We join our colleagues in thanking the Society for Cardiovascular Pathology (SCVP) Publication Committee for our receipt of the 2021 Margaret Billingham Award for the most important original paper published in 2020 in the Society's journal, Cardiovascular Pathology [1]. This is a particular honor because the Award is given in memory of the legendary Margaret Billingham who was a very respected cardiovascular pathologist and admirable person. In this Commentary, we provide context relevant to the genesis of our paper and subsequent pertinent developments.
In December 2019, a rapidly spreading respiratory illness arose in Wuhan, China. By January 2020, the virus was identified as a novel coronavirus based on its molecular sequencing [2]. The World Health Organization (WHO) designated the illness as a Public Health Emergency of International Concern on January 30, named the illness as coronavirus disease-19 (COVID-19) and the virus as SARS-CoV-2 on February 11, and then declared COVID-19 to be a pandemic on March 11, 2020. The virus spread rapidly from China to Europe with early hotspots in Northern Italy and subsequent high mortality rate in the Lombardy region [3], [4], [5]. From Northern Italy, the pandemic spread to other parts of Europe, the USA, and other countries [6].
The pandemic elicited an intense focus of governments, public health professionals, health care providers and scientists on prevention and treatment of the disease. Clinicians described a myriad of signs and symptoms and a spectrum from mild to severe illness in individuals with COVID-19 [6]. However, the clinical and public health efforts were hampered by a glaring deficiency of information about the mechanisms responsible for morbidity and mortality in COVID-19 [7].
Clearly there was an urgent need to obtain fundamental information about pathological changes responsible for the clinical features of the disease. Because of concern that the virus was highly transmissible, performance of bronchial biopsies by pulmonologists was considered unsafe. This meant that the autopsy was the only feasible way to obtain clinicopathological correlation about the disease and to obtain tissues for research. Early in 2020, a clarion call to action for autopsy studies was proclaimed, with an Epub on April 10, 2020 [8]. At that time, end April 2020, a literature search revealed that only seven of 7010 published papers included an analysis of tissue samples, with information from only five complete autopsies [9]. Multiple factors had contributed to a slowdown in the performance of autopsies, including safety concerns for autopsy personnel and the plethora of conflicting, changing and confusing guidelines from various governmental and professional organizations [7]. A cadre of concerned pathologists mobilized to put forward standards for performance of autopsies on COVID-19 decedents [10], [11], [12], [13], [14], [15]. This elicited the publication of revised guidelines and protocols [16]. Subsequently, it was shown that the infectious risk to autopsy personnel is eliminated with appropriate use of personal protective equipment (PPE).
The COVID-19 pandemic reached Houston in March 2020, and fatalities started to occur in April, 2020. In Houston, we took a proactive approach to moving forward with autopsies on COVID-19 patients. We joined the national autopsy listserv and interest group organized by Dr. Alex Williamson of Northwell Health [15]. We determined that our autopsy suites had appropriate negative pressure and airflow and we obtained appropriate PPE. Having dealt with local concerns and facility requirements, we commenced performing autopsies in our hospital autopsy suites on hospitalized patients while the county medical examiner performed autopsies on subjects who died outside the hospital. Decedents were determined to be COVID-19 based on positive PCR swab test for SARS-CoV-2 virus during life and/or at autopsy. By early May we were able to perform several autopsies on COVID-19 patients and review our findings and compare them to early reports from other US cities.
