Abstract
Background:
Since the introduction of Haemophilus influenzae serotype b (Hib) conjugate vaccines in the United States, invasive H. influenzae disease (Hi) epidemiology has changed and racial disparities have not been recently described.
Methods:
Active population- and laboratory-based surveillance for Hi was conducted through Active Bacterial Core surveillance (ABCs) at 10 U.S. sites. Data from 2008–2017 was used to estimate projected nationwide annual incidence in cases/100,000.
Results:
During 2008–2017, ABCs identified 7379 Hi cases. Of 6705 (90.9%) patients with reported race, 76.2% were White, 18.6% were Black, 2.8% were Asian/Pacific Islander (PI), and 2.4% were American Indian and Alaska Native (AI/AN). Nationwide annual incidence was 1.8 cases/100,000. By race, incidence was highest among AI/AN populations (3.1) and lowest among Asian/PI populations (0.8). Nontypeable Hi (NTHi) caused the largest incidence within all races (1.3), with no striking disparities identified. Among AI/AN children aged <5 years, incidence of Hi serotype a (Hia) was 16.7 times higher and Hib incidence was 22.4 times higher than among White children. Though Hia incidence was lower among White and Black populations compared to AI/AN, Hia incidence increased 13.6% annually among White children and 40.4% annually among Black children aged <5 years.
Conclusions:
While NTHi causes the largest Hi burden overall, AI/AN populations experience disproportionately high rates of Hia and Hib, with the greatest disparity among AI/AN children aged <5 years. Prevention tools are needed to reduce disparities affecting AI/AN children and address increasing Hia incidence in other communities.
Keywords: Haemophilus influenzae, Haemophilus influenzae vaccines, epidemiology, public health surveillance, minority health
Background
Haemophilus influenzae is an important bacterial cause of invasive disease (e.g., meningitis, bacteremia) and non-invasive disease (e.g., otitis media, sinusitis) in young children, older adults, and persons with certain medical conditions [1–3]. Both encapsulated (serotypes a [Hia], b [Hib], c [Hic], d [Hid], e [Hie], and f [Hif]) and unencapsulated (nontypeable [NTHi]) strains can cause infection.
Historically, Hib was the most common cause of invasive bacterial disease in children aged <5 years in the United States [4]. In the pre-Hib vaccine era, racial disparities in Hib disease were reported with incidence rates 3–4 times higher among Black children than among White children [5–10]. American Indian and Alaska Native (AI/AN) children experienced greater disparities, with an incidence of invasive Hib disease 5–10 times that among White children [11–13]. Among all ages, Black populations had invasive H. influenzae disease incidence 1.5–3.5 times higher than White populations [4, 7, 14].
Following Hib conjugate vaccine introduction into the childhood immunization schedule during the 1980s–1990s, Hib disease incidence in children aged <5 years declined dramatically among all racial groups [15, 16]. Since then, H. influenzae disease epidemiology has changed. NTHi currently accounts for >70% of invasive H. influenzae disease cases [3]. Additionally, from 2002–2015, incidence of Hia and NTHi increased annually by 13% and 3%, respectively [3].
With this changing epidemiology, it is important to monitor whether racial disparities in invasive H. influenzae disease persist. We analyzed data from active population- and laboratory-based surveillance from 2008–2017 to identify current racial disparities in invasive H. influenzae disease in the United States, to help inform the design and implementation of prevention strategies.
Methods
Surveillance
Invasive H. influenzae disease data were collected through Active Bacterial Core surveillance (ABCs), an active population- and laboratory-based surveillance system supported by the Centers for Disease Control and Prevention (CDC) as a part of the Emerging Infections Program [17]. Data from 2008 through 2017 were included in the analysis.
The surveillance areas included: California (3 San Francisco Bay-area counties); Colorado (5 Denver-area counties); Connecticut (statewide); Georgia (20 Atlanta-area counties, 2008–2009, statewide 2010–2017); Maryland (statewide); Minnesota (statewide); New Mexico (statewide); New York (15 Rochester- and Albany-area counties); Oregon (statewide); and Tennessee (11 counties 2008–2009, 20 counties 2010–2017). The population under surveillance represented 11.9% of the U.S. population in 2008 and 13.7% in 2017 [18]. Race was classified as White, Black, Asian/Pacific Islander (PI), or AI/AN. The racial distribution of the population under ABCs surveillance in 2008 was 77.1% White, 15.8% Black, 5.8% Asian/PI, and 1.3% AI/AN, and was 73.0% White, 18.4% Black, 7.1% Asian/PI, and 1.5% AI/AN in 2017.
A case of invasive H. influenzae disease was defined as isolation of H. influenzae from a normally sterile site (e.g., blood, pleural fluid, or cerebrospinal fluid [CSF]) in a surveillance-area resident. Epidemiologic and clinical information was abstracted from medical records. Infants with a gestational age ≤22 weeks were excluded. Outcome (alive/dead) was based on patient status at hospital discharge. A hierarchical definition was used to assign a single syndrome for cases: meningitis if a clinical diagnosis of meningitis was recorded in the medical record and H. influenzae was isolated from CSF or other sterile sites, bacteremic pneumonia if pneumonia was recorded in the medical record and H. influenzae was isolated from blood or pleural fluid, and primary bacteremia if H. influenzae was isolated from blood and the medical record did not note another clinical syndrome. All other clinical syndromes were classified based upon source of isolate or information noted in the medical record.
