Abstract
Dry mouth results from hypofunction of the salivary glands due to Sjögren's syndrome (SS), various medications, and radiation therapy for head and neck cancer. In severe cases of salivary gland hypofunction, sialagogues are not always effective due to the loss of salivary parenchyma. Therefore, regenerative medicine using stem cell therapy is a promising treatment for severe cases. Stem cells are classified into three groups: tissue stem cells, embryonic stem cells, and induced pluripotent stem cells. Tissue stem cells, such as hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs) and salivary stem/progenitor cells, could rescue irradiation-induced salivary gland hypofunction. Both HSCs and MSCs can rescue salivary gland hypofunction through soluble factors in a paracrine manner, while salivary stem/progenitor cells can reconstitute the damaged salivary glands. In fact, we clarified that CD133-positive cells in mouse submandibular glands showed stem cell features, which reconstituted the damaged salivary glands. Furthermore, we focused on the challenge of producing functional salivary glands that are three-dimensionally induced from mouse ES cells.
Keywords: Salivary gland hypofunction, Regenerative medicine, Stem cells, Cell therapy, Organoid
1. Introduction
Dry mouth is a common disorder, and the estimated number of people affected is increasing in Japan. Dry mouth results from hypofunction of the salivary glands due to SS, various medications, and radiation therapy for head and neck cancer (Table 1). In cases with severe symptoms, dry mouth possibly causes various oral diseases, including mucosal infections, dysphagia, and aspiration pneumonia, and leads to a declining quality of life because saliva has various important functions, such as antibacterial, mucosal protective, and digestive effects, and plays important roles in maintaining not only oral circumstances but also general health [1]. Artificial saliva and sialagogues are typically used for the treatment of dry mouth but are frequently ineffective in severe cases. In such cases, regenerative medicine is expected to be a promising method; that is, stem cells or differentiated cells derived from stem cells are supplied to restore the damaged functions.
Table 1.
Causes of dry mouth.
| Side effect |
| Medications |
| Radiation therapy to the head and neck |
| Chemotherapy |
| Nerve damage from a head or neck injury |
| Diseases |
| Sjögren's syndrome |
| IgG4-related disease |
| Diabetes mellitus |
| HIV infection |
| HCV infection/AIDS |
| Graft-versus-host disease |
| Psychogenic disorders |
| Dehydration |
| Impaired fluid intake |
| Vomiting/diarrhea |
| Physiological |
| Aging |
| Other conditions |
| Salivary gland agenesis or aplasia |
| Smoking |
| Mouth breathing |
| Decreased mastication |
2. Hypofunction of the salivary glands
Hypofunction of the salivary glands, especially in elderly people, is mainly caused by the side effects from drugs, such as anticholinergic, antidepressant, antihypertensive, and diuretic agents, which are used to treat systemic diseases, although aging is also involved in salivary gland hypofunction [1], [2]. There is no salivary gland damage in drug-induced salivary hypofunction. On the other hand, the salivary gland hypofunction due to an incurable autoimmune disease, SS or irradiation for the treatment of head and neck cancer is caused by severe damage to the salivary glands. Therefore, regenerative medicine using cell transfer is a promising method to restore salivary gland hypofunction by reconstituting the damaged salivary glands.
The saliva of dry mouth patients decreases in volume and becomes foamy and sticky. Oral symptoms in patients with dry mouth include dry mucosa, burning sensation, and difficulties in chewing, swallowing, tasting, or speaking. The dorsal surface of the tongue is frequently fissured with atrophic filiform papillae. Importantly, among elderly people, dry mouth patients suffer from considerable morbidity, including dental caries, dysphagia, mucosal infection, and aspiration pneumonia, which leads to a significant decrease in QOL [3]. The use of sialagogues, such as pilocarpine, effectively increases secretion in dry mouth patients. However, the application of a sialagogue is not always satisfactory because of its side effects, such as sweating, nausea, runny nose, and diarrhea [4]. Here, we focus on two diseases causing severe dry mouth as described below.
2.1. Irradiation for head and neck cancer
A majority of the salivary glands included in the fields of radiation are irreversibly damaged, and salivary flow decreases from 6 weeks to 3 years after the treatment [5]. The mechanisms of the irradiation-induced reduction of saliva secretion have been examined in detail using animal models. In the early stage after irradiation (until a week), there was no morphological change in irradiated salivary glands, although salivary secretion was reduced. The reduction of salivary secretion without morphological changes in the salivary glands can be explained by three mechanisms: 1) cell membrane damage through free radicals generated by irradiation, 2) abnormal signaling pathways involved in saliva secretion, such as calcium signaling, and 3) abnormal ion channels and transporters, such as Aqp5 [6]. On the other hand, in the late stage after irradiation, the volume of irradiated salivary glands is markedly reduced. Histological findings also reveal atrophy and loss of acinar cells, duct dilation, and infiltration of chronic inflammatory cells. Fibrosis and fatty degeneration increasingly progresses. Irradiation directly or indirectly damages DNA via free radicals in the nucleus and mitochondria, which result in acinar cell death, blood flow decrease by endothelial cell injury, and disordered epithelia organ regeneration mediated through parasympathetic neurons [7], [8], [9].
