinical assumptions using a large dataset.
RAO cases in the IRIS Registry can be sub-classified according to diagnosis codes into central retinal artery occlusion (CRAO), branch retinal arterial occlusion (BRAO), partial retinal arterial occlusion (PRAO), and transient retinal arterial occlusion (TRAO). PRAO indicates the incomplete disruption of blood flow,12,13 and in clinical practice, TRAO is commonly referred to as transient ischemic attack. Published estimates suggest that the incidence rates are 1.64 per 100,000 person-years for CRAO, 4.99 per 100,000 person-years for BRAO, and 6.63 per 100,000 person-years for RAO.10,11
RVO cases in the IRIS Registry can be sub-classified according to diagnostic code into central retinal vein occlusion (CRVO), branch retinal vein occlusion (BRVO), and venous engorgement (VE). Epidemiological analyses reveal that the global prevalence of RVO, BRVO, and CRVO reached 28.06 million (0.77%), 23.38 million (0.64%), and 4.67 million (0.13%) respectively in people aged 30–89 in 2015, while the pooled five- and ten-year cumulative incidences of RVO were 0.86% and 1.63%, respectively.12 Compared to RAO, the incidence rate for RVO is higher, with 48.31 per 100,000 person-years.13 Clearly, the widespread prevalence of RVO is of global significance.
Previous studies have investigated the etiology, demographic characteristics, and risk factors of retinal vascular occlusion. The etiology of RAO is embolus, thrombus, and less commonly arteritis, leading to blockage of the central retinal artery or one of its branches; blockage can be permanent in the case of CRAO, BRAO, and PRAO or transient in the case of TRAO.14–17 Identified risk factors for RAO include hypertension,18,19 hypercholesterolemia,17,19 carotid artery disease,20,21 cardiac disease,16,22 heart attack history,18,23 stroke history,15,21 and smoking.17,24
The etiology of CRVO is thought to be occlusion of the central retinal vein at or proximal to the lamina cribrosa, due to thrombosis.25 Abnormalities in blood clotting or blood viscosity due to a variety of systemic diseases may contribute to CRVO in some cases.26–28 Known disease associations with CRVO are systemic arterial hypertension, diabetes mellitus, and open-angle glaucoma.29 Oral contraceptive and diuretic use have also been identified as risk factors.30 The etiology of BRVO is thought to be a thrombus at arteriovenous crossings. Risk factors for BRVO are systemic arterial hypertension, cardiovascular disease, a history of glaucoma31 and advanced age.32–34
Of note, some studies found that RVO34,35 and RAO36 occurred more frequently in men while others report no difference between men and women, and so gender predilection is still uncertain.23,32,37 Few studies have compared the eye laterality at the onset of any subtype of retinal vascular occlusion.
Knowledge of laterality differences in vascular occlusion will provide a more comprehensive understanding of these disease processes’ pathogenesis and enable further studies to advance the diagnosis and treatment. Structural and anatomic asymmetry of the aortic arch may explain a right-eye laterality preference for RAO. Emboli causing RAO can originate from plaques within the blood vessels, often the carotid arteries, or within the heart.16,17 The central retinal artery is a branch of the ophthalmic artery, which gets its blood supply from the common carotid artery. However, the origin of right and left common carotid arteries are different. The right common carotid artery is a branch of the brachiocephalic trunk, while the left common carotid artery originates directly from the aortic arch. The brachiocephalic trunk is the first orifice of the aortic arch. Thus, emboli in the blood that flows out of the left ventricle will reach the brachiocephalic trunk first. As a result, emboli may have a higher chance of entering the right common carotid artery through the brachiocephalic trunk than entering the left common carotid artery in cases when the embolus originates from the heart.38 A 416-patient retrospective study reported a tendency of predominance in men and right-eye onset in RAO patients, but the gender and laterality differences were not statistically significant.39 In this paper, we report our analyses of the vast amount of data available in the IRIS Registry to examine gender and laterality in all subtypes of vascular occlusion. We aim to investigate previously held but unproven assumptions about the pathophysiology of vascular occlusion, specifically the effects of gender, age, and laterality on disease onset.
Methods
Data source
The IRIS Registry, as described above, was used as the data source in this analysis. The version published by the IRIS Registry on 07/26/2019 was accessed on 12/12/2019 and used to select cases for this study. The IRIS Registry is maintained by the American Academy of Ophthalmology and contains integrated data from numerous different electronic health record (EHR) systems provided by contracted healthcare providers.40 Patients’ information is de-identified during the data collection procedure, and only fully de-identified data were used in our study. As such, researchers have no access to identifying information for any particular patient. According to Partners Human Research Committee (PHRC) Policy Definition of Human-Subjects Research, the institutional review board of Massachusetts Eye and Ear classified this study as exempt because data were de-identified, which implied that the patients’ informed consent to this study was not necessary. This study adhered to the Declaration of Helsinki
Selection criteria and measurements
All patients with records between January 1, 2013, and December 31, 2017, in the IRIS Registry, who were diagnosed with TRAO, PRAO, BRAO, CRAO, VE, BRVO, and CRVO on the first visit, were included in the data analyses.
Diagnoses were stored as International Classification of Diseases, 9th revision and 10th revision (ICD-9 and ICD-10) codes in the IRIS Registry. They were identified and paired according to the description. That is, cases with codes H34.0*/362.34 were identified as TRAO, with codes H34.21*/362.33 as PRAO, with codes H34.23*/362.32 as BRAO, with codes H34.1*/362.31 as CRAO, with codes H34.82*/362.37 as VE, with codes H34.83*/362.36 as BRVO, and with codes H34.81*/362.35 as CRVO, in ICD-10/ICD-9 codes respectively, where an asterisk, “*”, indicates any number of any digits that may follow in the code from that point onward, i.e., it includes all possible “subcodes” of the respective base code.