Vaccination histories of children aged <5 years with Hib disease were abstracted from medical records and state vaccine registries. Children were considered age-appropriately vaccinated or fully vaccinated if vaccinated according to the recommended schedule for age and vaccine product [19]. Children who received at least one dose of Hib vaccine but not all recommended doses for their age and vaccine product were considered under-vaccinated. Children were considered unvaccinated if they had not received any Hib vaccine doses and were aged >2 months. Cases occurring in children aged ≤2 months were considered too young to receive vaccine. Vaccine type is reported as Hib antigen polyribosylribitol phosphate (PRP) conjugated to either meningococcal outer membrane protein (OMP), tetanus toxoid (T), or mutant diphtheria toxin (CRM).
Laboratory Methods
State public health laboratories serotyped and sent H. influenzae isolates to CDC’s Bacterial Meningitis Laboratory, where species and serotyping were confirmed for all isolates by real-time polymerase chain reaction and/or slide agglutination [3, 20, 21].
Statistical Analysis
Case-fatality ratios were calculated using the proportion of cases with known outcomes as the denominator. Kruskal-Wallis test was used to compare medians of continuous variables. Pearson’s χ2 test and Fisher’s exact test were used to compare categorical variables. For the ABCs sites, incidence rates were reported as cases/100,000 and calculated using National Center for Health Statistics’ bridged-race postcensal population estimates [18]; nationwide estimates were calculated by directly standardizing to the age and race distribution of the U.S. population. For race-stratified nationwide incidence estimates, missing race was multiply imputed using sequential regression multiple imputation [22]. Multiply imputed datasets were created using IVEware software (Institute for Social Research, University of Michigan, Ann Arbor). Variance estimates were calculated using standard combining rules for multiply imputed data. The 95% confidence intervals (CIs) around the directly standardized incidences were calculated using a method derived from the relationship between the Poisson distribution and the gamma distribution, whereas estimated age-, race-, and serotype-specific 95% CIs were calculated using exact CI for a Poisson random variable [23]. Incidences in Black, Asian/PI, and AI/AN populations were compared to those in White populations and incidence rate ratios (IRR) were calculated. A negative binomial model was used to estimate annual percentage changes in incidence.
This project was reviewed in accordance with CDC human research protection procedures and was determined to be nonresearch public health surveillance. At each ABCs site, it was deemed either a public health assessment or human subjects research, for which approval was granted by local institutional review boards.
Results
Patient Characteristics
From 2008–2017, 7379 cases of invasive H. influenzae disease were reported from ABCs sites. Of 6705 (90.9%) cases in patients with reported race, 76.2% were White, 18.6% were Black, 2.8% were Asian/PI, and 2.4% were AI/AN (Table 1). Median age was 68 years among White patients, 50 years among Black patients, 61 years among Asian/PI patients, and 24 years among AI/AN patients (p<0.0001). While children aged <5 years accounted for <20% of cases in White, Black, and Asian/PI patients, they represented almost half (46.6%) of cases in AI/AN patients.
Table 1.
Epidemiologic and Clinical Characteristics of Patients with Invasive H. influenzae Disease, by Race — Active Bacterial Core Surveillance, 2008–2017
| White | Black | Asian/PI | AI/AN | Totala | |
|---|---|---|---|---|---|
|
| |||||
| N (%) | 5106 (76.2) | 1248 (18.6) | 190 (2.8) | 161 (2.4) | 6705 |
