Abstract
Renal dysfunction is very common among patients with chronic liver disease, and concomitant liver disease can occur among patients with chronic kidney disease. The spectrum of clinical presentation and underlying etiology is wide when concomitant kidney and liver disease occur in the same patient. Management of these patients with dual onslaught is challenging and requires a team approach of hepatologists and nephrologists. No recent guidelines exist on algorithmic approach toward diagnosis and management of these challenging patients. The Indian National Association for Study of Liver (INASL) in association with Indian Society of Nephrology (ISN) endeavored to develop joint guidelines on diagnosis and management of patients who have simultaneous liver and kidney disease. For generating these guidelines, an INASL-ISN Taskforce was constituted, which had members from both the societies. The taskforce first identified contentious issues on various aspects of simultaneous liver and kidney diseases, which were allotted to individual members of the taskforce who reviewed them in detail. A round-table meeting of the Taskforce was held on 20–21 October 2018 at New Delhi to discuss, debate, and finalize the consensus statements. The evidence and recommendations in these guidelines have been graded according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system with minor modifications. The strength of recommendations (strong and weak) thus reflects the quality (grade) of underlying evidence (I, II, III). We present here the INASL-ISN Joint Position Statements on Management of Patients with Simultaneous Liver and Kidney Disease.
Keywords: hepatorenal syndrome, chronic kidney disease, cirrhosis, acute kidney injury
Introduction
Many patients with chronic kidney disease (CKD) or acute kidney disease also develop concomitant acute or chronic liver disease (CLD), and many patients with acute or CLD suffer from concomitant acute or chronic kidney dysfunction. The spectrum is wide and can involve etiologies that can cause both kidney and liver disease (e.g., metabolic syndrome), drugs that are both hepatotoxic and nephrotoxic (e.g., antitubercular therapy), and kidney diseases that occur in the setting of pre-existing liver disease (e.g., hepatorenal syndrome) and vice versa (e.g., hepatitis C infection in dialysis patients). Management of these patients with dual onslaught is challenging and requires a team approach of internal medicine physicians, hepatologists, nephrologists, transplant surgeons, and critical care specialists. No recent guidelines exist on algorithmic approach toward diagnosis and management of these challenging patients. The Indian National Association for Study of Liver (INASL) in association with Indian Society of Nephrology (ISN) endeavored to develop joint guidelines on diagnosis and management of patients who have simultaneous liver and kidney disease.
For generating these guidelines an INASL–ISN Taskforce was constituted, which had members from both the societies. The taskforce first identified contentious issues on various aspects of simultaneous liver and kidney diseases, which were allotted to individual members of the taskforce who reviewed them in detail. A round-table meeting of the Taskforce was held on 20–21 October 2018 at New Delhi to discuss, debate, and finalize the consensus statements. The evidence and recommendations in these guidelines have been graded according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system with minor modifications (Table 1). The strength of recommendations (strong and weak) thus reflects the quality (grade) of underlying evidence (I, II, III). We present here the INASL–ISN Joint Position Statements on Management of Patients with Simultaneous Liver and Kidney Disease.
Table 1.
Grading of Recommendations, Assessment, Development, and Evaluation (GRADE).
| Level of evidence | |
| I | Randomized, controlled trials |
| II-1 | Controlled trials without randomization |
| II-2 | Cohort or case–control analytical studies |
| II-3 | Multiple time series, dramatic uncontrolled experiments |
| III | Opinions of respected authorities, descriptive epidemiology |
| Grade of recommendation | |
| Strong | Factors influencing the strength of the recommendation included the quality of the evidence, presumed patient-important outcomes, and costs |
| Weak | Variability in preferences and values, or more uncertainty. Recommendation is made with less certainty: higher costs or resource consumption |
What is the prevalence of liver disease in patients with chronic kidney disease?
According to Western reports, the prevalence of chronic viral hepatitis (HBV and HCV) is greater in CKD patients than in the general population, especially in those with advanced CKD. CLD secondary to viral hepatitis has been associated with poor outcomes in patients with CKD. However, from India, there is no recent good quality data available for the prevalence of viral hepatitis in CKD patients. Recent small studies have shown that seroprevalence HCV infection ranges between 9.4% and 30.8% in India. Small single-center studies published from north India showed a prevalence of HCV infection from 9.4 to 33.5%1, 2, 3; while similar studies from south India, and east India showed prevalence of 30.8% and 12.1%, respectively.4,5 Hepatitis B prevalence ranges from 1.5 to 5.5%.1,5 Majority of available data are from hemodialysis (HD) (CKD stage 5 D) patient cohort. Higher prevalence of HCV in some of the studies is likely to be due to better screening in those centers. Recently, in the last decade, newer antiviral therapies have resulted in a significant reduction in the prevalence of viral hepatitis in this patient cohort.
The majority of individuals with HCV infection are asymptomatic, making screening necessary to detect infection in CKD patients; this is particularly true for HD patients in whom signs or symptoms of acute HCV infection are rarely recognized.6 In addition, HCV has been identified as an independent risk factor for both CKD onset and rapid CKD progression in multiple studies. Thus, HCV screening is recommended at the time of initial evaluation of CKD. HCV screening is also indicated for patients on maintenance hemodialysis (MHD). In dialysis units with a high prevalence of HCV, initial nucleic acid testing (NAT) should be considered.6
Recently, with the changing metabolic profile of CKD patients, nonalcoholic fatty liver disease (NAFLD) and associated CLD are also emerging as a new risk factor for CLD associated with CKD.7,8 Growing evidence suggests that NAFLD and CKD share common pathogenetic mechanisms.9 However, Indian data regarding the prevalence of NAFLD in CKD population are not available.
There is an urgent need for setting up regional or national collaborative networks for data collection to understand the prevalence of CLD in CKD patients.
Consensus statements
-
•
The prevalence of viral hepatitis is higher in CKD patients than in the general population. (Level of evidence: II-1)
-
•
Patients of CKD on dialysis should be tested for HCV by NAT initially and then every 6 months. (Level of evidence: II-1, strength of recommendation: Strong)
What is the prevalence of kidney disease in patients with chronic liver disease?
Renal failure is a frequent complication of patients with liver cirrhosis, which is associated with increased mortality and morbidity, occurring in one of every five patients with cirrhosis.10,11 Renal dysfunction is detected in 20–50% of patients who are admitted to the hospital with decompensated cirrhosis.12 However, the prevalence of renal dysfunction varies widely in reported studies based on criteria used in various studies.
There are different causes of renal dysfunction. Besides acute kidney injury (AKI), chronic kidney failure induced by comorbidities like diabetes mellitus, arterial hypertension, or specific causes such as immunoglobulin A nephropathy or glomerulopathy is frequent; however, the prevalence is still unknown. A large cohort study is needed to determine the precise incidence and prevalence of AKI based on the IAC criteria.
Consensus statements
-
•
Renal dysfunction occurs in approximately 20–50% of hospitalized patients with decompensated cirrhosis. However, the prevalence of renal dysfunction varies widely in reported studies based on variations on criteria to define renal dysfunction and on study population. (Level of evidence: II-2)
-
•
A well-designed large prospective multicenter cohort study among patients with CLD and cirrhosis is needed to determine the accurate incidence and prevalence of AKI based on the criteria defined by AKI network. (Strength of recommendation: Strong)
Pathophysiology and interpretation of renal function test in patients with liver disease
The kidney functions to excrete nitrogenous waste, that is, urea and creatinine, maintain water and salt balance and act as an endocrine organ secreting erythropoietin (EPO) and vitamin D. However, in patients with cirrhosis, the pathophysiology of renal functions is altered due to the effect of cirrhotic liver. The fibrotic liver tissue leads to an increased intrahepatic vascular resistance, causing portal hypertension, and vasodilators accumulate in the splanchnic area and, in the systemic circulation. This causes a pooling effect in the splanchnic vessels, leading to increased shear-wall stress and transudation of plasma into the abdominal cavity as ascites. As a consequence, effective circulating blood volume and mean arterial pressure are decreased. This activates the sympathetic nervous system, initiating a hyperdynamic circulation and also stimulating the renin–angiotensin–aldosterone system (RAAS). Excessive RAAS activation promotes water and sodium retention, thereby aggravating ascites formation and high levels of angiotensin II induce renal vasoconstriction. The higher prevalence of Systemic inflammatory response syndrome (SIRS) and sepsis in cirrhosis also supposedly lead to renal blood flow redistribution, resulting in ischemia and subsequent tubular injury. The LPS increases portal pressure and may induce hepatocyte death, thereby promoting hepatic decompensation.
Estimation of renal function is not accurate in the presence of cirrhosis. It is well known that serum creatinine, the most common marker of liver function, is neither a sensitive nor an accurate marker of kidney function in these patients. It causes overestimation of renal function and misclassification of kidney disease stage and delays the diagnosis and treatment of such patients.13,14 Creatinine, an endogenous compound derived from the creatinine in the muscle that is freely filtered by the glomerulus, is the most widely used surrogate marker of renal function. The sCr concentration is influenced not only by GFR but also by other physiological processes, collectively termed ‘‘non-GFR determinants,’’ including creatinine generation by muscle, dietary intake, tubular creatinine secretion, and extrarenal creatinine elimination by the gastrointestinal tract. In addition, in liver disease decreased production of creatine by the liver, protein-calorie malnutrition and low muscle mass reduce creatinine production. Body weight can be greatly affected by edema and ascites and reduces the creatinine value by hemodilution. Serum bilirubin being a chromogen interferes with the creatinine measurement my spectroscopy and can lead to falsely low creatinine measurements. Thus, on an individual basis, serum creatinine should be interpreted with caution in cirrhotic patients due to frequent overestimation of renal function.
Keeping these limitations in mind, we still recommend that renal function in patients with liver disease, serum creatinine should be measured by Jaffe reaction using alkaline picrate method. Expert professional bodies have recommended that all creatinine methods should become traceable to a reference method based on using the international standard reference traceable to isotope dilution mass spectrometry.15
The glomerular filtration rate (GFR) is the volume of fluid filtered from the kidney glomeruli into the Bowman's capsule per minute and is the best overall index of kidney function in health and diseases.16 In stable patients, GFR is used to define CKD, while the acute changes in markers of GFR are used to define AKI.17 While the GFR can be measured directly by clearance studies of exogenous markers, such as inulin, iohexol, iothalamate, and Cr51-EDTA, these procedures are costly and time-consuming and are not suited to the routine detection of kidney disease. In clinical practice, endogenous substance creatinine is more commonly used to estimate the GFR: the creatinine clearance (CrCl) test. CrCl is calculated from the creatinine concentration in the collected urine sample, urine flow rate, and the plasma concentration. CrCl is slightly higher than true GFR because creatinine is also secreted by the proximal tubule.15 Studies have also shown that, in cirrhotic patients, CrCl, compared with inulin clearance, overestimates true GFR by a mean of about 13 mL/min/1.73 m2 of surface area.18, 19, 20 Overestimation is highest in patients with low GFR.20
Measurement of GFR using the clearance of endogenous substance, such as creatinine (CrCl), still requires both serum and an accurately timed urine collection, so efforts have been directed at more convenient “urine-free” estimates of GFR.21 Estimated GFR (eGFR) is a mathematically derived entity based on a patient's serum creatinine level, age, sex, and race. A number of formulas have been devised to calculate the eGFR values on the basis of serum creatinine levels. Some of these formulas are Cockcroft–Gault formula; Modification of Diet in Renal Disease (MDRD) formula; CKD-EPI formula; Mayo Quadratic formula; and Schwartz formula. However, all eGFR equations overestimate the kidney function in the patients with liver disease. To use any equation, the renal function should be stable, and in AKI, these equations cannot be used. We recommend that in patients with liver disease, the eGFR should be assessed using MDRD-6-based equation.
Cystatin C (CysC) is a 13-kDa protein, produced by all nucleated cells at constant rate. CysC is almost completely filtered by the glomeruli and almost completely reabsorbed and catabolized by the proximal tubular cells. Thus, when GFR decreases, plasma CysC increases. Compared with SCr, CysC has several advantages, not being influenced by gender, ethnicity, sarcopenia, and/or liver diseases.22 CysC has been found to be an accurate biomarker of GFR in patients with cirrhosis, showing a better correlation with inulin clearance than SCr.17,23,24 We suggest CysC or Cystatin eGFR as confirmatory renal function testing in patients with liver disease.25
Blood urea nitrogen (BUN) is a waste product formed in the liver. The normal BUN levels are 7–20 mg/dl and are elevated in renal failure, urinary tract obstruction, congestive heart failure, recent heart attack, gastrointestinal bleeding, dehydration, shock, severe burns, and after high-protein diet. Although BUN is widely used in clinical practice, it has limited utility when used alone for the estimation of GFR. Indeed, in patients with advanced liver disease, BUN may increase due to impaired liver function with alterations of urea cycle, malnutrition, or gastrointestinal bleeding.22 However, BUN is still useful for the assessment of MDRD equation and still represents a parameter used for the timing of renal replacement therapy (RRT).16,17 The plasma BUN/creatinine ratio is usually 10 to 15:1 when both are expressed as mg/dl in normal individuals and in AKI but may be greater than 20:1 in prerenal AKI because of the increase in the passive reabsorption of urea, gastrointestinal bleeding, tissue and high catabolic status. The BUN/creatinine ratio was a better index than the MELD score in predicting in-hospital mortality in cirrhotic patients with normal renal function.26
Hyponatremia is frequently seen in patients with ascites secondary to advanced cirrhosis and portal hypertension and is defined when serum Na <130meq/L. Hyponatremia may be either with hypovolemia due to diuretics or with hypervolemia due to expanded extracellular fluid volume due to the inability of the kidneys to excrete solute-free water.
Albuminuria/proteinuria refers to abnormal loss of albumin/protein in the urine. Albuminuria or proteinuria is common in glomerular disease and rules out hepatorenal syndrome. Urinary proteinuria should be <500 mg per day to diagnose hepatorenal syndrome (HRS). Urine in liver disease usually shows no proteinuria or hematuria. We suggest initial testing of proteinuria by urine albumin-to-creatinine ratio (ACR) or urine protein-to-creatinine ratio. Sodium excretion is lower, and urinary osmolality is usually more than plasma osmolality. Twenty-four-h urine sodium excretion or measurement of urine spot Na/K may be used to predict diuretic resistance. Ascites leads to decreased effective intravascular volume, and urinary Na excretion reduces (FENa <1 or Urinary Na <10 mEq/l). Use of diuretics may increase the urinary sodium excretion. Patients who gain weight despite excreting more than 78 mEq Na/day are not compliant with the diet. A single, intravenous 80-mg dose of furosemide is given, and urinary sodium is measured in the next 8 h. Patients with diuretic resistance have sodium excretion <50 mEq/8 h. Spot urine Na/K ratio can be used as an easier alternative to 24-h urinary sodium excretion, with adequate accuracy.27 Urinary spot Na/K ratio is significantly lower in the diuretic-resistant group.
Consensus statements
-
•
Serum creatinine estimation is recommended as the initial test for assessment of renal function in patients with liver disease. (Level of evidence: II-2, strength of recommendation: strong)
-
•
The eGFR should be assessed using MDRD-6-based equation. (Level of evidence: II-2, strength of recommendation: strong)
-
•
The CrCl test overestimates GFR. (Level of evidence: II-2)
-
•
CysC or Cystatin eGFR can be used as confirmatory renal function testing in liver disease. (Level of evidence: II-3, strength of recommendation: weak)
-
•
The initial testing of proteinuria should be done by urine ACR or urine protein-to-creatinine ratio. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Twenty-four-h urine sodium excretion or measurement of urine spot Na/K may be used to predicts diuretic resistance. (Level of evidence: II-2, strength of recommendation: strong)
Evaluation of severity of liver disease in the presence of end-stage renal disease on hemodialysis
The evaluation of severity of liver disease should be done in patients with CKD with risk factors like hepatitis B, hepatitis C, alcohol abuse, or NAFLD.28, 29, 30 The aim of this assessment is to diagnose cirrhosis to decide about renal transplant alone or simultaneous liver–kidney transplantation. The gold standard for evaluation of the severity of liver disease in the presence of CKD is a liver biopsy. However, biopsy is invasive and associated with complications like postprocedural pain and intra-abdominal bleeding, especially in end-stage renal disease (ESRD) patients.31 The evaluation of the severity of liver disease by noninvasive methods is an appealing option; however, limited studies have assessed the efficacy of these markers in ESRD.32 The use of noninvasive biomarkers like aspartate aminotransferase–platelet ratio index (APRI), Fibrotest, FIB-4 index, and transient elastography (TE) are emerging as accurate tools in patients with CKD similar to the general population.33 In a study by Schiavon et al., the diagnostic value of noninvasive markers to stage liver fibrosis in 203 ESRD HCV-infected patients was studied, and 24% patients were diagnosed with significant fibrosis. This study identified AST and platelet count as independent predictors of significant fibrosis, and APRI had a high predictive value for assessment of liver fibrosis, obviating the need for a liver biopsy in a substantial proportion of patients.34 The noninvasive method of TE is sufficiently reliable to detect extensive fibrosis and/or cirrhosis obviating the need for a liver biopsy in patients with CKD. In a study by Liu et al., 284 HD patients with chronic hepatitis C (CHC) were assessed for liver fibrosis by TE, and APRI was performed before liver biopsy. TE was superior to APRI in determining the severity of hepatic fibrosis and can substantially decrease the need for staging liver biopsy in HD patients.28 Thus, it can be recommended that TE can be used with fair reliability for assessment of fibrosis; and APRI can be used as initial screening test and in places where TE is not available. Taneja et al. studied the optimal timing of TE for the assessment of liver fibrosis in CKD patients on HD. This study showed that there was a significant reduction in LSM after HD and LSM assessment after HD performed better at detecting significant fibrosis. They recommended that TE should be done after HD for fibrosis assessment.35
Consensus statements
-
•
Noninvasive tests are recommended as initial tests for the assessment of the liver fibrosis stage among patients with ESRD with liver disease. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Noninvasive tests like AST Platelet Ratio Index and TE or fibroscan have a good negative predictive value. (Level of evidence: II-2)
Liver biopsy in patients with chronic kidney disease: indications and safety
Assessment of activity of the liver disease is an important issue, which helps in deciding treatment and predicting response to treatment of liver disease. In patients with associated CKD and its advanced-stage ESRD, it further becomes important as advance liver disease makes patient unfit for isolated renal transplant or lowers patient position in priority list for isolated organ allocation. Two histological features define the liver disease status: necroinflammation and fibrosis. While necroinflammation is potentially reversible, fibrosis, in majority of situations, is irreversible and mostly progressive. It is also believed that fibrosis does not develop without necroinflammation.
There are reasons to believe that liver function in patients with advanced CKD and ESRD has to be differently viewed than without CKD. Firstly, CKD/ESRD being immunocompromised states, immune response to viral injury in terms of elevated liver enzymes is not proportional to liver injury, and in 25% of cases, enzymes may not be elevated in spite of definite histological necroinflammation. Due to same reason, in case of hepatitis B, it may be difficult to differentiate immune-tolerant state from active disease. Hence, liver biopsy for assessing disease activity in patients with compensated CLD and CKD is recommended, especially when noninvasive test results are discordant, or a coexisting other liver disease is suspected.36 Secondly, as CKD/ESRD often have an abnormal coagulation profile due to various reasons, risk of bleeding following liver biopsy in these patients is more. Thus, liver biopsy as such in these patients should be done carefully and by the person who is regularly doing liver biopsy in these patients. In patients with early (up to CKD-IIIa) percutaneous liver biopsy may be safe. However, if risk of bleeding is more, especially in patients with advanced CKD, approaches other than percutaneous, like transjugular or laproscopic, should be selected. It is also advisable to use 16G cutting sheathed biopsy needle for getting adequate biopsy core for interpretation.