We determined laterality for our analysis by integrating documentation from the IRIS Registry EHR-based laterality variable along with laterality based on diagnosis codes (if specified). We classified vascular occlusions as “bilateral” onset either when “bilateral” was specified in the diagnosis or when the disease was diagnosed separately for the right and left eye within the same calendar year of onset. This definition was based on the granularity of date only to year in the IRIS version used for this analysis. Our data analysis combined two diagnostic systems (ICD-9 and ICD-10 codes). While ICD-9 codes do not consider laterality, ICD-10 codes allow for the decoding of laterality within the diagnostic code. As this could have introduced different tendencies in reporting vs. not reporting laterality between the two systems, we additionally performed our main data analyses and compared the results in ICD-10 and ICD-9 codes separately and found little difference (SI Table 1–2, supplementary files are available at www.aaojournal.org), which supported our use of data from cases based on both ICD-10 and ICD-9 codes.
The study focused on the age, gender (men and women), and laterality (right eye, left eye, and both eyes) at the onset of the disease. There are two dates relevant to clinical diagnoses in the IRIS Registry. “Documentation year” specifies that a diagnosis was documented in the electronic medical record of the respective year. Typically, from the first time of the diagnosis forward, the diseases were reported every year in the database. “Onset year” explicitly refers to the year of the first diagnosis. While the “onset year” entry was not always available, each diagnostic entry contained at least one documentation year, and the first documentation year of disease is supposed to coincide with the disease onset year. Cases where the onset year was later than the documentation year were excluded. If an onset year was explicitly reported, it was selected for data analysis. If no onset year for the respective eye was explicitly specified, we selected the earliest documentation year as the onset year. Age was defined as the time between onset year/documentation year and year of birth. Age was categorized into five-year intervals from age 0 to age 84 and ≥ 85 when cases were 85 years old or older. Gender was categorized in the IRIS Registry as male, female, and unspecified. The entire set of IRIS Registry diagnoses included eye-related codes within ICD-9 and ICD-10.
Patients with missing or unspecified subtype diagnosis information (3.0%) were excluded from the data analysis. Cases without specified gender (0.2%) or laterality (35.1%) information were excluded when the relevant analyses of gender or laterality were conducted. Details of the exclusion process are presented in Figure 1. The baseline characteristics and the comparisons of selected and excluded cases are described in the supplementary material.
Figure 1. Selection of cases for analysis.

The number of cases retrieved from the entire Intelligent Research in Sight (IRIS) Registry database and included for analyses. The percentages of excluded cases were based on the 1,289,836 cases diagnosed with retinal vascular occlusion.
Statistical analysis
The overall comparisons of interest were: the association of onset frequency and age, the association of onset frequency and gender, the correlation between gender and age, the association of onset frequency and laterality, and the correlation between laterality and age. We present all parameters with both the number of cases and corresponding percentages. We use a Spearman test to test the correlation between onset and age, and between onset laterality and age. We use a binomial test to compare the onset proportion by gender (null hypothesis: the proportion of each gender was 0.5), unilateral onset proportion (null hypothesis: the proportion of unilateral onset of each subtype was that of the entire set of IRIS Registry), and onset proportion by laterality (only for unilateral onset cases, null hypothesis: the proportion of right-eye or left-eye onset was 0.5). We use a Z test to compare the proportion differences between men and women, between unilateral and bilateral onset, and between right-eye and left-eye onset. All procedures were performed in R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.01 was considered statistically significant. A 99% confidence interval (99% CI) and the odds ratio (OR) were given.
Results
Baseline Description
A total of 1,289,836 cases of retinal vascular occlusion identified were included from the entire set of IRIS Registry diagnoses (299,385,279 cases with ophthalmic diseases) as of December 31, 2017. We defined the date of onset by the date of the first visit at which a new diagnosis was given. The prevalence of retinal vascular occlusion among the entire set of IRIS Registry diagnoses, as defined by the date of onset, was 4.31%. According to the inclusion and exclusion criteria, after exclusions of 38,360 cases without specified subtype diagnosis, 1,251,476 cases of retinal vascular occlusion remained for analysis (Figure 1). The baseline characteristics overall and for each retinal vascular occlusion subtype are described in Table 1. According to their diagnosis, patients were classified into seven subtypes, with subtype frequency ranging from 21,075 for VE to 573,880 for BRVO. RAO cases made up 23.8% of retinal vascular occlusion cases (4.9% for TRAO, 4.5% for PRAO, 8.3% for BRAO, and 6.1% for CRAO), while RVO composed 76.2% of cases (1.7% for VE, 45.9% for BRVO, 28.6% for CRVO). The population distribution according to age intervals was presented in Figure 2 (overall view of the entire set of IRIS diagnoses, retinal vascular occlusion, RAO, and RVO), Figure 3 (subtypes of RAO), and Figure 4 (subtypes of RVO). As the gender and laterality information of some cases was unspecified, we excluded those cases when analyzing gender and laterality. The number of cases selected and excluded and exclusion criteria at each step are described in Figure 1. The baseline characteristics of the selected cases are described before each analysis.
Table 1.
The distribution of baseline characteristics of cases of retinal vascular occlusion selected for analysis.