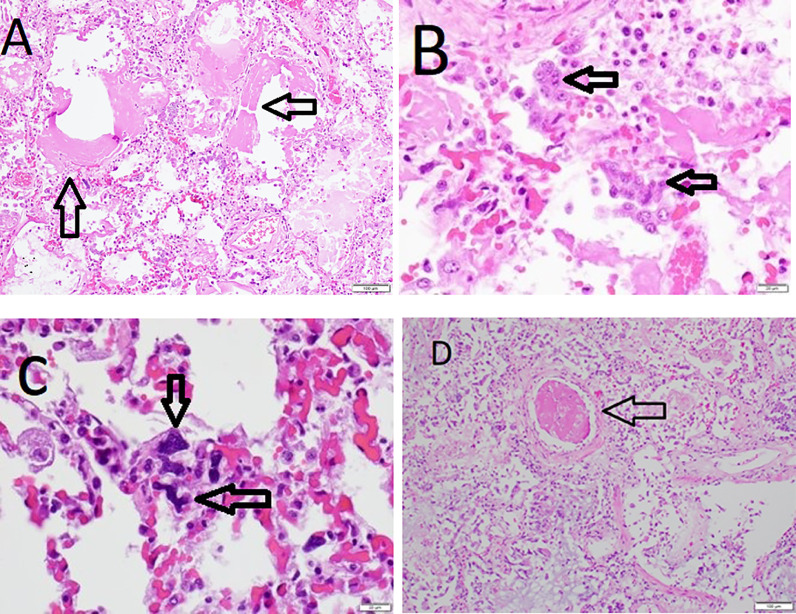
Our paper featured a detailed description of pathological changes in our first three COVID-19 autopsies in Houston and correlation with the pathological findings from initial published autopsy reports on 23 patients with COVID-19 from five centers in the United States [1]. Analysis of the 23 cases documented that COVID-19 is a systemic disease with major involvement of the lungs and cardiovascular system. Evaluation of our three cases included gross findings, histopathology, immunohistochemistry, and electron microscopy. Acute COVID-19 pneumonia was shown to have features of a distinctive acute interstitial pneumonia characterized by extensive diffuse alveolar damage (DAD) with hyaline membranes, type II pneumocyte hyperplasia, low grade lymphohistiocytic infiltrate, and microvasculopathy with intravascular and extravascular fibrin deposition and intravascular trapping of megakaryocytes, platelets and neutrophils in capillaries, and in some cases, microthrombi in arterioles (Fig. 1 ). Major pulmonary thromboemboli with pulmonary infarcts and/or hemorrhage occurred in five of the 23 patients. It was noted that some patients may exhibit a variant pattern of lung involvement known as acute fibrinous and organizing pneumonia (AFOP) characterized by interstitial pneumonitis without hyaline membranes and with intra-alveolar deposits of fibrin enclosed in granulation tissue. One of the three Houston cases had similar pulmonary pathology. The classic DAD and AFOP patterns have subsequently been confirmed in a review of pulmonary pathology in COVID-19 [17]. In addition to major pulmonary pathology, the three Houston cases had evidence of lymphocytic pericarditis, multifocal acute injury of cardiomyocytes without inflammatory cellular infiltrates, depletion of splenic white pulp, focal hepatocellular degeneration and rare glomerular capillary thrombosis. All 3 patients had evidence of obesity and cardiac disease with hypertensive left ventricular hypertrophy, and these comorbidities were common in the overall series of 23 patients. We concluded that the autopsy findings support the concept that the pathogenesis of severe COVID-19 disease involves initial viral-induced injury of multiple organs, including heart and lungs, coupled with an intense inflammatory reaction and a prothrombotic coagulopathy.
Fig. 1.
Histopathological features of lung involvement in COVID-19. A. Interstitial pneumonitis with edematous alveolar septae, mild lymphohistiocytic inflammation, and prominent hyaline membranes (arrows). B. Alveolus containing aggregates of type II pneumocytes (arrows), hyaline material, and inflammatory cells. C. Aggregate of megakaryocytes (arrows) in alveolar capillaries. D. Area of inflamed lung with microthrombus (arrow). Photomicrographs of hematoxylin and eosin stained sections with magnification scale bars.
Our publication was one of the first to provide a comprehensive analysis of pathological changes in severe COVID-19 infection. It has subsequently been cited nearly 200 times on Google Scholar and recognized as a highly cited paper and hot paper by Clarivate Web of Science. We now have performed over 50 autopsies on COVID-19 patients at our hospital-based autopsy service. Over 50 autopsies on COVID-19 patients also have been performed at the Harris County Institute of Forensic Sciences. We have submitted some of these cases for inclusion in the large multi-institutional autopsy survey study published by the COVID-19 autopsy interest group [18]. We also have presented our findings in 34 subjects at the recent meeting of the US and Canadian Academy of Pathology (USCAP) [19]. The duration of illness in our cases has ranged from 24 hours or less to over 12 weeks. The corresponding pulmonary pathology is characterized by the exudative, proliferative, and fibrotic phases of DAD, often with overlapping features of two or three phases in the same lungs. Utilizing IHC with antibodies to viral nucleocapsid protein and spike protein, we have found detectable virus in type II pneumocytes and endothelial cells only in cases dying with an illness of less than 2 weeks. These findings are consistent with the hypothesis that the initial phase of illness is driven by viral infection and the later phase by host response creating an immune mediated hyperinflammatory state [20], [21], [22].