2.2. SS
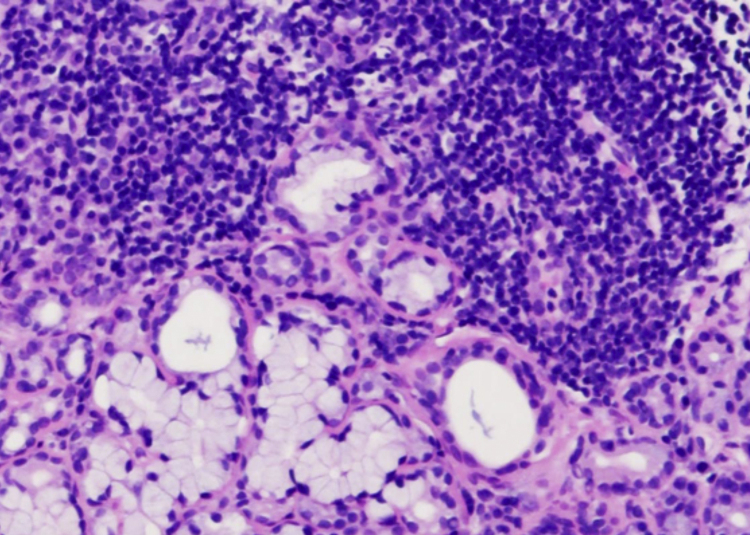
SS is a chronic autoimmune disease that results in dry mouth and dry eye (keratoconjunctivis sicca). SS is predominantly found in middle-aged adults at a female to male ratio of 9:1. SS is classified as primary or secondary. Primary SS occurs with no other autoimmune disorder, while secondary SS presents with other autoimmune disorders, including systemic lupus erythematosus. Histologically, CD4-positive T lymphocytes predominantly infiltrate the salivary gland with the destruction of acinar cells. A lymphoepithelial lesion is a characteristic finding. Lymphocyte infiltration surrounds intralobular ducts and diffusely spreads to whole salivary glands (Fig. 1). Lymphocyte infiltration also occurs in minor salivary glands, which are biopsied for the diagnosis of this disease. Secretory hypofunction is mainly caused by the loss of acinar cells. In contrast, secretory hypofunction occurs not only in the late stage with the loss of acinar cells but also in the early stage without the loss of acinar cells. In the early stages, anti-muscarinic acetylcholine receptor antibodies inhibit the translocation of Aqp5 and anti-Aqp5 antibodies inhibit the saliva secretion pathway [10], [11]. The frequency of anti-muscarinic acetylcholine receptor antibodies and anti-Aqp5 antibodies in patients with Sjogren syndrome is ranged from 9 to 90% and 73.2%, respectively.
Fig. 1.
Lip biopsy specimen of minor salivary glands in SS patient. Lymphocyte infiltration surrounds intralobular ducts and diffusely spreads to whole salivary glands. Scale bar: 100 μm.
2.3. IgG4-related disease
The igG4-related disease is a newly described fibroinflammatory disorder and characterized by lymphoplasmacytic infiltration and fibrosis in multiple organs accompanied by high serum IgG4 levels [12], [13]. Mikulicz's disease, which was likely associated with SS, is now classified as IgG4-related disease. Masaki et al. has compared IgG4-related disease including Mikulicz's disease with SS [14]. They raised several points:(1)IgG4-related disease occurs in both men and women whereas SS occurs mainly in women; (2)The patients with IgG4-related disease show significant enlargement of the lacrimal and salivary glands but relatively mild xerostomia and xerophthalmia; (3)Complications such as autoimmune pancreatitis frequently occurs in IgG4-related disease; (4)The level of serum IgG4 and IgG4 + plasma cell infiltration in tissues increased in patients with IgG4-related disease compared with SS patients; (5)The patients with IgG4-related disease show a better response to glucocorticoid therapy compared with SS patients. Besides, IgG4-related disease is known to predominantly occur in middle-aged and older people who are a little younger than SS patients [15].
Histologically, IgG4-related sialadenitis is usually found in the submandibular gland, but not in parotid and minor salivary glands. There is chronic sclerosing sialadenitis consisting of a heavy lymphoplasmacytic infiltrate including IgG4+ plasmacytes, hyperplastic lymphoid follicles, and acinar atrophy.
3. Application of stem cells for regenerative medicine
Cell transfer therapy with stem cells is expected to be the only method for the compensation of lost parenchymal cells in damaged organs. Stem cells have been used as sources of cell therapy because these cells have common functions of self-renewal and multipotency and are capable of generating the daughter cells constituting an organ for a lifetime. Stem cells are classified into three types: tissue stem cells, embryonic stem (ES) cells, and induced pluripotent stem (iPS) cells.