| RAO | RAO | RVO | RVO | Retinal vascular occlusion | Entire set of IRIS Registry diagnoses | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TRAO | PRAO | BRAO | CRAO | VE | BRVO | CRVO | |||||
| n (%a) |
61,241 (4.9) |
56,133 (4.5) |
103,942 (8.3) |
76,268 (6.1) |
297,584 (23.8) |
21,075 (1.7) |
573,880 (45.9) |
358,937 (28.6) |
953,892 (76.2) |
1,251,476 (100.0) |
299,385,279 |
| Age | |||||||||||
| 0–25 (%b) |
1,230 (2.0) |
160 (0.3) |
784 (0.8) |
567 (0.7) |
2,741 (0.9) |
288 (1.4) |
1,405 (0.3) |
1,725 (0.5) |
3,418 (0.4) |
6,159 (0.5) |
22,267,552 (7.4) |
| 25–45 (%b) |
3,701 (6.0) |
921 (1.6) |
3,987 (3.8) |
2,219 (2.9) |
10,828 (3.7) |
1,164 (5.5) |
10,605 (1.8) |
11,433 (3.2) |
23,202 (2.4) |
34,030 (2.7) |
24,628,180 (8.2) |
| 45–65 (%b) |
17,956 (29.3) |
12,478 (22.2) |
26,140 (25.1) |
17,587 (23.1) |
74,161 (24.9) |
6,944 (32.9) |
138,374 (24.1) |
84,738 (23.6) |
230,056 (24.1) |
304,217 (24.3) |
96,890,436 (32.4) |
| 65–85 (%b) |
37,715 (61.6) |
41,505 (74.0) |
71,292 (68.6) |
54,282 (71.2) |
204,794 (68.8) |
12,490 (59.3) |
410,171 (71.5) |
251,985 (70.2) |
674,646 (70.7) |
879,440 (70.3) |
152,780,390 (51.0) |
| ≥85 (%b) |
639 (1.1) |
1,069 (1.9) |
1,739 (1.7) |
1,613 (2.1) |
5,060 (1.7) |
189 (0.9) |
13,325 (2.3) |
9,056 (2.5) |
22,570 (2.4) |
27,630 (2.2) |
2,818,721 (1.0) |
| Gender | |||||||||||
| Men (%b) |
27,550 (45.0) |
29,964 (53.4) |
53,217 (51.2) |
40,036 (52.5) |
150,767 (50.7) |
9,256 (43.9) |
260,796 (45.4) |
180,570 (50.3) |
450,622 (47.3) |
601,389 (48.1) |
123,672,549 (41.3) |
| Women (%b) |
33,539 (54.8) |
26,063 (46.4) |
50,492 (48.6) |
36,026 (47.2) |
146,120 (49.1) |
11,788 (55.9) |
311,885 (54.4) |
177,474 (49.4) |
501,147 (52.5) |
647,267 (51.7) |
175,061,945 (58.5) |
| Unspecified (%b) |
152 (0.2) |
106 (0.2) |
233 (0.2) |
206 (0.3) |
697 (0.2) |
31 (0.2) |
1,199 (0.2) |
893 (0.3) |
2,123 (0.2) |
2,820 (0.2) |
650,785 (0.2) |
| Laterality | |||||||||||
| Right (%b) |
7,132 (11.6) |
17,672 (31.5) |
30,764 (29.6) |
23,849 (31.3) |
79,417 (26.7) |
2,239 (10.6) |
177,934 (31.0) |
107,859 (30.0) |
288,032 (30.2) |
367,449 (29.4) |
23,753,144 (7.9) |
| Left (%b) |
6,661 (10.9) |
14,839 (26.4) |
26,698 (25.7) |
20,773 (27.2) |
68,971 (23.2) |
2,556 (12.1) |
171,206 (29.8) |
111,939 (31.2) |
285,701 (29.9) |
354,672 (28.3) |
20,907,839 (7.0) |
| Both (%b) |
5,220 (8.5) |
2,023 (3.6) |
4,175 (4.0) |
2,959 (3.9) |
14,377 (4.8) |
7,416 (35.2) |
31,658 (5.5) |
22,517 (6.3) |
61,591 (6.5) |
75,968 (6.1) |
93,568,896 (31.3) |
| Unspecified (%b) |
42,228 (69.0) |
21,599 (38.5) |
42,305 (40.7) |
28,687 (37.6) |
134,819 (45.3) |
8,864 (42.1) |
193,082 (33.7) |
116,622 (32.5) |
318,568 (33.4) |
453,387 (36.2) |
161,155,400 (53.8) |
The proportion of each subtype cases in the selected retinal vascular occlusion population.
The proportion of age/gender/laterality group cases in the population of the corresponding diagnosis.
Abbr. IRIS Registry: Intelligent Research in Sight Registry; RAO: Retinal artery occlusion; TRAO: Transient retinal artery occlusion; PRAO: Partial retinal artery occlusion; BRAO: Branch retinal artery occlusion; CRAO: Central retinal artery occlusion; RVO: Retinal vein occlusion; VE: Venous engorgement; BRVO: Branch retinal vein occlusion; CRVO: Central retinal vein occlusion.
Figure 2. Distribution of the number of included cases by age, gender, and laterality at onset.

Distribution of numbers of cases by age, gender and laterality at first diagnosis. The numbers of men, women, and unspecified gender cases are presented inline in each age category. Each bar shows the cumulative case numbers of right, left, both, and unspecified eye at onset. A. The entire set of Intelligent Research in Sight (IRIS) Registry diagnoses (y-axis 200 times larger than other plots). B. Diagnoses of retinal vascular occlusions. C. Diagnoses of retinal artery occlusions. D. Diagnoses of retinal vein occlusions.
Figure 3. Distribution of the number of included cases of retinal artery occlusion by subtype, age, gender, and laterality at onset.

Distribution of numbers of cases by age and gender at first diagnosis. The numbers of men, women, and unspecified gender cases are presented inline in each age category. Each bar shows the cumulative case number of right, left, both, and unspecified eye at onset. A. Transient retinal artery occlusion. B. Partial retinal artery occlusion. C. Branch retinal artery occlusion. D. Central retinal artery occlusion.
Figure 4. Distribution of the number of included cases of retinal vein occlusion by subtype, age, gender, and laterality at onset.
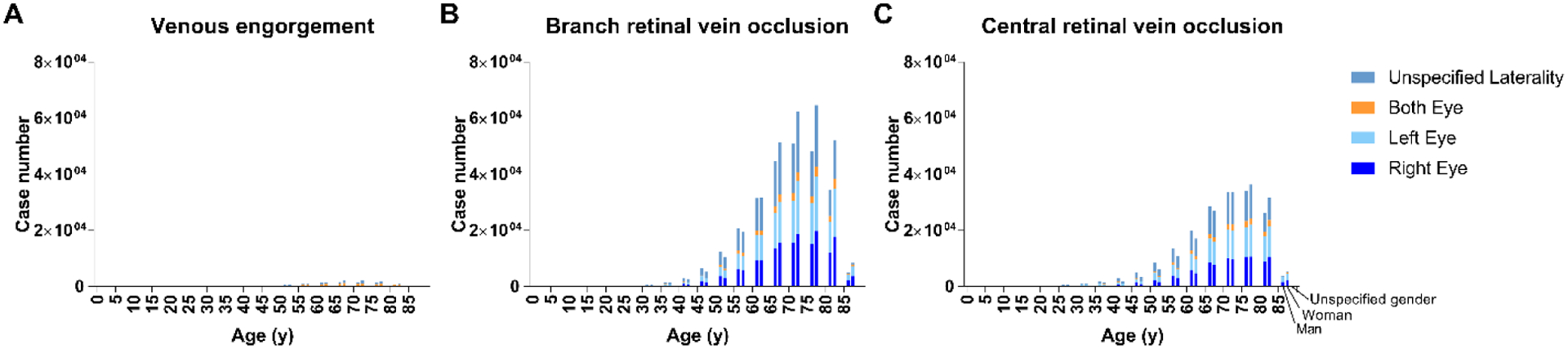
Distribution of numbers of cases by age and gender at first diagnosis. The numbers of men, women, and unspecified gender cases are presented inline in each age category. Each bar shows the cumulative case number of right, left, both, and unspecified eye at onset. A. Venous engorgement. B. Branch retinal vein occlusion. C. Central retinal vein occlusion.