In the last decade, for avoiding liver biopsy complications, which sometimes can be fatal, noninvasive tests are being developed so as to avoid liver biopsy completely. There are three groups of noninvasive tests: indirect biomarkers, direct biomarkers, and elastography. These tests have not been adequately validated in patients with CKD/ESRD. In addition, these tests do not adequately differentiate moderate degree of fibrosis from very mild to advanced fibrosis. These are good for extreme degree of fibrosis: either very mild or very advanced. It is a general impression that in CKD/ESRD patients, a combination of noninvasive tests may be better option than any single test alone. If patients already have obvious clinical features of advanced/decompensated liver disease, liver biopsy is usually not required.
Consensus statements
-
•We suggest liver biopsy for assessing patients with compensated CLD and CKD when:
-
○noninvasive test results are discordant or
-
○a coexisting other liver disease is suspected. (Level of evidence: II-2, strength of recommendation: strong)
-
○
-
•
In patients with early (up to CKD-IIIa) percutaneous liver biopsy may be safe; however, in advanced CKD, approaches other than percutaneous, like transjugular or laproscopic should be selected. (Level of evidence: II-3)
Hepatitis vaccination in patients with chronic kidney disease and after renal transplantation
Routine hepatitis B screening and vaccination are recommended for all patients on MHD or peritoneal dialysis (PD), including those on home dialysis, who are susceptible to HBV infection. Hepatitis B screening and vaccination are also recommended for persons with pre-ESRD who are not yet on dialysis. In such patients, vaccination is best begun before the onset of dialysis, in view of higher rate of hepatitis B seroconversion rate and higher antibody levels when vaccination is done before than after starting maintenance dialysis. Vaccination is not indicated if a person already has HBV infection or antibodies to HBV; however, testing for these is not required before vaccination is begun.
Patients with renal failure should receive a higher vaccine dosage (double the usual dose of HB vaccine or 40 μg intramuscular each [may be given as 20 μg each at two separate sites on each occasion], at 0, 1, and 6 months) or more (six) doses than usual. For patients on MHD, a faster vaccination schedule may be used; however, such cases should receive an additional 4th dose (0, 1, 2, 6, or 12 months).
Patients on MHD should be tested for level of antibody to hepatitis B surface antigen (anti-HBs) 1–2 months after administration of the last dose of the vaccine series. Those lacking protective levels (>10 mIU/mL) should receive a second vaccine series and retested 1–2 months after its last dose. Those who still do not achieve protective level should be tested for HBsAg. If HBsAg is positive, the person should be referred for appropriate management, and no further doses of vaccine should be given.
Patients on MHD should be tested annually for anti-HBs, and a single booster vaccine dose should be administered if and when anti-HBs levels decline to <10 mIU/mL.37
Kidney transplant recipients who lack protective anti-HBs level (>10 IU/mL) should receive a complete course of hepatitis B vaccination, preferably at a time of less intense immunosuppression (such as after the first 3 months of transplantation), followed by anti-HBs testing 1–3 months after the last dose.37
In kidney transplant recipients who have previously had an adequate response to hepatitis B vaccine, anti-HBs level should be checked every 6–12 months and booster doses administered if the levels fall below <10 IU/mL or are expected to fall below this cut-off in the next 3–6 months, if the risk of HB infection continues to be high.37
Consensus statements
-
•
Screening for hepatitis B infection is indicated in all CKD patients at the time of diagnosis and again prior to initiation of dialysis. (Level of evidence: I, strength of recommendation: strong)
-
•Routine hepatitis B vaccination is recommended for the following:
-
○All patients on MHD or PD, including those on home dialysis.
-
○Persons with pre-ESRD who are not on dialysis.
-
○Kidney transplant recipients who lack protective anti-HBs level (>10 IU/mL). (Level of evidence: I, strength of recommendation: strong)
-
○
-
•
Vaccination is not indicated if a person already has hepatitis B virus infection. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Patients with renal failure should receive higher vaccine dosages (double the usual dose of HB vaccine or 40 μg intramuscular, at 0, 1, and 6 months) or more (six) doses than usual. For patients on MHD, a faster vaccination schedule may be used; however, such cases should receive an additional 4th dose (0, 1, 2, 6, or 12 months). (Level of evidence: II-2, strength of recommendation: strong)
-
•Patients on MHD should be tested for level of antibody to hepatitis B surface antigen (anti-HBs) 1–2 months after administration of the last dose of the vaccine series.
-
○Those lacking protective levels (>10 mIU/mL) should receive a second vaccine series and be retested 1–2 months after its last dose. Those who still fail to achieve protective level should be tested for HBsAg and linked to treatment if positive. (Level of evidence: II-2, strength of recommendation: strong)
-
○
-
•
Patients on MHD should be tested annually for anti-HBs, and a booster vaccine dose administered if and when the levels decline to <10 mIU/mL. (Level of evidence: II-3, strength of recommendation: strong)
-
•
In kidney transplant recipients, anti-HBs level should be checked every 6–12 months and booster doses administered when the levels are below <10 IU/mL or are expected to fall below this cut-off in near future, if the risk of HB infection continues to be high. (Level of evidence: II-2, strength of recommendation: strong)
How do you prevent hepatitis B virus and hepatitis C virus in dialysis unit?
Patients undergoing dialysis treatment, and in particular HD, are at increased risk for contracting viral infections. This is due to their underlying impaired cellular immunity, which increases their susceptibility to infection. In addition, the process of HD requires blood exposure to infectious materials through the extracorporeal circulation for a prolonged period. Moreover, HD patients may require blood transfusion, frequent hospitalizations, and surgery, which increase opportunities for nosocomial infection exposure. The most frequent viral infections encountered in HD units are HBV, HCV and, to a lesser extent, human immunodeficiency virus (HIV) infection.38,39
Fortunately, the risk of transmission of these infections to dialysis patients has been reduced quite significantly in the last three decades. This has been due to opting vaccination for HBV to all CKD patients, decreased use of blood transfusion with increasing use of erythropoietin, improvement in transfusional safety, and implementation of universal precautions. However, despite stringent measures, failures of infection control mechanisms leading to isolated outbreaks of HBV infection in HD centers are still reported often.40 Further preventive strategies that have been developed over the past 25 years include the increased availability of disposable dialyzers, sophisticated machines with electronic fail-safe systems, the replacement of arteriovenous shunts with fistulae, durable synthetic grafts and cuffed indwelling venous catheters, the routine viral screening of blood donors and the launching of recombinant human erythropoietin in 1989 to substitute for or reduce the need for blood transfusions.38 HD facilities should adhere to standard infection-control procedures including hygienic precautions that effectively prevent transfer of blood and blood-contaminated fluids between patients to prevent transmission of blood-borne pathogens. Universal precautions are a standard set of guidelines aimed at preventing the transmission of blood-borne pathogens from exposure to blood and other potentially infectious materials. These procedures are now standard practice and include hand-washing after touching blood or body fluid, and the use of gowns and face shields when exposure is anticipated.41 For the prevention of blood-borne infection in the dialysis units “Universal Precautions” must be strictly adhered to 38,39,41, 42, 43. Regular audits of infection control procedures in HD units should be performed.
Isolation of dialysis patients and machines in separate rooms/halls, to prevent or reduce nosocomial transmission and seroconversion of viral hepatitis in HD units, remains a controversial issue. In view of the high incidence of transmission of HCV in dialysis units, it may be prudent to consider segregation of HCV-infected patients in units with high prevalence. Many studies have reported a significant drop in the prevalence of HCV-positive patients after applying an isolation policy.38,39,44 We also suggest preferably single use of dialyzer/dialyzers of HCV-infected patients. These can be reused if there is adherence to standard infection-control procedures. HD centers should track all new cases of HCV infections in their patients.
The guidelines for HBV infection control in dialysis units were published in 2001.45 These include HBV vaccination, screening of HD patients, and segregation of those that are infectious. Safe, sharp handling is advised, as is avoidance of multidose vials for intravenous drugs. Other measures that have contributed to a reduction in infection risk include a widespread move from reusable membranes toward disposable dialysers and the introduction of synthetic erythropoietin with a decrease in blood transfusion. There is strong epidemiological evidence that separation of HBV-infected dialysis patients reduces HBV transmission among patients in dialysis centers. Hence, we recommend the segregation of HBsAg-positive patients on dialysis and dedicated dialysis machines for HBsAg-positive patients.
Guidelines published by INASL in 2018 and by Indian Society Nephrology in 2016 recommend that all patients with CKD should be screened for HBsAg at diagnosis.37,46,47 Patients on HD should be screened on initiation of dialysis and subsequently every 6 months. All CKD patients who are HBsAg-negative should be vaccinated for HBV. CKD patients not on dialysis should receive standard dose (20 mcg) of recombinant vaccine at usual schedule of 0, 1, 2, and 6 months. CKD patients on dialysis or those with eGFR <30 mL/min should receive double dose (40 mcg) of recombinant vaccine at accelerated schedule of 0, 1, 2, and 6 months. The anti-HBs should be checked annually, and booster doses should be given when anti-HBs falls below 10 mU/mL46,47.
Response rates of patients on HD to HBV vaccination vary between 10% and 50%.48, 49, 50 Early vaccination before the onset of dialysis therapy is the most important determinant of high seroconversion rates. Male gender, older age, duration of dialysis therapy, dialysis adequacy, nutritional status, HCV positivity, diabetes mellitus, erythropoietin resistance, vitamin D inadequacy, and hyperparathyroidism are other well-known factors associated with seroconversion rate.48 However, strategies to improve vaccination response (adjuvants GMCSF/IL-2 injection or intradermal injection) are not recommended.
Consensus statements
-
•
For the prevention of blood-borne infection in the dialysis units “Universal Precautions” must be strictly adhered to. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Regular audits of infection control procedures in HD units should be performed. (Level of evidence: II-3, strength of recommendation: strong)
-
•
In view of the high incidence of transmission of HCV in dialysis units, it may be prudent to consider segregation of HCV-infected patients in units with high prevalence. (Level of evidence: II-3, strength of recommendation: strong)
-
•
We recommend the segregation of HBSAg-positive patients on dialysis and dedicated dialysis machines for HBsAg-positive patients. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Hepatitis B vaccination is recommended to every patient: 40 μg of Recombivax HB or 40 μg of Engerix B at 0, 1, 2, and 6 months should be administered. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Strategies to improve vaccination response (adjuvants GMCSF/IL-2 injection or intradermal injection) is not recommended. (Strength of recommendation: strong)
How do you manage chronic hepatitis B in patients with chronic kidney disease?
The HBV management guidelines for patients with CKD were recently published by the INASL.46,47 The following section is reproduced from the same.46
Hepatitis B positive patients with CKD/dialysis should be evaluated for the extent of liver disease and viral activity. This should include ALT level, HBe antigen status, HBV DNA viral load, and the presence of significant inflammation and/or fibrosis (by noninvasive methods or liver biopsy). Assessment of the degree of liver fibrosis helps to determine treatment and prognosis. Noninvasive tests such as TE can be used and are particularly useful in identifying those with cirrhosis but have not been tested much in patients with CKD/dialysis. Liver biopsy can assess both fibrosis and/or inflammation but carries a higher risk of complications in CKD patients.28,51 Additional parameters to consider are the minimal or no increase in transaminases and the lower viral load levels in these patients because of their clearance by dialysis.52,53
All HBsAg-positive CKD/dialysis patients should be considered for antiviral treatment if they have HBV DNA levels >2000 IU/mL, significant fibrosis on noninvasive tests (Fibroscan >8.0 kPa) or moderate inflammation and/or significant fibrosis on histology, irrespective of ALT levels.51,54,55
NAs are recommended as the first line of treatment. Entecavir (ETV) clears HBV DNA in up to 100% of naive patients at 24 months, with approximate time to undetectability and ALT normalization at a median of 12.6–15.7 months, without any significant toxicity. In LAM-resistant patients the response to ETV becomes less with a clearance rate of only 45% at 24 months.56,57 Tenofovir Disoproxil Fumarate (TDF) or Tenofovir alafenamide (TAF) is the preferred alternative in LAM-resistant patients, with an expected clearance rate of 86% at 96 weeks.51 Although there is potential nephrotoxicity with the use of TDF,58 this has not been widely studied in CKD patients. There are, however, documented reports of chronic tubular damage with hypophosphatemia, and a decline of GFR and bone mineral density in some patients (up to 15%).59 In renal transplant recipients, TDF appears to be safe, and small studies have shown that they were well tolerated with no significant renal toxicity.30,60 A combination of TDF and telbivudine has been shown to improve GFR and may be considered an alternative in CKD patients and renal transplant recipients; however, studies with this combination are limited.61
TAF is a promising alternative to tenofovir disoproxil, both in terms of efficacy and toxicity. Pivotal studies have shown that TAF is noninferior to TDF for DNA clearance and more importantly had a smaller reduction in CrCl at week 48 and week 96.62,63 TAF has not been tested in patients with CKD, on dialysis and after renal transplant; however, it remains a promising alternative, especially in those with LAM-resistant hepatitis B.
All doses of NAs should be adjusted according to eGFR or CrCl values in patients with eGFR <50 mL/min, except for TAF, which does not require dose adjustment if eGFR is >15 mL/min. For CrCl 30–49 mL/min: 300 mg of TDF 300 mg or ETV 0.5 mg should be given every 48 h. For CrCl 10–29 mL/min, TDF 300 mg or ETV 0.5 mg should be given every 72–96 h. For subjects receiving MHD, TDF 300 mg or ETV 0.5 mg administered every 7 days after a hemodialysis session is recommended.
All renal transplant recipients who are HBsAg positive should receive antiviral prophylaxis with NAs (prophylactic therapy, at least 2 weeks before renal transplant). ETV is the preferred option due to its renal safety, and TDF or TAF can be used in LAM-resistant patients. Treatment should be continued indefinitely. HbsAg-positive patients with low DNA (less than 2000 IU/mL) with evidence for liver disease or with a high DNA >2000 IU/mL should be treated immediately at the time of diagnosis. HBsAg-negative, anti-HBc-positive patients do not require antiviral treatment; they should be monitored for HBsAg reactivation every 6 months and treated only if there is a seroconversion.54,64 However, ABO-incompatible transplants are given higher immunosuppressants, such as rituximab. There are data to show that there is a significant risk of HBV flares with higher immunosuppressants like rituximab used for other diseases in patients with isolated anti-HBc positive.46 These patients should be given prophylactic antivirals for first 6 months of transplant, starting 2 weeks prior to transplant.
Consensus statements
-
•All CKD patients who are HBsAg positive:
-
○Should be evaluated for extent of liver disease and viral activity, and treatment should be based on standard guidelines for HBV patients. (Level of evidence: I, strength of recommendation: strong)
-
○NAs (dose according to GFR) are recommended as the first-line treatment. TAF, ETV, and TDF are the preferred choices. (Level of evidence: I, strength of recommendation: strong)
-
○For subjects receiving MHD, TDF 300 mg or ETV 0.5 mg administered every 7 days after a hemodialysis session is recommended. (Level of evidence: I, strength of recommendation: strong)
-
○
-
•All renal transplant recipients who are
-
○HBsAg-positive should receive life-long antiviral prophylaxis with TAF, ETV, or TDF. (Level of evidence: I, strength of recommendation: strong)
-
○HBsAg-negative and unvaccinated should receive HBV vaccination with double dose (40 mcg) at accelerated schedule (0, 1, 2, and 6 months). Their anti-HBs titers should be checked 1–2 months after vaccination and revaccinated if anti-HBs is below 10 IU/mL. (Level of evidence: II-1, strength of recommendation: strong)
-
○HBsAg-negative and previously vaccinated should have annual anti-HBs titers checked and booster doses be given if titers fall below 10 IU/mL. (Level of evidence: II-3, strength of recommendation: strong)
-
○HBsAg-negative, anti-HBc-positive should be monitored for HBsAg and ALT every 6 months. (Level of evidence: II-3, strength of recommendation: strong)
-
○
What is the management of chronic hepatitis C in patients with chronic kidney disease?
Patients with CHC and CKD are a special group not only because of high prevalence of CHC in CKD but also because these patients tend to have normal ALT; often have negative anti-HCV antibody with only HCV RNA positivity; and thus have difficulty in the evaluation of severity of their liver disease. Further, in contrast to non-CKD patients in India, patients with CKD in India, predominantly have genotype 1 infection.2
There are three major issues while treating CHC in CKD. First issue relates to deciding about kidney transplantation alone versus simultaneous liver kidney (SLK) transplantation depending on the severity of liver disease and the presence or absence of clinically significant portal hypertension (CSPH). This issue is discussed later. Second issue is regarding the use of various directly acting antivirals (DAAs) in CKD depending on the GFR and the availability of drugs. The third issue relates to treatment of CHC before or after renal transplantation. Even if the patient is not a candidate for renal transplantation, CHC should be treated in a patient with ESRD on dialysis because of the increased morbidity and mortality in these patients because of HCV.65
As per the recent recommendations of the American Association for the Study of Liver Diseases (AASLD), no dose adjustment in DAAs is required for treating CHC patients with CKD stages 1–3, and all drugs including sofosbuvir, daclatasvir, simeprevir, fixed-dose combination of ledipasvir/sofosbuvir, fixed-dose combination of sofosbuvir/velpatasvir, fixed-dose combination of elbasvir/grazoprevir, fixed-dose combination of glecaprevir/pibrentasvir, and fixed-dose combination of sofosbuvir/velpatasvir/voxilaprevir can be without dose modification, as used in non-CKD patients.66 Recommendations for treating CHC patients with CKD stage 4–5 include usage of fixed-dose combination of Elbasvir (50 mg)/Grazoprevir (100 mg) for 12 weeks for Genotype 1a, or 1 b, or 4 infection and use of Glecaprevir (300 mg)/Pibrentasvir (120 mg) for 8–16 weeks in Genotype 1, 2, 3, 4, 5, or 6 infection.66 These recommendations are based on the results of C-SURFER study67, which used grazoprevir plus elbasvir in genotype 1 patients with CKD stage 4–5 and Expedition 4 study68, which used glecaprevir and pibrentasvir in all HCV genotype patients with severe renal impairment and showed sustained virologic response at 12 weeks (SVR12) of 96–99% with no major side effects. Unfortunately, the DAAs recommended by these two studies67,68 (Grazoprevir/elbasvir and glecaprevir/pibrentasvir) are not available in India at present, and we have to rely on the sofosbuvir-based regimens used in combination with daclatasvir or ledipasvir or velpatasvir (drugs currently available in India).69,70 Sofosbuvir a NS5B inhibitor predominantly has renal excretion and pharmacokinetic studies have shown high serum levels of sofosbuvir and its metabolites in patients with severe renal insufficiency.71 But for some recent data, the clinical relevance of the high levels of sofosbuvir and its metabolites in patients with CKD stage 4–5, however, has not been established, and there are data on the use of both half-dose and full-dose sofosbuvir in patients with CKD stage 4−572, 73, 74, 75, 76, 77, 78, 79. Studies using both half-dose (200 mg daily or 400 mg alternate days) and full-dose (400 mg/day) sofosbuvir in combination with other drugs in patients with CKD stage 4–5 have shown SVR12 of 85–100% with minor side effects and worsening of renal functions in only one real-life study from the United States.74 In fact, one of the recent studies documented the efficacy of full-dose sofosbuvir and velpatasvir in patients undergoing dialysis with no treatment-related discontinuations or serious adverse events.80 Data from India suggest that sofosbuvir half-dose (200 mg/day) in combination with daclatasvir irrespective of the genotype in CHC patients with CKD stage 4–5 is as efficacious (SVR12 100%) as the full dose (400 mg/day) with minor side effects reported in both regimens.75, 76, 77, 78, 79 Based on the available data, both European Association for the Study of the Liver (EASL) and Asia–Pacific Association for the Study of the Liver (APASL) suggest the use of sofosbuvir-based regimens in patients with ESRD either with caution or with weak recommendations, respectively.81,82 However, some of the recent data do suggest the reduction of eGFR and occurrence of tubular injury with increasing neutrophil gelatinase-associated lipocalin (NGAL) in patients using sofosbuvir-based regimens especially in patients with risk factors for CKD.83,84 Hence, till we have more data, a caution needs to be exercised while using sofosbuvir-based regimens in patients with CKD.