3.1. Tissue stem cells
Tissue stem cells are organ-specific cells that reside in a tissue and have the potential to compensate for lost cells by turnover with new cells to maintain tissue homeostasis. In addition, tissue stem cells can proliferate and supply new cells when the tissue is injured. Tissue stem cells are located at a niche, which is necessary to maintain stemness. Stem cells divide asymmetrically to give rise to two daughter cells: one cell is the original stem cell, and the other cell is a daughter cell that can differentiate into tissue cells. In contrast, terminally differentiated cells also possess self-renewal activity and supply new cells in adult tissues. β-cells in the pancreas have the potential to self-renew [16]. Surprisingly, the acinar cells in salivary glands also have the potential to self-renew [17]. Therefore, the functions of tissue stem cells are still controversial and will need to be precisely examined in future studies.
The advantage of using tissue stem cells for cell therapy is that tissue stem cells can be individually obtained from the patient and there is no rejection of their transplantation. In addition, there are no ethical issues for transplantation.
HSCs and MSCs are representative tissue stem cells that have been well characterized. The transplantation of HSCs has been widely applied not only for patients with leukemia and hematologic disorders but also for those with myocardial infarction and autoimmune diseases [18], [19]. Friedenstein is the first to isolate MSCs as adherent fibroblast-like cells with the ability to grow rapidly in vitro from bone marrow. The International Society for Cellular Therapy (ISCT) defined the criteria of MSCs as follows: (1) the growth of cells as a population adhering to a substrate in vitro; (2) the presence of surface antigens for clusters of differentiation (CD) 73, CD90, and CD105, and the lack of CD45, CD34, CD14, CD11b, CD79a or CD19 or class II histocompatibility complex antigens; (3) the potential to differentiate into bone, cartilage, and adipose tissue [20]. Tissue stem cells reside in adipose tissue, synovial tissue, and umbilical cord tissue. In the oral region, tissue stem cells reside in dental pulp and the periodontal ligament [21]. Although the characterization of MSCs is different among organs, MSCs can be differentiated into bone, cartilage, adipose tissue, myoblast, and neuronal cells under specific conditions. Furthermore, MSCs secrete soluble factors, including cytokines, chemokines, growth factors and exosomes, which contain cytokines and growth factors, signaling lipids, mRNAs, and regulatory miRNAs [22]. Remarkably, MSCs secrete immunomodulatory proteins, such as TSG6[23]. Furtheremore, MSCs possess homing capacity toward injured tissue. Thus, MSCs have multitherapeutic functions and have already been applied to over 1,000 clinical trials for various diseases, including bone and cartilage defects, liver cirrhosis, chronic inflammatory diseases, systemic lupus erythematosus, Crohn's disease, and spinal cord injury [24].
3.2. ES cells
ES cells were initially established from the inner cell mass of a mouse blastocyst in 1981[25]. Subsequently, human ES cells were derived in 1998. ES cells have pluripotency and can be differentiated into three germ layers: ectoderm, mesoderm, and endoderm. As ES cells proliferate infinitely with a normal euploid karyotype in undifferentiated conditions, these cells are a promising cell source for regenerative medicine [26]. In Japan, the Ministry of Education, Culture, Sports, Science, and Technology established guidelines for the derivation and utilization of human ES cells in 2001. Nakatsuji and his research team at Kyoto University obtained approval to produce human ES cell lines in 2002 and have succeeded in establishing the first human ES cells in Japan. The Riken BRC cell bank and the JCRB cell bank supply human ES cells for only basic research.
Clinical trials using human ES cells have been reported since human ES cell-derived oligodendrocyte progenitor cells were transplanted into patients with acute spinal cord injury by the Geron Corporation (USA). Human ES cell-derived retinal pigment epithelium has been applied to clinical trials in patients with macular degeneration of the retina, and human ESC-derived cardiac-committed progenitors have been transplanted into the epicardium of infarcted areas [27]. Indeed, the genetic stability of human ES cells is advantageous for clinical application. However, several complications, such as clinical issues and rejection at transplantation, have remained unsolved.
3.3. iPS cells
iPS cells were first created from adult mouse tail-tip fibroblasts by Yamanaka at Kyoto University in 2006, and then human iPS cells were established from adult human fibroblasts in 2007[28], [29]. Human iPS cells have been an attractive cell source for regenerative medicine because human iPS cells possess ES cell-like proliferation ability and pluripotency and can be established from the patient's own somatic cells. iPS cells were established by introducing four reprogramming factors (Sox2, Oct3/4, c-Myc, and Klf4) into somatic cells. As the original reprogramming protocol used viral vectors and oncogenes, the risk of tumorigenesis from iPS cells has been seriously addressed in clinical use. Thereafter, nonintegrating viral (adenovirus and Sendai virus) and nonviral (protein and compound) reprogramming methods have been successfully developed [30]. Thus, iPS cells suitable for clinical use have been developed, and the technical issues have been solved. Indeed, Takahashi at RIKEN initiated a clinical trial to treat neovascular age-related macular degeneration by transplanting iPSC-derived retinal pigment epithelial sheets [31]. This author also recently presented confirmation that eye surgery using iPS cells from third-party donors can be safely performed. The iPS cells from HLA-homozygous donors at the iPS cell bank or iPS cells with artificial HLA molecules may precede the clinical application of the patient's own iPS cells because it costs too much and takes too much time to establish autologous iPS cells. Furthermore, disease-specific iPS cells may be a promising tool for disease investigation and drug discovery [32].