Association of Age with Retinal Vascular Occlusion Onset
For the total included retinal vascular occlusion population, the frequency of onset rose with increasing age between 0 to 84 years old (Table 1). Only 0.5% (0.3% to 2.0% by subtype) of cases were less than 25 years old, 2.7% (1.6% to 6.0% by subtype) of cases were between 25 and 45 years old, and 24.3% (22.2% to 32.9% by subtype) of cases were between 45 and 65 years old. Patients aged between 65 and 85 years old composed 70.3% (59.3% to 74.0% by subtype) of cases, which were the largest component. The percentage of patients equal to or above 85 years old was 2.2% (0.9% to 2.5% by subtype) due to the low enrollment of the very elderly patients in the IRIS Registry platform; the same percentage in the entire set of IRIS Registry diagnoses was 1.0%. The Spearman correlation coefficients and 99% CI for the onset of each subtype with age are given in SI Table 3; they range from 0.82 to 0.93 by subtype and demonstrate high positive correlations between subtypes of retinal vascular occlusion and age of onset; the correlation of the entire set of IRIS Registry diagnosis with age is 0.64.
Association of Gender with Retinal Vascular Occlusion Onset
A total of 1,248,656 cases were selected for gender-related analysis after 2,820 (0.2%) cases without specified gender were excluded. The baseline characteristics and comparison of selected and excluded cases were presented in SI Table 4.
The percentages of men and women among selected retinal vascular occlusion cases were 48.2% and 51.8%, respectively. PRAO, BRAO, CRAO, CRVO were more frequent among men, while TRAO, VE, and BRVO were more frequent among women. The frequency differences between men and women in each subtype were 7.0%, 2.6%, 5.3%, 0.9% for PRAO, BRAO, CRAO, CRVO, and −9.8%, −12.0%, −8.9% for TRAO, VE, BRVO (Table 2, p<0.0001 for each subtype).
Table 2.
Distribution of selected cases of retinal vascular occlusion in analysis cohort by gender and subtype together with estimates of the precision of differences and associations.
| Number of cases | % Mena | % Women | % Men – % women | 99% CI of % men – % womenb | ORc | 99% CI of ORc | |
|---|---|---|---|---|---|---|---|
| Retinal vascular occlusion | 1,248,656 | 48.2 | 51.8 | −3.7 | (−3.84, −3.51) | 0.93 | (0.93, 0.93) |
| RAO | 296,887 | 50.8 | 49.2 | 1.6 | (1.23, 1.90) | 1.03 | (1.02, 1.04) |
| TRAO | 61,089 | 45.1 | 54.9 | −9.8 | (−10.54, −9.07) | 0.82 | (0.80, 0.84) |
| PRAO | 56,027 | 53.5 | 46.5 | 7.0 | (6.19, 7.73) | 1.15 | (1.13, 1.18) |
| BRAO | 103,709 | 51.3 | 48.7 | 2.6 | (2.06, 3.19) | 1.05 | (1.04, 1.07) |
| CRAO | 76,062 | 52.6 | 47.4 | 5.3 | (4.61, 5.93) | 1.11 | (1.09, 1.13) |
| RVO | 951,769 | 47.3 | 52.7 | −5.3 | (−5.50, −5.12) | 0.90 | (0.89, 0.90) |
| VE | 21,044 | 44.0 | 56.0 | −12.0 | (−13.28, −10.78) | 0.79 | (0.76, 0.81) |
| BRVO | 572,681 | 45.5 | 54.5 | −8.9 | (−9.16, −8.68) | 0.84 | (0.83, 0.84) |
| CRVO | 358,044 | 50.4 | 49.6 | 0.9 | (0.56, 1.17) | 1.02 | (1.01, 1.02) |
H0 hypothesis states that the proportion of men in each subtype is 50.0%. Two-tailed p<0.0001 for all subtypes using the binomial test.
H0 hypothesis states that the proportions of men and women in each subtype are the same. Two-tailed p<0.0001 for all subtypes using the Z test.
The OR and 99% CI of OR when setting the population of the entire IRIS Registry diagnoses as control with a percentage of men as 50.0%.
Abbr. CI: confidence interval; OR: odds ratio; RAO: Retinal artery occlusion; TRAO: Transient retinal artery occlusion; PRAO: Partial retinal artery occlusion; BRAO: Branch retinal artery occlusion; CRAO: Central retinal artery occlusion; RVO: Retinal vein occlusion; VE: Venous engorgement; BRVO: Branch retinal vein occlusion; CRVO: Central retinal vein occlusion.
The spearman correlation of percentages of men with age was calculated to investigate the association of age and gender differences (SI Table 5). Only the gender distribution of RAO was slightly associated with age. The trends in the gender distribution of other subtypes (TRAO, PRAO, BRAO, CRAO, RVO, VE, BRVO, and CRVO) were not significantly correlated with the age category. Details are presented for the proportion of men and women with each subtype across 5-year age categories (SI Table 6). The frequency of all vascular occlusion subtypes increased with age, overall and separately for men and women.
Association of Laterality with Retinal Vascular Occlusion Onset
For analyses relevant to laterality, we excluded 453,387 cases without specified laterality information. Thus, 798,089 cases were selected. The baseline characteristics and comparison of the cases with/without specified laterality were presented in SI Table 7.