If cirrhosis with CSPH is excluded and an early renal transplantation is possible then CHC patients can also be treated with DAAs after the renal transplantation. The advantages of treating CHC before renal transplantation include reducing the HCV burden prior to transplant, thereby reducing the risk of fibrosing cholestatic hepatitis and drug interactions, which might occur between DAAs and the immunosuppressive drugs. However, the advantages of treating postrenal transplantation includes decrease in waiting time for the transplant; sometimes even allowing use of anti-HCV-positive donor; and the availability of wide range of DAAs in view of normal renal functions postrenal transplantation. Hence, the decision to treat prior or after the renal transplantation can be taken based on the feasibility of early renal transplantation and the local experience and practice.
As per the recent recommendations of the AASLD, postrenal transplant patients with CHC Genotype 1 or 4 infection should be treated with daily fixed-dose combination of glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks or daily fixed-dose combination of ledipasvir (90 mg)/sofosbuvir (400 mg) for 12 weeks.66 Patients with Genotype, 2, 3, 5, or 6 should be treated with glecaprevir (300 mg)/pibrentasvir (120 mg) for 12 weeks or daily daclatasvir (60 mg) plus sofosbuvir (400 mg) plus low initial dose of ribavirin (600 mg; increase as tolerated) for 12 weeks. Experience from India and abroad have shown good SVR12 with DAAs in postrenal transplant CHC patients with minor side effects.85, 86, 87, 88 Because of the availability of only four DAAs (sofosbuvir, daclatasvir, ledipasvir, and velpatasvir) in India, combination of these drugs can be used safely in these patients depending on the genotype and the severity of liver disease. Caution should be taken while using ribavirin in postrenal transplant CHC patients because of the severe anemia, which might occur.87
Consensus statements
-
•
CHC patients with mild to moderate renal impairment (CKD stage 1–3, CrCl >30 mL/min) can be treated as non-CKD patients with no dose modification required for sofosbuvir, daclatasvir, ledipasvir, and velpatasvir. (Level of evidence: I, strength of recommendation: strong)
-
•
CHC patients with severe renal impairment (CKD stage 4–5, CrCl < 30 mL/min) can be treated with a daily combination of sofosbuvir (200 mg) and daclatasvir (60 mg) for 12–24 weeks depending on the genotype and the presence or absence of cirrhosis. (Level of evidence: II-1, strength of recommendation: strong)
-
•
CHC patients can also be treated with DAAs after the renal transplantation if cirrhosis with CSPH is excluded and an early renal transplantation is possible. (Level of evidence: II-3, strength of recommendation: strong)
-
•
All patients with ESRD with CHC, who are not candidates for renal transplantation, should also receive treatment for HCV infection. (Level of evidence: II-2, strength of recommendation: strong)
Diagnosis and management of nonalcoholic fatty liver disease and metabolic syndrome in patients with chronic kidney disease
Fatty liver disease is the most common liver disease in the world. About 25% of adults in the Western population have fatty livers in the absence of excessive alcohol consumption, a condition termed NAFLD.89 Growing evidence suggests that NAFLD and CKD share common pathogenetic mechanisms,9 and further, NAFLD is an independent risk factor for development of CKD (stage ≥3). A large meta-analysis90 of 9 observational studies with 96,595 adult individuals (34.1% with NAFLD) of predominantly Asian descent, and 4653 cases of incident CKD stage ≥3 (i.e., defined as occurrence of estimated GFR<60 mL/min/1.73 m2) suggested that NAFLD (detected by biochemistry, fatty liver index, or ultrasonography [US]) is associated with a nearly 40% increase in the long-term risk of incident CKD. However, the observational nature of the eligible studies did not allow for proving causality.90 Similarly, NASH has also been shown to be associated with higher risk of incident CKD (stage ≥3).91 According to a meta-analysis on 11,109,003 participants from 66 studies, metabolic syndrome and its components are independently associated with the increased risk of CKD.92
In clinical practice, ultrasound is the first modality of choice to diagnose liver steatosis in patients with CKD. TE along with controlled attenuation parameter (CAP) is an emerging tool to assess steatosis.8,28,35 Liver biopsy is gold standard for the diagnosis of NAFLD/NASH; however, it is not commonly done in patients with CKD. Limited data suggest the utility of metabolic syndrome and other noninvasive tools like NAFLD fibrosis score (>1.45), fibrosis-4 index, FIB-4 (>3.25), TE for identifying NAFLD patients with higher likelihood of having advanced fibrosis in patients with CKD.36 Metabolic syndrome is most commonly diagnosed by National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria, as it is easy to use.93,94
Lifestyle modification including dietary modification, exercise, and weight loss improves NAFLD in patients of CKD. There are no data on use of pharamacotherapy for the treatment of NAFLD in CKD.95,96
Consensus statements
-
•
Ultrasound is the first modality of choice to diagnose liver steatosis in patients with CKD. (Level of evidence: II-2, strength of recommendation: strong)
-
•
TE along with CAP is an emerging tool to assess steatosis. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Liver biopsy is gold standard for the diagnosis of NASH. It is not commonly done in patients with CKD. (Level of evidence: II-2)
-
•
Limited data suggest the utility of metabolic syndrome and other noninvasive tools like NAFLD fibrosis score (>1.45), fibrosis-4 index, FIB-4 (>3.25), TE for identifying NAFLD patients with higher likelihood of having advanced fibrosis in patients with CKD. (Level of evidence: II-3, strength of recommendation: weak)
-
•
NAFLD is an independent risk factor for development of CKD (stage≥ 3). (Level of evidence: II-2)
-
•
NASH has been shown to be associated with higher risk of incident CKD (stage≥ 3). (Level of evidence: II-2)
-
•
Metabolic syndrome is most commonly diagnosed by National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria as easy to use. (Level of evidence: I, strength of recommendation: strong)
-
•
Metabolic syndrome according to different definitions, independent of its single component, diabetes status, study type, ethnicity, and sex, is associated with CKD risk. (Level of evidence: II-2)
-
•
Lifestyle modification improves NAFLD in patients of CKD. (Level of evidence: I, strength of recommendation: strong)
-
•
There are no data on use of pharamacotherapy for treatment of NAFLD in CKD. (Level of evidence: III)
Safety of drugs for portal hypertension in patients with chronic kidney disease?
Nonselective beta-blockers, terlipressin, somatostatin, and octreotide are the commonly used drugs for portal hypertension. According to a meta-analysis of eight trials, published by Badve et al. in 2011, it was found that treatment with beta-blockers improved all-cause mortality in patients with CKD and chronic systolic heart failure.97 Even though these patients were not of cirrhosis, it can still be concluded that beta-blockers can be safely used in patients with CKD who have portal hypertension.
Terlipressin is a vasopressin analogue, useful for management of portal hypertensive bleeding and hepatorenal syndrome. Terlipressin is converted to the lysine vasopressin in the circulation after the N-triglycyl residue is cleaved by endothelial peptidases.98 Only about 1% of the dose of terlipressin administered is excreted unchanged in the urine, which indicates almost complete metabolism by peptidases. Although terlipressin is the drug of choice for patients with AKI due to hepatorenal syndrome, its effect on patients of CKD has not been studied. Hence, it should be used with caution with strict monitoring in patients with CKD. Terlipressin use should be avoided in patients with history of coronary artery disease, cardiac arrhythmias, cardiomyopathies, obliterative arterial disease of the lower limbs, bronchial asthma, chronic obstructive pulmonary disease, cerebrovascular disease, and age >70 years.
Somatostatin is a cyclic hormone-release inhibitory peptide. Somatostatin significantly reduces portal and variceal pressure, and azygos flow is superior to placebo in controlling variceal hemorrhage. Somatostatin and its derivative octreotide are often used for the emergency treatment of bleeding esophageal varices in patients with cirrhosis of the liver.99 The effect of somatostatin and octreotide in patients of CKD has not been studied. For mild, moderate, or severe renal impairment (non-dialysis patients), there is no need to adjust the initial dose of octreotide; the maintenance dose should be adjusted based on clinical response and tolerability.
Consensus statements
-
•
Beta-blockers can be safely used in patients with CKD. (Level of evidence: I, strength of recommendation: strong)
-
•
Terlipressin has limited data in CKD and can be used with caution under strict monitoring. (Level of evidence: II-3, strength of recommendation: strong)
-
•
In patients with mild, moderate, or severe renal impairment (nondialysis patients), there is no need to adjust the initial dose of octreotide; the maintenance dose should be adjusted based on clinical response and tolerability. In patients with severe renal impairment (on dialysis), the data are limited on the use of octreotide. (Level of evidence: II-3, strength of recommendation: strong)
Evaluation and management of cirrhotic ascites in patients of chronic kidney disease
Ascites is the most common cause of decompensation in cirrhosis, as 5%–10% of patients with compensated cirrhosis per year develop this complication.100 The evaluation of cirrhotic ascites in patients with CKD is not different from patients without CKD. Diagnostic paracentesis including calculation of serum-ascites albumin gradient (SAAG) should be performed in patients with new onset or worsening of pre-existing ascites and in patients who are hospitalized with complications of cirrhosis. The calculation of SAAG is useful when the cause of ascites is not immediately evident, as SAAG ≥1.1 g/dl indicates that portal hypertension is involved in ascites formation with an accuracy of about 97%.100,101
A moderate restriction of sodium intake (80–120 mmol/day, corresponding to 4.6–6.9 g of salt) is recommended in patients with moderate, uncomplicated ascites. Salt restriction with loop diuretics like furosemide may be initiated in Grade I/II ascites in patients with CKD. Aldosterone antagonists are weak diuretics and should be avoided due the risk of hyperkalemia in patients with low GFR. In cirrhotic patients with CKD, large volume paracentesis with albumin infusion (8 g/l of ascites removed) may be used in-patient with gross ascites (Grade III ascites) causing marked abdominal distension. Caution must be exercised to avoid fluid overload.
TIPS cannot be recommended in view of absence of data in patients with CKD. Liver–kidney simultaneous transplantation should be considered in patients with refractory ascites in CKD.
Consensus statements
-
•
Diagnostic paracentesis including calculation of SAAG should be performed in patients with new onset or worsening of pre-existing ascites and in patients who are hospitalized with complications of cirrhosis. (Level of evidence: I, strength of recommendation: strong)
-
•
Salt restriction with loop diuretics like furosemide may be initiated in Grade I/II ascites in patients with CKD. (Level of evidence: II-1, strength of recommendation: strong)
-
•
Aldosterone antagonists are weak diuretics and should be avoided due the risk of hyperkalemia in patients with low GFR. (Level of evidence: II-2, strength of recommendation: strong)
-
•
In cirrhotic patients with CKD, large-volume paracentesis with albumin infusion (8 g/l of ascites removed) may be used in-patient with gross ascites (Grade III ascites) causing marked abdominal distension. (Level of evidence: I, strength of recommendation: strong)
-
•
Caution must be exercised to avoid fluid overload. (Strength of recommendation: strong)
-
•
TIPS cannot be recommended in view of absence of data in patients with CKD. (Level of evidence: III, strength of recommendation: weak)
-
•
SLK transplantation should be considered in patients with refractory ascites due to cirrhosis and concomitant CKD. (Level of evidence: II-3, strength of recommendation: strong)
Options for renal replacement therapy in decompensated cirrhosis with chronic kidney disease-5
RRT either in the form of HD/slow/sustained low efficiency dialysis or continuous veno-venous hemodiafiltration should be considered, particularly in the presence of intractable fluid overload, acidosis, uremic symptoms, and electrolyte abnormalities (i.e., hyperkalemia, hyponatremia, and hypercalcemia). Usage of RRT in patients who are not candidates for liver transplantation (LT) remains somewhat controversial as it is not likely to influence long-term outcomes. However, lack of transplant candidacy should not be used as the sole determinant of futility in patients with refractory HRS, and a trial of RRT may be given for a predefined duration. The decision between intermittent hemodialysis (IHD)/SLED or continuous renal replacement therapy (CRRT) is based on clinical characteristics of the patient, local availability, and the expertise at the center for different modalities. There is no documented superiority of CRRT versus SLED versus IHD in mortality or recovery of renal function. CRRT is tolerated better in hemodynamically unstable liver failure patients and is documented to show lower cerebral edema, common in patients with fulminant hepatic failure. Intraoperative and postoperative CRRT in liver transplant recipients should be done in the presence of renal failure, volume overload, and dyselectrolemia. Extracorporeal liver support systems such as extracorporeal albumin dialysis (MARS) and fractional plasma separation and adsorption with HD (Prometheus) do provide a bridge to liver transplant in severely decompensated cirrhotic patients—however, no improvement in mortality seen in randomized trials over standard of care treatment combined with routine RRTs.
Consensus statements
-
•
RRT and its type in patients with decompensated cirrhosis and CKD-5 should be decided case-to-case basis as per the clinical situation and indication. (Level of evidence: II-3, strength of recommendation: strong)
Anti-tubercular treatment (ATT) in patients with chronic liver disease and chronic kidney disease
CKD correlates with increased risk of pulmonary tuberculosis.102 The risk further increases in those receiving HD.103 All patients of CKD with active TB should be treated with four agents.104 The first-line treatment is with rifampicin, isoniazid, pyrazinamide, and either ethambutol or moxifloxacin with pyridoxine. Patients with renal disease have a higher incidence of adverse effects related to antituberculous drugs than patients with normal renal function and should be managed by physicians experienced in the management of TB. To avoid accumulation, changes to regimens that include pyrazinamide and ethambutol must be made for patients with advanced stages of CKD or on RRT. However, dose adjustment can lead to decreased efficacy as these drugs exhibit concentration-dependent activity. In view of this, increasing the dose interval to three times weekly is recommended in stages 4 and 5 CKD and in patients on HD, as evidence suggests increased efficacy using this approach. Both rifampicin and isoniazid can be given at the normal daily dose. Moxifloxacin is frequently substituted for ethambutol but is only suitable for daily dosing.104
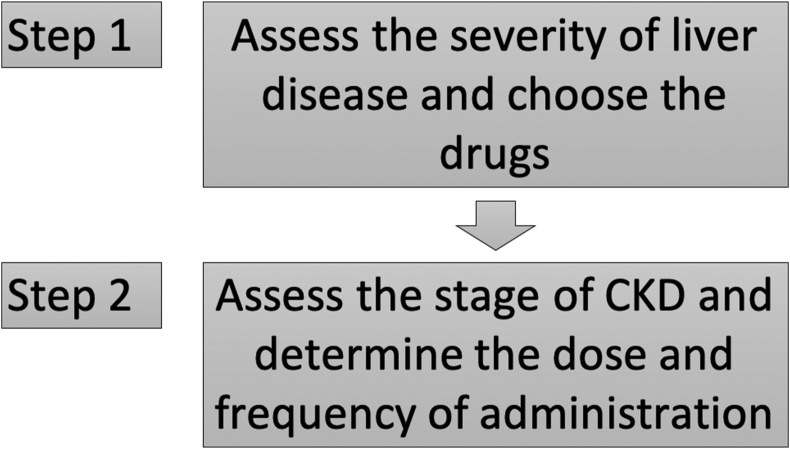
In patients with simultaneous CLD and CKD, the choice of antitubercular drugs should be based on the severity of liver disease, and the dose of the drugs should be based on severity of kidney disease105 (Figure 1). Pyrazinamide should be avoided in all patients with liver cirrhosis because liver injury may be severe and prolonged. In stable patients with liver cirrhosis (CTP score ≤7), regimens with two potentially hepatotoxic drugs (rifampicin and isoniazid) are likely to be well tolerated. In patients with advanced liver disease (CTP score 8–10), a regimen with only one potentially hepatotoxic drug is recommended; rifampicin is preferred over isoniazid. In the setting of severe unstable liver disease (CTP score >10), where hepatic decompensation and complications of cirrhosis are evident, a regimen with no hepatotoxic agents is recommended.105
Figure 1.
Algorithm for choosing anti-tubercular drugs for patients with chronic liver disease and chronic kidney disease.
Consensus statements
-
•
In patients with concomitant cirrhosis and CKD, the choice of antitubercular drugs should be based on the severity of liver disease, and the dose of the drugs should be based on severity of kidney disease. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Pyrazinamide should be avoided in all patients with liver cirrhosis because liver injury from this drug may be severe and prolonged. (Level of evidence: II-2, strength of recommendation: strong)
-
•
In stable patients with liver cirrhosis (CTP score ≤7), regimens with two potentially hepatotoxic drugs (rifampicin and isoniazid) are likely to be well tolerated. (Level of evidence: II-1, strength of recommendation: strong)
-
•
In patients with advanced liver disease (CTP score 8–10), a regimen with only one potentially hepatotoxic drug is recommended; rifampicin is preferred over isoniazid. (Level of evidence: II-1, strength of recommendation: strong)
-
•
In the setting of severe unstable liver disease (CTP score >10), where hepatic decompensation and complications of cirrhosis are evident, a regimen with no hepatotoxic agents is recommended. (Level of evidence: II-1, strength of recommendation: strong)
-
•
No dose adjustment is needed for isoniazid, rifampicin and moxifloxacillin for patients with stages 4 and 5 CKD. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Dosing intervals should be increased to three times weekly for ethambutol, pyrazinamide, and the aminoglycosides for patients with stages 4 and 5 CKD. (Level of evidence: II-2, strength of recommendation: strong)
What is the spectrum and definition of acute kidney injury in patients with cirrhosis?
Prevalence and spectrum of acute kidney injury in cirrhosis
The exact incidence and prevalence of renal disorders in patients with cirrhosis is largely unknown, and probably underestimated. In a prospective study from India, Prakash et al reported 24.5% prevalence of renal diseases in patients of cirrhosis at BHU Varanasi.106 The differential diagnosis of acute renal failure/AKI in their cirrhotic patients was acute tubular necrosis (ATN) (44.4%), prerenal failure (36.4%), and HRS (19.2%). Several other studies from Western countries, although mostly retrospective, reported similar AKI incidence rate with prerenal failure and ATN being most common etiologies.10,107, 108, 109
Definition of acute kidney injury in cirrhosis
Currently, studies on AKI in patients with cirrhosis showed that AKI defined by an absolute increase in serum creatinine ≥0.3 mg/dl and/or ≥50% increase from baseline are associated with a higher probability of the patients being transferred to the intensive care unit, a longer hospital stay, and an increased in-hospital as well as 90-day and mid-term mortality.110, 111, 112, 113, 114, 115, 116, 117 We propose the same definition as adapted by International Club of Ascites (ICA).118
Staging and course of acute kidney injury
AKI as diagnosed with modified KDIGO criteria has been shown to be associated with increased mortality in patients with cirrhosis who were hospitalized in regular wards in a modified KDIGO stage-dependent fashion. The progression of AKI through stages (e.g., from stage 1–2 or stage 2–3) was strongly correlated with an increased mortality in these patients.110, 111, 112 We propose the same staging and course classification as adapted by ICA118 (Table 2).