4. Regenerative medicine for salivary gland hypofunction
4.1. Therapeutic experiments using animal models with salivary gland hypofunction
4.1.1. Application of salivary gland stem cells
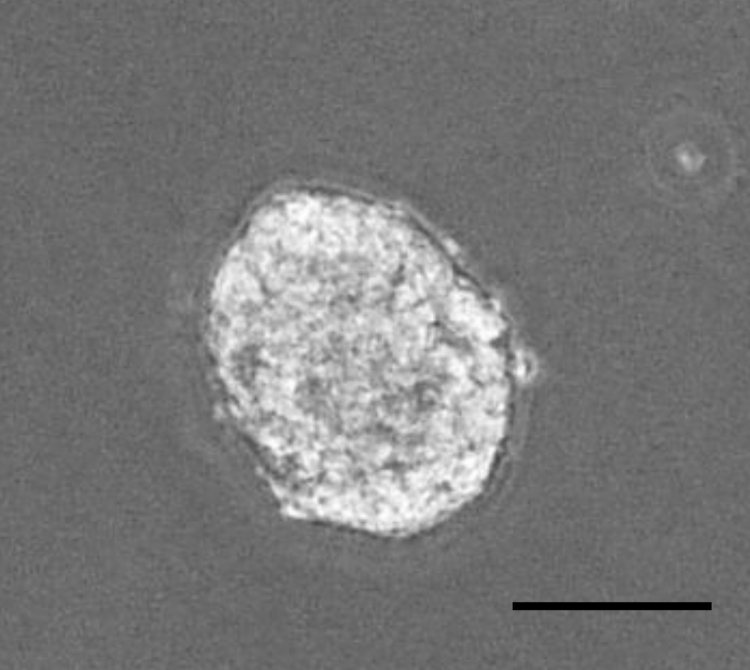
Transplantation of salivary gland stem cells has been reported to restore the function of salivary glands in irradiated mice, suggesting that cell transfer therapy using stem cells may be useful for the treatment of dry mouth patients. Nanduri et al. demonstrated that salivary gland stem cells isolated from mice formed neurosphere-like structures, referred to as salispheres (Fig. 2), in floating culture [33], [34]. Moreover, these authors reported that c-Kit-positive cells concentrated in the salisphere showed a stem cell-like ability to reconstitute salivary glands and had therapeutic potential for salivary gland hypofunction induced by irradiation. On the other hand, Hisatomi et al. reported that c-Kit + Sca-1+ cells, which appeared in salivary glands after duct ligation, showed multipotency and could be differentiated into salivary gland cells [35], [36]. Consistent with animal models, c-Kit+ cells also exist in human salivary glands, and these cells could produce salispheres and be cultured in vitro [37]. Human salivary gland stem cells express several cell surface markers in addition to c-Kit: CD44, CD29, CD49f, CD90, CD117, CD166, and ALDH [33], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. Thus, c-Kit seems to be a reliable marker to purify salivary gland stem cells, though this still remains controversial. Kwak et al. examined whether using c-KitCreERT2-based genetic labeling and lineage tracing, c-Kit-positive cells possess stem cell property, such as multipotency [47]. Long-term in vivo lineage tracing studies reveal that c-Kit marks ductal cell lineages, but not acinar cells. Therefore, they conclude that c-Kit is not a reliable marker for salivary gland stem cells. This conclusion is contradictory to previous reports which indicate that c-Kit is a marker for the adult salivary gland stem cells. However, Kwak et al. have also recently reported that c-Kit-positive cells can be differentiated ductal and acinar cells and contribute to the regeneration in injured salivary glands by ductal ligation [48]. Thus, c-Kit-positive cells can acquire multipotent stem cell-like property in the course of regeneration after injury, although the mechanisms remain unclear [49].
Fig. 2.
Salisphere formation in vitro. Salivary galnd cells were cultured in DMEM/F12 medium containing 20 ng/ml of EGF, 20 ng/ml of FGF2, N2, 10 mg/ml of insulin for 14 days. Salisphere was formed. Scale bar: 50 μm.
We recently identified CD133 as a stem cell marker of mouse salivary glands [50]. CD133+ cells are sparsely distributed in the intercalated and exocrine ducts and possess the stem cell properties, such as the ability of colony and sphere formation. Crucially, transplantation of CD133+ cells isolated from mouse submandibular glands into the submandibular gland can reconstitute gland structures, including functional acinar. Also, we clarified that the stem cell potential of CD133+ cells is mediated through Sox9.
Salivary gland stem cells might be isolated from lip biopsy specimens of the minor salivary glands using these markers, and these stem cells could be expanded using the salisphere technique. Patel et al. reported that bioengineered 3-O-HS binds to FGFR2b and stabilizes FGF10/FGFR2b complexes in a receptor and growth factor-specific manner. Therefore, 3-O-HS may be used to expand salivary gland stem cells in vitro for regenerative therapy [51].