Unilateral onset was significantly more frequent among retinal vascular occlusion cases (90.5%) than among the entire set of all IRIS Registry diagnoses (32.3%). The same trend was observed in each subtype (P<0.0001 for each subtype with 99% CI given in Table 3). As for the association of age and laterality preference by subtype, the Spearman test showed a positive correlation between unilateral onset with age in all subtypes except PRAO and VE, which displayed no significant correlation (SI Table 8 and the details in SI Table 9). The Spearman correlation coefficients ranged from 0.66 to 0.97.
Table 3.
Distribution of selected cases of retinal vascular occlusion in analysis cohort by unilateral/bilateral onset and subtype together with estimates of the precision of differences and associations.
| Number of cases with specified laterality | % Unilateral onseta | % Bilateral onset | % Unilateral onset – % bilateral onset | 99% CI of % unilateral onset – % bilateral onsetb | ORc | 99% CI of ORc | ||
|---|---|---|---|---|---|---|---|---|
| Entire set of IRIS Registry diagnoses | 138,229,879 | 32.3 | 67.7 | −35.4 | ||||
| Retinal vascular occlusion | 798,089 | 90.5 | 9.5 | 81 | (80.84, 81.08) | 19.92 | (19.77, 20.16) | |
| RAO | 162,765 | 91.2 | 8.8 | 82.3 | (82.08, 82.59) | 21.62 | (21.24, 22.22) | |
| TRAO | 19,013 | 72.5 | 27.5 | 45.1 | (43.91, 46.27) | 5.54 | (5.30, 5.76) | |
| PRAO | 34,534 | 94.1 | 5.9 | 88.3 | (87.82, 88.75) | 33.67 | (31.52, 35.45) | |
| BRAO | 61,637 | 93.2 | 6.8 | 86.5 | (86.08, 86.82) | 28.84 | (27.57, 29.94) | |
| CRAO | 47,581 | 93.8 | 6.2 | 87.6 | (87.16, 87.97) | 31.59 | (30.19, 33.3) | |
| RVO | 635,324 | 90.3 | 9.7 | 80.6 | (80.48, 80.75) | 19.52 | (19.30, 19.73) | |
| VE | 12,211 | 39.3 | 60.7 | −21.5 | (−23.08, −19.85) | 1.35 | (1.29, 1.42) | |
| BRVO | 380,798 | 91.7 | 8.3 | 83.4 | (83.21, 83.54) | 23.11 | (22.81, 23.51) | |
| CRVO | 242,315 | 90.7 | 9.3 | 81.4 | (81.20, 81.63) | 20.45 | (20.08, 20.81) | |
H0 hypothesis states that the proportion of unilateral onset cases in each subtype is 32.3% (the proportion of that in the entire set of IRIS Registry diagnoses). Two-tailed p<0.0001 for all subtypes using the binomial test.
H0 hypothesis states that the proportions of unilateral and bilateral onset cases in each subtype are the same. Two-tailed p<0.0001 for all subtypes using the Z test.
The OR and 99% CI of OR when setting the population of the entire IRIS Registry diagnoses as control.
Abbr. CI: confidence interval; OR: odds ratio; RAO: Retinal artery occlusion; TRAO: Transient retinal artery occlusion; PRAO: Partial retinal artery occlusion; BRAO: Branch retinal artery occlusion; CRAO: Central retinal artery occlusion; RVO: Retinal vein occlusion; VE: Venous engorgement; BRVO: Branch retinal vein occlusion; CRVO: Central retinal vein occlusion.
We examined the right-eye and left-eye differences among unilateral onset cases in each subtype of retinal vascular occlusion (Table 4). For BRVO and all four subtypes of RAO, right-eye onset was more frequent than left-eye onset. For VE and CRVO, onset was more frequent in the left eye than the right eye (p < 0.0001 for each of these seven subtypes). To investigate the association of age and right eye versus left eye onset by subtype, we calculated the percentages with right-eye onset among men, women, and cases with unspecified gender by 5-year age intervals (SI Figure 1). The Spearman correlation coefficients ranged from −0.03 to 0.57 between right-eye onset frequency and age, which did not suggest a meaningful correlation (SI Table 8).
Table 4.
Distribution of selected unilateral cases of retinal vascular occlusion in analysis cohort by right-eye/left-eye onset and subtype together with estimates of the precision of differences and associations.
| Number of unilateral onset cases | % Right-eyea | % Left-eye | % Right-eye – % left-eye | 99% CI of % right-eye – % left-eyeb | ORc | 99% CI of ORc | ||
|---|---|---|---|---|---|---|---|---|
| Retinal vascular occlusion | 722,121 | 50.9 | 49.1 | 1.8 | (1.55, 1.98) | 1.04 | (1.03, 1.04) | |
| RAO | 148,388 | 53.5 | 46.5 | 7 | (6.57, 7.51) | 1.15 | (1.14, 1.17) | |
| TRAO | 13,793 | 51.7 | 48.3 | 3.4 | (1.86, 4.97) | 1.07 | (1.02, 1.12) | |
| PRAO | 32,511 | 54.4 | 45.6 | 8.7 | (7.70, 9.72) | 1.19 | (1.16, 1.23) | |
| BRAO | 57,462 | 53.5 | 46.5 | 7.1 | (6.32, 7.84) | 1.15 | (1.13, 1.18) | |
| CRAO | 44,622 | 53.4 | 46.6 | 6.9 | (6.03, 7.76) | 1.15 | (1.12, 1.17) | |
| RVO | 573,733 | 50.2 | 49.8 | 0.4 | (0.17, 0.65) | 1.01 | (1.00, 1.01) | |
| VE | 4,795 | 46.7 | 53.3 | −6.6 | (−9.26, −3.97) | 0.88 | (0.81, 0.94) | |
| BRVO | 349,140 | 51.0 | 49 | 1.9 | (1.62, 2.24) | 1.04 | (1.03, 1.05) | |
| CRVO | 219,798 | 49.1 | 50.9 | −1.9 | (−2.25, −1.47) | 0.96 | (0.95, 0.98) | |
H0 hypothesis states that the proportion of right-eye onset cases in each subtype is 50.0%. Two-tailed p<0.0001 for all subtypes except RVO (p<0.01) using binomial test.