| Age in years – median (IQR) | 68 (52–81) | 50 (23–65) | 61 (25–78) | 24 (1–60) | 64 (44–79) |
| Age <5 years – No. (%) | 443 (8.7) | 212 (17.0) | 32 (16.8) | 75 (46.6) | 762 (11.4) |
| Sex – No. (%) | |||||
| Male | 2334 (45.7) | 552 (44.2) | 95 (50.0) | 78 (48.5) | 3059 (45.6) |
| Female | 2772 (54.3) | 696 (55.8) | 95 (50.0) | 83 (51.6) | 3646 (54.4) |
| Ethnicity – No. (%) | |||||
| Hispanic | 236 (4.6) | 13 (1.0) | 9 (4.7) | 8 (5.0) | 266 (4.0) |
| Non-Hispanic | 3679 (72.1) | 950 (76.1) | 160 (84.2) | 111 (68.9) | 4900 (73.1) |
| Unknown | 1191 (23.3) | 285 (22.8) | 21 (11.1) | 42 (26.1) | 1539 (23.0) |
| Serotype – No. (%) b | |||||
| NTHi | 3371 (73.5) | 750 (67.8) | 135 (82.3) | 56 (37.1) | 4312 (71.8) |
| Hia | 230 (5.0) | 57 (5.2) | 5 (3.1) | 57 (37.8) | 349 (5.8) |
| Hib | 70 (1.5) | 14 (1.3) | 3 (1.8) | 20 (13.3) | 107 (1.8) |
| Hic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.0) |
| Hid | 2 (0.0) | 2 (0.2) | 0 (0.0) | 0 (0.0) | 4 (0.1) |
| Hie | 220 (4.8) | 53 (4.8) | 3 (1.8) | 4 (2.7) | 280 (4.7) |
| Hif | 692 (15.1) | 231 (20.9) | 18 (11.0) | 13 (8.6) | 954 (15.9) |
| Clinical Syndrome – No. (%) c | |||||
| Bacteremic Pneumonia | 3079 (60.7) | 575 (46.5) | 94 (50.3) | 67 (41.9) | 3815 (57.3) |
| Bacteremia | 1506 (29.7) | 496 (40.1) | 73 (39.0) | 51 (31.9) | 2126 (31.9) |
| Meningitis | 327 (6.4) | 91 (7.4) | 4 (2.1) | 34 (21.3) | 456 (6.9) |
| Other | 160 (3.2) | 74 (6.0) | 16 (8.6) | 8 (5.0) | 258 (3.9) |
| Hospitalized – No. (%) d | 4789 (94.1) | 1129 (90.7) | 182 (95.8) | 147 (91.9) | 6274 (93.9) |
| Case fatality ratio – No. (%) e | 738 (14.6) | 142 (11.5) | 23 (12.2) | 23 (14.3) | 926 (13.9) |
Table excludes patients with unknown race (n=674, 9.1%)
Among cases with known serotype (n=6007, 89.6%)
Among cases with known syndrome (n=6655, 99.3%)
Among cases with known hospitalization status (n=6683, 99.7%)
Among cases with known outcome (n=6652, 99.2%)
Abbreviations: PI, Pacific Islander; AI/AN, American Indian and Alaska Native; IQR, interquartile range; NTHi, nontypeable H. influenzae; Hia, H. influenzae serotype a; Hib, H. influenzae serotype b; Hic, H. influenzae serotype c; Hid, H. influenzae serotype d; Hie, H. influenzae serotype e; Hif, H. influenzae serotype f
Among 6007 (89.6%) reported cases with serotyping data available: 71.8% were NTHi, 5.8% Hia, 1.8% Hib, 0.0% Hic, 0.1% Hid, 4.7% Hie, and 15.9% Hif (Table 1). NTHi was the predominant cause of invasive H. influenzae among White (73.5%), Black (67.8%), and Asian/PI (82.3%) patients. By contrast, NTHi (37.1%) and Hia (37.8%) predominated among AI/AN patients. Although Hib caused <2% of H. influenzae cases among White, Black, and Asian/PI patients, Hib caused significantly more H. influenzae disease in AI/AN patients (13.3%, p<0.0001).
Bacteremic pneumonia was the most common clinical syndrome among all races (57.3%; Table 1). A larger proportion of cases among AI/AN patients presented as meningitis (21.3%). Case fatality ranged from 11.5% in Black patients to 14.6% in White patients.
Incidence Trends
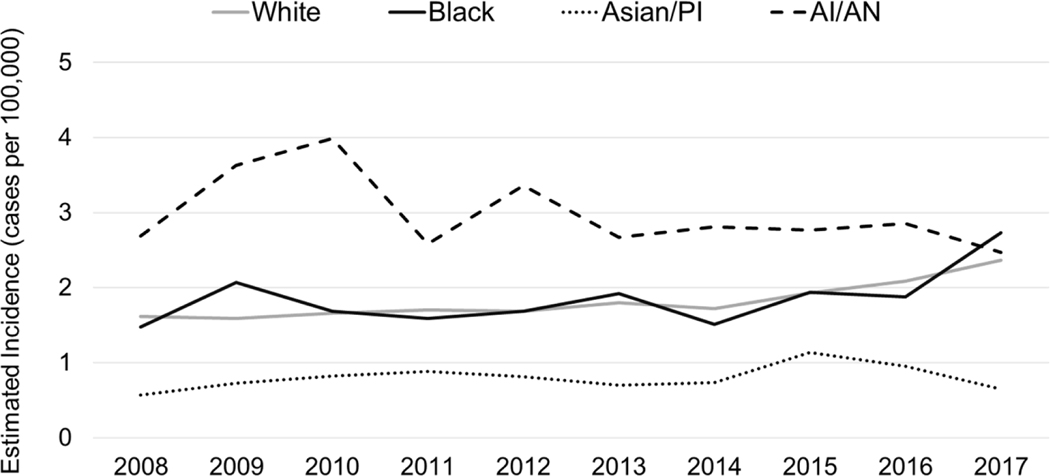
From 2008–2017, the average annual incidence of invasive H. influenzae disease was 1.8 cases/100,000 (Table 2). By race, incidence was highest among AI/AN populations (3.1 cases/100,000) and lowest among Asian/PI populations (0.8 cases/100,000). Incidences among White and Black populations were similar: 1.8 and 1.9 cases/100,000, respectively. AI/AN populations had the highest incidence throughout most of the analysis period (2008 to 2016, Figure 1), though incidence declined by 2.2% annually (95% CI: −4.0% to −0.3%) overall. During this period, incidence increased 3.9% annually (95% CI: 3.6%–4.2%) in White populations, 3.7% annually (95% CI: 2.9%–4.5%) in Black populations, and 3.0% annually (95% CI 1.1%–4.8%) in Asian/PI populations.
Table 2.