4.1.2. Application of other stem cells
Sumita et al. reported that bone marrow–derived cells had the potential to reconstitute salivary glands. Interestingly, in his study, the transplanted bone marrow-derived cells were directly differentiated into salivary gland epithelial cells, which rescued the irradiation-induced hypofunction of mouse salivary glands [52]. Furthermore, MSCs derived from both bone marrow and adipose tissue were also reported to have therapeutic potential in animal models [53]. In SS model mice (NOD), treatment with bone marrow-derived MSCs suppressed autoimmunity and restored salivary gland secretory function [54], [55]. Consistent with this result, we reported that dental pulp stem cells isolated from mice had the same effects [56]. However, it is still controversial whether these mesenchymal cells are directly differentiated into salivary gland epithelial cells or whether their soluble factors induce residual cell regeneration. Our study demonstrated that endothelial-like cells isolated from mouse salivary glands were involved in the mitigation of irradiation-induced hypofunction through the soluble factor, clusterin [57]. Therefore, it should be clarified whether MSCs derived from mesoderm can truly be differentiated into salivary gland epithelial cells derived from ectoderm. Further study is needed to answer this question.
4.2. Current clinical applications
Salivary gland stem cells have never been applied to clinical trials, while MSCs have been applied to several clinical trials. Adipose-tissue derived stem cells (ADSCs) have been used for clinical trials of irradiation-induced salivary gland hypofunction in head and neck cancer patients [58]. A Phase I/II Study to assess the safety and activity of cell therapy using ADSCs has been started. In addition, allogenic umbilical cord-derived MSCs suppressed autoimmunity and restored salivary gland secretory function in patients with primary SS [54]. Thus, although there have been few clinical trials using stem cells for the treatment of patients with dry mouth, cell therapy using MSCs will be promoted for the treatment of these patients.
5. Future perspectives
The majority of trials to generate salivary gland epithelial cells from stem cells have focused on using two-dimensional monolayer cultures. However, three-dimensional cultures are expected to recapitulate in vivo salivary glands and have been developed with extracellular matrix, such as Matrigel [53].
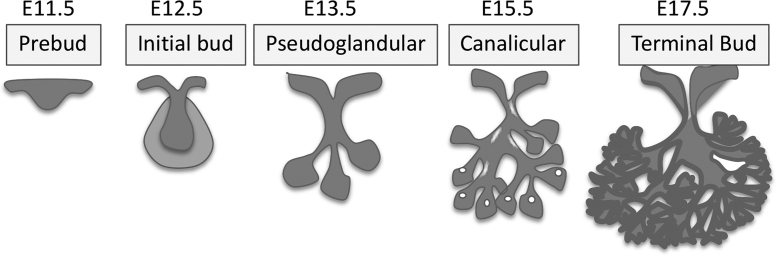
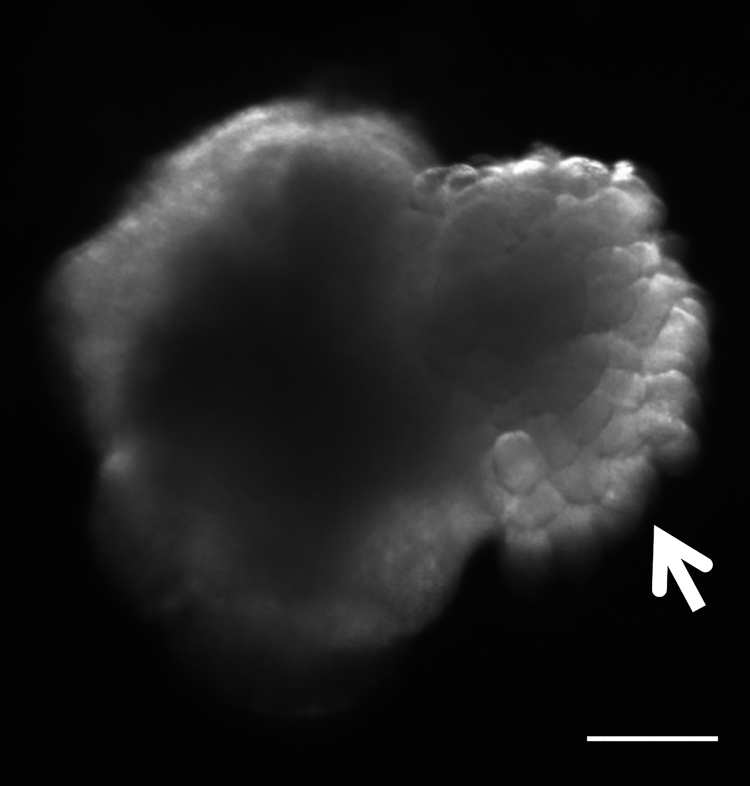
Sasai et al. at Riken was the first to obtain prepatterned 3D-like forebrain organoids by combining the SFEB system with free-floating and coated-dish culture (SFEBq) [59]. Various organoids, including pituitary, optic cup, inner ear, thyroid, intestine, liver, and kidney, have recently been generated from ES, iPS and tissue stem cells in specific three-dimensional cultures [60]. These organoids have been recently reported to retain physiological functions similar to those of the original organs. Therefore, organoids have great potential to model development and disease as a tool for drug screening and therapeutic approaches. In addition, these organoid techniques may make it possible to replace whole organs [61], [62]. To date, several studies have generated salivary gland cells from ES and iPS cells [63], [64]. However, there have been no reports about the organoids derived from these pluripotent stem cells. Hence, we tried to differentiate mouse ES cells into salivary gland organoids by recapitulating embryonic salivary gland development in vitro. The development of one of the major salivary glands, the submandibular gland (SMG), in mice begins with