Table 2.
International Club of Ascites (ICA-AKI) new definitions for the Diagnosis and Management of AKI in Patients with cirrhosis.
| Subject | Definition | ||
|---|---|---|---|
| Baseline sCr | A value of sCr obtained in the previous 3 months, when available, can be used as baseline sCr. In patients with more than one value within the previous 3 months, the value closest to the admission time to the hospital should be used. | ||
| In patients without a previous sCr value, the sCr on admission should be used as baseline. | |||
| Definition of AKI |
|
||
| Staging of AKI |
|
||
| Progression of AKI | Progression | Regression | |
| Progression of AKI to a higher stage and/or need for RRT | Regression of AKI to a lower stage | ||
| Response to treatment | No response | Partial response | Full response |
| No regression of AKI | Regression of AKI stage with a reduction of sCr to 0.3 mg/dl (26.5 μmol/l) above the baseline value | Return of sCr to a value within 0.3 mg/dl (26.5 μjmol/L) of the baseline value | |
AKI, acute kidney injury; RRT, renal replacement therapy; sCr, serum creatinine.
Consensus statements
-
•
Clinical renal diseases are common in decompensated cirrhotic patients, and AKI is the commonest kidney disease. (Level of evidence: II-2)
-
•
Spectrum of AKI in decompensated cirrhosis includes prerenal, ATN, and hepatorenal syndrome. (Level of evidence: II-2)
-
•AKI in cirrhosis is now defined as:
-
○Acute increase of SCr ≥0.3 mg/dl within 48 h
-
○A percentage increase SCr ≥50% from baseline, which is known, or presumed, to have occurred within the prior 7 days (Level of evidence: I)
-
○
-
•
The classification and course of AKI in cirrhosis are the same as described by the ICA criteria (Table 2). (Level of evidence: I)
Renal involvement in acute on chronic liver failure
Acute on chronic liver failure (ACLF) is a distinct clinical entity defined as acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed CLD.119,120 AKI is one of the major features of ACLF and a major component in grading the severity of ACLF.12 AKI is the defining feature of ACLF as per the EASL-CLIF definition121 and is not a defining feature as per the APASL definition.119,120 AKI can be broadly classified as HRS-AKI and non-HRS-AKI. The pattern of AKI observed in patients admitted to the hospital with ACLF is likely to be one of inflammatory kidney injury including acute tubular injury (non-HRS-AKI) rather than HRS-AKI.122 AKI in ACLF patients is more likely associated with structural kidney injury, and is more progressive, with a poorer response to terlipressin treatment and a worse prognosis than that in patients with decompensated cirrhosis.123
Consensus statements
-
•
AKI is common in patients with ACLF and is associated with poor outcomes. (Level of evidence: II-2)
How will you diagnose and treat HRS?
In the revised consensus of the ICA a modified version of the KDIGO criteria was used to define AKI in patients with cirrhosis. According to the revised consensus criteria, AKI was defined either by an increase in sCr of more than 0.3 mg/dl (≥26.5 μmol/l) within 48 h or by a percentage increase in sCr of more than 50% from the baseline, which is known, or presumed, to have occurred within the previous 7 days. Type 1 HRS according to the revised criteria is now defined as “HRS-AKI.” Further, in the revised consensus, the previous type 2 HRS was proposed to be considered as a type of CKD called as HRS-CKD. We recommend that for diagnosis of HRS-AKI we should use the same definition as ICA.
The other major change that was considered in the revised consensus criteria was removal of any cut-off value for sCr both from the definition of AKI and HRS. This is because even though sCr remains the most practical biomarker for the assessment and management of renal failure in patients with cirrhosis it is associated with several limitations. It lags behind the onset of disease process and is affected by numerous extrarenal factors such as race, age, body weight, total body volume, sex, drugs, muscle metabolism, and protein intake and is falsely recorded low in sarcopenic patients with advanced cirrhosis. Levels of sCr are also affected by increased tubular secretion of creatinine and the presence of hyperbilirubinemia in these patients. SCr also does not provide any relevant information regarding the etiology of kidney injury, that is, the presence of underlying structural kidney damage and therefore the response to various treatment modalities.118,124 It may be debatable whether it is worthwhile to retain a cut-off value for sCr along with the AKI criteria. At least two prospective studies in hospitalized patients with cirrhosis have recently shown the significance of retaining a cut-off value of sCr of 1.5 mg/dl to predict both progression and resolution of AKI.111,112 It was further reported that patients with AKI stage 1 could be substratified into two groups based on absolute value of sCr of 1.5 mg/dl. The short-term mortality of patients with peak sCr of less than 1.5 mg/dl was similar to those without AKI while those with peak sCr more than 1.5 mg/dl had a significantly higher mortality. Patients with AKI stage 2 and 3 had the highest mortality and also higher progression of AKI. Results of these two studies imply that AKI episodes with a peak sCr of less than 1.5 mg/dl may not be of clinical relevance. Contrary to this, Thalheimer and Burroughs and Wong et al. have reported that AKI with a peak sCr of less than 1.5 mg/dl is not a benign condition.125,126 Similarly, Tsien et al. reported worse prognosis for patients with AKI irrespective of the peak sCr.113 Therefore, future studies in large series of patients should address the question of whether a threshold of Scr of 1.5 mg/dl is of prognostic value. In patients with ACLF, there are further discrepancies in the absolute value of sCr and therefore even though 1.5 mg/dl signifies significant renal injury; however, interventions should not be delayed based on whether a patient meets the absolute value of sCr rather relative increases in sCr are relevant for management of AKI in patients with ACLF.127
According to the new consensus by the ICA for AKI a new algorithm for the management of AKI based on the revised criteria has been proposed. On the basis of this algorithm, it is recommended that patients with initial AKI stage 1 should initially be assessed for precipitants (careful review of medications, diuretics, nephrotoxic drugs, vasodilators or nonsteroidal anti-inflammatory drugs). In the second step, plasma volume expansion in patients with hypovolemia along with identification and early treatment of bacterial infections is needed. The choice of fluid could either be a crystalloid or albumin or even blood as indicated. Patients who respond with a decrease in sCr value of 0.3 mg/dl of the baseline value should be subsequently followed up for any new episodes of AKI. Patients with progression should be managed as ICA-AKI stage 2 and 3. In this group of patients, along with the institution of all measures as recommended for patients with stage 1 AKI, a workup for the differential diagnosis should be done on an immediate basis to identify whether it is HRS-AKI, intrinsic AKI, or postrenal cause. Patients with HRS-AKI are recommended to be managed with early use of vasoconstrictors based on the revised criteria for HRS-AKI.118,124 Combination of albumin and vasoconstrictors is superior to vasoconstrictor alone in HRS-AKI.128, 129, 130, 131 Terlipressin is the vasoconstrictor of choice, and it should be administered as continuous infusion. Other vasoconstrictors used in management of HRS-AKI are norepinephrine and midodrine plus octreotide. A comparison of noradrenaline with terlipressin in randomized controlled trials as well as in meta-analysis has shown noradrenaline to be as effective and safe as terlipressin in the management of HRS.132, 133, 134, 135, 136, 137, 138 However, in a recent randomized controlled trial in patients with ACLF with HRS-AKI diagnosed according to the revised consensus criteria terlipressin was shown to be superior to noradrenaline.139 Importantly, terlipressin was given as a continuous infusion in this trial. Adverse effects were more frequently noted with terlipressin compared with noradrenaline; however, most of these were mild and well tolerated. Comparison of octreotide and midodrine in a randomized controlled trial, however, showed a significantly higher rate of recovery of renal function with terlipressin (70.4%) compared with octreotide and midodrine (28.6%).140 The trial had to be stopped early after the interim analysis reported a superiority of the intervention (terlipressin) compared with the standard therapy (midodrine plus octreotide). In a recent randomized controlled trial, a combination of furosemide, albumin and dopamine was shown to be non-inferior to terlipressin and albumin in management of HRS.141 Further, terlipressin has a superior efficacy if given as continuous infusion compared with bolus infusion.
The response to vasoconstrictors in AKI-HRS has been reported in only 35–50% which is also associated with improved survival.142 Various predictors that predict nonresponse to treatment include a higher value of sCr and total serum bilirubin, failure to achieve an increase in the mean arterial pressure and cardiac output, and the presence of underlying tubular dysfunction.143 Patients with ACLF also have an inferior response to terlipressin possibly because of higher structural AKI or tubular dysfunction secondary to severe inflammation, increased severity of bacterial infections, and higher prevalence of extrarenal organ failures. Further, in context of ACLF severity of grade is associated with nonresponse to the drug.144 Rodriguez et al. recently evaluated patients with HRS associated with bacterial infections wherein poor response to terlipressin was directly related to the severity of liver failure as well as nonresolution of infection.145 A strong interaction was noted between resolution of bacterial infection as well as severity of liver failure and the extrahepatic organ failures.145,146 Similar to this, we have also reported an inferior response of terlipressin in patients with ACLF. However, it remains to be clarified whether the poor response to terlipressin in patients with ACLF is associated with a progression to acute tubular necrosis, an inadequate cardiac output secondary to bacterial infection, severity of systemic inflammation, or a different pathophysiological basis of kidney injury in these patients.145, 146, 147, 148, 149, 150
There is paucity of data on dialysis in patients with cirrhosis therefore no recommendations can be proposed regarding the dose, the intensity, duration, and time of initiation of dialysis in these patients.151 Further, it has been shown by various studies in the past that patients with cirrhosis with deteriorating kidney function who require dialysis while awaiting LT have a high mortality in the absence of transplantation.152, 153, 154, 155, 156
Patients with HRS-AKI should be considered for an early LT. The utility of artificial liver support in the management of patients with cirrhosis and ACLF with HRS also remains questionable because of the lack of adequately powered trials. Artificial liver support with its ability to transiently improve renal function could be used as a bridging therapy to LT in these patients.157, 158, 159 Finally, a focused and appropriate management of extrarenal organ failures in ACLF patients with AKI is also important as this might well impact the overall prognosis as well as response to vasoconstrictors.
Consensus statements
-
•
For diagnosis of HRS-AKI we should use the same definition as ICA. (Level of evidence: I, strength of recommendation: strong)
-
•
Vasoconstrictors should be combined with intravenous albumin for management of HRS-AKI. (Level of evidence: I, strength of recommendation: strong)
-
•
Terlipressin is the vasoconstrictor of choice. Terlipressin should be administered as continuous infusion in management of HRS. (Level of evidence: I, strength of recommendation: strong)
-
•
Noradrenaline has been shown to be as effective as terlipressin in the management of HRS in patients with cirrhosis. (Level of evidence: I, strength of recommendation: strong)
-
•
Patients with HRS-AKI should be considered for an early LT. (Level of evidence: II-3, strength of recommendation: strong)
-
•
High serum creatinine, bilirubin, and failure to achieve mean arterial pressure predict nonresponse in HRS. (Level of evidence: I)
-
•
Patients with ACLF have an inferior response to terlipressin possibly because of higher structural AKI or tubular dysfunction secondary to severe inflammation, increased severity of bacterial infections, and higher prevalence of extrarenal organ failures. (Level of evidence: II-2)
-
•
Artificial liver support needs further evaluation in management in patients with ACLF and HRS. (Level of evidence: III, strength of recommendation: weak)
Prevention of contrast-induced nephropathy in patients with chronic liver disease
Contrast-induced nephropathy (CIN) is defined as an increase in serum creatinine by 25% or by at least 0.5 mg/dl from the pre-exposure value. The timing of the assessment of renal function is typically after 48160 or 72 h161 but may be rarely delayed till day 5. A rise in creatinine in the initial 12 h may be predictive of occurrence of CIN.162 Although it is known that intravascular contrast is associated with CIN, the risks are more pronounced for intra-arterial procedures such as a coronary angiogram and are negligible for intravenous contrast exposure like contrast-enhanced CT scans.163,164 If the patients are critically ill, the risk is higher.165
In a retrospective study of 216 consecutive patients with cirrhosis who underwent CT scan with IV contrast those with ascites, undergoing contrast-related procedures, are at an increased risk of CIN (OR 3.38, 95% CI 1.55–7.34).166 However, when the benefit is likely to be greater than the risk, one may proceed with the desired investigation after taking informed consent. All patients of CLD who are planned for a contrast-related procedure should undergo risk assessment including a baseline creatinine. Use of lower doses of contrast agent is associated with lesser occurrence of CIN167,168; hence, for prevention of CIN, use the lowest possible effective dose of contrast and avoid performing repeated studies that are closely spaced (within 48–72 h). For repeated investigations, noncontrast alternative investigations should be considered, if possible. In normal individuals, the risk of CIN is similar with both high-osmolar and low-osmolar contrast media. However, low-osmolar contrast media is less nephrotoxic in those at high risk of CIN.169,170 Hence, in patients with CLD at increased risk of CIN, use either iso-osmolar or low-osmolar iodinated contrast media, rather than high-osmolar iodinated contrast media. Volume expansion with intravenous normal saline with careful monitoring of fluid status should be preferred over no hydration. Measures such as preprocedural administration of n-acetyl cysteine, fenoldopam, or theophylline have not been found to be useful, either alone or along with volume expansion. Post-contrast exposure HD or hemofiltration are also not useful to prevent CIN.
Consensus statements
-
•
CLD especially with ascites is a risk factor for development of AKI in patients undergoing procedures requiring contrast. (Level of evidence: II-2)
-
•
Use the lowest effective dose possible of contrast and avoid performing repeated studies that are closely spaced (within 48–72 h). (Level of evidence: II-2, strength of recommendation: strong)
-
•
Use either iso-osmolar or low-osmolar iodinated contrast media, rather than high-osmolar iodinated contrast media in patients at increased risk of CI-AKI. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Volume expansion with IV Normal saline with careful monitoring of fluid status should be preferred over no hydration. (Level of evidence: II-2, strength of recommendation: strong)
-
•
N acetyl cysteine has no additional advantage either alone or along with volume expansion. (Level of evidence: II-2)
-
•
No benefit of either prophylactic HD or hemofiltration in decreasing the incidence of CIN-AKI. (Level of evidence: II-2)
Transplantation in patients with chronic liver disease + chronic kidney disease or chronic liver disease+ acute kidney injury: single versus dual versus sequential
Kidney injury is frequently seen in patients with end-stage liver disease from cirrhosis and liver failure.171 Among select patients, SLK transplantation provides improved post-transplant graft and patient outcomes compared with LT alone. However, it is a challenge to determine the precise population, based on severity of liver disease and reversibility of renal dysfunction that would benefit from SLK. The criteria for SLK allocation are relatively homogeneous among patients with ESRD with cirrhosis and among patients with cirrhosis and CKD. However, these are quite heterogeneous among patients with cirrhosis and AKI, mainly due to inability to accurately differentiate cause of AKI especially hepatorenal syndrome versus intrarenal etiology.171
According to a consensus summit meeting172 in 2012, the following criteria were considered as an indication for SLK in patients who were on the liver transplant waitlist and had CKD for more than 3 months: eGFR ≤40 mL/min (MDRD–6 equation), proteinuria ≥2 g a day, kidney biopsy showing >30% global glomerulosclerosis/interstitial fibrosis, or metabolic disease. The summit attendees also considered the following criteria as an indication for SLK in patients who were on the liver transplant waitlist and have AKI: candidates with persistent AKI for ≥ 4 weeks with either stage 3 AKI as defined by modified RIFLE or eGFR ≤35 mL/min (MDRD–6 equation) or GFR ≤ 25 mL/min (iothalamate clearance). We adopted similar criteria for our Indian patients.
Consensus statements
-
•SLK is recommended in patients with AKI in the presence of
-
○eGFR of <25 mL per minute for >6 weeks and documented every 7 days, or
-
○On sustained dialysis for >6 weeks, or
-
○A combination of the above criteria for >6 weeks (Level of evidence: II-2, strength of recommendation: strong)
-
○
-
•Among patients with CKD on MHD and concomitant cirrhosis, SLK is recommended for
-
○Symptomatic portal hypertension or
-
○CSPH. (Level of evidence: II-2, strength of recommendation: strong)
-
○
-
•SLK should also be considered in patients with advanced liver disease with simultaneous CKD not on dialysis with:
-
○Estimated eGFR by MDRD 6 ≤ 40 mL/min or GFR≤ 30 mL/min by iothalamate clearance.
-
○Advanced histological findings on kidney biopsy with
- > 30% global glomerulosclerosis or
- > 30% interstitial fibrosis. (Level of evidence: II-2, strength of recommendation: Strong)
-
○
-
•SLK is also recommended for
-
○Metabolic disease: primary hyperoxaluria, atypical and hemolytic uremic syndrome, familial amyloidosis, and methylmalonic aciduria.