We should acknowledge that the lead author is also the editor-in-chief of Cardiovascular Pathology (CVP), the official journal of the Society for Cardiovascular Pathology. The review process for the manuscript was conducted by an Associate Editor. The publication history of our manuscript is: original submitted April 21, 2020, revision submitted April 27, 2020, accepted for publication April 28, 2020, published electronically May 7, 2020. This rapid turnaround reflects the priority given to dissemination of information regarding COVID-19 by the Editorial Board of CVP and Elsevier, the publisher of CVP. Several articles on COVID-19 have now been published in CVP and are collated in an ongoing special article collection [23].
Since April 2020, the void in knowledge has been partially rectified with the publication of findings from limited and complete autopsies now numbering around 400 [23]. Our findings have been confirmed in the subsequent autopsy studies [14,24]. We need to make one correction and that is the misidentification of normal structures for viral particles by electron microscopy, namely, our misidentification of normal subcellular structures, including multivesicular bodies and clathrin-coated vesicles, as coronavirus virions [25,26].
Our work has been part of a concerted effort by cardiovascular pathologists, individually and through their organizations, the Society for Cardiovascular Pathology (SCVP) and the Association for European Cardiovascular Pathology (AECVP), to obtain and disseminate credible information about the pathological basis for the diverse clinical manifestations of cardiovascular system involvement in COVID-19 [27], [28], [29].
Clinical studies have shown that patients with severe COVID-19 often have elevated serum troponin level. This finding was initially equated with myocarditis. However, cardiovascular pathologists have shown that there is a low incidence of overt myocarditis characterized by multifocal lymphohistiocytic infiltrates with associated cardiomyocyte damage (less than 10% of cases). However, it is noteworthy that at least one acute, potentially COVID-19 related cardiovascular histopathologic finding, such as focal cardiomyocyte necrosis, macro- or microvascular thrombi, inflammation, or intraluminal megakaryocytes, have been reported in nearly 50% of cases. Thus, pathological studies have documented that COVID-19 related cardiac histopathological findings are common, while myocarditis is infrequent.
Our paper was one of the first to provide evidence that important components of severe COVID-19 are persistent inflammation and microvasculopathy giving rise to a hypercoagulable state and a predisposition to in situ thrombosis in lungs and other organs as well as pulmonary thromboemboli. Autopsy studies firmly established that severe COVID-19 is a systemic disease and provided support for the important role of vascular involvement with endothelial dysfunction [30,31]. Autopsy findings have led to significant improvements in treatment protocols including the early institution of antithrombotic therapy and administration of corticosteroids [32,33]. While autopsy studies have been crucially important in understanding the pathobiology of the disease, application of virological and epidemiological methods have been majorly important in understanding the nature of the pandemic and in devising approaches to controlling it.
In the last 20 years, the world has been confronted with three serious coronavirus epidemics: Severe Acute Respiratory Syndrome (SARS) in 2002, Middle East Respiratory Syndrome (MERS) in 2012 and COVID-19. A comparative analysis of the SARS, MERS, and COVID-19 epidemics reveals commonalities and unique features of each disease. SARS and MERS affected thousands of individuals in a few countries; whereas, as of March 23, 2021, COVID-19 has affected over 123 million individuals worldwide [22,34]. The vastly greater magnitude of the COVID-19 pandemic is related to a combination of factors including a high rate of infectivity (reproduction number-R 2.5 or greater) and a longer incubation time with a negative serial interval during which asymptomatic individuals shed virus and can infect others, thereby making the spread of COVID-19 more difficult to contain than SARS and MERS.