epithelial thickening of oral mucosa at E11.5, and then, the epithelial invaginates into the underlying mesenchyme (Fig. 3). Therefore, transcription factors related to epithelial thickening at the initial stage of salivary gland development are expected to be useful for inducing salivary gland rudiment from oral epithelium differentiated from ESCs. To identify transcription factors that can induce SMG rudiment from ES-cell derived primitive oral epithelium, we investigated the transcription factors that were strongly expressed in the SMG rudiment and oral epithelium continuing to SMG rudiment compared with distant oral epithelium. The mandibles of E12.5 mice were separated into SMG epithelium, invaginating oral epithelium connected to the SMG, and oral epithelium distant from the SMG through laser microdissection, and the gene expression profiles of these three specimen types were then compared via RNA sequencing (RNA-seq). As a result, Sox9 and Foxc1 were selected as candidate genes to enable ES-cell-derived OE to differentiate into SMG rudiment, because both of them were confirmed to be strongly expressed in the SMG rudiment and oral epithelium connected to the SMG rudiment. Thus, we identified a specific combination of two transcription factors (Sox9 and Foxc1) responsible for the differentiation of mouse embryonic stem cell-derived oral ectoderm into the salivary gland rudiment in an organoid culture system [65]. We referred salivary gland organoid generated by organoid culture system using these genes to induced salivary gland primordium (iSG) (Fig. 4). Actually, α-SMA-single-positive cells appeared in the outer layer of the branching structures in iSG. Moreover, a stem/progenitor cell marker, c-Kit; two-lumen markers, Zo-1 and CD133 were detected in branching structures. AQP5-positive signals were observed in the cytosol and basolateral membrane of the end bud-like cells. In contrast, several acinar markers, such as PSP and Mist1, were not expressed, suggesting that the structure of iSG corresponded to normal SMG development between E15 and E18 in mice. Following the orthotopic transplantation of iSG into mice whose salivary glands have been removed, the induced salivary gland rudiment not only showed a similar morphology and gene expression profile to those of the embryonic salivary gland rudiment of normal mice but also exhibited characteristics of mature salivary glands, including saliva secretion.
Fig. 3.
Submandibular gland development. The development of one of the major salivary glands, the submandibular gland (SMG), in mice begins with epithelial thickening of oral mucosa at E11.5, and then, the epithelial invaginates into the underlying mesenchyme. The epithelium branching starts at E13.5. The ducts develop lumen at E15.5 and the canalicular stage from about E15.5. Terminal buds are formed at E17.5.
Fig. 4.
Generation of the salivary gland organoid in 3D ESC cultures. Bright-field view of the aggregate showing the epithelial branching structure that resembled to salivary gland primordium (arrow head). Scale bar: 300 μm.
The current study provides evidence of the successful replacement of a functional organ through orthotopic transplantation of a self-organized organ rudiment generated from pluripotent stem cells [66]. This study will contribute to the future development of next-generation organ replacement regenerative therapy using PSCs. The generated salivary glands are expected to be a promising tool for regenerative medicine and drug discovery in patients with salivary gland hypofunction.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgment
This work was supported by JSPS KAKENHI (Grant numbers: 15H05013, 15K15678, 23592718, 20592162).