-
○Genetic disease: ARPKD with extensive liver involvement. (Level of evidence: II-2, strength of recommendation: strong)
-
○
Transplantation in patients with chronic liver disease + acute kidney injury
LT in patients with preoperative renal insufficiency confers a poorer outcome than in those with normal renal function. Sharma et al. have shown that GFR at LT was the only independent predictor of post-LT outcome including development of CKDCRF.173 They have also shown that the survival benefit of LT significantly decreased as pretransplant creatinine increased for candidates within same MELD categories.174 Studies have also showed that in pre–liver transplant period, patients with AKI requiring RRT have a higher mortality rate than patients not requiring RRT.175 Hence, every effort should be made to identify the cause and treat aggressively AKI preoperatively. Aggressive treatment of pre-LT HRS with vasoconstrictor results in better survivals. Restuccia et al. showed that patients with HRS treated with vasopressin analogues before LT had a posttransplantation outcome similar to that of patients transplanted with normal renal function.176 Similarly, Moreau et al. showed that in patients with type 1 HRS, terlipressin-induced improved renal function was associated with an increase in survival post-LT.177 The etiology of AKI on outcomes following LT is also important; Nadim et al. have shown that the patient survival and renal outcomes 1 and 5 years after LT were significantly worse for those with pre-op ATN than HRS. At 5 years, the incidence of CKD (stage 4 or 5) was statistically higher in the ATN group versus the HRS group.178
As mentioned before, the estimation of renal function may not be accurate in the presence of cirrhosis. Since serum creatinine is neither a sensitive nor an accurate marker of kidney function in these patients, the diagnosis of AKI in the pretransplant setting maybe difficult and delayed.13,14 Human studies have shown that NGAL levels (either urine or serum) might be helpful to detect early-stage AKI in numerous clinical situations.22 A study by Gomaa et al. suggested the use of urinary NGAL (uNGAL) or uNGAL/urinary creatinine concentration (UCC) ratio alone or in combination with serum CysC is a valuable marker in the early detection of HRS in patients with decompensated liver cirrhosis.14 In patients with cirrhosis and infections, measurement of urinary NGAL at infection diagnosis is useful in predicting important clinical outcomes, specifically persistency and type of AKI, development of a second infection, and 3-month mortality.179 In subjects undergoing LT, a single plasma NGAL level measured within 2 h of reperfusion was shown to be highly predictive of subsequent AKI.180,181
In patients listed for LT and who have renal dysfunction, if intrinsic kidney disease (systemic disease or thrombotic microangiopathy) is suspected, a percutaneous kidney biopsy is recommended to decide who would benefit from simultaneous liver-kidney versus LT alone. The degree of glomerulosclerosis and interstitial fibrosis may predict who will not recover following LT. In general, a biopsy specimen with >30% interstitial fibrosis or 30% glomerulosclerosis is considered to show advanced and irreversible kidney disease, and in these cases, it may be best to consider combined liver kidney transplantation.182
Post LT AKI is an independent risk factor for complications affecting the outcome of LT. Studies have shown that early post-transplant AKI, even if transient, has been associated with a poor long-term survival, increased rates of acute rejection and infectious complications, longer ICU stays, greater hospital costs, and higher mortality rates independent of pretransplant renal function.183,184
Consensus statements
-
•
LT with preoperative AKI confers a poorer outcome than in those without AKI. (Level of evidence: II-2)
-
•
Every effort should be made to identify the cause of AKI in preoperative period and aggressive measures taken to treat the AKI. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Single measurement of plasma NGAL within 24 h predicted AKI and severe AKI with a high degree of accuracy and was superior to serum creatinine at determining which patients were at risk of post-LT AKI. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Kidney biopsy is recommended for patients with AKI if intrinsic kidney disease (systemic diseases or thrombotic microangiopathy) is suspected. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Post LT AKI is an independent risk factor for complications affecting the outcome of LT. (Level of evidence: II-2)
Transplantation in patients with acute liver failure + acute kidney injury
AKI is a frequent complication of acute liver failure (ALF) and is associated with worse prognosis with or without transplantation.185, 186, 187, 188, 189 Multiple factors contribute to AKI in patients with ALF that include direct toxic effect of an ingested agent (e.g., acetaminophen), volume depletion, systemic hypoperfusion, sepsis, and HRS. In a retrospective study of 1604 patients, 70% of patients had AKI and 30% received RRT. Patients with acetaminophen-induced ALF had AKI frequently.186 In another study of 2280 adult patients with ALF; 56% had renal dysfunction defined as eGFR <60 mL/min/1.73 m2 and mortality increased with increasing renal failure.187
As ALF is uncommon disease and patients with multiorgan failure are generally not taken up for LT, there are limited data on outcomes of LT in the presence of ALF with AKI. Randomized studies are not available and cannot be done, as control group cannot be denied of a potential lifesaving LT. Most of the data are available from cadaveric LT setting, as living donor LT centers may not take patients with high risk of adverse outcome for LT due to risk to donors. If dialysis is needed, then continuous mode is better than intermittent dialysis and CRRT may help in spontaneous recovery or bridge to LT.190, 191, 192
LT, although associated with somewhat lower survival in the presence of AKI, should not be deferred in patients with ALF and AKI, in the absence of other contraindications. Barshes et al. analyzed data of LT for ALF from the United States (UNOS database). The study group comprised of a modeling group (n = 972) and a cross-validation group (n = 486). The authors found that serum creatinine >2.0 mg/dl was independent factor for post LT mortality.188 Two other retrospective analyses also showed worse survival after LT in patients with renal failure in pretransplant period.189,193 Living donor LT studies have also shown that pretransplant renal dysfunction is associated with increased risk.194,195 Knight et al. studied the effect of pretransplant RRT on outcomes of LT for ALF.193 The authors compared outcomes of LT for 336 patients without RRT to 389 patients with RRT. While indications for RRT may be several; patients in RRT group had serum creatinine of 157 (117–237 μmol/l). The 3-year patient and graft survival in RRT group was 77.7% and 72.6%, which was somewhat inferior compared with 85.1% and 79.4% in non-RRT group, P values being significant for both. Patients with serum creatinine greater than 175 μmol/l in non-RRT had a significant risk of graft failure.193 Leithead et al. studied 101 patients, 53.5% fulfilled the criteria of AKI and majority of these underwent RRT before transplantation. Sixty-three patients of ALF received RRT after transplantation also. In most of patients, renal function (eGFR) recovered to values equal to patients transplanted with cirrhosis with better eGFR and no RRT at baseline. The authors identified older age, female gender, pretransplant diagnosed hypertension, and cyclosporine as immunosuppressive therapy (and not pretransplant AKI) to be predictors of CKD after transplantation. Patients transplanted for acetaminophen-related ALF were at lower risk of CKD in long-term follow-up than patients transplanted for other etiologies of ALF. The log-rank P value for survival of patients with or without AKI was 0.061189. The recovery of renal function should be related to the absence of CKD and short duration of renal injury in these patients. It should be noted that the data of LT in ALF patients with AKI is mainly available from Western world where acetaminophen is a main etiology of ALF and cadaveric LT is done.
Consensus statements
-
•
AKI is a common problem in ALF. AKI is associated with worse prognosis in patients with ALF. (Level of evidence: II-2)
-
•
LT, although associated with somewhat lower survival in the presence of AKI, should not be deferred in patients with ALF and AKI, in the absence of other contraindications. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Post LT renal function recovery may not depend on pretransplantation severity of AKI. (Level of evidence: II-2)
How will you monitor for calcineurin inhibitor–induced renal dysfunction in patients with liver transplantation?
Renal dysfunction after LT is common and increases with time. In a review of 2100 adults who underwent LT, approximately 3% had subsequent kidney biopsy for evaluation of renal dysfunction.196 The etiology of renal dysfunction was found to be attributable to one or more of the following etiologies: apparent calcineurin inhibitor (CNI) toxicity (48%), hypertensive vascular changes (44%), MPGN (17%), IgA nephropathy (9%), diabetic nephropathy (9%), proliferative glomerulonephritis with crescents (4%), and ATN (4%).196
CNIs are the mainstay of immunosuppression after LT, and their use has improved the outcomes of the LT recipients.197 However, CNIs are associated with significant nephrotoxicity and causes short- and long-term renal complications. The progressive structural changes can be irreversible in the long term, leading to chronic kidney dysfunction. The total avoidance of CNI from post-LT immunosuppressive regimens has been associated with unacceptably high rates of acute, steroid-resistant rejections, and late conversion from CNI to non-nephrotoxic immunosuppressant regimen failed to produce recovery of renal function. Early CNI minimization and conversion to non-nephrotoxic immunosuppressant improved GFR, although it had no effect on patient survival rates. Recently, mTOR inhibitors such as sirolimus and everolimus have been used with or without CNIs in LT recipients for their renal-sparing effect. The combination of everolimus and tacrolimus not only maintains immunosuppressive efficacy but also minimizes kidney injury. According to a meta-analysis198 everolimus use with CNI minimization in LT recipients was associated with improved GFR by 10.2 mL/min at 12 months. Further, everolimus use was not associated with an increased risk of biopsy-proven acute rejection, graft loss, or mortality. However, it was associated with an overall increased risk of infections (RR 1.45, 95% CI: 1.10–1.91).198 Thus, early CNI minimization after LT is the most rational approach for preserving post-transplant renal function, provided the increased frequency of infections is addressed in a timely manner.197
For prevention and timely identification of renal dysfunction after LT, renal functions should be closely monitored. Presently, serum creatinine levels and eGFR using serum creatinine-based equations should be used, bearing their limitations in mind. Efforts must be made to validate serum creatinine-based equations, for example, CKD-EPI for liver cirrhosis and after LT and to develop newer equations. More research is needed for validating use of serum CysC and equations based on it in liver cirrhosis and after LT. Kidney biopsy may be useful in selected patients.
Consensus statements
-
•
Renal dysfunction after LT is common and increases with time. (Level of evidence: II-2)
-
•
Renal function should be closely monitored after LT. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Renal dysfunction after LT is multifactorial; chronic CNI nephrotoxicity is responsible for a minority of cases. (Level of evidence: II-2)
-
•
Presently, serum creatinine levels and eGFR using serum creatinine-based equations should be used, bearing their limitations in mind. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Efforts must be made to validate serum creatinine-based equations, for example, CKD-EPI for liver cirrhosis and after LT and to develop newer equations. (Level of evidence: II-2, strength of recommendation: strong)
-
•
More research is needed for validating use of serum CysC and equations based on it in liver cirrhosis and after LT (Level of evidence: III, strength of recommendation: weak)
-
•
Kidney biopsy may be useful in selected patients (Level of evidence: III, strength of recommendation: weak)
Normal and abnormal transaminases in general population, in patients with chronic kidney disease and after renal transplantation
Normal transaminases in general population
Despite widespread use of liver transaminases in our clinical practice establishing the normal values has been difficult. This is due to variability in defining the healthy control population that can be used to assess the transaminases values. The popularly used upper limit of normal values for AST/ALT of 40 IU was not gender specific and was not derived from population studies. Subsequently, several population-based studies have attempted to establish normal ranges for AST and ALT. One of the proposed values for ULN for ALT was from a large study of 6835 blood donors with normal viral serology, and BMI under 24.9 kg/m2, to be 30 IU/l for men, and 19 IU/l for women.199 In another Korean study of 1105 potential liver donors with normal liver biopsies, the proposed ULN for ALT was 33 IU/l for men and 25 IU/l for women.200 From the database of National Health and Nutrition Examination Survey (NHANES) 1999–2002 and 2005–2008, after eliminating subjects with viral hepatitis, significant alcohol use, diabetes, BMI >25, or enlarged waist circumference, and using statistical analysis, the calculated “maximum correct classification” for ULN of ALT was found to be 29 IU/l for men and 22 IU/l for women.201 In the recent American College of Gastroenterology guidelines202 the recommendations have been as follows: (i) a true healthy normal ALT level in prospectively studied populations without identifiable risk factors for liver disease ranges from 29 to 33 IU/l for males and 19–25 IU/l for females, and levels above this should be evaluated by physicians and (ii) elevated ALT or AST above the ULN in a population without identifiable risk factors is associated with increased liver-related mortality.202
Normal transaminases in chronic kidney disease population
It has been observed for the last nearly three decades that serum AST and ALT levels are decreased in CKD patients undergoing HD. Several causative factors have been hypothesized. These include removal of aminotransferases during the HD session, high lactate serum levels, which, during biochemical dosages, would rapidly consume nicotinamide adenine dinucleotide phosphate and result in low levels of aminotransferases, the presence of uremic factors that would inhibit the activity of these enzymes; and the deficiency of pyridoxine, a cofactor for the synthesis of the aminotransferases.203 Ray et al., 2015, in a hospital-based retrospective analysis, compared the levels of serum AST, ALT, and ALP among three groups: CKD patients without ESRD; patients with ESRD; and healthy controls. It was found that levels of serum aminotransferases were low in CKD with and without ESRD and the levels become lower as the severity of CKD increases. The authors of this study from a medical college in south India emphasized the need for separate reference ranges of serum aminotransferases in different stages of CKD in our population.204
Normal transaminases in renal transplant recipients
There are surprisingly few reports on the prevalence and implications of elevated liver enzyme levels after renal transplantation. Einollahi et al.205 performed a retrospective study on 1589 kidney transplants, who were negative for HBsAg and HCV antibody and had no other liver diseases, to detect the prevalence of elevated liver enzymes and the risk factors in these patients. In this cohort of patients after kidney transplantation, ALT was the liver enzyme abnormality with the highest prevalence (34.3%) followed by AST (6.7%). The levels of ALT and AST were significantly elevated within the first 3 months after transplantation, followed by the 4–12-month period (P < 0.001). There was an inverse correlation between liver enzyme levels and renal allograft function in both univariate and linear regression analyses. This correlation increased over time. There was also a significant relation between cyclosporine blood levels and liver enzyme values in the univariate analysis. The authors suggested the need for serial monitoring of aminotransferases, particularly ALT, in all patients after kidney transplantation.205 In another study from Israel, the authors retrospectively evaluated the prevalence, risk factors, and clinical importance of abnormal elevation in liver enzyme levels after renal transplantation in 62 children. Liver enzyme levels were abnormal in 67.7% during the first 6 months following, with a peak on day 14 (P < 0.001). In all the seven patients who had elevated ALT levels before transplant, the post-transplant ALT levels were high. Seroconversion was documented during the first post-transplantation year in nine patients (14.5%), of whom eight (89%) were Cytomegalovirus (CMV) IgG negative. An abnormally elevated liver enzyme level was significantly correlated with high blood tacrolimus level, but only on post-transplant day 3 (P <0.001)206. In an another report from Turkey Dizdar et al. analyzing liver function test abnormalities in 107 kidney transplant recipients over a 7-year period implicated drug toxicity due to MMF and pretransplant positive CMV IgM tests as a significant independent risk factor for hepatotoxicity in multiple analysis.207 Elevated ALT/AST levels are of much clinical significance occurring during postrenal transplant. The causative factors in these reported studies are mutt factorial namely drug hepatotoxicity, previous liver injury, and acute viral infection or reactivation.
Consensus statements
-
•
The reference range values for ALT and AST need to be defined in patients with various stages of CKD and posttransplantation. (Level of evidence: III, strength of recommendation: strong)
-
•
Transient elevated liver enzymes especially ALT is common and multifactorial in postrenal transplant individuals, which needs further evaluation. (Level of evidence: II-2, strength of recommendation: strong)
Polycystic liver and kidney disease
Polycystic liver disease (PLD) is an inherited ciliopathy/cholangiopathy characterized by multiple cysts, which take up at least half of the volume of the liver parenchyma.208,209 PLD usually occurs as an extrarenal manifestation of autosomal-dominant polycystic kidney disease (ADPKD). Estimations of the prevalence of PLD in patients with ADPKD range from 20% to 94%.208
The most common methods for the diagnosis of PLD are US and computed tomography (CT), and MRI; however, ultrasonography (USG) is the diagnostic modality of choice. Diagnosis is dependent on age and the presence of at least two or three cysts. CT or MRI at diagnosis are used assess total kidney (and liver) volume. Genetic testing is not routinely recommended. However, it is indicated in patients with renal failure without renomegaly, early and severe PKD, and markedly asymmetric PKD.
Hypertension and progression to ESRD is typical in ADPKD as well as non-ADPKD, so use of an agent to inhibit the RAAS are indicated with target BP of <130/80 mmHg. All patients with ADPKD with eGFR >60 mL/min should be advised to consume at least 3 L of water everyday (unless contraindicated). Avoidance of estrogen-containing contraceptive pills is recommended in PLD. The liver cysts in patients with ADPKD do not require any treatment if they are asymptomatic.
Several RCTs have demonstrated that with the use of somatostatin analogues octreotide and lanreotide there was a reduction in total liver volume in the treatment group compared with an increase in the placebo group.210, 211, 212 Hence, somatostatin analogue should be considered in moderate (liver volume >1600 mL) to severe PLD with impairment of quality of life (QOL) and annual liver volume increase >5% and/or complications.
Cyst-causing symptoms can be treated with aspiration sclerotherapy (>8 cm and percutaneously accessible); fenestration (multiple large cysts in the anterior segments of liver and laparoscopically accessible); or resection (clustered cysts in few segments, fenestration/AS not feasible).
Consensus statements
-
•
Diagnosis of polycystic kidney and liver disease is done using ultrasound examination based on age-specific number of cysts. (Level of evidence: II-2, strength of recommendation: strong)
-
•Genetic testing is NOT routinely recommended. It is indicated in the following:
-
○Renal failure without renomegaly,
-
○Early and severe PKD,
-
○Markedly asymmetric PKD (Level of evidence: II-2, strength of recommendation: strong)
-
○
-
•
Hypertension should be treated with target BP of <130/80 mmHg. (Level of evidence: II-2, strength of recommendation: strong)
-
•
RAAS inhibitors are the antihypertensives of choice unless there are contraindications. (Level of evidence: II-3, strength of recommendation: strong)
-
•
All patients with ADPKD with eGFR >60 mL/min should be advised to consume at least 3 L of water everyday (unless contraindicated). (Level of evidence: II-3, strength of recommendation: strong)
-
•
Avoidance of estrogen-containing contraceptive pills is recommended in PLD. (Level of evidence: II-3, strength of recommendation: strong)
-
•
The liver cysts in patients with ADPKD do not require any treatment if they are asymptomatic. (Level of evidence: II-3, strength of recommendation: strong)
How do you manage hepatitis B virus–related kidney disease?
HBV-related nephropathy is one of the most common extrahepatic manifestations affecting 3%–5% of patients with chronic HBV infection.213,214 Several morphological patterns of renal disease, including membranoproliferative glomerulonephritis (MPGN), membranous nephropathy, IgA nephropathy (IgAN), and focal segmental glomerulosclerosis have been described in association with chronic HBV infections.215 For diagnosis of HBV-related kidney disease, kidney biopsy with demonstration of HBV antigen in histology is recommended.
The guidelines for management of HBV-related extrahepatic manifestations including glomerulonephritis were recently published by the INASL.47 The management of such cases can be challenging. HBsAg-positive patients with extrahepatic manifestations and active HBV replication may respond to antiviral therapy. PegIFN-α can worsen some immune-mediated extrahepatic manifestations and should not be administered in HBV-infected patients with immune-related extrahepatic manifestations. Patients with replicative HBV infection and extrahepatic manifestations should receive antiviral treatment with NA. Prefer entecavir or tenofovir alafenamide to tenofovir disoproxil fumarate because it is associated with a lower risk of renal toxicity. Adefovir, lamivudine, and telbivudine are not used for initial treatment due to a high rate of resistance and weak antiviral activity. The optimal duration of antiviral therapy for HBV-associated nephropathy has not been determined. Long-term antiviral treatment is required to avoid relapses after initial remission.
Plasmapheresis, corticosteroids, and potentially other immune-suppressive drugs during the initial phase can be useful in addition to NA therapy in special cases.216 Immunosuppression may lead to enhanced viral replication and flare-up of the hepatic disease and hence should not be used without concomitant NA therapy.
Consensus statements
-
•
For diagnosis of HBV-related kidney disease, kidney biopsy with demonstration of HBV antigen in histology is recommended. (Level of evidence: II-3, strength of recommendation: strong)
-
•
Entecavir or tenofovir alafenamide are the preferred drugs in view of their antiviral efficacy, low propensity for drug resistance, and excellent safety profile. (Level of evidence: II-3, strength of recommendation: strong)
How do you manage hepatitis C virus–related kidney disease?
HCV infection has been associated with a large spectrum of glomerular lesions in both native and transplanted kidneys.217 The most common HCV-associated renal disease is type I MPGN usually, but not invariably, in the context of type II mixed cryoglobulinemia (MC). HCV infection is also the major cause of MC, a systemic vasculitis characterized by involvement of small and, less frequently, medium-sized vessels. Conflicting data exist on the treatment of HCV-associated glomerular disease.217
Various approaches have been recommended for the treatment of HCV-related glomerular disease, including immunosuppressive therapy (corticosteroids, cytotoxic agents, and mAbs) and antiviral therapy. These regimens should be considered according to the level or proteinuria and kidney failure. Immunosuppressive agents are recommended in patients with nephrotic syndrome and/or rapidly progressive kidney failure. Sofosbuvir plus ledipasvir and sofosbuvir plus daclatasvir have been shown to be effective in achieving SVR (>90%); complete immunological response (50%); clinical response (50%). However, the choice of DAA combination (SL; SD; SV) and dosages should be dependent on Genotype and GFR.
Consensus statements
-
•
HCV itself is an uncommon cause of glomerular disease. (Level of evidence: III)
-
•
Treatment with DAAs is the treatment of choice for HCV-induced MC/GN. (Level of evidence: II-3, strength of recommendation: strong)
-
•
If there is deterioration despite DAAs, patients may require immunosuppressive therapy. (Level of evidence: II-3, strength of recommendation: weak)
Renal replacement therapy in patients with acute liver failure
Patients of ALF with AKI carry high mortality and up to 50% of these patients require RRT.186,218 The RRT for ALF-associated AKI should be initiated according to the standard indications (e.g., refractory hyperkalemia/metabolic acidosis, fluid overload, uremic encephalopathy) as in the general patients.219 In view of high mortality, the futility of RRT in these patients should be guided by a clear goal of hepatic management: either there should be chances of reversibility, or possibility of LT in case of irreversibility.220 Early initiation of RRT may be required in selected cases to decrease ammonia levels and intracranial edema.