Early on, the Italian experience provided stark lessons regarding the difficulties in controlling COVID-19 [3], [4], [5]. The first detected cases were reported on 21 February 2020 in two Italian towns: Vo' Euganeo in the Province of Padua, Veneto region, and Codogno, in the Province of Lodi, Lombardy region. In the next weeks the epidemic spread quickly across the country but mainly in the north of Italy. The two regions, Veneto and Lombardy, implemented different strategies to control the viral spread. In Veneto, health personnel tested both symptomatic and asymptomatic subjects, while in Lombardy only symptomatic cases were investigated. While the population of Lombardy is twice that of the Veneto (10 M vs. 4.5 M), the number of tests per capita performed in Veneto was almost two times higher than in Lombardy. The death rate due to COVID-19 in Veneto was two times lower than in Lombardy (181.3 and 344.9 cases per million population). The analysis of the evolution of the epidemic in these regions showed that testing both symptomatic and asymptomatic cases is a more effective strategy to mitigate the epidemic impact. This experience led to recommendations for decision-makers: ensure early isolation of symptomatic patients and rapid identification of their contacts; maximize testing rapidly, especially among people with multiple daily contacts with infected populations, high exposure to the public in essential services; rapidly increase diagnostic capacity by mobilizing trained personnel capable of performing rRT-PCR on respiratory samples; equip the population with protective masks. Variable application of containment strategies is reflected by order of confirmed cases (USA, India, Brazil, France, Russia, Turkey, United Kingdom, Italy, Spain, Germany, Argentina, and Poland) and deaths (USA, Brazil, Mexico, India, United Kingdom, Italy, Russia, France, Germany, Spain, Columbia, and Iran) [35].
Since April, 2020, knowledge concerning the pulmonary and extrapulmonary pathology of COVID-19 has rapidly accumulated. However, more well-designed and well-controlled studies are needed to address the many unanswered questions regarding the nature of the virus-host interactions in COVID-19, including the basis for the postacute sequelae of SARS-CoV-2 infection (PASC) or long tail COVID-19 [36]. While autopsy has been essential in establishing our current knowledge of the disease, autopsy findings on patients who died from acute or subacute SARS-CoV-2 infection need to be probed for their applicability to those patients who recover from their illness. We will need to continue to investigate the pathology related to the emerging mutant variants of SARS-CoV-2. Nevertheless, the spectacular achievement of vaccine development against the SARS-Cov-2 virus has provided much needed optimism that the pandemic can be brought under control [37].
Footnotes
Conflict of interest: None of the authors have a conflict of interest regarding this manuscript.
References
- 1.Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. Epub 2020 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. Epub 2020 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuite AR, Ng V, Rees E, Fisman D. Estimation of COVID-19 outbreak size in Italy. Lancet Infect Dis. 2020;20(5):537. doi: 10.1016/S1473-3099(20)30227-9. Epub 2020 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odone A, Delmonte D, Scognamiglio T, Signorelli C. COVID-19 deaths in Lombardy, Italy: data in context. Lancet Public Health. 2020;5(6):e310. doi: 10.1016/S2468-2667(20)30099-2. Epub 2020 Apr 25. Erratum in: Lancet Public Health. 2020 Jun;5(6):e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagnani P, Gnone G, Guzzi F, Negrini S, Guastalla A, Annunziato F, et al. The COVID-19 infection: lessons from the Italian experience. J Public Health Policy. 2020;41(3):238–244. doi: 10.1057/s41271-020-00229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 7.Ledford H. Autopsy slowdown hinders quest to determine how coronavirus kills. Nature. 2020 May 7 doi: 10.1038/d41586-020-01355-z. [DOI] [PubMed] [Google Scholar]
- 8.Barth RF, Xu X, Buja LM. A call to action: the need for autopsies to determine the full extent of organ involvement associated with COVID-19. Chest. 2020;158(1):43–44. doi: 10.1016/j.chest.2020.03.060. Epub 2020 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salerno M, Sessa F, Piscopo A, Montana A, Torrisi M, Patanè F, et al. No autopsies on COVID-19 deaths: a missed opportunity and the lockdown of science. J Clin Med. 2020;9(5):1472. doi: 10.3390/jcm9051472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73(5):239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 11.Baj J, Ciesielka M, Buszewicz G, Maciejewski R, Budzyńska B, Listos P, et al. COVID-19 in the autopsy room-requirements, safety, recommendations and pathological findings. Forensic Sci Med Pathol. 2021;17(1):101–113. doi: 10.1007/s12024-020-00341-1. Epub 2021 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperhake JP. Autopsies of COVID-19 deceased? Absolutely! Leg Med (Tokyo) 2020;47 doi: 10.1016/j.legalmed.2020.101769. Epub 2020 Jul 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacy JM, Brooks EG, Akers J, Armstrong D, Decker L, Gonzalez A, et al. COVID-19: postmortem diagnostic and biosafety considerations. Am J Forensic Med Pathol. 2020;41(3):143–151. doi: 10.1097/PAF.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuone D, Sinard J, Gill JR, Masters A, Liu C, Morotti R, et al. Autopsy services and emergency preparedness of a tertiary academic hospital mortuary for the COVID-19 public health emergency: the Yale plan. Adv Anat Pathol. 2020;27(6):355–362. doi: 10.1097/PAP.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 15.Williamson AK. Creation and benefits of the "COVID autopsy listserve". Arch Pathol Lab Med. 2020;144(10):1160–1161. doi: 10.5858/arpa.2020-0300-LE. [DOI] [PubMed] [Google Scholar]
- 16.Amended COVID-19 autopsy guideline statement from the CAP Autopsy Committee. Updated 2/2/21: CAP Website. Available at: https://documents.cap.org/documents/COVID-Autopsy-Statement.pdf. Accessed April 21, 2022.