Estimated Average Annual Incidence of Invasive H. influenzae Disease, by Age Group and Race — United States, 2008–2017
| Age (years) |
White | Black | Asian/PI | AI/AN | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Incidencea | IRR | Incidencea | IRR (95% CI) |
Incidencea | IRR (95% CI) |
Incidencea | IRR (95% CI) |
Incidencea | |
| <1 | 8.0 | Ref | 11.2 | 1.4 (1.1–1.7) |
5.7 | 0.7 (0.4–1.2) |
34.9 | 4.4 (3.1–6.2) |
8.9 |
| 1–4 | 1.2 | Ref | 2.3 | 1.9 (1.5–2.4) |
1.1 | 0.9 (0.6–1.6) |
8.8 | 7.1 (5.0–10.0) |
1.6 |
| 5–17 | 0.3 | Ref | 0.5 | 1.6 (1.2–2.1) |
0.3 | 0.8 (0.4–1.5) |
0.3 | 0.9 (0.3–2.6) |
0.4 |
| 18–34 | 0.4 | Ref | 0.9 | 2.3 (1.9–2.8) |
0.2 | 0.6 (0.4–1.1) |
0.8 | 2.2 (1.2–4.0) |
0.5 |
| 35–49 | 0.7 | Ref | 1.3 | 2.0 (1.7–2.4) |
0.4 | 0.6 (0.4–1.0) |
1.9 | 3.0 (1.9–4.7) |
0.7 |
| 50–64 | 1.8 | Ref | 2.6 | 1.5 (1.3–1.7) |
0.8 | 0.4 (0.3–0.6) |
3.5 | 2.0 (1.4–2.9) |
1.8 |
| ≥65 | 6.8 | Ref | 5.6 | 0.8 (0.7–0.9) |
3.5 | 0.5 (0.4–0.7) |
9.4 | 1.4 (1.0–2.0) |
6.6 |
| Total | 1.8 | Ref | 1.9 | 1.0 (1.0–1.1) |
0.8 | 0.4 (0.4–0.5) |
3.1 | 1.7 (1.5–2.0) |
1.8 |
Cases per 100,000 persons per year
Abbreviations: PI, Pacific Islander; AI/AN, American Indian and Alaska Native; IRR, Incidence Rate Ratio; CI, confidence interval; Ref, Reference category for IRR calculation
Figure 1. Trends in Estimated Annual Incidence of Invasive H. influenzae Disease, by Race — United States, 2008–2017.
Abbreviations: PI, Pacific Islander; AI/AN, American Indian and Alaska Native
Age trends for each racial group followed a similar pattern, with the highest average annual incidences observed among children aged <1 year and adults aged ≥65 years (Table 2). Incidence among children aged <1 year was highest among AI/AN children (34.9 cases/100,000), 4.4 times (95% CI: 3.1–6.2) the incidence for White children. Among adults aged ≥65 years, incidence was also highest among AI/AN (9.4 cases/100,000), though not significantly higher than incidence among White older adults (IRR: 1.4; 95% CI: 1.0–2.0). In every age group, Asian/PI persons had the lowest incidence.
By serotype, trends for White, Black, and Asian/PI populations were similar, with the highest incidence of disease due to NTHi, followed by Hif, then Hia and Hie, and lastly Hib (Figure 2, Supplementary Table 1). NTHi incidence increased notably among White and Black populations, with average annual increases of 4.4% (95% CI: 4.0%–4.8%) and 4.6% (95% CI: 3.7%–5.6%), respectively.
Figure 2. Trends in Estimated Annual Incidence of Invasive H. influenzae Disease, by Race and Serotype — United States, 2008–2017.
Note: Incidence of invasive H. influenzae serotypes c, d, and e were omitted from Figure due to low incidence (<0.2 cases per 100,000) among all races
Abbreviations: PI, Pacific Islander; AI/AN, American Indian and Alaska Native; NTHi, nontypeable H. influenzae; Hia, H. influenzae serotype a; Hib, H. influenzae serotype b; Hif, H. influenzae serotype f
Serotype-specific incidence among AI/AN populations followed a different pattern than all other races, though annual incidence was unsteady throughout the analysis period due to small case counts and population sizes (Figure 2). Among AI/AN populations, NTHi and Hia contributed the highest burden, with incidences of 1.4 cases/100,000 and 1.0 cases/100,000, respectively (Supplementary Table 1). Hib incidence in AI/AN populations (0.4 cases/100,000) was substantially higher than in White, Black, and Asian/PI populations, which remained <0.1 cases/100,000.
Racial Disparities among Children Aged <5 Years
From 2008–2017, 823 cases of invasive H. influenzae disease among children aged <5 years were reported from ABCs sites. Of 766 (93.1%) cases with serotyped isolates, 56.5% were NTHi, 21.7% Hia, 5.2% Hib, 2.2% Hie, and 14.4% Hif.
By race, AI/AN children had the highest incidence of H. influenzae disease (13.8 cases/100,000), 5.3 times (95% CI: 4.2–6.8) the incidence among White children (2.6 cases/100,000; Table 3). Black children experienced an incidence of 4.1 cases/100,000, 1.6 times (95% CI: 1.4–1.9) higher than White children.
Table 3.