There are various forms of RRT like IHD, PD, and CRRT.221 CRRT may be preferable over IHD as it has better cardiovascular tolerability.222 There is limited evidence for the role of PD in these patients because it can increase the chances of peritonitis and portal vein thrombosis and may create problem in future LT. Slow/SLED is a modification of IHD with decreased blood flow rate, dialysate rate and increased duration. SLED is a reasonable alternative to IHD (better cardiovascular tolerability) and CRRT (less complicated and less expensive). Both IHD (including SLED) and CRRT are equivalent in terms of survival benefit. Other modification that has been tried especially in Japan is high-flow continuous hemodiafiltration (HFCHDF) with or without slow plasma exchange. The decision of choice of RRT should be based on clinical characteristics of the patient, availability, and local expertise at the center.
ALF patients have decreased production of both procoagulants and anticoagulant factors. Although there is coagulopathy (and thus bleeding tendency), there are increased chances of circuit clotting also. Thus, some form of anticoagulation is required to prevent circuit clotting unless there is evidence of active bleeding. Various options for anticoagulation are standard unfractionated heparin, “tight” heparin (half the dose of heparin), heparin free with saline flushes and regional citrate anticoagulation (RCA). RCA is contraindicated in patients with ALF due to the risk of citrate accumulation; however, its safe use has been demonstrated in few studies.223,224 In patients with high risk of bleeding, “tight heparin” or “heparin free with saline flushes” are reasonable options.
Dose of CRRT has no effect on mortality: higher CRRT dose (40 mL/kg/hr) is not better than the low-dose CRRT (25 mL/kg/hr) in terms of mortality.225
Liver support therapy (LST) provides the support for detoxification role of the liver. They can be in the form of single-pass albumin dialysis, Molecular Adsorbent Recirculating System (MARS), Prometheus system or plasma exchange.226 Experimental therapies include Dialive and Bioartificial Liver. There are no data to suggest that one form of LST is better than other. Various LSTs maybe considered for selected group of patients, for example, as a bridge to LT or those cases who are potentially recoverable. However, large trials have not shown any significant benefit of these therapies. HELIOS trial157 on Prometheus system in post-hoc analysis showed that the survival of patients with type 1 HRS when treated with Fractionated Plasma Separation and Adsorption (FPSA) was better compared with SMT. While the RELIEF trial158 with use of MARS concluded that the proportion of patients with a serum creatinine below 1.5 mg/dl at day 4 tended to be higher in those treated with MARS (p = 0.07). However, both these trials took some 7–9 years for completion; hence, a heterogeneous group of patients were enrolled. Thus, the use of artificial LST remains questionable because of the lack of adequately powered trials. It improves bilirubin, HE, and HRS in ALF patients but has shown no survival benefits. Moreover, the therapy is expensive and costly, and potential benefits may not overweigh the risks. LST can be used as a bridge to transplantation or recovery. High-flow plasma exchange has been shown to be beneficial in these patients.227 There is also a role of RRT in the management of acute hyperammonemia in the setting of ALF.228 In a large cohort of ALF patients, hyperammonemia was associated with high-grade HE and worse 21-day transplant-free survival. CRRT was associated with a reduction in serum ammonia level and improvement of 21-day transplant-free survival in these patients.192
Consensus statements
-
•
The RRT for ALF-associated AKI should be initiated according to the standard indications (e.g., refractory hyperkalemia/metabolic acidosis, fluid overload, uremic encephalopathy), as in the general patients. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Early initiation of RRT may be required in selected cases to decrease ammonia levels and intracranial edema. (Level of evidence: II-2, strength of recommendation: strong)
-
•
Both IHD (including SLED) and CRRT are equivalent in terms of survival benefit. (Level of evidence: II-2)
-
•
CRRT may be preferable over IHD as it has better cardiovascular tolerability. The decision should be based on clinical characteristics of the patient, availability and local expertise at the center. (Level of evidence: II-3, strength of recommendation: strong)
-
•
RCA is contraindicated in patients with ALF-AKI, although there is some evidence of its safe use. In patients with high risk of bleeding, “tight heparin” or “heparin free with saline flushes” are reasonable options. (Level of evidence: III, strength of recommendation: weak)
-
•
Dose of CRRT has no effect on mortality. (Level of evidence: III)
-
•
Various LSTs maybe considered for selected group of patients, for example, as a bridge to LT or those cases who are potentially recoverable. There are no data to suggest that one form of LST is better than other. (Level of evidence: II-3, strength of recommendation: strong)
Management of simultaneous liver and kidney involvement in patients with acute febrile illness
Acute febrile illness, manifested as rapid onset of high fever and symptoms such as headache, chills or muscle and joint pains, is common in the tropics and subtropics and can be caused by diverse pathogens.229,230 The common causes of acute febrile illness are malaria, typhoid, dengue, leptospirosis, scrub typhus, immunological reactions, and other sepsis syndromes.231 Liver and kidney involvement (often simultaneous) in acute febrile illness are common in tropical and subtropical environments and are multifactorial. There is insufficient evidence about the exact prevalence of acute febrile illness in community, as well as about the prevalence of simultaneous liver and kidney involvement in such illness. Involvement of these vital organs is often associated with sepsis syndrome. Before labeling a patient as having liver and kidney involvement due to acute febrile illness, it is essential to exclude those patients who have known liver and kidney disease and have developed acute febrile illness. Many of these illnesses presenting as fever, jaundice, and renal dysfunction have been recently reviewed.232, 233, 234
Management of febrile illness with SLK involvement requires management of primary disease with support for these organs as dictated by the clinical condition. Malaria, typhoid, dengue, leptospirosis, scrub typhus and immunological reactions need to be managed with specific treatment prescribed for them. Liver and kidney involvement will need supportive management till primary disease is taken care of. Acute liver injury or ALF needs to be managed as per published guidelines with intensive care where indicated.235,236 LT is usually not considered an option unless the primary disease and sepsis have been stabilized. All causes of prerenal acute renal failure need to be managed with resuscitation and volume replacement followed by specific treatment of etiology.16,237 In case of worsening renal failure or gross electrolyte/acid base disturbance, supportive treatment of dialysis/CRRT can be given to these patients.
Consensus statements
-
•
Acute febrile illnesses with simultaneous liver and kidney involvement are common in tropical and subtropical environments and are multifactorial. The common causes are malaria, typhoid, dengue, leptospirosis, scrub typhus, and immunological reactions. (Level of evidence: II-2)
-
•
Management of febrile illness with simultaneous liver and kidney involvement requires management of primary disease with support for these organs as dictated by the clinical condition. (Level of evidence: II-2, strength of recommendation: strong)
-
•
In case of worsening renal failure or gross electrolyte/acid base disturbance, supportive treatment of dialysis/CRRT can be given to these patients. (Level of evidence: III, strength of recommendation: strong)
-
•
LT is usually not considered an option unless the primary disease and sepsis has been stabilized. (Level of evidence: III, strength of recommendation: weak)
CRediT authorship contribution statement
Anil Arora: Conceptualization, Methodology, Writing - review & editing, Formal analysis, Supervision. Ashish Kumar: Methodology, Writing - original draft. Narayan Prasad: Expert Panel Member, Writing - review & editing. Ajay Duseja: Expert Panel Member, Writing - review & editing. Subrat K. Acharya: Expert Panel Member, Writing - review & editing. Sanjay K. Agarwal: Expert Panel Member, Writing - review & editing. Rakesh Aggarwal: Expert Panel Member, Writing - review & editing. Anil C. Anand: Expert Panel Member, Writing - review & editing. Anil K. Bhalla: Expert Panel Member, Writing - review & editing. Narendra S. Choudhary: Expert Panel Member, Writing - review & editing. Yogesh K. Chawla: Expert Panel Member, Writing - review & editing. Radha K. Dhiman: Expert Panel Member, Writing - review & editing. Vinod K. Dixit: Expert Panel Member, Writing - review & editing. Natarajan Gopalakrishnan: Expert Panel Member, Writing - review & editing. Ashwani Gupta: Expert Panel Member, Writing - review & editing. Umapati N. Hegde: Expert Panel Member, Writing - review & editing. Sanjiv Jasuja: Expert Panel Member, Writing - review & editing. Vivek Jha: participated in the consensus meeting and reviewed the manuscript, Writing - review & editing. Vijay Kher: participated in the consensus meeting and reviewed the manuscript, Writing - review & editing. Ajay Kumar: Expert Panel Member, Writing - review & editing. Kaushal Madan: Expert Panel Member, Writing - review & editing. Rakhi Maiwall: Expert Panel Member, Writing - review & editing. Rajendra P. Mathur: Expert Panel Member, Writing - review & editing. Suman L. Nayak: Expert Panel Member, Writing - review & editing. Gaurav Pandey: Expert Panel Member, Writing - review & editing. Rajendra Pandey: Expert Panel Member, Writing - review & editing. Pankaj Puri: Expert Panel Member, Writing - review & editing. Ramesh R. Rai: Expert Panel Member, Writing - review & editing. Sree B. Raju: Expert Panel Member, Writing - review & editing. Devinder S. Rana: Expert Panel Member, Writing - review & editing. Padaki N. Rao: Expert Panel Member, Writing - review & editing. Manish Rathi: Expert Panel Member, Writing - review & editing. Vivek A. Saraswat: Expert Panel Member, Writing - review & editing. Sanjiv Saxena: Expert Panel Member, Writing - review & editing. Shalimar: Expert Panel Member, Writing - review & editing. Praveen Sharma: Expert Panel Member, Writing - review & editing. Shivaram P. Singh: Expert Panel Member, Writing - review & editing. Ashwani K. Singal: Expert Panel Member, Writing - review & editing. Arvinder S. Soin: Expert Panel Member, Writing - review & editing. Sunil Taneja: Expert Panel Member, Writing - review & editing. Santosh Varughese: Expert Panel Member, Writing - review & editing.
Conflicts of interest
All authors have none to declare.
References
- 1.Malhotra R., Soin D., Grover P., Galhotra S., Khutan H., Kaur N. Hepatitis B virus and hepatitis C virus co-infection in hemodialysis patients: a retrospective study from a tertiary care hospital of North India. J Nat Sci Biol Med. 2016;7:72–74. doi: 10.4103/0976-9668.175076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora A., Bansal N., Sharma P. Hepatitis C virus infection in patients with end-stage renal disease: a study from a tertiary care centre in India. J Clin Exp Hepatol. 2016;6:21–25. doi: 10.1016/j.jceh.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahajan S., Nayak S.L., Gupta E. Utility of hepatitis C virus RNA as the screening test for diagnosing hepatitis C virus infection in hemodialysis patients. J Lab Physicians. 2017;9:345. doi: 10.4103/JLP.JLP_99_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somsundaram N., Rani R.V. Seroprevalence of hepatitis C virus infection among chronic kidney disease patients on maintenance hemodialysis in a tertiary care hospital. Int J Curr Microbiol Appl Sci. 2017;6:1416–1420. doi: 10.20546/ijcmas.2017.607.169. [DOI] [Google Scholar]
- 5.Bhaumik P., Debnath K. Prevalence of hepatitis B and C among hemodialysis patients of Tripura, India. In: Ozkan H., Rahman S., editors. Euroasian J Hepato-Gastroenterol. vol. 2. 2012. pp. 10–13. [DOI] [Google Scholar]
- 6.Jadoul M., Berenguer M.C., Doss W. Executive summary of the 2018 KDIGO Hepatitis C in CKD Guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94:663–673. doi: 10.1016/j.kint.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Targher G., Chonchol M.B., Byrne C.D. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis Off J Natl Kidney Found. 2014;64:638–652. doi: 10.1053/j.ajkd.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Mikolasevic I., Racki S., Bubic I., Jelic I., Stimac D., Orlic L. Chronic kidney disease and nonalcoholic Fatty liver disease proven by transient elastography. Kidney Blood Press Res. 2013;37:305–310. doi: 10.1159/000350158. [DOI] [PubMed] [Google Scholar]
- 9.Musso G., Cassader M., Cohney S. Fatty liver and chronic kidney disease: novel mechanistic insights and therapeutic opportunities. Diabetes Care. 2016;39:1830–1845. doi: 10.2337/dc15-1182. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Tsao G., Parikh C.R., Viola A. Acute kidney injury in cirrhosis. Hepatol Baltim Md. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 11.Appenrodt B., Lammert F. Renal failure in patients with liver cirrhosis: novel classifications, biomarkers, treatment. Vis Med. 2018;34:246–252. doi: 10.1159/000492587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huelin P., Piano S., Solà E. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2017;15:438–445. doi: 10.1016/j.cgh.2016.09.156. e5. [DOI] [PubMed] [Google Scholar]
- 13.Sherman D.S., Fish D.N., Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis Off J Natl Kidney Found. 2003;41:269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 14.Gomaa S.H., Shamseya M.M., Madkour M.A. Clinical utility of urinary neutrophil gelatinase-associated lipocalin and serum cystatin C in a cohort of liver cirrhosis patients with renal dysfunction: a challenge in the diagnosis of hepatorenal syndrome. Eur J Gastroenterol Hepatol. 2019 doi: 10.1097/MEG.0000000000001347. Published online January 4. [DOI] [PubMed] [Google Scholar]
- 15.Myers G.L., Miller W.G., Coresh J. Recommendations for improving serum creatinine measurement: a report from the laboratory working group of the national kidney disease education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Piano S., Brocca A., Angeli P. Renal function in cirrhosis: a critical review of available tools. Semin Liver Dis. 2018;38:230–241. doi: 10.1055/s-0038-1661372. [DOI] [PubMed] [Google Scholar]
- 18.DeSanto N.G., Anastasio P., Loguercio C. Creatinine clearance: an inadequate marker of renal filtration in patients with early posthepatitic cirrhosis (Child A) without fluid retention and muscle wasting. Nephron. 1995;70:421–424. doi: 10.1159/000188639. [DOI] [PubMed] [Google Scholar]
- 19.Roy L., Legault L., Pomier-Layrargues G. Glomerular filtration rate measurement in cirrhotic patients with renal failure. Clin Nephrol. 1998;50:342–346. [PubMed] [Google Scholar]
- 20.Proulx N.L., Akbari A., Garg A.X., Rostom A., Jaffey J., Clark H.D. Measured creatinine clearance from timed urine collections substantially overestimates glomerular filtration rate in patients with liver cirrhosis: a systematic review and individual patient meta-analysis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2005;20:1617–1622. doi: 10.1093/ndt/gfh839. [DOI] [PubMed] [Google Scholar]
- 21.Peake M., Whiting M. Measurement of serum creatinine--current status and future goals. Clin Biochem Rev. 2006;27:173–184. [PMC free article] [PubMed] [Google Scholar]
- 22.Francoz C., Nadim M.K., Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809–824. doi: 10.1016/j.jhep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Woitas R.P., Stoffel-Wagner B., Flommersfeld S. Correlation of serum concentrations of cystatin C and creatinine to inulin clearance in liver cirrhosis. Clin Chem. 2000;46:712–715. [PubMed] [Google Scholar]
- 24.Gerbes A.L., Gülberg V., Bilzer M., Vogeser M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50:106–110. doi: 10.1136/gut.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D., Feng J.-F., Wang A.-Q., Yang Y.-W., Liu Y.-S. Role of Cystatin C and glomerular filtration rate in diagnosis of kidney impairment in hepatic cirrhosis patients. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000006949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y.-W., Wu C.-J., Chang C.-W., Lee S.-Y., Sun F.-J., Chen H.-H. Renal function in patients with liver cirrhosis. Nephron Clin Pract. 2011;118:c195–c203. doi: 10.1159/000321384. [DOI] [PubMed] [Google Scholar]
- 27.El-Bokl M.A., Senousy B.E., El-Karmouty K.Z. Spot urinary sodium for assessing dietary sodium restriction in cirrhotic ascites. World J Gastroenterol. 2009;15:3631–3635. doi: 10.3748/wjg.15.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C.-H., Liang C.-C., Huang K.-W. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol CJASN. 2011;6:1057–1065. doi: 10.2215/CJN.04320510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadoul M., Horsmans Y. Impact of liver fibrosis staging in hepatitis C virus (HCV) patients with kidney failure. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2014;29:1108–1110. doi: 10.1093/ndt/gfu047. [DOI] [PubMed] [Google Scholar]
- 30.Pipili C.L., Papatheodoridis G.V., Cholongitas E.C. Treatment of hepatitis B in patients with chronic kidney disease. Kidney Int. 2013;84:880–885. doi: 10.1038/ki.2013.249. [DOI] [PubMed] [Google Scholar]
- 31.Rockey D.C., Caldwell S.H., Goodman Z.D., Nelson R.C., Smith A.D. American association for the study of liver diseases. Liver biopsy. Hepatol Baltim Md. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 32.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;(109):S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 33.Wijarnpreecha K., Thongprayoon C., Scribani M., Ungprasert P., Cheungpasitporn W. Noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver in USA. Eur J Gastroenterol Hepatol. 2018;30:404–410. doi: 10.1097/MEG.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 34.Schiavon L.L., Schiavon J.L.N., Filho R.J.C. Simple blood tests as noninvasive markers of liver fibrosis in hemodialysis patients with chronic hepatitis C virus infection. Hepatol Baltim Md. 2007;46:307–314. doi: 10.1002/hep.21681. [DOI] [PubMed] [Google Scholar]
- 35.Taneja S., Borkakoty A., Rathi S. Assessment of liver fibrosis by transient elastography should Be done after hemodialysis in end stage renal disease patients with liver disease. Dig Dis Sci. 2017;62:3186–3192. doi: 10.1007/s10620-017-4777-6. [DOI] [PubMed] [Google Scholar]
- 36.Shiha G., Ibrahim A., Helmy A. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int. 2017;11:1–30. doi: 10.1007/s12072-016-9760-3. [DOI] [PubMed] [Google Scholar]
- 37.Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol. 2016;26:15. [Google Scholar]
- 38.Bernieh B. Viral hepatitis in hemodialysis: an update. J Transl Intern Med. 2015;3:93–105. doi: 10.1515/jtim-2015-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karkar A., Abdelrahman M., Ghacha R., Malik T.Q. Prevention of viral transmission in HD units: the value of isolation. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2006;17:183–188. [PubMed] [Google Scholar]
- 40.Tang S., Lai K.N. Chronic viral hepatitis in hemodialysis patients. Hemodial Int Int Symp Home Hemodial. 2005;9:169–179. doi: 10.1111/j.1492-7535.2005.01129.x. [DOI] [PubMed] [Google Scholar]
- 41.Broussard I.M., Bhimji S.S. StatPearls. StatPearls Publishing. 2018. Universal precautions.http://www.ncbi.nlm.nih.gov/books/NBK470223/ Accessed. [PubMed] [Google Scholar]
- 42.Centers for Disease Control (CDC) Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health-care settings. MMWR Morb Mortal Wkly Rep. 1988;37:377–382. 387-388. [PubMed] [Google Scholar]