References
- 1.Guggenheimer J., Moore P.A. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134:61–69. doi: 10.14219/jada.archive.2003.0018. quiz 118-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith C.H., Boland B., Daureeawoo Y., Donaldson E., Small K., Tuomainen J. Effect of aging on stimulated salivary flow in adults. J Am Geriatr Soc. 2013;61:805–808. doi: 10.1111/jgs.12219. [DOI] [PubMed] [Google Scholar]
- 3.Sreebny L., Chambers M.S., Fleming T.J., Martin J.W., Toth B.B. Xerostomia: managing a complex condition. Interview by Phillip Bonner. Dent Today. 1997;16:66–67. 86-87. [PubMed] [Google Scholar]
- 4.Frydrych A.M., Davies G.R., Slack-Smith L.M., Heywood J. An investigation into the use of pilocarpine as a sialagogue in patients with radiation induced xerostomia. Aust Dent J. 2002;47:249–253. doi: 10.1111/j.1834-7819.2002.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 5.Eisbruch A., Ten Haken R.K., Kim H.M., Marsh L.H., Ship J.A. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 6.Coppes R.P., Meter A., Latumalea S.P., Roffel A.F., Kampinga H.H. Defects in muscarinic receptor-coupled signal transduction in isolated parotid gland cells after in vivo irradiation: evidence for a non-DNA target of radiation. Br J Cancer. 2005;92:539–546. doi: 10.1038/sj.bjc.6602365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotrim A.P., Sowers A., Mitchell J.B., Baum B.J. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007;15:2101–2106. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- 8.Lombaert I.M., Brunsting J.F., Wierenga P.K., Kampinga H.H., de Haan G., Coppes R.P. Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res. 2008;14:7741–7750. doi: 10.1158/1078-0432.CCR-08-1449. [DOI] [PubMed] [Google Scholar]
- 9.Knox S.M., Lombaert I.M., Haddox C.L., Abrams S.R., Cotrim A., Wilson A.J. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumida T., Tsuboi H., Iizuka M., Hirota T., Asashima H., Matsumoto I. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjogren's syndrome: a critical review. J Autoimmun. 2014;51:44–50. doi: 10.1016/j.jaut.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Alam J., Koh J.H., Kwok S.K., Park S.H., Park K., Choi Y. Functional epitopes for anti-aquaporin 5 antibodies in Sjogren syndrome. J Dent Res. 2017 doi: 10.1177/0022034517717965. 22034517717965. [DOI] [PubMed] [Google Scholar]
- 12.Umehara H., Okazaki K., Masaki Y., Kawano M., Yamamoto M., Saeki T. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1–14. doi: 10.1007/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto M., Takahashi H., Shinomura Y. Mechanisms and assessment of IgG4-related disease: lessons for the rheumatologist. Nat Rev Rheumatol. 2014;10:148–159. doi: 10.1038/nrrheum.2013.183. [DOI] [PubMed] [Google Scholar]
- 14.Masaki Y., Dong L., Kurose N., Kitagawa K., Morikawa Y., Yamamoto M. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68:1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 15.Tsuboi H., Honda F., Takahashi H., Ono Y., Abe S., Kondo Y. Pathogenesis of IgG4-related disease. Comparison with Sjogren's syndrome. Mod Rheumatol. 2020;30:7–16. doi: 10.1080/14397595.2019.1650694. [DOI] [PubMed] [Google Scholar]
- 16.Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 17.Aure M.H., Konieczny S.F., Ovitt C.E. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell. 2015;33:231–237. doi: 10.1016/j.devcel.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathur A., Arnold R., Assmus B., Bartunek J., Belmans A., Bonig H. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: rationale and design of the BAMI trial. Eur J Heart Fail. 2017;19:1545–1550. doi: 10.1002/ejhf.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander T., Farge D., Badoglio M., Lindsay J.O., Muraro P.A., Snowden J.A. Hematopoietic stem cell therapy for autoimmune diseases – clinical experience and mechanisms. J Autoimmun. 2018;92:35–46. doi: 10.1016/j.jaut.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Squillaro T., Peluso G., Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 22.Jiang B., Yan L., Wang X., Li E., Murphy K., Vaccaro K. Concise review: mesenchymal stem cells derived from human pluripotent cells, an unlimited and quality-controllable source for therapeutic applications. Stem Cells. 2019;37:572–581. doi: 10.1002/stem.2964. [DOI] [PubMed] [Google Scholar]
- 23.Lee R.H., Yu J.M., Foskett A.M., Peltier G., Reneau J.C., Bazhanov N. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci U S A. 2014;111:16766–16771. doi: 10.1073/pnas.1416121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrzejewska A., Lukomska B., Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019 doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman L.M., Carpenter M.K. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 27.Ilic D., Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17–25. doi: 10.1002/stem.2450. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez F., Boue S., Izpisua Belmonte J.C. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet. 2011;12:231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 31.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y., Inoue H., Wu J.C., Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombaert I.M., Brunsting J.F., Wierenga P.K., Faber H., Stokman M.A., Kok T. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pringle S., Nanduri L.S., van der Zwaag M., van Os R., Coppes R.P. Isolation of mouse salivary gland stem cells. J Vis Exp. 2011 doi: 10.3791/2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hisatomi Y., Okumura K., Nakamura K., Matsumoto S., Satoh A., Nagano K. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39:667–675. doi: 10.1002/hep.20063. [DOI] [PubMed] [Google Scholar]