- 17.Polak SB, Van Gool IC, Cohen D, von der Thusen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper JE, Padera RF, Dolhnikoff M, da Silva LFF, Duarte-Neto AN, Kapp ME, et al. A postmortem portrait of the coronavirus disease 2019 (COVID-19) pandemic: a large multiinstitutional autopsy survey study. Arch Pathol Lab Med. 2021 doi: 10.5858/arpa.2020-0786-SA. [DOI] [PubMed] [Google Scholar]
- 19.McDonald M, Zhang S, Buja LM, Zhao B. COVID-19 autopsy findings: a case series from Houston, TX. Mod Pathol. 2021;34(Suppl 2):19–21. [Google Scholar]
- 20.Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol. 2021;191(1):4–17. doi: 10.1016/j.ajpath.2020.08.009. Epub 2020 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth RF, Buja LM, Parwani AV. The spectrum of pathological findings in coronavirus disease (COVID-19) and the pathogenesis of SARS-CoV-2. Diagn Pathol. 2020;15(1):85. doi: 10.1186/s13000-020-00999-9. PMID: 32665025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth RF, Buja LM, Barth AL, Carpenter DE, Parwani AV. A comparison of the clinical, pathologic, virologic, and immunologic features of SARS, MERS, and COVID-19 viral diseases. Arch Pathol Lab Med. 2021 doi: 10.5858/arpa.2020-0820-SA. In press. [DOI] [PubMed] [Google Scholar]
- 23.COVID-19 publications in CVP. Available at: https://www.sciencedirect.com/journal/cardiovascular-pathology/special-issue/10LS1SM3NG4. Accessed 8 June 2021.
- 24.Maiese A, Manetti AC, La Russa R, Di Paolo M, Turillazzi E, Frati P, Fineschi V. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. Jun 2021;17(2):279–296. doi: 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannico GA, Miller SE. Electron microscopy identification of SARS-COV-2: what is the evidence? Letter to the Editor. Cardiovasc Pathol. 2021;52 doi: 10.1016/j.carpath.2021.107338. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buja LM. Electron microscopic identification of SARS-CoV-2. Letter to the Editor. Cardiovasc Pathol. 2021;52 doi: 10.1016/j.carpath.2021.107337. Epub Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, MC Aubry, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buja LM, Stone JR. A novel coronavirus meets the cardiovascular system: Society for Cardiovascular Pathology Symposium 2021. Editorial. Cardiovasc Pathol. 2021;53 doi: 10.1016/j.carpath.2021.107336. Epub Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqi HK, Libby P, Ridker PM. COVID-19—a vascular disease. Trends Cardiovasc Med. 2021;31(1):1–5. doi: 10.1016/j.tcm.2020.10.005. Epub 2020 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Liu S. The management of coronavirus disease 2019 (COVID-19) J Med Virol. 2020;92(9):1484–1490. doi: 10.1002/jmv.25965. Epub 2020 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Mehta S, Sarangdhar N, Ray A, Sinha S, Wig N. Management of COVID-19 from the pulmonologist's perspective: a narrative review. Expert Rev Respir Med. 2021;15(4):519–535. doi: 10.1080/17476348.2021.1853529. Epub 2020 Dec 17. [DOI] [PubMed] [Google Scholar]
- 34.da Costa VG, Moreli ML, Saivish MV. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch Virol. 2020;165(7):1517–1526. doi: 10.1007/s00705-020-04628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int. Accessed April 25, 2021.
- 36.Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. The long tail of Covid19′ – the detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020;9:1349. doi: 10.12688/f1000research.27287.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desmond A, Offit PA. On the shoulders of giants – from Jenner's cowpox to mRNA COVID vaccines. New Engl J Med. 2021;384:1081–1083. doi: 10.1056/NEJMp2034334. [DOI] [PMC free article] [PubMed] [Google Scholar]