- 43.Bierer B.E. Universal precautions: necessary safety procedures when handling human blood, body fluids, and specimens. Curr Protoc Im. 2017;118 doi: 10.1002/cpim.29. A.3P.1-A.3P.3. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed W.Z. Prevention of hepatitis C virus in hemodialysis patients: five years experience from a single center. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2010;21:548–554. [PubMed] [Google Scholar]
- 45.Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 46.Arora A., Anand A.C., Kumar A. INASL guidelines on management of hepatitis B virus infection in patients receiving chemotherapy, biologicals, immunosupressants, or corticosteroids. J Clin Exp Hepatol. 2018;8:403–431. doi: 10.1016/j.jceh.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arora A., Singh S.P., Kumar A. INASL position Statements on prevention, diagnosis and management of hepatitis B virus infection in India: the Andaman Statements. J Clin Exp Hepatol. 2018;8:58–80. doi: 10.1016/j.jceh.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sit D., Esen B., Atay A.E., Kayabaşı H. Is hemodialysis a reason for unresponsiveness to hepatitis B vaccine? Hepatitis B virus and dialysis therapy. World J Hepatol. 2015;7:761–768. doi: 10.4254/wjh.v7.i5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grzegorzewska A.E., Wobszal P.M., Sowińska A., Mostowska A., Jagodziński P.P. Association of the interleukin-12 polymorphic variants with the development of antibodies to surface antigen of hepatitis B virus in hemodialysis patients in response to vaccination or infection. Mol Biol Rep. 2013;40:6899–6911. doi: 10.1007/s11033-013-2809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin S.-Y., Liu J.-H., Wang S.-M. Association of response to hepatitis B vaccination and survival in dialysis patients. BMC Nephrol. 2012;13:97. doi: 10.1186/1471-2369-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarin S.K., Kumar M., Lau G.K. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabrizi F., Messa P., Dixit V., Martin P. Therapy with nucleos(t)ide analogues: current role in dialysis patients. Int J Artif Organs. 2010;33:329–338. [PubMed] [Google Scholar]
- 53.Tseng G.-Y., Lin H.-J., Fang C.-T. Hemodialysis reduces the viral load in uremic patients with chronic hepatitis B infection. Ren Fail. 2008;30:1000–1005. doi: 10.1080/08860220802406377. [DOI] [PubMed] [Google Scholar]
- 54.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Pipili C., Cholongitas E., Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39:35–46. doi: 10.1111/apt.12538. [DOI] [PubMed] [Google Scholar]
- 56.Yap D.Y.H., Yung S., Tang C.S.O. Entecavir treatment in kidney transplant recipients infected with hepatitis B. Clin Transplant. 2014;28:1010–1015. doi: 10.1111/ctr.12410. [DOI] [PubMed] [Google Scholar]
- 57.Hu T.-H., Tsai M.-C., Chien Y.-S. A novel experience of antiviral therapy for chronic hepatitis B in renal transplant recipients. Antivir Ther. 2012;17:745–753. doi: 10.3851/IMP2097. [DOI] [PubMed] [Google Scholar]
- 58.Tsai H.J., Chuang Y.W., Lee S.W., Wu C.Y., Yeh H.Z., Lee T.Y. Using the chronic kidney disease guidelines to evaluate the renal safety of tenofovir disoproxil fumarate in hepatitis B patients. Aliment Pharmacol Ther. 2018;47:1673–1681. doi: 10.1111/apt.14682. [DOI] [PubMed] [Google Scholar]
- 59.Lampertico P., Chan H.L.Y., Janssen H.L.A., Strasser S.I., Schindler R., Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016;44:16–34. doi: 10.1111/apt.13659. [DOI] [PubMed] [Google Scholar]
- 60.Daudé M., Rostaing L., Sauné K. Tenofovir therapy in hepatitis B virus-positive solid-organ transplant recipients. Transplantation. 2011;91:916–920. doi: 10.1097/TP.0b013e3182100f59. [DOI] [PubMed] [Google Scholar]
- 61.Amarapurkar D.N., Patel N. Increased eGFR with telbivudine in combination therapy of chronic hepatitis B infection. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 2014;33:89–91. doi: 10.1007/s12664-013-0325-2. [DOI] [PubMed] [Google Scholar]
- 62.Buti M., Gane E., Seto W.K. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 63.Agarwal K., Brunetto M., Seto W.K., GS-US-320-0110; GS-US-320-0108 Investigators 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672–681. doi: 10.1016/j.jhep.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 64.Ridruejo E. Antiviral treatment for chronic hepatitis B in renal transplant patients. World J Hepatol. 2015;7:189–203. doi: 10.4254/wjh.v7.i2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fabrizi F., Takkouche B., Lunghi G., Dixit V., Messa P., Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta-analysis of observational studies. J Viral Hepat. 2007;14:697–703. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 66.AASLD-IDSA HCV Guidance Panel Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67:1477–1492. doi: 10.1093/cid/ciy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roth D., Nelson D.R., Bruchfeld A. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet Lond Engl. 2015;386:1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 68.Gane E., Lawitz E., Pugatch D. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. 2017;377:1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
- 69.Gupta V., Kumar A., Sharma P., Arora A. Newer direct-acting antivirals for hepatitis C virus infection: perspectives for India. Indian J Med Res. 2017;146:23–33. doi: 10.4103/ijmr.IJMR_679_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tmu N., Kumar A., Sharma P., Singla V., Bansal N., Arora A. Results of sofosbuvir plus ribavirin in patients with hepatitis C related decompensated cirrhosis. J Clin Exp Hepatol. 2018 doi: 10.1016/j.jceh.2018.02.009. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smolders E.J., de Kanter C.T.M.M., van Hoek B., Arends J.E., Drenth J.P.H., Burger D.M. Pharmacokinetics, efficacy, and safety of hepatitis C virus drugs in patients with liver and/or renal impairment. Drug Saf. 2016;39:589–611. doi: 10.1007/s40264-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhamidimarri K.R., Kalyan Ram B., Czul F. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of Hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63:763–765. doi: 10.1016/j.jhep.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Nazario H.E., Ndungu M., Modi A.A. Sofosbuvir and simeprevir in hepatitis C genotype 1-patients with end-stage renal disease on haemodialysis or GFR <30 ml/min. Liver Int Off J Int Assoc Study Liver. 2016;36:798–801. doi: 10.1111/liv.13025. [DOI] [PubMed] [Google Scholar]
- 74.Saxena V., Koraishy F.M., Sise M.E. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int Off J Int Assoc Study Liver. 2016;36:807–816. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taneja S., Duseja A., De A. Low-dose sofosbuvir is safe and effective in treating chronic hepatitis C in patients with severe renal impairment or end-stage renal disease. Dig Dis Sci. 2018;63:1334–1340. doi: 10.1007/s10620-018-4979-6. [DOI] [PubMed] [Google Scholar]
- 76.Goel A., Bhadauria D.S., Kaul A. Daclatasvir and reduced-dose sofosbuvir: an effective and pangenotypic treatment for hepatitis C in patients with eGFR <30 ml/min. Nephrol Carlton Vic. 2018 doi: 10.1111/nep.13222. Published online January 12. [DOI] [PubMed] [Google Scholar]
- 77.Kumar null Manoj, Nayak S.L., Gupta E., Kataria A., Sarin S.K. Generic sofosbuvir-based direct-acting antivirals in hepatitis C virus-infected patients with chronic kidney disease. Liver Int Off J Int Assoc Study Liver. 2018;38:2137–2148. doi: 10.1111/liv.13863. [DOI] [PubMed] [Google Scholar]
- 78.Singh A., Kumari S., Kumar P., De A., Singh V. Sofosbuvir with NS5A inhibitors in hepatitis C virus infection with severe renal insufficiency. J Viral Hepat. 2018;25:1501–1506. doi: 10.1111/jvh.12983. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal S.K., Bagchi S., Yadav R.K. Hemodialysis patients treated for hepatitis C using a sofosbuvir-based regimen. Kidney Int Rep. 2017;2:831–835. doi: 10.1016/j.ekir.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sofosbuvir/velpatasvir for 12 Weeks is safe and effective in patients undergoing dialysis. http://www.natap.org/2018/AASLD/AASLD_19.htm
- 81.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 82.Kanda T., Lau G.K.K., Wei L. APASL clinical practice recommendation: how to treat HCV-infected patients with renal impairment? Hepatol Int. 2019;13:103–109. doi: 10.1007/s12072-018-9915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strazzulla A., Coppolino G., Barreca G.S. Evolution of glomerular filtration rates and neutrophil gelatinase-associated lipocalin during treatment with direct acting antivirals. Clin Mol Hepatol. 2018;24:151–162. doi: 10.3350/cmh.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mallet V., Parlati L., Dorval O. Estimated glomerular filtration rate variations and direct acting antivirals treatment for chronic hepatitis C: a retrospective longitudinal study. J Hepatol. 2018;68:S22. doi: 10.1016/S0168-8278(18)30262-9. [DOI] [Google Scholar]
- 85.Sawinski D., Kaur N., Ajeti A. Successful treatment of hepatitis C in renal transplant recipients with direct-acting antiviral agents. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2016;16:1588–1595. doi: 10.1111/ajt.13620. [DOI] [PubMed] [Google Scholar]
- 86.Lin M.V., Sise M.E., Pavlakis M. Efficacy and safety of direct acting antivirals in kidney transplant recipients with chronic hepatitis C virus infection. PloS One. 2016;11 doi: 10.1371/journal.pone.0158431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taneja S., Duseja A., De A. Successful treatment of chronic hepatitis C infection with directly acting antivirals in renal transplant recipients. Nephrol Carlton Vic. 2018;23:876–882. doi: 10.1111/nep.13109. [DOI] [PubMed] [Google Scholar]
- 88.Goel A., Bhadauria D.S., Kaul A. Experience with direct acting anti-viral agents for treating hepatitis C virus infection in renal transplant recipients. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 2017;36:137–140. doi: 10.1007/s12664-017-0745-5. [DOI] [PubMed] [Google Scholar]
- 89.Diehl A.M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 90.Mantovani A., Zaza G., Byrne C.D. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism. 2018;79:64–76. doi: 10.1016/j.metabol.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Yasui K., Sumida Y., Mori Y. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism. 2011;60:735–739. doi: 10.1016/j.metabol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 92.Alizadeh S., Ahmadi M., Ghorbani Nejad B., Djazayeri A., Shab-Bidar S. Metabolic syndrome and its components are associated with increased chronic kidney disease risk: evidence from a meta-analysis on 11 109 003 participants from 66 studies. Int J Clin Pract. 2018 doi: 10.1111/ijcp.13201. Published online May 23. [DOI] [PubMed] [Google Scholar]
- 93.Samson S.L., Garber A.J. Metabolic syndrome. Endocrinol Metab Clin N Am. 2014;43:1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 94.Misra A., Chowbey P., Makkar B.M. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Phys India. 2009;57:163–170. [PubMed] [Google Scholar]
- 95.Kashi M.R., Torres D.M., Harrison S.A. Current and emerging therapies in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:396–406. doi: 10.1055/s-0028-1091984. [DOI] [PubMed] [Google Scholar]
- 96.Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatol Baltim Md. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 97.Badve S.V., Roberts M.A., Hawley C.M. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 98.Kam P.C.A., Williams S., Yoong FFY Vasopressin and terlipressin: pharmacology and its clinical relevance. Anaesthesia. 2004;59:993–1001. doi: 10.1111/j.1365-2044.2004.03877.x. [DOI] [PubMed] [Google Scholar]
- 99.Gøtzsche P.C., Hróbjartsson A. Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database Syst Rev. 2005;1:CD000193. doi: 10.1002/14651858.CD000193.pub2. [DOI] [PubMed] [Google Scholar]
- 100.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 101.Runyon B.A., Montano A.A., Akriviadis E.A., Antillon M.R., Irving M.A., McHutchison J.G. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215–220. doi: 10.7326/0003-4819-117-3-215. [DOI] [PubMed] [Google Scholar]
- 102.Cheng K.-C., Liao K.-F., Lin C.-L., Liu C.-S., Lai S.-W. Chronic kidney disease correlates with increased risk of pulmonary tuberculosis before initiating renal replacement therapy: a cohort study in Taiwan. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000012550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moran E., Baharani J., Dedicoat M. Risk factors associated with the development of active tuberculosis among patients with advanced chronic kidney disease. J Infect. 2018;77:291–295. doi: 10.1016/j.jinf.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Myall K., Milburn H.J. An update on the management of latent tuberculosis infection and active disease in patients with chronic kidney disease. Pol Arch Intern Med. 2017;127:681–686. doi: 10.20452/pamw.4093. [DOI] [PubMed] [Google Scholar]
- 105.Dhiman R.K., Saraswat V.A., Rajekar H., Reddy C., Chawla Y.K. A guide to the management of tuberculosis in patients with chronic liver disease. J Clin Exp Hepatol. 2012;2:260–270. doi: 10.1016/j.jceh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prakash J., Mahapatra A.K., Ghosh B., Arora P., Jain A.K. Clinical spectrum of renal disorders in patients with cirrhosis of liver. Ren Fail. 2011;33:40–46. doi: 10.3109/0886022X.2010.541582. [DOI] [PubMed] [Google Scholar]
- 107.Bucsics T., Krones E. Renal dysfunction in cirrhosis: acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep. 2017;5:127–137. doi: 10.1093/gastro/gox009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hartleb M., Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol. 2012;18:3035–3049. doi: 10.3748/wjg.v18.i24.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Russ K.B., Stevens T.M., Singal A.K. Acute kidney injury in patients with cirrhosis. J Clin Transl Hepatol. 2015;3:195–204. doi: 10.14218/JCTH.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Belcher J.M., Garcia-Tsao G., Sanyal A.J. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatol Baltim Md. 2013;57:753–762. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piano S., Rosi S., Maresio G. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 112.Fagundes C., Barreto R., Guevara M. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 113.Tsien C.D., Rabie R., Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131–137. doi: 10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- 114.de Carvalho J.R., Villela-Nogueira C.A., Luiz R.R. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol. 2012;46:e21–e26. doi: 10.1097/MCG.0b013e31822e8e12. [DOI] [PubMed] [Google Scholar]
- 115.Wong F., O'Leary J.G., Reddy K.R. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145:1280–1288. doi: 10.1053/j.gastro.2013.08.051. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Altamirano J., Fagundes C., Dominguez M. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10:65–71. doi: 10.1016/j.cgh.2011.09.011. e3. [DOI] [PubMed] [Google Scholar]
- 117.Angeli P., Rodríguez E., Piano S. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64:1616–1622. doi: 10.1136/gutjnl-2014-307526. [DOI] [PubMed] [Google Scholar]
- 118.Angeli P., Ginès P., Wong F. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968–974. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 119.Sarin S.K., Kumar A., Almeida J.A. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sarin S.K., Kedarisetty C.K., Abbas Z. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 121.Moreau R., Jalan R., Gines P. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1-9. [DOI] [PubMed] [Google Scholar]
- 122.Davenport A., Sheikh M.F., Lamb E., Agarwal B., Jalan R. Acute kidney injury in acute-on-chronic liver failure: where does hepatorenal syndrome fit? Kidney Int. 2017;92:1058–1070. doi: 10.1016/j.kint.2017.04.048. [DOI] [PubMed] [Google Scholar]
- 123.Jiang Q.-Q., Han M.-F., Ma K. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol. 2018;24:2300–2310. doi: 10.3748/wjg.v24.i21.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Angeli P., Gines P., Wong F. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 125.Thalheimer U., Burroughs A.K. To close the stable door before the horse has bolted. J Hepatol. 2014;60:678–679. doi: 10.1016/j.jhep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 126.Wong F., O'Leary J.G., Reddy K.R. A cut-off serum creatinine value of 1.5 mg/dl for AKI--to be or not to be. J Hepatol. 2015;62:741–743. doi: 10.1016/j.jhep.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 127.Maiwall R., Kumar G., Bharadwaj A. AKI persistence at 48 h predicts mortality in patients with acute on chronic liver failure. Hepatol Int. 2017;11:529–539. doi: 10.1007/s12072-017-9822-1. [DOI] [PubMed] [Google Scholar]
- 128.Sanyal A.J., Boyer T., Garcia-Tsao G. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martín-Llahí M., Pépin M.-N., Guevara M. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 130.Neri S., Pulvirenti D., Malaguarnera M. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830–835. doi: 10.1007/s10620-007-9919-9. [DOI] [PubMed] [Google Scholar]
- 131.Sanyal A.J., Boyer T.D., Frederick R.T. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther. 2017;45:1390–1402. doi: 10.1111/apt.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sagi S.V., Mittal S., Kasturi K.S., Sood G.K. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25:880–885. doi: 10.1111/j.1440-1746.2009.06132.x. [DOI] [PubMed] [Google Scholar]
- 133.Fabrizi F., Dixit V., Messa P., Martin P. Terlipressin for hepatorenal syndrome: a meta-analysis of randomized trials. Int J Artif Organs. 2009;32:133–140. [PubMed] [Google Scholar]
- 134.Gluud L.L., Christensen K., Christensen E., Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatol Baltim Md. 2010;51:576–584. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 135.Alessandria C., Ottobrelli A., Debernardi-Venon W. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 136.Sharma P., Kumar A., Shrama B.C., Sarin S.K. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689–1697. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 137.Nassar Junior A.P., Farias A.Q., D’ Albuquerque L.A.C., Carrilho F.J., Malbouisson L.M.S. Terlipressin versus norepinephrine in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. PloS One. 2014;9 doi: 10.1371/journal.pone.0107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saif R.U., Dar H.A., Sofi S.M., Andrabi M.S., Javid G., Zargar S.A. Noradrenaline versus terlipressin in the management of type 1 hepatorenal syndrome: a randomized controlled study. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 2018;37:424–429. doi: 10.1007/s12664-018-0876-3. [DOI] [PubMed] [Google Scholar]
- 139.Arora V., Maiwall R., Vijayaraghavan R. Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure. Hepatol Baltim Md. 2018 doi: 10.1002/hep.30208. Published online August 3. [DOI] [PubMed] [Google Scholar]
- 140.Cavallin M., Kamath P.S., Merli M. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatol Baltim Md. 2015;62:567–574. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 141.Srivastava S., null Shalimar, Vishnubhatla S. Randomized controlled trial comparing the efficacy of terlipressin and albumin with a combination of concurrent dopamine, furosemide, and albumin in hepatorenal syndrome. J Clin Exp Hepatol. 2015;5:276–285. doi: 10.1016/j.jceh.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cavallin M., Piano S., Romano A. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatol Baltim Md. 2016;63:983–992. doi: 10.1002/hep.28396. [DOI] [PubMed] [Google Scholar]
- 143.Boyer T.D., Sanyal A.J., Garcia-Tsao G. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315–321. doi: 10.1016/j.jhep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Piano S., Schmidt H.H., Ariza X. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2018;16:1792–1800. doi: 10.1016/j.cgh.2018.01.035. e3. [DOI] [PubMed] [Google Scholar]
- 145.Rodríguez E., Elia C., Solà E. Terlipressin and albumin for type-1 hepatorenal syndrome associated with sepsis. J Hepatol. 2014;60:955–961. doi: 10.1016/j.jhep.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 146.Barreto R., Fagundes C., Guevara M. Type-1 hepatorenal syndrome associated with infections in cirrhosis: natural history, outcome of kidney function, and survival. Hepatol Baltim Md. 2014;59:1505–1513. doi: 10.1002/hep.26687. [DOI] [PubMed] [Google Scholar]