Estimated Average Annual Incidence of Invasive H. influenzae Disease among Children Aged <5 years, by Serotype and Race — United States, 2008–2017
| White | Black | Asian/PI | AI/AN | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Incidencea | IRR | Incidencea | IRR (95% CI) |
Incidencea | IRR (95% CI) |
Incidencea | IRR (95% CI) |
Incidencea | |
| NTHi | 1.6 | Ref | 2.4 | 1.5 (1.2–1.9) |
1.4 | 0.9 (0.6–1.4) |
2.0 | 1.2 (0.7–2.3) |
1.7 |
| Hia | 0.5 | Ref | 0.6 | 1.3 (0.9–1.9) |
0.1 | 0.1 (0.0–0.8) |
8.4 | 16.7 (11.7–24.0) |
0.7 |
| Hib | 0.1 | Ref | 0.1 | 0.4 (0.1–1.3) |
0.0 | N/A | 2.9 | 22.4 (11.9–42.1) |
0.2 |
| Hie | 0.1 | Ref | 0.1 | 2.8 (1.0–7.1) |
0.1 | 1.2 (0.1–8.9) |
0.0 | N/A | 0.1 |
| Hif | 0.3 | Ref | 0.9 | 2.8 (1.9–4.2) |
0.5 | 1.7 (0.9–3.5) |
0.6 | 1.9 (0.6–5.7) |
0.4 |
| Total | 2.6 | Ref | 4.1 | 1.6 (1.4–1.9) |
2.0 | 0.8 (0.6–1.1) |
13.8 | 5.3 (4.2–6.8) |
3.0 |
Note: No cases of invasive disease due to H. influenzae serotypes c or d were reported among children aged <5 years.
Cases per 100,000 persons per year
Abbreviations: PI, Pacific Islander; AI/AN, American Indian and Alaska Native; IRR, Incidence Rate Ratio; CI, confidence interval; Ref, Reference category for IRR calculation; NTHi, nontypeable H. influenzae; Hia, H. influenzae serotype a; Hib, H. influenzae serotype b; Hie, H. influenzae serotype e; Hif, H. influenzae serotype f
By serotype, NTHi accounted for the highest incidence of H. influenzae disease among children aged <5 years (1.7 cases/100,000; Table 3). NTHi incidence was highest among Black children (2.4 cases/100,000); 1.5 times (95% CI: 1.2–1.9) the incidence among White children. The largest disparity between Black and White children was in Hif disease (IRR: 2.8; 95% CI: 1.9–4.2).
Hib and Hia were the primary causes of invasive H. influenzae disease among AI/AN children aged <5 years. Hib incidence was highest among AI/AN children (2.9 cases/100,000), 22.4 times higher than White children (95% CI: 11.9–42.1, Table 3). Hia incidence was highest among AI/AN children (8.4 cases/100,000), 16.7 times higher than White children (95% CI: 11.7–24.0). However, incidence of Hia in AI/AN children declined 11.7% annually (95% CI: −15.2% to −8.1%) during this period. Though Hia incidence was relatively low (<1 cases/100,000) among White and Black children, recent increases were observed: 13.6% annual increase (95% CI: 10.7%–16.5%) among White children and 40.4% annual increase (95% CI: 32.1%–49.2%) among Black children (Figure 3, Supplementary Figure 1).
Figure 3. Trends in Estimated Annual Incidence of Invasive H. influenzae serotype a Disease among Children Aged <5 years, by Race — United States, 2008–2017.
Note: Supplementary Figure 1 includes a more detailed view of trends in estimated annual incidence of invasive H. influenzae serotype a disease among white and black children aged <5 years
Abbreviations: PI, Pacific Islander; AI/AN, American Indian and Alaska Native
We examined Hib vaccination status of Hib cases in children aged <5 years reported to ABCs. Among 40 cases reported, 40% were age-appropriately or fully vaccinated. By race, 53.3% of Hib cases among AI/AN children were age-appropriately or fully vaccinated, compared to 32.0% among White and Black children (p=0.20, Supplementary Figure 2). Of Hib cases in age-appropriately and fully vaccinated children, 44% received PRP-OMP, 31% received PRP-T, and 13% received PRP-CRM (Supplementary Table 2). Of Hib cases in age-appropriately or fully vaccinated AI/AN children, 75% received PRP-OMP.
Incidence Trends among Adults Aged ≥65 years
From 2008–2017, 45.4% (3352/7379) of all invasive H. influenzae disease cases reported from ABCs sites occurred among adults aged ≥65 years. Following children aged <1 year, adults aged ≥65 years had the second highest incidence of invasive H. influenzae disease (6.6 cases/100,000; Table 2). Within each racial group, NTHi caused the highest incidence in this oldest age group (range: 3.2–5.7 cases/100,000; Supplementary Table 3). NTHi incidence in adults aged ≥65 years increased among White and Black populations, with average annual increases of 2.9% (95% CI: 2.4%–3.4%) and 3.4% (95% CI: 1.7%–5.1%), respectively, though no pronounced racial disparities were identified. Hia and Hib incidences were significantly higher among AI/AN adults aged ≥65 years when compared to White populations (Hia IRR: 9.3, 95% CI: 3.5–21.8; Hib IRR: 14.2, 95% CI: 4.6–52.0).
Discussion
In the Hib vaccine era, racial disparities in invasive H. influenzae disease persist. In the ABCs surveillance population, disparities affecting AI/AN populations were most evident, with the greatest disparity among AI/AN children aged <5 years who experienced elevated Hia and Hib incidence. White and Black populations showed similar disease trends, with incidence increasing in recent years, especially for Hia and NTHi. Asian/PI populations consistently had the lowest burden of invasive H. influenzae disease.
NTHi was the most common type of invasive H. influenzae disease within all racial groups. Though observed disparities in NTHi incidence were limited, Black children aged <5 years experienced an incidence of NTHi 1.5 times that of White children. A vaccine targeting NTHi could have substantial impact on invasive disease in all racial groups. Recently, a candidate NTHi vaccine (GSK2838504A) finished phase 2 clinical trials in adults at risk for acute exacerbations of chronic obstructive pulmonary disease [24]. While the candidate vaccine demonstrated an acceptable safety profile and was immunogenic, whether it can reduce NTHi carriage and prevent invasive disease remains to be determined.