H0 hypothesis states that the proportions of right-eye and left-eye onset cases in each subtype are the same. Two-tailed p<0.0001 for all subtypes using Z test.
The OR and 99% CI of OR when setting the population of the entire IRIS Registry diagnoses as control with a percentage of right-eye onset as 50.0%.
Abbr. CI: confidence interval; OR: odds ratio; RAO: Retinal artery occlusion; TRAO: Transient retinal artery occlusion; PRAO: Partial retinal artery occlusion; BRAO: Branch retinal artery occlusion; CRAO: Central retinal artery occlusion; RVO: Retinal vein occlusion; VE: Venous engorgement; BRVO: Branch retinal vein occlusion; CRVO: Central retinal vein occlusion.
Discussion
In this study, we used the IRIS Registry, a large national database. In accordance with previous publications,10,11,13 there was a significantly higher rate of venous occlusions than arterial occlusions in this dataset. We observed a positive correlation between onset frequency and age in each subtype. The onset differences related to gender and laterality were heterogeneous in each subtype.
Age is a well-known risk factor of retinal vascular occlusion; disease onset increased with age for all subtypes of retinal vascular occlusion (SI Table 3 and Figure 2–4).32 This trend may be due to worsening cardiovascular disease with age;18,30 unfortunately, we do not currently have access to co-morbidities in the IRIS Registry, but this area warrants future study.
Previous studies have suggested a predominance for RVO and RAO in men.34–36 In our study, there were slightly more women in TRAO, VE, and BRVO, and more men in PRAO, BRAO, CRAO, and CRVO (Table 2). The gender differences in all subtypes were significant (all p values < 0.0001). Past studies have suggested that venous thromboembolism occurs more commonly in women under age 55 because of pregnancy, postpartum status, and oral contraceptive medication. But in older populations, cases among men tend to be more common, perhaps due to cardiovascular risk factors.41,42 We observed this trend (SI Table 6) even though we found no significant correlation between age and percentages of men (SI Table 5). The proportion of women trended up in older age categories, which may be related to the longevity of women.43 Again, information on medication use and co-morbidities are not currently available in the IRIS Registry, but when added, will further elucidate the relationships between retinal vascular occlusion and gender.
Unilateral onset was more common than bilateral onset in all retinal vascular occlusion subtypes, except for VE. Of note, all subtypes, including VE, had a significantly higher unilateral onset rate than the entire set of IRIS Registry (Table 3). Given the pathophysiology of all arterial occlusions, BRVO, and CRVO, it is not surprising that a unilateral onset is most frequent. By comparison, the VE more commonly presented bilaterally (VE 60.1% vs. retinal vascular occlusion 9.5% for bilateral presentation). As VE indicates the incipient or partial occlusion of retinal veins, which could be asymptomatic and latent, doctors may be inclined to recognize the structural changes in both eyes and make a bilateral diagnosis simultaneously. Bilateral presentation is consistent with the known etiology of VE as a manifestation of systemic diseases, such as hyperviscosity syndrome.44
Eye laterality onset difference in retinal vascular occlusion has not been comprehensively analyzed in the past. Our analyses demonstrate a left-eye preference in most subtypes of RVO except BRVO, as well as a right-eye preference in BRVO and all four subtypes of RAO (Table 4). To our knowledge, our study is the first to demonstrate a laterality deviation in all subtypes of retinal vascular occlusion. Arterial occlusions are frequently caused by emboli, which can arise from blood vessel walls or cardiac valves. Given the different anatomy of the vasculature leading to the central retinal arteries,38 there is a pathophysiologic reason to expect that arterial occlusions would present more commonly in the right eye, as the emboli from the heart would be more likely to block the right central retinal artery, in agreement with our findings of a right-eye preference for all arterial occlusions. Of course, it is essential to consider alternative explanations for this observation; in particular, most people are right-eye dominant, leading to reporting bias of right-eye disease. If right-eye bias were the explanation, one would expect it to be apparent for venous occlusion as well, but it was not.
Although there was a statistically significant left-eye preference for BRVO and right-eye preference for CRVO, there is no clear pathologic explanation for this difference. Thrombus formation would be expected to develop unilaterally but in each eye with a similar frequency. Notably, the right-eye preference of BRVO and the left-eye preference of CRVO were only 51.0% and 50.9%, respectively. By comparison, other etiologies demonstrated an approximately 3–8% difference in preference between right and left eye. This slight difference for venous occlusion may not be clinically significant.
It is known that the embolic etiology of stroke (cardiac versus vascular) differs among age groups.45 We observed a positive correlation between age and unilateral onset in all subtypes except PRAO and VE (SI Table 8 and 9). Younger patients may have particular risk factors, such as coagulation abnormalities, leading to the early onset of retinal vascular occlusion in both eyes.37 However, we found no correlation between age and right-eye onset in all subtypes of vascular occlusion, suggesting that if the vascular anatomy does account for the laterality difference in arterial occlusion onset overall, that right-eye and left-eye difference does not change with age.
There were some limitations to this study. The results rely on accurate coding of ICD-9/ICD-10 diagnoses, documented onset age, gender, and onset laterality. Patients visiting different institutions could cause duplication of diagnoses. However, the ICD codes are related to the insurance reimbursement claims and of high-level accuracy. As we only analyzed the principal diagnosis, most cases included were unique. Based on the large number of cases, the imprecision in coding and documentation can be slight and neglectable.
The IRIS Registry platform aggregates the collection, arrangement, and de-identification of EHR information from more than 18,000 healthcare providers. Due to the size of the Registry, there is a delay in the release of data. At the time of the data analysis, we only had access to the 2017 version of the database and could not include later cases.
In addition, we were unable to associate systemic measurements or other co-morbidities with diagnosis due to the current limitations of the IRIS Registry data set. An additional analysis that combines systemic and ophthalmic disease would be beneficial for a better understanding of retinal vascular occlusions. Similarly, medication information was not available; future analysis using medication data would further inform our findings.