- 147.Francoz C., Durand F. Type-1 hepatorenal syndrome in patients with cirrhosis and infection vs. sepsis-induced acute kidney injury: what matters? J Hepatol. 2014;60:907–909. doi: 10.1016/j.jhep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 148.Shah N., Mohamed F.E., Jover-Cobos M. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int Off J Int Assoc Study Liver. 2013;33:398–409. doi: 10.1111/liv.12047. [DOI] [PubMed] [Google Scholar]
- 149.Shah N., Dhar D., El Zahraa Mohammed F. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047–1053. doi: 10.1016/j.jhep.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 150.Wong B.T., Chan M.J., Glassford N.J. Mean arterial pressure and mean perfusion pressure deficit in septic acute kidney injury. J Crit Care. 2015;30:975–981. doi: 10.1016/j.jcrc.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 151.Angeli P., Morando F., Cavallin M., Piano S. Hepatorenal syndrome. Contrib Nephrol. 2011;174:46–55. doi: 10.1159/000329235. [DOI] [PubMed] [Google Scholar]
- 152.Simpson N., Cho Y.W., Cicciarelli J.C., Selby R.R., Fong T.-L. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS Database. Transplantation. 2006;82:1298–1303. doi: 10.1097/01.tp.0000241104.58576.e6. [DOI] [PubMed] [Google Scholar]
- 153.Ikegami T., Shirabe K., Soejima Y. The impact of renal replacement therapy before or after living donor liver transplantation. Clin Transplant. 2012;26:143–148. doi: 10.1111/j.1399-0012.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 154.Xing T., Zhong L., Chen D., Peng Z. Experience of combined liver-kidney transplantation for acute-on-chronic liver failure patients with renal dysfunction. Transplant Proc. 2013;45:2307–2313. doi: 10.1016/j.transproceed.2013.02.127. [DOI] [PubMed] [Google Scholar]
- 155.Northup P.G., Argo C.K., Bakhru M.R., Schmitt T.M., Berg C.L., Rosner M.H. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2010;16:440–446. doi: 10.1002/lt.22008. [DOI] [PubMed] [Google Scholar]
- 156.Lenhart A., Hussain S., Salgia R. Chances of renal recovery or liver transplantation after hospitalization for alcoholic liver disease requiring dialysis. Dig Dis Sci. 2018;63:2800–2809. doi: 10.1007/s10620-018-5170-9. [DOI] [PubMed] [Google Scholar]
- 157.Kribben A., Gerken G., Haag S. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782–789. doi: 10.1053/j.gastro.2011.12.056. e3. [DOI] [PubMed] [Google Scholar]
- 158.Bañares R., Nevens F., Larsen F.S. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatol Baltim Md. 2013;57:1153–1162. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 159.Wong F., Raina N., Richardson R. Molecular adsorbent recirculating system is ineffective in the management of type 1 hepatorenal syndrome in patients with cirrhosis with ascites who have failed vasoconstrictor treatment. Gut. 2010;59:381–386. doi: 10.1136/gut.2008.174615. [DOI] [PubMed] [Google Scholar]
- 160.Harjai K.J., Raizada A., Shenoy C. A comparison of contemporary definitions of contrast nephropathy in patients undergoing percutaneous coronary intervention and a proposal for a novel nephropathy grading system. Am J Cardiol. 2008;101:812–819. doi: 10.1016/j.amjcard.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 161.Thomsen H.S., Morcos S.K. Contrast media and the kidney: European Society of Urogenital Radiology (ESUR) guidelines. Br J Radiol. 2003;76:513–518. doi: 10.1259/bjr/26964464. [DOI] [PubMed] [Google Scholar]
- 162.Ribichini F., Graziani M., Gambaro G. Early creatinine shifts predict contrast-induced nephropathy and persistent renal damage after angiography. Am J Med. 2010;123:755–763. doi: 10.1016/j.amjmed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 163.Bruce R.J., Djamali A., Shinki K., Michel S.J., Fine J.P., Pozniak M.A. Background fluctuation of kidney function versus contrast-induced nephrotoxicity. AJR Am J Roentgenol. 2009;192:711–718. doi: 10.2214/AJR.08.1413. [DOI] [PubMed] [Google Scholar]
- 164.McDonald J.S., McDonald R.J., Carter R.E., Katzberg R.W., Kallmes D.F., Williamson E.E. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271:65–73. doi: 10.1148/radiol.13130775. [DOI] [PubMed] [Google Scholar]
- 165.Polena S., Yang S., Alam R. Nephropathy in critically Ill patients without preexisting renal disease. Proc West Pharmacol Soc. 2005;48:134–135. [PubMed] [Google Scholar]
- 166.Lodhia N., Kader M., Mayes T., Mantry P., Maliakkal B. Risk of contrast-induced nephropathy in hospitalized patients with cirrhosis. World J Gastroenterol. 2009;15:1459–1464. doi: 10.3748/wjg.15.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen M.L., Lesko L., Williams R.L. Measures of exposure versus measures of rate and extent of absorption. Clin Pharmacokinet. 2001;40:565–572. doi: 10.2165/00003088-200140080-00001. [DOI] [PubMed] [Google Scholar]
- 168.Nyman U., Björk J., Aspelin P., Marenzi G. Contrast medium dose-to-GFR ratio: a measure of systemic exposure to predict contrast-induced nephropathy after percutaneous coronary intervention. Acta Radiol Stockh Swed 1987. 2008;49:658–667. doi: 10.1080/02841850802050762. [DOI] [PubMed] [Google Scholar]
- 169.Goldfarb S., Spinler S., Berns J.S., Rudnick M.R. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(suppl 5):S7–S10. doi: 10.1097/00004424-199311001-00003. discussion S11-12. [DOI] [PubMed] [Google Scholar]
- 170.Barrett B.J., Carlisle E.J. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188:171–178. doi: 10.1148/radiology.188.1.8511292. [DOI] [PubMed] [Google Scholar]
- 171.Singal A.K., Ong S., Satpathy S., Kamath P.S., Wiesner R.H. Simultaneous liver kidney transplantation. Transpl Int Off J Eur Soc Organ Transplant. Published online December. 2018;10 doi: 10.1111/tri.13388. [DOI] [PubMed] [Google Scholar]
- 172.Nadim M.K., Sung R.S., Davis C.L. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2012;12:2901–2908. doi: 10.1111/j.1600-6143.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 173.Sharma P., Welch K., Eikstadt R., Marrero J.A., Fontana R.J., Lok A.S. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2009;15:1142–1148. doi: 10.1002/lt.21821. [DOI] [PubMed] [Google Scholar]
- 174.Sharma P., Schaubel D.E., Guidinger M.K., Merion R.M. Effect of pretransplant serum creatinine on the survival benefit of liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2009;15:1808–1813. doi: 10.1002/lt.21951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gonwa T.A., Mai M.L., Melton L.B. Renal replacement therapy and orthotopic liver transplantation: the role of continuous veno-venous hemodialysis. Transplantation. 2001;71:1424–1428. doi: 10.1097/00007890-200105270-00012. [DOI] [PubMed] [Google Scholar]
- 176.Restuccia T., Ortega R., Guevara M. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140–146. doi: 10.1016/j.jhep.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 177.Moreau R., Durand F., Poynard T. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923–930. doi: 10.1053/gast.2002.32364. [DOI] [PubMed] [Google Scholar]
- 178.Nadim M.K., Genyk Y.S., Tokin C. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2012;18:539–548. doi: 10.1002/lt.23384. [DOI] [PubMed] [Google Scholar]
- 179.Barreto R., Elia C., Solà E. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol. 2014;61:35–42. doi: 10.1016/j.jhep.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 180.Niemann C.U., Walia A., Waldman J. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2009;15:1852–1860. doi: 10.1002/lt.21938. [DOI] [PubMed] [Google Scholar]
- 181.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomarkers Med. 2010;4:265–280. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eason J.D., Gonwa T.A., Davis C.L., Sung R.S., Gerber D., Bloom R.D. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK) Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2008;8:2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 183.Hilmi I.A., Damian D., Al-Khafaji A. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth. 2015;114:919–926. doi: 10.1093/bja/aeu556. [DOI] [PubMed] [Google Scholar]
- 184.Karapanagiotou A., Kydona C., Dimitriadis C. Acute kidney injury after orthotopic liver transplantation. Transplant Proc. 2012;44:2727–2729. doi: 10.1016/j.transproceed.2012.09.096. [DOI] [PubMed] [Google Scholar]
- 185.Dhiman R.K., Jain S., Maheshwari U. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-Stage Liver Disease (MELD) and King's College Hospital criteria. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2007;13:814–821. doi: 10.1002/lt.21050. [DOI] [PubMed] [Google Scholar]
- 186.Tujios S.R., Hynan L.S., Vazquez M.A. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015;13:352–359. doi: 10.1016/j.cgh.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Urrunaga N.H., Magder L.S., Weir M.R., Rockey D.C., Mindikoglu A.L. Prevalence, severity, and impact of renal dysfunction in acute liver failure on the US liver transplant waiting list. Dig Dis Sci. 2016;61:309–316. doi: 10.1007/s10620-015-3870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barshes N.R., Lee T.C., Balkrishnan R., Karpen S.J., Carter B.A., Goss J.A. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation. 2006;81:195–201. doi: 10.1097/01.tp.0000188149.90975.63. [DOI] [PubMed] [Google Scholar]
- 189.Leithead J.A., Ferguson J.W., Bates C.M., Davidson J.S., Simpson K.J., Hayes P.C. Chronic kidney disease after liver transplantation for acute liver failure is not associated with perioperative renal dysfunction. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2011;11:1905–1915. doi: 10.1111/j.1600-6143.2011.03649.x. [DOI] [PubMed] [Google Scholar]
- 190.Davenport A., Will E.J., Davidson A.M. Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med. 1993;21:328–338. doi: 10.1097/00003246-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 191.Deep A., Stewart C.E., Dhawan A., Douiri A. Effect of continuous renal replacement therapy on outcome in pediatric acute liver failure. Crit Care Med. 2016;44:1910–1919. doi: 10.1097/CCM.0000000000001826. [DOI] [PubMed] [Google Scholar]
- 192.Cardoso F.S., Gottfried M., Tujios S., Olson J.C., Karvellas C.J. US Acute Liver Failure Study Group. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatol Baltim Md. 2017 doi: 10.1002/hep.29488. Published online August 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Knight S.R., Oniscu G.C., Devey L., Simpson K.J., Wigmore S.J., Harrison E.M. Use of renal replacement therapy may influence graft outcomes following liver transplantation for acute liver failure: a propensity-score matched population-based retrospective cohort study. PloS One. 2016;11 doi: 10.1371/journal.pone.0148782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim T.-S., Kim J.M., Kwon C.H.D., Kim S.J., Joh J.-W., Lee S.-K. Prognostic factors predicting poor outcome in living-donor liver transplantation for fulminant hepatic failure. Transplant Proc. 2017;49:1118–1122. doi: 10.1016/j.transproceed.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 195.Jin Y.-J., Lim Y.-S., Han S., Lee H.C., Hwang S., Lee S.G. Predicting survival after living and deceased donor liver transplantation in adult patients with acute liver failure. J Gastroenterol. 2012;47:1115–1124. doi: 10.1007/s00535-012-0570-7. [DOI] [PubMed] [Google Scholar]
- 196.O'Riordan A., Dutt N., Cairns H. Renal biopsy in liver transplant recipients. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2009;24:2276–2282. doi: 10.1093/ndt/gfp112. [DOI] [PubMed] [Google Scholar]
- 197.Zhang W., Fung J. Limitations of current liver transplant immunosuppressive regimens: renal considerations. Hepatobiliary Pancreat Dis Int HBPD INT. 2017;16:27–32. doi: 10.1016/s1499-3872(16)60167-4. [DOI] [PubMed] [Google Scholar]
- 198.Lin M., Mittal S., Sahebjam F., Rana A., Sood G.K. Everolimus with early withdrawal or reduced-dose calcineurin inhibitors improves renal function in liver transplant recipients: a systematic review and meta-analysis. Clin Transplant. 2017;31 doi: 10.1111/ctr.12872. [DOI] [PubMed] [Google Scholar]
- 199.Prati D., Taioli E., Zanella A. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 200.Lee J.K., Shim J.H., Lee H.C. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatol Baltim Md. 2010;51:1577–1583. doi: 10.1002/hep.23505. [DOI] [PubMed] [Google Scholar]
- 201.Ruhl C.E., Everhart J.E. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatol Baltim Md. 2012;55:447–454. doi: 10.1002/hep.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kwo P.Y., Cohen S.M., Lim J.K. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 203.Sette L.H.B.C., Almeida Lopes E.P. d.e. Liver enzymes serum levels in patients with chronic kidney disease on hemodialysis: a comprehensive review. Clin Sao Paulo Braz. 2014;69:271–278. doi: 10.6061/clinics/2014(04)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ray L., Nanda S.K., Chatterjee A., Sarangi R., Ganguly S. A comparative study of serum aminotransferases in chronic kidney disease with and without end-stage renal disease: need for new reference ranges. Int J Appl Basic Med Res. 2015;5:31–35. doi: 10.4103/2229-516X.149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Einollahi B., Ghadian A., Ghamar-Chehreh E., Alavian S.M. Non-viral related liver enzymes elevation after kidney transplantation. Hepat Mon. 2014;14 doi: 10.5812/hepatmon.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alfandary H., Davidovits M., Dagan A. Transient abnormal liver enzyme level in the early stage after renal transplantation in children. Indian J Transplant. 2018;12:90. doi: 10.4103/ijot.ijot_6_17. [DOI] [Google Scholar]
- 207.Dizdar O.S., Ersoy A., Aksoy S., Ozel Coskun B.D., Yildiz A. Analysis of liver function test abnormalities in kidney transplant recipients: 7 year experience. Pak J Med Sci. 2016;32:1330–1335. doi: 10.12669/pjms.326.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wong M.Y., McCaughan G.W., Strasser S.I. An update on the pathophysiology and management of polycystic liver disease. Expet Rev Gastroenterol Hepatol. 2017;11:569–581. doi: 10.1080/17474124.2017.1309280. [DOI] [PubMed] [Google Scholar]
- 209.Drenth J.P.H., Chrispijn M., Nagorney D.M., Kamath P.S., Torres V.E. Medical and surgical treatment options for polycystic liver disease. Hepatol Baltim Md. 2010;52:2223–2230. doi: 10.1002/hep.24036. [DOI] [PubMed] [Google Scholar]
- 210.Hogan M.C., Masyuk T.V., Page L.J. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol JASN. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Caroli A., Antiga L., Cafaro M. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol CJASN. 2010;5:783–789. doi: 10.2215/CJN.05380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.van Keimpema L., Nevens F., Vanslembrouck R. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–1668. doi: 10.1053/j.gastro.2009.07.052. e1-2. [DOI] [PubMed] [Google Scholar]
- 213.Gupta A., Quigg R.J. Glomerular diseases associated with hepatitis B and C. Adv Chron Kidney Dis. 2015;22:343–351. doi: 10.1053/j.ackd.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 214.Javaid M.M., Khatri P., Subramanian S. Should antiviral monotherapy with nucleotide analogs be the primary treatment option for focal segmental glomerulosclerosis-related nephrotic syndrome in chronic hepatitis B infection? Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2018;29:714–718. doi: 10.4103/1319-2442.235166. [DOI] [PubMed] [Google Scholar]
- 215.Kupin W.L. Viral-associated GN: hepatitis B and other viral infections. Clin J Am Soc Nephrol CJASN. 2017;12:1529–1533. doi: 10.2215/CJN.09180816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.03.021. Published online April 18. [DOI] [Google Scholar]
- 217.Fabrizi F., Martin P., Cacoub P., Messa P., Donato F.M. Treatment of hepatitis C-related kidney disease. Expet Opin Pharmacother. 2015;16:1815–1827. doi: 10.1517/14656566.2015.1066333. [DOI] [PubMed] [Google Scholar]
- 218.Betrosian A.-P., Agarwal B., Douzinas E.E. Acute renal dysfunction in liver diseases. World J Gastroenterol. 2007;13:5552–5559. doi: 10.3748/wjg.v13.i42.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Davenport A. Management of acute kidney injury in liver disease. Contrib Nephrol. 2010;165:197–205. doi: 10.1159/000313759. [DOI] [PubMed] [Google Scholar]
- 220.Cerdá J., Tolwani A., Gibney N., Tiranathanagul K. Renal replacement therapy in special settings: extracorporeal support devices in liver failure. Semin Dial. 2011;24:197–202. doi: 10.1111/j.1525-139X.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- 221.Hoste E.A.J., Dhondt A. Clinical review: use of renal replacement therapies in special groups of ICU patients. Crit Care Lond Engl. 2012;16:201. doi: 10.1186/cc10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Davenport A. Continuous renal replacement therapies in patients with liver disease. Semin Dial. 2009;22:169–172. doi: 10.1111/j.1525-139X.2008.00539.x. [DOI] [PubMed] [Google Scholar]
- 223.Lahmer T., Messer M., Rasch S. Sustained low-efficiency dialysis with regional citrate anticoagulation in medical intensive care unit patients with liver failure: a prospective study. J Crit Care. 2015;30:1096–1100. doi: 10.1016/j.jcrc.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 224.Pertica N., Cicciarella L., Carraro A. Safety and efficacy of citrate anticoagulation for continuous renal replacement therapy for acute kidney injury after liver transplantation: a single-center experience. Transplant Proc. 2017;49:674–676. doi: 10.1016/j.transproceed.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 225.O'Brien Z., Cass A., Cole L. Higher versus lower continuous renal replacement therapy intensity in critically ill patients with liver dysfunction. Blood Purif. 2018;45:36–43. doi: 10.1159/000480224. [DOI] [PubMed] [Google Scholar]
- 226.Shinozaki K., Oda S., Abe R., Tateishi Y., Yokoi T., Hirasawa H. Blood purification in fulminant hepatic failure. Contrib Nephrol. 2010;166:64–72. doi: 10.1159/000314854. [DOI] [PubMed] [Google Scholar]
- 227.Larsen F.S., Schmidt L.E., Bernsmeier C. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016;64:69–78. doi: 10.1016/j.jhep.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 228.Kamboj M., Kazory A. Expanding the boundaries of combined renal replacement therapy for non-renal indications. Blood Purif. 2018:1–4. doi: 10.1159/000493179. Published online September 18. [DOI] [PubMed] [Google Scholar]
- 229.Acestor N., Cooksey R., Newton P.N. Mapping the aetiology of non-malarial febrile illness in Southeast Asia through a systematic review--terra incognita impairing treatment policies. PloS One. 2012;7 doi: 10.1371/journal.pone.0044269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Capeding M.R., Chua M.N., Hadinegoro S.R. Dengue and other common causes of acute febrile illness in Asia: an active surveillance study in children. PLoS Neglected Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Salagre K.D., Sahay R.N., Pazare A.R., Dubey A., Marathe K.K. A study of clinical profile of patients presenting with complications of acute febrile illnesses during monsoon. J Assoc Phys India. 2017;65:37–42. [PubMed] [Google Scholar]
- 232.Kumar A., Arora A. Non-viral infective and non-infective mimics of hepatitis. In: Anand A.C., editor. Viral Hepatitis in Indian Subcontinent. 1st ed. Indian College of Physicians; Mumbai, India: 2017. pp. 91–118. [Google Scholar]
- 233.Anand A.C., Garg H.K. Approach to clinical syndrome of jaundice and encephalopathy in tropics. J Clin Exp Hepatol. 2015;5(suppl 1):S116–S130. doi: 10.1016/j.jceh.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kumar A., Arora A. Liver involvement in common febrile illnesses. Curr Med Res Pract. 2018;8:170–176. doi: 10.1016/j.cmrp.2018.08.001. [DOI] [Google Scholar]
- 235.European Association for the Study of the Liver EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 236.Flamm S.L., Yang Y.-X., Singh S., Falck-Ytter Y.T., AGA Institute Clinical Guidelines Committee American gastroenterological association institute guidelines for the diagnosis and management of acute liver failure. Gastroenterology. 2017;152:644–647. doi: 10.1053/j.gastro.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 237.Moore P.K., Hsu R.K., Liu K.D. Management of acute kidney injury: core curriculum 2018. Am J Kidney Dis Off J Natl Kidney Found. 2018;72:136–148. doi: 10.1053/j.ajkd.2017.11.021. [DOI] [PubMed] [Google Scholar]