- 36.Okumura K., Nakamura K., Hisatomi Y., Nagano K., Tanaka Y., Terada K. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–113. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- 37.Feng J., van der Zwaag M., Stokman M.A., van Os R., Coppes R.P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009;92:466–471. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Nanduri L.S., Maimets M., Pringle S.A., van der Zwaag M., van Os R.P., Coppes R.P. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99:367–372. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 39.Banh A., Xiao N., Cao H., Chen C.H., Kuo P., Krakow T. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin Cancer Res. 2011;17:7265–7272. doi: 10.1158/1078-0432.CCR-11-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rugel-Stahl A., Elliott M.E., Ovitt C.E. Ascl3 marks adult progenitor cells of the mouse salivary gland. Stem Cell Res. 2012;8:379–387. doi: 10.1016/j.scr.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kishi T., Takao T., Fujita K., Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340:544–552. doi: 10.1016/j.bbrc.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 42.David R., Shai E., Aframian D.J., Palmon A. Isolation and cultivation of integrin alpha(6)beta(1)-expressing salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng Part A. 2008;14:331–337. doi: 10.1089/tea.2007.0122. [DOI] [PubMed] [Google Scholar]
- 43.Neumann Y., David R., Stiubea-Cohen R., Orbach Y., Aframian D.J., Palmon A. Long-term cryopreservation model of rat salivary gland stem cells for future therapy in irradiated head and neck cancer patients. Tissue Eng Part C Methods. 2012;18:710–718. doi: 10.1089/ten.TEC.2012.0013. [DOI] [PubMed] [Google Scholar]
- 44.Sato A., Okumura K., Matsumoto S., Hattori K., Hattori S., Shinohara M. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells. 2007;9:191–205. doi: 10.1089/clo.2006.0054. [DOI] [PubMed] [Google Scholar]
- 45.Maria O.M., Maria A.M., Cai Y., Tran S.D. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis. 2012;18:162–168. doi: 10.1111/j.1601-0825.2011.01858.x. [DOI] [PubMed] [Google Scholar]
- 46.Palmon A., David R., Neumann Y., Stiubea-Cohen R., Krief G., Aframian D.J. High-efficiency immunomagnetic isolation of solid tissue-originated integrin-expressing adult stem cells. Methods. 2012;56:305–309. doi: 10.1016/j.ymeth.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Kwak M., Ninche N., Klein S., Saur D., Ghazizadeh S. c-Kit(+) cells in adult salivary glands do not function as tissue stem cells. Sci Rep. 2018;8:14193. doi: 10.1038/s41598-018-32557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ninche N., Kwak M., Ghazizadeh S. Diverse epithelial cell populations contribute to the regeneration of secretory units in injured salivary glands. Development. 2020:147. doi: 10.1242/dev.192807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocchi C., Barazzuol L., Coppes R.P. The evolving definition of salivary gland stem cells. NPJ Regen Med. 2021;6:4. doi: 10.1038/s41536-020-00115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka J., Mabuchi Y., Hata K., Yasuhara R., Takamatsu K., Kujiraoka S. Sox9 regulates the luminal stem/progenitor cell properties of salivary glands. Exp Cell Res. 2019;382:111449. doi: 10.1016/j.yexcr.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Patel V.N., Lombaert I.M., Cowherd S.N., Shworak N.W., Xu Y., Liu J. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev Cell. 2014;29:662–673. doi: 10.1016/j.devcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumita Y., Liu Y., Khalili S., Maria O.M., Xia D., Key S. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol. 2011;43:80–87. doi: 10.1016/j.biocel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lombaert I., Movahednia M.M., Adine C., Ferreira J.N. Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cells. 2017;35:97–105. doi: 10.1002/stem.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J., Wang D., Liu D., Fan Z., Zhang H., Liu O. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruan G.F., Zheng L., Huang J.S., Huang W.X., Gong B.D., Fang X.X. Effect of mesenchymal stem cells on Sjogren-like mice and the microRNA expression profiles of splenic CD4+ T cells. Exp Ther Med. 2017;13:2828–2838. doi: 10.3892/etm.2017.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamura Y., Yamada H., Sakurai T., Ide F., Inoue H., Muramatsu T. Treatment of salivary gland hypofunction by transplantation with dental pulp cells. Arch Oral Biol. 2013;58:935–942. doi: 10.1016/j.archoralbio.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Mishima K., Inoue H., Nishiyama T., Mabuchi Y., Amano Y., Ide F. Transplantation of side population cells restores the function of damaged exocrine glands through clusterin. Stem Cells. 2012;30:1925–1937. doi: 10.1002/stem.1173. [DOI] [PubMed] [Google Scholar]
- 58.Gronhoj C., Jensen D.H., Glovinski P.V., Jensen S.B., Bardow A., Oliveri R.S. First-in-man mesenchymal stem cells for radiation-induced xerostomia (MESRIX): study protocol for a randomized controlled trial. Trials. 2017;18:108. doi: 10.1186/s13063-017-1856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 61.Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12:520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 62.Ogawa M., Oshima M., Imamura A., Sekine Y., Ishida K., Yamashita K. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2498. doi: 10.1038/ncomms3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawakami M., Ishikawa H., Tachibana T., Tanaka A., Mataga I. Functional transplantation of salivary gland cells differentiated from mouse early ES cells in vitro. Hum Cell. 2013;26:80–90. doi: 10.1007/s13577-013-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ono H., Obana A., Usami Y., Sakai M., Nohara K., Egusa H. Regenerating salivary glands in the microenvironment of induced pluripotent stem cells. Biomed Res Int. 2015;2015:293570. doi: 10.1155/2015/293570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka J., Ogawa M., Hojo H., Kawashima Y., Mabuchi Y., Hata K. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat Commun. 2018;9:4216. doi: 10.1038/s41467-018-06469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikeda E., Ogawa M., Takeo M., Tsuji T. Functional ectodermal organ regeneration as the next generation of organ replacement therapy. Open Biol. 2019;9:190010. doi: 10.1098/rsob.190010. [DOI] [PMC free article] [PubMed] [Google Scholar]