Another potential limitation arises from the large number of cases with unreported eye laterality. It was the most frequent cause for excluding cases from our laterality-relevant analyses (SI Table 7). The cases with unspecified laterality accounted for 53.8% of cases in the entire set of IRIS Registry, and 36.2% of cases in retinal vascular occlusive diseases, which was lower than that in the entire set of IRIS Registry except for TRAO. As TRAO is a temporary ischemic attack, patients could have difficulty recalling the onset situation, which would result in a high proportion of unspecified laterality information. Considering our study purpose, it was imperative for us to know the laterality for each patient for accurate estimation of the association of laterality with retinal vascular occlusion onset. Therefore, the necessary exclusion of all unspecified laterality cases led to a one-third reduction in the number of cases. The baseline characteristics of excluded cases are described in SI Table 7. For analyses related to age and gender, we kept those cases and included laterality distribution in baseline descriptions (Table 1 and SI Table 4). Our future efforts aim to prompt doctors to keep thorough records in clinical practice and ensure accurate estimation of the laterality of diseases. Exact laterality information would help mitigate this issue and allow for larger sample sizes for laterality-relevant analysis.
In another limitation, TRAO and PRAO are pathologies that may not be well defined, and these diagnoses may not be objectively confirmed by testing like fluorescein angiography. Instead, this terminology is used variably, and diagnoses may be listed based on symptomatology instead of exam findings. As demonstrated, laterality data was lower for TRAO than overall IRIS Registry diagnoses, which may further support the uncertainty of this diagnosis. However, TRAO and PRAO did account for 4.9% and 4.5% of vascular occlusion diagnoses in the data set. 4.9% and 4.5% of those included in the gender-relevant analysis; 2.4% and 4.3% of those included in the laterality-relevant analysis, respectively. Given that these diagnoses were therefore not rare, it seemed important to include them in this overall analysis of vascular occlusion, but inclusion may be a limitation of the paper if the diagnoses were not certain.
The strength of our analysis is the large population size that represents a diverse array of patients from across the United States. Thus, we conclude that we have provided valuable information about the associates of age, gender, and laterality effects on the onset of retinal vascular occlusion across all subtypes. This study indicates the differences in these fields and can serve as a direction and foundation for future research. More targeted investigation and experiments should be conducted worldwide to understand better the association of demographics and laterality with retinal vascular occlusion.
Supplementary Material
Acknowledgments
We acknowledge Mengyu Wang and Raymond C. S. Wong for assistance in data collection and analysis. We acknowledge the IRIS Registry team for the clinical data collection and platform construction.
Financial Support:
The authors had no financial support for the preparation of this article.
Abbreviations and Acronyms:
- RAO
retinal artery occlusion
- RVO
retinal vein occlusion
- IRIS Registry
Intelligent Research in Sight Registry
- TRAO
transient retinal artery occlusion
- PRAO
partial retinal artery occlusion
- BRAO
branch retinal artery occlusion
- CRAO
central retinal artery occlusion
- VE
venous engorgement
- BRVO
branch retinal vein occlusion
- CRVO
central retinal vein occlusion
- EHR
electronic health record
- HIPAA
Health Insurance Portability and Accountability Act
- ICD-9
International Classification of Diseases, 9th revision
- ICD-10
International Classification of Diseases, 10th revision
- 99% CI
99% confidence interval
- OR
odds ratio
Footnotes
Meeting Presentation: The Association for Research in Vision and Ophthalmology, Baltimore, MD, 2020 (Poster)
Online-only material: This article contains additional online-only material. The following should appear online-only (available at www.aaojournal.org): SI Figure 1 and SI Table 1–9.
References:
- 1.Hayreh SS. Ocular vascular occlusive disorders: Natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mlaco A, Mlaco N, Bejtovic D, Dzubur A, Spuzic M. Venous Thromboembolism During Ten-year Follow up on Clinical Center University of Sarajevo. Materia Socio Medica. 2019;31(2):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pershing S, Lum F, Hsu S et al. Endophthalmitis after Cataract Surgery in the United States. Ophthalmology. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atchison EA, Wood KM, Mattox CG et al. The Real-World Effect of Intravitreous Anti–Vascular Endothelial Growth Factor Drugs on Intraocular Pressure. Ophthalmology. 2018;125(5):676–682 [DOI] [PubMed] [Google Scholar]
- 5.Chiang MF, Sommer A, Rich WL, Lum F, Parke DW. The 2016 American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) Database. Ophthalmology. 2018;125(8):1143–1148 [DOI] [PubMed] [Google Scholar]
- 6.Parke DW, Lum F. Return to the Operating Room after Macular Surgery. Ophthalmology. 2018;125(8):1273–1278 [DOI] [PubMed] [Google Scholar]
- 7.Rao P, Lum F, Wood K et al. Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti–VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology. 2018;125(4):522–528 [DOI] [PubMed] [Google Scholar]
- 8.Repka MX, Lum F, Burugapalli B. Strabismus, Strabismus Surgery, and Reoperation Rate in the United States. Ophthalmology. 2018;125(10):1646–1653 [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Owen JP, Yanagihara RT et al. Smoking Is Associated with Higher Intraocular Pressure Regardless of Glaucoma: A Retrospective Study of 12.5 Million Patients Using the Intelligent Research in Sight (IRIS®) Registry. Ophthalmology Glaucoma. 2020;(In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Y, Jan R, Weng S et al. Retinal Artery Occlusion and the 3-Year Risk of Stroke in Taiwan: A Nationwide Population-Based Study. Am J Ophthalmol. 2012;154(4):645–652.e1 [DOI] [PubMed] [Google Scholar]