Pronounced racial disparities in invasive Hia disease were identified. Consistent with other reports of increased Hia incidence among AI/AN communities [25–29], AI/AN children aged <5 years had the largest burden of Hia disease, nearly 17 times the incidence among White children. Hia incidence was lower but rising 13.6% annually among White children and 40.4% annually among Black children. These results, combined with the similarities in clinical presentation and severity between Hia and Hib [30], indicate a growing need for prevention tools. In Canada, a similarly high incidence of Hia disease among indigenous populations prompted the National Research Council of Canada and the Public Health Agency of Canada to develop a Hia vaccine [31, 32], though clinical trials are awaiting funding [33].
In the pre-Hib vaccine era, invasive Hib incidence in Black and AI/AN children aged <5 years was estimated to be 3–4 times and 5–10 times higher, respectively, than White children. Hib incidence estimates ranged from 24–66 cases/100,000 among White children [5, 9], 104–219 cases/100,000 among Black children [5, 9] and 214–705 cases/100,000 among AI/AN children [34, 35]. In the Hib vaccine era, invasive Hib incidence in children aged <5 years declined dramatically (>99%) among all races. Our analysis demonstrates that in the Hib vaccine era, racial disparities in invasive Hib disease have been eliminated among Black children but not among AI/AN children. According to these ABCs surveillance estimates, Hib incidence in AI/AN children remains 2.9 cases/100,000, more than 22 times that of White children and more than 10 times the Healthy People 2020 goal of 0.27 cases/100,000 [36]. The persistence of Hib disease among AI/AN children in the Hib vaccine era may reflect ongoing high nasopharyngeal colonization and transmission.
To address remaining Hib disease and disparities, evaluation of the current vaccination strategy is needed. Data from National Immunization Surveys indicate coverage with the full Hib vaccine series in children aged 19–35 months is not significantly different among racial groups, though no racial group has met the Healthy People 2020 goal of 90% coverage [36]. In our analysis, most Hib cases among White and Black children were unvaccinated or under-vaccinated, highlighting the need to increase vaccine uptake. More than half of Hib cases among AI/AN children were age-appropriately vaccinated, primarily with PRP-OMP, suggesting susceptibility to Hib disease despite vaccination. The Advisory Committee on Immunization Practices recommends AI/AN children preferentially receive a Hib vaccine primary series containing PRP-OMP [19]. While PRP-OMP elicits protective antibodies after the first dose, the overall immunogenicity of the PRP-OMP series is lower when compared to the PRP-T series [37]. Further evaluations are needed to determine duration of protection in AI/AN children receiving the PRP-OMP vaccine series, and whether there is a way to improve protection against invasive Hib disease.
To address racial disparities in invasive H. influenzae disease, updated evaluations of risk factors explaining these disparities are needed. Continued high rates of household crowding [38], poverty [39], and limited access to running water [40, 41] may be contributing factors to the elevated burden of disease among AI/AN populations. Inversely, the observed low incidence of invasive H. influenzae disease among Asian/PI populations may be partially due to lower reported rates of poverty [42].
This analysis had several limitations. First, race data was abstracted from medical charts rather than patient interview; for 9.1% of cases where race was missing, race was multiply imputed. Second, it is difficult to draw clear conclusions about disease trends among racial minorities due to small case counts and unsteady incidence. Lastly, though ABCs includes a similar proportion of AI/AN persons (1.5% in 2017) as the general U.S. population (1.4% in 2017) [18], incidence of invasive H. influenzae is not uniform in all AI/AN communities. Notably, reported Hia incidence in AI/AN children aged <5 years in Alaska (27.7 cases/100,000) [29] is more than 3 times that estimated by ABCs (8.4 cases/100,000). However, similar estimates of Hib incidence were noted in Alaska (2.8 cases/100,000) [26] and ABCs (2.9 cases/100,000). Despite these limitations, ABCs offers the ability to make national incidence projections, highlighting disparities in invasive H. influenzae disease for further investigation.
Our results indicate that racial disparities in invasive H. influenzae disease persist in the United States in the Hib vaccine era, despite remarkable reductions in Hib disease overall. AI/AN communities continue to experience disproportionately high rates of Hib disease. Additionally, Hia disease disproportionately affects AI/AN communities, though Hia incidence among White and Black communities has recently increased. Evaluation of current Hib prevention strategies, efforts to increase Hib vaccine uptake, and new prevention tools for Hia and NTHi could help reduce invasive H. influenzae disease burden overall and address remaining disparities.
Supplementary Material
Key points:
Invasive Haemophilus influenzae serotype a (Hia) incidence increased among White and Black children. American Indian and Alaska Native populations experienced disproportionally high rates of invasive Hia and serotype b (Hib) disease, with the greatest disparity among children aged <5 years.
Acknowledgments
The authors are grateful to the following individuals for their contributions to the establishment and maintenance of the ABCs system. California Emerging Infections Program: Susan Brooks and Hallie Randel. Colorado Emerging Infections Program: Benjamin White, Deborah Aragon, Meghan Barnes, and Jennifer Sadlowski. Connecticut Department of Public Health: Matt Cartter, Carmen Marquez, and Michelle Wilson. Georgia Emerging Infections Program: Stephanie Thomas, Amy Tunali, Wendy Baughman, Ashley Moore, Lauren Lorentzson, and Melissa Tobin-D’Angelo. Maryland Emerging Infections Program: Joanne Benton, Terresa Carter, Rosemary Hollick, Kim Holmes, and Kathleen Shutt. Minnesota Emerging Infections Program: Kathryn Como-Sabetti, Corinne Holtzman, Richard Danila, and Kerry MacInnes. New Mexico Emerging Infections Program: Kathy Angeles, Joseph Bareta, Lisa Butler, Nicole Espinoza, Sarah Khanlian, Robert Mansmann, Megin Nichols, and Lisa Onischuk. New York Emerging Infections Program: Suzanne McGuire, Alison Muse, Glenda Smith, Nancy Spina, and Rachel Wester. Oregon Emerging Infections Program: Mark Schmidt, Jamie Thompson, and Tasha Poissant. Tennessee Emerging Infections Program: Brenda Barnes, Karen Leib, Katie Dyer, Tiffanie Markus, and Lura McKnight. Arctic Investigations Program, CDC: Debby Hurlburt, Danielle Lecy, Gail Thompson, Sara Seeman, Alisa Reasonover, and Carolynn Debyle. Division of Bacterial Diseases, CDC: Melissa Arvay, Olivia Almendares, Huong Pham, the Bacterial Meningitis Laboratory.
Financial support
This work was supported by a cooperative agreement with the Emerging Infections Program of the CDC (CDC-RFA-CK12-120205CONT16).
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest
Lee H. Harrison has served as a consultant to GSK, Merck, Pfizer, and Sanofi Pasteur. William Schaffner has served as a consultant to Merck, Pfizer, and Roche Diagnostics. All other authors: No reported conflicts.
References
- 1.MacNeil JR, Cohn AC, Farley M, et al. Current epidemiology and trends in invasive Haemophilus influenzae disease--United States, 1989–2008. Clin Infect Dis 2011; 53(12): 1230–6. [DOI] [PubMed] [Google Scholar]
- 2.Blain A, MacNeil J, Wang X, et al. Invasive Haemophilus influenzae Disease in Adults >/=65 Years, United States, 2011. Open Forum Infect Dis 2014; 1(2): ofu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soeters HM, Blain A, Pondo T, et al. Current Epidemiology and Trends in Invasive Haemophilus influenzae Disease-United States, 2009–2015. Clin Infect Dis 2018; 67(6): 881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenger JD, Hightower AW, Facklam RR, Gaventa S, Broome CV. Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. The Bacterial Meningitis Study Group. J Infect Dis 1990; 162(6): 1316–23. [DOI] [PubMed] [Google Scholar]
- 5.Granoff DM, Basden M. Haemophilus influenzae infections in Fresno County, California: a prospective study of the effects of age, race, and contact with a case on incidence of disease. J Infect Dis 1980; 141(1): 40–6. [DOI] [PubMed] [Google Scholar]
- 6.Parke JC Jr., Schneerson R, Robbins JB. The attack rate, age incidence, racial distribution, and case fatality rate of Hemophilus influenzae type b meningitis in Mecklenburg County, North Carolina. J Pediatr 1972; 81(4): 765–9. [DOI] [PubMed] [Google Scholar]
- 7.Fraser DW, Darby CP, Koehler RE, Jacobs CF, Feldman RA. Risk factors in bacterial meningitis: Charleston County, South Carolina. J Infect Dis 1973; 127(3): 271–7. [DOI] [PubMed] [Google Scholar]
- 8.Floyd RF, Federspiel CF, Schaffner W. Bacterial meningitis in urban and rural Tennessee. Am J Epidemiol 1974; 99(6): 395–407. [DOI] [PubMed] [Google Scholar]
- 9.Tarr PI, Peter G. Demographic factors in the epidemiology of hemophilus influenzae meningitis in young children. J Pediatr 1978; 92(6): 884–8. [DOI] [PubMed] [Google Scholar]
- 10.Murphy TV, Osterholm MT, Pierson LM, et al. Prospective surveillance of Haemophilus influenzae type b disease in Dallas County, Texas, and in Minnesota. Pediatrics 1987; 79(2): 173–80. [PubMed] [Google Scholar]
- 11.Coulehan JL, Michaels RH, Williams KE, et al. Bacterial meningitis in Navajo Indians. Public Health Rep 1976; 91(5): 464–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Ward JI, Margolis HS, Lum MK, Fraser DW, Bender TR, Anderson P. Haemophilus influenzae disease in Alaskan Eskimos: characteristics of a population with an unusual incidence of invasive disease. Lancet 1981; 1(8233): 1281–5. [DOI] [PubMed] [Google Scholar]
- 13.Losonsky GA, Santosham M, Sehgal VM, Zwahlen A, Moxon ER. Haemophilus influenzae disease in the White Mountain Apaches: molecular epidemiology of a high risk population. Pediatr Infect Dis 1984; 3(6): 539–47. [DOI] [PubMed] [Google Scholar]
- 14.Schlech WF 3rd, Ward JI, Band JD, Hightower A, Fraser DW, Broome CV. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA 1985; 253(12): 1749–54. [PubMed] [Google Scholar]
- 15.Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis 1998; 4(2): 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singleton R, Hammitt L, Hennessy T, et al. The Alaska Haemophilus influenzae type b experience: lessons in controlling a vaccine-preventable disease. Pediatrics 2006; 118(2): e421–9. [DOI] [PubMed] [Google Scholar]