- 11.Chang Y, Ho C, Chu C et al. Risk of retinal artery occlusion in patients with diabetes mellitus: A retrospective large-scale cohort study. Plos One. 2018;13(8):e0201627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song P, Xu Y, Zha M, Zhang Y, Rudan I. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J Glob Health. 2019;9(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SJ, Choi N, Park KH, Woo SJ. Nationwide Incidence of Clinically Diagnosed Retinal Vein Occlusion in Korea, 2008 through 2011. Ophthalmology. 2014;121(6):1274–1280 [DOI] [PubMed] [Google Scholar]
- 14.Glueck CJ, Ping W, Hutchins R, Petersen MR, Golnik K. Ocular vascular thrombotic events: central retinal vein and central retinal artery occlusions. Clin Appl Thromb Hemost. 2008;14(3):286–94 [DOI] [PubMed] [Google Scholar]
- 15.Egan RA, Lutsep HL. Prevalence of Retinal Emboli and Acute Retinal Artery Occlusion in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2020;29(2):104446. [DOI] [PubMed] [Google Scholar]
- 16.Yen J, Lin H, Hsu C, Li YJ, Hsu M. Atrial Fibrillation and Coronary Artery Disease as Risk Factors of Retinal Artery Occlusion: A Nationwide Population-Based Study. Biomed Res Int. 2015;2015:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcucci R, Sodi A, Giambene B et al. Cardiovascular and thrombophilic risk factors in patients with retinal artery occlusion. Blood Coagul Fibrinolysis. 2007;18(4):321–6 [DOI] [PubMed] [Google Scholar]
- 18.Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116(10):1928–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risimic D, Nikolic D, Simeunovic D et al. Correlation of atherogenic risk factors with retinal artery occlusion in adults. Med Glas (Zenica). 2014;11(1):110–4 [PubMed] [Google Scholar]
- 20.Callizo J, Feltgen N, Pantenburg S et al. Cardiovascular Risk Factors in Central Retinal Artery Occlusion. Ophthalmology. 2015;122(9):1881–1888 [DOI] [PubMed] [Google Scholar]
- 21.Xiao YY, Wei WB, Wang YX et al. Correlation of the history of stroke and the retinal artery occlusion: a nested case-control study. Int J Ophthalmol. 2020;13(3):431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang Y, Weng S, Chang C et al. Risk of Retinal Artery Occlusion in Patients With End-Stage Renal Disease. Medicine. 2016;95(14):e3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim LL, Cheung N, Wang JJ et al. Prevalence and risk factors of retinal vein occlusion in an Asian population. Brit J Ophthalmol. 2008;92(10):1316–1319 [DOI] [PubMed] [Google Scholar]
- 24.Schorr EM, Rossi KC, Stein LK et al. Characteristics and Outcomes of Retinal Artery Occlusion. Stroke. 2020;51(3):800–807 [DOI] [PubMed] [Google Scholar]
- 25.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(10):1243–7 [DOI] [PubMed] [Google Scholar]
- 26.Bucciarelli P, Passamonti SM, Gianniello F, Artoni A, Martinelli I. Thrombophilic and cardiovascular risk factors for retinal vein occlusion. Eur J Intern Med. 2017;44:44–48 [DOI] [PubMed] [Google Scholar]
- 27.Li D, Zhou M, Peng X, Sun H. Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism, and risk of retinal vein occlusion: an updated meta-analysis. Bmc Ophthalmol. 2014;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong N, Xu B, Tang X. Plasma homocysteine concentrations in acute and convalescent changes of central retinal vein occlusion in a Chinese population. Invest Ophthalmol Vis Sci. 2014;55(7):4057–62 [DOI] [PubMed] [Google Scholar]
- 29.Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1996;114(5):545–54 [PubMed] [Google Scholar]
- 30.Ponto KA, Scharrer I, Binder H et al. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions. J Hypertens. 2019;37(7):1372–1383 [DOI] [PubMed] [Google Scholar]
- 31.Risk factors for branch retinal vein occlusion. The Eye Disease Case-control Study Group. Am J Ophthalmol. 1993;116(3):286–96 [PubMed] [Google Scholar]
- 32.Rogers S, McIntosh RL, Cheung N et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–9.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keel S, Xie J, Foreman J et al. Prevalence of retinal vein occlusion in the Australian National Eye Health Survey. Clinical & Experimental Ophthalmology. 2018;46(3):260–265 [DOI] [PubMed] [Google Scholar]
- 34.Thapa R, Bajimaya S, Paudyal G et al. Prevalence, pattern and risk factors of retinal vein occlusion in an elderly population in Nepal: the Bhaktapur retina study. Bmc Ophthalmol. 2017;17(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponto KA, Elbaz H, Peto T et al. Prevalence and risk factors of retinal vein occlusion: the Gutenberg Health Study. J Thromb Haemost. 2015;13(7):1254–1263 [DOI] [PubMed] [Google Scholar]
- 36.Schwaber EJ, Fogelman N, Sobol EK et al. Associations with retinal vascular occlusions in a diverse, urban population. Ophthal Epidemiol. 2018;25(3):220–226 [DOI] [PubMed] [Google Scholar]
- 37.Kolar P Risk Factors for Central and Branch Retinal Vein Occlusion: A Meta-Analysis of Published Clinical Data. J Ophthalmol. 2014;2014:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malone F, McCarthy E, Delassus P et al. Embolus Analog Trajectory Paths Under Physiological Flowrates Through Patient-Specific Aortic Arch Models. Journal of Biomechanical Engineering. 2019;141(10) [DOI] [PubMed] [Google Scholar]
- 39.Schmidt D, Hetzel A, Geibel-Zehender A, Schulte-Monting J. Systemic diseases in non-inflammatory branch and central retinal artery occlusion--an overview of 416 patients. Eur J Med Res. 2007;12(12):595–603 [PubMed] [Google Scholar]
- 40.Parke DW II, Lum F, Rich WL. The IRIS®-Registry Purpose and perspective. Der Ophthalmologe. 2016;113(6):463–468 [DOI] [PubMed] [Google Scholar]
- 41.Silverstein Marc D., Heit John A., Mohr David N. et al. Trends in the Incidence of Deep Vein Thrombosis and Pulmonary Embolism. Archives of Internal Medicine. 1998;(158):585–593 [DOI] [PubMed] [Google Scholar]
- 42.Kappert K, Böhm M, Schmieder R et al. Impact of Sex on Cardiovascular Outcome in Patients at High Cardiovascular Risk. Circulation. 2012;126(8):934–941 [DOI] [PubMed] [Google Scholar]
- 43.Ho JY, Hendi AS. Recent trends in life expectancy across high income countries: retrospective observational study. BMJ. 2018:k2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone MJ. Waldenström’s Macroglobulinemia: Hyperviscosity Syndrome and Cryoglobulinemia. Clinical Lymphoma & Myeloma. 2009;9(1):97–99 [DOI] [PubMed] [Google Scholar]
- 45.Murphy SJ, Werring DJ. Stroke: causes and clinical features. Medicine. 2020;48(9):561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