- 17.Langley G, Schaffner W, Farley MM, et al. Twenty Years of Active Bacterial Core Surveillance. Emerg Infect Dis 2015; 21(9): 1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridged-Race Population Estimates, United States. National Center for Health Statistics
- 19.Briere EC, Rubin L, Moro PL, et al. Prevention and control of haemophilus influenzae type b disease: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2014; 63(RR-01): 1–14. [PubMed] [Google Scholar]
- 20.Wang X, Mair R, Hatcher C, et al. Detection of bacterial pathogens in Mongolia meningitis surveillance with a new real-time PCR assay to detect Haemophilus influenzae. Int J Med Microbiol 2011; 301(4): 303–9. [DOI] [PubMed] [Google Scholar]
- 21.Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae, 2nd edition. Geneva: World Health Organization, 2011. [Google Scholar]
- 22.Raghunathan TE, Lepkowski JM, Van Hoewyk J, and Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology 2001; 27(1): 85–95. [Google Scholar]
- 23.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997; 16(7): 791–801. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson TMA, Schembri S, Brightling C, et al. Non-typeable Haemophilus influenzae protein vaccine in adults with COPD: A phase 2 clinical trial. Vaccine 2019; 37(41): 6102–11. [DOI] [PubMed] [Google Scholar]
- 25.Millar EV, O’Brien KL, Watt JP, et al. Epidemiology of invasive Haemophilus influenzae type A disease among Navajo and White Mountain Apache children, 1988–2003. Clin Infect Dis 2005; 40(6): 823–30. [DOI] [PubMed] [Google Scholar]
- 26.Bruce MG, Zulz T, DeByle C, et al. Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983–2011. Emerg Infect Dis 2013; 19(6): 932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruce MG, Deeks SL, Zulz T, et al. Epidemiology of Haemophilus influenzae serotype a, North American Arctic, 2000–2005. Emerg Infect Dis 2008; 14(1): 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plumb ID, Lecy KD, Singleton R, et al. Invasive Haemophilus influenzae Serotype a Infection in Children: Clinical Description of an Emerging Pathogen-Alaska, 2002–2014. Pediatr Infect Dis J 2018; 37(4): 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soeters HM, Oliver SE, Plumb ID, et al. Epidemiology of Invasive Haemophilus influenzae Serotype a Disease — United States, 2008–2017 Clin Infect Dis [in press] 2020. [DOI] [PMC free article] [PubMed]
- 30.Bozio CH, Blain A, Edge K, et al. Clinical severity and sequelae of invasive Haemophilus influenzae serotype a cases — United States, 2011–2015. Clin Infect Dis [in press] 2020. [DOI] [PubMed]
- 31.Cox AD, Barreto L, Ulanova M, Bruce MG, Tsang RSW. Developing a vaccine for Haemophilus influenzae serotype a: Proceedings of a workshop. Can Commun Dis Rep 2017; 43(5): 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreto L, Cox AD, Ulanova M, Bruce MG, Tsang RSW. The emerging Haemophilus influenzae serotype a infection and a potential vaccine: Implementation science in action. Can Commun Dis Rep 2017; 43(3): 85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuchman M. Development of vaccine that could save lives of children in the North at a standstill. CMAJ 2019; 191(10): E293–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coulehan JL, Michaels RH, Hallowell C, Schults R, Welty TK, Kuo JS. Epidemiology of Haemophilus influenzae type B disease among Navajo Indians. Public Health Rep 1984; 99(4): 404–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Ward JI, Lum MK, Hall DB, Silimperi DR, Bender TR. Invasive Haemophilus influenzae type b disease in Alaska: background epidemiology for a vaccine efficacy trial. J Infect Dis 1986; 153(1): 17–26. [DOI] [PubMed] [Google Scholar]
- 36.Healthy People 2020. Available at: https://www.healthypeople.gov/. Accessed 1/3/2020.
- 37.Bulkow LR, Wainwright RB, Letson GW, Chang SJ, Ward JI. Comparative immunogenicity of four Haemophilus influenzae type b conjugate vaccines in Alaska Native infants. Pediatr Infect Dis J 1993; 12(6): 484–92. [DOI] [PubMed] [Google Scholar]
- 38.Pettit KLS, Kingsley GT, Biess J, Bertumen K, Pindus N, Narducci C, Budde A. Continuity and Change: Demographic, Socioeconomic, and Housing Conditions of American Indians and Alaska Natives. In: Development USDoHaU. Washington, D.C., 2014. [Google Scholar]
- 39.Maccartney S, Bishaw A, Fontenot K. Poverty Rates for Selected Detailed Race and Hispanic Groups by State and Place: 2007–2011. In: Bureau USC. Washington, D.C., 2013. [Google Scholar]
- 40.Wenger JD, Zulz T, Bruden D, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J 2010; 29(3): 251–6. [DOI] [PubMed] [Google Scholar]
- 41.Hennessy TW, Ritter T, Holman RC, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health 2008; 98(11): 2072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Proctor BD, Semega JL, Kollar MA. Income and Poverty in the United States: 2015. In: Bureau USC. Washington, D.C.: U.S. Government Printing Office, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.